Abstract
BACKGROUND:
Alloimmunization to platelet rich plasma (PRP) transfusions can cause adverse reactions such as platelet refractoriness or transplant rejection. Pathogen reduction treatment with ultraviolet light and riboflavin (UV+R) of allogeneic PRP was shown to reduce allogeneic antibody responses and confer partial antigen-specific immune tolerance to subsequent transfusions in mice. Studies have shown UV+R was effective both at rapidly killing donor white blood cells (WBCs) and reducing their ability to stimulate an allogeneic response in vitro. However, the manner in which UV+R induces WBC death and its associated role in the immune response to treated PRP is unknown.
METHODS AND MATERIALS:
This study evaluates whether UV+R causes WBC apoptosis by examining phosphatidylserine exposure on the plasma membrane, membrane asymmetry, caspase activity and chromatin condensation by flow cytometry. The immunogenicity of WBCs killed with UV+R versus apoptotic or necrotic pathways was also examined in vivo.
RESULTS:
WBCs after UV+R exhibited early apoptotic-like characteristics including phosphatidylserine exposure on the outer leaflet of the plasma membrane and loss of membrane asymmetry, but unlike canonical apoptotic cells, caspase activity and chromatin condensation were not apparent. However, in vivo studies demonstrated, unlike untreated or necrotic WBCs, both apoptotic WBCs and UV+R treated WBCs failed to prime alloantibody responses to subsequent untreated transfusions.
CONCLUSION:
Overall, the mechanism of WBC death following UV+R treatment shares some membrane characteristics of early apoptosis, but is distinct from classic apoptosis. Despite these differences, UV+R treated and apoptotic WBCs both offer some protection from alloimmunization.
Keywords: pathogen reduction, apoptosis, cell death, ultraviolet B, riboflavin
INTRODUCTION:
Alloimmunization is a common complication to platelet rich plasma (PRP) transfusion that can negatively impact subsequent transfusions and transplants.1–7 The advent of leukoreduction has significantly reduced, but not eliminated, the risk of alloimmunization.8–14 Pathogen reduction treatment (PRT) with ultraviolet (UV) B light and the photoactivator riboflavin (UV+R; Mirasol, Terumo BCT, Lakewood, CO), primarily designed to reduce the risk of transfusion-transmission related infectious agents, was shown to reduce allogeneic antibody responses in animal models.15–19 Moreover, transfusion of UV+R treated PRP conferred partial immune tolerance to subsequent exposures of untreated PRP in a white blood cell (WBC)-dependent, antigen specific manner in mice.15 In vitro studies with human peripheral blood mononuclear cells (PBMCs) showed that UV+R results in rapid cell death and down-regulation of surface adhesion molecules, which prevents allogeneic T cell responses in mixed lymphocyte reactions.20
Cell death pathways are complex and diverse, and the manner in which cells die can impact how they are perceived by the immune system, with apoptotic cells generally tolerated, and necrotic cells associated with inflammation.21 Apoptosis is a coordinated process of cell death that involves regulated morphological and biochemical cellular change.22 In live cells, anionic phosphatidylserine (PS) is distributed on the cytoplasmic surface of the cell membrane.22 One of the earliest indicators of apoptosis is the translocation of PS from the internal to the external leaflet of the plasma membrane.21,22 Committed apoptotic cells also undergo caspase activation, chromatin condensation, and loss of cell membrane asymmetry, among other cellular changes.21,22
UVB light induces apoptosis in many different mouse and human cell types by various mechanisms.23–30 Different wavelengths and doses of UV light have different effects, with lower doses generally inducing apoptosis and higher doses inducing necrosis.29 The cell death pathway induced also depends on the cell type and is related to the extent of DNA damage. This paper focuses on WBCs, as contaminating lymphocytes in transfusion products are the most potent inducers of anti-MHC alloimmunization.30–32 UVB light can induce apoptosis in lymphocytes,24,25,29,33 but as doses and wavelengths of UV light can vary considerably, it is unclear if these findings will translate to blood products treated with commercially available PRT systems. There is some evidence to suggest that the Mirasol system may induce apoptosis. Yang et al. described PS exposure in human lymphocytes treated with riboflavin and an in-house source of UVB light,34 and Asano et al. reported elevated PS exposure in rat WBCs following Mirasol treatment.19 Neither of these studies, however, evaluated indicators of apoptosis beyond PS exposure.
UV treated cells have been shown to induce tolerance in humans and animal models. Extracorporeal photochemotherapy, in which autologous blood is treated with UVA light and psoralen before reinfusion, has been shown to regulate inflammatory immune responses in autoimmune diseases and graft-versus-host disease.35–38 Multiple infusions of allogeneic PBMCs treated with UVB light (lower doses than used in UV+R), can control donor specific humoral responses, and tolerance in this model can be conferred to naïve mice by adoptive transfer of CD4+ CD25+ T cells.39,40
Treatment of PRP with UV+R reduces alloimmunization and provides WBC-dependent partial protection from subsequent exposure.15,16 As treatment induces rapid WBC death20 and apoptotic cells are suggested to have immune tolerant properties,41 we sought to determine the manner of death induced by UV+R treatment in both human and mouse WBCs. PS exposure, membrane asymmetry, caspase activity and chromatin condensation were evaluated in WBCs isolated from pathogen reduced PRP and compared with WBCs treated with known apoptosis or necrosis inducers. The immunogenicity of these cells was also compared to apoptotic and necrotic controls in vivo.
MATERIALS AND METHODS:
Participants and samples
Healthy male and female subjects were recruited to participate in the study. Small volumes of blood were collected into a final volume of 14% citrate phosphate dextrose anti-coagulant (CPDA-1) that was aliquoted from a 500-mL WBF double CPDA-1 blood bag unit (Haemonetics, Braintree, MA). Written informed consent and sample collection were in accordance with institutional review board–approved protocols per the Declaration of Helsinki.
Mice and samples
Male and female BALB/cJ (BALB/c) and C57Bl/6J (B6) mice (The Jackson Laboratory, Bar Harbor, ME) between 8 to 11 weeks of age for recipient mice and 6 weeks to 6 months of age for donor mice were housed and maintained in a specific pathogen free vivarium under barrier conditions at our institute in accordance with the Guide for the Care and Use of Laboratory Animals. Mice acclimated for a minimum of a week prior to use. Blood was collected by exsanguination via orbital enucleation under inhalation anesthesia (isoflurane). Samples were pooled into tubes with a final volume of 14% CPDA-1. Research was performed with approval and oversight of the Institutional Animal Care and Use Committee at Covance Laboratories, Inc. (San Carlos, CA) under Animal Welfare Assurance A3367–01.
Sample Processing
PRP fractions were isolated by gentle centrifugation (two 15 min spins each at 200 X g). The remaining fraction was diluted in phosphate buffered saline (PBS), centrifuged over Lymphocyte Separation Medium (Cellgro, Mediatech, Manassas, VA) to isolate WBCs and washed with sterile PBS. The WBCs were used alone or added back to a portion of the PRP to generate a WBC-enriched PRP, i.e. non-leukoreduced PRP.
UV+R treatment
Non-leukoreduced PRP, prepared as described above, underwent pathogen reduction treatment (UV+R) with the Mirasol Pathogen Reduction Technology System (Terumo BCT, Lakewood, CO). A scaled version of the Mirasol system was adapted for mice as previously described.13,15,16 Treated samples were transferred to sterile tubes and kept at room temperature for injection into mice or evaluated in vitro as described below.
Apoptosis
WBCs from non-leukoreduced PRP with and without UV+R treatment were evaluated for indicators of apoptosis. Empirically, we observed that human WBCs were more resistant to induction of PS exposure than mouse cells when treated with the same concentration of ethanol (EtOH). Therefore, to generate experimental controls, an aliquot was treated with 5% total volume ethanol (mice) or 10% total volume ethanol (human) for 90 minutes at 37°C for the induction of apoptosis.42–44 A separate aliquot was treated with a 10% total volume ethanol (mice), 20% total volume ethanol (human), or a cycle of freeze/thaw in dry ice and placed in 37°C incubation for the induction of cell necrosis.42,45 Cells were washed prior to analysis. PS exposure was measured by affinity to Annexin V conjugated to allophycocyanin (APC; Life Technologies, Carlsbad, CA). Membrane asymmetry was measured with the Ratiometric Membrane Asymmetry (VRMA) Probe 4’-N,N-diethylamino-6-(N,N,N-dodecyl-methylamino-sulfopropyl)-methyl-3-hydroxyflavone (F2N12S) (Molecular Probes, Eugene, OR). Effector caspase 3 and/or caspase 7 expressions were measured by CellEvent Caspase-3/7 Green Flow Cytometry Assay Kit (Life Technologies, Carlsbad, CA). Total caspase activity was measured by Vybrant FAM Poly Caspases Assay Kit (Molecular Probes, Eugene, OR). Chromatin condensation was measured with the cell permeable nuclear dye Vybrant DyeCycle Violet Stain (Molecular Probes, Eugene, OR). Reagents were used per manufacturer’s instructions. Cells were analyzed immediately after treatment and staining, which was between 3–5 hours post UV+R treatment, depending on the experiment.
Transfusion
For primary transfusion, leuko-poor PRP was treated with UV+R and used as a vehicle for administration of WBCs that were either untreated, UV+R treated, 5% ethanol treated, or freeze/thaw treated. Ethanol, freeze/thaw, and untreated WBCs were washed and resuspended in UV+R treated leuko-poor PRP prior to transfusion into mice. Secondary transfusions consisted of untreated non-leukoreduced PRP. PRP products were stored at room temperature prior to administration of 100 μL volume by intravenous tail vein injection within seven hours of collection for the first transfusion and 5 hours for the second transfusion. For primary transfusion, the number of WBCs was normalized to 5 × 105 cells/transfusion, and the number of WBCs in the secondary transfusion of non-leukoreduced untreated PRP ranged from 5.8 – 7.0 × 105 cells/transfusion. Total platelets (PLT) were between 1.1 × 107 to 7.4 × 107 cells/transfusion.
Alloantibody responses and flow cytometry
Alloantibody responses were measured by flow cytometry as previously described using B6 splenocytes as target cells.15,16 Median fluorescence intensity (MFI) values were normalized by dividing individual MFI by the mean MFI of the non-transfused controls for that experiment.
Anti-mouse antibodies used for flow cytometry from BD Biosciences (San Diego, CA) included phycoerythrin (PE)-CD61, biotin-conjugated anti-mouse Igκ, PE-Cy5–conjugated anti-mouse TCRβ, pacific blue (PB)-conjugated anti-mouse B220, APC-conjugated streptavidin and brilliant violet (BV) 421-CD45.2, and anti-mouse CD16/32 for blocking. Anti-human PE-CD14 antibody was from Caltag (Molecular Probes, Eugene, OR). Live/dead discrimination was determined by staining with propidium iodide (PI) or Sytox AAdvanced (Life Technologies, Carlsbad, CA), which are functionally interchangeable for the detection of live/dead cells. Absolute counts of cell populations were made with BD Trucount Tubes (BD Biosciences, San Diego, CA). Samples were run on a BD FACS LSR II (BD Biosciences, San Diego, CA) and acquired with BD FACSDiva software. Cytometry data was analyzed on FlowJo software version 10 (FlowJo, LLC).
Statistical analysis
Treatment groups were compared using one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnet’s multiple comparison post-test as indicated, assuming normal distribution unless otherwise noted. Analysis and graphing were performed using Prism version 7 (GraphPad Software, Inc., La Jolla, CA). Power analysis suggested a minimum sample size of 5 for figure 1 and a minimum sample size of 10 for figure 5 where power = 0.8 and type I error rate = 5%.
Figure 1: UV+R treatment significantly enriches PS exposure on outer plasma membrane.
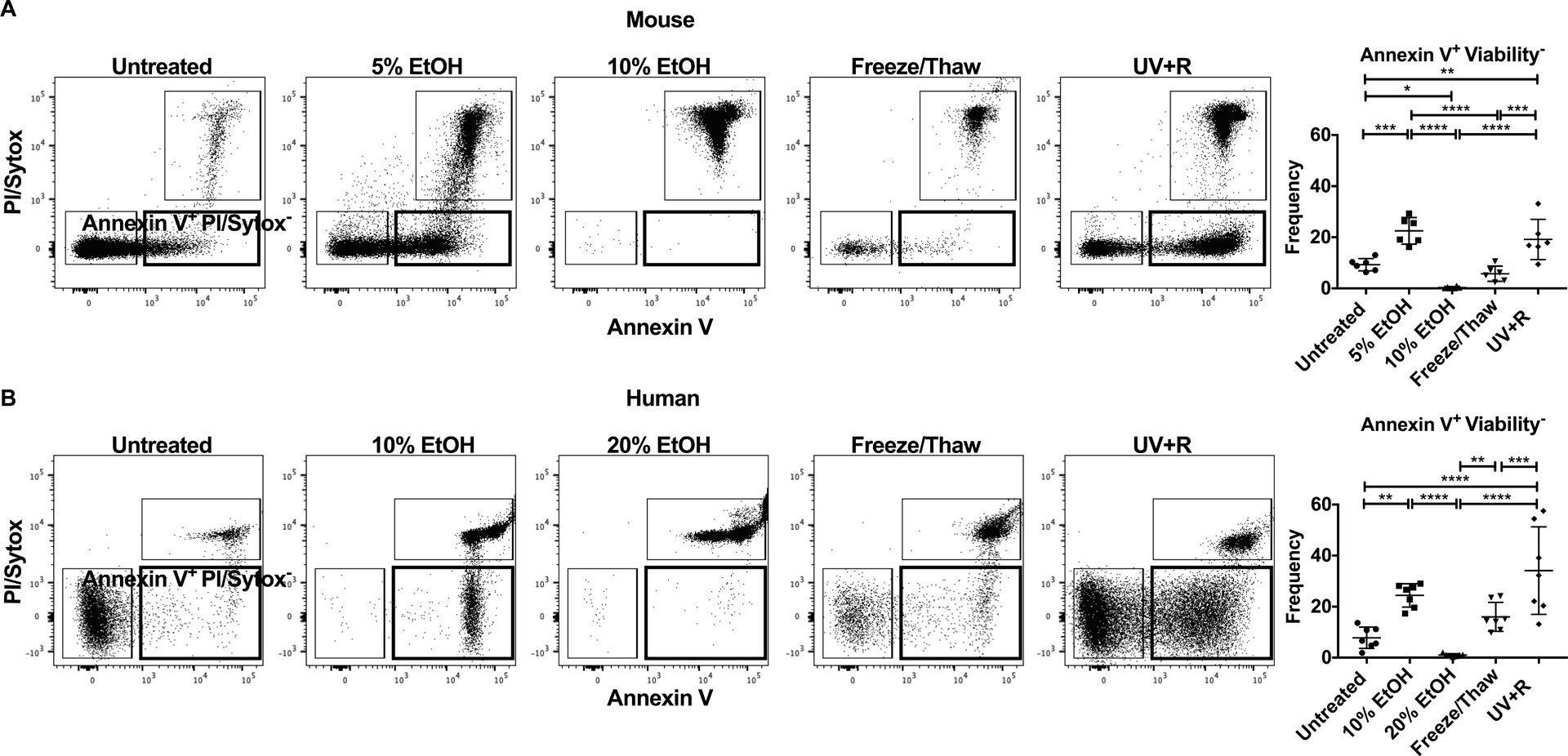
The presence of PS on the outer leaflet of the plasma membrane was measured by staining with Annexin V in WBCs derived from (A) B6 mice and (B) healthy human donors. WBCs were untreated, or treated with 5% EtOH, 10% EtOH, 20% EtOH, a freeze/thaw cycle or pathogen reduction with UV+R. Samples were analyzed within 3–5 hours of UV+R treatment. Representative gating by flow cytometry for each treatment sample selected for single lymphocytes (CD14− for human samples). Live/dead markers used were propidium iodide (PI) or Sytox AADvanced dead cell stain kit. Graphical representation of data shows the frequency of Annexin V+ PI/Sytox AADvanced− (gate in bold) (N=6 or 7). Groups were compared by ANOVA with Tukey’s post-test. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Figure 5: UV+R treated WBCs fail to prime increased alloantibody responses to secondary allo-exposure, similar to apoptotic WBCs.
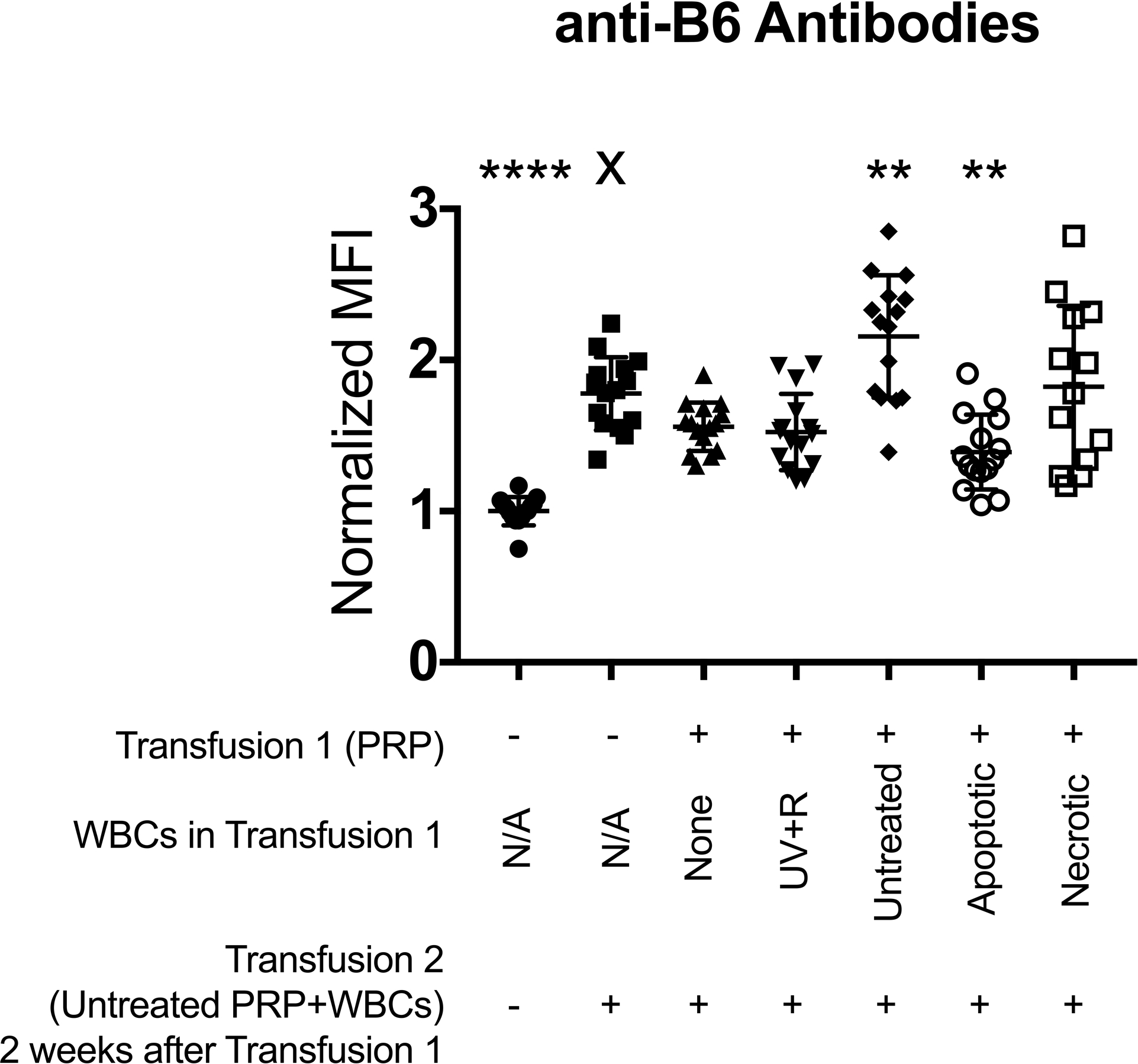
BALB/c recipient mice were 1) not transfused (negative control ●), 2) given a single transfusion with non-leukoreduced untreated B6 PRP (single transfusion control ■), or transfused with non-leukoreduced untreated B6 PRP 2 weeks after initial transfusion with either 3) leukoreduced UV+R treated PRP alone (π), 4) UV+R treated PRP containing UV+R treated WBCs (θ), 5) UV+R treated PRP containing untreated WBCs (◆), 6) UV+R treated PRP containing 5% EtOH-treated WBCs (apoptotic ○), or 7) UV+R treated PRP containing freeze/thaw-treated WBCs (necrotic □), all from allogeneic B6 donors. Two weeks after last transfusion, serum from recipients was screened for antibodies against B6 target cells by flow cytometry. Shown is combined data from three independent experiments (N=13–15 per group). Groups were compared by ANOVA with Dunnet’s post-test to compare each group to the single untreated PRP transfusion control (X). **p<0.01 and ****p<0.0001.
RESULTS:
Exposure of PS on the outer plasma membrane is an early indicator of apoptosis. To evaluate PS exposure following pathogen reduction, WBCs isolated from UV+R treated PRP were stained with Annexin V and compared with WBCs killed under conditions known to induce either apoptosis or necrosis. In WBCs from mice (Figure 1A) and healthy human donors (Figure 1B), PS exposure did not differ between UV+R and apoptosis-induced controls (5% EtOH for mice and 10% EtOH for human; apoptotic cells). Both did show significantly enhanced PS exposure when compared to untreated cells or necrotic cells (10% EtOH for mice, 20% EtOH for human, and freeze/thaw). For human samples, the apoptotic control showed an increased trend of PS exposure when compared to necrotic cells treated with a cycle of freeze/thaw but the difference was not significant. Therefore, UV+R significantly induced PS exposure similar to apoptotic mouse and human WBCs.
To evaluate whether membrane asymmetry, another indicator of apoptosis, was compromised after UV+R, the charge distribution of the outer plasma membrane was measured with the VRMA probe F2N12S. Loss of membrane asymmetry is associated with a reduced ratio between 585 nm/530 nm fluorescence from F2N12S. Mouse WBCs (Figure 2A) and healthy human donors (Figure 2B) showed more than a two fold increase in the loss of membrane asymmetry among apoptotic cells and following UV+R when compared with untreated or necrotic cells. Therefore, UV+R, like apoptotic cells, induced a greater loss of membrane asymmetry in mouse and human WBCs.
Figure 2: Loss of plasma membrane asymmetry is elevated following UV+R treatment.
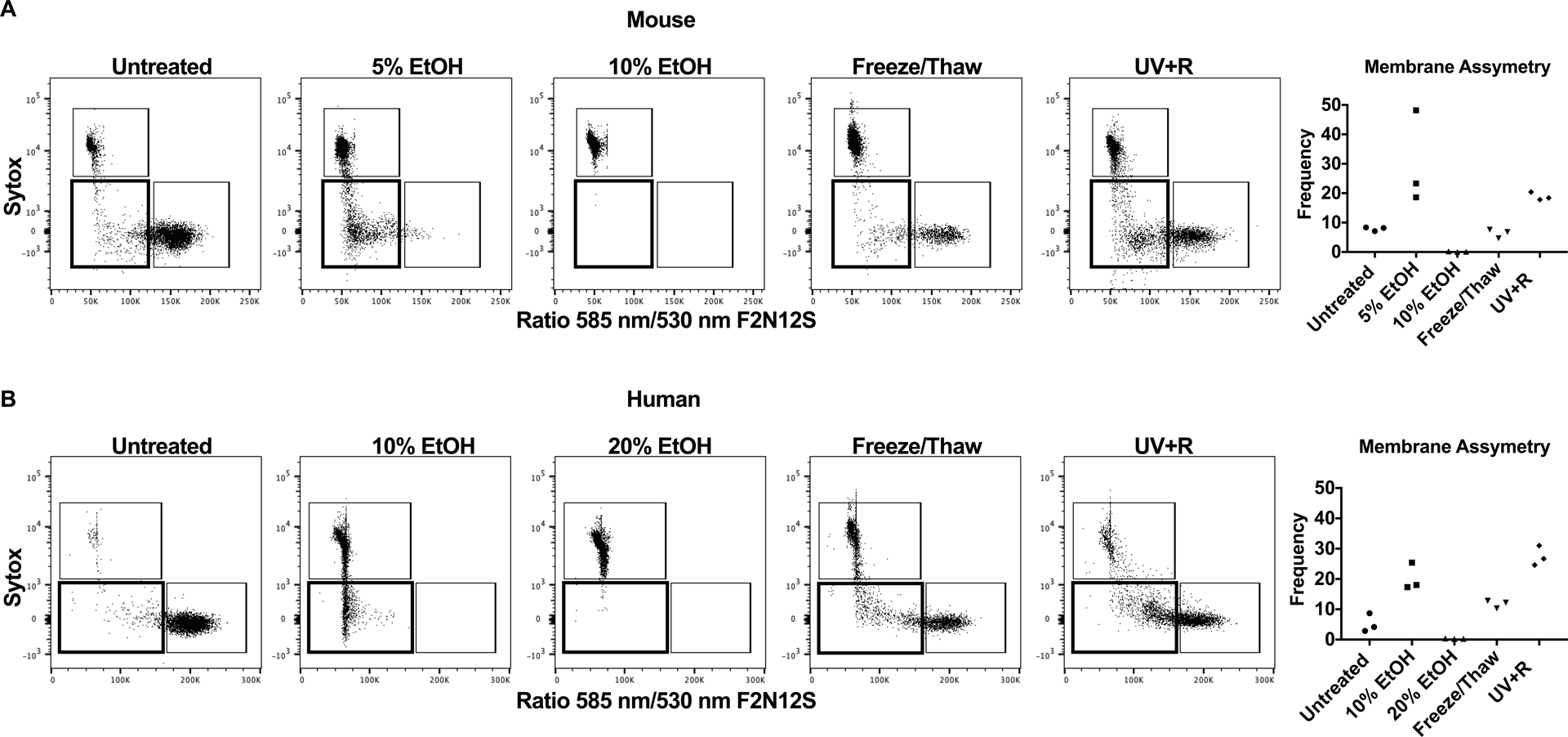
Membrane asymmetry was determined by shift in surface charge measured using the Violet Ratiometric Membrane Asymmetry Probe (F2N12S) by flow cytometry. Representative gating for WBCs that were untreated, or treated with 5% EtOH, 10% EtOH, 20% EtOH, a freeze/thaw cycle or pathogen reduction with UV+R from (A) B6 mice and (B) healthy human donors selected for single lymphocytes (CD14− for human samples). Samples were analyzed within 3 hours of UV+R treatment. Sytox AADvanced measured live/dead cells. The ratiometric response of emission bands corresponding to 530 nm and 585 nm from the dual fluorescence of the probe measured loss of membrane asymmetry. Graphical representation of data shows the frequency of F2N12S Ratio 585 nm/530 nm low Sytox AADvanced− (gate in bold) from the combined data of three independent experiments (N=3).
Since caspase activation is an established indicator of apoptosis, the effector caspases 3 and 7, as well as total caspase activity were measured.21,22 Expression of caspase was measured in the Annexin V− PI/Sytox− subset and the Annexin V+ PI/Sytox− subset as illustrated by the respective cytometry plots (Figure 3). Necrotic cells induced by ethanol treatment were not examined due to insufficient PI/Sytox− events (see Figure 1). Among WBCs from mice (Figure 3A, C) and humans (Figure 3B, D), the apoptotic cells expressed total caspase and effector caspase 3 and 7 in the Annexin V+ PI/Sytox− subset population, but not in the Annexin V− PI/Sytox− subset of cells. The human necrotic sample from a single freeze/thaw also showed caspase activity. Strikingly, little to no total caspase or effector caspase 3 and 7 activity was observed after UV+R in either mouse or human WBCs.
Figure 3: Little to no caspase activity is detected after UV+R treatment.
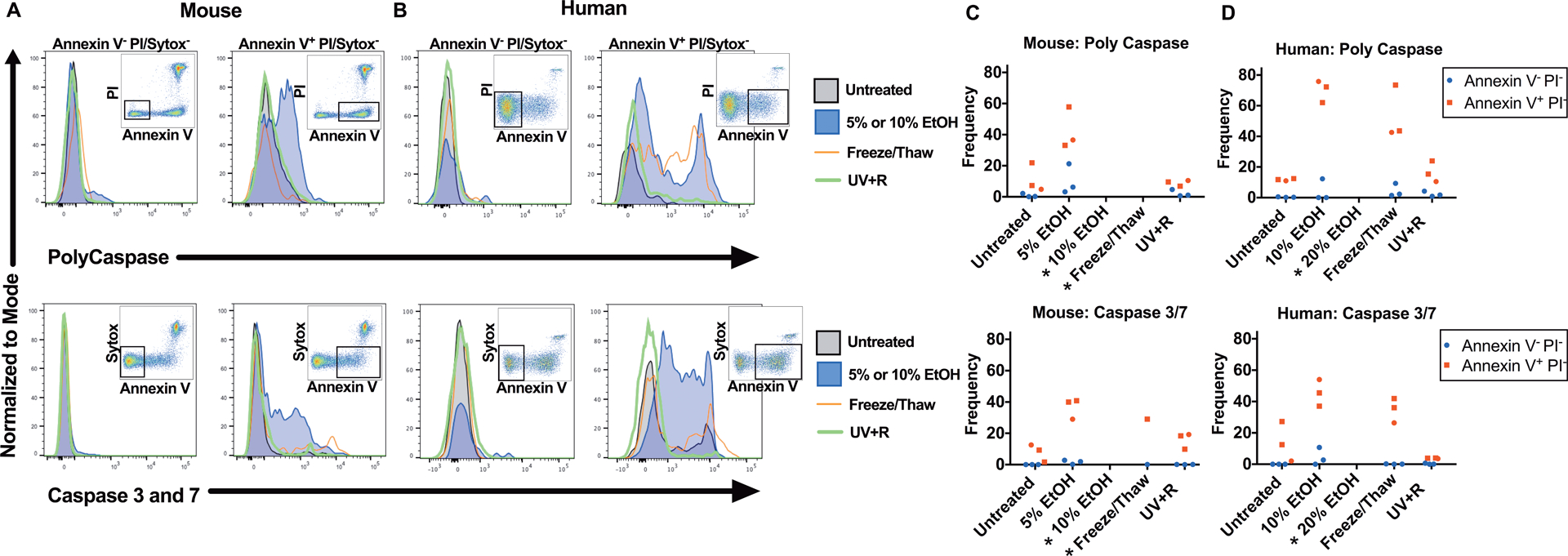
Total caspase (top panel) and effector caspase 3 and caspase 7 (bottom panel) activity were measured in WBCs from (A) B6 mice or (B) healthy human donors. WBCs were untreated, or treated with 5% EtOH, 10% EtOH, a freeze/thaw cycle or pathogen reduction with UV+R. Samples were analyzed within 4–5 hours of UV+R treatment. Gating by flow cytometry selected for single lymphocytes (CD14− for human samples) and for Annexin V− PI/Sytox AADvanced− or Annexin V+ PI/Sytox AADvanced−, respectively. Live/dead markers used were PI or Sytox AADvanced dead cell stain kit. Representative flow cytometry plots are from the UV+R treated group of each respective experiment and highlight the population selected for the histogram. Graphical representation of compiled data from three independent experiments in (C) mice and (D) humans (N=3). (*) Data below 200 events not shown.
To examine a late indicator of apoptosis, chromatin condensation was measured with the cell permeable nuclear dye Vybrant® DyeCycle™ Violet Stain which stains DNA and increases fluorescent intensity with increased proximity, associated with chromatin condensation. Samples were gated for Annexin V− PI− (black), Annexin V+ PI− (blue) and Annexin V+ PI+ (red) subsets and examined for Vybrant Dye Cycle signal intensity (Figure 4, first column). For mouse (Figure 4A) and human (Figure 4B) WBCs, the apoptotic control exhibited relatively greater intensity of Vybrant Dye Cycle signal from the Annexin V+ PI− subset compared to untreated or necrotic controls (dotted arrow). In the UV+R sample, the Annexin V+ PI− subset also exhibited moderate enhancement of the Vybrant Dye Cycle signal but not to the same degree as the apoptotic control (dashed arrow). Moreover, only a small fraction of events showed elevated Vybrant Dye Cycle signal in the human UV+R sample (dashed arrow). A moderately increased Vybrant Dye Cycle signal was observed in the Annexin V− PI− subset in the UV+R group when compared to untreated and apoptotic control (solid arrows). Therefore, UV+R does not induce chromatin condensation in mice or humans.
Figure 4: UV+R treatment may not be inducing chromatin condensation.
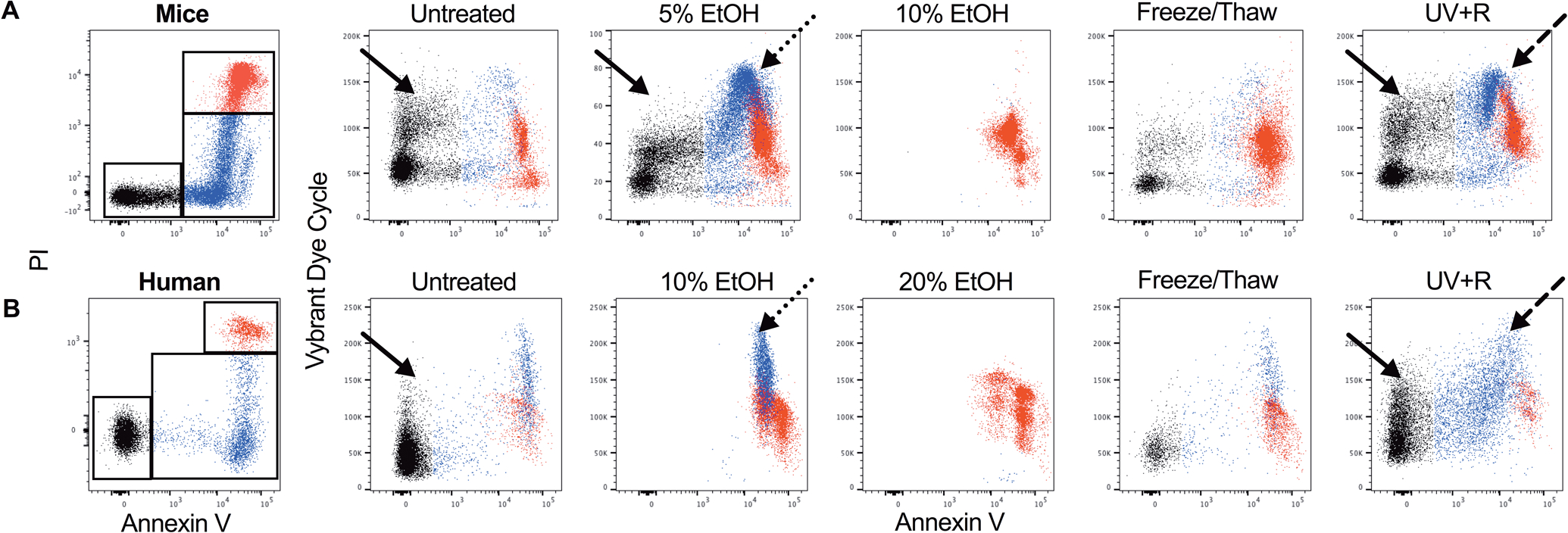
Chromatin condensation was determined by staining with the nuclear dye Vybrant Dye Cycle Violet Stain in WBCs from (A) B6 mice or (B) healthy human donors. WBCs were untreated, or treated with 5% EtOH, 10% EtOH, 20% EtOH, a freeze/thaw cycle or pathogen reduction with UV+R. Samples were analyzed within 4–5 hours of UV+R treatment. Gating selected for single nucleated lymphocytes (CD14− for human samples) that were Annexin V− PI− (black), Annexin V+ PI− (blue) and Annexin V+ PI+ (red) populations (first column). These populations were then examined for Vybrant Dye Cycle expression for each group. Solid arrows indicate chromatin condensation for the Annexin V− PI− population. Chromatin condensation for the Annexin V+ PI− population is indicated by the dotted arrow (apoptotic control) and dashed arrow (UV+R group). PI stained for live/dead cells. Plots shown are representative of three independent experiments.
UV+R treated, untreated, apoptotic, and necrotic WBCs were evaluated for the ability to prime alloantibody responses in vivo (Figure 5). BALB/c recipients were either given no transfusion or primed with a transfusion of allogeneic B6 WBCs that were either UV+R treated, untreated, 5% EtOH treated (apoptotic control) or freeze/thaw treated (necrotic control). All WBCs were suspended in UV+R treated leukoreduced B6 PRP with equal numbers of WBCs used for each treatment. An additional control group was primed with UV+R treated leukoreduced B6 PRP alone. Two weeks later, recipients received no transfusion (negative control) or were challenged with non-leukoreduced untreated B6 PRP transfusion to evaluate the primed response. Alloantibody was screened another two weeks after the final transfusion (3 independent experiments). All groups were compared to mice receiving only the second untreated B6 PRP transfusion (single non-leukoreduced untreated B6 PRP transfusion) to determine if exposure to the variously treated WBCs primed an enhanced response. Mice given untreated WBCs in the initial transfusion had significantly higher anti-B6 antibody responses than the single exposure control group, indicating a primed response. Alloantibody responses in mice given UV+R treated cells were equivalent to those of mice given only a single exposure to untreated PRP, demonstrating no primed response. Mice given apoptotic cells had a slightly reduced response compared with the single exposure group, suggesting a slight protective effect. The concentration of WBCs in the necrotic group was diminished relative to the other groups due to cell loss from the freeze/thaw and wash steps, which may have introduced variability in the results. Nonetheless, the primed responses of the necrotic groups showed an increased trend when compared to the response to a single allogeneic transfusion.
DISCUSSION:
Prior in vitro studies showed UV+R treated human PBMCs underwent rapid cell death, but the manner in which death progressed was unclear.20 Here we show that UV+R induces apoptotic-like characteristics in both human and mouse WBCs including externalization of PS and loss of membrane asymmetry, but unlike canonical apoptosis, caspase expression and chromatin condensation were not apparent. Therefore PRT with UV+R does not induce conventional apoptosis as originally thought, but instead induces a quasi-apoptotic state in blood leukocytes.34 Despite these differences, an allogeneic transfusion of UV+R treated cells, similar to apoptotic cells, failed to prime enhanced alloantibody responses to secondary allo-exposure.
Caspase expression was not observed in WBCs following UV+R pathogen reduction treatment, suggesting these cells underwent an alternative caspase-independent apoptotic pathway. Reports using varying doses of UV exposure suggested WBCs generally undergo conventional apoptosis, i.e., caspase activation, upregulation of GADD45α, CD95 (FAS/APO-1), Bax, and/or p73, and poly-ADP ribose polymerase (parp) cleavage.24,29,34,46–48 There are, however, reports of caspase-independent apoptosis occurring in WBCs in response to other stimuli, or in other cell types in response to UV exposure, providing some precedence for our findings.22,48–50 Platelets also demonstrated caspase 9 and 3 activity after UVB exposure with riboflavin using the Mirasol system, but differences were observed five days post storage.30,51 Caspase 3 was also identified in platelets after exposure to UVA and psoralen.31 In contrast, UV+R treated WBCs showed little to no caspase activity whether it was from the effector caspases 3 and 7 or from total caspase activity. Collectively, these data suggest that in many cell types, UV exposure can induce caspase dependent mechanisms of apoptosis, but exposure to UVB light and riboflavin with the Mirasol system uniquely induces caspase independent apoptosis in WBCs. Alternatively, the absence of measurable caspase activity with UV+R treatment may suggest a non-apoptotic pathway that includes some apoptotic-like characteristics such as PS exposure and loss of membrane asymmetry. Increased PS exposure in the absence of apoptosis can be induced due to elevated cytosolic Ca2+ or depletion of ATP52–54 Also, caspase expression could be transient so caspase activity could have disappeared by the time of measurement, though all groups were measured at the same time post treatment initiation.21,22
Similarly, UV+R treated WBCs did not exhibit chromatin condensation to the same degree as the apoptotic control. The UV component of UV+R induces DNA crosslinking, which may explain the moderate increase in fluorescent activity observed. This may be similar to reports of UVB and UVC exposure in different cell lines that have been described to exhibit chromatin condensation with a “moth eaten” appearance which was dissimilar to chromatin condensation found in classic apoptotic cells.46,50 Overall, chromatin condensation after UV+R was distinct from that of the ethanol-treated apoptotic cells.
Exposure of PS on the outer membrane may be sufficiently apoptotic from the perspective of the immune system. Elevated PS on the outer plasma membrane of cells facilitates recognition and uptake of apoptotic cells by antigen presenting cells such as macrophages.55,56 This may explain why exposure to UV+R treated WBCs leads to down-modulated cytokine responses to subsequent exposures,15 as UV+R treated cells may be perceived by antigen presenting cells as apoptotic when processed, leading to some tolerizing signals to T cells during indirect presentation of alloantigens.
In clinical practice, leukoreduction reduces, but may not completely eliminate, WBCs from PRP transfusions and the residual WBCs may contribute to alloimmunization over repeat exposures.8–14 Earlier studies have shown that the presence of WBCs significantly increases the alloresponse to PRP transfusion, with optimal protection observed using a combination of leukoreduction and UV+R.2,13,16,17 Furthermore, we have found that MHC antigens from WBCs are critical for induction of the partially tolerogenic phenotype observed with UV+R treatment.15,32 Therefore, WBCs were added back into the leukoreduced PRP in our experimental model to simulate a worst-case scenario of alloimmunization. To determine the immunogenicity of WBCs in different states of cell death, PRP transfusions were enriched with WBCs killed in different ways, and their capacity to prime alloantibody responses in vivo was evaluated. Prior exposure to untreated WBCs led to primed (increased) responses to subsequent untreated transfusion, compared with controls. In contrast, a prior exposure to UV+R treated WBCs or apoptotic WBCs led to similar or reduced responses when compared to a single allogeneic transfusion. This indicates UV+R treated allogeneic WBCs are perceived similarly to apoptotic WBCs and may be more tolerated by the immune system. Overall, UV+R may be inducing caspase-independent apoptosis in WBCs that provides partial protection against primed alloantibody responses in subsequent transfusions. In contrast, UV+R appears to induce classical apoptosis in platelets including PS exposure and caspase activation, and is thought to enhance platelet immunogeneicity, suggesting that differences in the mechanism of cell death and cellular origin of alloantigens may be critical in regulation of the alloresponse.30,31,51,57 Platelets and leukocytes can also induce very different allogeneic responses, which may help to explain differences in study outcomes. UVB treated leukocytes have been shown to induce tolerance in a different mouse model, but when plasma and platelets were included with the treated leukocytes, it interfered with this tolerance.39
UV+R has been shown to abrogate binding and stimulation to allogeneic cells in in vitro studies and prevent alloimmunization in animal models.15–20,58 In the clinic, PRT provides graft-versus-host disease prophylaxis and can prevent formation of alloantibodies.59–62 However, conflicting clinical reports have suggested otherwise. A quantitative systematic review that combined clinical data from several studies of hemato-oncological patients and different PRT technologies concluded that PRT increased the risk of platelet refractoriness due to alloimmunization.63 A recent Pathogen Reduction Evaluation and Predictive Analytical Rating Score (PREPAReS) trial of hemato-oncological patients similarly indicated PRT does not prevent alloimmunization after platelet transfusion.64 However, confounding factors that could increase alloimmunization risk such as concurrent exposure to other untreated allogeneic blood products, storage prior to transfusion and use of gamma irradiation have complicated interpretation of these clinical studies.65 Most of the participants in the trials to date have received red cells in addition to their treated or untreated platelet products, introducing an alternative source of alloantigen.63–65 PRT treatment of red cells is more complicated as the hemoglobin absorbs much of the UV light energy, requiring either higher doses of UV light or alternative approaches.66 As these approaches have not yet been approved for use, all red cells given in these trials are untreated.63–65 Also, increased storage prior to transfusion of PRT treated PRP has been reported to elevate caspase-dependent apoptosis in platelets, which may have inflammatory effects.30,51,57 As our animal studies have used freshly treated products and most of the clinical trials have used products stored for a few days after treatment, this may help to explain some of the discrepancies. The PREPAReS trial included off-protocol transfusions, including RBC transfusions, and up to 7 days of storage prior to transfusion that could have increased platelet storage lesion.64 Furthermore, while PRT has been shown to be an effective replacement for gamma irradiation in preventing graft-versus-host disease59,67, some institutions practice tandem treatment with PRT and gamma irradiation, and gamma irradiation was utilized in all arms of the PREPAReS trial.68 Gamma irradiation does not induce apoptosis in leukocytes and does not enhance immunologic suppression when treated along with UVB.17,39,46 Moreover, tandem treatment with PRT and gamma irradiation could promote cell necrosis and ultimately incite more inflammatory responses. Ultimately, more studies will be needed to determine the impact of interacting variables on cell death and alloimmunization to help further reconcile differences observed between pre-clinical studies and clinical outcomes. Understanding the mechanism that regulates the response to PRT may help guide future strategies to prevent the risk of transplant rejection and platelet refractoriness.
ACKNOWLEDGMENTS:
NIH R01 HL133024 supported this work, and Terumo BCT provided materials for pathogen reduction. Special thanks to Inderdeep Singh from Vitalant Research Institute, San Francisco for donor sample collection.
Source of support:
NIH R01 HL133024 supported this work, and Terumo BCT provided materials for pathogen reduction.
Footnotes
Disclaimers:
The authors have no disclaimers.
Conflict of interest disclosures:
The authors have no conflict of interest.
REFERENCES:
- 1.Howard JE, Perkins HA. The natural history of alloimmunization to platelets. Transfusion. 1978;18(4):496–503. [DOI] [PubMed] [Google Scholar]
- 2.Jackman RP, Deng X, Bolgiano D, et al. Low-level HLA antibodies do not predict platelet transfusion failure in TRAP study participants. Blood. 2013;121(16):3261–3266; quiz 3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105(10):4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itescu S, Tung TC, Burke EM, et al. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation. 1998;98(8):786–793. [DOI] [PubMed] [Google Scholar]
- 5.Massad MG, Cook DJ, Schmitt SK, et al. Factors influencing HLA sensitization in implantable LVAD recipients. Ann Thorac Surg. 1997;64(4):1120–1125. [DOI] [PubMed] [Google Scholar]
- 6.Moazami N, Itescu S, Williams MR, et al. Platelet transfusions are associated with the development of anti-major histocompatibility complex class I antibodies in patients with left ventricular assist support. J Heart Lung Transplant. 1998;17(9):876–880. [PubMed] [Google Scholar]
- 7.Tsau PH, Arabia FA, Toporoff B, et al. Positive panel reactive antibody titers in patients bridged to transplantation with a mechanical assist device: risk factors and treatment. ASAIO J. 1998;44(5):M634–637. [DOI] [PubMed] [Google Scholar]
- 8.Andreu G, Dewailly J, Leberre C, et al. Prevention of HLA immunization with leukocyte-poor packed red cells and platelet concentrates obtained by filtration. Blood. 1988;72(3):964–969. [PubMed] [Google Scholar]
- 9.Fisher M, Chapman JR, Ting A, et al. Alloimmunisation to HLA antigens following transfusion with leucocyte-poor and purified platelet suspensions. Vox Sang. 1985;49(5):331–335. [DOI] [PubMed] [Google Scholar]
- 10.Murphy MF, Metcalfe P, Thomas H, et al. Use of leucocyte-poor blood components and HLA-matched-platelet donors to prevent HLA alloimmunization. Br J Haematol. 1986;62(3):529–534. [DOI] [PubMed] [Google Scholar]
- 11.Schiffer CA, Dutcher JP, Aisner J, et al. A randomized trial of leukocyte-depleted platelet transfusion to modify alloimmunization in patients with leukemia. Blood. 1983;62(4):815–820. [PubMed] [Google Scholar]
- 12.van Marwijk Kooy M, van Prooijen HC, Moes M, et al. Use of leukocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective, randomized trial. Blood. 1991;77(1):201–205. [PubMed] [Google Scholar]
- 13.Jackman RP, Deng X, Bolgiano D, et al. Leukoreduction and ultraviolet treatment reduce both the magnitude and the duration of the HLA antibody response. Transfusion. 2014;54(3):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sintnicolaas K, van Marwijk Kooij M, van Prooijen HC, et al. Leukocyte depletion of random single-donor platelet transfusions does not prevent secondary human leukocyte antigen-alloimmunization and refractoriness: a randomized prospective study. Blood. 1995;85(3):824–828. [PubMed] [Google Scholar]
- 15.Jackman RP, Muench MO, Heitman JW, et al. Immune modulation and lack of alloimmunization following transfusion with pathogen-reduced platelets in mice. Transfusion. 2013;53(11):2697–2709. [DOI] [PubMed] [Google Scholar]
- 16.Muench MO, Heitman JW, Inglis H, et al. Reduced alloimmunization in mice following repeated transfusion with pathogen-reduced platelets. Transfusion. 2016;56(6):1419–1429. [DOI] [PubMed] [Google Scholar]
- 17.Slichter SJ, Pellham E, Bailey SL, et al. Leukofiltration plus pathogen reduction prevents alloimmune platelet refractoriness in a dog transfusion model. Blood. 2017;130(8):1052–1061. [DOI] [PubMed] [Google Scholar]
- 18.Marschner S, Goodrich R. Pathogen Reduction Technology Treatment of Platelets, Plasma and Whole Blood Using Riboflavin and UV Light. Transfus Med Hemother. 2011;38(1):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asano H, Lee CY, Fox-Talbot K, et al. Treatment with riboflavin and ultraviolet light prevents alloimmunization to platelet transfusions and cardiac transplants. Transplantation. 2007;84(9):1174–1182. [DOI] [PubMed] [Google Scholar]
- 20.Jackman RP, Heitman JW, Marschner S, et al. Understanding loss of donor white blood cell immunogenicity after pathogen reduction: mechanisms of action in ultraviolet illumination and riboflavin treatment. Transfusion. 2009;49(12):2686–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojeda F, Guarda MI, Lovengreen C, et al. Ultraviolet exposure of thymocytes: selective inhibition of apoptosis. Int J Radiat Biol. 2004;80(6):445–450. [DOI] [PubMed] [Google Scholar]
- 24.Narbutt J, Cebula B, Lesiak A, et al. The effect of repeated exposures to low-dose UV radiation on the apoptosis of peripheral blood mononuclear cells. Arch Dermatol. 2009;145(2):133–138. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney JF, Nguyen PK, Omann GM, et al. Ultraviolet irradiation accelerates apoptosis in human polymorphonuclear leukocytes: protection by LPS and GM-CSF. J Leukoc Biol. 1997;62(4):517–523. [DOI] [PubMed] [Google Scholar]
- 26.Aragane Y, Kulms D, Metze D, et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140(1):171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naik E, Michalak EM, Villunger A, et al. Ultraviolet radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J Cell Biol. 2007;176(4):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guhl S, Hartmann K, Tapkenhinrichs S, et al. Ultraviolet irradiation induces apoptosis in human immature, but not in skin mast cells. J Invest Dermatol. 2003;121(4):837–844. [DOI] [PubMed] [Google Scholar]
- 29.Caricchio R, Reap EA, Cohen PL. Fas/Fas ligand interactions are involved in ultraviolet-B-induced human lymphocyte apoptosis. J Immunol. 1998;161(1):241–251. [PubMed] [Google Scholar]
- 30.Chen Z, Schubert P, Culibrk B, et al. p38MAPK is involved in apoptosis development in apheresis platelet concentrates after riboflavin and ultraviolet light treatment. Transfusion. 2015;55(4):848–857. [DOI] [PubMed] [Google Scholar]
- 31.Stivala S, Gobbato S, Infanti L, et al. Amotosalen/ultraviolet A pathogen inactivation technology reduces platelet activatability, induces apoptosis and accelerates clearance. Haematologica. 2017;102(10):1650–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran JQ, Muench MO, Heitman JW, et al. Allogeneic major histocompatibility complex antigens are necessary and sufficient for partial tolerance induced by transfusion of pathogen reduced platelets in mice. Vox Sang. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arlett CF, Lowe JE, Harcourt SA, et al. Hypersensitivity of human lymphocytes to UV-B and solar irradiation. Cancer Res. 1993;53(3):609–614. [PubMed] [Google Scholar]
- 34.Yang P, Wen H, Zhong T, et al. GADD45alpha is involved in the apoptosis of lymphocytes induced by riboflavin and ultraviolet light. Transfusion. 2017;57(3):646–656. [DOI] [PubMed] [Google Scholar]
- 35.Babic AM. Extracorporeal photopheresis: Lighting the way to immunomodulation. Am J Hematol. 2008;83(7):589–591. [DOI] [PubMed] [Google Scholar]
- 36.Knobler R Extracorporeal photochemotherapy--present and future. Vox Sang. 2000;78 Suppl 2:197–201. [PubMed] [Google Scholar]
- 37.Oliven A, Shechter Y. Extracorporeal photopheresis: a review. Blood Rev. 2001;15(2):103–108. [DOI] [PubMed] [Google Scholar]
- 38.Russo GG, Mullen C. Cutaneous and noncutaneous disorders treated with extracorporeal photopheresis. Int J Dermatol. 2001;40(2):89–100. [DOI] [PubMed] [Google Scholar]
- 39.Kao KJ. Induction of humoral immune tolerance to major histocompatibility complex antigens by transfusions of UVB-irradiated leukocytes. Blood. 1996;88(11):4375–4382. [PubMed] [Google Scholar]
- 40.Kao KJ, Huang ES, Donahue S. Characterization of immunologic tolerance induced by transfusion of UV-B--irradiated allogeneic mononuclear leukocytes. Blood. 2001;98(4):1239–1245. [DOI] [PubMed] [Google Scholar]
- 41.Morelli AE, Larregina AT. Concise Review: Mechanisms Behind Apoptotic Cell-Based Therapies Against Transplant Rejection and Graft versus Host Disease. Stem Cells. 2016;34(5):1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castilla R, Gonzalez R, Fouad D, et al. Dual effect of ethanol on cell death in primary culture of human and rat hepatocytes. Alcohol Alcohol. 2004;39(4):290–296. [DOI] [PubMed] [Google Scholar]
- 43.Lamb RG, Koch JC, Snyder JW, et al. An in vitro model of ethanol-dependent liver cell injury. Hepatology. 1994;19(1):174–182. [PubMed] [Google Scholar]
- 44.Higuchi H, Kurose I, Kato S, et al. Ethanol-induced apoptosis and oxidative stress in hepatocytes. Alcohol Clin Exp Res. 1996;20(9 Suppl):340A–346A. [PubMed] [Google Scholar]
- 45.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehemtulla A, Hamilton CA, Chinnaiyan AM, et al. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1). J Biol Chem. 1997;272(41):25783–25786. [DOI] [PubMed] [Google Scholar]
- 47.Tomimori Y, Ikawa Y, Oyaizu N. Ultraviolet-irradiated apoptotic lymphocytes produce interleukin-10 by themselves. Immunol Lett. 2000;71(1):49–54. [DOI] [PubMed] [Google Scholar]
- 48.Nicolo C, Tomassini B, Rippo MR, et al. UVB-induced apoptosis of human dendritic cells: contribution by caspase-dependent and caspase-independent pathways. Blood. 2001;97(6):1803–1808. [DOI] [PubMed] [Google Scholar]
- 49.Ferraro-Peyret C, Quemeneur L, Flacher M, et al. Caspase-independent phosphatidylserine exposure during apoptosis of primary T lymphocytes. J Immunol. 2002;169(9):4805–4810. [DOI] [PubMed] [Google Scholar]
- 50.Salucci S, Burattini S, Battistelli M, et al. Ultraviolet B (UVB) irradiation-induced apoptosis in various cell lineages in vitro. Int J Mol Sci. 2012;14(1):532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schubert P, Johnson L, Marks DC, et al. Ultraviolet-Based Pathogen Inactivation Systems: Untangling the Molecular Targets Activated in Platelets. Front Med (Lausanne). 2018;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bevers EM, Williamson PL. Getting to the Outer Leaflet: Physiology of Phosphatidylserine Exposure at the Plasma Membrane. Physiol Rev. 2016;96(2):605–645. [DOI] [PubMed] [Google Scholar]
- 53.Segawa K, Suzuki J, Nagata S. Constitutive exposure of phosphatidylserine on viable cells. Proc Natl Acad Sci U S A. 2011;108(48):19246–19251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elliott JI, Surprenant A, Marelli-Berg FM, et al. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol. 2005;7(8):808–816. [DOI] [PubMed] [Google Scholar]
- 55.Fadok VA, Voelker DR, Campbell PA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–2216. [PubMed] [Google Scholar]
- 56.Fadok VA, Laszlo DJ, Noble PW, et al. Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. J Immunol. 1993;151(8):4274–4285. [PubMed] [Google Scholar]
- 57.Carvalho H, Alguero C, Santos M, et al. The combined effect of platelet storage media and intercept pathogen reduction technology on platelet activation/activability and cellular apoptosis/necrosis: Lisbon-RBS experience. Transfus Apher Sci. 2006;34(2):187–192. [DOI] [PubMed] [Google Scholar]
- 58.Fast LD, Dileone G, Li J, et al. Functional inactivation of white blood cells by Mirasol treatment. Transfusion. 2006;46(4):642–648. [DOI] [PubMed] [Google Scholar]
- 59.Kaiser-Guignard J, Canellini G, Lion N, et al. The clinical and biological impact of new pathogen inactivation technologies on platelet concentrates. Blood Rev. 2014;28(6):235–241. [DOI] [PubMed] [Google Scholar]
- 60.Ambruso DR, Thurman G, Marschner S, et al. Lack of antibody formation to platelet neoantigens after transfusion of riboflavin and ultraviolet light-treated platelet concentrates. Transfusion. 2009;49(12):2631–2636. [DOI] [PubMed] [Google Scholar]
- 61.Norris PJ, Kaidarova Z, Maiorana E, et al. Ultraviolet light-based pathogen inactivation and alloimmunization after platelet transfusion: results from a randomized trial. Transfusion. 2018;58(5):1210–1217. [DOI] [PubMed] [Google Scholar]
- 62.Trial to Reduce Alloimmunization to Platelets Study G. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337(26):1861–1869. [DOI] [PubMed] [Google Scholar]
- 63.Estcourt LJ, Malouf R, Hopewell S, et al. Pathogen-reduced platelets for the prevention of bleeding. Cochrane Database Syst Rev. 2017;7:CD009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saris A, Kerkhoffs JL, Norris PJ, et al. The role of pathogen-reduced platelet transfusions on HLA alloimmunization in hemato-oncological patients. Transfusion. 2018. [DOI] [PubMed] [Google Scholar]
- 65.Stolla M Pathogen reduction and HLA alloimmunization: more questions than answers. Transfusion. 2019;59(3):1152–1155. [DOI] [PubMed] [Google Scholar]
- 66.Jackman RP, Muench MO, Inglis H, et al. Reduced MHC alloimmunization and partial tolerance protection with pathogen reduction of whole blood. Transfusion. 2017;57(2):337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marschner S, Fast LD, Baldwin WM 3rd, et al. White blood cell inactivation after treatment with riboflavin and ultraviolet light. Transfusion. 2010;50(11):2489–2498. [DOI] [PubMed] [Google Scholar]
- 68.Ypma PF, van der Meer PF, Heddle NM, et al. A study protocol for a randomised controlled trial evaluating clinical effects of platelet transfusion products: the Pathogen Reduction Evaluation and Predictive Analytical Rating Score (PREPAReS) trial. BMJ Open. 2016;6(1):e010156. [DOI] [PMC free article] [PubMed] [Google Scholar]


