Key Points
Priapism is a common morbidity of sickle cell disease that is often undisclosed to care providers and hence is mostly unrecognized.
The prevalence of erectile dysfunction is 2.5-fold higher in men with sickle cell disease than in those without SCD.
Abstract
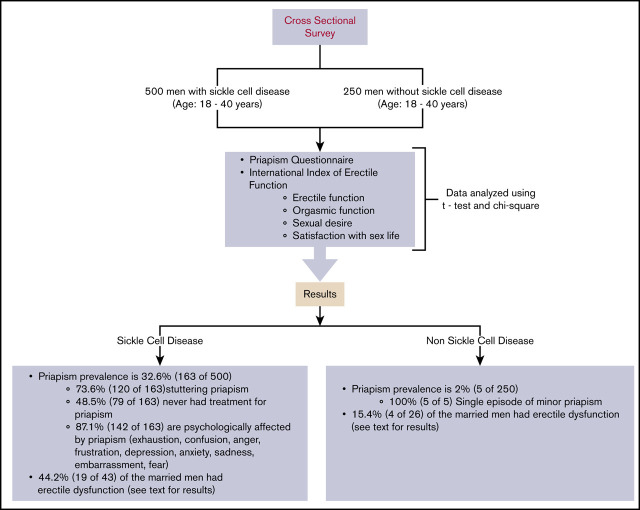
Recurrent ischemic priapism is a common complication of sickle cell disease (SCD). We assessed the burden, characteristics, and types of priapism, including sexual dysfunction, in a cohort of men with and those without SCD, to test the hypothesis that sexual dysfunction is more prevalent in men with SCD. In Kano, Nigeria, we conducted a comparative cross-sectional survey that included 500 and 250 men 18 to 40 years of age, with and without SCD, respectively. The survey used the Priapism Questionnaire and the International Index of Erectile Function for sexual function assessment. All eligible participants approached for the study gave informed consent and were enrolled. Stuttering and major priapism were defined based on the average duration of priapism experiences that lasted ≤4 and >4 hours, respectively. The prevalence of priapism was significantly higher in men with SCD than in those without it (32.6% vs 2%; P < .001). Stuttering priapism accounted for 73.6% of the priapism episodes in men with SCD. Nearly 50% of the participants with SCD-related priapism had never sought medical attention for this complication. The majority of the men with SCD-related priapism used exercise as a coping mechanism. Priapism affected the self-image of the men with SCD, causing sadness, embarrassment, and fear. The percentage of the men with SCD who had erectile dysfunction was more than twofold higher than that of those without SCD who had erectile dysfunction (P = .01). The men with SCD had a higher prevalence of priapism and sexual dysfunction than the men without SCD.
Visual Abstract
Introduction
Sickle cell disease (SCD), which disproportionately affects people of African descent, is one of the most common autosomal recessive disorders.1 The disease distribution coincides with places where falciparum malaria is endemic, particularly in sub-Saharan Africa, the Middle East, India, and the Mediterranean.1,2 Of the estimated 300 000 newborns with SCD in the world annually, ∼50% are in Nigeria.2
Recent advances in the care of individuals with SCD have improved the childhood survival rate in both high- and low-income countries.3 As a result of the improved survival, the full spectrum of morbidities associated with SCD is becoming more prominent, resulting in a shift of the current research focus toward reducing morbidity and improving quality of life.1,3 Priapism, defined as a painful, persistent, and purposeless erection,4 is often a chronic complication reported in 32% to 40% of individuals with SCD5,6; with a cumulative incidence of 60% by the age of 40 years.7 Priapism in SCD is predominantly ischemic (low flow) and is classified as stuttering or major priapism based on the duration of an episode lasting ≤4 or >4 hours, respectively.8 Adeyoju et al5 documented that 24% of surveyed men with SCD reported having sexual dissatisfaction, mainly due to fear of experiencing priapism.
Despite the very high burden of priapism and its time-dependent adverse effect on erectile function,9 few studies have comprehensively defined the clinical epidemiology of this complication nor have any compared sexual dysfunction in men with SCD with that in those without it. Evidence-based treatment strategies for recurrent ischemic priapism in SCD are currently lacking.10 As part of the ongoing Priapism in Nigeria study, intended to comprehensively address the knowledge gap in the clinical epidemiology and treatment of recurrent ischemic priapism, we sought to determine the prevalence and pattern of priapism, and sexual dysfunctions in men with SCD compared with age- and race-matched men without SCD. We tested the hypothesis that men with SCD, when compared with those without it, would have a higher prevalence of priapism and sexual dysfunction.
Methods
Study design
We conducted a comparative cross-sectional survey that involved men aged 18 to 40 years who had confirmed SCD diagnosed with hemoglobin electrophoresis or high-performance liquid chromatography. Only participants with HbSS phenotype were included in the survey. Men with SCD were matched by age and race with men without known histories of SCD. The study was conducted at 2 sites: Aminu Kano Teaching Hospital and Murtala Mohammed Specialist Hospital. These 2 hospitals are located in Kano metropolis, a city with ∼3 million inhabitants,11 and have a combined recruitment pool of nearly 5000 individuals with SCD. Participants with SCD were recruited from the adult SCD clinics of both hospitals. Participants without SCD were recruited from the general outpatient clinics of the same hospitals. The non-SCD participants were selected when they presented to the general outpatient clinics with mild complaints, such as catarrh, dyspepsia, or headache. Potential participants without SCD were excluded if they had a history of hypertension, diabetes, or other chronic disease that could cause erectile dysfunction (ED).
All eligible participants who were approached for the survey provided informed consent and were enrolled in the study. No potential participant refused to answer the questions. Major priapism was defined based on the average duration of priapism experience that lasted for >4 hours. Stuttering priapism was defined as a recurrent experience, with each episode lasting for ≤4 hours with an intervening period of complete detumescence. A single episode of priapism lasting ≤4 hours was considered to be a rare type in this study. The participants were interviewed with a comprehensive priapism survey questionnaire and a validated International Index of Erectile Function (IIEF) questionnaire.12 In this study, the sexual function assessments with the latter were restricted to married men with or without SCD. Nonmarried men were excluded because of the cultural belief that engaging in sex before marriage is not acceptable in the Hausa Muslim community, and the validity of the IIEF questionnaire rests on having sexual activity within the past 4 weeks from the date of the interview. Consent for the study and the survey data were collected electronically on an iPad directly into REDCap, with the server hosted by Vanderbilt University Medical Center. REDCap is a robust, secure, and Health Insurance Portability and Accountability Act–compliant online data management platform. The study protocol received Institutional Review Board approval from Vanderbilt University (no. 181434), Aminu Kano Teaching Hospital (no. 2344), and Kano State Ministry of Health (no. 980). The survey commenced in February 2019 and was completed in November 2019.
Description of study procedures and questionnaires
The study participants completed the following 2 questionnaires:
The Priapism Questionnaire is a self-administered survey explicitly designed to provide comprehensive information on the nature, pattern, and associated factors of priapism (example in the supplemental Appendix).
The IIEF is a validated patient-reported outcome questionnaire that assesses male sexual function.12 The questionnaire has 15 questions, with scoring split into 5 domains. Each question has different answer choices and corresponding numerical values ranging from 1 to 5. The domains and possible scores for various domains are as follows: erectile function (6-30), intercourse satisfaction (3-15), orgasmic function (2-10), sexual desire (2-10), and overall satisfaction (2-10). A higher score for each domain of the questionnaire indicates higher sexual function. The erectile function domain was further subclassified into normal score (26-30), mild ED (22-25), mild-to-moderate ED (17-21), moderate ED (11-16), and severe ED (6-10).
Statistical analysis
Given the absence of prior studies comparing priapism and sexual dysfunction in men with and without SCD, we sought 500 men with SCD and 250 men without it. We considered this sample size large enough to give us a reliable estimate of the prevalence of priapism and sexual dysfunction.
A 2-sample Student t test and Pearson’s χ2 test were used to compare demographic data (age, marital status, educational level, and job) between those with and those without SCD. The total scores and domain-specific scores of the IIEF were compared between the 2 groups by Student t test. We used the Mann-Whitney U test where necessary to compare scores of the IIEF between those with stuttering and major priapism and between those with a history of priapism that lasted 72 hours and those without such a history. For the IIEF questionnaire analysis, we limited the analysis to only sexually active married men who had had sexual activity during the 4 weeks before the interview, as recommended.12 The prevalence of priapism was reported as a percentage and compared between the 2 groups by using the χ2 test or Fisher’s exact test for expected small frequencies. All other categorical variables were also reported as percentages. We considered the α level of significance to be <.05 in this analysis. Analyses were performed with STATA, version 13 (STATA Corp, College Station, TX).
Results
Basic demographic information of men with and those without SCD
The basic demographic information on the study participants with and those without SCD was similar in age (P = .43), marital status (P = .43), and education (P = .31). However, more men with SCD had a higher rate of unemployment than those without it (P < .001). The men without SCD reported smoking cigarettes more than those who had SCD (P < .001; Table 1).
Table 1.
Demographic information on study participants: a cross-sectional analysis of participants with and those without SCD identified in outpatient clinics
| SCD (n = 500), n (%) | Non-SCD (n = 250), n (%) | P | |
|---|---|---|---|
| Age, mean, y | 23.6 | 23.9 | .425* |
| Marital status | .421† | ||
| Married | 43 (8.6) | 26 (10.4) | |
| Single | 457 (91.4) | 224 (89.6) | |
| Educational level | .305† | ||
| Primary/none | 27 (5.4) | 10 (4.0) | |
| Secondary | 330 (66) | 156 (62.4) | |
| Tertiary | 143 (28.6) | 84 (33.6) | |
| Employment | <.001† | ||
| Employed | 284 (61.9) | 188 (76.7) | |
| Unemployed | 73 (15.9) | 20 (8.2) | |
| Other | 102 (22.2) | 37 (15.1) | |
| Smoking | <.001† | ||
| Yes | 12 (2.4) | 20 (8.0) | |
| No | 486 (97.6) | 230 (92) |
Student t test.
Pearson’s χ2 test
Recurrent ischemic priapism is a common morbidity among men with SCD
Priapism was more common in the men with SCD (32.6%) than in those without it (2.0%; P < .001; Table 2). The predominant type of priapism reported by the men with SCD and those without it was stuttering (73.62%; 120 of 163) and rare (a single episode of priapism lasting ≤4 hours; 100%; 5 of 5; P = .330), respectively, with monthly recurrence (31.3%; 51 of 163) being the most typical pattern when compared with the single episodes reported by only 2.0% (5 of 250) of the men without SCD.
Table 2.
Experiences of priapism and associated conditions in a nonselect group of men in outpatient clinics, with or without SCD
| SCD, n (%) | Non-SCD, n (%) | P | |
|---|---|---|---|
| Priapism experience | (N = 500) | (N = 250) | <.001* |
| Yes | 163 (32.6) | 5 (2.0) | |
| No | 337 (67.4) | 245 (98) | |
| Typical priapism | (n = 163) | (n = 5) | .330† |
| Major | 43 (26.4) | 0.0 | |
| Stuttering/rare | 120 (73.6) | 5 (100) | |
| Age at priapism onset, y | (n = 163) | (n = 5) | .721† |
| Childhood, <12 | 14 (8.6) | 0.0 | |
| Teenaged, 13-17 | 51 (31.3) | 1 (20.0) | |
| Young adult, 18-25 | 75 (46.0) | 4 (80.0) | |
| Adult, >25 | 23 (14.1) | 0.0 | |
| Priapism frequency | (n = 163) | (n = 5) | NA |
| Daily | 13 (8.0) | 0.0 | |
| Every other day | 14 (8.6) | 0.0 | |
| Weekly | 31 (19.0) | 0.0 | |
| Monthly | 51 (31.3) | 0.0 | |
| Other (rare) | 54 (33.1) | 5 (100) | |
| Precipitants | (n = 163) | (n = 5) | .002† |
| Sexual arousal | 16 (9.8) | 1 (20.0) | |
| Sexual intercourse | 1 (0.6) | 0.0 | |
| Sleep | 146 (89.6) | 1 (20) | |
| Other | 11 (6.7) | 3 (60) | |
| Episode ≥72 h | (n = 163) | (n = 5) | .587† |
| Yes | 30 (18.4) | 0.0 | |
| No | 133 (81.6) | 5 (100) | |
| Penis deformity | (n = 161) | (n = 5 | .588† |
| Yes | 37 (22.98) | 0.0 | |
| No | 124 (77.02) | 5 (100) | |
| Sought treatment | (n = 163) | (n = 5) | .059† |
| Yes | 84 (51.5) | 0.0 | |
| No | 79 (48.5) | 5 (100) | |
| Present condition | (n = 161) | (n = 5) | .464† |
| Better | 103 (64.0) | 5 (100) | |
| Worse | 17 (10.6) | 0.0 | |
| About the same | 41 (25.5) | 0.0 | |
| Worsening erection | (n = 227) | (n = 109) | .091* |
| Yes | 31 (13.7) | 8 (7.3) | |
| No | 196 (86.3) | 101 (92.7) | |
| Affecting self-image | (n = 163) | (n = 5) | <.001† |
| Yes | 142 (87.1) | 0.0 | |
| No | 21 (12.9) | 5 (100) |
Data are number (percentage) of total responses in each category. The number of participants who responded is shown beside each category.
NA, not applicable; N, total sample size; n, sample of participants with priapism who responded.
Pearson’s χ2 test.
Fisher’s exact test.
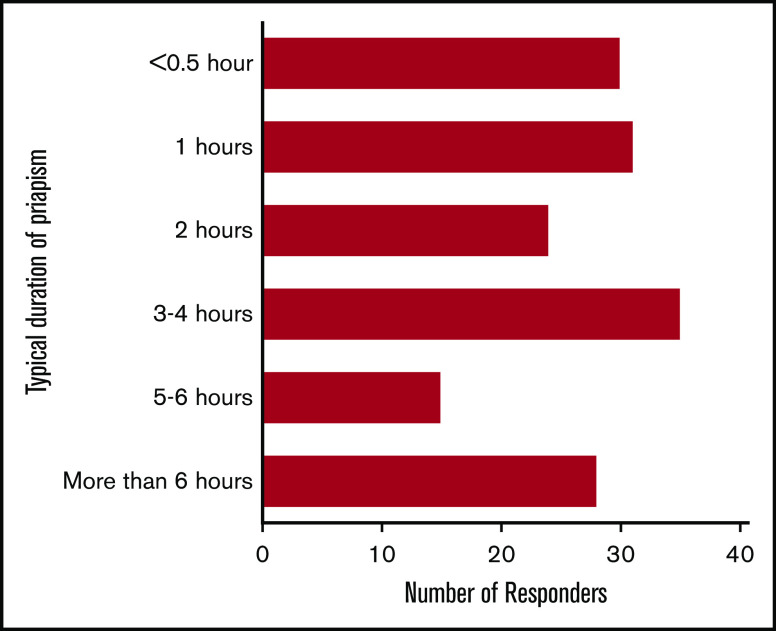
The most common priapism duration was 3 to 4 hours (21.5%; 35 of 163), and the least frequently reported duration was 5 to 6 hours (9.2%; 15 of 163; Figure 1). Young adults (18-25 years) was the most commonly reported age group at first onset of priapism in participants with SCD and those without it (46.0% [75 of 163] vs 80% [4 of 5], respectively; P = .721; Table 2). Some men with SCD-related priapism had had at least 1 episode of priapism lasting 72 hours (3 days; 18.4%; 30 of 163); those without SCD had none (0%; 0 of 5; P = .587). Deformity of the penis or scarring occurred in 22.9% (37 of 161) of those with SCD-related priapism and in 0% (0 of 5) of those without it (P = .588; Table 2). There was an association between perceived penile deformity and 72-hour priapism (36.7% vs 19.8%; P = .048), but no association between perceived penile deformity and stuttering or major priapism (23.33% vs 21.95%; P = .86; Table 3).
Figure 1.
Self-reported durations of typical priapism episodes in men with SCD. In those who participated (n = 163), the most typical durations of priapism were 3 to 4 hours (n = 35; 21.5%), 1 hour (n = 31; 19%), <0.5 hour (n = 30; 18.4%), >6 hours (n = 28; 17.2%), and 2 hours (n = 24; 14.7%), and the least reported duration was 5 to 6 hours (n = 15; 9.2%).
Table 3.
Association between perceived penile deformity and ED with or without 3-day priapism and priapism durations
| 3-d priapism | No 3-d priapism | P | Stuttering | Major | P | |
|---|---|---|---|---|---|---|
| Penis deformity, n (%) | .048* | |||||
| Yes | 11 (36.67) | 26 (19.85) | 28 (23.33) | 9 (21.95) | .856* | |
| No | 19 (63.33) | 105 (84.68) | 92 (76.67) | 32 (78.05) | ||
| ED, n (%) | .189† | |||||
| Yes | 2 (100) | 17 (41.46) | 5 (38.46) | 3 (42.86) | .99† | |
| No | 0.0 | 24 (58.54) | 8 (61.54) | 4 (57.14) | ||
Pearson’s χ2 test.
Fisher’s exact test.
Most of the men with SCD-related priapism (89.6%; 146 of 163) reported sleep as the precipitant of an episode compared with 20% (1 of 5) without SCD, and only 9.8% (16 of 163) of the men with SCD attributed their priapism to sexual desire compared with 20% (1 of 5) without SCD (P = .002 for overall comparison of precipitants of priapism; Table 2).
Priapism psychologically affects the lives of men with SCD
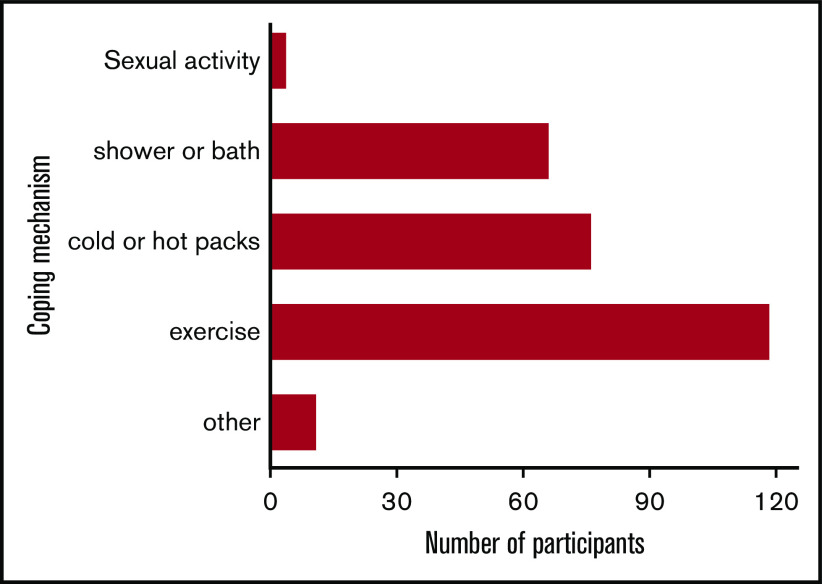
The men with SCD experienced significant impairment in mental health associated with priapism. Most participants with SCD-related priapism (87.1%; 142 of 163) reported its effect on their self-image, whereas none of those without SCD reported a similar effect (P < .001). The psychological effects of priapism were typically sadness (66.9%; 95 of 142), embarrassment (62%; 88 of 142), fear (43.7%; 62 of 142), and exhaustion (38.7%; 55 of 142), among others (Figure 2). Despite the high level of mental duress associated with priapism, 48.5% (79 of 163) of the men with SCD-related priapism never sought medical treatment for the condition. Because priapism is recurrent, men with SCD developed various coping mechanisms. Exercise (n = 90; 79.7%) was the most common nonmedicinal strategy to relieve priapism, followed by cold or hot packs (n = 76; 51.4%) administered to the penis, and a cold shower (n = 66; 44.6%; Figure 3).
Figure 2.
The wide range of psychological feelings experienced by the participants with SCD-related priapism. The participants (n = 142) were given multiple options to express the most appropriate feelings reflecting what they have experienced. They reported feeling sad (n = 95; 66.9%), embarrassed (n = 88; 62%), frightened (n = 62; 43.7%), frustrated (n = 60; 42.3%), exhausted (n = 55; 38.7%), depressed (n = 51; 35.9%), confused (n = 50; 35.2%), anxious (n = 32; 22.5%), and angry (n = 27; 19%).
Figure 3.
Coping mechanisms used by participants with SCD-related priapism. Responses (n = 163) included exercise (n = 90; 79.7%), cold or hot packs (n = 52; 51.4%), shower or bath (n = 41; 44.6%), sexual activity (n = 4, 3%), and other (n = 4, 3%).
Men with SCD and priapism underwent a wide range of treatment regimens in the hospitals
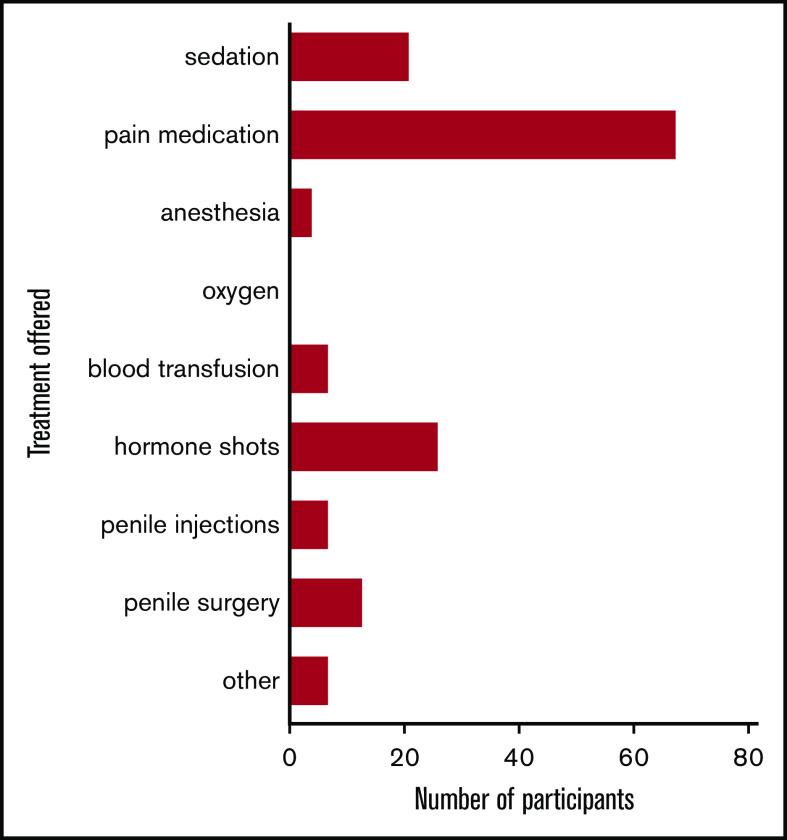
Treatment was heterogeneous among the 84 participants with SCD-related priapism who had sought medical treatment and included prescribed pain medication (84.8%), antitestosterone therapy (32.1%), penile surgery (16%), penile injections (8.6%), and blood transfusions (8.6%), among others (Figure 4).
Figure 4.
Various treatments offered by physicians to the study participants with SCD-related priapism. Patients (n = 163) were given multiple options because of the recurrent nature of the priapism: pain medication (n = 68; 84%), hormone shots (n = 26; 32.1%), sedation (n = 21; 25.9%), penile surgery (n = 13; 16%), penile injections (n = 7; 8.6%), blood transfusion (n = 7; 8.6%), anesthesia (n = 4; 4.9%), and other (n = 7; 8.6%) were reported treatments received by the participants.
Only 10.6% (17 of 161) of the men with SCD reported worsening of the present priapism condition, whereas the majority of them believed that their current situation showed improvement (64%; 103 of 161) or had remained the same (25.5%; 41 of 161); however, all the men without SCD reported that their condition was getting better (5 of 5; P = .464; Table 2).
Men with SCD report worse erectile and sexual dysfunction
The percentage of the men with SCD who perceived progressive worsening of erectile function over time for wanted sexual situations was not different from that of the men without SCD (13.7% vs 7.3%; P = .091; Table 2). Based on the IIEF questionnaire, the prevalence of ED, defined by an Erectile Function domain score of <26, was significantly higher in married men with SCD than in those without SCD (44.2% vs 15.4%; P = .014; Table 4). The percentage of severe ED was more than twofold higher in married men with SCD than in those without it (27.9% vs 11.5%), but the overall difference in severity was not significant (P = .105; Table 4).
Table 4.
IIEF domain-specific sexual function assessment among married men with and without SCD in outpatient clinics
| SCD (n = 43) | Non-SCD (n = 26) | P | |
|---|---|---|---|
| International Index of Erectile Function | 54.6 ± 23.7 | 69.7 ± 16.3 | .005* |
| Erectile function | 22.9 ± 9.9 | 29.5 ± 7.8 | .006* |
| Orgasmic function | 8.4 ± 4.3 | 10.0 ± 3.1 | .109* |
| Sexual desire | 6.3 ± 2.1 | 8.3 ± 1.5 | .001* |
| Intercourse satisfaction | 10.5 ± 5.3 | 13.5 ± 4.2 | .019* |
| Overall satisfaction with sex life | 6.3 ± 3.3 | 8.3 ± 1.6 | .005* |
| ED, n (%) | .014† | ||
| Normal | 24 (55.8) | 22 (84.6) | |
| ED score (<26) | 19 (44.2) | 4 (15.4) | |
| Severity of ED, n (%) | .105† | ||
| Normal | 24 (55.8) | 22 (84.6) | |
| Mild | 5 (11.6) | 1 (3.85) | |
| Mild-to-moderate | 2 (4.65) | 0 (0.0) | |
| Moderate | 0 0 (0.0) | 0 (0.0) | |
| Severe | 12 (27.9) | 3 (11.5) | |
Data are means ± standard deviation. Erectile domain scores: normal (26-30), mild ED (22-25), mild-to-moderate ED, (17-21) moderate ED (11-16), and severe ED (6-10).
NA, not applicable (contains cells with zero value).
Student t test.
Fisher’s exact test.
Compared with married men without SCD, those with SCD had significantly lower mean scores for erectile function (22.9 vs 29.5; P = .006), sexual desire (6.37 vs 8.38; P = .001), intercourse satisfaction (10.5 vs 13.5; P = .019), and overall satisfaction with sex life (6.34 vs 8.38; P = .005). However, there was no significant difference for orgasmic function between men with and those without SCD (8.46 vs 10.0; P = .109; Table 4).
The results of a subanalysis of married men with SCD-related priapism for sexual function based on stuttering vs major priapism revealed that those with stuttering compared with major priapism were not different in erectile function (25.23 vs 23.0; P = .811), orgasmic function (9.84 vs 8.42; P = .443), intercourse satisfaction (11.76 vs 10.0; P = .408), overall satisfaction with sex life (7.53 vs 6.71; P = .91), and sexual desire (6.31 vs 6.71; P = .776; Table 5). There was no significant association between ED and priapism duration (stuttering and major; 38.46% vs 42.86%; P = .99; Table 3). Similarly, married men without a history of priapism that lasted for 72 hours compared with those who had priapism episodes of 72 hours had higher mean erectile function scores (23.26 vs 15.5; P = .31), orgasmic function (8.53 vs 7.0; P = .855), sexual desire (6.46 vs 4.5; P = .185), intercourse satisfaction (10.68 vs 6.5; P = .242), and overall satisfaction with sex life (6.48 vs 3.5; P = .21; Table 5). These observed differences were not statistically significant.
Table 5.
Summary results of sexual function assessment using IIEF among married men with stuttering vs major priapism and 3-day priapism vs no 3-day priapism
| 3-d priapism (n = 2) | No 3-d priapism (n = 41) | P * | Stuttering (n = 13) | Major (n = 7) | P * | |
|---|---|---|---|---|---|---|
| Erectile function | 15.5 | 23.2 | .310 | 25.2 | 23 | .811 |
| Orgasmic function | 7.0. | 8.5 | .855 | 9.8 | 8.4 | .443 |
| Sexual desire | 4.5 | 6.4 | .185 | 6.3 | 6.7 | .776 |
| Intercourse satisfaction | 6.5 | 10.6 | .242 | 11.7 | 10.0 | .408 |
| Overall satisfaction with sex life | 3.5 | 6.4 | .210 | 7.5 | 6.7 | .914 |
Data are means.
Mann-Whitney U test.
Discussion
Priapism is a major complication in men with SCD. However, because of the sensitivity of discussing priapism and sexual dysfunction, many men with SCD do not mention these morbidities to their health care providers.13,14 Furthermore, most studies in men with SCD have been completed in high-income settings where SCD is a rare disease, thus limiting the inferences from these studies because of small sample sizes and lack of comparison groups. To address these challenges, we conducted the largest comparative survey of priapism and sexual dysfunction in a group of 500 men with SCD and 250 men without SCD. Also, to improve the generalizability of our results, we used the IIEF (a widely validated, reproducible tool) for assessment of sexual dysfunction in men with SCD and those without SCD.12 We report results showing a high prevalence of priapism (32.6%) in men with SCD. Among married men with SCD and those without SCD, we report that 44.2% and 15.4%, respectively, were found to have ED. These results, taken together, highlight the significant health challenges of priapism and sexual dysfunction in men with SCD in comparison with men without it.
A major observation from the results of this study is that ∼50% of the men with SCD-related priapism never sought medical attention for the complication. These data indicate that the burden of priapism is far greater than is acknowledged in the literature. Our study did not elicit why participants never sought medical attention for this complication. However, other studies have proffered reasons that include lack of knowledge of the relationship between priapism and SCD, the embarrassment associated with the morbidity, and the self-limiting nature of stuttering priapism.13,14 Appropriate behavioral changes in communication strategies and health education awareness campaigns are needed to improve the health-seeking behavior of men with SCD-related priapism.
In our study, young adulthood (18-25 years of age) was the most common period of initial onset of priapism in men with SCD, with the second most common period being early adolescence (13-17 years of age). Several other studies have reported a similar age range for onset of priapism in SCD.5,6,13 The most common age at onset typically coincides with the period of puberty, but there is no clear evidence of association with testosterone surge.15 Given the high rate of priapism in teenaged and young men, anticipatory guidance can improve self-awareness, particularly in preparing to transition from a pediatric to an adult care provider. Internists and pediatricians require higher vigilance to screen for priapism in men and adolescents with SCD.
Risk factors of SCD-related priapism are still poorly understood. Our study and others documented sleep as a major factor related to the occurrence of priapism.15 Of the men with SCD-related priapism, ∼90% stated that sleep is associated with the onset of priapism. We can only postulate as to the association between sleep and priapism. Perhaps there is a sleep-induced neural response, which often leads to the awakening of individuals with erections.16 Another biologically plausible explanation is sleep-disordered breathing with potential increased nocturnal hemoglobin oxygen desaturation, which may predispose to cellular sickling and vaso-occlusion within cavernosal sinusoids.16 The design of this cross-sectional study did not allow for the elucidation of the mechanistic relationship between onset of sleep and priapism.
ED is an unrecognized, common problem in men with SCD. Using a widely validated tool for sexual function assessment, we report the prevalence of ED among married men with SCD to be as high as 2.5-fold greater than among age-matched married men without SCD. This prevalence (44.2%) of ED is similar to the 47.5% (19 of 40) reported by Anele and Burnett17 in a retrospective study, but higher than the 21% (10 of 46) reported by Adeyoju et al.5
We also demonstrate that men with SCD, when compared with the general population, have significantly lower sexual desire, sexual intercourse satisfaction, and global satisfaction with sex life. Most likely because of small samples, our results suggest, but do not confirm, that longer priapism durations contribute to erectile and sexual dysfunction. Any intervention for relieving priapism lasting 48 to 72 hours is unlikely to salvage erectile function.9 Adeyoju et al5 have reported high sexual dissatisfaction among individuals with SCD because of fear of a priapism episode. This high level of sexual dysfunction can further compound the low quality of life in individuals with SCD. In summary, our results support that further research is needed to elucidate the etiology and optimal treatment of sexual dysfunction in men with SCD.
The multiple strengths of this study include a large sample size, a comprehensive survey of priapism, and sexual dysfunction in men with and in those without SCD, the use of a validated tool (IIEF) for data collection, and an easily reproducible design. As expected in any cross-sectional study, our study had limitations, which include the inability to document the real-time occurrences of priapism events and the incidence of sexual dysfunction. We also could not establish a temporal relationship between clinical risk factors and ED. Further, we were unable to assess biological risk factors for priapism, such as baseline hemoglobin level, lactate dehydrogenase level, and reticulocyte level. To address these limitations, we plan to perform a prospective study to ascertain further the other factors that influence priapism and sexual dysfunction in men with SCD.
Priapism and sexual dysfunction are significant morbidities in men with SCD in comparison with those without it. Our results provide compelling evidence for greater vigilance by physicians to inquire about priapism in young men with SCD. Given the burden of recurrent ischemic priapism and sexual dysfunction, definitive therapeutic interventions are necessary.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all members of M.R.D.’s research team in Kano, Nigeria, and his research laboratory at Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease for their untiring support and all participants with SCD and their families for their voluntary participation in this study. I.M.I. thanks the American Society of Hematology Clinical Research Training Institute for providing a mentored fellowship award on the priapism project.
I.M.I. was supported by a VECD (Vanderbilt-Emory-Cornell-Duke) Global Health Fellowship, funded by National Institutes of Health (NIH), Fogarty International Center (FIC) grant D43 TW009337. The work was also supported by Afolabi.
The views expressed in this publication are solely those of the authors and do not necessary represent the views of the NIH.
Footnotes
The original data may be obtained by e-mail request to the corresponding author Michael R. DeBaun (m.debaun@vumc.org).
Authorship
Contribution: M.R.D. and I.M.I. designed the study; I.M.I. wrote the manuscript; I.M.I., A.A., S.A.M., and J.A.G., performed the study; A.L.B. and S.A.G. critically reviewed the manuscript; M.R.D., I.M.I., and N.H. performed the statistical analyses; and M.R.D., A.L.B., and I.M.I. interpreted the data.
Conflict-of-interest disclosure: M.R.D. and his institution are the sponsors of 2 externally funded research investigator–initiated projects. Global Blood Therapeutics (GBT) is providing funding for the cost of the clinical studies, but will not be a cosponsor of either study. M.R.D. is not receiving any compensation for the conduct of these 2 investigator-initiated observational studies. He is a member of the GBT advisory board for a proposed randomized controlled trial for which he receives compensation, and he is the chairperson of a steering committee for an industry supported phase 2 trial (SPARTAN) for prevention of priapism in SCD. A.L.B. is a consultant for Novartis and Coloplast and has received research support from Boston Scientific. The remaining authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Vanderbilt-Meharry Sickle Cell Disease Center of Excellence, Vanderbilt University Medical Center, 2525 West End Ave, Suite 750, Nashville, TN 37203; e-mail: m.debaun@vumc.org.
References
- 1.Makani J, Williams TN, Marsh K. Sickle cell disease in Africa: burden and research priorities. Ann Trop Med Parasitol. 2007;101(1):3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piel FB, Hay SI, Gupta S, Weatherall DJWT, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi S, DeBaun MR. Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: the last 40 years. Am J Hematol. 2016;91(1):5-14. [DOI] [PubMed] [Google Scholar]
- 4.Halls JE, Patel DV, Walkden M, Patel U. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(spec iss 1):S79-S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeyoju AB, Olujohungbe ABK, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications - an international multicentre study. BJU Int. 2002;90(9):898-902. [DOI] [PubMed] [Google Scholar]
- 6.Emond AM, Holman R, Hayes RJSG, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140(11):1434-1437. [PubMed] [Google Scholar]
- 7.Serjeant G, Hambleton I. Priapism in homozygous sickle cell disease: a 40-year study of the natural history. West Indian Med J. 2015;64(3):175-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kheirandish P, Chinegwundoh F, Kulkarni S. Treating stuttering priapism. BJU Int. 2011;108(7):1068-1072. [DOI] [PubMed] [Google Scholar]
- 9.Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7(1 Pt 2):476-500. [DOI] [PubMed] [Google Scholar]
- 10.Chinegwundoh FI, Smith S, Anie KA. Treatments for priapism in boys and men with sickle cell disease. Cochrane Database Syst Rev. 2020;(4):CD004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustapha I. Affordable housing provision in Kano north western Nigeria: the imperative for the creation of sustainable city. Int J Manag Soc Sci Res. 2013;2(8):189-198. [Google Scholar]
- 12.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830. 4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 13.Adediran A, Wright K, Akinbami A, et al. Prevalence of priapism and its awareness amongst male homozygous sickle cell patients in Lagos, Nigeria. Adv Urol. 2013;2013:890328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBaun MR. Hydroxyurea therapy contributes to infertility in adult men with sickle cell disease: a review. Expert Rev Hematol. 2014;767-773. [DOI] [PubMed] [Google Scholar]
- 15.Fowler JE Jr., Koshy M, Strub M, Chinn SK. Priapism associated with the sickle cell hemoglobinopathies: prevalence, natural history and sequelae. J Urol. 1991;145(1):65-68. [DOI] [PubMed] [Google Scholar]
- 16.Burnett AL. Pathophysiology of priapism: dysregulatory erection physiology thesis. J Urol. 2003;170(1):26-34. [DOI] [PubMed] [Google Scholar]
- 17.Anele UA, Burnett AL. Erectile dysfunction after sickle cell disease-associated recurrent ischemic priapism: profile and risk factors. J Sex Med. 2015;12(3):713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.