Abstract
Background
The ferocious global assault of COVID-19 continues. Critically ill patients witnessed significantly higher mortality than severe and moderate ones. Herein, we aim to comprehensively delineate clinical features of COVID-19 and explore risk factors of developing critical disease.
Methods
This is a Mini-national multicenter, retrospective, cohort study involving 2,387 consecutive COVID-19 inpatients that underwent discharge or death between January 27 and March 21, 2020. After quality control, 2,044 COVID-19 inpatients were enrolled. Electronic medical records were collected to identify the risk factors of developing critical COVID-19.
Findings
The severity of COVID-19 climbed up straightly with age. Critical group was characterized by higher proportion of dyspnea, systemic organ damage, and long-lasting inflammatory storm. All-cause mortality of critical group was 85•45%, by contrast with 0•58% for severe group and 0•18% for moderate group. Logistic regression revealed that sex was an effect modifier for hypertension and coronary heart disease (CHD), where hypertension and CHD were risk factors solely in males. Multivariable regression showed increasing odds of critical illness associated with hypertension, CHD, tumor, and age ≥ 60 years for male, and chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), tumor, and age ≥ 60 years for female.
Interpretation
We provide comprehensive front-line information about different severity of COVID-19 and insights into different risk factors associated with critical COVID-19 between sexes. These results highlight the significance of dividing risk factors between sexes in clinical and epidemiologic works of COVID-19, and perhaps other coronavirus appearing in future.
Funding
National Science Foundation of China.
Keywords: Covid-19, Risk factors, Severity of disease, Sex
Research in context.
Evidence before this study
We searched PubMed for articles up to May 23, 2020, using the keywords '2019 novel coronavirus', 'COVID-19′, 'SARS-CoV-2′, and 'coronavirus' for articles published without any language restrictions. We found that the mortality of critically ill COVID-19 patients is significantly greater than that of severely and moderately ill patients. However, risk factors of developing critical COVID-19 remain enigmas, especially their difference between sexes.
Added value of this study
We found that the clinical features and course of COVID-19 were similar between moderate and severe group, but critical patients have dramatically distinct features. We revealed risk factors of developing critical COVID-19 differ between sexes, where hypertension and CHD remarkably increased the risk of critical disease in male, but barely affected female patients.
Implications of all the available evidence
These results highlight the significance of dividing risk factors between sexes in clinical and epidemiologic works of COVID-19, and perhaps other coronavirus appearing in future.
Alt-text: Unlabelled box
1. Introduction
From December 2019, a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), outbroke in Wuhan, China and ferociously spread around the world, causing major public health emergency of global concern. The WHO named the pneumonia caused by SARS-CoV-2 as coronavirus disease 2019 (COVID-19) [1], which has begun behaving greatly like a once-in-a-century pandemic [2]. As of June 18, 2020, there have been 8242,999 confirmed cases of COVID-19, including 445,535 deaths, reported to WHO globally [3].
Though clinical characteristics and risk factors for mortality of COVID-19 have been portrayed [4], [5], [6], [7], [8], [9], we feel the necessity to delineate them more comprehensively. Available clinical information well reflected the early stage of COVID-19 outbreak, but witnessed limited patients with definite outcomes. Clinical data that depict the peak of the pandemic and document the clinical course from admission to discharge or death will contribute to better understanding of progression and remission of COVID-19. Moreover, mortality of critically ill patients is 49.0%, by contrast with 2.3% for overall COVID-19 patients [5]. unraveling risk factors associated with developing into critical illness will facilitate early identification of high-risk patients.
Here, we provide detailed clinical information of COVID-19 patients admitted to two branches of Tongji Hospital, the unique designed hospital for severely and critically ill patients in Wuhan and the epicenter of COVID-19 pandemic, where 40 first-class medical teams from 13 provinces cooperated and engaged with COVID-19 at the peak of COVID-19 crisis in China. We aim to delineate comprehensive features of COVID-19 patients with known outcomes (death or discharge) as of March 21, 2020 and explore risk factors accounting for developing into severe/critical illness.
2. Methods
2.1. Study design and participants
This study was carried out in two branches of Tongji hospital (Optical Valley Branch and Sino-French New City Branch), which were reinvented imminently and designated at the peak of outbreak in Wuhan to receive moderate, severe, and critical COVID-19 patients. 40 medical teams from 13 provinces worked here simultaneously, each of whom in charge of one ward unit independently with slightly different therapeutic regimen, making this study as a mini-national multi-center study. In this retrospective cohort study, we included 2387 consecutive patients that underwent discharge or death between January 27 (the admission date of the first COVID-19 patients) and March 21, 2020 (the beginning of transferring patients to other hospitals). We believe that this study period could best reflect the outbreak period of the disease and best eliminate potential bias.
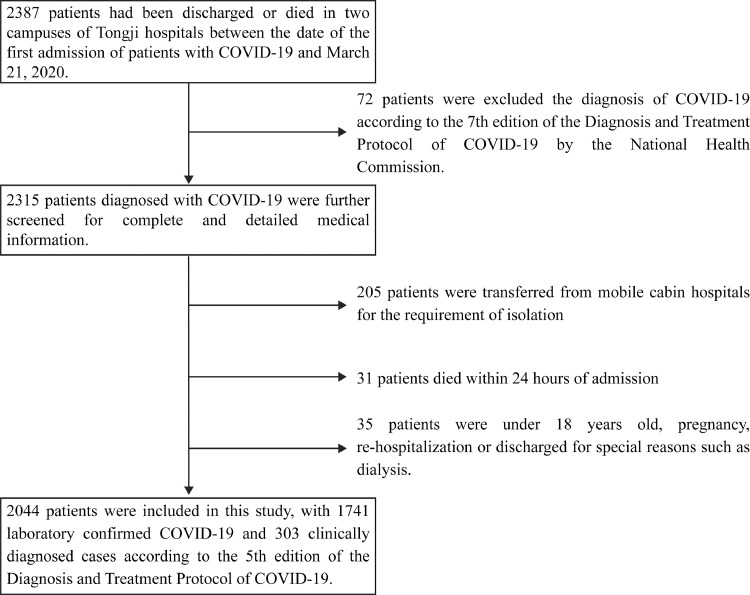
72 patients failed to meet diagnostic criteria and were excluded according to Guidance for COVID-19 (7th edition) released by the National Health Commission of China [10]. We further excluded 205 patients transferred from mobile cabin hospitals for isolation, 31 patients died within 24 hrs of admission lacking blood test and medical history, and 35 patients with ages of less than 18, pregnant, re-hospitalization or discharge for special reasons such as dialysis. Finally, 2044 patients were included, with 1741 laboratory-confirmed and 303 clinically-diagnosed cases (before antibody tests were available) according to Guidance for COVID-19 (5th edition) released by the National Health Commission of China [11]. The flowchart of the study is shown in Fig. 1. The study was approved by the Medical Ethical Committee of Tongji Hospital (TJIRB20200406) with written informed consent waived. The trial has been registered in Chinese Clinical Trial Registry (ChiCTR2000032161).
Fig. 1.
the flowchart of this study.
2.2. Data collection and oversight
Epidemiological, demographic, laboratory, radiological, treatment, nursing, and outcome data were obtained from electronic medical record using a designed data collection form. Experienced clinicians from Tongji Hospital analyzed patients’ medical records. Trained and experienced researchers entered and double-checked data in a computerized database. All authors reviewed the manuscript and guarantee the accuracy and completeness of the data.
2.3. Laboratory procedures and treatment
Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) Assay for SARS-CoV-2 is interpreted elsewhere [12,13]. Briefly, RT-PCR testing was used according to the recommended protocol [12]. The primers and probes for SARS-CoV-2 detection were selected according to the National Pathogen Resource Center (National Institute for Viral Disease Control and Prevention, China CDC) [12]. Serum quantitative and qualitative SARS-CoV-2 IgM and IgG antibodies were detected using commercial chemiluminescence kit (iFlash-SARS-CoV-2 IgM (Cat. No. C86095M), iFlash-SARS-CoV-2 IgG (Cat. No. C86095G), SHENZHEN YHLO BIOTECH CO., LTD.). Blood tests, radiology (Chest radiographs or CT scan) were performed on admission, and the time point of re-examinations and treatments were determined by physicians according to guidance aforementioned and individual differences.
2.4. Criteria of COVID-19 severity
COVID-19 severity of each patient was classified based on the most severe condition during the entire course of the disease. According to the Guidance for Coronavirus Disease 2019 (7th edition) released by the National Health Commission of China [10], COVID-19 was diagnosed and divided into four groups (mild, moderate, severe and critical) [12,14]. In mild group, the symptoms are mild and had no abnormal radiological findings. For the three groups involved in our study, patients in moderate group have symptoms including fever and respiratory tract symptoms, and an imaging finding of pneumonia, but fails to meet the criteria for severe group and critical group. COVID-19 patients that meet any of the following criteria belong to severe group: 1) dyspnea or respiratory rate ≥ 30 breaths/min; 2) SPO2 ≤ 93•00% at a rest state; 3) arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg; 4) patients with > 50.00% lesion progression in lung imaging within 48 hrs. Critical group is defined as patients that meet any of the following criteria: 1) presence of respiratory failure and mechanical ventilation is required; 2) occurrence of shock; 3) complicated with other organ failure that requires admission to ICU.
2.5. Definitions
Fever was defined as axillary temperature ≥ 37.30 °C (high fever ≥ 39.00 °C). Illness onset referred to the appearance of pneumonia-related symptoms, such as fever, cough, dyspnea, etc. Number of comorbidities was referred to the number of different complications including hypertension, diabetes, coronary heart disease (CHD), chronic liver disease (CLD), chronic kidney disease (CKD) and chronic obstructive pulmonary disease (COPD). Sepsis and septic shock were defined according to the 2016 Third International Consensus Definition for Sepsis and Septic Shock [13]. Secondary infection was diagnosed when patient had a positive culture of a new pathogen obtained from lower respiratory tract specimens (qualified sputum, endotracheal aspirate, or bronchoalveolar lavage fluid) or blood samples after admission. Acute kidney injury was diagnosed according to the KDIGO guidelines [15]. Acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin Definition [16]. Acute cardiac injury was diagnosed if serum level of cardiac troponin I was above the 99th percentile upper reference limit. Coagulopathy was defined as a 3-second extension of prothrombin time (PT) or a 10-second extension of activated partial thromboplastin time (APTT). Hypoproteinaemia was defined as serum level albumin ≤ 30 g/L. Sequential (Sepsis-related) Organ Failure Assessment (SOFA) score was referred to previous reported [13]. Acute heart failure was diagnosed with typical symptoms (e.g. breathlessness) and accompanied signs (e.g. elevated jugular venous pressure) caused by a cardiac abnormality [17]. Acute liver injury was defined as serum level of total bilirubin ≥ 3 mg/dl and an acute increase in ALT of at least five times the upper limit of the normal range and/or an increase in alkaline phosphatase of at least twice the upper limit of the normal [12].
2.6. Statistical analysis
Continuous variables were expressed as medians and interquartile ranges (IQR). Categorical variables were summarized as n (%) in each category. Kruskal-Wallis test, chi-square test, and Fisher's exact test were applied where appropriate. Uni- and multivariable binary logistic regression were used to explore the risk factors. Dyspnea and laboratory indexes were excluded from regression analysis, as they were considered to be reflections of disease severity. Any variable with P < 0•25 in univariate analysis was included in the model building of multivariable logistic regression, and then a backward elimination strategy was used to delete variables that contribute the least until the final model. The odds ratio (OR) along with the 95% confidence interval (95% CI) was reported. Variables with no differences, too small to analysis odds ratios, and colinear with the severity of disease were excluded from analysis. The effect modification between sexes and other factors were tested using logistic regression models containing the interaction terms (sex and hypertension, etc.). The missing data of demographics, clinical, laboratory and radiological characteristics were dealt as missing values, while there were limited missing data in logistic regression analysis (2041 records of symptoms and 2039 records of comorbidities). A two-sided P value of less than 0•05 was considered statistically significant. Statistical analyses were done using the STATA software (version 15.1).
2.7. Role of funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (QLG) had full access to all data in the study and final responsibility for the study.
3. Results
3.1. Demographics and characteristics in different severity of COVID-19 patients
Demographics and characteristics of COVID-19 patients are provided in Table 1. Exact cases in charge by each medical team are shown in Fig. 2. Among 2044 patients, there were 1087 (53%) moderate, 689 (34%) severe, and 268 (13%) critical cases. The median age of critical group (69•0, IQR: 62•0–77•0) was significantly greater (P = 0•001) than that of severe group (64•0, IQR: 54•0–71•0) and moderate group (59.0, IQR: 46•0–67•0). 80•22% of patients in critical group was aged ≥ 60, by contrast with 61.54% for severe group and 49•49% for moderate group. Generally, 57•63% of all patients and 79•7% of critical group had at least one comorbidity. Critical group witnessed more comorbidities (hypertension, 56•02%; diabetes, 22•56%; coronary heart disease (CHD), 17•29%; chronic kidney disease (CKD), 3•01%; tumor, 7•14%; chronic obstructive pulmonary disease (COPD), 3•01%). For symptoms, fever was the most prevalent symptom in all patients (80•55%). Cough, dyspnea, and disorders of consciousness were more prevalent in critical group.
Table 1.
Characteristics of patients with different disease severity of COVID-19 on admission.
| Disease severity | |||||
|---|---|---|---|---|---|
| Total (n = 2044) | Moderate (n = 1087) | Severe (n = 689) | Critical (n = 268) | p value | |
| Age, years | 62•0 (51•0–70•0) | 59•0 (46•0–67•0) | 64•0 (54•0–71•0) | 69•0 (62•0–77•0) | 0•0001 |
| ≥60 | 1177 (57•58%) | 538 (49•49%) | 424 (61•54%) | 215 (80•22%) | <0•001 |
| Sex | ‥ | ‥ | ‥ | ‥ | <0•001 |
| Female | 1044 (51•08%) | 612 (56•30%) | 340 (49•35%) | 92 (34•33%) | ‥ |
| Male | 1000 (48•92%) | 475 (43•70%) | 349 (50•65%) | 176 (65•67%) | ‥ |
| Presence of comorbidity | 1175/2039 (57•63%) | 540/1086 (49•72%) | 423/687 (61•57%) | 212/266 (79•70%) | <0•001 |
| Hypertension | 810/2039 (39•73%) | 3567/1086 (32•87%) | 304/687 (44•25%) | 149/266 (56•02%) | <0•001 |
| Diabetes | 341/2039 (16•72%) | 140/1086 (12•89%) | 141/687 (20•52%) | 60/266 (22•56%) | <0•001 |
| CHD | 199/2039 (9•76%) | 79/1086 (7•27%) | 74/687 (10•77%) | 46/266 (17•29%) | <0•001 |
| CLD | 78/2041 (3•82%) | 44/1086 (4•05%) | 22 (3•19%) | 12/266 (4•51%) | 0•538 |
| Tumor | 63/2039 (3•09%) | 25/1086 (2•30%) | 19/687 (2•77%) | 19/266 (7•14%) | <0•001 |
| CKD | 32/2039 (1•57%) | 14/1086 (1•29%) | 10/687 (1•46%) | 8/266 (3•01%) | 0•124 |
| COPD | 18/2039 (0•88%) | 3/1086 (0•28%) | 7/687 (1•02%) | 8/266 (3•01%) | <0•001 |
| Number of comorbidities | 1•0 (0•0–2•0) | 1•0 (0•0–1•0) | 1•0 (0•0–2•0) | 1•0 (1•0–2•0) | 0•0001 |
| Temperature, °C | 38•3 (37•5–39•0) | 38•0 (37•2–38•9) | 38•5 (37•8–39•0) | 38•3 (37•8–38•9) | 0•0001 |
| Fever ≥37•0 | 1644/2041 (80•55%) | 834/1086 (76•80%) | 589/688 (85•61%) | 221/267 (82•77%) | <0•001 |
| High fever ≥39•0 | 521/2027 (25•70%) | 243/1077 (22•56%) | 213/688 (30•96%) | 65/262 (24•81%) | <0•001 |
| Cough | 1487/2041 (72•86%) | 755/1086 (69•52%) | 517/688 (75•15%) | 215/267 (80•52%) | <0•001 |
| Dyspnea | 870/2041 (42•63%) | 351/1086 (32•32%) | 338/688 (49•13%) | 181/267 (67•79%) | <0•001 |
| Sputum | 805/2041 (39•44%) | 388/1086 (35•73%) | 302/688 (43•90%) | 115/267 (43•07%) | 0•001 |
| Fatigue | 736/2041 (36•06%) | 370/1086 (34•07%) | 258/688 (37•50%) | 108/267 (40•45%) | 0•095 |
| Diarrhea | 488/2041 (23•91%) | 231/1086 (21•27%) | 195/688 (28•34%) | 62/267 (23•22%) | 0•003 |
| Myalgia | 406/2041 (19•89%) | 227/1086 (20•90%) | 131/688 (19•04%) | 48/267 (17•98%) | 0•444 |
| Vomiting | 93/2041 (4•56%) | 46/1086 (4•24%) | 35/688 (5•09%) | 12/267 (4•49%) | 0•703 |
| Disorders of consciousness at admission | 63 (3•08%) | 4 (0•37%) | 6 (0•87%) | 53 (19•78%) | <0•001 |
| Respiratory rate, per min | 21•0 (20•0–24•0) | 20•0 (20•0–22•0) | 22•0 (20•0–24•0) | 29•0 (22•0–33•0) | 0•0001 |
| ≥24 | 525/2039 (25•75%) | 117/1086 (10•77%) | 223 (32•37%) | 185/264 (70•08%) | <0•001 |
| Mean arterial pressure, mmHg | 96•7 (88•7–105•7) | 96•7 (88•7–105•7) | 96•0 (88•7–105•0) | 97•0 (89•7–106•7) | 0•4391 |
| <70 | 20/2041 (0•98%) | 6/1085 (0•55%) | 6/688 (0•87%) | 8 (2•99%) | 0•001 |
| SpO2,% | 95•0 (92•0–97•0) | 96•0 (95•0–98•0) | 92•0 (90•0–95•0) | 86•0 (76•0–92•0) | 0•0001 |
| ≤93 | 562/2038 (27•58%) | 0/1084 (0•00%) | 352/687 (51•24%) | 210/267 (78•65%) | <0•001 |
| SOFA score at admission | 1•0 (0•0–2•0) | 0•0 (0•0–0•0) | 1•0 (1•0–2•0) | 5•0 (3•5–6•5) | 0•0001 |
| 0–1 | 594/1211 (49•05%) | 177/255 (69•41%) | 416/688 (60•47%) | 1/268 (0•37%) | <0•001 |
| 2–3 | 372/1211 (30•72%) | 69/255 (27•06%) | 237/688 (34•45%) | 66 /268(24•63%) | ‥ |
| ≥4 | 245/1211 (20•23%) | 9/255 (3•53%) | 35/688 (5•09%) | 201/268 (75•00%) | ‥ |
| Time from illness onset to hospital visit, days | 6•0 (3•0–11•0) | 7•0 (3•0–12•0) | 6•0 (3•0–9•0) | 5•0 (2•0–9•0) | 0•0001 |
| Time from illness onset to hospital admission, days | 12•0 (8•0–18•0) | 13•0 (8•0–20•0) | 11•0 (8•0–16•0) | 11•0 (7•0–16•0) | 0•0001 |
| Laboratory findings | |||||
| White blood cell count, × 109 per L | 5•7 (4•4–7•4) | 5•3 (4•2–6•7) | 5•6 (4•5–7•2) | 9•1 (6•3–12•9) | 0•0001 |
| >10 | 192/2042 (9•40%) | 28/1086 (2•58%) | 50 (7•26%) | 114/267 (42•70%) | <0•001 |
| Lymphocyte count, × 109 per L | 1•1 (0•8–1•6) | 1•4 (1•0–1•8) | 1•0 (0•7–1•4) | 0•6 (0•4–0•8) | 0•0001 |
| <0•8 | 555/2041 (27•19%) | 154/1085 (14•19%) | 214 (31•06%) | 187/267 (70•04%) | <0•001 |
| Hemoglobin, g/L | 126•0 (116•0–137•0) | 126•0 (116•0–137•0) | 126•0 (116•0–136•0) | 128•0 (115•0–143•0) | 0•3030 |
| Anemia | 392/2041 (19•21%) | 193/1085 (17•79%) | 132 (19•16%) | 67/267 (25•09%) | 0•025 |
| Platelet count, × 109 per L | 222•0 (167•0–291•5) | 229•0 (179•5–297•0) | 225•0 (170•0–296•0) | 165•5 (117•0–235•0) | 0•0001 |
| <100 | 96/2036 (4•72%) | 28/1084 (2•58%) | 18/686 (2•62%) | 50/266 (18•80%) | <0•001 |
| Alanine aminotransferase, U/L | 22•0 (14•5–37•0) | 20•0 (14•0–34•0) | 24•0 (15•0–41•0) | 27•5 (18•0–43•0) | 0•0001 |
| >40 | 446 (21•82%) | 198 (18•22%) | 173 (25•11%) | 75 (27•99%) | <0•001 |
| Aspartate aminotransferase, U/L | 25•0 (19•0–38•0) | 22•0 (18•0–31•0) | 28•0 (20•0–41•0) | 39•0 (27•5–59•0) | 0•0001 |
| >40 | 430/2036 (21•12%) | 130/1083 (12•00%) | 173/685 (25•26%) | 127 (47•39%) | <0•001 |
| Albumin, g/L | 35•5 (32•0–39•4) | 37•5 (34•3–40•9) | 34•2 (31•3–37•6) | 31•0 (27•9–34•0) | 0•0001 |
| ≤30 | 292/2041 (14•31%) | 64/1085 (5•90%) | 113/688 (16•42%) | 115 (42•91%) | <0•001 |
| Total bilirubin, μmol/L | 8•7 (6•5–12•1) | 8•0 (6•1–10•8) | 8•9 (6•6–12•3) | 12•1 (8•5–17•5) | 0•0001 |
| ≥20 | 97/2041 (4•75%) | 20/1085 (1•84%) | 30/688 (4•36%) | 47 (17•54%) | <0•001 |
| Lactate dehydrogenase, U/L | 262•0 (203•0–351•0) | 224•0 (187•0–283•0) | 290•0 (232•0–374•0) | 480•5 (371•0–634•0) | 0•0001 |
| ≥245 | 1156/2038 (56•72%) | 426/1086 (39•23%) | 478/686 (69•68%) | 252/266 (94•74%) | <0•001 |
| Blood urea nitrogen, mmol/L | 4•4 (3•4–5•8) | 4•1 (3•3–5•1) | 4•3 (3•3–5•5) | 8•0 (5•5–12•1) | 0•0001 |
| ≥10 | 144 (7•05%) | 20 (1•84%) | 31 (4•50%) | 93 (34•70%) | <0•001 |
| Creatinine, μmol/L | 68•0 (57•0–85•0) | 65•0 (56•0–80•0) | 69•0 (57•0–85•0) | 83•0 (66•0–109•0) | 0•0001 |
| ≥110 | 161 (7•88%) | 47 (4•32%) | 49 (7•11%) | 65 (24•25%) | <0•001 |
| Uric acid, μmol/L | 255•3 (196•2–332•0) | 259•0 (206•0–325•0) | 245•0 (187•0–309•0) | 263•0 (184•5–363•0) | 0•0006 |
| Prothrombin time, s | 13•8 (13•3–14•5) | 13•6 (13•1–14•1) | 13•9 (13•3–14•5) | 15•1 (14•1–16•6) | 0•0001 |
| ≥17 | 75/2007 (3•74%) | 9/1062 (0•85%) | 10/680 (1•47%) | 56/265 (21•13%) | <0•001 |
| Activated partial thromboplastin time, s | 38•8 (35•8–42•7) | 38•4 (35•7–41•9) | 39•3 (36•3–43•5) | 39•7 (35•9–45•4) | 0•0001 |
| ≥52 | 76/1839 (4•13%) | 23/994 (2•31%) | 24/615 (3•90%) | 29/230 (12•61%) | <0•001 |
| D-dimer, μg/mL | 0•73 (0•35–1•74) | 0•47 (0•26–1•05) | 0•89 (0•48–1•81) | 4•16 (1•39–21•00) | 0•0001 |
| >1 | 794/1969 (40•33%) | 274/1046 (26•20%) | 307/668 (45•96%) | 213/255 (83•53%) | <0•001 |
| High-sensitivity cardiac troponin I, pg/mL | 4•4 (1•9–10•6) | 2•7 (1•9–5•9) | 5•0 (2•5–11•7) | 29•1 (9•1–175•2) | 0•0001 |
| Male>34•2 Female>15•6 |
244/1725 (14•14%) | 40/914 (4•38%) | 68/584 (11•64%) | 136/227 (59•91%) | <0•001 |
| NT-proBNP, pg/mL | 119•0 (44•5–353•0) | 67•0 (29•0–169•0) | 146•0 (62•0–370•0) | 768•0 (291•5–2186•5) | 0•0001 |
| >450 | 330/1608 (20•52%) | 63/821 (7•67%) | 116/555 (20•90%) | 151/232 (65•09%) | <0•001 |
| C reactive protein, mg/L | 17•2 (2•5–64•8) | 5•1 (1•2–26•5) | 34•4 (6•6–79•6) | 98•2 (59•3–151•9) | 0•0001 |
| >10 | 1110/1936 (57•33%) | 404/1022 (39•53%) | 467/661 (70•65%) | 239/253 (94•47%) | <0•001 |
| Erythrocyte sedimentation rate, mm/h | 32•0 (15•0–58•5) | 24•0 (11•0–47•0) | 40•0 (22•0–65•0) | 40•0 (22•0–67•0) | 0•0001 |
| Ferritin, ug/L | 554•7 (313•8–1067•3) | 421•5 (229•5–619•8) | 634•9 (354•1–1134•4) | 1353•2 (798•0–2162•4) | 0•0001 |
| >600 | 535/1159 (46•16%) | 155/561 (27•63%) | 219/407 (53•81%) | 161/191 (84•29%) | <0•001 |
| Procalcitonin, ng/mL | 0•06 (0•04–0•11) | 0•05 (0•03–0•06) | 0•06 (0•04–0•12) | 0•21 (0•11–0•67) | 0•0001 |
| ≥0•25 | 219/1749 (12•52%) | 28/896 (3•13%) | 71/595 (11•93%) | 120/258 (46•51%) | <0•001 |
| IL-2R | 570•0 (363•0–871•0) | 469•0 (318•0–673•0) | 615•0 (412•0–909•0) | 1098•0 (768•0–1565•0) | 0•0001 |
| >710 | 589/1654 (35•61%) | 186/862 (21•58%) | 234/577 (40•55%) | 169/215 (78•60%) | <0•001 |
| IL-6, pg/mL | 5•4 (2•0–22•5) | 3•3 (1•5–8•7) | 7•4 (2•6–26•4) | 54•9 (22•0–137•1) | 0•0001 |
| ≥7 | 750/1663 (45•10%) | 257/870 (29•54%) | 295/578 (51•04%) | 198/215 (92•09%) | <0•001 |
| ≥14 | 547/1663 (32•89%) | 156/870 (17•93%) | 213/578 (36•85%) | 178/215 (82•79%) | <0•001 |
| IL-8 | 10•7 (6•1–20•9) | 9•0 (5•6–15•8) | 10•8 (6•4–20•3) | 25•7 (13•8–55•5) | 0•0001 |
| ≥62 | 110/1653 (6•65%) | 34/862 (3•94%) | 29/576 (5•03%) | 47/215 (21•86%) | <0•001 |
| IL-10 | 5•0 (5•0–5•7) | 5•0 (5•0–5•0) | 5•0 (5•0–5•6) | 8•6 (5•0–14•9) | 0•0001 |
| TNF-α | 7•7 (5•8–10•3) | 7•2 (5•4–9•2) | 8•3 (5•9–10•8) | 11•1 (7•4–15•5) | 0•0001 |
| ≥8•1 | 762/1651 (46•15%) | 310/862 (35•96%) | 299/575 (52•00%) | 153/214 (71•50%) | <0•001 |
| Radiologic findings | |||||
| Bilateral pulmonary infiltration | 1833/1981 (92•53%) | 960/1077 (89•14%) | 655/680 (96•32%) | 218/224 (97•32%) | <0•001 |
| Ground-glass opacity | 1030/1903 (54•13%) | 557/1066 (52•25%) | 381/676 (56•36%) | 92/161 (57•14%) | 0•177 |
Data are median (IQR), n (%) or n/N (%). p values were calculated by Kruskal-Wallis test, χ² test or Fisher's exact test, as appropriate. CHD=coronary heart disease. CLD=chronic liver disease. CKD=chronic kidney disease. COPD=chronic obstructive pulmonary disease. SOFA=Sequential Organ Failure Assessment. NT-proBNP=N-terminal pro B-type natriuretic peptide. IL-2R=interleukin-2 receptor. IL-6=interleukin-6. IL-8=interleukin-8. IL-10= interleukin-10. TNF-α=tumor necrosis factor-α.
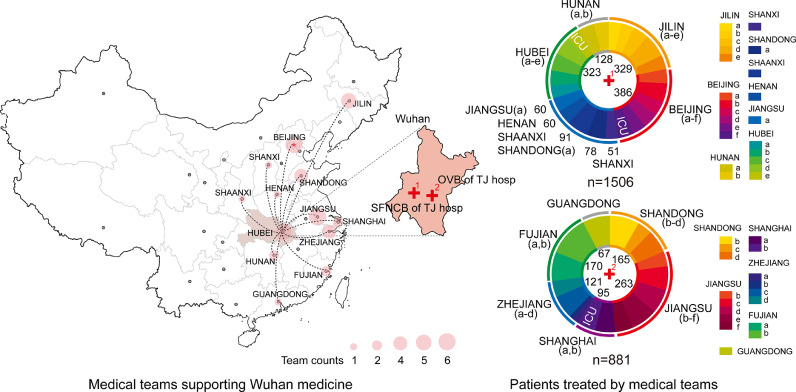
Fig. 2.
Medical teams supporting Wuhan and the number of patients they are in charge in two branches of Tongji Hospital.
OVB=Optical Valley Branch, SFNCB= Sino-French New City Branch, TJ= Tongji.
Vital signs were also recorded for all patients. More patients in critical group experienced the Sequential (Sepsis-related) Organ Failure Assessment (SOFA) score ≥ 4 at admission than those in severe group and moderate group (critical vs. severe vs. moderate: 75•00% vs. 5•09% vs. 3•53%). The median time from illness onset to hospital visit and from illness onset to hospital admission were both shorter (P = 0•001; P = 0•001) in severe (6•0, IQR: 3•0–9•0; 11•0, IQR: 8•0–16•0) /critical (5•0, IQR: 2•0–9•0; 11•0, IQR: 7•0–16•0) groups than in moderate group (7•0, IQR: 3•0–12•0; 13•0, IQR: 8•0–20•0).
3.2. Laboratory and radiological findings
We observed substantial differences in laboratory findings among patients in three groups (Table 1). Leukocytosis, lymphopenia, thrombocytopenia, hyperbilirubinemia, and hypoalbuminemia were more commonly seen in critical group than severe group and moderate group. Significantly increased PT (P = 0•001), APTT (P = 0•001), cTnI (P = 0•001), NT-proBNP (P = 0•001), ferritin (P = 0•001), and procalcitonin (P = 0•001) were observed in critical group when compared with severe group and moderate group. For radiologic findings, we observed comparable ground glass opacity between three groups, more bilateral pulmonary infiltration in severe/critical groups than moderate group, and more consolidation in severe group than moderate and critical group.
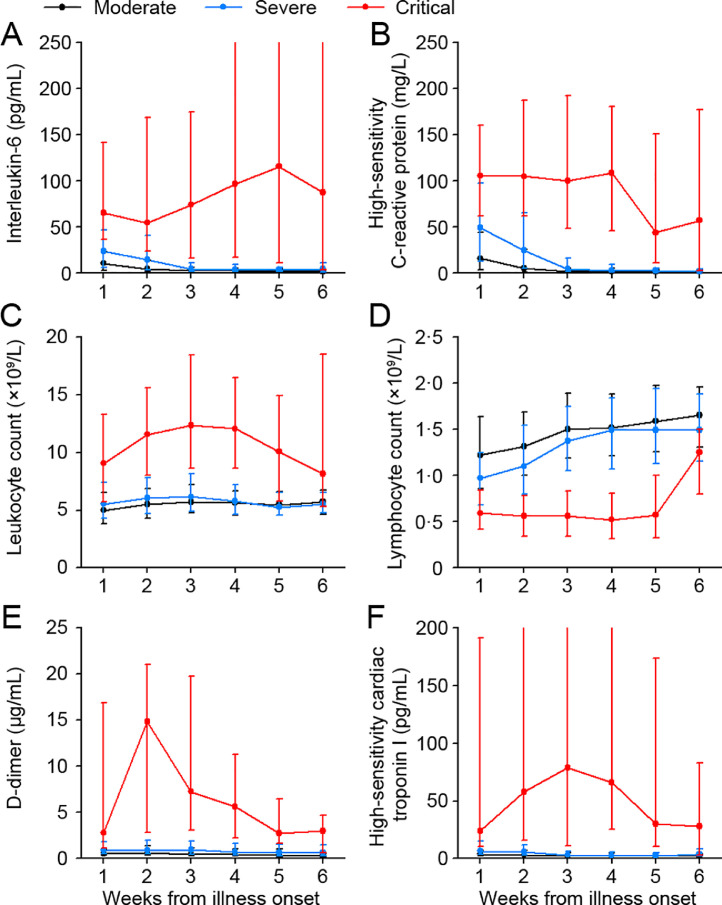
Additionally, serum levels of IL-2R, IL-6, IL-8, IL-10, and TNF-α were prominently higher in critical group on admission (Table 1). Several pivotal laboratory indicators IL-6, CRP, d-dimer, leukocyte count, and cTnI were significantly higher, whereas lymphocyte counts were lower in critical group than severe group and moderate group, with P < 0•05 for all time points shown throughout the clinical course (Fig. 3). In severe group and moderate group, most of the index were mildly abnormal and were restored to the normal range at the 3rd week after illness onset. In critical group, d-dimer, cTnI, and leukocyte increased to the peak at 2–3 weeks after illness onset and then decreased, while IL-6, IL-8, and TNF-α were consistently elevated at high levels and were continued to rise for as long as five weeks (Fig. 3 and Supplementary Figure 1).
Fig. 3.
Dynamic changes in laboratory markers from illness onset in patients with COVID-19.
Figure shows temporal changes in the median and IQR of interleukin-6 (A), high-sensitivity C-reactive protein (B), leukocyte count (C), lymphocyte count (D), D-dimer (E), and high-sensitivity cardiac troponin I (F). The overall differences among moderate, severe and critical group were significant for all timepoints shown. COVID-19=coronavirus disease 2019.
3.3. Treatments and outcomes of patients with different disease severity
Critical group received significantly fewer (P < 0•001) antiviral treatments, while more (P < 0•001) antibiotics compared with severe group and moderate group though lopinavir/ritonavir and ribavirin therapy were comparable in three groups (Table 2). Critical group tended to receive more interferon, glucocorticoid, and intravenous immunoglobin therapy. The duration of using all antiviral treatments, interferon, and glucocorticoid were shorter in critical group. Notably, critical group harbored a higher possibility of receiving non-invasive and invasive mechanical ventilation or ECMO than severe group and moderate group (critical vs. severe vs. moderate; non-invasive: 43•66% vs. 0•00% vs. 0•00%; invasive: 47•76% vs. 0•16% vs. 0•00%).
Table 2.
Treatments and outcomes of patients with different disease severity
| Disease severity | |||||
| Total (n = 2044) | Moderate (n = 1087) | Severe (n = 689) | Critical (n = 268) | p value | |
| Treatments | |||||
| Antibiotics | 1618/2043 (79•20%) | 782 (71•94%) | 577 (83•74%) | 259/267 (97•00%) | <0•001 |
| Carbostyrl | 1469/2043 (71•90%) | 728 (66•97%) | 527 (76•49%) | 214/267 (80•15%) | <0•001 |
| Cephalosporin | 463/2043 (22•66%) | 162 (14•90%) | 166 (24•09%) | 135/267 (50•56%) | <0•001 |
| Broad-spectrum antibiotics | 233/2043 (11•40%) | 40 (3•68%) | 43 (6•24%) | 150/267 (56•18%) | <0•001 |
| Antiviral treatments | 1892 (92•56%) | 1022 (94•02%) | 650 (94•34%) | 220 (82•09%) | <0•001 |
| Arbidol hydrochloride | 1653 (80•87%) | 916 (84•27%) | 569 (82•58%) | 168 (62•69%) | <0•001 |
| Duration of use, days | 9•0 (6•0–12•0) | 9•0 (7•0–13•0) | 10•0 (6•0–12•0) | 5•0 (3•0–7•0) | 0•0001 |
| Oseltamivir | 605 (29•60%) | 354 (32•57%) | 202 (29•32%) | 49 (18•28%) | <0•001 |
| Duration of use, days | 6•0 (3•0–10•0) | 6•0 (4•0–10•0) | 6•0 (4•0–10•0) | 5•0 (2•0–7•0) | 0•0086 |
| Lopinavir/ritonavir | 422 (20•71%) | 216 (19•91%) | 159 (23•14%) | 47 (17•67%) | 0•111 |
| Duration of use, days | 8•0 (4•0–11•0) | 7•0 (4•0–10•0) | 9•0 (6•0–11•0) | 5•0 (2•0–9•0) | 0•0001 |
| Ribavirin | 191 (9•34%) | 98 (9•02%) | 60 (8•71%) | 33 (12•31%) | 0•196 |
| Duration of use, days | 5•0 (0•5–8•0) | 5•0 (0•0–8•0) | 5•0 (3•0–10•0) | 3•0 (1•5–4•5) | 0•0298 |
| Interferon | 215 (10•53%) | 107 (9•85%) | 68 (9•88%) | 40 (14•93%) | 0•042 |
| Duration of use, days | 3•0 (1•0–7•0) | 3•5 (1•0–7•0) | 3•5 (2•0–7•5) | 2•0 (1•0–3•0) | 0•0079 |
| Corticosteroids | 667 (32•63%) | 206 (18•95%) | 237 (34•40%) | 224 (83•58%) | <0•001 |
| Duration of use, days | 7•0 (4•0–12•0) | 8•0 (5•0–12•0) | 8•0 (5•0–13•0) | 5•0 (3•0–10•0) | 0•0001 |
| Intravenous immunoglobin | 468 (22•90%) | 176 (16•19%) | 144 (20•90%) | 148 (55•22%) | <0•001 |
| High-flow nasal cannula oxygen therapy | 75/1705 (4•40%) | 20/809 (2•47%) | 44/628 (7•01%) | 11 (4•10%) | <0•001 |
| Non-invasive mechanical ventilation | 117/1705 (6•86%) | 0/809 (0•00%) | 0/628 (0•00%) | 117 (43•66%) | < 0•001 |
| Invasive mechanical ventilation/ECMO | 129/1705 (7•57%) | 0/809 (0•00%) | 1/628 (0•16%) | 128 (47•76%) | <0•001 |
| Outcomes | |||||
| The highest SOFA Score | 1•0 (0•0–2•0) | 0•0 (0•0–1•0) | 1•0 (1•0–2•0) | 14•0 (12•0–17•0) | 0•0001 |
| 0–1 | 1321 (64•63%) | 968 (89•05%) | 353 (51•23%) | 0 (0•00%) | <0•001 |
| 2–3 | 385 (18•84%) | 96 (8•83%) | 272 (39•48%) | 17 (6•34%) | ②‥ |
| ≥4 | 338 (16•54%) | 23 (2•12%) | 64 (9•29%) | 251 (93•66%) | ‥ |
| Acute liver injury | 773 (37•82%) | 330 (30•36%) | 301 (43•69%) | 142 (52•99%) | <0•001 |
| Sepsis | 711 (34•78%) | 110 (10•12%) | 333 (48•33%) | 268 (100•00%) | <0•001 |
| Hypoproteinaemia | 474/2041 (23•22%) | 103/1085 (9•49%) | 173/688 (25•15%) | 198 (73•88%) | <0•001 |
| ARDS | 446 (21•82%) | 29 (2•67%) | 149 (21•63%) | 268 (100•00%) | <0•001 |
| Acute cardiac injury | 309/1831 (16•88%) | 45/940 (4•79%) | 72/635 (11•34%) | 192/256 (75•00%) | <0•001 |
| Respiratory failure | 273 (13•36%) | 5 (0•46%) | 13 (1•89%) | 255 (95•15%) | <0•001 |
| Acute kidney injury | 250 (12•23%) | 63 (5•80%) | 59 (8•56%) | 128 (47•76%) | <0•001 |
| Coagulopathy | 243 (11•89%) | 42 (3•86%) | 45 (6•53%) | 156 (58•21%) | <0•001 |
| Septic shock | 242 (11•84%) | 4 (0•37%) | 8 (1•16%) | 230 (85•82%) | <0•001 |
| Admission to ICU | 163 (7•97%) | 5 (0•46%) | 3 (0•44%) | 155 (57•84%) | <0•001 |
| Heart failure | 134/2041 (6•57%) | 6/1086 (0•55%) | 7/688 (1•02%) | 121/267 (45•32%) | <0•001 |
| Secondary infection | 27 (1•32%) | 1 (0•09%) | 6 (0•87%) | 20 (7•46%) | <0•001 |
| Time from illness onset to ICU admission, days | 15•0 (11•0–22•0) | 25•0 (14•0–27•0) | 12•5 (12•0–13•0) | 15•0 (11•0–22•0) | 0•5010 |
| ICU length of stay, days | 7•0 (3•0–11•0) | 7•0 (1•0–12•0) | 13•0 (9•0–27•0) | 7•0 (3•0–11•0) | 0•1847 |
| Time from illness onset to death or discharge, days | 34•0 (26•0–42•0) | 34•0 (27•0–43•0) | 36•0 (29•0–43•0) | 24•0 (17•0–35•0) | 0•0001 |
| Hospital length of stay of survivors, days | 20•0 (14•0–28•0) | 18•0 (12•0–25•0) | 23•0 (17•0–30•0) | 32•0 (27•0–38•0) | 0•0001 |
| Hospital length of stay of non-survivors, days | 9•0 (5•0–16•0) | 8•0 (1•0–15•0) | 9•0 (8•0–13•5) | 9•0 (5•0–16•0) | 0•8011 |
| Duration of viral shedding after COVID-19 onset, days | 24•0 (19•0–31•0) | 24•0 (19•0–32•0) | 24•0 (19•0–30•0) | 23•0 (19•0–30•5) | 0•8407 |
| Non-survivors | 235 (11•50%) | 2 (0•18%) | 4 (0•58%) | 229 (85•45%) | <0•001 |
Data are median (IQR), n (%) or n/N (%). p values were calculated by Kruskal-Wallis test, χ² test or Fisher's exact test, as appropriate. ECMO=extracorporeal membrane oxygenation. SOFA=Sequential Organ Failure Assessment. ARDS=acute respiratory distress syndrome. ICU=intensive care unit. COVID-19=coronavirus disease 2019. Interferon, indicating Recombinant Human Interferon α−2b inhalation or Interferon α−1b inhalation.
The median value of the highest SOFA score in clinical course was dramatically higher in critical group than in severe and moderate groups (critical vs. severe vs. moderate: 14•0 vs. 1•0 vs. 0•0; Table 2). Critical group exhibited a higher risk to develop multi-organ damage including sepsis (100%), ARDS (100%), respiratory failure (95•15%), and septic shock (85•82%), resulting in more admission to ICU (57•84%) and more death (85•45%). Longer hospital stays of survivors, shorter hospital stays and time from illness onset to death or discharge were observed in critical group. Moreover, the median duration of viral shedding was 24 days, being comparable in three groups. The three longest duration of viral shedding in all patients were 76, 70, and 51 days since symptom onset. The top three duration of positive SARS-CoV-2 RNA were 46, 43, and 43 days. Basic information of patients admitted to ICU and general wards taken over by different medical teams are shown in Supplementary Tables 1 and 2, respectively.
3.4. Risk factors associated with disease severity
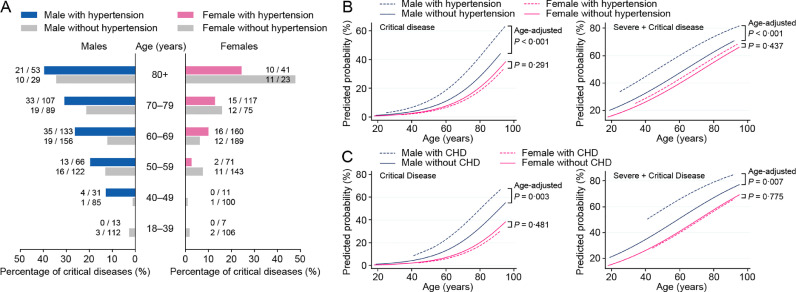
The severity of disease climbed up straightly with age (Supplementary Figure 2A). On the other hand, males of all ages had significantly higher proportion of more serious diseases than females, with overall P < 0•05 (chi-square test) for all age groups (Supplementary Figure 2A). The odds ratios comparing male to female were 2•24 (95% CI: 1•70–2•96; P < 0•001) for critical illness and 1•61 (95% CI: 1•34–1•93; P < 0•001) for severe/critical illness after adjusting for ages (Supplementary Figure 2B and 2C). Interestingly, we found that sex was an effect modifier for hypertension and CHD. In univariable analysis, hypertension (OR:2•76, 95% CI: 1•97–3•86, P < 0•001) and CHD (OR:2•75, 95% CI: 1•76–4•29, P < 0•001) were remarkable risk factors in male patients, but they did not affect the odds of developing into critical illness in female (OR:1•42, 95% CI: 0•92–2•18, P = 0•113; OR:1•40, 95% CI: 0•72–2•73, P = 0•001). In our study population, males with hypertension had an increased proportion of critical illness across ages (Fig. 4A). Patients with hypertension or CHD had an increased probability to develop into critical or severe/critical diseases only in male but not in female (Fig. 4B and 4C). The significant differences of ORs for hypertension and CHD between different sexes were further confirmed using logistic regression models containing the interaction terms (sex and hypertension, sex and CHD, Table 3). As a result, a single model that combined male and female is inappropriate, so we reported models based on different sexes separately (Table 3 and Supplementary Table 3).
Fig. 4.
Different effects of hypertension and CHD in male and female patients with COVID-19.
A. The proportions of critical disease in male and female patients with or without hypertension in different age groups were shown. B. The probabilities of developing into critical or severe/critical disease were predicted using logistic regression models containing age, gender, hypertension, and the interaction term of gender and hypertension. Age-adjusted P values for hypertension in different genders were shown. C. The probabilities of developing into critical or severe/critical disease were predicted using logistic regression models containing age, gender, CHD, and the interaction term of gender and CHD. Age-adjusted P values for CHD in different genders were shown. COVID-19=coronavirus disease 2019. CHD=coronary heart disease.
Table 3.
Risk factors associated with critical disease (vs. moderate and severe disease).
| Univariable Analysis | Multivariable Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||||
| OR (95% CI) | p value | OR (95% CI) | p value | Effect modification p valuea | OR (95% CI) | p value | OR (95% CI) | p value | |
| Factors with effect modification | |||||||||
| Hypertension | 2•76 (1•97–3•86) | <0•001 | 1•42 (0•92–2•18) | 0•113 | 0•007 | 2•05 (1•42–2•95) | <0•001 | 0•90 (0•56–1•45) | 0•677 |
| CHD | 2•75 (1•76–4•29) | <0•001 | 1•40 (0•72–2•73) | 0•322 | 0•043 | 1•65 (1•02–2•66) | 0•041 | ‥ | ‥ |
| COPD | 3•01 (0•97–9•32) | 0•056 | 15•99 (2•64–96•99) | 0•003 | 0•068 | ‥ | ‥ | 11•32 (1•63–78•77) | 0•014 |
| Characteristics | |||||||||
| Age, yearsb | 1•06 (1•04–1•07) | <0•001 | 1•07 (1•05–1•09) | <0•001 | ‥ | ‥ | ‥ | ‥ | ‥ |
| ≥50 | 8•28 (4•01–17•10) | <0•001 | 8•93 (2•80–28•49) | <0•001 | ‥ | ‥ | ‥ | ‥ | ‥ |
| ≥60 | 3•41 (2•31–5•02) | <0•001 | 3•78 (2•17–6•58) | <0•001 | ‥ | 2•63 (1•76–3•95) | <0•001 | 3•89 (2•17–6•95) | <0•001 |
| Presence of comorbidity | 4•01 (2•64–6•08) | <0•001 | 2•40 (1•48–3•89) | <0•001 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Diabetes | 1•39 (0•94–2•06) | 0•098 | 1•61 (0•94–2•76) | 0•080 | ‥ | ‥ | ‥ | 1•32 (0•74–2•35) | 0•353 |
| Tumor | 2•83 (1•36–5•86) | 0•005 | 3•32 (1•39–7•97) | 0•007 | ‥ | 2•35 (1•09–5•08) | 0•029 | 3•76 (1•50–9•41) | 0•005 |
| CLD | 1•13 (0•54–2•38) | 0•751 | 1•11 (0•33–3•73) | 0•864 | ‥ | ‥ | ‥ | ‥ | ‥ |
| CKD | 1•36 (0•44–4•18) | 0•593 | 4•28 (1•31–13•92) | 0•016 | ‥ | ‥ | ‥ | 3•58 (0•98–13•06) | 0•054 |
| Number of comorbiditiesb | 1•83 (1•57–2•14) | <0•001 | 1•58 (1•30–1•92) | <0•001 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Fever | 1•02 (0•64–1•63) | 0•920 | 1•08 (0•65–1•80) | 0•754 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Temperature, °Cb | 1•03 (0•87–1•22) | 0•698 | 1•07 (0•86–1•33) | 0•566 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Temp(max)≥39 °C | 0•85 (0•59–1•23) | 0•389 | 0•86 (0•50–1•50) | 0•601 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Cough | 1•63 (1•09–2•44) | 0•018 | 1•63 (0•96–2•78) | 0•073 | ‥ | 1•70 (1•12–2•59) | 0•013 | 1•54 (0•89–2•65) | 0•122 |
| Sputum | 1•28 (0•92–1•78) | 0•142 | 1•07 (0•69–1•66) | 0•756 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Vomiting | 1•18 (0•54–2•62) | 0•676 | 0•84 (0•30–2•37) | 0•738 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Diarrhea | 0•96 (0•64–1•42) | 0•826 | 1•04 (0•64–1•69) | 0•875 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Fatigue | 1•36 (0•97–1•89) | 0•074 | 1•11 (0•72–1•73) | 0•630 | ‥ | ‥ | ‥ | ‥ | ‥ |
| Myalgia | 0•87 (0•57–1•32) | 0•503 | 0•88 (0•51–1•53) | 0•662 | ‥ | ‥ | ‥ | ‥ | ‥ |
OR=odds ratio. CI=confidence interval. CHD=coronary heart disease. COPD=chronic obstructive pulmonary disease. CLD=chronic liver disease. CKD=chronic kidney disease. a Test of effect modification between gender and other factors using logistic regression models containing the interaction terms (gender and hypertension, gender and CHD, gender and COPD). b Per 1 unit increase.
In univariable analysis, cough (OR:1•63, 95% CI: 1•09–2•44, P = 0•018) in addition to hypertension (OR:2•76, 95% CI: 1•97–3•86, P < 0•001) and CHD (OR:2•75, 95% CI: 1•76–4•29, P < 0•001) were associated with critical illness in male. COPD (OR:15•99, 95% CI: 2•64–96•99, P = 0•003) and CKD (OR:4•28, 95% CI: 1•31–13•92, P = 0•016) were associated with critical illness in female. While age, tumor, the presence of comorbidity, and number of comorbidities were associated with critical illness in both male and female patients. In multivariable analysis of critical illness, we found that hypertension (OR:2•05, 95% CI: 1•42–2•95, P < 0•001), CHD (OR:1•65, 95% CI: 1•02–2•66, P = 0•041), tumor (OR:2•35, 95% CI: 1•09–5•08, P = 0•029), age ≥ 60 years (OR:2•63, 95% CI: 1•76–3•95, P < 0•001), and cough (OR:1•70, 95% CI: 1•12–2•59, P = 0•013) were independent risk factors for male patients, and COPD (OR:11•32, 95% CI: 1•63–78•77, P = 0•014), tumor (OR:3•76, 95% CI: 1•50–9•41, P = 0•005), and age ≥ 60 years (OR:3•89, 95% CI: 2•17–6•95, P < 0•001) were independent risk factors for female patients. Additionally, CKD reached marginally significant (OR=3•58, 95% CI: 0•98–13•06, P = 0•054) in multivariable analysis for female. Cough, however, can be caused by many different conditions, including COPD, heart failure, ACEI side effect, and COVID-19 itself, etc. In analysis of critical illness in male, cough was no longer a statistically significant risk factor after adjusting for heart failure (OR=1•52, 95% CI: 0•92–2•50, P = 0•099). Therefore, cough was not yet considered to be an independent risk factor for developing critical cases. Risk factors of developing into severe/critical illness were showed in Supplementary Table 4 and 5.
4. Discussion
To our knowledge, this report is the largest multi-center, retrospective cohort study that comprehensively describes the clinical features of COVID-19. Meanwhile, it is the first study that elucidates the disparity of risk factors of developing severe/critical diseases between sexes. Via analyzing clinical data of 2044 adult inpatients with definite outcomes, we discovered that gender was an effect modifier for hypertension and coronary heart disease (CHD), where hypertension and CHD remarkably increased the risk of critical disease in male, but barely affected female patients.
In accordance with previous descriptions, clinical features and course of COVID-19 were similar between moderate group and severe group [18,19]. For critical group, patients exhibited dramatically distinct features characterized by higher proportion of dyspnea and disorders of consciousness, persistent SOFA score ≥ 4, systemic organ damage, and lasting inflammatory storm manifested as increased IL-6, CRP, d-dimer, cTnI, leukocyte count, and decreased lymphocyte count. We observed a higher mortality for critical patients in this study than previous report (85•45% vs. 78•00%) [13], which may attribute to delayed access to medical care in the peak of outbreak in Wuhan, the epicenter of COVID-19 crisis in China, and definition of COVID-19 severity as the most severe condition during the entire course of the disease. We enrolled discharged/dead patients including patients with critical illness on admission and those deteriorating into critical diseases. Noticeably, severe patients had lower mortality than previously reported (0•58% vs. 22•00%) [13]. We owed this to contributions of numerous experts that rushed to Wuhan from the whole country, which represents the highest level of critical care in China.
Critical group experienced more multiorgan damage including ARDS, sepsis, coagulopathy, and acute liver, kidney, and cardiac injury. The underlying mechanisms for SARS-CoV-2-related multiorgan damage remain unclear. Angiotensin-converting enzyme 2 (ACE2) gene is broadly expressed in lung alveolar cells, vascular endothelial cells, kidneys, heart, and other tissues [20]. The multiorgan damage was partially induced by direct attack of SARS-CoV-2. 21Meanwhile, this and previous studies observed significantly higher levels of pro-inflammatory cytokines and chemokines including IL-2R, IL-6, IL-8, IL-10, and TNF-α in critical group [7,20,21]. Innate and adaptive immune responses were activated after virus infection, dysregulation of which could result in cytokine release syndrome (CRS) and subsequent multi-organ damage, even death [21,22]. CRS was more prevalently seen in critical group. Immunosuppression therapies such as tocilizumab and glucocorticoids were recommended for patients with grade 3 or 4 CRS, where tocilizumab, an anti-human IL-6R mAb, specifically inhibits IL-6 signaling, and corticosteroids have more widespread effects on immune system [23]. Whether direct attacks of SARS-CoV-2 or CRS or a combination of both lead to multiorgan damage is still unclear. However, exploration of the underlying causes of multiorgan damage is crucial because they determined the clinical decision-making of treatment to attenuate multiorgan damage.
Advanced age and male sex had been reported as risk factors of mortality in SARS, MERS, and COVID-19 [12,13,24]. Here, we reported that advanced age was highly associated with critical disease in COVID-19 patients. More importantly, we found that the effects of multiple critical factors on COVID-19 progression were completely different between sexes. Hypertension and CHD dramatically increased the risk of critical disease in male, but barely affected female patients. COPD and CKD seemed more likely to affect females than males. However, hypertension and CHD have much higher prevalence rates than COPD and CKD, which might be a basic reason for more cases of serious COVID-19 in males. Sex differences were also reported in hypertension with depressive order and coronary artery disease [25,26], which represented that male had a higher risk of suffering from hypertension beyond protective role of estrogens [27]. Meantime, female smokers had accelerated decline in forced expiratory volume in one second (FEV1), who were more vulnerable to COPD and had a high risk of hospitalization and death with age [28,29]. ACE2 polymorphism, androgen mediated SARS-CoV-2 infection, and estrogen protective role might also account for sex differences [30], [31], [32]. Similar trend in sex differences were observed in certain reports of SARS and MERS, albeit poorly investigated [24].
The prevalence of COPD in this study was only 0.88%. Guan WJ, et al. reported 1.8% (24/1566) COVID-19 patients with COPD, while the data were extracted from 575 hospitals in 31 province/autonomous regions/provincial municipalities across mainland China [4]. It was reported that the prevalence of COPD differed by geographic region, with the lowest in central China, where Wuhan loacted [33]. Therefore, even if COPD is a risk factor for critical disease of female COVID-19 patients, it will not be of great importance in the present study, considering the low prevalence of COPD in female.
This study has some limitations. First, though data used for analysis are comprehensive, a few patients did not receive all above-mentioned laboratory tests, and several information such as symptoms and comorbidities are documented in accordance with personal narratives. For instance, patient outcomes could be different with the different frequency and severity, chronic and acute onset of cough, which was not clear. Second, COVID-19 cases with comorbidities such as COPD and CKD are limited. Further investigations including more COVID-19 patients with comorbidities will consolidate our findings. Third, patients in our study are primarily locals in Wuhan. Larger nationwide or worldwide cohort studies are needed to support our results.
In summary, this multi-center retrospective cohort study provides comprehensive front-line information about different severity of COVID-19 and insights into the different risks associated with developing into serious disease. The fundamental differences in disease progression between sexes highlight the importance of dividing sexes in clinical and epidemiologic works, because it might be inappropriate to combine males and female as one single population. These findings also highlight the importance in further investigating the molecular and genetic mechanism underlying the differences between sexes to understand the nature of the disease, and perhaps other coronavirus appearing in future.
Declaration of Competing Interest
We declare no conflicts of interests for all authors.
Acknowledgments
Contributors
DL, PC, SZ, SW, XF, YG, and SX contributed equally to this work. SZ, SW, YG, SX, RY, YW, and YY collected the clinical data. RL, XJ, JC, JL, YY, XZ, CS, NJ, WG, XL and GC double-checked and loaded the data into database. DL, PC, XF, CL, and QG analyzed the clinical records. PC, SZ, SW, XF, YG, SX, and HL drafted the manuscript. DL, QG, PC, SZ, SW, XF, YG, and SX analyzed and interpreted the data. CL advised on the conception and design of the study. DL and QG conceptualized and designed the study, supervised the project, and revised the manuscript. All authors vouch for the respective data and analysis, revised, approved the final version, and agreed to publish the manuscript.
Acknowledgments
We thank all health-care workers and people involved in fighting against COVID-19.
Funding
The study was supported by the National Science and Technology Major Sub-Project (2018ZX10301402–002), the Technical Innovation Special Project of Hubei Province (2018ACA138), the National Natural Science Foundation of China (81772787, 81873452, and 81974405), and the Fundamental Research Funds for the Central Universities (2019kfyXMBZ024).
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100471.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO Emergencies Press Conference On novel coronavirus-11 February 2020. https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-full-press-conference-11feb2020-final.pdf?sfvrsn=e2019136_2 (Accessed 1 May 2020).
- 2.Gates B. Responding to Covid-19 — A Once-in-a-Century Pandemic? New England Journal of Medicine. 2020;382(18):1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus June;(COVID-19). 2020. https://covid19.who.int/ (Accessed 19 June 2020).
- 4.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. (Accessed 4 April 2020) [DOI] [PubMed] [Google Scholar]
- 6.McMichael T.M., Currie D.W., Clark S. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. New England J Medicine. 2020 doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G., Zangrillo A., Zanella A. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudbjartsson D.F., Helgason A., Jonsson H. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 2020 doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Commission of China . National Health Commission of China. (7th edn) 2020. Guidance for COVID-19.http://117.128.6.32/cache/www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf?ich_args2=465-14165911033078_f32a0b9f969d8670abb9ee3d42d8e898_10001002_9c896d2ed6c1f4d09f3c518939a83798_0ad7c3e0d3161a7f69a04f61b3ce861b (in Chinese) (Accessed 5 May 2020) [Google Scholar]
- 11.National Health Commission of China . National Health Commission of China. (5th edn) 2020. Guidance for COVID-19.http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf (in Chinese) (Accessed 8 February 2020) [Google Scholar]
- 12.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan A., Liu L., Wang C. Association of Public Health Interventions With the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kdigo A. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 16.Ranieri V.M., Rubenfeld G.D., Thompson B.T. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y., Ling Y., Bai T. COVID-19 with Different Severity: a Multi-center Study of Clinical Features. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. Journal of Allergy and Clinical Immunology. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G., Wu D., Guo W. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai C.T., Hwang J.J., Lai L.P., Wang Y.C., Lin J.L., Chiang F.T. Interaction of gender, hypertension, and the angiotensinogen gene haplotypes on the risk of coronary artery disease in a large angiographic cohort. Atherosclerosis. 2009;203(1):249–256. doi: 10.1016/j.atherosclerosis.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Kao W.-.T., Chang C.-.L., Lin C.-.H., Wu S.-.L., Lin S.-.L., Lung F.-.W. Gender Disparity in the Risk of Hypertension in Subjects With Major Depressive Disorder. Front Psychiatry. 2019;10 doi: 10.3389/fpsyt.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onat A., Karadeniz Y., Tusun E., Yüksel H., Kaya A. Advances in understanding gender difference in cardiometabolic disease risk. Expert Rev Cardiovasc Ther. 2016;14(4):513–523. doi: 10.1586/14779072.2016.1150782. [DOI] [PubMed] [Google Scholar]
- 28.Sorheim I.C., Johannessen A., Gulsvik A., Bakke P.S., Silverman E.K., DeMeo D.L. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–485. doi: 10.1136/thx.2009.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gan W.Q., Man S.F.P., Postma D.S., Camp P., Sin D.D. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7(1):52. doi: 10.1186/1465-9921-7-52. -52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. New England Journal of Medicine. 2020 doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wambier C.G., Goren A. SARS-COV-2 infection is likely to be androgen mediated. J. Am. Acad. Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding T., Zhang J., Wang T. A Multi-hospital Study in Wuhan, China:Protective Effects of Non-menopause and Female Hormones on SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1101/2020.03.26.20043943. 2020.03.26.20043943. published online March 30, 2020(priprint) [DOI] [Google Scholar]
- 33.Fang L., Gao P., Bao H. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018 Jun;(2213–2619 (Electronic)) doi: 10.1016/S2213-2600(18)30103-6. (Accesed 17 June 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.