Abstract
The 22q11.2 deletion syndrome (22q11.2DS) is a known risk factor for development of schizophrenia and is characterized by a complex neuropsychological profile. To date, a quantitative meta-analysis examining cognitive functioning in 22q11.2DS has not been conducted. A systematic review of cross-sectional studies comparing neuropsychological performance of individuals with 22q11.2DS with age-matched healthy typically developing and sibling comparison subjects was carried out. Potential moderators were analyzed. Analyses included 43 articles (282 effects) that met inclusion criteria. Very large and heterogeneous effects were seen for global cognition (d = − 1.21) and in specific neuropsychological domains (intellectual functioning, achievement, and executive function; d range = − 0.51 to − 2.43). Moderator analysis revealed a significant role for type of healthy comparison group used (typically developing or siblings), demographics (age, sex) and clinical factors (externalizing behavior). Results revealed significant differences between pediatric and adult samples, with isolated analysis within the pediatric sample yielding large effects in several neuropsychological domains (intellectual functioning, achievement, visual memory; d range = − 0.56 to − 2.50). Large cognitive deficits in intellectual functioning and specific neuropsychological variables in individuals with 22q11.2DS represent a robust finding, but these deficits are influenced by several factors, including type of comparison group utilized, age, sex, and clinical status. These findings highlight the clinical relevance of characterizing cognitive functioning in 22q11.2DS and the importance of considering demographic and clinical moderators in future analyses.
Keywords: 22q11.2 Deletion syndrome, Velocardiofacial syndrome, Meta-analysis, Neuropsychology, Cognitive
Introduction
The 22q11.2 deletion syndrome (22q11.2DS) is the most common microdeletion genetic disorder known in humans, with prevalence rates of 1:2000 to 1:4000 live births (Botto et al. 2003). The microdeletion of about 30–40 genes in the long arm of chromosome 22 is associated with a heterogeneous phenotype of clinical and behavioral characteristics. Typical features include congenital cardiovascular symptoms, immunological deficiencies, hypocalcemia, craniofacial abnormalities, cleft palate anomalies, and cognitive deficits (Ryan et al. 1997; Shprintzen 2000; McDonald-McGinn et al. 2015). The 22q11.2DS phenotype is also associated with a high prevalence of neuropsychiatric disorders, including adolescent and adult onset schizophrenia (Bassett and Chow 1999; Murphy et al. 1996; Shprintzen et al. 1992). Currently, 22q11.2DS is the most common known genetic risk factor for development of schizophrenia (Baker and Skuse 2005; Gothelf et al. 2005; Murphy et al. 1999). Studies estimate that about 30% of children with 22q.11.2DS develop psychosis in early adulthood (Murphy 2002; Murphy et al. 1999). 22q11.2DS is one of the most common causes of learning and intellectual disability in children (Gothelf and Lombroso 2001; Moss et al. 1999). The complex neuropsychological phenotype of 22q11.2DS has received attention in the literature, and is important to understand given the impact of cognitive deficits in 22q11.2DS on everyday functioning (Addington et al. 2005).
A review of cross-sectional studies has indicated that nonverbal intellectual abilities are significantly more impaired than verbal intellectual abilities in children with 22q11.2DS (Andersson et al. 2008; Moss et al. 1999; Scherer et al. 1999, 2001). The neuropsychological profile of 22q11.2DS (Gur et al. 2014) includes impairments in executive functioning (Bish et al. 2005; Henry et al. 2002; Rockers et al. 2009; Sobin et al. 2004; Van Aken et al. 2010; Woodin et al. 2001), nonverbal memory (Bearden et al. 2001; Henry et al. 2002; Lajiness-O’Neill et al. 2005), verbal memory (Majerus et al. 2007), attention (Bearden et al. 2001; Swillen et al. 1999a, b; Woodin et al. 2001), visuospatial functioning (Antshel et al. 2008; Cabaral et al. 2012; Simon et al. 2005), visual-motor functioning (Howley et al. 2012), and working memory (Lajiness-O’Neill et al. 2005). Academic achievement is another area of noted impairment in 22q11.2DS. Language development is often delayed and falls significantly below other school-aged children (Moss et al. 1999). However, inconsistency exists regarding the specific nature of language impairments that have been characterized in 22q11.2DS. Abilities for sentence repetition, word reading, and phonological processing have been shown to be intact, while reading comprehension and expressive language difficulties have also been observed (Solot et al. 2001). In addition to language delays, deficits in mathematics have been noted (Woodin et al. 2001), including calculation, word problem solving (De Smedt et al. 2006), and numerical magnitude judgment (Simon et al. 2005).
Additional characterization of cognitive functioning in 22q11.2DS is needed given the methodological limitations suffered by many studies to date. These include use of small sample sizes and the absence of age-matched comparison groups. Most studies examining cognitive functioning in children have restricted assessment to a narrow range of neuropsychological domains, providing limited comparisons of performance across domains to identify the most robust impairments in 22q11.2DS. Furthermore, the majority of studies characterizing cognitive functioning have focused on younger populations. Given that 25–30% of children diagnosed with 22q11.2DS develop schizophrenia in adulthood (Murphy 2002), an understanding of associated cognitive impairments throughout the lifespan could also inform a neurodevelopmental model of schizophrenia.
In the current study, we conduct a quantitative meta-analysis of existing studies examining cognitive functioning in children and adults with 22q11.2DS. A meta-analytic approach allowed for the combination of results across studies to provide a more powerful estimate of true population differences. We examined intellectual, academic, and neuropsychological functioning in both children and adults with 22q11.2DS as compared with healthy age-matched typically developing controls, sibling comparison groups, and mixed groups of typically developing and sibling controls. Further, we sought to identify the impact of various potential moderators, such as demographic and clinical variables that have been previously identified as different between patient and healthy comparison groups.
Materials and methods
Literature search strategy
Articles were identified through a computerized literature search using PubMed, PsychINFO, and MEDLINE Web of Science databases to find relevant studies with the search terms “22q11.2 Deletion syndrome, Velo-Cardio-Facial syndrome, DiGeorge syndrome, and neuropsychology, cognitive, attention, achievement, executive, motor, memory, intelligence, and visual perceptual.” The search was limited to English articles published between 1990 and February 2017 that dealt with human subjects. Additionally, a thorough manual review of articles was performed utilizing cross-references from identified original articles and reviews. This search procedure yielded 69 articles that addressed neurocognitive function in 22q11.2DS patients. Studies to be included in the meta-analysis were reviewed by five of the authors (M.J.R, C.L.M., A.C.G., K.L.V., & P.J.M.), and followed these criteria: (1) a focus on standard or experimental tasks of intellectual or neuropsychological function in patients with 22q11.2DS, (2) had an age-matched comparison group of either typically developing healthy participants or healthy siblings of patients, and (3) provided data or statistical information that allowed for the calculation of effect size.
Study selection
Titles and abstracts were reviewed and retained if they reported cognitive data in individuals with 22q11.2DS. Figure 1 provides a flow chart of search and selection criteria.
Fig. 1.
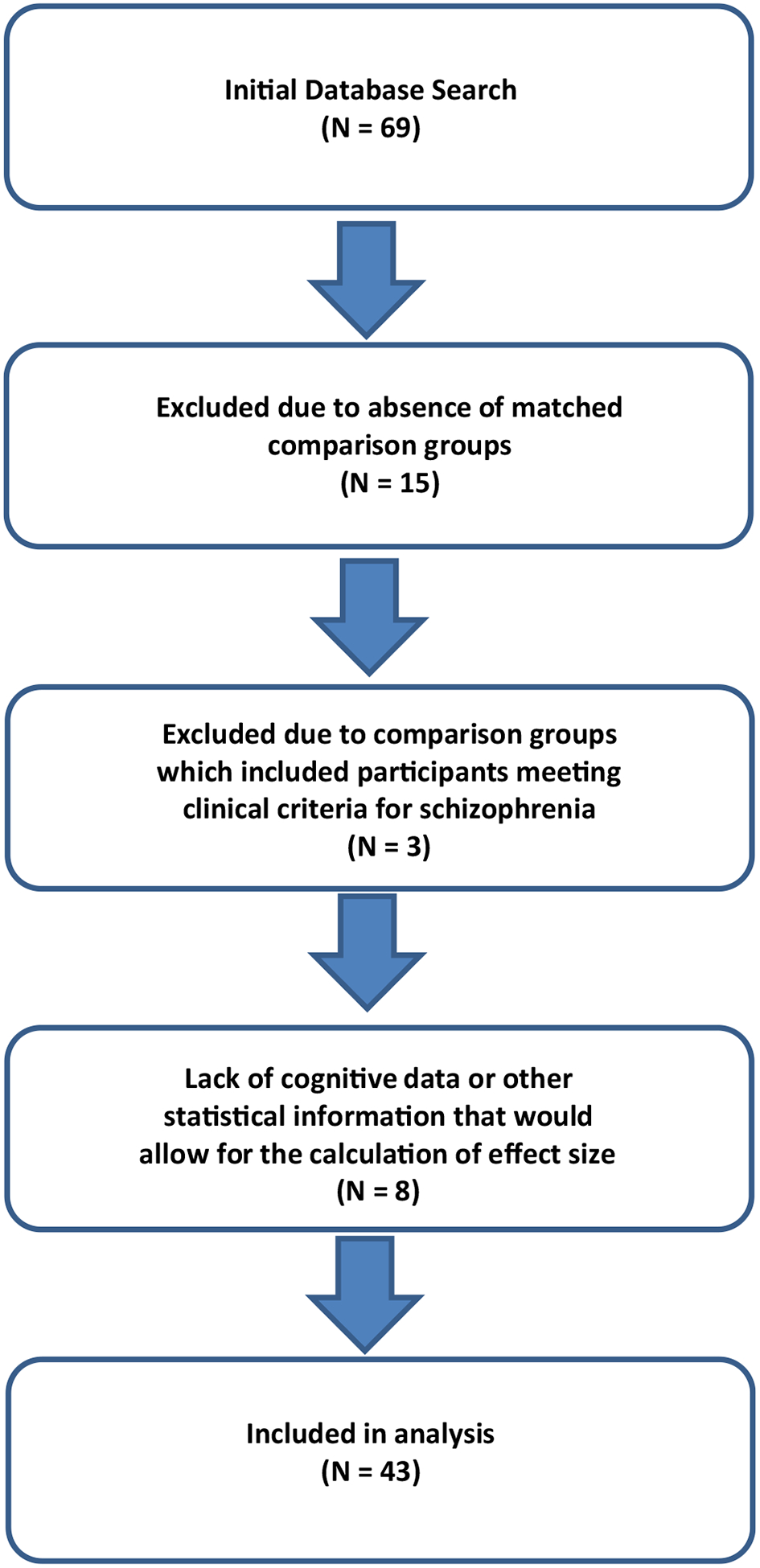
Search and selection criteria
After review, 26 articles of the original 69 were excluded, resulting in 43 publications totaling 282 effects for analysis that had reported comparative results of neurocognitive testing (see Supplement for complete list of included articles). Reasons for exclusion included: (1) absence of matched comparison groups (N = 15); (2) comparison groups which included participants meeting clinical criteria for schizophrenia (N = 3) and, (4) lack of data or other statistical information that would allow for the calculation of effect size (N = 8).
Data extraction and management
Participant types
Studies eligible for inclusion focused on the comparison of intellectual and neuropsychological functioning in patients with 22q11.2DS relative to those of: (1) typically developing healthy participants, (2) healthy siblings of patients with 22q11.2DS, or (3) a mixture of sibling and typically-developing controls. We relied upon the diagnostic criteria and probes i.e., fluorescent in situ hybridization (FISH) used in the source articles for the diagnosis of 22q11.2DS as well as the screening criteria used for healthy typically developing and sibling groups. If a selected article provided longitudinal data, baseline assessment data were extracted.
Outcome measure type
We sought to define neurocognitive functioning broadly, looking at effect size across thirteen basic domains including: (1) overall intellectual ability (i.e., Full-Scale IQ), (2) verbal intelligence, (3) nonverbal intelligence, (4) academic achievement, (5) executive functioning, (6) attention, (7) motor skills, (8) processing speed, (9) visual memory, (10) verbal memory, (11) visuospatial abilities, (12) emotional functioning, and (13) language. Assignment of neurocognitive tests to selected domains was guided by the classifications made in source articles and consensus of the authors. In the absence of assignment in source articles, tests were assigned to domain based on discussions between authors (P.J.M., M.J.R., C.L.M., and V.K.).
Moderator variable type
In the event of significant effect heterogeneity over the studies examined, categorical moderator analysis of the 13 specific neurocognitive domains was undertaken. Within the patient population, the following demographic and clinical moderator variables were coded: (1) mean age at the time of testing, (2) sex (i.e., percentage of the sample that was male) and, (3) externalizing and internalizing scores from the Child Behavioral Checklist (CBCL; Achenbach 1991). Other clinical scales were considered, but no other scale was reported with enough consistency to be included in analysis. The included articles were searched for additional demographic characteristics, including ethnic background and education level of participants; however, these data were not sufficiently reported to be included in formal analyses. Any disagreements concerning moderator inclusion were resolved through discussion.
Statistical analyses
Meta-analyses were conducted with Comprehensive Meta-analysis Version 2.0 software (Borenstein et al. 2005). The mean difference in scores between studies contrasting 22q11.2DS patients and healthy comparison subjects on measures of intellectual and neuropsychological functioning were standardized by calculating Cohen’s d in a multivariate random-effect model. Effect sizes were calculated based on the difference between the two raw means divided by the pooled standard deviation (SD), and were categorized as small (d ≥ 0.2), medium (d ≥ 0.5), or large (d ≥ 0.8) consistent with Cohen’s metric (Cohen 1988). Significance level of mean effect sizes was computed using random-effects models. Worse performance or cognitive deficits in the 22q11.2DS patients were defined by negative values for ease of understanding. As an example of this metric in the current meta-analysis, an effect size ≤ − 0.8 indicates that the neurocognitive test score of the average 22q11.DS subject falls below 79% of their same age- and sex-matched healthy comparison peers.
When means and SDs were not available, d was calculated from reported univariate F-tests, t statistics, or p values. The confidence intervals (CI) and z-transformations of the effect size were used to determine whether mean effect sizes were statistically significant. To assess homogeneity of the effect sizes across studies for each neuropsychological domain, the Cochran Q-statistic was used (Hedges and Olkin 1985). If analysis of the Q-statistic revealed significant within-group heterogeneity, we used a multivariate random-effects model to compute the significance level of the mean effect sizes. In addition to visual/graphic examination of the funnel-plot, mathematical methods for the evaluation of possible publication bias included those recommended by Begg and Mazumdar (1994), Egger, Smith, Schneider, and Minder (1997), as well as Duval and Tweedy (2000).
In categorical domains with significant heterogeneity, potential effect size moderators were assessed with the Q-statistic. Analysis was performed on all intellectual and neuropsychological performance differences for eligible studies. The effects of demographic moderator variables such as age and sex (i.e., percentage of the sample that was male), and clinical variables, including internalizing and externalizing scales from the CBCL were analyzed with meta-regression methods. Further analysis comprised of comparison of studies by task type, as well as pertinent demographic and clinical characteristics.
Results
Overall meta-analysis results in 22q11.2DS
Analysis of effect sizes across neurocognitive domains for the entire sample revealed a large overall effect size (N = 282 effects, d = − 1.21, 95% CI − 1.32 < δ < − 1.10) that was significantly heterogeneous (QB[281] = 2256.22, p < .001). Given that the variability in effect sizes between patient and healthy comparison groups differed more than would be expected from sampling error alone, analysis of potential moderator variables was undertaken.
Publication bias
Analysis for the presence of possible response bias revealed an asymmetric funnel plot and significant Begg (p < .001, 1-tailed) and Egger (p < .001, 1-tailed) tests, suggesting a potential “file drawer” problem and/or publication bias in this literature. To address the latter possibility, we imputed the potentially missing studies using the Duval and Tweedie (2000) “trim and fill” method. This procedure indicated that 0 studies are missing from analysis, and generated an imputed point estimate that is slightly smaller (d = − 1.09, 95% CI − 1.12 < δ < − 1.05) than the original Cohen’s d of − 1.21. In addition, calculation of a fail-safe N revealed that 246,068 “null” studies would need to be found and incorporated in the analysis to negate the presented effect. As such, these methods converge in supporting the notion that the current data accurately represent the extant literature concerning neurocognitive function in patients with a 22q11 Deletion syndrome.
Moderator analysis
Comparison group type
Categorical moderator analysis was performed examining whether type of comparison group used (i.e., unrelated typically developing subjects (TD), sibling subjects, or a mix of TD & sibling subjects) had impact on the observed heterogeneity. This analysis revealed that comparing 22q11.2DS patients to unrelated TD subjects produced significantly smaller effect sizes (d = − 1.02, 95% CI − 1.15 < δ < − 0.90) than when contrasting them to either siblings only (d = − 1.49, 95% CI − 1.68 < δ < − 1.29) (QB[1] = 14.29, p < .001) or a mixture of TD and siblings (d = − 1.81, 95% CI − 2.21 < δ < − 1.41) (QB[1] = 13.55, p < .001). The sibling and mixed groups did not differ from each other.
Neuropsychological domain
Moderator analysis across the 13 specific domains of neuropsychological function revealed significant heterogeneity among effects (QB[12] = 322.95, p < .001). As can be seen in Fig. 2, extremely large effect sizes were seen in basic intellectual functions, including Full Scale IQ (FSIQ) (d = − 2.53, 95% CI − 2.74 < δ < − 2.32), Performance IQ (PIQ) (d = − 2.44, 95% CI − 2.78 < δ < − 2.09), and Verbal IQ (VIQ) (d = − 1.96, 95% CI − 2.28 < δ < − 1.64). While no significant differences between verbal and nonverbal intellectual scores were seen (p = .06), the marked deficit in global intellectual function was significantly greater than that seen in achievement, attention, emotion, executive functioning, language, motor, processing speed, verbal memory, visual memory and visuospatial skills. Excluding intellectual impairments, among the defined neuropsychological domains, motor functions were the next most impaired ability (d = − 1.20, 95% CI − 1.97 < δ < − .48). In addition, effect sizes for tests of achievement (d = − 0.96, 95% CI − 1.16 < δ < − 0.77) were significantly larger than those for attention, processing speed, and visual perceptual skills. Executive function (d = − 0.97, 95% CI − 1.19 < δ < − 0.75), in turn, was also more impaired relative to attention, processing speed, and visuospatial skills. The remainder of the contrasts between neuropsychological domains did not reveal any significant differences.
Fig. 2.
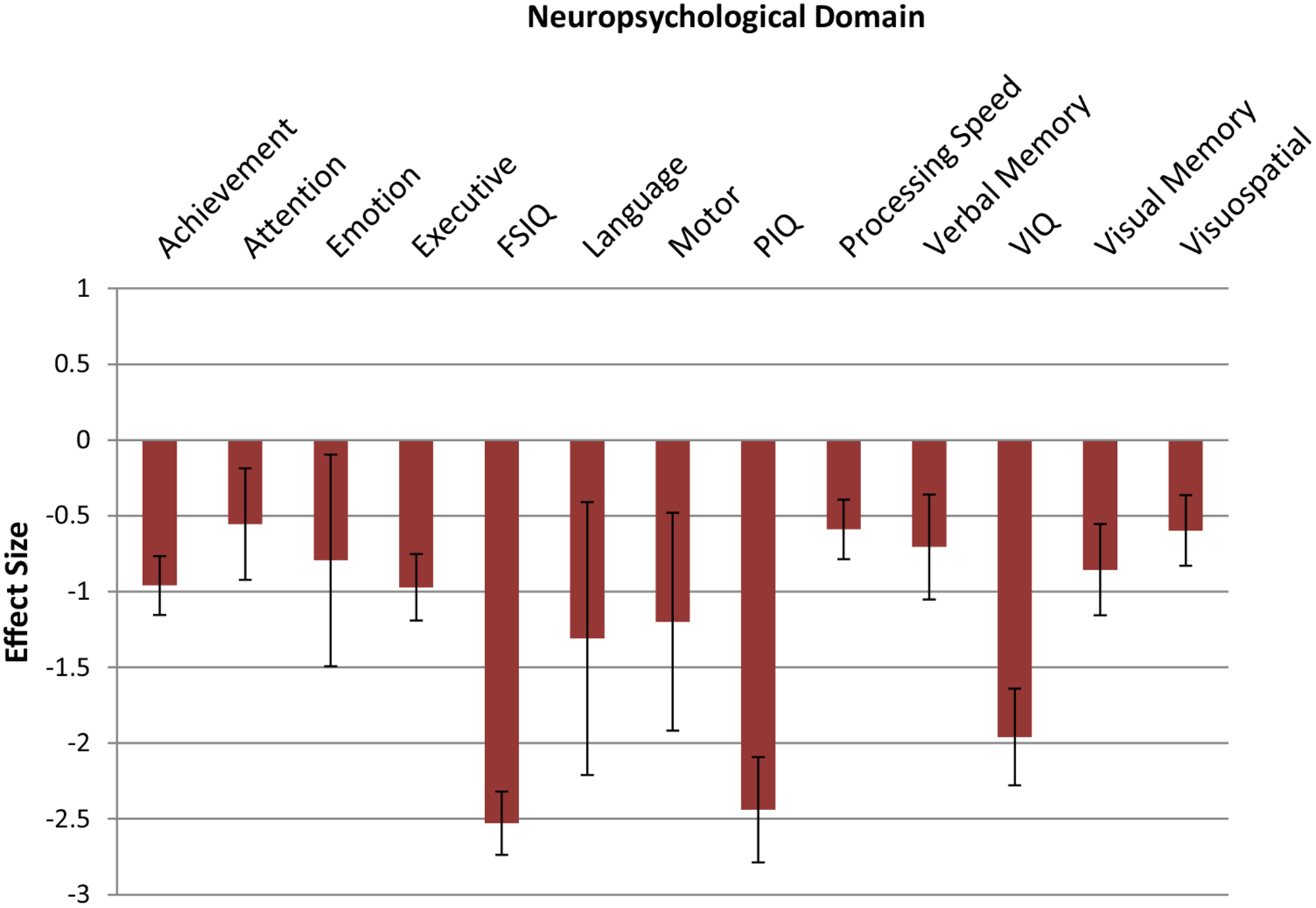
Effect sizes for neuropsychological domains for entire sample. Error bars represent 95% Confidence Intervals. Key: FSIQ > achievement, attention, emotion, executive function, language, motor, processing speed, verbal memory, VIQ, visual memory, visuospatial, p < .05. VIQ > achievement, attention, emotion, executive function, motor, processing speed, verbal memory, visual memory, visuospatial, p < .05. PIQ > achievement, attention, emotion, executive function, motor, processing speed, verbal memory, visual memory, visuospatial, p < .05. Executive function > attention, processing speed, visuospatial, p < .05. Achievement > attention, processing speed, visuospatial, p < .05
Meta-regression of demographic characteristics
Age
The average age of patients was 14.5 years, and meta-regression revealed a strong relationship between older age and better global cognitive abilities (N = 260 effects, Z = 3.47, p < .001). Visual inspection for outliers, however, revealed two publications that yielded smaller effect sizes (N = 31 effects). Additional review of these publications revealed that the subjects in the outlying studies were older than in many of the remaining studies. For this reason, additional analyses were undertaken by separating 5 studies with a mean patient age over 20 years (mean age = 26.6; N = 33 effects) from the remaining studies with pediatric samples (mean age = 12.6). When examining the studies comprised solely of younger patients, the opposite pattern was observed. That is, older children tended to showed greater neuropsychological impairment (N = 147 effects, Z = − 6.21, p < .001). Based on these differences, effect sizes for the 13 neuropsychological domains across studies of pediatric patients were also calculated. Results of these analyses are described below in the section “Pediatric Meta-Analysis Results” following analysis of the remaining moderating variables for the overall sample (both adults and children).
Sex
Meta-regression analysis of sex composition of the overall sample revealed that in patients, a larger magnitude of neurocognitive deficit was seen in those studies with a greater proportion of men (N = 208 effects, Z = − 2.51, p = .01).
Clinical characteristics
CBCL, internalizing/externalizing
The relationship between internalizing behaviors and neuropsychological performance in the 22q11.2 deletion syndrome sample was not observed to be significant (N = 9 effects, Z = 0.46, p = .64). In contrast, a significant positive relationship was seen between effect size and externalizing behaviors (N = 9 effects, Z = 2.88, p < .004), with higher externalizing scores being related to lesser cognitive deficit.
Pediatric meta-analysis results
An exploratory analysis of effect sizes across pediatric samples for the 13 neuropsychological domains revealed a large overall effect size (N = 228 effects, d = − 1.19, 95% CI − 1.31 < δ < − 1.07) that was significantly heterogeneous (QB[227] = 1823.05, p < .001). The mean effect sizes across the 13 neuropsychological domains can be seen in Fig. 3. Consistent with the results seen in the overall sample, the largest effects were seen on tests of basic intellectual functions including, FSIQ (d = − 2.50, 95% CI − 2.74 < δ < − 2.26), PIQ (d = − 2.66, 95% CI − 3.06 < δ < − 2.26), and VIQ (d = − 2.04, 95% CI − 2.41 < δ < − 1.69). In contrast to the results seen in the overall sample, a significant split between verbal and performance IQ was observed, in favor of the former (QB[1] = 5.70, p = .017). Once again, the extremely large effect sizes seen in overall global intellectual ability reflected significantly greater impairment relative to all of the other specific neuropsychological domains. Of specific neuropsychological abilities independent of IQ, motor abilities were again the next most impaired (d = − 1.20, 95% CI − 1.91 < δ < − 0.49) followed by visual memory deficits (d = − 1.01, 95% CI − 1.34 < δ < − 0.67).
Fig. 3.
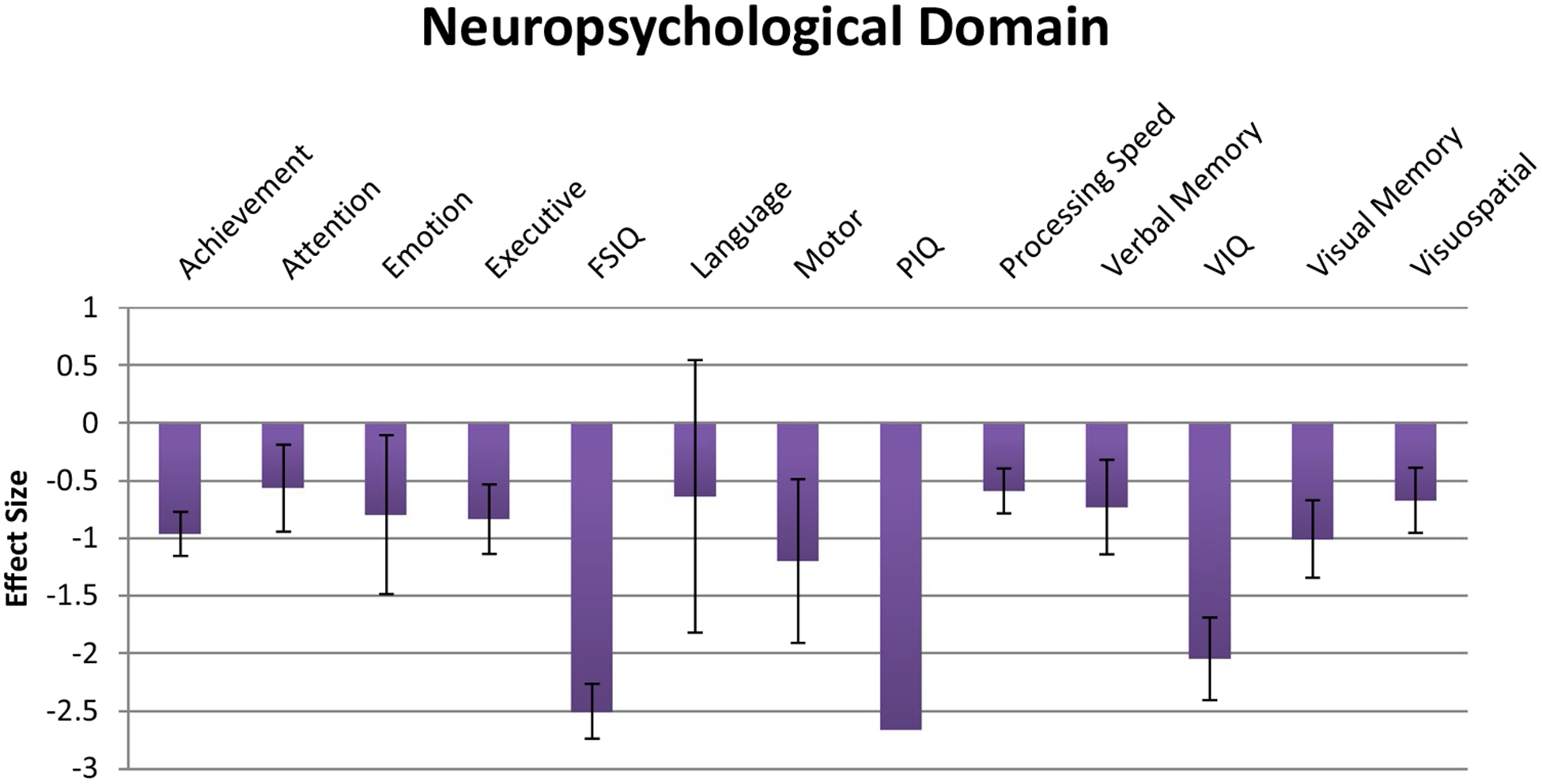
Effect sizes for neuropsychological domains in pediatric-only sample. Error bars represent 95% Confidence Intervals. FSIQ > achievement, attention, emotion, executive function, language, motor, processing speed, verbal memory, visual memory, visuospatial, p < .05. VIQ > achievement, attention, emotion, executive function, language, motor, processing speed, verbal memory, visual memory, visuospatial, p < .05. PIQ > achievement, attention, emotion, executive function, language, motor, processing speed, verbal memory, visual memory, visuospatial, p < .05. Achievement > attention, processing speed, p < .05. Visual memory > attention, processing speed, p < .05
Meta-regression of demographic moderator variables was also analyzed in the pediatric sample. In contrast to the analysis with the total sample, moderator analysis of age revealed a significant relationship between greater age and worse global cognitive deficit (N = 227 effects, Z = − 10.53, p < .001) in younger patients. Effects of sex composition in the pediatric sample were not significantly associated with neurocognitive performance (p = .30).
Discussion
The present meta-analytic review extends the current literature on neurocognitive functioning in patients with 22q11.2DS by quantifying the magnitude of cognitive impairment relative to healthy comparison subjects as well as specifying potential moderator variables that influence neuropsychological performance. Prior studies of cognition in 22q11.2DS have been limited by a restricted examination of neuropsychological domains as well as inadequate elucidation of cognition in adults with the disorder. Our study extended previous findings to a sample of both pediatric and adult patients with 22q11.2DS, and examined performance across a range of neurocognitive domains including, intellectual functioning, achievement, attention, executive function, emotional functioning, language, motor functioning, visuospatial functioning, processing speed, verbal memory, and visual memory.
Results from an analysis of 43 publications (282 effects) revealed a large overall effect size for cognition globally (d = − 1.21). This overall effect was heterogeneous and was significantly influenced by a number of moderating variables, including type of healthy comparison group used, neuropsychological domain assessed, age, and sex. There has been some debate in the extant literature about the most appropriate comparison group for 22q11.2DS patients, with a number of authors arguing that siblings without the chromosomal deletion have the advantage as a comparison group of being: (1) easily accessible, (2) relatively similar with regard to genetic factors other than the deletion (i.e., siblings share approximately 50% of genes), and (3) sharing environmental factors, including the same parents (Arnold et al. 2001). More broadly, contrasts resulting from sibling studies are thought to adjust for a wide range of unmeasured variables, and therefore may be particularly relevant for genetic disorders like 22q.11.2DS (Frisell et al. 2012). In order to more fully explore this issue, we conducted moderator analysis on studies that exclusively studied typically developing healthy comparison subjects to those studies that used only sibling comparison subjects. Analysis revealed that unrelated TD subjects produced significantly smaller effect sizes (d = − 1.02, 95% CI − 1.15 < δ < − 0.90) than when contrasting them to either siblings only (d = − 1.49, 95% CI − 1.68 < δ < − 1.29) (QB[1] = 14.29, p < .001) or a mixture of TD and siblings (d = − 1.81, 95% CI − 2.21 < δ < − 1.41) (QB[1] = 13.55, p < .001). These data support the notion that by incorporating a sibling comparison group, other environmental factors such as educational attainment, socioeconomic status, nutrition, etc., are held relatively constant and may better reflect true deficits in 22q11.2DS.
As noted previously, results of an outlier analysis revealed publications that were largely comprised of older patients (i.e., mean age = 26.6 years). Following removal of these outliers, the remaining samples were generally pediatric in nature (i.e., mean age = 12.6 years). As such, we first conducted an analysis of the entire sample and subsequently conducted additional exploratory analyses in the pediatric sample alone in order to discern any age cohort differences. Examination of moderator variable analysis in the pediatric sample revealed slightly different results with regard to the profile of neuropsychological deficits (e.g., after removal of studies with older aged participants, no remaining studies included measures of executive functioning), as well as slight variation in the strength and direction of the meta-regression results. While some differing patterns of neurocognitive performance were seen across adult and pediatric patients with 22q11.2DS, overarching and significant cognitive impairments were seen in the areas of intellectual abilities and motor functioning. Additional details about these results and differences between the age groups are discussed below.
Intellectual functions
Consistent with the extant literature concerning intellectual functions in patients with 22q11.2DS (Bearden et al. 2001; Moss et al. 1995), very large and robust effect sizes were found for global estimates of IQ across both younger and older patients with the disorder. Similar impairments have been consistently demonstrated in previous studies, though a number of authors have identified relatively greater deficits in nonverbal IQ relative to verbal skills, a pattern thought to be more closely aligned with a nonverbal learning disorder (Golding-Kushner et al. 1985; Moss et al. 1999; Swillen et al. 1997). It is notable that in the current meta-analysis such a verbal/nonverbal discrepancy was not seen in the overall sample, but rather emerged only when the studies comprised of pediatric patients were examined in isolation (QB[1] = 5.70, p = .017). While the current cross-sectional data cannot address possible changes in intellectual functions with age, it may be that such verbal/nonverbal discrepancies in IQ are minimized due to brain maturation, specific cognitive remediation, or academic and behavioral interventions over a child’s lifetime (Moss et al. 1995). Additionally, a possible resolution of the observed verbal/nonverbal IQ discrepancy over time could also reflect a regression of verbal skills due to impending, or onset of, psychosis as patients mature. These hypotheses will require longitudinal studies to examine possible individual changes.
Neuropsychological functions
In addition to significant impairments in intellectual abilities, large and consistent deficits were found for motor skills (d = − 1.17), achievement (d = − 1.04), and executive functioning (d = − 0.90). Significant motor delays in individuals with 22q11.2DS have been well documented regardless of an individual’s history of cardiac involvement, neonatal surgery, and hypotonia (Swillen et al. 2005). Indeed, in a study of 37 primary school children with 22q11.2DS, Van Aken and colleagues (2007) found that 62% of the children fell below the 5th percentile on tests of motor function and 78% met cut-offs for the initiation of physio- and/or psychomotor therapy. The current data extend these findings by documenting the very large effect sizes seen in this domain and highlight the importance of motor disability in the clinical and neuropsychological profile of these patients. The motor impairments seen in 22q11.2DS are thought to be stable across development, as indicated by a small longitudinal study of children with 22q11.2DS, where observed neuromotor deficits remained stable over 3 years (Sobin et al. 2006). A developmental pattern of motor deficits has also been shown in the extant schizophrenia literature, where impaired motor function prior to age 16 has been identified as a significant vulnerability for development of schizophrenia (Dickson et al. 2012).
With regard to executive deficits, our analysis also revealed moderate to large impairments in basic executive functions. This finding is consistent with a study by Goldenberg and colleagues (2012) where, in a computerized battery of neurocognitive functioning, individuals diagnosed with 22q11.2DS were shown to demonstrate greater impairment in abstraction and mental flexibility in relation to low risk individuals, first-degree family members of schizophrenia patients, individuals with prodromal symptoms, and patients diagnosed with schizophrenia. The finding of greater impairment in motor and executive measures, while generally consistent with the neuropsychological and clinical literature, stands in contrast to a meta-analysis of structural MRI findings in 22q11.2DS where a rostro-caudal gradient was observed for effect sizes across studies: frontal < temporal < cerebellar < occipital. As described in more detail below, Kates et al. (2005) noted that male 22q11.2DS patients showed decrements in frontal lobe volume, while females showed a relative preservation of this brain region. We speculate that the finding of significant executive impairments in the current meta-analysis is being largely driven by an interaction with sex. While it is not possible to test out a potential interaction via meta-analytic techniques, future prospective studies may help further inform this possibility.
Sex effects
In addition to age, sex was also an influential moderator, with greater neurocognitive impairment associated with a higher percentage of males in any given sample. Among individuals with 22q11.2DS, males have been shown to account for 67% of total impaired neuropsychological scores (Sobin et al. 2004). Phenotypic differences in neuroanatomical development between males and females with 22q11.2DS has also been demonstrated, with whole brain volume and frontal lobe volume being preserved in females and reduced in males (Kates et al. 2005). These findings are consistent with other reports in the literature showing neuroanatomical and neuropsychological phenotypes in other prototypical developmental disorders, such as ADHD (Mostofsky et al. 2002) and Tourette’s syndrome (Kurlan 1992) where a similar preservation of function in females is also seen. Similarly, studies investigating the relationship between sex and cognitive functioning in schizophrenia have demonstrated that females present with better premorbid functioning and cognitive ability as well as experience a better response to treatment and better clinical course (Canuso and Pandina 2007; Seeman 2000).
Clinical measures
Contrary to expectations based on the extant literature in 22q11.2DS, moderator analysis revealed greater levels of externalizing behavior, as measured by the CBCL, were associated with a smaller magnitude of cognitive impairment. In comparison, internalizing behaviors did not significantly moderate overall effect size. A study examining differences in psychopathology between children with 22q11.2DS and those with developmental delays, found that significantly lower rates of externalizing behaviors, while still within the normal range of behavior as defined by the CBCL, were evident in 22q11.2DS youth (Feinstein et al. 2002). On the CBCL, the externalizing composite score is comprised of destructive and aggressive behaviors. While studies of children diagnosed with 22q11.2DS have documented difficulties with mood lability, social interaction, impulsivity, and disorganization (Gerdes et al. 1999; Golding-Kushner et al. 1985; Swillen et al. 1999a, b), it is less clear why the presence of more externalizing behaviors would be associated with better cognitive outcomes (i.e. smaller effect sizes) in patients with 22q11.2DS. It is notable that the CBCL also can reflect normal patterns of behavior that are not indicative of gross pathology, and as the CBCL is a parent-report measure that reflects a parent’s perception of their child’s behavior it may be that those higher functioning (i.e., better cognitive function) children with 22q11.2DS also have parents that have higher expectations for behavior. Unfortunately, other clinical scales that may have shed light on the latter possibility (or others) were either not used or reported in the literature.
Age
As noted earlier, age was found to be an influential moderator in the overall sample, with increasing age being associated with better neurocognitive function (N = 260 effects, Z = 3.47, p < .001). It has been noted previously that in some neurodevelopmental disorders, brain changes over the course of development may induce a “disappearance” of dysfunctions that were present at an earlier age (Hadders-Algra 2004). Along this same line, the reverse can also be seen, where deficits may not be seen in children, but later emerge as impairments due to an age-related increase in the complexity of neural connections and/or functions. Indeed, outlier analysis revealed that this “enhancing” moderator effect was being largely driven by the inclusion of older 22q11.2DS patients. When the effect of age was examined in the pediatric sample alone, the moderating effect of age was inverted, with poorer neurocognitive abilities being associated with older age. Two cross-sectional studies of brain morphology in 22q11.2DS may shed light on this differential pattern. Kates et al. (2004) found reductions in parietal lobe volume in children with 22q11.2DS whereas another study found larger parietal lobe volume in adults with this disorder (van Amelsvoort et al. 2001). This initial evidence may suggest one temporal pathway of brain development over the lifespan that influences cognitive ability in 22q11.2DS. It is also possible that observed age-related improvement in cognition could be attributed to beneficial effects of maturation and supportive educational efforts over the lifespan aimed at improving cognitive ability and academic achievement in 22q11.2DS. Lastly, it is well-known that patients with 22q11.2DS are more likely to develop psychosis as they enter adulthood (Gothelf et al. 2005; Tang et al. 2014). As such, the relationship with age may reflect the possible exclusion of more impaired individuals in adult samples due to the emergence of psychotic symptoms or other psychiatric disorder. Consequently, the literature’s representation of older individuals with 22q11.2DS may be a biased sample of individuals with fewer psychotic symptoms and better functional outcomes.
Limitations
The current study is characterized by some limitations worthy of discussion. First, our analysis was comprised of studies that only included a healthy comparison, sibling group, or a mixture of the two. While this allowed for comparisons of effect sizes between patient and age-matched healthy comparison and sibling comparison groups, it omitted studies that relied on normative data to calculate patients’ cognitive performance, and studies that characterized performance in relation to individuals with schizophrenia vulnerability or diagnosis. In making this decision, we sought to use the most straightforward and commonly used approach in meta-analyses, as the calculation of effect sizes on disparate populations of comparison subjects, patients and family members can increase the variance and make interpretation of effects difficult. Secondly, there are limitations in the moderating variables that were examined. For example, as only a few studies reported results for education level, ethnicity, and notably, socioeconomic status, our analyses cannot shed light on the likely important moderating impact of these factors on cognition in 22q11.2DS. Third, our analysis was limited in its inclusion of only cross-sectional studies. The relative lack of longitudinal studies in the literature likely reflects the inherent practical challenges in following children throughout development and into adulthood. In the current meta-analysis, differences between adult and pediatric cohorts point to the importance of longitudinal studies to track the anatomical, behavioral, and cognitive sequelae of 22q11.2DS. Lastly, the examination of multiple effect sizes from different cognitive measures in a single study can be potentially problematic in data interpretation. The non-independence of measures is an issue in most meta-analyses in the literature, but the available solutions can be very hard to utilize due to the variability of what is reported in the publications themselves (i.e., correlations between neuropsychological measures) or the lack of information in the general literature or test manuals concerning these inter-correlations. Indeed, in most cases the meta-analyst does not know the correlations between multiple measures used in a particular study. We had considered consulting previous studies or manuals from test publishers to impute the correlations between measures. Theoretically, such an approach makes sense. However, this approach created more problems than it solved. For example, when a study measures outcomes using standardized tests, the correlations between them might be available from test publishers or in the research literature, but the extent to which these correlations generalize beyond the normative sample to a special population (such as 22q11. DS patients) or to other cognitive measures not in the validation sample is almost never documented. Lastly, even when the within-study correlations are available, it is often difficult to estimate the between study correlation and it is often estimated as 1 or − 1, at the boundary of its parameter space, causing an upward bias in the between-study variance estimates (see Jackson et al. 2011). Overall, we decided that the multivariate random-effects model we used is the current and most accepted method to deal with these concerns.
Conclusions
Increased emphasis on the importance of understanding cognition in 22q11.2DS has helped inform the neurodevelopmental model of schizophrenia. 22q11.2DS is the most common known genetic risk factor for development of schizophrenia, with about a third of children showing signs of psychosis in late adolescence and early adulthood. This has led to an increased focus on identifying the neuropsychological phenotype of 22q11.2Ds, aiming to improve functional outcomes for individuals with 22q11.2DS. To date, studies characterizing neuropsychological functioning in 22q11.2DS have been limited by small sample sizes, examination of a restricted range of neurocognitive domains, and a narrow focus on pediatric cohorts to the exclusion of adults with 22q11.2DS. The use of a quantitative meta-analytic approach allowed us to combine results across several studies to examine effect sizes estimates of differences in cognition in 22q11.2DS. Additionally, given the range of 22q11.2DS phenotypes, a meta-analytic approach is advantageous in allowing for wider inclusion of a range of polymorphisms in non-deleted alleles that are associated with phenotypic differences. Our results indicated large and generalized impairments across pediatric and adult patients with 22q11.2DS in overall intellectual functioning, verbal intellectual functioning, nonverbal intellectual functioning, executive functioning, and motor functioning. These effect size estimates were moderated by a number of sample characteristics, including older age, male sex, and externalizing behavior profile. Exploratory analysis of pediatric samples showed significant impairments in overall intellectual functioning, verbal intellectual functioning, nonverbal intellectual functioning, motor functioning, and verbal memory. Consistent with findings in the literature, among the pediatric samples, nonverbal intellectual functioning was significantly more impaired than verbal intellectual functioning, and age was a significant moderator characteristic. This highlights the need to properly consider these moderators when examining cognitive functioning across the lifespan in 22q11.2DS. Future research in this area needs to focus on a longitudinal approach for examination of cognition in 22q11.2DS, measure the impact of education and ethnicity on cognitive functioning, and pay close attention to the significant moderators outlined in this study.
Supplementary Material
Acknowledgements
The authors thank The Philadelphia Center for providing internship placement and educational planning for Ms. Richman and Mr. Tsering as part of their academic and training program. We also thank Ms. Kelly Chadwick for her help in acquisition and entry of the sibling data.
Funding This work was supported by the National Institutes of Health Grant MH087626 to R.E.G.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10519–018-9903–5) contains supplementary material, which is available to authorized users.
Conflict of interest P.J. Moberg, M.J. Richman, D.R. Roalf, C.L. Morse, A.C. Graefe, L. Brennan, K. Vickers, W. Tsering, V. Kamath, B.I. Turetsky, R.C. Gur, and R.E. Gur declare that they have no conflict of interest.
Ethical Approval All procedures performed in this study were in accordance with the ethical standards of the institutional review board of the University of Pennsylvania and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and Animal Rights This article does not contain any studies with animals performed by any of the authors.
Informed consent As this study examined group-level data from the peer-reviewed, published literature on neuropsychological function in 22q11.2DS, informed written consent was not required or necessary.
References
- Achenbach TM (1991) Manual for the child behavior checklist/4–18 and 1991 profile. Department of Psychiatry, University of Vermont, Burlington [Google Scholar]
- Addington J, Saeedi H, Addington D (2005) The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr Res 78(1):35–43 [DOI] [PubMed] [Google Scholar]
- Andersson F, Glaser B, Spiridon M, Debbane M, Vuilleumier P, Eliez S (2008) Impaired activation of face processing networks revealed by functional magnetic resonance imaging in 22q11.2 deletion syndrome. Biol Psychiatry 63(1):49–57 [DOI] [PubMed] [Google Scholar]
- Antshel KM, Peebles J, AbdulSabur N, Higgins AM, Roizen N, Shprintzen R, et al. (2008) Associations between performance on the Rey-Osterrieth complex figure and regional brain volumes in children with and without velocardiofacial syndrome. Dev Neuropsychol 33(5):601–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PD, Siegel-Bartelt J, Cytrynbaum C, Teshima I, Schachar R (2001) Velo-cardio-facial syndrome: implications of microdeletion 22q11 for schizophrenia and mood disorders. Am J Med Genet 105(4):354–362 [DOI] [PubMed] [Google Scholar]
- Baker KD, Skuse DH (2005) Adolescents and young adults with 22q11 deletion syndrome: psychopathology in an at-risk group. Br J Psychiatry 186:115–120 [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW (1999) 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry 46(7):882–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, McDonald-McGinn D, Zackai E, et al. (2001) The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol 23(4):447–464 [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101 [PubMed] [Google Scholar]
- Bish JP, Ferrante SM, McDonald-McGinn D, Zackai E, Simon TJ (2005) Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11.2 deletion syndrome. Dev Sci 8(1):36–43 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H (2005) Comprehensive meta-analysis version 2. Biostat, Englewood [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, et al. (2003) A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics 112(1 Pt 1):101–107 [DOI] [PubMed] [Google Scholar]
- Cabaral MH, Beaton EA, Stoddard J, Simon TJ (2012) Impaired multiple object tracking in children with chromosome 22q11.2 deletion syndrome. J Neurodev Disord 4(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuso CM, Pandina G (2007) Gender and schizophrenia. Psychopharmacol Bull 40(4):178–190 [PubMed] [Google Scholar]
- Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn Erlbaum, Hillsdale [Google Scholar]
- De Smedt B, Swillen A, Devriendt K, Fryns JP, Verschaffel L, Ghesquiere P (2006) Mathematical disabilities in young primary school children with velo-cardio-facial syndrome. Genet Couns 17(3):259–280 [PubMed] [Google Scholar]
- Dickson H, Laurens K, Cullen A, Hodgins S (2012) Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med 42:743–755 [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2):455–463 [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein C, Eliez S, Blasey C, Reiss AL (2002) Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biol Psychiatry 51(4):312–318 [DOI] [PubMed] [Google Scholar]
- Frisell T, Öberg S, Kuja-Halkola R, Sjölander A (2012) Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology 23(5):713–720 [DOI] [PubMed] [Google Scholar]
- Gerdes M, Solot C, Wang PP, Moss E, LaRossa D, Randall P et al. (1999) Cognitive and behavior profile of preschool children with chromosome 22q11. 2 deletion. Am J Med Genet 85(2):127–133 [PubMed] [Google Scholar]
- Goldenberg PC, Calkins ME, Richard J, McDonald-McGinn D, Zackai E, Mitra N et al. (2012) Computerized neurocognitive profile in young people with 22q11.2 deletion syndrome compared to youths with schizophrenia and at-risk for psychosis. Am J Med Genet B 159B(1):87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding-Kushner KJ, Weller G, Shprintzen RJ (1985) Velo-cardio-facial syndrome: language and psychological profiles. J Craniofac Genet Dev Biol 5(3):259–266 [PubMed] [Google Scholar]
- Gothelf D, Lombroso PJ (2001) Genetics of childhood disorders: XXV. Velocardiofacial syndrome. J Am Acad Child Adolesc Psychiatry 40(4):489–491 [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C et al. (2005) COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci 8(11):1500–1502 [DOI] [PubMed] [Google Scholar]
- Gur RE, Yi J, McDonald-McGinn D, Tang S, Calkins M, Whinna D, Souders M, Savitt A, Zackai E, Moberg PJ, (2014) Neurocognitive development in 22q11. 2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Mol Psychiatry 19:1205–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M (2004) General movements: a window for early identification of children at high risk for developmental disorders. J Pediatr 145(2):S12–S18 [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic Press, Amsterdam [Google Scholar]
- Henry JC, van Amelsvoort T, Morris RG, Owen MJ, Murphy DG, Murphy KC (2002) An investigation of the neuropsychological profile in adults with velo-cardio-facial syndrome (VCFS). Neuropsychologia 40(5):471–478 [DOI] [PubMed] [Google Scholar]
- Howley SA, Prasad SE, Pender NP, Murphy KC (2012) Relationship between reaction time, fine motor control, and visual-spatial perception on vigilance and visual-motor tasks in 22q11.2 deletion syndrome. Res Dev Disabil 33(5):1495–1502 [DOI] [PubMed] [Google Scholar]
- Jackson D, Riley R, White IR (2011) Multivariate meta-analysis: potential and promise. Stat Med 30:2481–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Bessette BA, Folley BS, Strunge L, Jabs EW, Pearlson GD (2004) Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2). J Child Neurol 19(5):337–342 [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Willhite R, Bessette BA, AbdulSabur N, Marie Higgins A (2005) Gender-moderated dorsolateral prefrontal reductions in 22q11. 2 deletion syndrome: implications for risk for schizophrenia. Child Neuropsychol 11(1):73–85 [DOI] [PubMed] [Google Scholar]
- Kurlan R (1992) The pathogenesis of Tourette’s syndrome: a possible role for hormonal and excitatory neurotransmitter influences in brain development. Arch Neurol 49(8):874. [DOI] [PubMed] [Google Scholar]
- Lajiness-O’Neill RR, Beaulieu I, Titus JB, Asamoah A, Bigler ED, Bawle EV, Pollack R (2005) Memory and learning in children with 22q11.2 deletion syndrome: evidence for ventral and dorsal stream disruption? Child Neuropsychol 11(1):55–71 [DOI] [PubMed] [Google Scholar]
- Majerus S, Van der Linden M, Braissand V, Eliez S (2007) Verbal short-term memory in individuals with chromosome 22q11.2 deletion: specific deficit in serial order retention capacities? Am J Ment Retard 112(2):79–93 [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE (2015) 22q11. 2 deletion syndrome. Nat Rev Dis Primers 1:15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EM, Wang PP, McDonald-McGinn DM, Gerdes M, DaCosta AM, Christensen KM et al. (1995) Characteristic cognitive profile in patients with a 22q11. 2 deletion: verbal IQ exceeds nonverbal IQ. Am J Hum Genet 57:42 [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA et al. (1999) Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. J Pediatr 134(2):193–198 [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE (2002) Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry 52:785–794 [DOI] [PubMed] [Google Scholar]
- Murphy KC (2002) Schizophrenia and velo-cardio-facial syndrome. Lancet 359(9304):426–430 [DOI] [PubMed] [Google Scholar]
- Murphy KC, Cardno AG, McGuffin P (1996) The molecular genetics of schizophrenia. J Mol Neurosci 7(2):147–157 [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ (1999) High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry 56(10):940–945 [DOI] [PubMed] [Google Scholar]
- Rockers K, Ousley O, Sutton T, Schoenberg E, Coleman K, Walker E, Cubells JF (2009) Performance on the modified card sorting test and its relation to psychopathology in adolescents and young adults with 22q11.2 deletion syndrome. J Intellect Disabil Res 53(7):665–676 [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, Aurias A (1997) Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet 34(10):798–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer NJ, D’Antonio LL, Kalbfleisch JH (1999) Early speech and language development in children with velocardiofacial syndrome. Am J Med Genet 88(6):714–723 [PubMed] [Google Scholar]
- Scherer NJ, D’Antonio LL, Rodgers JR (2001) Profiles of communication disorder in children with velocardiofacial syndrome: comparison to children with Down syndrome. Genet Med 3(1):72–78 [DOI] [PubMed] [Google Scholar]
- Seeman MV (2000) Women and schizophrenia. Medscape Women’s Health 5(2):2. [PubMed] [Google Scholar]
- Shprintzen RJ (2000) Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Ment Retard Dev Disabil Res Rev 6(2):142–147 [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW (1992) Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet 42(1):141–142 [DOI] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn DM, Zackai E (2005) Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex 41(2):145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Blundell M, Anyane-Yeboa K, Karayiorgou M (2004) Networks of attention in children with the 22q11 deletion syndrome. Dev Neuropsychol 26(2):611–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Monk SH, Kiley-Brabeck K, Khuri J, Karayiorgou M (2006) Neuromotor deficits in children with the 22q11 deletion syndrome. Mov Disord 21(12):2082–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solot CB, Gerdes M, Kirschner RE, McDonald-McGinn DM, Moss E, Woodin M et al. (2001) Communication issues in 22q11.2 deletion syndrome: children at risk. Genet Med 3(1):67–71 [DOI] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP (1997) Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J Med Genet 34(6):453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Vandeputte L, Cracco J, Maes B, Ghesquiere P, Devriendt K, Fryns JP (1999a) Neuropsychological, learning and psychosocial profile of primary school aged children with the velo-cardio-facial syndrome (22q11 deletion): evidence for a nonverbal learning disability? Child Neuropsychol 5(4):230–241 [DOI] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Prinzie P, Vogels A, Ghesquiere P, Fryns JP (1999b) The behavioural phenotype in velo-cardio-facial syndrome (VCFS): from infancy to adolescence. Genet Couns 10(1):79–88 [PubMed] [Google Scholar]
- Swillen A, Feys H, Adriaens T, Nelissen L, Mertens L, Gewillig M et al. (2005) Early motor development in young children with 22q.11 deletion syndrome and a conotruncal heart defect. Dev Med Child Neurol 47(12):797–802 [DOI] [PubMed] [Google Scholar]
- Tang S, Yi J, Calkins M, Whinna D, Kohler C, Souders M, McDonald-McGinn D, Zackai E, Emanuel B, Gur R (2014) Psychiatric disorders in 22q11. 2 deletion syndrome are prevalent but undertreated. Psychol Med 44:1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsvoort T, Daly E, Robertson D, Suckling J, Ng V, Critchley H et al. (2001) Structural brain abnormalities associated with deletion at chromosome 22q11: quantitative neuroimaging study of adults with velo-cardio-facial syndrome. Br J Psychiatry 178:412–419 [DOI] [PubMed] [Google Scholar]
- Van Aken K, De Smedt B, Van Roie A, Gewillig M, Devriendt K, Fryns JP et al. (2007) Motor development in school-aged children with 22q11 deletion (velocardiofacial/DiGeorge syndrome). Dev Med Child Neurol 49(3):210–213 [DOI] [PubMed] [Google Scholar]
- Van Aken K, Swillen A, Beirinckx M, Janssens L, Caeyenberghs K, Smits-Engelsman B (2010) Prospective control abilities during visuo-manual tracking in children with 22q11.2 deletion syndrome compared to age- and IQ-matched controls. Res Dev Disabil 31(3):634–641 [DOI] [PubMed] [Google Scholar]
- Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E (2001) Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet Med 3(1):34–39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


