Abstract
In detection, treatment, and follow-up, male breast cancer has historically lagged behind female breast cancer. On the whole, breast cancer is less common among men than among women, limiting utility of screening, yet the incidence of male breast cancer is rising, and there are men at high risk for breast cancer. While women at high risk for breast cancer are well characterized, with clearly established guidelines for screening, supplemental screening, risk prevention, counseling, and advocacy, men at high risk for breast cancer are poorly identified and represent a blind spot in public health. Today, more standardized genetic counseling and wider availability of genetic testing are allowing identification of high-risk male relatives of women with breast cancer, as well as men with genetic mutations predisposing to breast cancer. This could provide a new opportunity to update our approach to male breast cancer. This article reviews male breast cancer demographics, risk factors, tumor biology, and oncogenetics; recognizes how male breast cancer differs from its female counterpart; highlights its diagnostic challenges; discusses the implications of the widening clinical use of multigene panel testing; outlines current National Comprehensive Cancer Network guidelines (version 1, 2018) for high-risk men; and explores the possible utility of targeted screening and surveillance. Understanding the current state of male breast cancer management and its challenges is important to shape future considerations for care. Shifting the paradigm of male breast cancer detection toward targeted precision medicine may be the answer to improving clinical outcomes of this uncommon disease.
Introduction
Despite continual progress in diagnosing female breast cancer (1), the approach to male breast cancer has changed little. While it is estimated that 35% fewer women now die from breast cancer each year due to early detection via screening and better treatment (2), it is less clear whether, and how, this translates to male breast cancer outcomes. Although male breast cancer accounts for only 1% of all breast cancers, its incidence has increased by 20%–25% in the past few decades and continues to rise (3,4). In the United States, compared with 900 cases diagnosed in 1991, the American Cancer Society projects that 2550 cases of male breast cancer will occur in 2018, and 480 men are projected to die of breast cancer (5). However, some of these deaths may be avoidable by focusing on early disease detection among those with identifiable risk factors—in other words, targeted screening. Widening use of genetic testing and counseling in recent years is beginning to identify men at increased risk for breast cancer, which makes targeted screening possible.
To bridge the gap between male and female breast cancer diagnosis and treatment, it is essential that we understand the current scope of the disparity and recognize the challenges unique to male breast cancer. To that end, important differences between male and female breast cancers are reviewed in terms of epidemiology, risk factors, tumor biology, and oncogenetics. The clinical and imaging diagnostic challenges associated with male breast cancer are considered. The potential utility of targeted male breast cancer screening is illustrated, and the current 2018 National Comprehensive Cancer Network guidelines for high-risk men are discussed (6).
Male versus Female Breast Cancer: An Imperfect Overlap
Male Breast Cancer: Current Scope of Knowledge
Current understanding of male breast cancer is lacking and is limited to the findings from small retrospective series. Although the improvement in survival of patients with female breast cancer has been attributed to early diagnosis through screening and improved treatment (7,8), such improvement, if any, in patients with male breast cancer is likely to be seen to a lesser extent, owing to a lack of screening and less tailored therapy (9). Because male breast cancer is uncommon (Table 1) (5), poor accrual of patients has been a major barrier to prospective randomized trials (10). For this reason, treatment strategies often rely on extrapolation of data derived from clinical trials in women with breast cancer, which limits the ability to tailor therapy to individual patients. Although larger multicenter prospective trial efforts are currently under way (11), completing these studies will take time. On the other hand, although early detection has not been the focus in the diagnosis of male breast cancer, the benefits of early detection with targeted screening in men have been anecdotally reported (12), and screening in select high-risk individuals is a clinically actionable step at the patient level that could potentially improve individual outcomes.
Table 1:
Estimated New Breast Cancer Cases and Deaths in the United States in 2018 Grouped by Sex
| Breast Cancer | Both Sexes | Males | Females |
|---|---|---|---|
| No. of new cases | 268 670 | 2550 | 266 120 |
| No. of deaths | 41 400 | 480 | 40 920 |
Source.—Reference 5.
Male breast cancer, although uncommon, can be deadly. More men will die of breast cancer (480 deaths in 2550 cases) than of testicular cancer (400 deaths in 9310 cases) in the United States in 2018, according to the estimates of the American Cancer Society (5). Thus, an unmet need exists to better understand male breast cancer, to optimize its treatment and follow-up.
Male Breast Cancer: Current State of Disparity
It should come as no surprise that disparity exists between the survival outcomes of male breast cancer patients and female breast cancer patients, owing to a lack of screening and less tailored therapy in men (13–16), but the gap has continued to widen since screening programs were put in place for female breast cancer in the 1990s (15). In pooled data from multiple studies comparing stage-matched disease-free and overall survival between male (n = 885) and female (n = 88 727) breast cancer patients, men demonstrated significantly poorer long-term survival as compared to women (Table 2) (13–15).
Table 2:
Stage-matched Comparison of Median 10-year Survival of Male and Female Breast Cancer Patients
| Study | Year | Male 10-year Disease-Free Survival (%) | Female 10-year Disease-Free Survival (%) | Male 10-year Overall Survival (%) | Female 10-year Overall Survival (%) | No. of Male Patients | No. of Female Patients |
|---|---|---|---|---|---|---|---|
| Chen et al (13) | 2013 | 40.1 | 51.5 | 53.9 | 68.5 | 150 | 300 |
| Iorfida et al (14) | 2014 | 51.7 | 66.5 | 70.7 | 84.2 | 99 | 198 |
| Lautrup et al (15) | 2018 | NA | NA | 31.7 | 59.3 | 636 | 88 229 |
Note.—Numbers of male and female patients are the numbers of individual patients with a cancer diagnosis included in the study. NA = not available.
In a large nationwide population-based registry comparison of male and female breast cancer in Denmark, where access to high-quality health care is free, a marked disparity in overall survival was identified between male and female breast cancer patients: 5-year and 10-year survival rates of 55.1% and 31.7%, respectively, for male breast cancer patients, compared with 76.8% and 59.3% for female breast cancer patients (15). While female breast cancer survival saw significant improvement over time (span of 30 years) (P < .0001), there was no change in male breast cancer survival (15). Along the same line, in the findings from a different study, investigators noted that death rates from male breast cancer have not decreased since 1975, despite a concurrent decline in mortality from female breast cancer (17). At a time when discussion of overdiagnosis of female breast cancer is necessary (18), the opposite conversation is likely needed for male breast cancer, which typically manifests only when clinically evident, usually with a larger tumor size, a more advanced disease stage, and a higher likelihood of lymph node involvement (4,16) (Fig 1).
Figure 1.
Juxtaposition of typical clinical manifestations of male (a, b) and female (c) breast cancer. (a, b) Mediolateral oblique (MLO) (a) and craniocaudal (CC) (b) mammographic views of the left breast of a 65-year-old man presenting with a palpable irregular mass in the left breast show the mass (arrow) causing nipple retraction, findings consistent with an invasive cancer. (c) CC mammographic view of the right breast of a 45-year-old asymptomatic woman shows screening-identified calcifications (arrows), findings consistent with in situ cancer.
Data suggest that the survival disparity between men and women with breast cancer may be greatest in early disease. In the results of a study of patients in the Veterans Affairs Central Cancer Registry that compared male breast cancer patients (n = 612) with female breast cancer patients (n = 2413), investigators found that the largest survival disparity was in patients with stage I or stage II disease; in fact, in lymph node–negative patients, the median survival was only 6.1 years for male breast cancer patients, compared with 14.6 years for female breast cancer patients (16), a finding that highlights the need for early detection of male breast cancer.
Additional data exist to suggest that even in the setting of recurrent disease, men fared worse than women, with a male median survival of only 1 year, compared with a female median survival of 2 years, from recurrent breast cancer (19). Finally, in the results of an analysis of data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, investigators suggested that the continued increase in the incidence of male breast cancer and a concurrent decline in the incidence of female breast cancer in the United States are not related to fluctuationsin breast cancer risk factors shared by men and women, but rather are more likely attributed to the currently divergent approach to the diagnosis and treatment of male and female breast cancers (3).
Male Breast Cancer: A Distinct Disease
The stage-matched disparity in survival of men and women with breast cancer is not accounted for by the more advanced manifestation of male breast cancer alone. Emerging data suggest that male breast cancer and female breast cancer likely differ in tumor biology and genetic signatures (20–22) (Table 3). Compared with female breast cancer, male breast cancer is overwhelmingly ER positive (>90%), and the most common histologic subtype is the ER-positive, PR-positive, human epidermal growth factor receptor 2 (HER2)–negative subtype. Despite this fact, not all male patients with ER-positive breast cancer receive tamoxifen treatment (11). While triple-negative breast cancer comprises 10%–15% of female breast cancer (23), it is exceedingly rare in male breast cancer (<0.5%) (Fig 2). Similarly, male breast cancers are predominantly ductal in origin, and lobular cancers are rare (<0.5%) becausethe male breast intrinsically lacks lobules. A small preponderance of papillary cancers has also been described in the setting of male breast cancer (11).
Table 3:
Representative Differences between Male and Female Breast Cancers
| Variable | Male Breast Cancer | Female Breast Cancer |
|---|---|---|
| Histologic types | >90% IDC, <0.5% ILC | ≤90% IDC, 10% ILC |
| Molecular markers | >90% ER+, >80% PR+, <0.5%TN | 75% ER+, 65% PR+, 10%−15% TN |
| Tumor grade | No correlation with survival | Yes; correlation with survival |
| PIK3CA and TP53 mutations | Infrequent | Frequent |
Note.—ER = estrogen receptor, IDC = invasive ductal carcinoma, ILC = invasive lobular carcinoma, PIK3CA = phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene, PR = progesterone receptor, TN = triple negative, TP53 = tumor protein p53 gene.
Figure 2.
Triple-negative breast cancer in a 54-year-old man who presented with an enlarging left axillary mass that was first palpated 3 years earlier. (a, b) Diagnostic MLO (a) and CC (b) mammographic views of the left breast show that an irregular mass (arrows on a) in the left axilla was only depicted on the MLO view. No other mass was depicted in either breast. (c) Gray-scale US image in sagittal projection shows a corresponding irregular mass with central areas of necrosis in the left axilla. The findings from histopathologic examination and testing of the specimen from core biopsy of the mass disclosed triple-negative (ER-negative, PR-negative, HER2-negative) invasive ductal carcinoma. The patient has a family history of breast cancer diagnosed in his mother when she was 52 years old; he has no known genetic mutation.
In female breast cancer, the histologic grade of the tumor is an established prognostic indicator that has been correlated with disease-free survival and overall survival (24). However, the histologic grade of the tumor is not necessarily correlated with clinical outcomes in male breast cancer, as shown by the results of a recent large multicenter trial with central pathology review of 1483 cases of male breast cancer (20).
Similarly, male breast cancer oncogenetics differs from that of female breast cancer. Although from a clinicopathologic standpoint, male breast cancer (most are ER positive and HER2 negative) should resemble ER-positive HER2-negative luminal molecular subtypes of female breast cancer, which commonly harbor somatic genetic alterations such as PIK3CA and TP53 mutations, these genetic alterations are found less frequently in male breast cancer (22). Similarly, mutations of the MAP3K4 gene (mitogen-activated protein kinase kinase kinase 4 gene) and of the NCOR1 gene (nuclear receptor corepressor 1 gene), which are frequently found in female breast cancer, are not encountered in male breast cancer (22). Other genetic mutations affect male breast cancer disproportionately. Although mutation of the PALB2 gene (partner and localizer of BRCA2 gene) is found in only 1% of female breast cancers, it is seen in 16% of male breast cancers (22). Amplification of the EMSY gene (EMSY, BRCA1 interacting transcriptional repressor gene) is found in 13% of female breast cancers but in 35% of male breast cancers (22).
These findings are suggestive of a distinct repertoire of somatic changes driving the disease process of male breast cancer. Breast cancer in men is not the same disease as in women, and male breast cancer–specific data are needed to optimize diagnostic and treatment strategies (10). Of note, specific functions of the aforementioned genes can be found in the Genetics Home Reference database of the National Institutes of Health (25).
Male Breast Cancer: Diagnostic Challenges
Clinical Diagnostic Challenges
Male breast cancer diagnosis is challenging not only because of the lack of screening but also because of limited awareness and education, as well as a general inclination among men to delay care (26). Frequently, the diagnosis of already advanced manifestations of breast cancer is further delayed among men because of a lack of or inappropriate clinical care (Fig 3). Indeed, investigators have shown that men with breast cancer typically seek medical care and evaluation after a considerable delay (mean duration of symptoms, 10–30 months) (27–29), which is consistent with the fact that more than 40% of male breast cancers manifest as stage III or stage IV disease (29). This delay in no small way reflects a lack of awareness and understanding of male breast cancer. In a 2010 interview survey of men with a family history of breast cancer, nearly 80% of them were not aware that men could get breast cancer, 40% voiced concerns that a diagnosis of breast cancer would be demasculinizing, and 0% reported ever discussing male breast cancer with a clinical provider (30).
Figure 3.
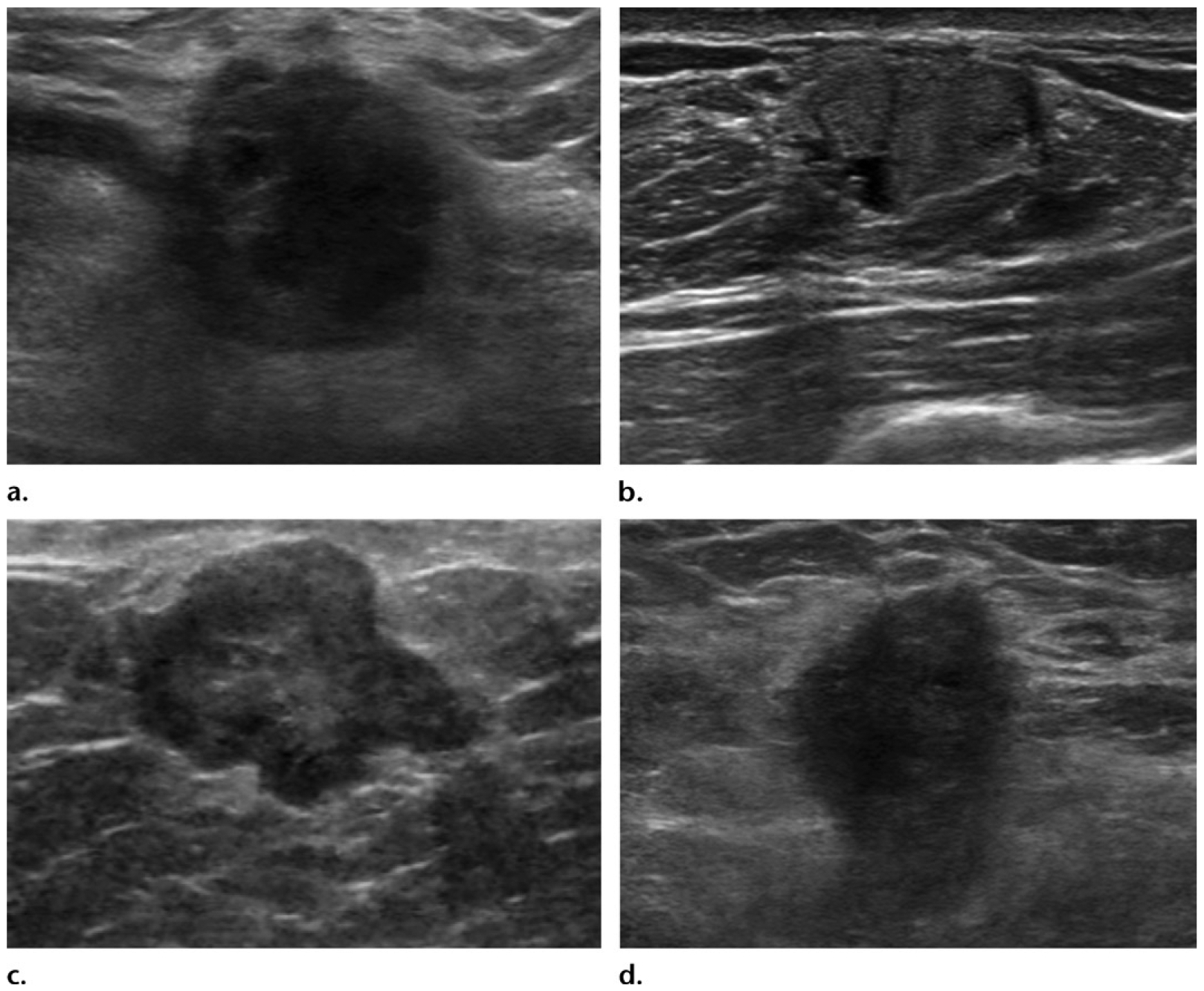
Typical clinical manifestations of male breast cancer as palpable irregular breast masses depicted on static US images in four distinct patients. (a) Delay in diagnosis in a 54-year-old man who had a palpable breast mass for 3 years before presentation: US image shows the breast mass, with findings consistent with an invasive ductal carcinoma. (b) Delay in diagnosis in a 53-year-old man who had a palpable breast mass for the previous 3.5 years and presented with the more recent onset of bloody nipple discharge: US image shows the breast mass, with findings consistent with a papillary carcinoma. (c) US image shows a palpable breast mass in a 76-year-old man who initially presented to the dermatology department for evaluation. Invasive ductal carcinoma was later diagnosed. (d) US image shows a palpable breast mass in a 54-year-old man who initially presented to the musculoskeletal department for evaluation. Invasive ductal carcinoma was ultimately diagnosed.
Beyond breast cancer, it is well known that men tend to avoid or delay clinical care in general, compared with women. In developed countries, men not only use health services less than women but also are less healthy than women and have higher mortality rates. In fact, being male is an independent risk factor for early mortality (26,31).
For all of these reasons, the current point of care for male breast cancer at clinical manifestation is poorly situated and highly limited, and strategies for earlier diagnosis are needed. At the same time, wider public awareness of male breast cancer is important. Despite burgeoning community-based efforts to advocate for awareness of male breast cancer (32), more can be done to educate the public, modeling after the successful campaigns to advocate for awareness of breast cancer in women.
Radiologic Diagnostic Challenges
Anatomic Considerations.—
Important anatomic differences exist between male and female breasts, which have diagnostic and prognostic implications. The relative paucity of breast tissue in men places tumors close to the skin and nipple, increasing the likelihood of dermal lymphatic spread and early regional metastasis. Anatomically, male breast tissue closely abuts the skin and pectoral fascia and shares lymphatic drainage with the skin by way of the subareolar plexus and also into the axilla (33,34). In comparison, the primary lymphatic network in the female breast is centered in the substance of the breast in a typically larger volume of fibroglandular tissue, with drainage into the axilla, and is less dependent on the superficial or deep tributaries of lymphatic pathways. Because there is usually a larger intervening volume of tissue encasing the tumor in women than in men, dermal involvement is less common in female breast cancer, compared with male breast cancer. This disparity is further exacerbated by the often delayed clinical care and the larger tumor size at diagnosis in men (27) (Fig 4).
Figure 4.
Multiple breast masses in a 58-year-old man with a family history of breast cancer (diagnosed in his sister at 48 years old) and no known genetic mutation, who presented for evaluation of a palpable area of concern in the left breast. (a, b) Diagnostic MLO (a) and CC (b) mammographic views of the left breast show multiple masses (solid arrows) in the upper outer quadrant and concurrent subareolar gynecomastia. The masses were associated with calcifications (dashed arrow). (c) Mediolateral (ML) magnification mammographic view best shows the calcifications. (d) Gray-scale US image shows corresponding clustered masses (arrows) spanning 5–6 cm, which reflects a large disease burden. US-guided biopsy was performed, and the findings from histopathologic examination and testing of the biopsy specimen yielded a diagnosis of ER-positive, PR-positive, HER2-negative invasive ductal carcinoma and ductal carcinoma in situ.
In comparison with female breast cancer, male breast cancer has been shown to have a higher propensity for lymphatic invasion (63%) (33). Male breast cancer involves the skin more frequently than female breast cancer (40% vs 5%–10%), invades the nipple more frequently (48% vs 5%–10%), and is more commonly associated with axillary nodal metastasis (60%–80% vs 10%–15%), all of which have been shown to be important predictors of adverse survival (33,35–39) (Fig 5). However, male breast cancer is also more likely than female breast cancer to be luminal A–like and luminal B–like molecular subtypes that are ER receptor positive and is less likely to be of the basal-like subtype, all of which are good prognostic indicators (9). Less aggressive molecular subtypes and more indolent tumor growth may in part contribute to delayed manifestation despite the fact that a male breast cancer should be easily detected if it comes to clinical attention. All things considered, earlier diagnosis of male breast cancer should be able to provide considerable improvement in individual clinical outcomes.
Figure 5.
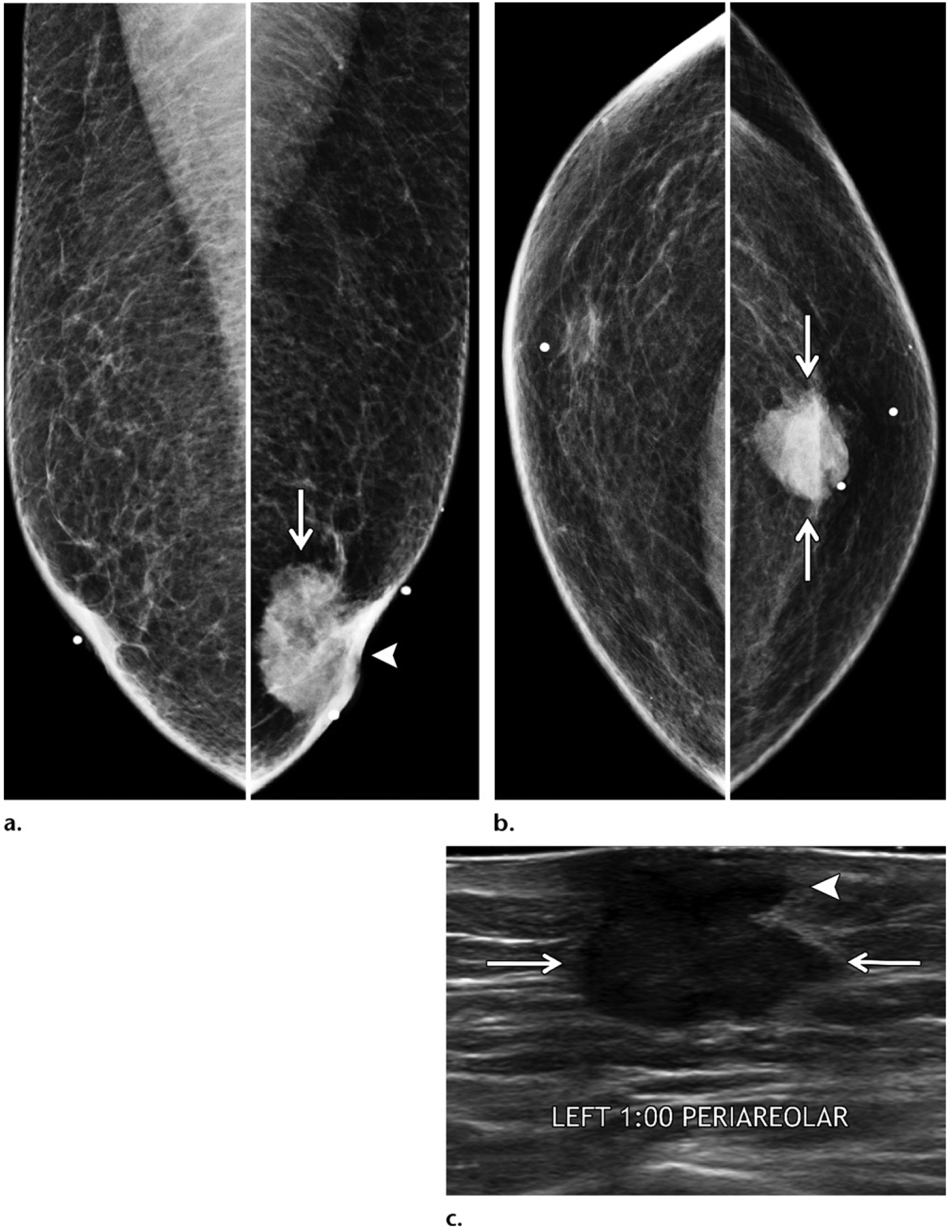
Invasive ductal carcinoma in a 70-year-old man of Ashkenazi Jewish ancestry who had a family history of breast cancer in multiple sisters and a maternal aunt, as well as a mutation of the CDKN2A gene (cyclin dependent kinase inhibitor 2A gene), who presented with a palpable mass in the left breast. (a, b) MLO (a) and CC (b) diagnostic mammographic views show an irregular retroareolar mass (arrows) in the left breast, causing nipple retraction (arrowhead on a). (c) Gray-scale US image shows a corresponding irregular mass (arrows) in the retroareolar area of the left breast, with direct invasion (arrowhead) of the overlying skin and nipple-areolar complex. US-guided biopsy was performed, and histopathologic analysis showed ER-positive, PR-positive, HER2-negative invasive ductal carcinoma. Mastectomy was performed, and the histopathologic results confirmed the presence of nipple involvement and lymphovascular invasion.
Central Location versus Eccentric Location.—
The classic teaching is that a central location compared with an eccentric location of a mass in a male breast usually differentiates cancer from gynecomastia. In reality, because the volume of the male breast is fairly small compared with that of the female breast, male breast cancer more often than not (58%) is subareolar and relatively central in location (Fig 6) (40). This location is also prevalent because male breast cancer is predominantly ductal in origin and most often arises from central ducts (11). Nevertheless, when a mass is eccentric in location in the male breast, suspicion should be raised for cancer (Fig 7). Along the spectrum of benign male breast disease more commonly encountered in the diagnostic setting, gynecomastia is typically central in location; and other benign conditions, including sebaceous cyst, oil cyst, lipoma, hematoma, or lymphoma, are most likely to be eccentric (41). Finally, if a mass has an unusual manifestation for breast cancer (Fig 8), consider other primary malignancies or secondary metastatic involvement of the breast, both of which are statistically more common than breast cancer in older men (5). Because male breast cancer is diagnosed late in life (median age at diagnosis of breast cancer, 70 years in men vs 60 years in women), it is important to be aware of any personal history of extramammary malignancy, and metastasis should always be considered (15,16).
Figure 6.
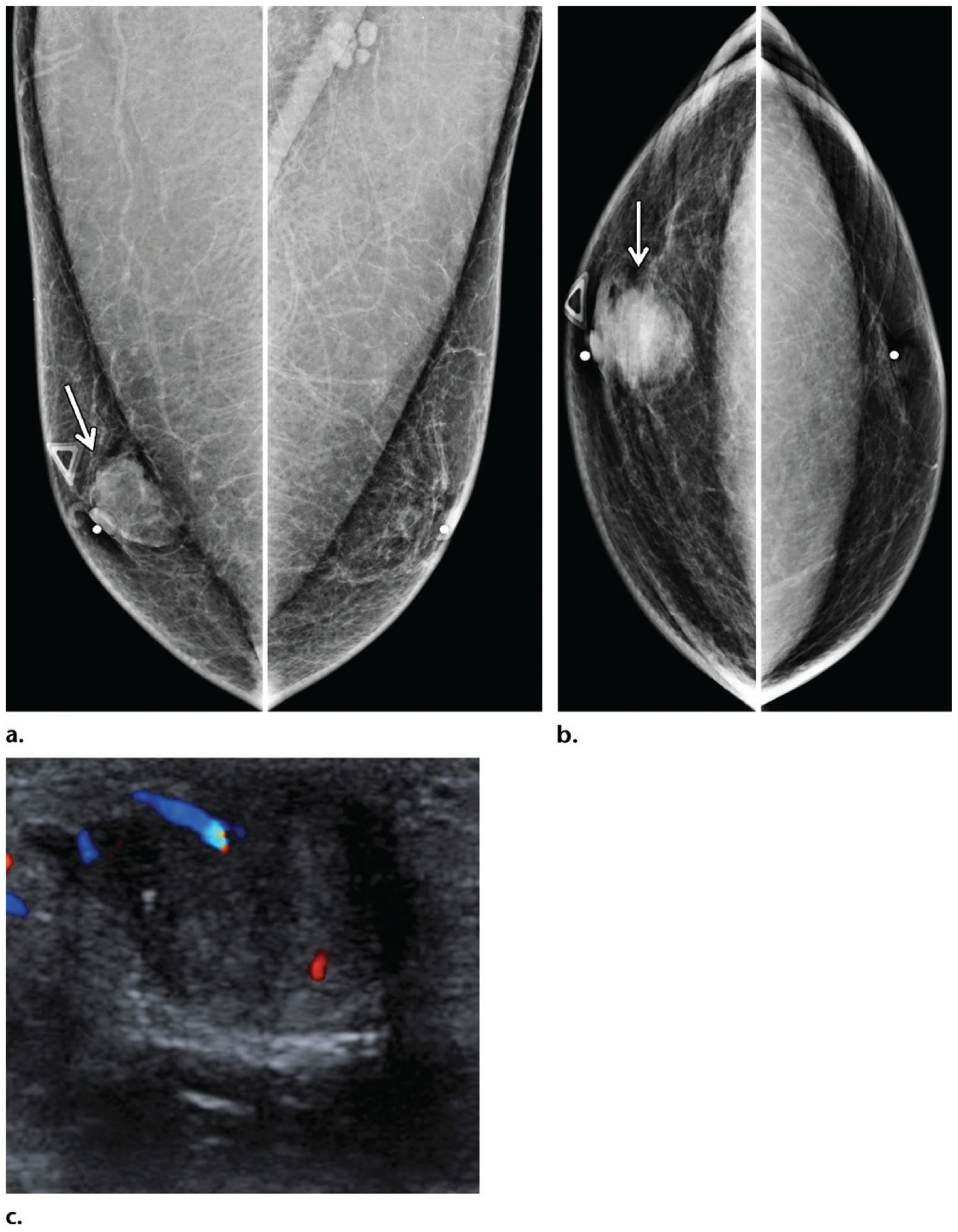
Retroareolar invasive ductal carcinoma in a 54-year-old Ashkenazi Jewish man who presented with a palpable mass in the right breast and who had a family history of breast cancer diagnosed in a sister (at 51 years old), a maternal aunt (at 60 years old), and his maternal grandmother (at 65 years old), as well as melanoma diagnosed in his father (at 65 years old). (a, b) MLO (a) and CC (b) mammographic views show a centrally located retroareolar mass (arrow) in the right breast. (c) Color Doppler US image also shows the mass. Biopsy of the mass was performed, and histopathologic analysis showed an ER-positive, PR-positive, HER2-negative invasive ductal carcinoma. Despite the considerable family history, this patient tested negative for any known genetic mutations.
Figure 7.
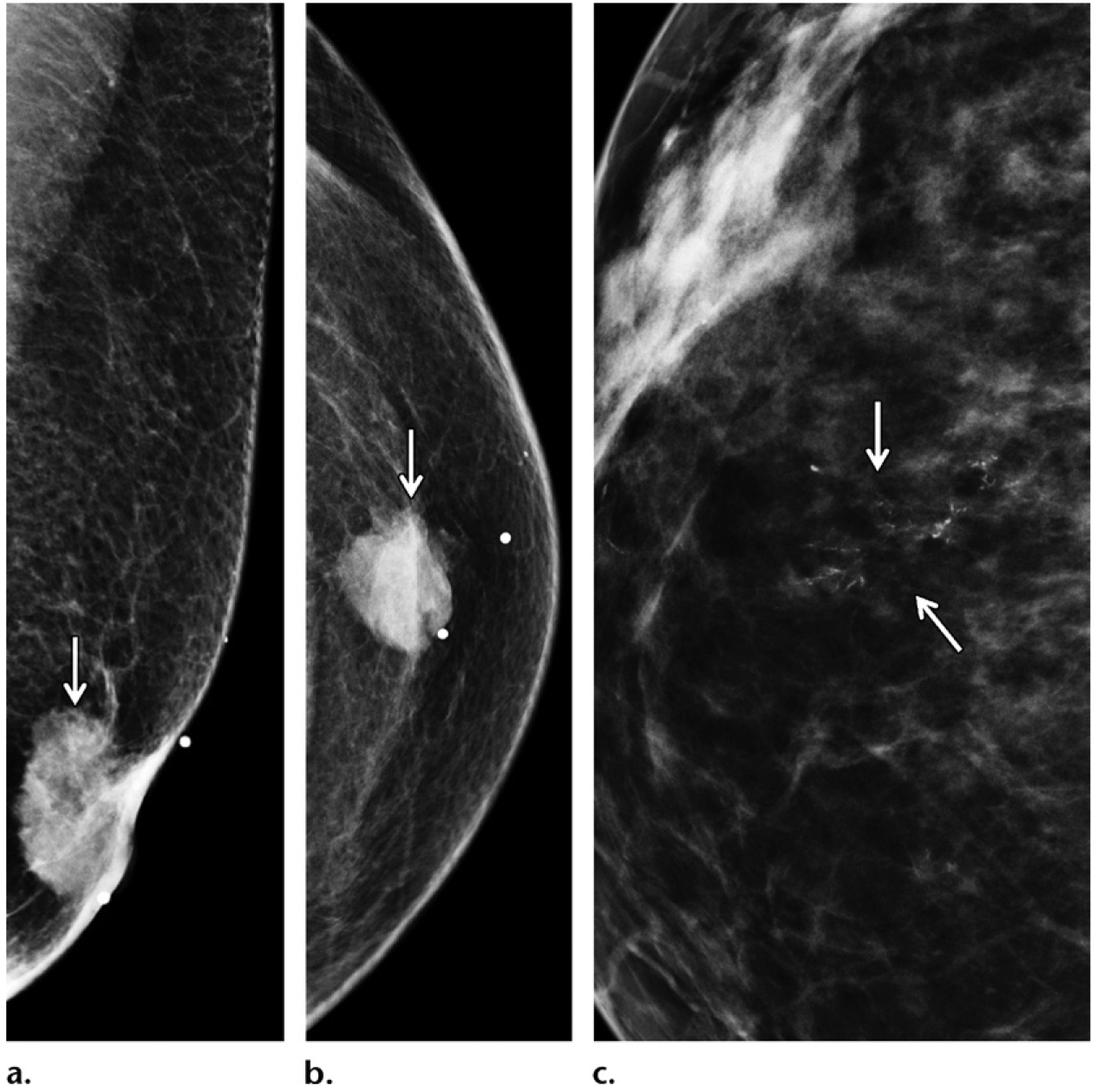
Eccentric invasive ductal carcinoma in a 50-year-old man with a family history of breast cancer (diagnosed in his mother at 45 years old), who presented with recent bloody nipple discharge, as well as a left breast mass that had been palpable for more than 3 years. (a, b) MLO (a) and CC (b) diagnostic mammographic views show an irregular periareolar mass (arrow) eccentric to the left nipple. (c) Magnification mammographic view shows that the mass is associated with calcifications (arrows). (d) Gray-scale US image shows a periareolar mass (arrow) that corresponded to the mammographic mass and is either a complex cystic and solid mass or an intracystic mass. A biopsy was performed, and histopathologic analysis showed an invasive ductal carcinoma with papillary features.
Figure 8.
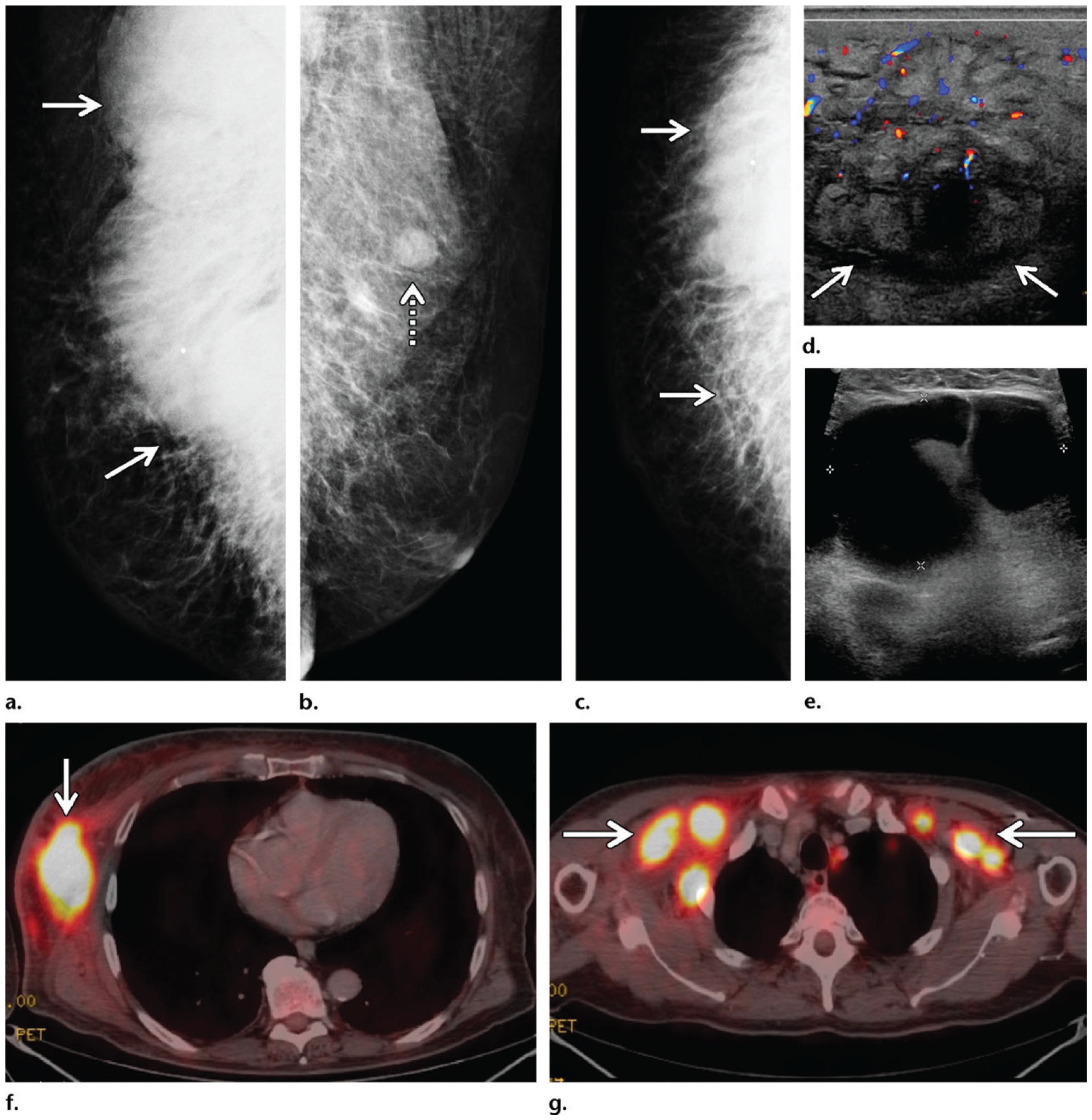
Mycosis fungoides in a 72-year-old man who presented with general malaise, weight loss, a palpable mass in the right breast, and bilateral palpable axillary masses. (a–c) Diagnostic mammography: MLO views of the right (a) and left (b) breasts and CC view of the right breast (c) show a large radiopaque mass (solid arrows on a, c) in the posterior part of the right breast extending into the axilla, with associated stranding and dermal thickening. An enlarged lymph node (dashed arrow on b) is depicted in the left axilla. (d) Color Doppler US image shows a 12-cm hypervascular right breast mass (arrows) with edema and overlying skin thickening, along with bilateral enlarged axillary lymph nodes. (e) Gray-scale US image shows a representative example of the bilateral enlarged axillary lymph nodes. (f) Subsequent axial PET/CT image at the level of the breasts shows a highly FDG-avid right breast mass (arrow) with mildly FDG-avid overlying skin. Marked systemic enlargement of lymph nodes was found throughout the nodal stations in the body. (g) Axial PET/CT image at the level of the axillae shows enlarged lymph nodes (arrows) in both axillae. Biopsy of the breast mass and select lymph nodes was performed, and histopathologic analysis showed mycosis fungoides, a common form of cutaneous T-cell lymphoma.
Gynecomastia.—
Gynecomastia is benign proliferation of rudimentary breast tissue in men, including ductal hyperplasia and periductal stromal proliferation. On the basis of the mammographic appearance, there are three subtypes of gynecomastia: the nodular, dendritic, and diffuse forms (Fig 9). Nodular gynecomastia appears as a subareolar mass and may mimic cancer (Fig 9d). Dendritic gynecomastia has a classic flame- or fan-shaped configuration, which is diagnostic at mammography (Fig 9c, 9d). Diffuse gynecomastia is most often seen in patients with exogenous estrogen exposure and resembles dense fibroglandular tissue in the female breast (Fig 9b). Gynecomastia is often asymmetric. It can be physiologic or may be due to any of a number of possible known causes (Table 4). Gynecomastia is exceedingly common, found in 40%–55% of all men in one autopsy study, and has a trimodal age distribution when it is physiologic, with the rate of occurrence peaking in the neonatal, pubertal, and senescent populations (42).
Figure 9.
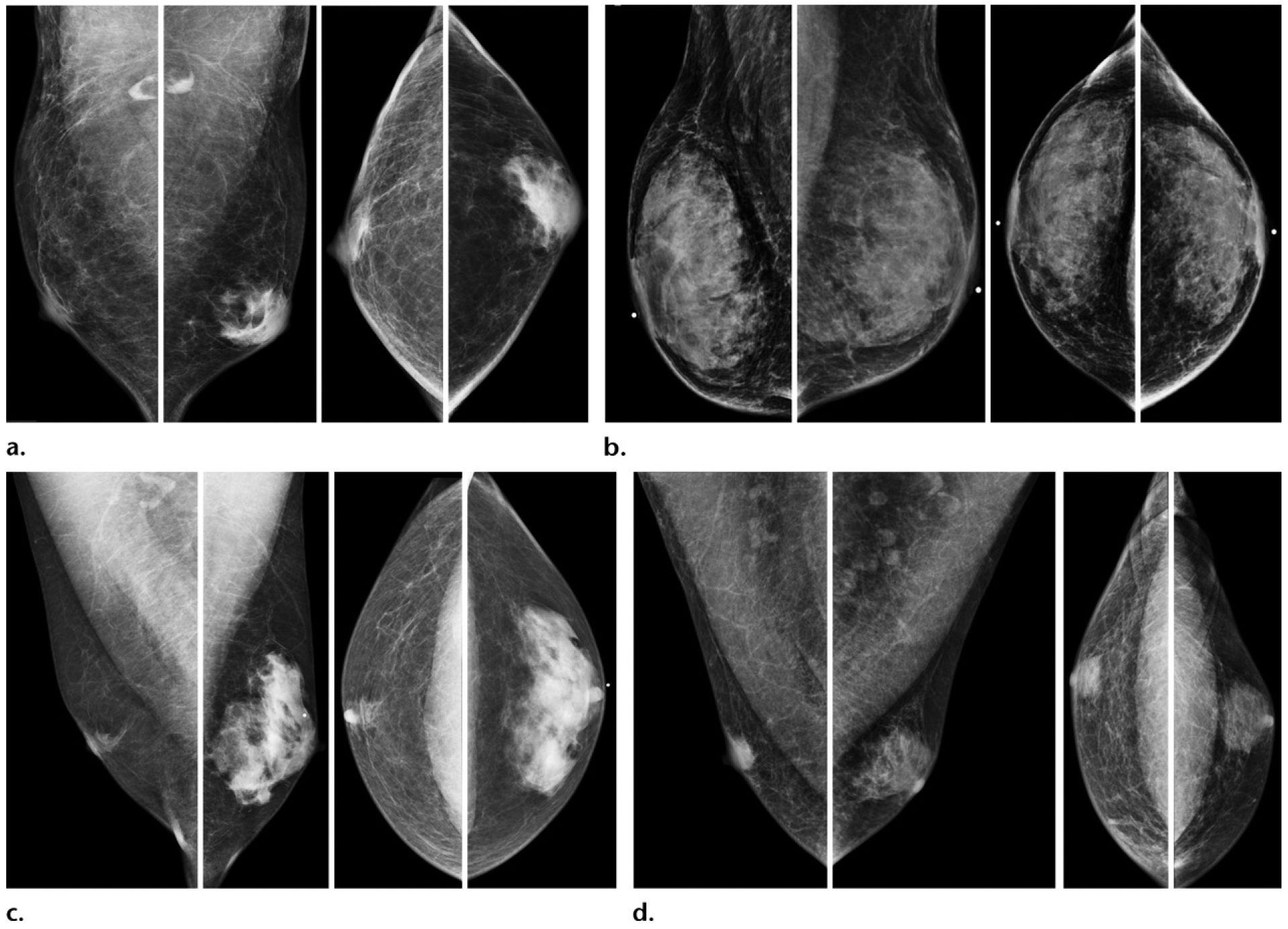
Gynecomastia depicted as increased retroareolar fibroglandular tissue in four separate patients: Bilateral MLO and CC mammographic views of a 39-year-old man (a), a 75-year-old man (b), a 41-year-old man (c), and a 60-year-old man (d). Gynecomastia can often be asymmetric (a, c, d). Three subtypes of gynecomastia may be seen. Dendritic gynecomastia has a classic flame-shaped form, as seen in the right breast on c and the left breast on d. Nodular gynecomastia is depicted as a retroareolar mass in the right breast on d. Diffuse gynecomastia is seen as diffusely increased breast tissue bilaterally, mimicking female breasts, as depicted in both breasts on b. In this case (b), diffuse gynecomastia was a result of end-stage liver failure.
Table 4:
Common Representative Causes of Gynecomastia
| Etiologies | Specific Causes |
|---|---|
| High estrogen level | Testicular tumors (germ cell tumor, Leydig cell tumor, Sertoli cell tumor); adrenal, liver, lung, and renal tumors; exogenous estrogen exposure; high body mass index; hyper- or hypothyroidism; starvation and refeeding; occupational exposure; use of isoflavones; exposure to phytoestrogens (in cosmetics, soy products, beer, tea tree oil, or lavender oil) |
| Low androgen level | Testicular trauma, orchitis, Klinefelter syndrome (47, XXY); pituitary disease; chemo-therapy; neurologic disease, spinal cord injury |
| Substance exposure | Alcohol, anabolic steroids, amphetamines, cocaine, heroin, marijuana |
| Pharmacologic agents | Alkylating chemotherapy agents, amiodarone, captopril, verapamil, cimetidine, diazepam, digitalis, haloperidol, isoniazid, metronidazole, nifedipine, omeprazole, phenytoin, spironolactone, tricyclic antidepressants, thiazide diuretics |
Clinical assessment is the mainstay for diagnosing gynecomastia, and confirmation with imaging is usually not recommended (43), yet gynecomastia is the most common reason for diagnostic examinations in men because of the often unilateral symptoms and indeterminate findings at physical examination. Although gynecomastia is a benign entity, questions have been raised about whether there might be a possible association between gynecomastia and male breast cancer because of the shared hormonal risk factors (high-estrogen or low-testosterone states). Of interest, gynecomastia was found to be a significant risk factor for male breast cancer in the results of a large series of cases from the National Institutes of Health Male Breast Cancer Pooling Project (n = 2405) (odds ratio, 9.78; 95% confidence interval: 7.52, 12.7) (44), as well as in the results of a separate study that used the U.S. Department of Veterans Affairs medical care system (n = 642) (odds ratio, 5.86; 95% confidence interval: 3.74, 9.17) (45).
The greatest barrier to screen detection of breast cancer in women is the masking of cancer by fibroglandular tissue at mammography—a barrier reflected by the way breast density is currently categorized in the Breast Imaging Reporting and Data System (BI-RADS) lexicon (46) and by the ongoing quest to improve diagnostic accuracy by minimizing overlapping tissue (47). The same challenge, to a much lesser extent, is encountered in diagnosing breast cancer in men with gynecomastia. In most cases of small-volume gynecomastia, mammography is sufficient to exclude other abnormalities, and US is generally obviated because the sonographic appearance of gynecomastia may overlap with that of cancer. Small cancers central to the nipple, however, can be masked by gynecomastia at mammography (Fig 10), and US is helpful in these cases to delineate possible masses. Similarly, in select cases of high clinical suspicion for malignancy and a substantial presence of gynecomastia, US can be of benefit (Fig 11). More recent implementation of digital breast tomosynthesis, which improves mammographic evaluation, likely also helps increase diagnostic confidence when evaluating gynecomastia.
Figure 10.
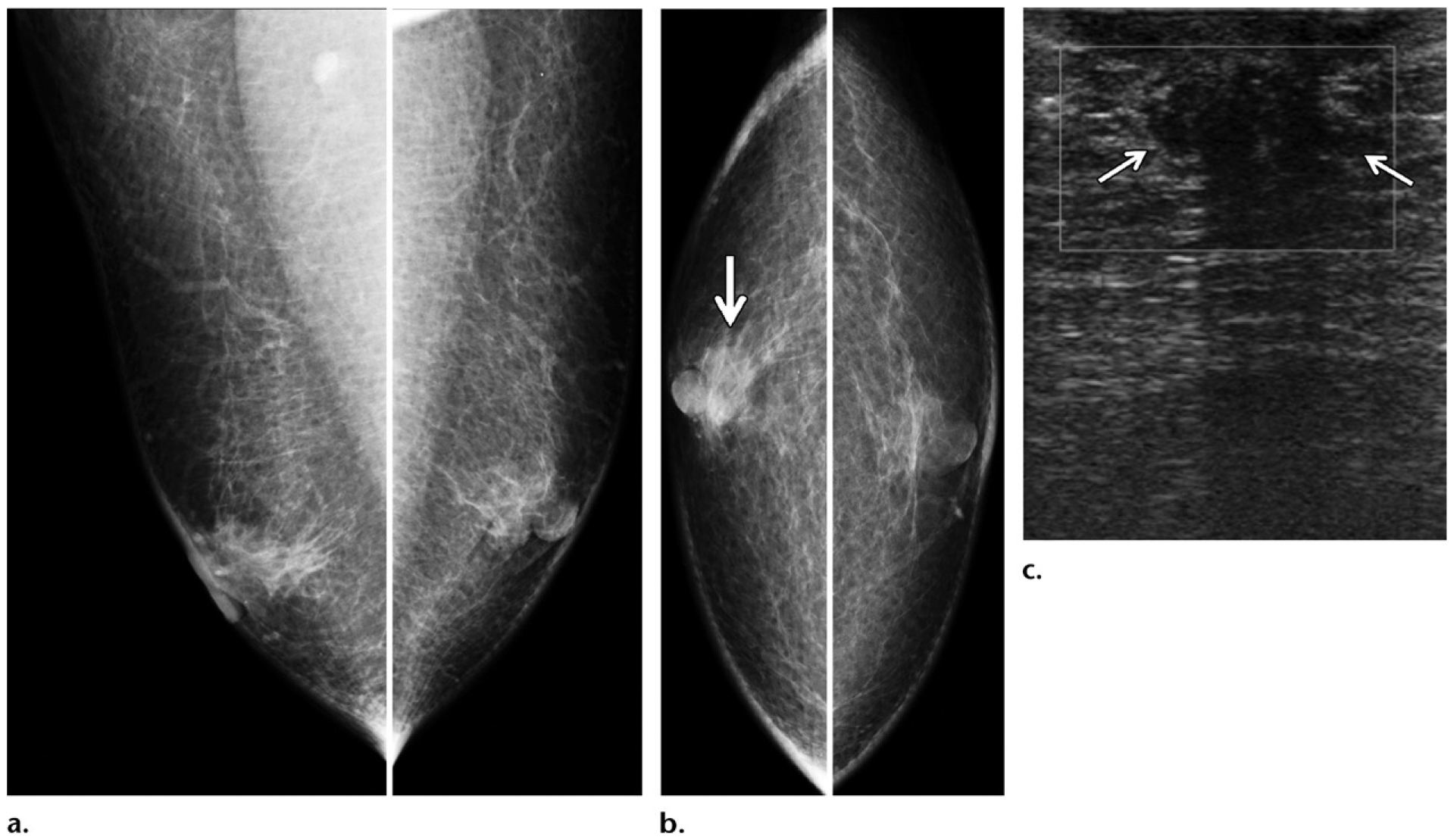
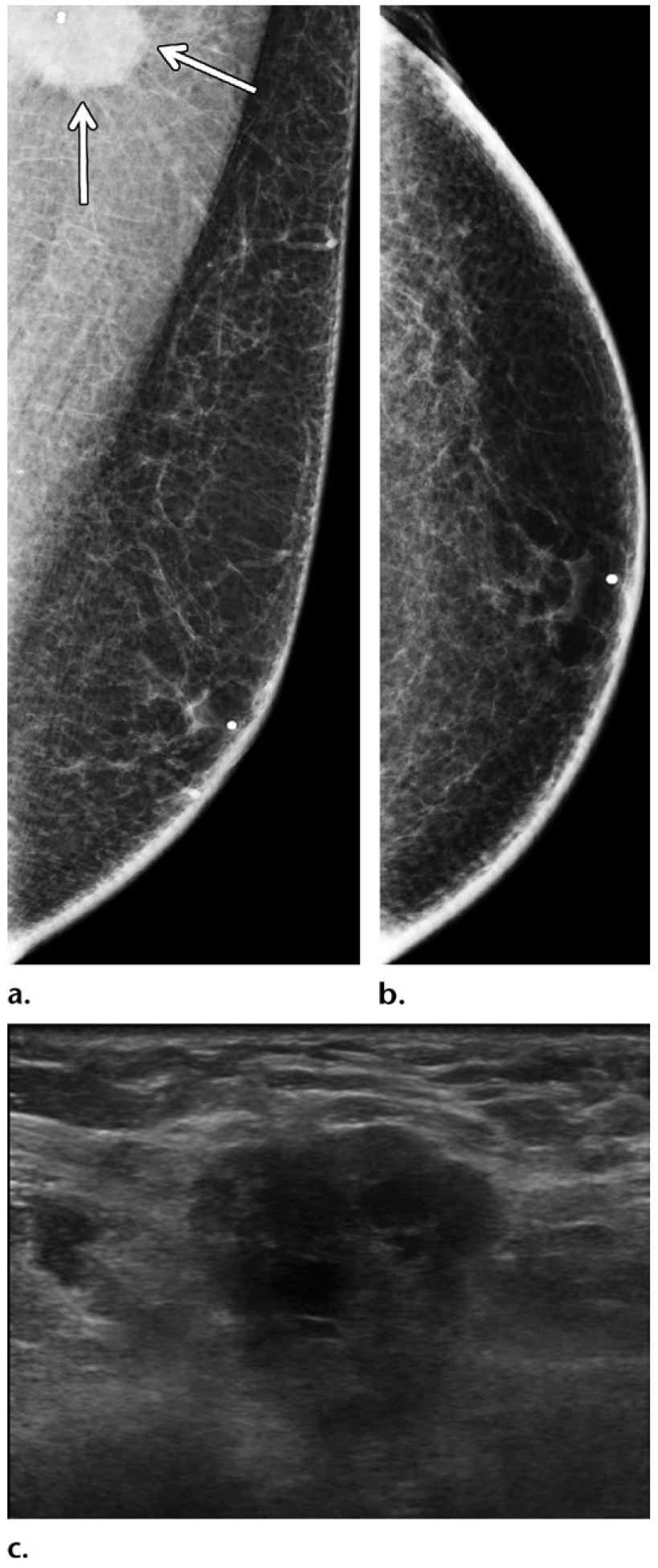
Gynecomastia masking a small cancer in a 75-year-old man presenting with a palpable right retroareolar mass, who had a history of chest irradiation for lymphoma treatment but no known family history or genetic mutation. (a, b) Diagnostic MLO (a) and CC (b) mammographic views show bilateral flame-shaped dendritic gynecomastia on both views. In the retroareolar area of the right breast, a minimally increased density (arrow on b) was depicted on the CC view. (c) Targeted gray-scale US image of the retroareolar region of the right breast shows an irregular mass (arrows) central to the nipple. Biopsy of this mass was performed, and histopathologic analysis showed a diagnosis of ER-positive, PR-positive, HER2-negative invasive ductal carcinoma.
Figure 11.
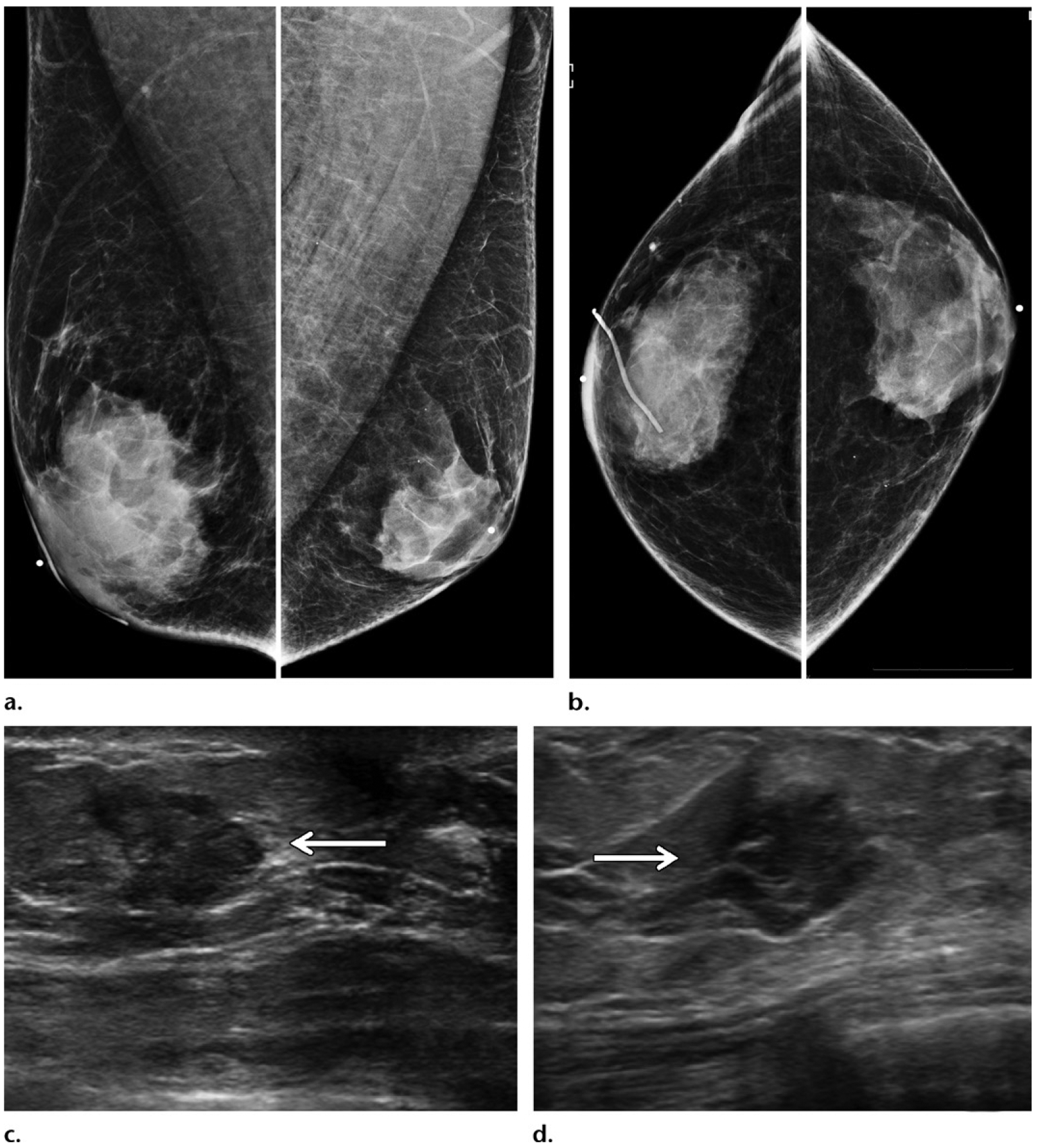
Benefits of US in a case at high clinical suspicion for malignancy. A 50-year-old man of Ashkenazi Jewish ancestry who had bloody right nipple discharge and normal initial imaging workup underwent central duct excision at another institution, with surgical pathology results showing ductal carcinoma in situ with positive margins. This patient subsequently presented to our institution for further care. (a, b) Diagnostic MLO (a) and CC (b) mammographic views show the site of previous surgery in the right breast, as demarcated by a scar marker (a, b). Moderate bilateral gynecomastia was depicted as dense subareolar tissue in both breasts. No mass was seen at mammography. (c, d) Gray-scale US images show an irregular mass (arrow) in transverse (c) and sagittal (d) projections in the periareolar region of the left breast. A postoperative seroma was seen in the right breast (not shown). The patient underwent bilateral mastectomy, and surgical pathology results showed ER-positive, PR-positive, HER2-negative invasive ductal carcinoma in the left breast and ER-positive, PR-positive, HER2-negative ductal carcinoma in situ in the right breast.
Calcifications.—
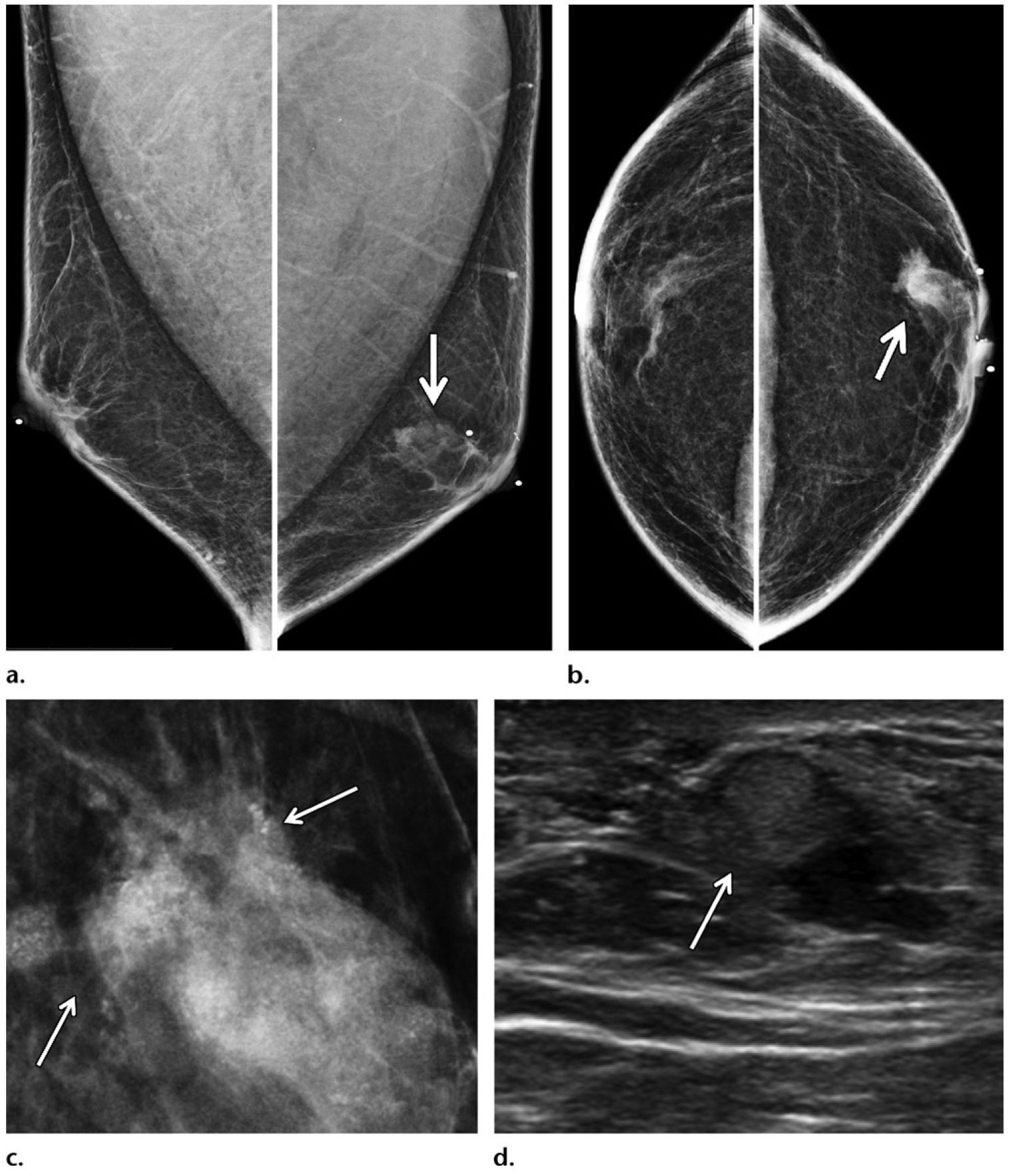
Calcifications are less commonly encountered than masses in male breast cancer patients, compared with female breast cancer patients (33), a finding that is consistent with the fact that male breast cancer is typically diagnosed later in the disease process as compared to female breast cancer (Fig 1). In male breast cancer, calcifications can be seen either in association with masses (Figs 4, 7)or alone (Fig 12). Male breast cancer is more likely to assume a pseudobenign appearance as compared to female breast cancer, with masses more likely to be round or oval (Fig 6) and with calcifications more likely to be larger, rounder, coarser, and less numerous (Fig 12). In contrast, the typical morphology of female breast cancer includes spiculated and irregular masses and pleomorphic fine calcifications.
Figure 12.
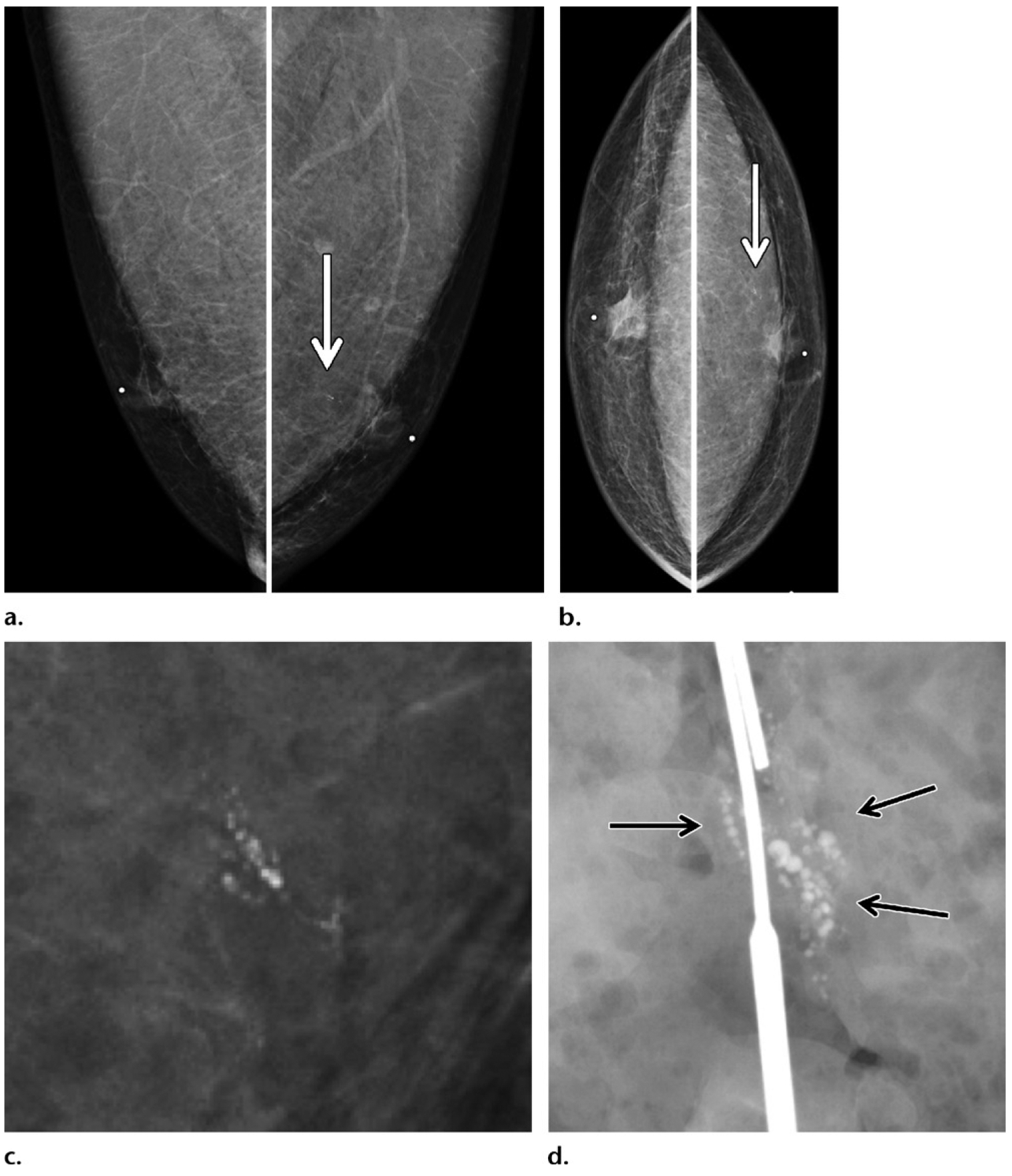
Calcifications depicted on screening mammogram of a 53-year-old Ashkenazi Jewish man with extensive family history of breast cancer (diagnosed in his father, two sisters, and paternal grandmother), who recently tested positive for BARD1 gene mutation (a BRCA1-associated RING domain 1 gene mutation). (a, b) Screening MLO (a) and CC (b) mammographic views show round calcifications (arrow) in a linear branching distribution in the retroareolar region of the left breast. (c) Magnification mammographic view obtained at a subsequent diagnostic examination best shows the calcifications. Needle localization and excisional biopsy of the calcifications were performed. (d) Surgical specimen radiograph shows the targeted calcifications (arrows). Histopathologic analysis of the biopsy specimen revealed invasive ductal carcinoma and ductal carcinoma in situ (ER positive, PR positive, HER2 negative), with positive surgical margins. A left mastectomy was performed, and tamoxifen therapy was initiated.
Because most male breast cancer is ductal in origin (90%) (11) and because ductal carcinoma in situ is the most common precursor to male breast cancer (98%) (48), mammographic imaging earlier in the development of male breast cancer is likely to show calcifications more frequently. Although the current American Society of Clinical Oncology Multidisciplinary Guidelines for men at high risk for breast cancer include possible consideration of mammographic screening only when gynecomastia or glandular tissue is demonstrated at baseline (49), high-risk men may benefit from screening regardless of presence of breast tissue, because ductal carcinoma in situ manifests as calcifications, which are well delineated at mammography whether tissue is present or not (Fig 12).
Male Breast Cancer: Delineating Risk Factors
Definition of High Risk in Men
Although high-risk status in women is clearly associated with a lifetime risk of breast cancer of 20%–25% or more (50), what constitutes high risk in men is less well defined. The notion of “average risk” for development of breast cancer differs greatly between women and men (lifetime risk of 1/8 vs 1/833) (51), making it difficult to model how we categorize risk in male breast cancer after how we categorize risk in female breast cancer. Although many of the predisposing risk factors for male breast cancer are known, limited data exist with regard to the exact disease incidence and the prevalence of breast cancer and its prognosis among the subgroups of men at risk, because male breast cancer on the whole is so uncommon. For this reason, for the purpose of our discussion and throughout this article, we define high risk as any risk above average. As research moves forward, evidence-based risk assessment will certainly be needed to more clearly delineate the level of breast cancer risk in men, to further tailor screening frequency, as well as therapeutic strategies and risk reduction strategies.
Who Is at Risk for Male Breast Cancer?
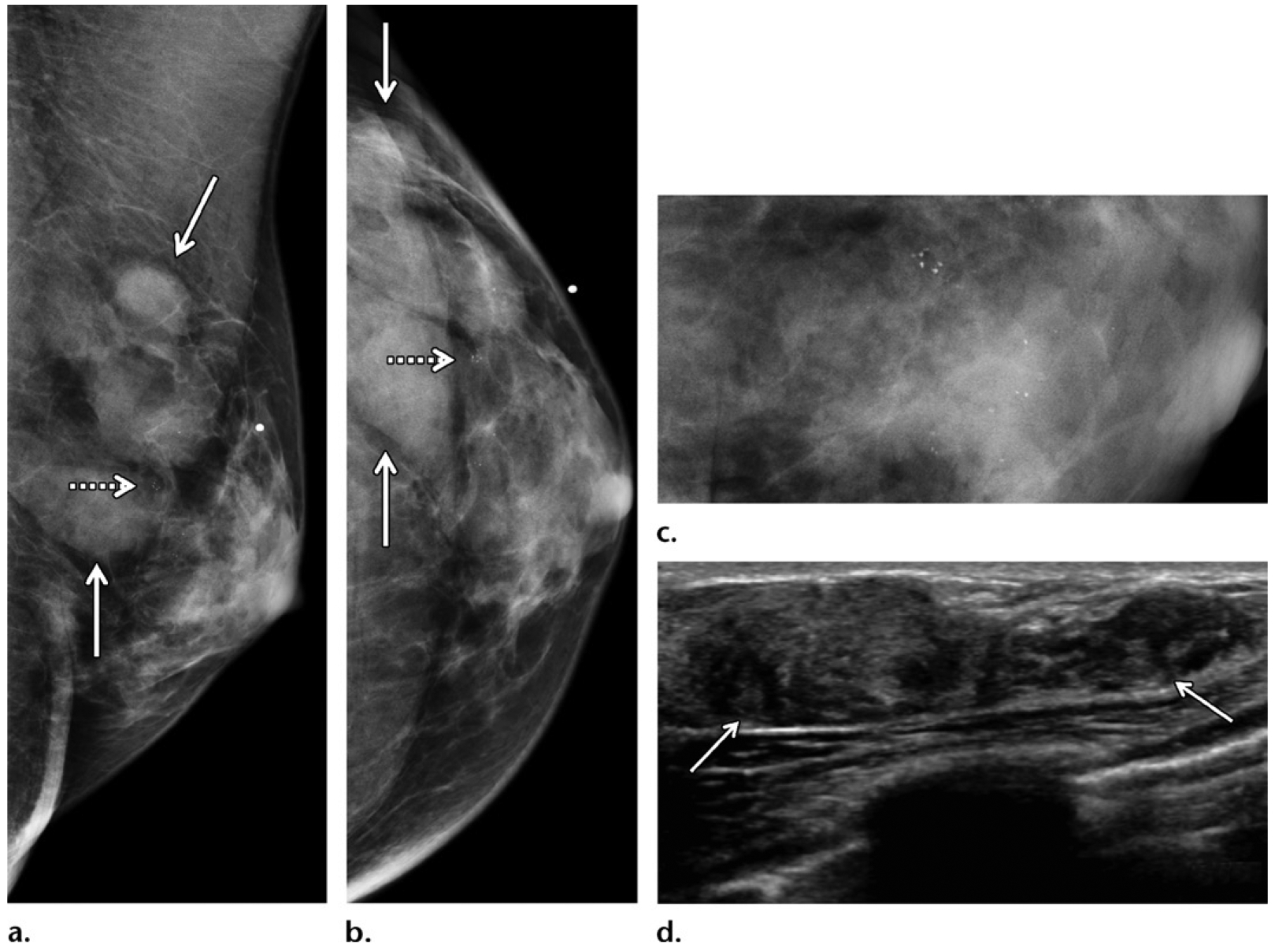
As with all cancers, age is an important risk factor for male breast cancer, which peaks at 75 years of age (49). However, in the clinical setting, male breast cancer is frequently encountered earlier in life, when additional risk factors are present (Figs 2, 4, 6, 7, 11–15) (Table 5). It is estimated that up to 20% of men with male breast cancer report a family history of breast or ovarian cancer, and 10% have an identifiable deleterious genetic mutation, most commonly a BRCA2 mutation. Less commonly implicated genes include PTEN, CHEK2, and BARD1 (Figs 12, 15). Therefore, all men who are diagnosed with breast cancer currently undergo genetic testing as recommended by the American Society of Clinical Oncology, and in part serve as sentinel cases indicating an elevated risk for their kin (49).
Figure 15.
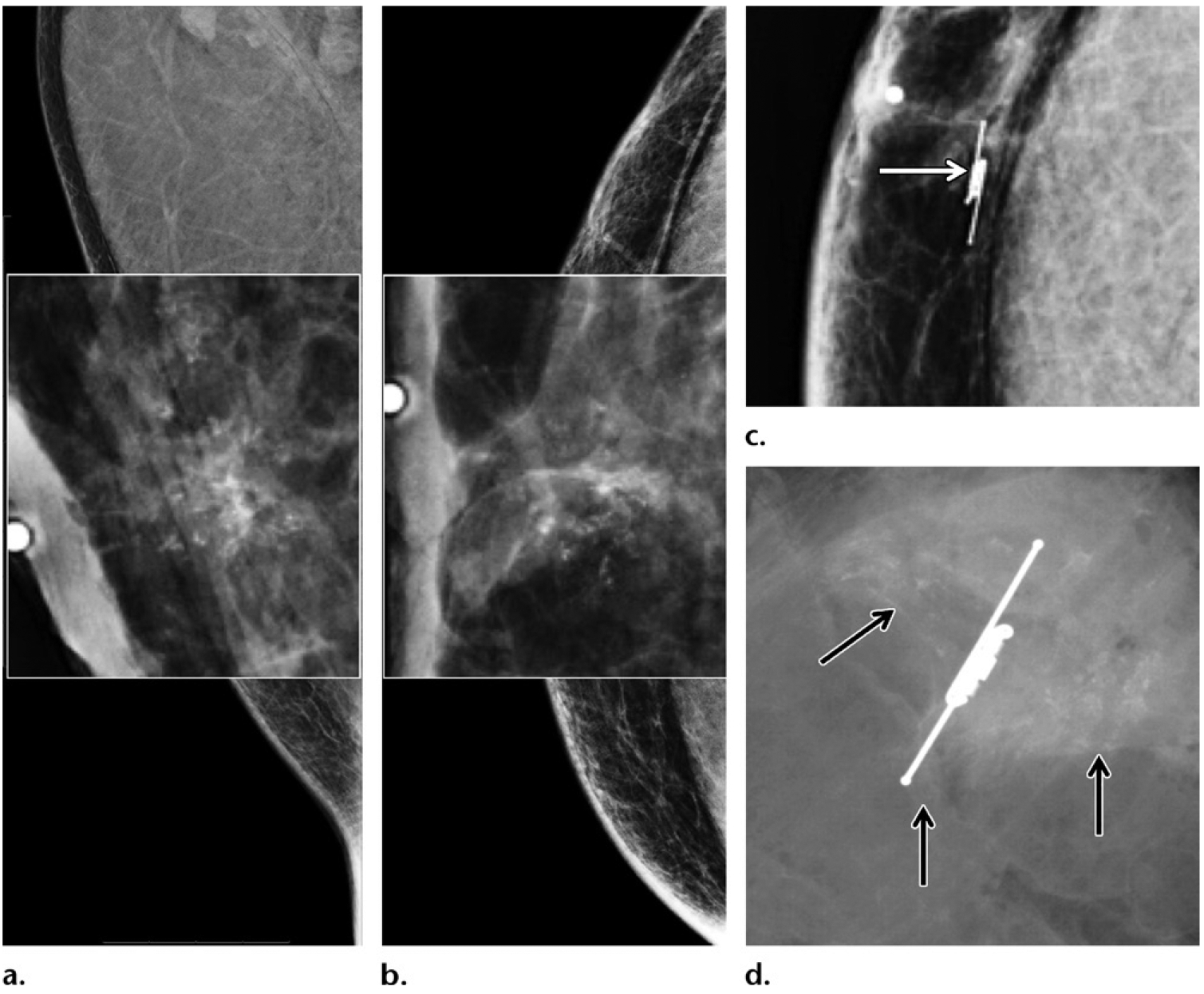
Screening and/or surveillance mammogram in a 54-year-old Ashkenazi Jewish man with a personal history of left breast cancer diagnosed 1 year prior and treated with mastectomy (same patient as in Fig 12), who tested positive for BARD1 mutation and had a family history of breast cancer (diagnosed in his father, two sisters, and paternal grandmother). (a, b) MLO (a) and CC (b) mammographic views of the contralateral right breast show new faint calcifications in the subareolar breast on the magnified views (at center). The patient underwent reflector placement for radar localization (Savi Scout; Cianna Medical, Aliso Viejo, Calif) of the calcifications prior to breast surgery. (c) Postprocedure mammogram in CC projection shows the calcifications demarcated by the reflector (arrow), to ensure removal. Right mastectomy was performed. (d) Radiograph of the surgical specimen confirmed presence of the reflector and the targeted calcifications (arrows). The histopathologic analysis showed ER-positive, PR-positive, HER2-negative ductal carcinoma in situ. This patient was therefore diagnosed with bilateral breast cancer early in the disease process on the basis of screening mammograms obtained within a 2-year span.
Table 5:
Risk Factors for Male Breast Cancer
| Risk Factor Category | Examples of Risk Factors |
|---|---|
| High estrogen level | Exogenous estrogen exposure, Klinefelter syndrome, body mass index ≥ 30, cirrhosis, pituitary adenoma |
| Low androgen level | Cryptorchidism, orchitis, orchiectomy |
| Genetic mutations | BRCA2, BRCA1, CHEK2, CYP17A1, PTEN |
| Exposures | Radiation, electromagnetic fields, polycyclic aromatic hydrocarbons, high ambient temperature, exhaust |
| Family history | First-degree relative with breast or ovarian cancer |
| Personal history | Breast cancer |
| Race and ethnicity | Ashkenazi Jew, African American, Saharan African |
Note.—BRCA1 = BRCA1, DNA repair associated gene; BRCA2 = BRCA2, DNA repair associated gene; CHEK2 = checkpoint kinase 2 gene; CYP17A1 = cytochrome P450 family 17 subfamily A member 1 gene; PTEN = phosphatase and tensin homolog gene.
In addition, a hormonal imbalance between the levels of estrogen and androgen (relative excess of estrogen vs androgen) owing to many causes—including exogenous estrogen exposure (trans-gender women), obesity (body mass index ≥ 30), heavy alcohol consumption, liver and testicular damage, or Klinefelter syndrome (47, XXY)—has been associated with male breast cancer (27). Various exposures, including prior mantle radiation therapy and occupational exposures to polycyclic aromatic hydrocarbons, exhaust emission, electromagnetic fields, and high ambient temperature, among others, are further risk factors associated with breast cancer (27,45) (Fig 10).
Racial and Ethnic Considerations
Male breast cancer is known to be particularly prevalent among certain racial and ethnic groups. Data from the California Cancer Registry and the National Cancer Database disclosed that the male breast cancer incidence is higher among African American men, who are more likely to be diagnosed at a younger age and are less likely to undergo treatment, compared with their white counterparts (52,53). In fact, not only are African American men at increased risk for male breast cancer, younger African American men (<65 years old) have a 76% higher risk of death from breast cancer compared with similarly aged white men, after adjusting for other clinical factors (52,53). This difference is likely attributable, at least in part, to disparity in socioeconomic status and access to care, which has been widely documented in the setting of female breast cancer among different racial and ethnic groups.
Men of Ashkenazi Jewish descent are another group at increased risk for breast cancer, largely owing to a high prevalence of founder germline mutations in BRCA1 and BRCA2 (54). African American men and men of Ashkenazi Jewish descent are more likely than the general population to be diagnosed with male breast cancer, and the latter group is more likely than other members of the Jewish community to have breast cancer. In the results of a study from the Israel Cancer Registry, investigators found that 78% of all breast cancers in Jewish men were identified in those with Ashkenazi ancestry, and Ashkenazi Jewish men were significantly more likely than Sephardic Jewish men to harbor breast cancer (odds ratio, 1.8; P = .001) (55). In comparison with non-Jewish men with breast cancer, 10% of whom carry BRCA1 or BRCA2 mutations, 20% of Jewish men with breast cancer carry such mutations (54).
Multigene Panel Testing: A Step Forward
Revolutionary advances in genomics in the past 2 decades have made it possible to perform genetic testing for clinical assessment of personal cancer risks. The advent of next-generation sequencing has dramatically improved throughput of DNA sequencing. Around 1990–2003, sequencing the first human genome (the Human Genome Project) took 13 years and cost more than a billion dollars. Today, human genome sequencing is available for clinical use, takes 1–2 days, and costs several thousand dollars. This change laid the foundation for commercially available multigene panel testing, which has been widely adopted in genetic testing of women at high risk for hereditary breast cancer (56). These tests require either blood or saliva samples and must be performed at facilities approved in accordance with the Clinical Laboratory Improvement Amendments of 1988. Test results are usually returned within 2–4 weeks, and costs of the tests are covered by most insurance plans owing to the federal Genetic Information Nondiscrimination Act of 2008. As a result of such improved genetic testing, women at risk are able to be tested for multiple genetic mutations predisposing to female breast cancer simultaneously, including BRCA1, BRCA2, the ATM gene (ATM serine/threonine kinase gene), CHEK2, PALB2, TP53 (Li-Fraumeni syndrome), PTEN (Cowden syndrome), the CDH1 gene (cadherin 1 gene), and the STK11 gene (serine/threonine kinase 11 gene), among others (6,57,58) (Table 6).
Table 6:
Commonly Panel-tested High-Risk Genes for Female Breast Cancer
| High-Risk Gene* | Relative Risk of Breast Cancer | Absolute Risk of Breast Cancer (%) | Other Cancers | Syndrome |
|---|---|---|---|---|
| BRCA1(AD) | 11.4 | 75–82 by age 80 y | Ovary | Hereditary breast cancer |
| BRCA2(AD) | 11.7 | 76–82 by age 80 y | Ovary, prostate, pancreas | Breast-ovarian cancer syndrome |
| TP53(AD) | 105 (62–165)† | 95 by age 70 y | Childhood sarcoma, adrenocortical cancer, brain tumors | Li-Fraumeni syndrome |
| PTEN(AD) | No reliable data | 85 by age 80 y | Thyroid, endometrial | Hamartoma tumor syndrome, Bannayan-Riley-Ruvalcaba syndrome, Cowden syndrome |
| CDH1(AD) | 6.6 (2.2–19.9)† | 53 by age 80 y; lobular cancer specific | Diffuse gastric cancer | Hereditary diffuse gastric cancer |
| STK11(AD) | Unknown | 32 by age 60 y | Colon, pancreas, ovarian sex cord stromal tumors | Peutz-Jeghers syndrome |
Because of more systematic genetic counseling and risk stratification among women with breast cancer who are at increased risk for breast cancer (ie, family history, known mutations), their male relatives who are at elevated risk for breast cancer are being increasingly identified. Wider clinical use of genetic testing (multigene panel) is allowing men with genetic mutations to be recognized before development of breast cancer, which makes targeted screening feasible. At the same time, as our knowledge of genetic alterations in male breast cancer evolves, the gene panels are likely to look different for male breast cancer and female breast cancer in the future (22).
Targeted Screening and Surveillance in Men
BRCA1 and BRCA2 Mutations
BRCA1 and BRCA2 are highly penetrant autosomal dominant tumor suppressor genes strongly as sociated with early development of breast cancer, both in women and in men. Genetic mutations involving BRCA1 or BRCA2 and polymorphisms are well categorized in the Breast Cancer Information Core open-access online database run by the National Human Genome Research Institute (59).
In women, BRCA1 or BRCA2 germline deleterious mutations confer significantly increased risks for breast and ovarian cancers. Absolute lifetime risk for breast cancer is 65% in female BRCA1 carriers and 40% in female BRCA2 carriers, compared with 12% in the average woman. BRCA1 mutation has been associated with the more-aggressive triple receptor–negative tumor molecular subtype (ER negative, PR negative, and HER2 negative) (60), concurrent early disease onset, and a family history of breast cancer, particularly in those with Jewish ancestry, further amplifying the already increased risks (6,54). Women with BRCA1 or BRCA2 mutations therefore not only undergo annual mammographic screening but also are recommended to undergo additional supplemental annual screening with contrast material–enhanced breast MRI (50). Fundamental risk reduction strategies of prophylactic mastectomy and salpingo-oophorectomy are routinely carried out in women to minimize risks.
Male carriers of BRCA1 or BRCA2 mutations are similarly at elevated risk for breast cancer, with BRCA2 mutation conferring higher risk than BRCA1 mutation. Absolute lifetime risk for breast cancer is 2% in male BRCA1 carriers and 8% in male BRCA2 carriers, compared with 0.1% in the average man, although risks can be substantially higher when combined with a family history of breast cancer (61). Even though the absolute risk of developing cancer in male BRCA1 or BRCA2 mutation carriers is much lower than in their female counterparts, the relative increase in risk from baseline is greater in men (increased by 20-fold for BRCA1 and 80-fold for BRCA2 mutation carriers), compared with that in women (increased by fourfold for BRCA1 and threefold for BRCA2 mutation carriers). Because survival in male breast cancer patients is poor on the whole and because breast cancer–specific mortality is especially high in men younger than 50 years old (11), selective mammographic screening in men with BRCA1 or BRCA2 mutations may be warranted. It is also important to mention that male BRCA1 or BRCA2 mutation carriers are at increased risk for prostate cancer, pancreatic cancer, and melanoma. Of note, BRCA2 mutation is associated with more-aggressive prostate cancer (higher histologic grade and increased metastasis) (6).
The current National Comprehensive Cancer Network guidelines (v 1, 2018) for male carriers of BRCA1 or BRCA2 mutations include (a) annual clinical breast examination and self-examination to begin at 35 years old and (b) prostate cancer screening to begin at 45 years old (6). Mammographic screening is not recommended owing to the limited data supporting imaging in men (Table 7) (6). Nevertheless, many male carriers of BRCA1 or BRCA2 mutations who have a concurrent family history and other risk factors often decide to pursue mammographic screening, in consultation with their clinicians (Fig 13).
Table 7:
Current National Comprehensive Cancer Network Guidelines (v 1, 2018) for Men Testing Positive for BRCA1 or BRCA2 Mutations
| Annual clinical breast examination starting at 35 y old |
| Annual breast self-examination starting at 35 y old |
| Regular mammography not recommended |
| Screening for prostate cancer starting at 45 y old |
Source.—Reference 6.
Figure 13.
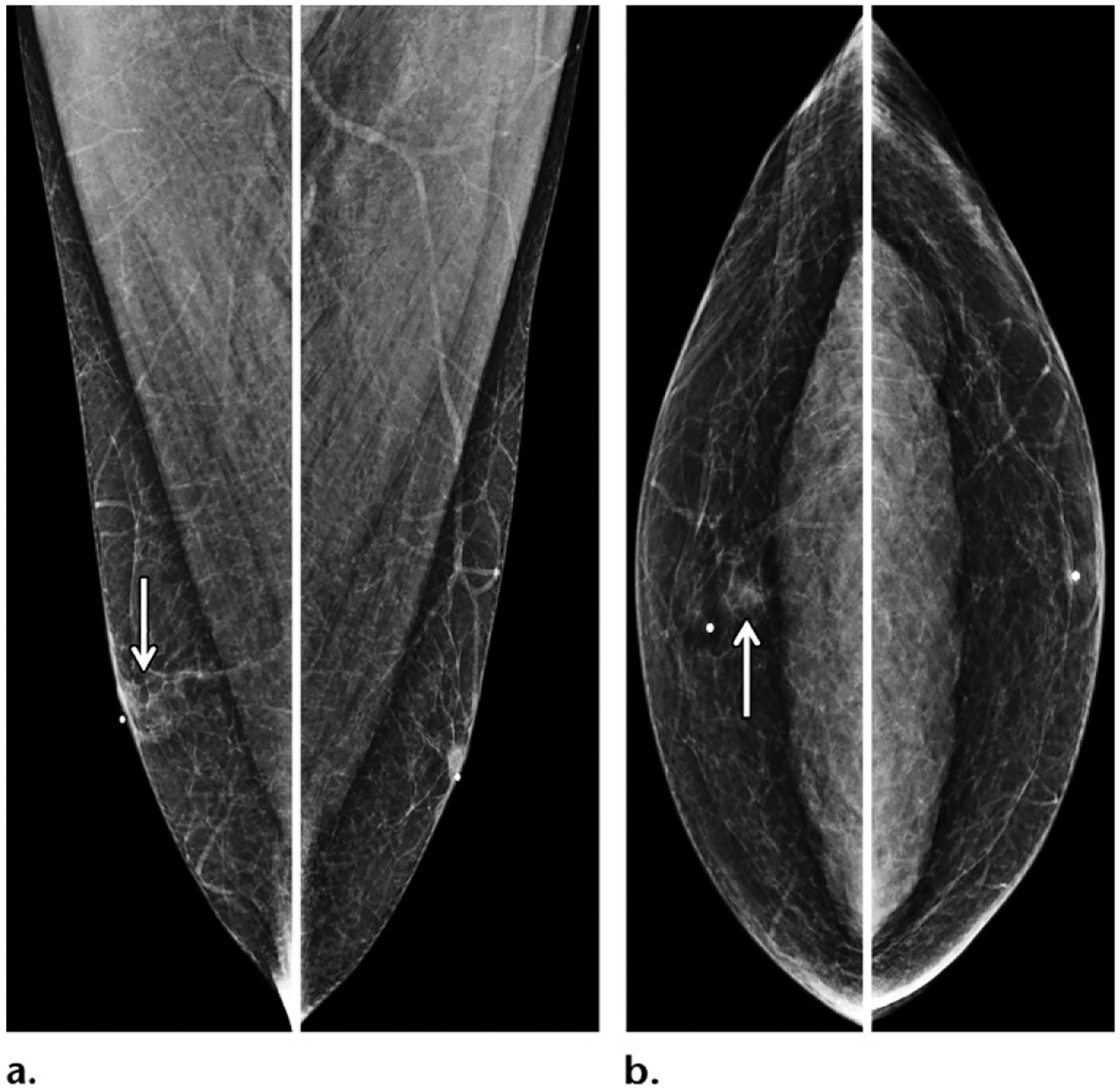
Baseline screening mammogram of a 50-year-old Ashkenazi Jewish male BRCA2 mutation carrier with a family history of breast cancer (diagnosed in his mother at 60 years old). Screening MLO (a) and CC (b) mammographic views show no evidence of malignancy and minimal gynecomastia (arrow) of the right breast.
Klinefelter Syndrome
Klinefelter syndrome is characterized by the addition of at least one X chromosome to the normal XY karyotype (47, XXY), which results in an elevated gonadotropin level and a decreased testosterone level, as well as an estrogen level up to twice that of normal men. This increase in the relative estrogen-to-androgen ratio in these individuals is associated with testicular dysgenesis, gynecomastia, and a markedly increased risk of breast cancer. Compared with average men (46, XY), those with Klinefelter syndrome have a 50-fold increase in the relative risk of developing breast cancer, as well as a 60-fold higher breast cancer–specific mortality (62,63). For these reasons, although no formal breast screening guideline currently exists for patients with Klinefelter syndrome, targeted screening in this group may be of benefit (Fig 14). Patients with Klinefelter syndrome are also at substantially increased risks for non-Hodgkin lymphoma and lung cancer (62).
Figure 14.
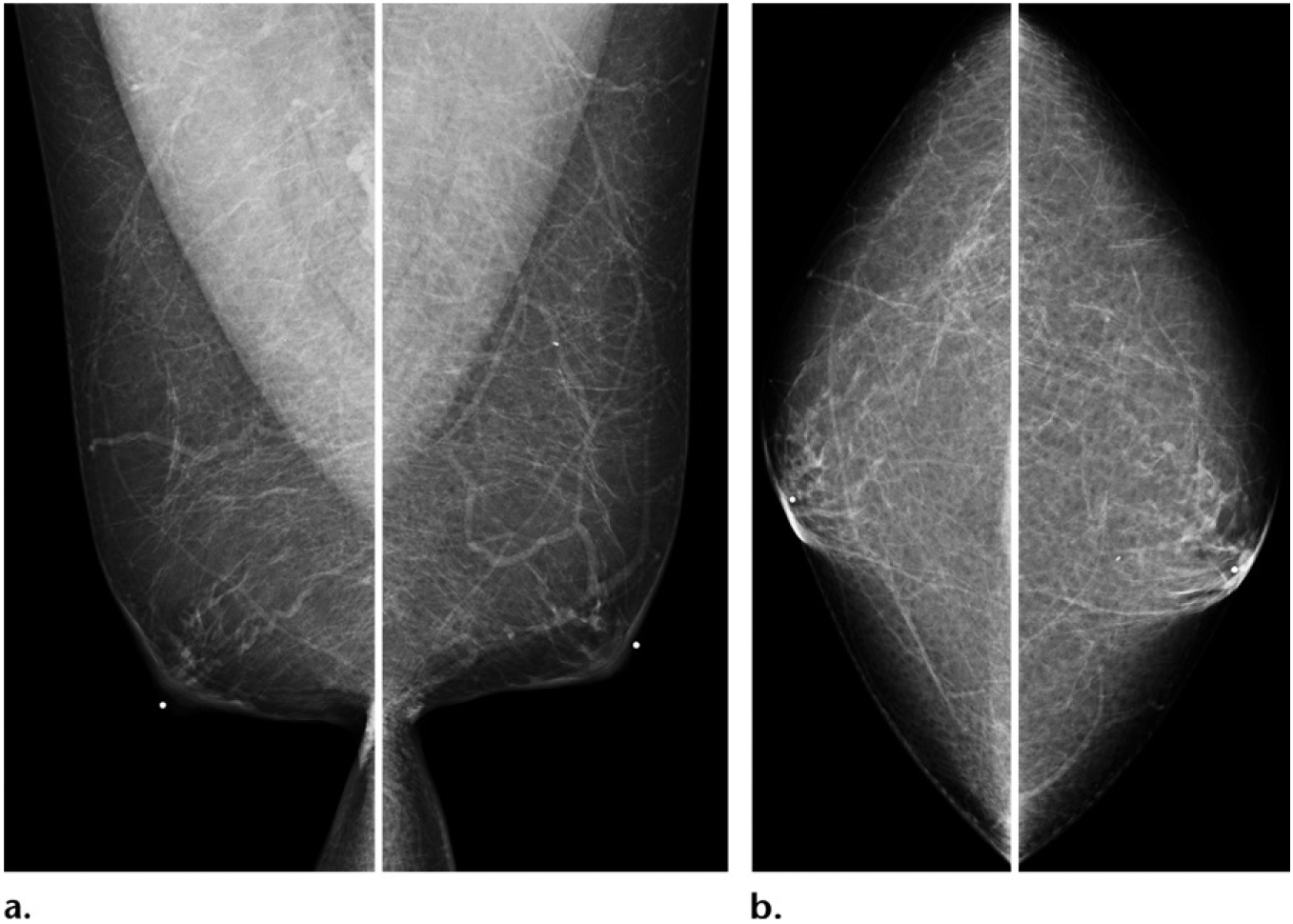
Screening mammogram of a 55-year-old man with Klinefelter syndrome (47, XXY). Annual screening MLO (a) and CC (b) mammographic views show normal bilateral findings.
Personal History
Because a personal history of male breast cancer is an indication for genetic counseling and testing owing to its high association with hereditary factors predisposing to breast cancer, it is not unexpected that men with a diagnosis of breast cancer would have an elevated risk for recurrence. In the results of a study assessing the risk of subsequent cancer among 1788 men unselected for other risk factors who had a diagnosis of primary breast cancer and who were registered with the National Cancer Institute Surveillance, Epidemiology, and End Results program (mean follow-up, 4 years), investigators found that the risk of developing a contralateral second breast cancer was increased by nearly 30-fold, compared with average men, with the highest risk seen in men who had breast cancer diagnosed earlier in life (<50 years old) (increased by 110-fold) (64). This increased risk is in contrast to that in women with a personal history of breast cancer, whose subsequent risk is increased by only two- to fourfold compared with the general population (64).
In the results of a separate study from the Swedish Family-Cancer Database, investigators found that a personal history of male breast cancer confers a 93-fold increased risk of subsequent breast cancer in men, compared with only a threefold increases risk in the female counterparts in this population. Of interest, the risk of recurrence was highest in men who were more than 10 years past their initial breast cancer diagnosis (65). Although the absolute risk of recurrence of male breast cancer is lower than that of female breast cancer, the relative risk of developing a second breast cancer was substantially higher in men, compared with women. For these reasons, mammographic screening should be considered, particularly when a family history and a genetic predisposition or other identifiable risk factors are present concurrently, factors that likely increase the stakes (Figs 12, 15).
Family History
A family history of breast and/or ovarian cancer is a significant risk factor for male breast cancer, and up to 20% of men with breast cancer report such a history. In the results of a prospective cohort study of more than 300 000 men in the National Institutes of Health–American Association of Retired Persons diet and health study, investigators showed that a family history of a first-degree relative with breast cancer is significantly associated with an increased risk of male breast cancer (relative risk, 1.92; confidence interval: 1.19, 3.09) (66). This risk was accentuated if the affected first-degree relative was a sister (relative risk, 2.25) and was even further enhanced if multiple relatives were afflicted, with a relative risk of 9.73 when both a mother and a sister had breast cancer (66) (Figs 12, 15).
A family history invariably overlaps with genetic mutations and is more common in families with germline mutations such as BRCA1 or BRCA2 mutations. For example, in the results of one study, investigators found that male breast cancer in association with a family history of breast and/or ovarian cancer was predictive of a BRCA2 mutation 77% of the time (67). This association is further complicated by aggregation of several highly prevalent founder mutations of BRCA1 or BRCA2 in the Ashkenazi Jewish population, which accounts for why a family history of breast cancer poses greater risks for Jewish men, compared with non-Jewish men (54). No guideline currently exists for mammographic screening in men with a family history of breast and/or ovarian cancer; however, those with multiple affected first-degree family members, concurrent genetic mutations, and other risk factors likely have an overall higher lifetime risk of developing male breast cancer and may benefit from screening and early cancer detection (Figs 12, 15).
Transgender
Discussion of male breast cancer in the context of breast cancer in biologically or phenotypically male patients is important. Transgender people represent a complex heterogeneous group of patients who are increasingly being referred for breast imaging evaluation. Male-to-female transgender patients are thought to be at increased risk for breast cancer owing to their often prolonged exposure to exogenous feminizing hormones and androgen suppression. Female-to-male transgender patients who do not undergo mastectomy remain at risk for breast cancer because their genotype is female. The individual family history and the genetic predisposition (mutations) further increase the risk of developing breast cancer in certain individuals.
Androgen insufficiency and exogenous estrogen exposure are established risk factors for breast cancer in numerous medical conditions (27). Although exposure to exogenous estrogen and a high cumulative lifetime exposure to estrogen have been associated with an increased risk of developing breast cancer (68,69), and although cases of breast cancer have been reported in transgender patients, the results of available retrospective cohort studies have not shown an increased incidence of breast cancer among transgender individuals, compared with the general population of the natal sex (70,71). This finding may be in part due to limitations in the cohort size and the study duration, given low baseline incidence of breast cancer in natal males (or male-to-female transgender women). Interestingly, evidence exists to suggest that transgender patients (specifically the male-to-female group) frequently present with late-stage breast cancers, which are most commonly ductal in origin, a finding that parallels the typical manifestation of male breast cancer in the general population (70,71). Among female-to-male transgender individuals, mastectomy and androgen supplementation are generally protective and reduce the risk of breast cancer. Rare cases of breast cancer in postmastectomy and androgen-treated patients have been reported, owing to residual tissue and the theoretical mechanism of aromatization of androgen to estrogen in the breast (72). Clinically, because evidence is limited and the results are mixed, transgender men and women, in consultation with their physicians, often pursue breast cancer screening on the basis of the presence of breast tissue (Fig 16).
Figure 16.
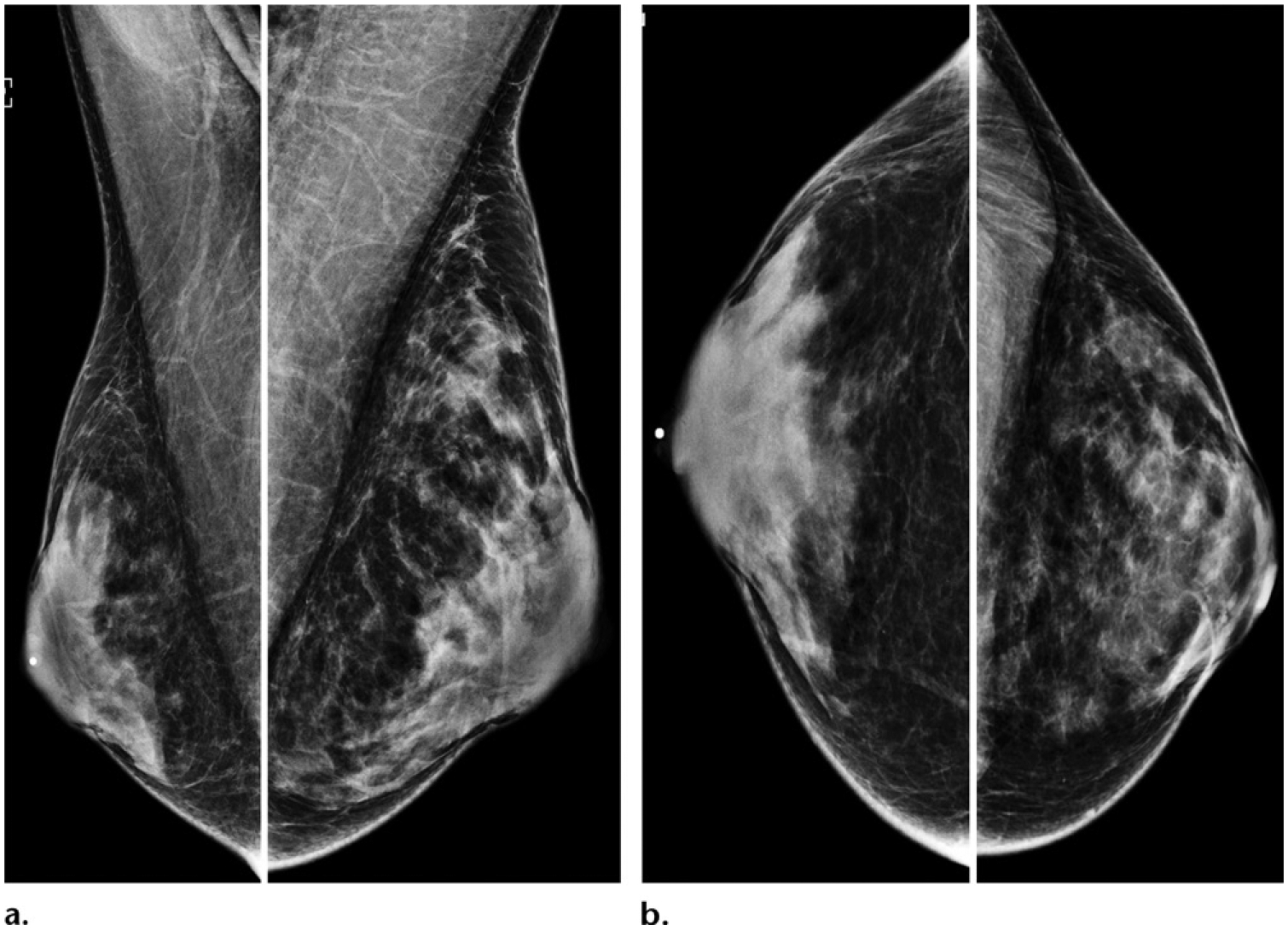
Screening mammogram in a 35-year-old male-to-female transgender person with a 5-year history of exogenous estrogen therapy who presented for screening. Bilateral MLO (a) and CC (b) mammographic views show moderately increased subareolar tissues that appear heterogeneously dense, a finding consistent with the sequelae of hormonal therapy.
Despite a lack of supporting evidence, there are currently published screening recommendations both from the Center of Excellence for Transgender Health at the University of California, San Francisco (73), and Fenway Health (Boston, Mass) (74), which in combination with recent evidence of high adherence to mammographic screening among transgender individuals (75), may reflect improved awareness and advocacy for these patients. Because more than 60% of transgender women (male-to-female) demonstrate mammographic density, screening may be of benefit (76). Current guidelines suggest screening mammography every 2 years starting at the age of 50 years in transgender patients who have been exposed to exogenous hormones for 5 years or longer (Table 8). Providers are also advised to assess additional individual risk factors such as a family history or a high body mass index (>35) and discuss the risks of excessive screening (73). As we improve access and quality of care for transgender patients, prospective studies are needed to help guide screening practices.
Table 8:
Current Screening Guidelines for Transgender Women (Male to Female)
| Age ≥ 50 y and ≥5 y of hormonal therapy required for beginning screening |
| Screening mammography every 2 y |
| Provider should discuss with the patient the risks of excessive screening and assess the individual risk factors (ie, family history, body mass index > 35). |
Therapy and Survivorship
Male breast cancer has emerged with distinct biologic features, many of which have yet to translate into updates in treatment practices. Recent data from the International Male Breast Cancer Program, enrolling 1822 male patients in 23 centers from nine countries, showed that although male breast cancer was ER positive more than 99% of the time, tailored adjuvant endocrine therapy was only received in 77% of cases (11). Although many male breast cancers are diagnosed at an advanced stage, the use of adjuvant radiation therapy has been controversial and inconsistent, particularly in the setting of mastectomy (77). Modified mastectomy currently represents the most common form of surgical care for male breast cancer (70%); and lumpectomy, with or without radiation therapy, is rarely performed (1%–13%) (78). It is possible that, given the relatively nonaggressive biologic profile (predominantly ductal, ER positive, HER2 negative, luminal A like or luminal B like, and, rarely, basal like) of male breast cancer, early detection with screening when the disease burden is small may allow a safe and less-extensive surgical approach in the future. As genetic profiling and molecular profiling become more sophisticated, the many uniquely affected genes in male breast cancer represent potential targets for tailored therapeutic interventions (22), some of which are already beginning to take shape (79). Therapy tailored to male breast cancer, instead of extrapolated from female breast cancer, will likely optimize care and improve outcomes.
Male breast cancer survivors have rather limited resources to deal with the aftermath of breast cancer diagnosis and treatment. Because breast cancer is so much more common in women than in men, male breast cancer survivors often feel isolated and stigmatized by their diagnosis. When male breast cancer survivors were asked about their feelings regarding a diagnosis of male breast cancer in one study, 30% of them reported embarrassment and 25% reported anxiety related to their diagnosis (80). Similarly, for men who have undergone treatment, discussion and support are limited with regard to disfigurement and the side effects of endocrine therapy, including sexual dysfunction. Regular contact with social workers, psychologists, and support groups has been found to be of benefit. Tailored survivorship guidelines for men with breast cancer are needed to engage open and regular dialogue in this unique group of patients (81).
Conclusion
An uncommon disease calls for an uncommon approach. In the case of male breast cancer, practice-based evidence is needed to pave the way for evidence-based practice. Although mammographic screening has no role in the general screening of men, owing to a paucity of disease, such screening may be of benefit among select groups that are at high risk for breast cancer. For the first time, improved genetic counseling and wider availability of genetic testing are allowing high-risk men to be recognized before they present with breast cancer, providing an opportunity for targeted screening, early diagnosis, and improved survival. A shift in our approach to male breast cancer, including better individual risk assessment, education, and outreach, is urgently needed to bridge the gap between male and female breast cancer.
SA-CME LEARNING OBJECTIVES.
After completing this journal-based SA-CME activity, participants will be able to:
Describe the current disparity in the diagnosis and treatment of male breast cancer and female breast cancer, as well as the implications of such disparity in survival outcomes.
Recognize that male and female breast cancers are not exactly the same disease and that a more tailored approach to male breast cancer may improve care.
Discuss the challenges in diagnosing breast cancer in men, as well as use of targeted screening in the setting of improved genetic testing and counseling.
TEACHING POINTS.
Although male breast cancer accounts for only 1% of all breast cancers, its incidence has increased by 20%–25% in the past few decades and continues to rise.
The stage-matched disparity in survival of men and women with breast cancer is not accounted for by the more advanced manifestation of male breast cancer alone. Emerging data suggest that male breast cancer and female breast cancer likely differ in tumor biology and genetic signatures.
Male breast cancer is more likely to assume a pseudobenign appearance as compared to female breast cancer, with masses more likely to be round or oval and with calcifications more likely to be larger, rounder, coarser, and less numerous.
Current guidelines suggest screening mammography every 2 years starting at the age of 50 years in transgender patients who have been exposed to exogenous hormones for 5 years or longer. Providers are also advised to assess additional individual risk factors such as a family history or a high body mass index (>35) and discuss the risks of excessive screening.
Although mammographic screening has no role in the general screening of men, owing to a paucity of disease, such screening may be of benefit among select groups that are at high risk for breast cancer. For the first time, improved genetic counseling and wider availability of genetic testing are allowing high-risk men to be recognized before they present with breast cancer, providing an opportunity for targeted screening, early diagnosis, and improved survival.
Abbreviations:
- CC
craniocaudal
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- MLO
mediolateral oblique
- PR
progesterone receptor
Footnotes
Recipient of a Certificate of Merit award for an education exhibit at the 2017 RSNA Annual Meeting.
References
- 1.Tabár L, Vitak B, Chen HH, Yen MF, Duffy SW, Smith RA. Beyond randomized controlled trials: organized mammographic screening substantially reduces breast carcinoma mortality. Cancer 2001;91(9):1724–1731. [DOI] [PubMed] [Google Scholar]
- 2.Kopans DB. An open letter to panels that are deciding guidelines for breast cancer screening. Breast Cancer Res Treat 2015;151(1):19–25. [DOI] [PubMed] [Google Scholar]
- 3.Stang A, Thomssen C. Decline in breast cancer incidence in the United States: what about male breast cancer? Breast Cancer Res Treat 2008;112(3):595–596. [DOI] [PubMed] [Google Scholar]
- 4.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer 2004;101(1):51–57. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): genetic/ familial high-risk assessment—breast and ovarian, version 1.2018. https://www.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Updated October 3, 2017. Accessed July 1, 2018.
- 7.Engel JM, Stankowski-Drengler TJ, Stankowski RV, Liang H, Doi SA, Onitilo AA. All-cause mortality is decreased in women undergoing annual mammography before breast cancer diagnosis. AJR Am J Roentgenol 2015;204(4): 898–902. [DOI] [PubMed] [Google Scholar]
- 8.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 2006;98(18):1285–1291. [DOI] [PubMed] [Google Scholar]
- 9.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol 2010;28(2):232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano SH. Male breast cancer: it’s time for evidence instead of extrapolation [editorial]. Onkologie 2008;31(10): 505–506. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol 2018;29(2):405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman BC, Keto J, Rosenbaum Smith SM. Screening mammography in men with BRCA mutations: is there a role? Breast J 2012;18(1):73–75. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Liu X, Zhang L, Li S, Shi Y, Tong Z. Poorer survival of male breast cancer compared with female breast cancer patients may be due to biological differences. Jpn J Clin Oncol 2013;43(10):954–963. [DOI] [PubMed] [Google Scholar]
- 14.Iorfida M, Bagnardi V, Rotmensz N, et al. Outcome of male breast cancer: a matched single-institution series. Clin Breast Cancer 2014;14(5):371–377. [DOI] [PubMed] [Google Scholar]
- 15.Lautrup MD, Thorup SS, Jensen V, et al. Male breast cancer: a nation-wide population-based comparison with female breast cancer. Acta Oncol 2018;57(5):613–621. [DOI] [PubMed] [Google Scholar]
- 16.Nahleh ZA, Srikantiah R, Safa M, Jazieh AR, Muhleman A, Komrokji R. Male breast cancer in the Veterans Affairs population: a comparative analysis. Cancer 2007;109(8): 1471–1477. [DOI] [PubMed] [Google Scholar]
- 17.Nahleh ZA. Hormonal therapy for male breast cancer: a different approach for a different disease. Cancer Treat Rev 2006;32(2):101–105. [DOI] [PubMed] [Google Scholar]
- 18.Morris E, Feig SA, Drexler M, Lehman C. Implications of overdiagnosis: impact on screening mammography practices. Popul Health Manag 2015;18(suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriques Abreu M, Henriques Abreu P, Afonso N, Pereira D, Henrique R, Lopes C. Patterns of recurrence and treatment in male breast cancer: a clue to prognosis? Int J Cancer 2016;139(8):1715–1720. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen MA, Slaets L, Cardoso F, et al. Pathological characterisation of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur J Cancer 2017;82:219–227. [DOI] [PubMed] [Google Scholar]
- 21.Yu XF, Feng WL, Miao LL, Chen B, Yang HJ. The prognostic significance of molecular subtype for male breast cancer: a 10-year retrospective study. Breast 2013;22(5): 824–827. [DOI] [PubMed] [Google Scholar]
- 22.Piscuoglio S, Ng CK, Murray MP, et al. The genomic landscape of male breast cancers. Clin Cancer Res 2016;22(16): 4045–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106(5):dju055. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4580552/pdf/dju055.pdf. Published April 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elston CW, Ellis IO. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19(5):403–410. [DOI] [PubMed] [Google Scholar]
- 25.U.S. National Library of Medicine. Genetics Home Reference: your guide to understanding genetic conditions. Genetics Home Reference website; https://ghr.nlm.nih.gov/. Published June 19, 2018. Accessed July 1, 2018. [Google Scholar]
- 26.Smith JA, Braunack-Mayer A, Wittert G. What do we know about men’s help-seeking and health service use? Med J Aust 2006;184(2):81–83. [DOI] [PubMed] [Google Scholar]
- 27.Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet 2006;367(9510):595–604. [DOI] [PubMed] [Google Scholar]
- 28.Sachs MD. Carcinoma of the male breast. Radiology 1941;37 (4):458–467. [Google Scholar]
- 29.Donegan WL, Redlich PN, Lang PJ, Gall MT. Carcinoma of the breast in males: a multiinstitutional survey. Cancer 1998;83(3):498–509. [DOI] [PubMed] [Google Scholar]
- 30.Thomas E Original research: men’s awareness and knowledge of male breast cancer. Am J Nurs 2010;110(10):32–37, 39–40; quiz 41–42. [DOI] [PubMed] [Google Scholar]
- 31.Kruger DJ, Nesse RM. Sexual selection and the male:female mortality ratio. Evol Psychol 2004;2(1):66–85. [Google Scholar]
- 32.Male Breast Cancer Coalition website. http://malebreastcancercoalition.org/. Accessed July 1, 2018.
- 33.Joshi MG, Lee AK, Loda M, et al. Male breast carcinoma: an evaluation of prognostic factors contributing to a poorer outcome. Cancer 1996;77(3):490–498. [DOI] [PubMed] [Google Scholar]
- 34.Crichlow RW, Galt SW. Male breast cancer. Surg Clin North Am 1990;70(5):1165–1177. [DOI] [PubMed] [Google Scholar]
- 35.Laronga C, Kemp B, Johnston D, Robb GL, Singletary SE. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol 1999;6(6):609–613. [DOI] [PubMed] [Google Scholar]
- 36.Simmons RM, Brennan M, Christos P, King V, Osborne M. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol 2002;9(2):165–168. [DOI] [PubMed] [Google Scholar]
- 37.Foerster R, Schroeder L, Foerster F, et al. Metastatic male breast cancer: a retrospective cohort analysis. Breast Care (Basel) 2014;9(4):267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Port ER, Tan LK, Borgen PI, Van Zee KJ. Incidence of axillary lymph node metastases in T1a and T1b breast carcinoma. Ann Surg Oncol 1998;5(1):23–27. [DOI] [PubMed] [Google Scholar]
- 39.Rakha EA, Martin S, Lee AH, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012;118(15):3670–3680. [DOI] [PubMed] [Google Scholar]
- 40.Mathew J, Perkins GH, Stephens T, Middleton LP, Yang WT. Primary breast cancer in men: clinical, imaging, and pathologic findings in 57 patients. AJR Am J Roentgenol 2008;191(6):1631–1639. [DOI] [PubMed] [Google Scholar]
- 41.Iuanow E, Kettler M, Slanetz PJ. Spectrum of disease in the male breast. AJR Am J Roentgenol 2011;196(3): W247–W259. [DOI] [PubMed] [Google Scholar]
- 42.Andersen JA, Gram JB. Male breast at autopsy. Acta Pathol Microbiol Immunol Scand A 1982;90(3):191–197. [DOI] [PubMed] [Google Scholar]
- 43.Mainiero MB, Lourenco AP, Barke LD, et al. ACR Appropriateness Criteria evaluation of the symptomatic male breast. J Am Coll Radiol 2015;12(7):678–682. [DOI] [PubMed] [Google Scholar]
- 44.Brinton LA, Cook MB, McCormack V, et al. Anthropo-metric and hormonal risk factors for male breast cancer: Male Breast Cancer Pooling Project results. J Natl Cancer Inst 2014;106(3):djt465. https://academic.oup.com/jnci/article/106/3/djt465/986640. Published February 19, 2014. [Published correction appears in J Natl Cancer Inst 2014;106(5):dju117.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brinton LA, Carreon JD, Gierach GL, McGlynn KA, Gridley G. Etiologic factors for male breast cancer in the U.S. Veterans Affairs medical care system database. Breast Cancer Res Treat 2010;119(1):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography In: D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS atlas: Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 47.McDonald ES, Oustimov A, Weinstein SP, Synnestvedt MB, Schnall M, Conant EF. Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol 2016;2(6):737–743. [DOI] [PubMed] [Google Scholar]
- 48.Doebar SC, Slaets L, Cardoso F, et al. Male breast cancer precursor lesions: analysis of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Mod Pathol 2017;30(4):509–518. [DOI] [PubMed] [Google Scholar]
- 49.Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010;28(12):2114–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 51.American Cancer Society. Key statistics for breast cancer in men. https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html. Updated April 27, 2018. Accessed June 27, 2018.
- 52.Sineshaw HM, Freedman RA, Ward EM, Flanders WD, Jemal A. Black/white disparities in receipt of treatment and survival among men with early-stage breast cancer. J Clin Oncol 2015;33(21):2337–2344. [DOI] [PubMed] [Google Scholar]
- 53.O’Malley C, Shema S, White E, Glaser S. Incidence of male breast cancer in California, 1988–2000: racial/ethnic variation in 1759 men. Breast Cancer Res Treat 2005;93(2):145–150. [DOI] [PubMed] [Google Scholar]
- 54.Rubinstein WS. Hereditary breast cancer in Jews. Fam Cancer 2004;3(3–4):249–257. [DOI] [PubMed] [Google Scholar]
- 55.Brenner B, Fried G, Levitzki P, et al. Male breast carcinoma in Israel: higher incidence but possibly better prognosis in Ashkenazi Jews. Cancer 2002;94(8):2128–2133. [DOI] [PubMed] [Google Scholar]
- 56.Desmedt C, Voet T, Sotiriou C, Campbell PJ. Next-generation sequencing in breast cancer: first take home messages. Curr Opin Oncol 2012;24(6):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 2015;372(23):2243–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol 2015;26(7):1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Human Genome Research Institute. Breast Cancer Information Core: an open access on-line breast cancer notation data base [database online]. National Human Genome Research Institute website; https://research.nhgri.nih.gov/bic/. Updated May 5, 2017. Accessed February 3, 2018. [Google Scholar]
- 60.Tun NM, Villani G, Ong K, Yoe L, Bo ZM. Risk of having BRCA1 mutation in high-risk women with triple-negative breast cancer: a meta-analysis. Clin Genet 2014;85(1):43–48. [DOI] [PubMed] [Google Scholar]
- 61.Mavaddat N, Barrowdale D, Andrulis IL, et al. ; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 2012;21(1):134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swerdlow AJ, Schoemaker MJ, Higgins CD, et al. ; UK Clinical Cytogenetics Group. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst 2005;97(16):1204–1210. [DOI] [PubMed] [Google Scholar]
- 63.Evans DB, Crichlow RW. Carcinoma of the male breast and Klinefelter’s syndrome: is there an association? CA Cancer J Clin 1987;37(4):246–251. [DOI] [PubMed] [Google Scholar]
- 64.Auvinen A, Curtis RE, Ron E. Risk of subsequent cancer following breast cancer in men. J Natl Cancer Inst 2002;94(17):1330–1332. [DOI] [PubMed] [Google Scholar]
- 65.Dong C, Hemminki K. Second primary breast cancer in men. Breast Cancer Res Treat 2001;66(2):171–172. [DOI] [PubMed] [Google Scholar]
- 66.Brinton LA, Richesson DA, Gierach GL, et al. ; Breast Cancer Linkage Consortium. Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst 2008;100(20):1477–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ford D, Easton DF, Stratton M, et al. ; Breast Cancer Linkage Consortium. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 1998;62(3):676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res 2003;5(5):239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med 2013;10(12): 3129–3134. [DOI] [PubMed] [Google Scholar]
- 71.Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat 2015;149(1):191–198. [DOI] [PubMed] [Google Scholar]
- 72.Gooren LJ. Care of transsexual persons. N Engl J Med 2011;364(13):1251–1257. [DOI] [PubMed] [Google Scholar]
- 73.Deutsch MB. Screening for breast cancer in transgender women. Center of Excellence for Transgender Health website; http://transhealth.ucsf.edu/tcoe?page=guidelines-breast-cancer-women. Published June 17, 2016. Accessed February 4, 2018. [Google Scholar]
- 74.Fenway Health. Radiology: mammography. Fenway Health website; http://fenwayhealth.org/care/medical/radiology/. Accessed July 1, 2018. [Google Scholar]
- 75.Narayan A, Lebron-Zapata L, Morris E. Breast cancer screening in transgender patients: findings from the 2014 BRFSS survey. Breast Cancer Res Treat 2017;166(3):875–879. [DOI] [PubMed] [Google Scholar]
- 76.Weyers S, Villeirs G, Vanherreweghe E, et al. Mammography and breast sonography in transsexual women. Eur J Radiol 2010;74(3):508–513. [DOI] [PubMed] [Google Scholar]
- 77.Losurdo A, Rota S, Gullo G, et al. Controversies in clinicopathological characteristics and treatment strategies of male breast cancer: a review of the literature. Crit Rev Oncol Hematol 2017;113:283–291. [DOI] [PubMed] [Google Scholar]
- 78.Cutuli B Strategies in treating male breast cancer. Expert Opin Pharmacother 2007;8(2):193–202. [DOI] [PubMed] [Google Scholar]
- 79.Humphries MP, Sundara Rajan S, Droop A, et al. A case-matched gender comparison transcriptomic screen identifies eIF4E and eIF5 as potential prognostic markers in male breast cancer. Clin Cancer Res 2017;23(10):2575–2583. [DOI] [PubMed] [Google Scholar]
- 80.Kipling M, Ralph JE, Callanan K. Psychological impact of male breast disorders: literature review and survey results. Breast Care (Basel) 2014;9(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferzoco RM, Ruddy KJ. Optimal delivery of male breast cancer follow-up care: improving outcomes. Breast Cancer (Dove Med Press) 2015;7:371–379. https://www.dovepress.com/optimal-delivery-of-male-breast-cancer-follow-up-care-improving-outcom-peer-reviewed-fulltext-article-BCTT. Published November 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


