Abstract
Reactive oxygen species (ROS) are continuously generated as a by-product of normal aerobic metabolism. Elevated ROS formation leads to potential damage of biological structures and is implicated in various diseases. Astaxanthin, a xanthophyll carotenoid, is a secondary metabolite responsible for the red-orange color of a number of marine animals and microorganisms. There is mounting evidence that astaxanthin has powerful antioxidant, anti-inflammatory, and antiapoptotic activities. Hence, its consumption can result in various health benefits, with potential for therapeutic application. Astaxanthin contains both a hydroxyl and a keto group, and this unique structure plays important roles in neutralizing ROS. The molecule quenches harmful singlet oxygen, scavenges peroxyl and hydroxyl radicals and converts them into more stable compounds, prevents the formation of free radicals, and inhibits the autoxidation chain reaction. It also acts as a metal chelator and converts metal prooxidants into harmless molecules. However, like many other carotenoids, astaxanthin is affected by the environmental conditions, e.g., pH, heat, or exposure to light. It is hence susceptible to structural modification, i.e., via isomerization, aggregation, or esterification, which alters its physiochemical properties. Here, we provide a concise overview of the distribution of astaxanthin in tissues, and astaxanthin structures, and their role in tackling singlet oxygen and free radicals. We highlight the effect of structural modification of astaxanthin molecules on the bioavailability and biological activity. These studies suggested that astaxanthin would be a promising dietary supplement for health applications.
1. Introduction
Carotenoids are a class of bioactive natural products synthesized by plants and certain photosynthetic microorganisms. Many carotenoids are directly involved in the photosynthesis process, while others protect the host from photooxidation and related damage [1]. Astaxanthin is a carotenoid that has garnered significant interest in recent years. It is a red xanthophyll carotenoid found predominantly in marine microorganisms and animals [2, 3]. Astaxanthin is ranked as the second most important carotenoid on the global market after capsanthin. Its market size exceeded US$ 288.7 million in 2017 and will reach US$ 426.9 million by 2022, dominating the carotenoid industry, with a predicted 8.1% compound annual growth rate until 2022 [4]. Natural astaxanthin has been approved as generally recognized as safe (GRAS). It can be sold as a dietary supplement and has been also approved as a food colorant (E161j) by the European Commission for use in the food and beverage industries [5].
Astaxanthin supplementation has been investigated in a broad range of clinical applications in humans and was shown to exert multiple pharmacological effects [3, 6]. The beneficial effects of astaxanthin are often associated with its antioxidative [7–9], anti-inflammatory [10–13], and antiapoptotic properties [10, 14–17]. As demonstrated in several studies, astaxanthin stimulates the immune response, e.g., by increasing interferon and interleukin 2 production without inducing cytotoxicity [18–21]. It potentially plays a neuroprotective role in neurological disorders, such as brain ischemic or traumatic injury, and subarachnoid bleeding [22–28]. It also exhibits a novel cardioprotective potential suppressing homocysteine-induced cardiotoxicity by alleviating mitochondrial dysfunction and oxidative damage [29]. Furthermore, astaxanthin effectively suppresses metastasis of the colon and cell lung cancers [30–32], prevents binding of the human papillomavirus L1 protein to the human sperm membrane [33], improves stem cell potency by increasing the proliferation of neural progenitor cells [34]; prevents liver damage in carbon tetrachloride-induced toxicity [35], inhibits membrane peroxidation in human endothelial cells [36], and exerts antiaging effects [37–40]. Some in vitro and in vivo studies of the biological role of astaxanthin are presented in Table 1.
Table 1.
Biological activities of astaxanthin evaluated in vitro and in vivo, with the study model, target, and dosage specified.
| Model | Main effect | Dosage | Target | Disease | Ref. |
|---|---|---|---|---|---|
| SD rat | Antioxidant | 10 μM | Nrf2/ARE | Diabetic nephropathy | [7] |
| C57BL/6 mice | Antioxidant | 0.02% (w/w) | Nrf2, HO-1, BALF | Chronic obstructive pulmonary melanogster | [8] |
| ETS mouse | Anti-inflammatory | 40 and 80 mg/kg | p38 MAPK, NF-κB-p65, PSD-95, IL-6, TNF-α, MDA, SOD, GSH, CAT | Cognitive impairment | [10] |
| H. Pylori-free BALB/c female mice | Anti-inflammatory | 10 or 40 mg/d | IFN-γ, IL-2, IL-10 | Gastrointestinal disease | [21] |
| Drosophila melanogaster (fruit fly) | Antiaging | 10 or 20 mg/mL | SOD1, SOD2, CAT | No detailed disease | [39] |
| Wistar rat | Antiaging | 10 mg/kg/d | IL-1β, IL-10 | Brain aging | [40] |
| Wistar rat | Anticancer | 15 mg/kg/d | ERK-2, NF-κB-p65, COX-2, MMPs-2/9, AKT, ERK-2 | Colon cancer | [31] |
| Nonsmall cell lung cancer type A549 and H1703 | Anticancer | 2.5–20 μM | Rad51, AKT | Lung cancer | [32] |
| Male BALB/c mice | Immunomodulatory | 0.28, 1.4, and 7 mg/kg/d | IFN-γ, IL-2, Con A, LPS | No detailed disease | [18] |
| HPV16-L1 | Anti-HPV | 2 μM | CTB, Lyn, Tyr-P, ARC | HPV | [33] |
| Male SPF C57BL/J mice | Antiobesity | 0.005% or 0.01%/diet powder | Weight gain, energy intake, fat index, plasma triacylglycerol and cholesterol, liver triacylglycerol and cholesterol | Obesity | [41] |
| Wistar rat | Neuroprotective | 10 mg/kg | GPx, MDA | Alzheimer's disease | [23] |
| HT22 cells | Neuroprotective | 1.25–5 μM | HO-1, Nrf2, Bcl-2, Bax, AIF, Cyto-c, p-Akt and p-GSK-3β | Alzheimer's disease | [24] |
| Wistar rat | Neuroprotective | 10 μL of 0.2 mM | Bax, Bcl-2, cleaved-caspase-3 | Spinal cord injury | [25] |
| Human cell line SH-SY5Y | Antiapoptotic | 1–20 μM | 6-OHDA | Parkinson's disease | [15] |
| Balb/C mice | Antiapoptotic | 20 and 40 mg/kg | TNF-α, Bcl-2 | Autoimmune hepatitis | [17] |
| Neural progenitor cells | Proproliferative | 1, 5, and 10 ng/mL | PI3K, MEK | Alzheimer's disease | [34] |
| H9c2 rat myocardial cells | Cardioprotective | 0.5–8 μM | Bcl-2 | Cardiovascular diseases | [29] |
| Mouse | Cardioprotective | 5 mg/kg/d | GSH-Rs and MDA | Cardiovascular diseases | [29] |
| Albino Wistar rat | Hepatoprotective | 100 and 250 μg/kg | SGPT, SGOT, ALP | No specific disease | [35] |
Note: SD: Sprague-Dawley; Nrf2/ARE: nuclear factor-erythroid 2-related factor 2/antioxidant response element; HO-1: heme oxygenase-1; BALF: bronchoalveolar lavage fluid; p38 MAPK: p38mitogen-activated protein kinase; NF-κB-p65: nuclear factor-kappa B-p65; PSD-95: postsynaptic density protein 95; IL-6: interleukin-6; TNF-α: tumor necrosis factor; MDA: malondialdehyde; SOD: superoxide dismutase; GSH: glutathione; CAT: catalase; IFN-γ: interferon gamma; IL-2: interleukin 2; IL-10: interleukin 10; IL-1β: interleukin 1 beta; ERK-2: extracellular signal-regulated kinase-2; COX-2: cyclooxygenase-2; MMPs2/9: matrixmetallo proteinases; Akt: protein kinase B; Rad51: Rad51 genes; Con A: concanavalin A; LPS: lipopolysaccharide; CTB: cholera toxin subunit B; Lyn: tyrosine kinase Lyn; ARC: acrosome-reacted cells; GPx: glutathione peroxidase enzyme; MDA: malondialdehyde; AIF: apoptosis-inducing factor; Cyto-c: cytochrome-c; GSK-3β: glycogen synthase kinase-3β; Bax: Bcl-2 associated X protein; Bcl-2: B-cell lymphoma 2; GSH-Rs: reduced glutathione; SGPT: serum glutamate transaminase; SGOT: serum glutamate oxaloacetate; ALP: alkaline phosphatase.
Reactive oxygen species (ROS) have attracted attention as novel signal mediators involved in the modulation of cell survival, cell death, differentiation, cell signaling, and inflammation-related factor production [42]. Large quantities of ROS are generated during mitochondrial oxidative metabolism, as well as during cellular response to xenobiotics, cytokines, and bacterial invasion [43, 44]. Cellular imbalance caused by ROS or oxidants that exceed the cellular capability to mount an effective antioxidant response is called oxidative stress. It results in macromolecular damage and is implicated in various diseases. The key function of astaxanthin as a potent antioxidant depends on its ability to scavenge singlet oxygen [45] and free radicals [46].
The ability of astaxanthin to attack active oxygen species is reportedly 10-fold higher than that of zeaxanthin, lutein, tunaxanthin, cathaxanthin, and β-carotene, and 100-fold higher than that of α-tocopherol [47]. The unique molecular structure of astaxanthin, the characteristics of its excited state and isomeric forms, and the tendency to aggregate in different solvents impact its biological activity and bioavailability. In the present review, we provide information on astaxanthin sources, structural diversity, and mechanism of action related to its interaction with ROS.
2. Sources of Astaxanthin
The natural sources of astaxanthin include green algae, bacteria, fungi, archaea, chromista, shrimp, crawfish, crabs, lobster, Antarctic krill, marine copepoda, and salmonids, as presented in Table 2.
Table 2.
Natural sources of astaxanthin.
| Source | Example of species | Astaxanthin concentration (mg kg−1)∗ |
Primary optical isomer | Reference |
|---|---|---|---|---|
| Microorganism | ||||
| Phytoplankton | Haematococcus pluvialis NIES-144 | 98,000 d.w. | 3S,3′S | [48] |
| Haemotococcus lacustris | 43,100 d.w. | 3S,3′S | [49] | |
| Haematococcus pluvialis CCAP-34/7 | 22,700 d.w. | 3S,3′S | [50] | |
| Neochloris wimmeri CCAP-213/4 | 19,200 d.w. | — | [50] | |
| Protosiphon botryoides SAG-731/1a | 14,300 d.w. | — | [50] | |
| Scotiellopsis oocystiformis SAG-277/1 | 10,900 d.w. | — | [50] | |
| Chlorella zofingiensis SAG-211/14 | 6800 d.w. | — | [50] | |
| Scenedesmus vacuolatus SAG-211/15 | 2700 d.w. | — | [50] | |
| Chlorococcum sp. | 4230 d.w. | — | [51] | |
| Chromochloris zofingiensis | 13,100 d.w. | — | [52] | |
| Euglena sanguinea | — | 3S,3′S | [53] | |
|
| ||||
| Zooplankton | Calanus helgolandicus | 50–220 d.w. | — | [54] |
| Acartia bifilosa | 477.4 d.w. | — | [55] | |
| Calanus finmarchicus | 100–500 d.w. | 3S,3′S | [56, 57] | |
| Calanus glacialis | 100–500 d.w. | — | [56] | |
| Calanus hyperboreus | 100–500 d.w. | — | [56] | |
| Calanus pacificus | — | — | [58] | |
| Diaptomus nevadensis | — | — | [58] | |
| Neocalanus tonsus | — | — | [58] | |
| Amphiascoides atopus | 619 d.w. | — | [59] | |
| Acartia bifilosa | 293–487 d.w. | — | [60] | |
| Pseudocalanus acuspes | 239–305 d.w. | — | [60] | |
| Idotea metallica | — | — | [61] | |
|
| ||||
| Bacteria | Agrobacterium aurantiacum | 140 d.w. | 3S,3′S | [62] |
| Brevundimonas sp. strain N-5 | 837 d.w. | 3S,3′S | [63] | |
| Sphingomicrobium astaxanthinifaciens | 40 d.w. | — | [64] | |
| Paracoccus bogoriensis | 400 w.w. | — | [65] | |
| Brevundimonas spp. | 27.6–365 d.w. | 3S,3′S | [66] | |
| Brevundimonas sp. M7 | 1300 d.w. | 3S,3′S | [66] | |
| Sphingomonas astaxanthinifaciens | 690 d.w. | — | [66] | |
| Altererythrobacter ishigakiensis | — | — | [67] | |
| Paracoccus haeundaensis | — | — | [68] | |
| Paracoccus carotinifaciens | — | 3S,3′S | [69] | |
|
| ||||
| Yeast | Phaffia rhodozyma PR 190 | 970 d.w. | 3R,3′R | [70] |
| Phaffia rhodozyma UCD 67-210 | 387 d.w. | 3R,3′R | [71] | |
| Candida utilis | 400 d.w. | — | [72] | |
|
| ||||
| Archaea | Halobacterium salinarium NRC-1 | 265 d.w. | — | [73] |
| Haloarcula hispanica ATCC 33960 | 17 d.w. | — | [73] | |
| Chromista | Thraustochytrium sp. CHN-3 | 2800 d.w. | — | [74] |
|
| ||||
| Crustaceans | ||||
| Shrimp | Marsupenaeus japonicus | 418 d.w. | — | [75] |
| Litopenaeus setiferus | 48.3 w.w. | — | [76] | |
| Penaeus sp. | 0.96 w.w. (only head) | — | [77] | |
| Litopenaeus vannamei | 11.3–31.8 w.w. | 3R,3′S | [57, 78] | |
| Penaeus monodon | 24.9–67.5 d.w. | 3R,3′S | [57, 79] | |
| Pandalus borealis | 30.9–147.7 w.w. | 3R,3′S | [57, 80, 81] | |
|
| ||||
| Crawfish | Procambarus clarkii | 78.5–197.9 d.w. (only shell) | — | [82] |
| Crabs | Pleuroncodes planipes | — | — | [83] |
| Eriocheir sinensis | 3.5–4.7 d.w. (only carapace) | — | [84] | |
| Chionoecetes opilio | 119.6 d.w. | — | [81] | |
|
| ||||
| Lobster | Jasus lalandii | 13 w.w. | — | [85] |
| Antarctic krill | Euphausia superba | — | 3R,3′R | [80] |
|
| ||||
| Fishes | ||||
| Salmonids | Atlantic salmon (Salmo salar) | 6–8 w.w. | 3S,3′S | [3] |
| Sockeye salmon (Oncorhynchus nerka) | 26–38 w.w. | 3S,3′S | [86] | |
| Chinook salmon (Oncorhynchus tshawytscha) | 5.4 w.w. | 3S,3′S | [86] | |
| Chum salmon (Oncorhynchus keta) | 3–5 w.w. | 3S,3′S | [86] | |
| Coho salmon (Oncorhynchus kisutch) | 10–21 w.w. | 3S,3′S | [86] | |
| Masu salmon (Oncorhynchus masou) | 4.6 w.w. | 3S,3′S | [86] | |
| Pink salmon (Oncorhynchus gorbuscha) | 4–7 w.w. | 3S,3′S | [86] | |
| Rainbow trout (Oncorhynchus mykiss) | 24 w.w. | 3S,3′S | [86] | |
| Arctic char (Salvelinus alpinus) | 8.6 w.w. | — | [86] | |
∗ d.w., dry weight; w.w., wet weight.
Astaxanthin is ubiquitous in marine organisms. It is responsible for the well-known red-orange color of the skin and flesh of shrimp and crayfish, and the flesh of salmon. Crustaceans and fish cannot synthesize astaxanthin de novo, and thereby rely on the supply of astaxanthin precursors through the consumption of algae and other microorganisms [87]. Likewise, human is unable to synthesize carotenoids, and they have to be obtained from the diet. The main source of astaxanthin for humans is seafood, with salmon, for example, wild Sockeye salmon (Oncorhynchus nerka), containing the most astaxanthin (26–38 mg kg–1 wet weight) [86]. The rainbow trout (Oncorhynchus mykiss) is also a good source of astaxanthin (24 mg kg–1 wet weight) [86]. Currently, approximately 72% of the world's salmon production is farmed [88]. Flesh pigmentation is a key commercial trait of farmed salmon, and it is often associated with product quality. In the aquaculture sector, commercial astaxanthin food additives can account for up to 25% of feed costs [89, 90]. The origin of astaxanthin sources can be monitored by high-performance liquid chromatography (HPLC) of the isomers present in the flesh. For instance, in salmon fed yeast-derived astaxanthin, the 3R,3′R isomer is the major component, while in salmon fed synthetic astaxanthin, the 3R,3′S isomer is abundant [91]. Of note, currently, over 95% of astaxanthin available on the market is derived synthetically from petrochemicals; on the other hand, Haematococcus pluvialis-derived natural astaxanthin accounts for <1% of commercialized astaxanthin [92]. However, safety concerns have arisen regarding the use of synthetic astaxanthin for human consumption [93].
3. Astaxanthin Structures
3.1. Basic Structure and Isomers of Astaxanthin
Astaxanthin is a xanthophyll carotenoid because it contains oxygen. Astaxanthin (3,3′-dihydroxy-β,β′-carotene-4,4′-dione; CAS no. 472-61-7; Mw = 596.8 g mol–1; ε468 nm = 125 × 103 mol–1 cm–1 in hexane) consists of two terminal β-ionone–type rings joined by a polyene chain. It has two asymmetric carbons located at the 3,3′-position of the β-ionone ring, with a hydroxyl group (-OH) on either end of the molecule (Figure 1). Oxygen is present in the ring system as both a hydroxyl and a keto (C=O) group.
Figure 1.
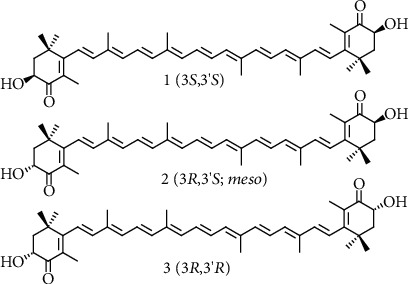
Structures of the optical isomers all-E-(3S,3′S) (1), all-E-(3R,3′S; meso) (2), and all-E-(3R,3′R) (3) astaxanthin.
Astaxanthin can be esterified, which increases its solubility in the cell and makes it more stable to oxidation [94]. The hydroxy group on one or both rings can bind to different fatty acids, such as palmitic, oleic, estearic, or linoleic acid, to form mono- or diesters, accordingly. Astaxanthin also exists in a free form, i.e., with the hydroxyl group not esterified, and in a chemical complex with proteins or lipoprotein. Further, stereoisomers and geometric isomers of astaxanthin are identified [81]. Figure 1 shows three configurational isomers of the compound: two enantiomers (3R,3′R and 3S,3′S) and a meso form (3R,3′S). The alga Haematococcus synthesizes mainly the 3S,3′S isomer, which is also predominant in wild Atlantic salmon, and occurs mainly in the free form [93]. The Antarctic krill (Euphausia superba) produces the 3R,3′R as the primary isomer [3]. Whereas the three optical stereoisomers exist in nature in variable ratios, synthetic astaxanthin is a racemic mixture of the two enantiomers and the meso form [81]. Synthetic astaxanthin contains the 3S,3′S, 3R,3′S, and 3R,3′R isomers in a 1 : 2 : 1 ratio, respectively. It is not esterified, while natural astaxanthin mostly occurs in esterified form, or in a complex with proteins or lipids [81].
Astaxanthin also exists as trans and cis (E and Z) geometrical isomers (Figure 2), depending on the configuration of the double bonds in the polyene chain. All-trans astaxanthin (all-E-astaxanthin) is the dominant isomer, although at least two cis-isomers (9-cis and 13-cis) also occur in nature, depending on the host species and body part, and are also found in synthetic preparations [95]. In rainbow trout (O. mykiss), most astaxanthin is present as all-trans astaxanthin (97%), followed by 9-cis (0.4%), 13-cis (1.5%), and other isomers (1.1%) [95, 96]. Further, 15-cis and di-cis isomers were identified in addition to all-trans, 13-cis, and 9-cis isomers, in various wild and cultured shrimps in China, Trachysalambria curvirostris, Penaeus monodon, Fenneropenaeus chinensis, Litopenaeus vannamei, and Exopalaemon carinicauda [97, 98].
Figure 2.

Structure of all-trans (1a, 2a, 3a), 9-cis (1a, 2b, 3c), 13-cis (1c, 2c, 3c), and 15-cis-astaxanthin (1d, 2d, 3d). Note: 1, 3S,3′S; 2, 3R,3′S; meso; 3, 3R,3′R.
The isomerization of trans-astaxanthin to cis-isomers has been investigated in various organic solvents [99]. All-trans natural astaxanthin is readily isomerized to cis-trans mixtures, especially the 9-cis and 13-cis isomers. Increased temperature, exposure to light, or the presence of acids can result in the formation of cis-isomer [99, 100]. Isomerization of all-trans astaxanthin is challenging because of low stereochemical stability, as well as other factors, such as microwave radiation, ultrasonic vibration, organic solvents, presence of fatty acids, and Cu (II) ion, which affect the formation yield of different astaxanthin isomers [99–103]. Therefore, isomerization of trans-astaxanthin and cis-isomers has to be minimized during the preparation of pure isomeric forms, i.e., during pigment extraction, astaxanthin ester saponification, and isomer purification. Reversed-phase HPLC can be used to separate the trans and cis-isomers of astaxanthin [100].
3.2. Structure of Astaxanthin Aggregates
Biomolecular aggregation is important in medical treatments. Like many other carotenoids, astaxanthin is expected to self-assemble in hydrated polar solvents to form aggregates [104]. Water concentration in these mixtures impacts the aggregate morphology and greatly affects their photophysical properties. Based on the spectroscopic analysis, the aggregate absorption spectra are blue-shifted or red-shifted compared with those of monomers, depending on the aggregation conditions [105–107]. Specifically, the red-shift is attributed to the formation of J-aggregate and the blue-shift is attributed to the formation of H-aggregate. J-aggregate consists of astaxanthin molecules arranged head-to-tail and forming a relatively loosely packed aggregate, while H-aggregate is characterized by tight “card-pack” stacking where the polyene chains are more or less parallel to each other [108].
Aggregation of astaxanthin in ethanol–water solution has been studied by ultraviolet/visible and fluorescence spectrometry. After the addition of water, astaxanthin immediately forms tightly packed stacks of individual molecules, with a maximum blue shift of 31 nm; the J-aggregate forms in 1 : 3 (v/v) ethanol–water solution after an hour [107]. In the case of dimethyl sulfoxide (DMSO), an organic solvent that is usually used for delivering carotenoid in cell culture, the addition of water for a ratio of 1 : 1 (v/v), generates a red-shifted J-aggregate with a maximum absorption band at 570 nm. On the other hand, the DMSO/water ratio of 1 : 9 (v/v) produces blue-shifted H-aggregate with maximum absorption bands at 386 nm and 460 nm [105]. Of note, the formation of astaxanthin aggregates also changes the excited-state dynamic of the compound, i.e., in DMSO/water solution, it results in a longer S1 lifetime than that of the corresponding monomer, with the astaxanthin triplet generated exclusively in the astaxanthin aggregate [105]. In nature, astaxanthin also forms monoesters and diesters with fatty acids. Ester formation completely prevents aggregation [109].
3.3. Structure and Bioavailability
As mentioned above, bioavailability of astaxanthin in aquatic animals has been well researched. The all-trans isomer is predominantly found in most body parts of the aquatic animals, including fish and crustaceans. The 13-cis isomer is present in the fish liver at relatively high amounts [95, 96]. In other words, the configuration of the astaxanthin molecule may influence its absorption, as reported for rainbow trout fed cold-pelleted diets containing select astaxanthin isomers [96]. This resulted in the formulation of the following hypotheses [57]: (1) an isomer that is easily ingested and absorbed accumulates faster than other isomers in an aquatic animal; (2) the isomer is selectively transported in the plasma and between tissues or organs; and (3) astaxanthin may undergo metabolic conversion before deposition in the animal. The higher absorption of all-trans astaxanthin than that of the cis-isomer may be explained by the relatively lower ability of the sterically bulkier cis-isomer to permeate the lipid membrane [110].
Bioavailability of natural astaxanthin stereoisomers from wild and farmed salmon in healthy men was assessed in a randomized and double-blind trial involving 28 volunteers [111]. The participants were given 250 g of wild or aquaculture salmon (5 μg g−1) daily, for 4 weeks. The plasma astaxanthin concentration and isomer distribution were monitored by HPLC. On day 28, the 3S,3′S isomer was predominant in the plasma (80%) of individuals in the wild salmon intake group, whereas the meso form (3R,3′S) was prevalent (48%) in the farmed salmon intake group. Although the all-trans astaxanthin has been the subject of many studies, according to recent research, the 9-cis and 13-cis isomers are selectively absorbed by human plasma. Bioavailability of synthetic astaxanthin was also assessed in the plasma and lipoprotein fractions in three middle-aged male volunteers after ingestion of a single meal containing a 100 mg dose of astaxanthin mixture of all-trans (74%), 9-cis (9%), and 13-cis isomers (17%) [110]. Astaxanthin was present mainly in very low-density lipoproteins containing chylomicrons (VLDL/CM; 36-64%), whereas low-density lipoprotein (LDL) and high-density lipoprotein (HDL) contained 29% and 24% of total astaxanthin, respectively. The astaxanthin isomers in HDL, LDL, and VLDL/CM ranged from 62.1–66.9% for all-trans isomer, 13.2–14.5% for 9-cis isomer, and 20.4–24.3% for 13-cis isomer, respectively.
Recently, bioaccessibility and bioavailability of all-trans, 9-cis, and 13-cis astaxanthins was investigated using an in vitro digestion model and human intestinal Caco-2 cells [112]. Indeed, the 13-cis astaxanthin was more bioaccessible than the 9-cis and all-trans forms during in vitro digestion, and 9-cis astaxanthin was transported in human intestinal Caco-2 cells more efficiently than the all-trans and 13-cis isomers [112]. In terms of absorption efficiency of all isomers during transport across differentiated Caco-2 cell monolayers, cellular uptake was pronounced after 3 h, and the absorption efficiency increased drastically after 12 h and continued to increase until the 24 h time point. The absorption efficiency of all-trans isomer was the highest, followed by those of 9-cis and 13-cis isomers, indicating that the linear structure of the isomer initially facilitates its permeation of the cell membrane, as compared with the sterically bulky cis-isomers. In the experiment, cis-astaxanthin was more readily isomerized to all-trans isomer than to another cis-isomer, although all-trans astaxanthin tended to be isomerized to 13-cis astaxanthin slightly more readily than to 9-cis astaxanthin [112]. In the experiment, DMSO was used to deliver astaxanthin to the cultured cells. The observations highlight the possible effect of structure on the biological role of these isomers, especially in cellular uptake and transport.
4. Interaction of Astaxanthin with Reactive Species
Harmful effects of reactive species are well established. As determined in studies involving animal models and human subjects, reactive species participate in the pathogenesis of acute and chronic diseases. That is because nucleic acids (RNA and DNA), and proteins are the main targets of free radicals [113, 114].
Inflammation widely contributes to multiple chronic diseases and is closely linked with oxidative stress. For example, elevated levels of prooxidants and various markers of oxidative stress, and cell and tissue damage are linked to the pathogenesis of cancer, cardiovascular disease, neurodegenerative diseases, reproductive system diseases, and aging [115]. Accumulation of an antioxidant in a cell can reduce or prevent oxidation of oxidizable substrates. An ideal antioxidant should be readily absorbed, eliminate free radicals, and chelate redox metals at physiologically relevant levels [116].
Free radicals contain one or more unpaired electrons. This feature makes them particularly reactive and is also responsible for their ability to trigger chain reactions, propagating the associated molecular damage. Most free radicals are, or arise from, reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS). ROS include oxygen-based free radicals, i.e., the superoxide radical anion (O2•–), and hydroxyl (HO•), alkoxyl (RO•), organic peroxyl (ROO•), and hydroperoxyl (HOO•) radicals. RNS comprise peroxynitrite (ONOO–), nitric oxide (NO•), and nitrogen dioxide (NO2•). The most common RSS are thiyl radicals (RS•), sulfenic acids (RSOH), and disulfide-S-oxides [RS(O)2SR]. Concerning reactivity, HO• is the most reactive and dangerous species among ROS. ROO• is significantly less reactive than HO•, which allows them to diffuse to remote cellular locations [117]. For RNS, the chemical reactivity of NO• is low, but it reacts with O2•– yielding peroxynitrite, a highly damaging species, to react with lipids, protein, and DNA [117].
Since RNS and RSS are less reactive than the reactive oxygen species, below, we focus on ROS.
4.1. Generation of ROS
In the cell, ROS are produced by enzymes of different origin, mainly by the cytoplasmic membrane NADPH oxidase; the enzyme complex of the mitochondrial respiratory chain; and such enzymes as xanthine oxidase, lipo- and cyclooxygenase, and cytochromes P450 in the endoplasmic reticulum, peroxisomes, and others [43]. The mitochondrial respiratory chain is a main contributor, as 85% of oxygen metabolized the mitochondrion and partially reduced oxygen intermediates are produced therein [118]. In this organelle, O2•– and H2O2 participate in redox signaling [119], but their production is greatly enhanced during oxidative stress, for example in response to various diseases or stimuli.
Oxidative stress reflects an imbalance between the production of ROS and the action of the antioxidant defense system in charge of their neutralization. The defense system includes superoxide dismutase, which reduces O2•– to H2O2 [120], and catalase, glutathione peroxidase, and thioredoxin reductase, which regulate H2O2 levels by converting it to H2O and O2 [121, 122]. The production of HO• was usually obtained by Fenton reaction, with Fe2+ reducing H2O2 to HO• and HO– (Figure 3). In this case, ROS are generated by a metal-catalyzed reaction and the resulting oxidative damage is often site-directed, particularly when the biomolecules coordinate metal ions [123].
Figure 3.
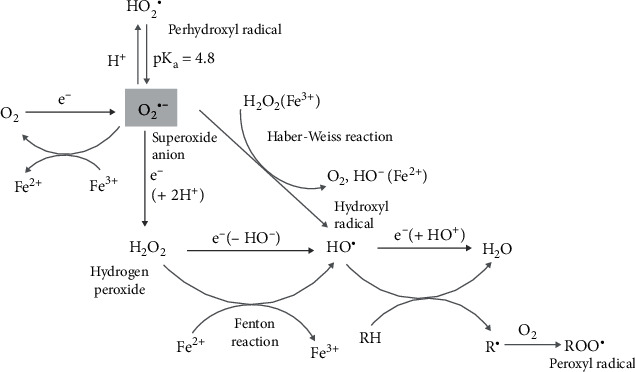
Chemical players in the generation of reactive oxygen species (ROS): superoxide anion (O2•–) radical, perhydroxyl (HO2•) radical, metal-catalyzed conversion of superoxide anion (O2•–) and hydrogen peroxide (H2O2) into hydroxyl (HO•) radical, and peroxyl radical (ROO•).
In a biological context, the toxicity of O2•– is indirect since the species is involved in the generation of highly reactive secondary species (HO•) [124]. The latter is generated by the reaction of superoxide (O2•–) and hydrogen peroxide (H2O2) (the Haber-Weiss reaction) (Figure 3). The reaction is thermodynamically, but not kinetically, favorable, and has to be catalyzed by a metal ion, for example, ferrous iron [125].
Further, H2O as an oxidant is not thermodynamically favorable under biological conditions [E°(O2•–/H2O2) = 0.93 V and E°(H2O2/HO•) = 0.30 V] [126]. The highest oxidizing potential of this molecule is achieved indirectly, when it is converted into HO• radicals via the metal-catalyzed Fenton and Haber-Weiss reactions (Figure 3).
The hydroxyl radical is a powerful oxidant among ROS, with a potential of E°(HO•/H2O) = 2.34 V [126]. It can be converted to a relatively less reactive oxide radical, O•–, at very low pH [pKa(HO•/O•–) = 11.9] [127]; however, this conversion is not relevant at physiological pH.
The HO• radical is electrophilic and has a strong affinity for electron-rich sites of molecules [127]. The radical targets numerous and various biomolecules via H-abstraction, leading to the formation of carbon-centered radicals that rapidly react with molecular oxygen to generate the peroxyl radical (ROO•). The latter reacts faster than the superoxide anion with numerous biological substrates (DNA, lipids, and proteins) with the rate constant ranging from 102 to 108 L mol–1 s–1 [128].
4.2. Astaxanthin Structure and Antioxidant Activity
A qualified antioxidant should be involved in one or more of the following processes to protect a biological system against oxidative damage [2]: (i) oxygen depletion; (ii) quenching of singlet oxygen molecules; (iii) scavenging of ROS or termination of a chain reaction of oxidation propagation; (iv) chelation of metal ion that otherwise could catalyze ROS formation; or (v) repair of oxidative damage. A range of ROS is found in the human body. Accordingly, the essential role of astaxanthin as an antioxidant is to deactivate reactive oxidants, such as singlet oxygen and ROS (processes 1-3), in particular, peroxyl radical intermediates [47, 80, 129].
The scavenging reaction not only depends on the carotenoid structure but also on the properties of free radicals [130]. Indeed, certain radicals elicit electron transfer, while others only engage in an addition reaction [131]. The environment plays an important role as well, with the solvent polarity leading to different mechanisms of radical–carotenoid interactions [131]. In general, three possible mechanisms of carotenoid (Car in the equations below) interactions with ROS exist: (i) hydrogen abstraction or hydrogen atom transfer from the side chain, i.e., C4-position of the cyclohexene ring (Equation (1)); (ii) radical addition to the polyene chain (Equation (2)); and (iii) electron transfer yielding a carotenoid radical cation (Equation (3)) [132, 133].
| (1) |
| (2) |
| (3) |
| (4) |
Many well-documented examples of reaction mechanisms corresponding to the reactions described by Equations (2) and (3) are known; however, the majority concern β-carotene [132–135]. Astaxanthin is a carotenoid with the best scavenging capacity for peroxyl and hydroxyl radicals [136, 137]. The HO• scavenging capacity of astaxanthin is 66% and 17% greater than that of Trolox and quercetin, respectively, and only 6% less potent than that of α-tocopherol [136]. However, with respect to ROO•, astaxanthin is 16% more potent than α-tocopherol [136]. Hypothetically, the keto group potentially activates the hydroxyl group, resulting in the formation of an ortho-dihydroxy–conjugate polyene system that facilitates hydrogen transfer (Equation (1)) to the peroxyl radical, in a manner similar to that of the hydroxyl group of α-tocopherol [138]. As determined in an electrochemical study of carotenoid in solution using cyclic voltammetry, upon electron transfer from the carotenoid molecule, the radical cation Car•+ is formed at an oxidation potential E1°. The second lost electron forms the dication Car2+ at an oxidation potential E2°. The electrochemical measurement of astaxanthin, and astaxanthin monoester and diester, indicated that astaxanthin [E1°(Car/Car•+) = 0.768 V] has a higher oxidation potential than other carotenoids, such as β-carotene [E1°(Car/Car•+) = 0.634 V], zeaxanthin [E1°(Car/Car•+) = 0.63 V], and lycopene [E1°(Car/Car•+) = 0.60 V] [135, 139]. Furthermore, electrochemical in combination with electron paramagnetic resonance (EPR) spin trapping studies demonstrated that with an increasing first oxidation potential of the carotenoid molecule, the relative scavenging ability also increases, towards peroxyl radicals HOO• formed in a Fenton reaction via the reaction between H2O2 and HO• [139, 140]. The antioxidative effects of astaxanthin were evaluated in 35 individuals who underwent bilateral cataract surgery by monitoring the changes in superoxide scavenging activity, and hydrogen peroxide and total hydroperoxide levels in human aqueous humor [141]. After astaxanthin intake, the superoxide scavenging activity was greatly elevated, while the total hydroperoxide levels were significantly lowered [141].
Based on in vitro studies, astaxanthin esters act as powerful quenchers of singlet oxygen under both hydrophilic and hydrophobic conditions [142]. Kinetic studies of the quenching reaction of singlet molecular oxygen (1O2) by carotenoids and food extracts in solution revealed that carotenoids with 11 carbon atoms involved in the π-conjugation (n), such as astaxanthin, quench 1O2 most effectively [143, 144]. The quenching mechanism was proposed to involve an energy transfer from the singlet oxygen to produce the carotenoid triplet state (3Car) (Equation (4)) because of the two oxo groups in the conjugated astaxanthin system [145–147]. Astaxanthin in the energy-rich triplet state can return to the ground state by dissipating the energy as heat [146]. Recent quantum dynamic calculations and ab initio calculations suggest that once 1O2 and carotenoids make a van der Waals contact, the strong electronic coupling induces an ultrafast 1O2 quenching, which overcomes the large energy gap to the Car•+/O2•– intermediate states [148]. Effects of astaxanthin supplementation on aging have been examined in the model organism Caenorhabditis elegans. Indeed, astaxanthin effectively quenched 1O2, thus protecting the mitochondrion and nucleus from oxidative injury and extending the lifespan [149].
The antioxidant capacity of astaxanthin can be determined from the quenching rate constant of singlet oxygen. A fast quenching rate constant indicates efficient singlet oxygen quenching. The quenching rate constants of singlet oxygen for astaxanthin determined by various methods and in various environments are shown in Table 3. The rate constants of astaxanthin in organic solvents (18–240 × 108 M–1 s–1) are higher than those in micelle (71.1 × 108 M–1 s–1) and liposome (0.19–5.9 × 108 M–1 s–1). The rate constants in heterogeneous environment are lower than those in organic solvents, which can be explained by a slower diffusion of singlet oxygen from the aqueous phase to the micelle or lipid membrane in these environments [150]. In addition, the quenching rate constants of astaxanthin using Rose Bengal- and 12-(1-pyrene)-dodecanoic acid-sensitized photooxidation methods are 100–667-fold (in ethanol) and 1–8-fold (liposomes) higher than those of α-tocopherol [151].
Table 3.
Second-order quenching rate constants for the quenching of singlet oxygen by astaxanthin.
| Quenching rate constant (kq, 108 M–1 s–1) |
Method | Solvent | Ref. |
|---|---|---|---|
| 5.9 | Rose Bengal-sensitized photooxidation | DPP liposomes | [150] |
| 0.19 | Rose Bengal-sensitized photooxidation | Stearylamine and dimyristoylphosphatidylcholine liposomes | [151] |
| 0.19 | 12-(1-Pyrene)-dodecanoic acid-sensitized photooxidation | Dimyristoylphosphatidylcholine liposomes | |
| 140 | Phenazine sensitization | Benzene | [152] |
| 71.1 | Thermal decomposition of 3-(1,4-epidioxy-4-methyl-1,4-dihydro-1-naphthyl) propionic acid as endoperoxide | Triton X-100 solution (5 wt%; 0.02 M phosphate buffer, pH 7.4) | [153] |
| 118 | Thermal decomposition of 3-(1,4-epidioxy-4-methyl-1,4-dihydro-1-naphthyl) propionic acid as endoperoxide | Ethanol:chloroform:D2O (50 : 50 : 1, v/v/v) | [154] |
| 240 | Thermodissociation of the endoperoxide of NDPO2 | Ethanol/chloroform/H2O (50 : 50 : 1, v/v) | [155] |
| 22 | Thermodissociation of the endoperoxide 1,4-dimethyl-naphthalene | CDCl3 | [156] |
| (18) | (CDCl3/CD3OD (2 : 1, v/v)) |
The molecular structure of astaxanthin endows it with unique properties. Hydroxyl and keto groups are present at positions C3 (C3′) and C4 (C4′), respectively, on each ionone ring. The two adjacent oxygen atoms on the cyclohexene ring enable the formation of stable complexes with metal ions (Figure 4), as is also observed in many α-hydroxyketones and hydroxyquinones [157, 158]. The generation of highly reactive oxygen species (HO•) via Fenton and photo-Fenton reactions in vitro can be completely inhibited by the chelation of metal ions Fe2+ or Cu2+ [159]. According to a density functional theory study, astaxanthin may form metal ion complexes with such metal ions as Ca2+, Cu2+, Pb2+, Zn2+, Cd2+, and Hg2+ [160]. Indeed, the interaction of astaxanthin with metal ion complexes, i.e., Ca2+, Zn2+, and Fe2+, was evaluated by mass spectrometry and nuclear magnetic resonance. The analysis revealed that the two oxygen atoms at the terminal cyclohexene rings chelate the metal to form 1 : 1 complexes at low Ca(ClO4)2, Zn(ClO4)2, and Fe(ClO4)2 salt concentrations; at high salt concentrations (>0.2 mM), a 2 : 1 salt to astaxanthin complex is formed [161]. Furthermore, the stability constant of 1 : 1 astaxanthin complex with Fe(ClO4)2 was calculated to be K1 = 3000 M–1, whereas for 1 : 1 complex with Ca(ClO4)2, the stability constants are K1 = 23,000 M–1 and K2 = 5000 M–1. The ability to form chelate complexes with metals could be related to the efficiency with which astaxanthin acts as a protective agent, particularly in inhibiting hydroxyl radicals [135].
Figure 4.
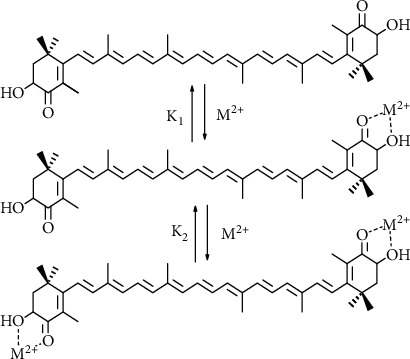
Complexation of astaxanthin with metal ions (adaptatrd from [161]).
The geometrical isomers of astaxanthin play an important role in its antioxidant activity. A microassay evaluating the peroxyl radical scavenging capacity of 15 carotenoid standards has been developed and validated, specifically to study the structure–activity relationship [162]. The values for ROO• scavenging capacity were then calculated using α-tocopherol as a reference compound. Among the carotenoids studied, all-trans astaxanthin was identified as a highly efficient ROO• scavenger (6.50 ± 0.62, α-tocopherol relative) [162]. As determined by a radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity test and lipid peroxidation test, the cis isomers of astaxanthin, especially the 9-cis isomer, showed a higher antioxidant potential in vitro than all-trans astaxanthin [163]. Furthermore, among all astaxanthin isomers, the 9-cis isomer also most effectively inhibits the generation of ROS induced by 6-hydroxydopamine in human neuroblastoma SH-SY5Y cells, as well as the degradation of collagen type II induced by docosahexaenoic acid (DHA) and linoleic acid hydroperoxides [163]. The 13-cis astaxanthin had higher antioxidant activity than all-trans and 9-cis in oxygen radical absorbing capacity assay for lipophilic compounds, photochemiluminescence, and cellular antioxidant activity assay [164]. It was shown that all isomers were relatively stable between pH 2.0–11.6, except for 9-cis and 13-cis astaxanthin at pH 2. Different methods of antioxidant evaluation and antioxidant activity of astaxanthin and cis-trans isomers are summarized in Table 4.
Table 4.
Antioxidant assays and methods for the determination of antioxidant activity for astaxanthin and its isomers.
| Type | Method | Antioxidant activity | Unit | Reference |
|---|---|---|---|---|
| Astaxanthin | Free-radical scavenging activity (DPPH) | 17.5 ± 3.6 | μg/mL | [165] |
| Astaxanthin | 39.1 ± 1.14 | [166] | ||
| All-trans | 5.06 | TE/mg | [164] | |
| 13-cis | 6.49 | |||
| 9-cis | 8.85 | |||
| All-trans | Oxygen radical absorbing capacity for lipophilic compounds | 7.65 | TE/mg | |
| 13-cis | 13.22 | |||
| 9-cis | 11.16 | |||
| All-trans | Photochemiluminescence | 92.22 | μmol TE/g | |
| 13-cis | 117.01 | |||
| 9-cis | 103.41 | |||
| Astaxanthin | Radical scavenging activity (ABTS) | 7.7 ± 0.6 | EC50, μg/mL | [165] |
| Astaxanthin | β-Carotene bleaching activity | 15.1 ± 1.9 | ||
| Astaxanthin | Singlet oxygen scavenging activity | 9.2 ± 0.5 | ||
| Astaxanthin | Ferric reducing antioxidant power | 0.5 | mol α-TE/mol | [167] |
| Astaxanthin | ABTS bleaching assay (αTEAC) | 0.8 | ||
| Astaxanthin | Luminol-chemiluminescence based Peroxyl radical scavenging capacity (LPSC) | 26.3 |
Note: ABTS: 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid; DPPH: 2,2-diphenyl-1-picrylhydrazyl; LPSC: luminal-chemiluminescence peroxyl radical scavenging; mol α-TE/mol: mol α-tocopherol equivalent per mol; TE: Trolox equivalent; αTEAC: α-tocopherol equivalent antioxidant capacity.
5. Conclusion
In the last decade, studies on astaxanthin as a potent therapeutic agent have shown encouraging results. Astaxanthin can potentially be used to address various human health issues, including cancer, cardiovascular and neurodegenerative diseases, and aging. These diseases are mostly associated with inflammation caused by an interaction of nucleic acids and proteins with harmful reactive species. These effects are related to the unique properties of astaxanthin molecular structure that allow it to scavenge reactive species and quench singlet oxygen. Recent experiments have uncovered the contribution of different astaxanthin isomers to antioxidant activities, both in vitro and in vivo. However, there is a lack of research on astaxanthin and its metabolism in biological systems. Future research should focus on the physicochemical properties of different astaxanthin structures, their uptake mechanisms, and the facility of incorporation into metabolic pathways. Molecular studies involving in vitro and in vivo models should also be performed to investigate the nutraceutical and pharmaceutical applications of various astaxanthin isomers.
Acknowledgments
This work was supported by the Ministry of Research and Technology of Indonesia/National Research and Innovation Agency through Basic Research Scheme (grant number 9/E1/KPT/2020) and the Fundamental Research Grant (grant number 005/PER-P2M/UPJ-DIKTI/04.19).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Frank H. A., Cogdell R. J. The photochemistry and function of carotenoids in photosynthesis. In: Young A. J., Britton G., editors. Carotenoids in Photosynthesis. Dordrecht: Springer; 1993. pp. 252–326. [Google Scholar]
- 2.Galasso C., Corinaldesi C., Sansone C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants. 2017;6(4):p. 96. doi: 10.3390/antiox6040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambati R. R., Moi P. S., Ravi S., Aswathanarayana R. G. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications - a review. Marine Drugs. 2014;12(1):128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McWilliams A. FOD025F the Global Market for Carotenoids BCC Research Report Overview the Global Market for Carotenoids. Massachusetts: BBC Research; 2018. [Google Scholar]
- 5.Solymosi K., Latruffe N., Morant-Manceau A., Schoefs B. Food colour additives of natural origin. In: Scotter M. J., editor. Woodhead Publishing Series in Food Science, Technology and Nutrition. Oxford: Woodhead Publishing; 2015. pp. 3–34. [Google Scholar]
- 6.Guerin M., Huntley M. E., Olaizola M. _Haematococcus_ astaxanthin: applications for human health and nutrition. Trends in Biotechnology. 2003;21(5):210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 7.Xie X., Chen Q., Tao J. Astaxanthin promotes Nrf2/ARE signaling to inhibit hg-induced renal fibrosis in GMCs. Marine Drugs. 2018;16(4):117–117. doi: 10.3390/md16040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubo H., Asai K., Kojima K., et al. Astaxanthin suppresses cigarette smoke-induced emphysema through Nrf2 activation in mice. Marine Drugs. 2019;17(12):p. 673. doi: 10.3390/md17120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J. P., Shin J. H., Seo S. H., Kim S. G., Lee S. H., Shin E. H. Effects of antioxidants in reducing accumulation of fat in hepatocyte. International Journal of Molecular Sciences. 2018;19(9, article 2563) doi: 10.3390/ijms19092563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Guo A. L., Pang Y. P., et al. Astaxanthin attenuates environmental tobacco smoke-induced cognitive deficits: a critical role of p38 MAPK. Marine Drugs. 2019;17(1):p. 24. doi: 10.3390/md17010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassett R. G., Coombes J. S. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiology. 2009;5(4):333–342. doi: 10.2217/fca.09.19. [DOI] [PubMed] [Google Scholar]
- 12.Ohgami K., Shiratori K., Kotake S., et al. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investigative Ophthalmology and Visual Science. 2003;44(6):2694–2701. doi: 10.1167/iovs.02-0822. [DOI] [PubMed] [Google Scholar]
- 13.Solomonov Y., Hadad N., Levy R. The combined anti-inflammatory effect of Astaxanthin, Lyc-O-Mato and Carnosic acid in vitro and in vivo in a mouse model of peritonitis. Journal of Nutrition & Food Sciences. 2018;8:1–7. doi: 10.4172/2155-9600.1000653. [DOI] [Google Scholar]
- 14.Lin X., Zhao Y., Li S. Astaxanthin attenuates glutamate-induced apoptosis via inhibition of calcium influx and endoplasmic reticulum stress. European Journal of Pharmacology. 2017;806:43–51. doi: 10.1016/j.ejphar.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y., Tsuji S., Satoh A., Ishikura M., Shirasawa T., Shimizu T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. Journal of Neurochemistry. 2008;107(6):1730–1740. doi: 10.1111/j.1471-4159.2008.05743.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang H. Q., Sun X. B., Xu Y. X., Zhao H., Zhu Q. Y., Zhu C. Q. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Research. 2010;1360:159–167. doi: 10.1016/j.brainres.2010.08.100. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Xia Y., Liu T., et al. Protective effects of astaxanthin on conainduced autoimmune hepatitis by the JNK/p-JNK pathway-mediated inhibition of autophagy and apoptosis. PLoS One. 2015;10(3, article e0120440) doi: 10.1371/journal.pone.0120440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin K. H., Lin K. C., Lu W. J., Thomas P. A., Jayakumar T., Sheu J. R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and Il-2 secretion in primary cultured lymphocytes in vitro and ex vivo. International Journal of Molecular Sciences. 2016;17:1–10. doi: 10.3390/ijms17010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J. S., Chyun J. H., Kim Y. K., Line L. L., Chew B. P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutrition and Metabolism. 2010;7(1):p. 18. doi: 10.1186/1743-7075-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhinaut J., Balourdet A., Teixeira M., Chogne M., Moret Y. A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Scientific Reports. 2017;7(1):p. 12429. doi: 10.1038/s41598-017-12769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davinelli S., Melvang H. M., Andersen L. P., Scapagnini G., Nielsen M. E. Astaxanthin from shrimp cephalothorax stimulates the immune response by enhancing IFN-γ, IL-10, and IL-2 secretion in splenocytes of helicobacter pylori-infected mice. Marine Drugs. 2019;17(7):382–389. doi: 10.3390/md17070382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nai Y., Liu H., Bi X., Gao H., Ren C. Protective effect of astaxanthin on acute cerebral infarction in rats. Human & Experimental Toxicology. 2017;37(9):929–936. doi: 10.1177/0960327117745693. [DOI] [PubMed] [Google Scholar]
- 23.Taksima T., Chonpathompikunlert P., Sroyraya M., Hutamekalin P., Limpawattana M., Klaypradit W. Effects of astaxanthin from shrimp shell on oxidative stress and behavior in animal model of Alzheimer’s disease. Marine Drugs. 2019;17(11):p. 628. doi: 10.3390/md17110628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen X., Huang A., Hu J., et al. Neuroprotective effect of astaxanthin against glutamate-induced cytotoxicity in HT22 cells: involvement of the Akt/GSK-3β pathway. Neuroscience. 2015;303:558–568. doi: 10.1016/j.neuroscience.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Masoudi A., Dargahi L., Abbaszadeh F., et al. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behavioural Brain Research. 2017;329:104–110. doi: 10.1016/j.bbr.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Fakhri S., Dargahi L., Abbaszadeh F., Jorjani M. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Research Bulletin. 2018;143:217–224. doi: 10.1016/j.brainresbull.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Zhang M., Cui Z., Cui H., Cao Y., Zhong C., Wang Y. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neuroscience. 2016;17(1):60–69. doi: 10.1186/s12868-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji X., Peng D., Zhang Y., et al. Astaxanthin improves cognitive performance in mice following mild traumatic brain injury. Brain Research. 2017;1659:88–95. doi: 10.1016/j.brainres.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Fan C. D., Sun J. Y., Fu X. T., et al. Astaxanthin attenuates homocysteine-induced cardiotoxicity in vitro and in vivo by inhibiting mitochondrial dysfunction and oxidative damage. Frontiers in Physiology. 2017;8, article 1041 doi: 10.3389/fphys.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H. Y., Kim Y. M., Hong S. Astaxanthin suppresses the metastasis of colon cancer by inhibiting the MYC- mediated downregulation of microRNA-29a-3p and microRNA-200a. Scientific Reports. 2019;9(1):p. 9457. doi: 10.1038/s41598-019-45924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagendraprabhu P., Sudhandiran G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investigational New Drugs. 2011;29(2):207–224. doi: 10.1007/s10637-009-9342-5. [DOI] [PubMed] [Google Scholar]
- 32.Ko J. C., Chen J. C., Wang T. J., et al. Astaxanthin down-regulates Rad51 expression via inactivation of AKT kinase to enhance mitomycin C-induced cytotoxicity in human non-small cell lung cancer cells. Biochemical Pharmacology. 2016;105:91–100. doi: 10.1016/j.bcp.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Donà G., Andrisani A., Tibaldi E., et al. Astaxanthin prevents human papillomavirus L1 protein binding in human sperm membranes. Marine Drugs. 2018;16(11):p. 427. doi: 10.3390/md16110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J. H., Nam S. W., Kim B. W., et al. Astaxanthin improves stem cell potency via an increase in the proliferation of neural progenitor cells. International Journal of Molecular Sciences. 2010;11(12):5109–5119. doi: 10.3390/ijms11125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao A. R., Sarada R., Shylaja M. D., Ravishankar G. A. Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. Journal of Food Science and Technology. 2015;52(10):6703–6710. doi: 10.1007/s13197-015-1775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuluaga M., Barzegari A., Letourneur D., Gueguen V., Pavon-Djavid G. Oxidative stress regulation on endothelial cells by hydrophilic Astaxanthin complex: chemical, biological, and molecular antioxidant activity evaluation. Oxidative Medicine and Cellular Longevity. 2017;2017:15. doi: 10.1155/2017/8073798.8073798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davinelli S., Nielsen M. E., Scapagnini G. Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients. 2018;10(4):p. 522. doi: 10.3390/nu10040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Luo Q., Rakariyatham K., et al. Antioxidation and anti-ageing activities of different stereoisomeric astaxanthin in vitro and in vivo. Journal of Functional Foods. 2016;25:50–61. doi: 10.1016/j.jff.2016.05.009. [DOI] [Google Scholar]
- 39.Huangfu J., Liu J., Sun Z., et al. Antiaging effects of astaxanthin-rich Alga Haematococcus pluvialis on fruit flies under oxidative stress. Journal of Agricultural and Food Chemistry. 2013;61(32):7800–7804. doi: 10.1021/jf402224w. [DOI] [PubMed] [Google Scholar]
- 40.Balietti M., Giannubilo S. R., Giorgetti B., et al. The effect of astaxanthin on the aging rat brain: gender-related differences in modulating inflammation. Journal of the Science of Food and Agriculture. 2016;96(2):615–618. doi: 10.1002/jsfa.7131. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., Liu S., Wang H., et al. Xanthophyllomyces dendrorhous-derived astaxanthin regulates lipid metabolism and gut microbiota in obese mice induced by a high-fat diet. Marine Drugs. 2019;17(6):p. 337. doi: 10.3390/md17060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dayem A. A., Hossain M., Lee S., et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. International Journal of Molecular Sciences. 2017;18(1):120–121. doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sies H., Jones D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Reviews Molecular Cell Biology. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 44.Ray P. D., Huang B. W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stahl W., Sies H. Antioxidant activity of carotenoids. Molecular Aspects of Medicine. 2003;24(6):345–351. doi: 10.1016/S0098-2997(03)00030-X. [DOI] [PubMed] [Google Scholar]
- 46.Jørgensen K., Skibsted L. H. Carotenoid scavenging of radicals. Effect of carotenoid structure and oxygen partial pressure on antioxidative activity. Z. Lebensm. Unters. Forschung. 1993;196:423–429. doi: 10.1007/BF01190806. [DOI] [PubMed] [Google Scholar]
- 47.Miki W. Biological functions and activities of animal carotenoids. Pure and Applied Chemistry. 1991;63(1):141–146. doi: 10.1351/pac199163010141. [DOI] [Google Scholar]
- 48.Domínguez-Bocanegra A. R., Guerrero Legarreta I., Martinez Jeronimo F., Tomasini Campocosio A. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresource Technology. 2004;92:209–214. doi: 10.1016/j.biortech.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y.-K., Soh C.-W. Accumulation of Astaxanthin in Haematococcus lacustris (Chlorophyta) Journal of Phycology. 1991;27(5):575–577. doi: 10.1111/j.0022-3646.1991.00575.x. [DOI] [Google Scholar]
- 50.Orosa M., Valero J. F., Herrero C., Abalde J. Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnology Letters. 2001;23(13):1079–1085. doi: 10.1023/A:1010510508384. [DOI] [Google Scholar]
- 51.Zhang D. H., Lee Y. K. Enhanced accumulation of secondary carotenoids in a mutant of the green alga, Chlorococcum sp. Journal of Applied Phycology. 1997;9(5):459–463. doi: 10.1023/A:1007902103419. [DOI] [Google Scholar]
- 52.Chen J.-h., Wei D., Lim P. E. Enhanced coproduction of astaxanthin and lipids by the green microalga Chromochloris zofingiensis: Selected phytohormones as positive stimulators. Bioresource Technology. 2020;295, article 122242 doi: 10.1016/j.biortech.2019.122242. [DOI] [PubMed] [Google Scholar]
- 53.Grung M., Liaaen-Jensen S. Algal carotenoids 52∗; secondary carotenoids of algae 3; carotenoids in a natural bloom of Euglena sanguinea. Biochemical Systematics and Ecology. 1993;21(8):757–763. doi: 10.1016/0305-1978(93)90088-9. [DOI] [Google Scholar]
- 54.Sommer F., Agurto C., Henriksen P., Kiørboe T. Astaxanthin in the calanoid copepod Calanus helgolandicus: dynamics of esterification and vertical distribution in the German bight, North Sea. Marine Ecology Progress Series. 2006;319:167–173. doi: 10.3354/meps319167. [DOI] [Google Scholar]
- 55.Lotocka M., Styczyńska-Jurewicz E. Astaxanthin, canthaxanthin and astaxanthin esters in the copepod Acartia bifilosa (Copepoda, Calanoida) during ontogenetic development. Oceanologia. 2001;43:487–497. [Google Scholar]
- 56.Hylander S., Kiørboe T., Snoeijs P., Sommaruga R., Nielsen T. Concentrations of sunscreens and antioxidant pigments in A rctic C alanus spp. in relation to ice cover, ultraviolet radiation, and the phytoplankton spring bloom. Oceanography. 2015;60(6):2197–2206. doi: 10.1002/lno.10194. [DOI] [Google Scholar]
- 57.Yu W., Liu J. Astaxanthin isomers: Selective distribution and isomerization in aquatic animals. Aquaculture. 2019;520, article 73491510.1016/j.aquaculture.2019.734915 [Google Scholar]
- 58.Juhl A. R., Ohman M. D., Goericke R. Astaxanthin in Calanus pacificus: assessment of pigment-based measures of omnivory. Limnology and Oceanography. 1996;41(6):1198–1207. doi: 10.4319/lo.1996.41.6.1198. [DOI] [Google Scholar]
- 59.Caramujo M. J., De Carvalho C. C. C. R., Silva S. J., Carman K. R. Dietary carotenoids regulate astaxanthin content of copepods and modulate their susceptibility to UV light and copper toxicity. Marine Drugs. 2012;10(12):998–1018. doi: 10.3390/md10050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.ŁOtocka M., Styczyńska-Jurewicz E., BŁȩdzki L. A. Changes in carotenoid composition in different developmental stages of copepods: Pseudocalanus acuspes Giesbrecht and Acartia spp. Journal of Plankton Research. 2004;26(2):159–166. doi: 10.1093/plankt/fbh021. [DOI] [Google Scholar]
- 61.Herring P. J. Pigmentation and carotenoid metabolism of the marine isopodIdotea metallica. Journal of the Marine Biological Association of the United Kingdom. 1969;49(3):767–779. doi: 10.1017/S0025315400037279. [DOI] [Google Scholar]
- 62.Yokoyama A., Izumida H., Miki W. Production of astaxanthin and 4-ketozeaxanthin by the marine bacterium, agrobacterium aurantiacum. Bioscience, Biotechnology, and Biochemistry. 2014;58:1842–1844. doi: 10.1271/bbb.58.1842. [DOI] [Google Scholar]
- 63.Asker D. Isolation and characterization of a novel, highly selective Astaxanthin-producing marine bacterium. Journal of Agricultural and Food Chemistry. 2017;65(41):9101–9109. doi: 10.1021/acs.jafc.7b03556. [DOI] [PubMed] [Google Scholar]
- 64.Shahina M., Hameed A., Lin S. Y., et al. Sphingomicrobium astaxanthinifaciens sp. nov., an astaxanthin-producing glycolipid-rich bacterium isolated from surface seawater and emended description of the genus Sphingomicrobium. International Journal of Systematic and Evolutionary Microbiology. 2013;63, Part 9:3415–3422. doi: 10.1099/ijs.0.047704-0. [DOI] [PubMed] [Google Scholar]
- 65.Osanjo G. O., Muthike E. W., Tsuma L., et al. A salt lake extremophile, Paracoccus bogoriensis sp nov., efficiently produces xanthophyll carotenoids. African Journal of Microbiology Research. 2009;3:426–433. [Google Scholar]
- 66.Barredo J.-L. Microbial carotenoids from Bacteria and microalgae. Methods and Protocols. 2012;892 [Google Scholar]
- 67.Matsumoto M., Iwama D., Arakaki A., et al. Altererythrobacter ishigakiensis sp. nov., an astaxanthin-producing bacterium isolated from a marine sediment. International Journal of Systematic and Evolutionary Microbiology. 2011;61(12):2956–2961. doi: 10.1099/ijs.0.024729-0. [DOI] [PubMed] [Google Scholar]
- 68.Lee J. H., Kim Y. S., Choi T. J., Lee W. J., Kim Y. T. Paracoccus haeundaensis sp. nov., a Gram-negative, halophilic, astaxanthin-producing bacterium. International Journal of Systematic and Evolutionary Microbiology. 2004;54(5):1699–1702. doi: 10.1099/ijs.0.63146-0. [DOI] [PubMed] [Google Scholar]
- 69.Tsubokura A., Yoneda H., Mizuta H. Paracoccus carotinifaciens sp. nov., a new aerobic gram-negative astaxanthin-producing bacterium. International Journal of Systematic and Evolutionary Microbiology. 1999;49(1):277–282. doi: 10.1099/00207713-49-1-277. [DOI] [PubMed] [Google Scholar]
- 70.Kusdiyantini E., Gaudin P., Goma G., Blanc P. J. Growth kinetics and astaxanthin production of Phaffia rhodozyma on glycerol as a carbon source during batch fermentation. Biotechnology Letters. 1998;20(10):929–934. [Google Scholar]
- 71.Johnson E. A., Lewis M. J. Astaxanthin formation by the yeast Phaffia rhodozyma. Journal of General Microbiology. 1979;115(1):173–183. doi: 10.1099/00221287-115-1-173. [DOI] [Google Scholar]
- 72.Miura Y., Kondo K., Saito T., Shimada H., Fraser P. D., Misawa N. Production of the carotenoids lycopene, β-carotene, and astaxanthin in the food yeast Candida utilis. Applied and Environmental Microbiology. 1998;64(4):1226–1229. doi: 10.1128/AEM.64.4.1226-1229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calo P., Miguel T., Sieiro C., Velasquez J. B., Vilia T. G. Ketocarotenoids in halobacteria: 3-hydroxy-echinenone and trans-astaxanthin. Journal of Applied Bacteriology. 1995;79(3):282–285. doi: 10.1111/j.1365-2672.1995.tb03138.x. [DOI] [Google Scholar]
- 74.Yamaoka Y. Microorganism and production of carotinoid compounds thereby. US Patent. US 7,374,908 B2, 2008.
- 75.Chien Y. H., Shiau W. C. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus Bate. Journal of Experimental Marine Biology and Ecology. 2005;318(2):201–211. doi: 10.1016/j.jembe.2004.12.016. [DOI] [Google Scholar]
- 76.Pu J., Bechtel P. J., Sathivel S. Extraction of shrimp astaxanthin with flaxseed oil: effects on lipid oxidation and astaxanthin degradation rates. Biosystems Engineering. 2010;107(4):364–371. doi: 10.1016/j.biosystemseng.2010.10.001. [DOI] [Google Scholar]
- 77.Armenta-Lopez R., Guerrero L. I., Huerta S. Astaxanthin Extraction From Shrimp Waste by Lactic Fermentation and Enzymatic Hydrolysis of the Carotenoprotein Complex. Journal of Food Science. 2002;67(3):1002–1006. doi: 10.1111/j.1365-2621.2002.tb09443.x. [DOI] [Google Scholar]
- 78.Parisenti J., Beirão L. H., Maraschin M., et al. Pigmentation and carotenoid content of shrimp fed with Haematococcus pluvialis and soy lecithin. Aquaculture Nutrition. 2011;17(2):e530–e535. doi: 10.1111/j.1365-2095.2010.00794.x. [DOI] [Google Scholar]
- 79.Pan C. H., Chien Y. H. Concentration and composition of astaxanthin in black tiger prawn penaeus monodon postlarvae fed Artemia sp. Nauplii or mauxia Shrimsp acetes intermedius. Journal of the World Aquaculture Society. 2003;34(1):57–65. doi: 10.1111/j.1749-7345.2003.tb00039.x. [DOI] [Google Scholar]
- 80.Visioli F., Artaria C. Astaxanthin in cardiovascular health and disease: mechanisms of action, therapeutic merits, and knowledge gaps. Food & Function. 2017;8(1):39–63. doi: 10.1039/c6fo01721e. [DOI] [PubMed] [Google Scholar]
- 81.Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F. M. Astaxanthin: a review of its chemistry and applications. Critical Reviews in Food Science and Nutrition. 2006;46(2):185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 82.Charest D. J., Balaban M. O., Marshall M. R., Cornell J. A. Astaxanthin extraction from crawfish shells by supercritical CO2with ethanol as Cosolvent. Journal of Aquatic Food Product Technology. 2001;10(3):81–96. doi: 10.1300/J030v10n03_08. [DOI] [Google Scholar]
- 83.Coral-Hinostroza G. N., Bjerkeng B. Astaxanthin from the red crab langostilla (Pleuroncodes planipes): optical R/S isomers and fatty acid moieties of astaxanthin esters. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology. 2002;133(3):437–444. doi: 10.1016/S1096-4959(02)00186-0. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z., Cai C.-f., Cao X.-m., et al. Supplementation of dietary astaxanthin alleviated oxidative damage induced by chronic high pH stress, and enhanced carapace astaxanthin concentration of Chinese mitten crab Eriocheir sinensis. Aquaculture. 2018;483:230–237. doi: 10.1016/j.aquaculture.2017.10.006. [DOI] [Google Scholar]
- 85.Auerswald L., Gäde G. The west coast rock lobsterJasus lalandiias a valuable source for chitin and astaxanthin. African Journal of Marine Science. 2005;27(1):257–264. doi: 10.2989/18142320509504084. [DOI] [Google Scholar]
- 86.Lim K. C., Yusoff F. M., Shariff M., Kamarudin M. S. Astaxanthin as feed supplement in aquatic animals. Reviews in Aquaculture. 2018;10:738–773. doi: 10.1111/raq.12200. [DOI] [Google Scholar]
- 87.Andersson M., Van Nieuwerburgh L., Snoeijs P. Pigment transfer from phytoplankton to zooplankton with emphasis on astaxanthin production in the Baltic Sea food web. Marine Ecology Progress Series. 2003;254:213–224. doi: 10.3354/meps254213. [DOI] [Google Scholar]
- 88.Marine Harvest. Salmon Farming Industry Handbook 2019. 2016.
- 89.Buttle L. G., Crampton V. O., Williams P. D. The effect of feed pigment type on flesh pigment deposition and colour in farmed Atlantic salmon, Salmo salar L. Aquaculture Research. 2001;32(2):103–111. doi: 10.1046/j.1365-2109.2001.00536.x. [DOI] [Google Scholar]
- 90.Lehnert S. J., Christensen K. A., Vandersteen W. E., et al. Carotenoid pigmentation in salmon: variation in expression at BCO2-l locus controls a key fitness trait affecting red coloration. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1913):p. 20191588. doi: 10.1098/rspb.2019.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Megdal P. A., Craft N. A., Handelman G. J. A simplified method to distinguish farmed (Salmo salar) from wild salmon: fatty acid ratios versus astaxanthin chiral isomers. Lipids. 2009;44(6):569–576. doi: 10.1007/s11745-009-3294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah M. M. R., Liang Y., Cheng J. J., Daroch M. Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brendler T., Williamson E. M. Astaxanthin: how much is too much? A safety review. Phytotherapy Research. 2019;33(12):3090–3111. doi: 10.1002/ptr.6514. [DOI] [PubMed] [Google Scholar]
- 94.Udayan A., Arumugam M., Pandey A. Chapter 4 - Nutraceuticals. In: Rastogi R. P., Madamwar D., Pandey A. B. T.-A. G. C., editors. Algae and Cyanobacteria. Amsterdam: Elsevier; 2017. pp. 65–89. [DOI] [Google Scholar]
- 95.Østerlie M., Bjerkeng B., Liaaen-Jensen S. Accumulation of Astaxanthin all-E, 9Z and 13Z geometrical isomers and 3 and 3′RS optical isomers in rainbow trout (Oncorhynchus mykiss) is selective. The Journal of Nutrition. 1999;129(2):391–398. doi: 10.1093/jn/129.2.391. [DOI] [PubMed] [Google Scholar]
- 96.Bjerkeng B., Følling M., Lagocki S., Storebakken T., Olli J. J., Alsted N. Bioavailability of all-E-astaxanthin and Z-isomers of astaxanthin in rainbow trout (Oncorhynchus mykiss) Aquaculture. 1997;157(1-2):63–82. doi: 10.1016/S0044-8486(97)00146-4. [DOI] [Google Scholar]
- 97.Su F., Liu J. The carotenoid characteristics of the important wild shrimp Trachysalambria curvirostris (Stimpson, 1860) in China. Journal of Oceanology and Limnology. 2019;37(2):706–712. doi: 10.1007/s00343-019-8018-z. [DOI] [Google Scholar]
- 98.Su F., Huang B., Liu J. The carotenoids of shrimps (Decapoda: Caridea and Dendrobranchiata) cultured in China. Journal of Crustacean Biology. 2018;38(5):523–530. doi: 10.1093/jcbiol/ruy049. [DOI] [Google Scholar]
- 99.Yuan J., Chen F. Isomerization of trans-Astaxanthin to cis-Isomers in organic solvents. Journal of Agricultural and Food Chemistry. 1999;47(9):3656–3660. doi: 10.1021/jf981319u. [DOI] [PubMed] [Google Scholar]
- 100.Yuan J. P., Chen F. Kinetics for the reversible isomerization reaction of trans-astaxanthin. Food Chemistry. 2001;73(2):131–137. doi: 10.1016/S0308-8146(01)00107-8. [DOI] [Google Scholar]
- 101.Zhao L., Zhao G., Chen F., Wang Z., Wu J., Hu X. Different effects of microwave and ultrasound on the stability of (all-E)-astaxanthin. Journal of Agricultural and Food Chemistry. 2006;54(21):8346–8351. doi: 10.1021/jf061876d. [DOI] [PubMed] [Google Scholar]
- 102.De Bruijn W. J. C., Weesepoel Y., Vincken J. P., Gruppen H. Fatty acids attached to all-trans-astaxanthin alter its cis-trans equilibrium, and consequently its stability, upon light-accelerated autoxidation. Food Chemistry. 2016;194:1108–1115. doi: 10.1016/j.foodchem.2015.08.077. [DOI] [PubMed] [Google Scholar]
- 103.Zhao L., Chen F., Zhao G., Wang Z., Liao X., Hu X. Isomerization of trans-Astaxanthin induced by copper(II) ion in ethanol. Journal of Agricultural and Food Chemistry. 2005;53(24):9620–9623. doi: 10.1021/jf0517750. [DOI] [PubMed] [Google Scholar]
- 104.Köpsel C., Möltgen H., Schuch H., et al. Structure investigations on assembled astaxanthin molecules. Journal of Molecular Structure. 2005;750(1-3):109–115. doi: 10.1016/j.molstruc.2005.02.038. [DOI] [Google Scholar]
- 105.Fuciman M., Durchan M., Šlouf V., Keşan G., Polívka T. Excited-state dynamics of astaxanthin aggregates. Chemical Physics Letters. 2013;568-569:21–25. doi: 10.1016/j.cplett.2013.03.009. [DOI] [Google Scholar]
- 106.Giovannetti R., Alibabaei L., Pucciarelli F. Kinetic model for astaxanthin aggregation in water–methanol mixtures. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2009;73:157–162. doi: 10.1016/j.saa.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 107.Lu L., Hu T., Xu Z. Structural characterization of astaxanthin aggregates as revealed by analysis and simulation of optical spectra. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2017;185:85–92. doi: 10.1016/j.saa.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 108.Zajac G., Machalska E., Kaczor A., Kessler J., Bouř P., Baranska M. Structure of supramolecular astaxanthin aggregates revealed by molecular dynamics and electronic circular dichroism spectroscopy. Physical Chemistry Chemical Physics. 2018;20(26):18038–18046. doi: 10.1039/c8cp01742e. [DOI] [PubMed] [Google Scholar]
- 109.Polyakov N. E., Magyar A., Kispert L. D. Photochemical and optical properties of water-soluble xanthophyll antioxidants: aggregation vs complexation. Journal of Physical Chemistry B. 2013;117(35):10173–10182. doi: 10.1021/jp4062708. [DOI] [PubMed] [Google Scholar]
- 110.Østerlie M., Bjerkeng B., Liaaen-Jensen S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin1. The Journal of Nutritional Biochemistry. 2000;11(10):482–490. doi: 10.1016/S0955-2863(00)00104-2. [DOI] [PubMed] [Google Scholar]
- 111.Rüfer C. E., Moeseneder J., Briviba K., Rechkemmer G., Bub A. Bioavailability of astaxanthin stereoisomers from wild (Oncorhynchus spp.) and aquacultured (Salmo salar) salmon in healthy men: a randomised, double-blind study. British Journal of Nutrition. 2008;99(5):1048–1054. doi: 10.1017/S0007114507845521. [DOI] [PubMed] [Google Scholar]
- 112.Yang C., Zhang H., Liu R., Zhu H., Zhang L., Tsao R. Bioaccessibility, cellular uptake, and transport of Astaxanthin isomers and their Antioxidative effects in human intestinal epithelial Caco-2 cells. Journal of Agricultural and Food Chemistry. 2017;65(47):10223–10232. doi: 10.1021/acs.jafc.7b04254. [DOI] [PubMed] [Google Scholar]
- 113.Trüeb R. M. The impact of oxidative stress on hair. International Journal of Cosmetic Science. 2015;37:25–30. doi: 10.1111/ics.12286. [DOI] [PubMed] [Google Scholar]
- 114.Anderson A. P., Luo X., Russell W., Yin Y. W. Oxidative damage diminishes mitochondrial DNA polymerase replication fidelity. Nucleic Acids Research. 2020;48(2):817–829. doi: 10.1093/nar/gkz1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kruk J., Aboul-Enein H. Y., Kladna A., Bowser J. E. Oxidative stress in biological systems and its relation with pathophysiological functions: the effect of physical activity on cellular redox homeostasis. Free Radical Research. 2019;53(5):497–521. doi: 10.1080/10715762.2019.1612059. [DOI] [PubMed] [Google Scholar]
- 116.Kurutas E. B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutrition Journal. 2015;15:1–22. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galano A. Free Radicals Induced Oxidative Stress at a Molecular Level: The Current Status, Challenges and Perspectives of Computational Chemistry Based Protocols. Journal of the Mexican Chemical Society. 2017;59(4):231–262. doi: 10.29356/jmcs.v59i4.81. [DOI] [Google Scholar]
- 118.Halliwell B., Gutteridge J. M. C. Free Radicals in Biology and Medicine. 5th. Oxford: Oxford University Press; 2015. [Google Scholar]
- 119.Brand M. D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radical Biology and Medicine. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 120.Oury T. D., Crapo J. D., Valnickova Z., Enghild J. J. Human extracellular superoxide dismutase is a tetramer composed of two disulphide-linked dimers: a simplified, high-yield purification of extracellular superoxide dismutase. The Biochemical Journal. 1996;317(1):51–57. doi: 10.1042/bj3170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stanley B. A., Sivakumaran V., Shi S., et al. Thioredoxin reductase-2 is essential for keeping low levels of H2O2 Emission from isolated heart mitochondria. Journal of Biological Chemistry. 2011;286(38):33669–33677. doi: 10.1074/jbc.M111.284612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kudin A. P., Augustynek B., Lehmann A. K., Kovács R., Kunz W. S. The contribution of thioredoxin-2 reductase and glutathione peroxidase to H2O2 detoxification of rat brain mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2012;1817(10):1901–1906. doi: 10.1016/j.bbabio.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 123.Peng Y., Wang C., Xu H. H., Liu Y. N., Zhou F. Binding of α-synuclein with Fe(III) and with Fe(II) and biological implications of the resultant complexes. Journal of Inorganic Biochemistry. 2010;104(4):365–370. doi: 10.1016/j.jinorgbio.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. International Journal of Molecular Sciences. 2019;20(10, article 2407) doi: 10.3390/ijms20102407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kehrer J. P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149(1):43–50. doi: 10.1016/S0300-483X(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 126.Wardman P. Reduction potentials of one Electron couples involving free radicals in aqueous solution. Journal of Physical and Chemical Reference Data. 1989;18(4):1637–1755. doi: 10.1063/1.555843. [DOI] [Google Scholar]
- 127.Buxton G. V., Greenstock C. L., Helman W. P., Ross A. B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. Journal of Physical and Chemical Reference Data. 1988;17(2):513–886. doi: 10.1063/1.555805. [DOI] [Google Scholar]
- 128.Neta P., Huie R. E., Ross A. B. Rate constants for reactions of Peroxyl radicals in fluid solutions. Journal of Physical and Chemical Reference Data. 1990;19(2):413–513. doi: 10.1063/1.555854. [DOI] [Google Scholar]
- 129.Goto S., Kogure K., Abe K., et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2001;1512(2):251–258. doi: 10.1016/S0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 130.Mortensen A., Skibsted L. H., Sampson J., Rice-Evans C., Everett S. A. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Letters. 1997;418(1-2):91–97. doi: 10.1016/S0014-5793(97)01355-0. [DOI] [PubMed] [Google Scholar]
- 131.Edge R., McGarvey D. J., Truscott T. G. The carotenoids as anti-oxidants — a review. Journal of Photochemistry and Photobiology B: Biology. 1997;41(3):189–200. doi: 10.1016/S1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 132.Young A. J., Lowe G. M. Antioxidant and prooxidant properties of carotenoids. Archives of Biochemistry and Biophysics. 2001;385(1):20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 133.Woodall A. A., Lee S. W. M., Weesie R. J., Jackson M. J., Britton G. Oxidation of carotenoids by free radicals: relationship between structure and reactivity. Biochimica et Biophysica Acta (BBA) - General Subjects. 1997;1336(1):33–42. doi: 10.1016/S0304-4165(97)00006-8. [DOI] [PubMed] [Google Scholar]
- 134.Han R. M., Zhang J. P., Skibsted L. H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules. 2012;17(2):2140–2160. doi: 10.3390/molecules17022140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Focsan A. L., Polyakov N. E., Kispert L. D. Photo protection of haematococcus pluvialis algae by astaxanthin: unique properties of astaxanthin deduced by epr, optical and electrochemical studies. Antioxidants. 2017;6(4):p. 80. doi: 10.3390/antiox6040080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodrigues E., Mariutti L. R. B., Mercadante A. Z. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Marine Drugs. 2012;10:1784–1798. doi: 10.3390/md10081784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang J., Sun Z., Sun P., Chen T., Chen F. Microalgal carotenoids: beneficial effects and potential in human health. Food & Function. 2014;5(3):413–425. doi: 10.1039/c3fo60607d. [DOI] [PubMed] [Google Scholar]
- 138.Naguib Y. M. A. Antioxidant activities of astaxanthin and related carotenoids. Journal of Agricultural and Food Chemistry. 2000;48(4):1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 139.Focsan A. L., Pan S., Kispert L. D. Electrochemical study of astaxanthin and Astaxanthinn-Octanoic monoester and diester: tendency to form radicals. The Journal of Physical Chemistry B. 2014;118(9):2331–2339. doi: 10.1021/jp4121436. [DOI] [PubMed] [Google Scholar]
- 140.Polyakov N. E., Kruppa A. I., Leshina T. V., Konovalova T. A., Kispert L. D. Carotenoids as antioxidants: spin trapping EPR andoptical study. Free Radical Biology and Medicine. 2001;31(1):43–52. doi: 10.1016/S0891-5849(01)00547-0. [DOI] [PubMed] [Google Scholar]
- 141.Hashimoto H., Arai K., Hayashi S., Okamoto H., Takahashi J. Isoflavone intake inhibits the development of 7,12-dimethylbenz(a)anthracene(DMBA)-induced mammary tumors in normal and ovariectomized rats. Journal of Clinical Biochemistry and Nutrition. 2014;54:31–38. doi: 10.3164/jcbn.13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kobayashi M., Sakamoto Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnology Letters. 1999;21(4):265–269. doi: 10.1023/A:1005445927433. [DOI] [Google Scholar]
- 143.Ouchi A., Aizawa K., Iwasaki Y., et al. Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. Development of a singlet oxygen absorption capacity (SOAC) assay method. Journal of Agricultural and Food Chemistry. 2010;58:9967–9978. doi: 10.1021/jf101947a. [DOI] [PubMed] [Google Scholar]
- 144.Böhm F., Edge R., Truscott G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: potential effects for human health. Molecular Nutrition & Food Research. 2012;56(2):205–216. doi: 10.1002/mnfr.201100222. [DOI] [PubMed] [Google Scholar]
- 145.Edge R., George Truscott T. Carotenoid Radicals and the Interaction of Carotenoids with Active Oxygen Species. In: Frank H. A., Young A. J., Britton G., Cogdell R. J., editors. The Photochemistry of Carotenoids. Dordrecht: Springer; 1999. pp. 223–234. (Advances in Photosynthesis and Respiration). [DOI] [Google Scholar]
- 146.Sandmann G. Antioxidant protection from UV- and light-stress related to carotenoid structures. Antioxidants. 2019;8(7):p. 219. doi: 10.3390/antiox8070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nielsen B. R., Jørgensen K., Skibsted L. H. Triplet—triplet extinction coefficients, rate constants of triplet decay and rate constant of anthracene triplet sensitization by laser flash photolysis of astaxanthin, β-carotene, canthaxanthin and zeaxanthin in deaerated toluene at 298 K. Journal of Photochemistry and Photobiology A: Chemistry. 1998;112(2-3):127–133. doi: 10.1016/S1010-6030(97)00285-2. [DOI] [Google Scholar]
- 148.Tamura H., Ishikita H., Accepted J. Quenching of singlet oxygen by carotenoids via ultrafast super-exchange dynamics. The Journal of Physical Chemistry A. 2020;124(25):5081–5088. doi: 10.1021/acs.jpca.0c02228. [DOI] [PubMed] [Google Scholar]
- 149.Yazaki K., Yoshikoshi C., Oshiro S., Yanase S. Supplemental cellular protection by a carotenoid extends lifespan via ins/IGF-1 signaling in caenorhabditis elegans. Oxidative Medicine and Cellular Longevity. 2011;2011:9. doi: 10.1155/2011/596240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cantrell A., McGarvey D. J., Truscott T. G., Rancan F., Böhm F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Archives of Biochemistry and Biophysics. 2003;412(1):47–54. doi: 10.1016/S0003-9861(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 151.Fukuzawa K., Inokami Y., Tokumura A., Terao J., Suzuki A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and α-tocopherol in liposomes. Lipids. 1998;33(8):751–756. doi: 10.1007/s11745-998-0266-y. [DOI] [PubMed] [Google Scholar]
- 152.Conn P. F., Schalch W., Truscott T. G. The singlet oxygen and carotenoid interaction. Journal of Photochemistry and Photobiology B: Biology. 1991;11(1):41–47. doi: 10.1016/1011-1344(91)80266-K. [DOI] [PubMed] [Google Scholar]
- 153.Mukai K., Ouchi A., Azuma N., Takahashi S., Aizawa K., Nagaoka S. Development of a singlet oxygen absorption capacity (SOAC) assay method. Measurements of the SOAC values for carotenoids and α-tocopherol in an aqueous triton X-100 micellar solution. Journal of Agricultural and Food Chemistry. 2017;65(4):784–792. doi: 10.1021/acs.jafc.6b04329. [DOI] [PubMed] [Google Scholar]
- 154.Iwasaki Y., Takahashi S., Aizawa K., Mukai K. Development of singlet oxygen absorption capacity (SOAC) assay method. 4. Measurements of the SOAC values for vegetable and fruit extracts. Bioscience, Biotechnology, and Biochemistry. 2014;79:280–291. doi: 10.1080/09168451.2014.972329. [DOI] [PubMed] [Google Scholar]
- 155.Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Archives of Biochemistry and Biophysics. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 156.Shimidzu N., Goto M., Miki W. Carotenoids as singlet oxygen quenchers in marine organisms. Fisheries Science. 1996;62(1):134–137. doi: 10.2331/fishsci.62.134. [DOI] [Google Scholar]
- 157.Kumbhar A., Padhye S., Ross D. Cytotoxic properties of iron-hydroxynaphthoquinone complexes in rat hepatocytes. Biometals. 1996;9(3):235–240. doi: 10.1007/BF00817921. [DOI] [PubMed] [Google Scholar]
- 158.Gokhale N., Padhye S., Newton C., Pritchard R. Hydroxynaphthoquinone metal complexes as antitumor agents X: synthesis, structure, spectroscopy and in vitro antitumor activity of 3-methyl-phenylazo lawsone derivatives and their metal complexes against human breast cancer cell line MCF-7. Metal-Based Drugs. 2000;7(3):128. doi: 10.1155/MBD.2000.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Timoshnikov V. A., Kobzeva T. V., Polyakov N. E., Kontoghiorghes G. J. Inhibition of Fe2+- and Fe3+- induced hydroxyl radical production by the iron-chelating drug deferiprone. Free Radical Biology and Medicine. 2015;78:118–122. doi: 10.1016/j.freeradbiomed.2014.10.513. [DOI] [PubMed] [Google Scholar]
- 160.Hernández-Marin E., Barbosa A., Martínez A. The metal cation chelating capacity of astaxanthin. Does this have any influence on antiradical activity? Molecules. 2012;17(1):1039–1054. doi: 10.3390/molecules17011039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Polyakov N. E., Focsan A. L., Bowman M. K., Kispert L. D. Free radical formation in novel carotenoid metal ion complexes of astaxanthin. Journal of Physical Chemistry B. 2010;114(50):16968–16977. doi: 10.1021/jp109039v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rodrigues E., Mariutti L. R. B., Chisté R. C., Mercadante A. Z. Development of a novel micro-assay for evaluation of peroxyl radical scavenger capacity: application to carotenoids and structure-activity relationship. Food Chemistry. 2012;135(3):2103–2111. doi: 10.1016/j.foodchem.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 163.Liu X., Osawa T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochemical and Biophysical Research Communications. 2007;357(1):187–193. doi: 10.1016/j.bbrc.2007.03.120. [DOI] [PubMed] [Google Scholar]
- 164.Yang C., Zhang L., Zhang H., et al. Rapid and efficient conversion of all-E-astaxanthin to 9Z- and 13Z-isomers and assessment of their stability and antioxidant activities. Journal of Agricultural and Food Chemistry. 2017;65(4):818–826. doi: 10.1021/acs.jafc.6b04962. [DOI] [PubMed] [Google Scholar]
- 165.Chintong S., Phatvej W., Rerk-Am U., Waiprib Y., Klaypradit W. In vitro antioxidant, antityrosinase, and cytotoxic activities of astaxanthin from Shrimp Waste. Antioxidants. 2019;8(5):p. 128. doi: 10.3390/antiox8050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Santhose I., Kanna R. Antioxidant and anti-skin cancer potential of a Ketocarotenoid pigment Astaxanthin isolated from a green microalga Haematococcus pluvialis Flotow. International Journal of Scientific and Engineering Research. 2016;7:902–916. [Google Scholar]
- 167.Müller L., Fröhlich K., Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chemistry. 2011;129(1):139–148. doi: 10.1016/j.foodchem.2011.04.045. [DOI] [Google Scholar]


