Key Points
Question
What are the contemporary outcomes of SAPIEN 3 (Edwards Lifesciences) transcatheter mitral valve-in-valve (MViV) replacement?
Findings
In this cohort study of 1529 high-risk patients who received SAPIEN 3 MViV, procedural technical success was 96.8% and all-cause mortality was 5.4% at 30 days and 16.7% at 1 year. Transseptal access was associated with lower 1-year all-cause mortality than transapical access
Meaning
In this study, the transseptal SAPIEN 3 MViV was associated with rare procedural complications and low mortality and should be considered an option for most patients with failed surgical bioprosthetic mitral valves.
Abstract
Importance
Bioprosthetic mitral valves are implanted with increasing frequency but inevitably degenerate, leading to heart failure. Reoperation is associated with high morbidity and mortality. Transcatheter mitral valve-in-valve (MViV) using balloon-expandable transcatheter valves has emerged as an alternative for high–surgical risk patients.
Objective
To assess contemporary outcomes of SAPIEN 3 (Edwards Lifesciences) MViV replacement.
Design, Setting, and Participants
In this registry-based prospective cohort study of SAPIEN 3 MViV, patients entered in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry from June 2015 to July 2019 were analyzed. US Centers for Medicare and Medicaid linkage ensured comprehensive collection of death and stroke data.
Exposures
Mitral valve-in-valve for degenerated bioprosthetic mitral valves.
Main Outcomes and Measures
The primary efficacy end point was 1-year mortality. The primary safety end point was procedural technical success as defined by the Mitral Valve Academic Research Consortium criteria. Secondary end points included 30-day mortality, New York Heart Association–defined heart failure, and mitral valve performance.
Results
A total of 1529 patients (mean [SD] age, 73.3 [11.84] years; 904 women [59.1%]) underwent transseptal or transapical MViV implant at 295 hospitals between June 2015 and July 2019. The mean (SD) Society of Thoracic Surgeons predicted risk of mortality was 11.1% (8.7%). Procedural technical success was achieved for 1480 of 1529 patients (96.8%). All-cause mortality was 5.4% at 30 days and 16.7% at 1 year. Transseptal access was associated with lower 1-year all-cause mortality than transapical access (15.8% vs 21.7%; P = .03). Transcatheter MViV led to early, sustained, and clinically meaningful improvements in heart failure (class III/IV New York Heart Association heart failure of 87.1% at baseline vs 9.7% at 1 year). The mean (SD) mitral valve gradient at 1 year was 7 (2.89) mm Hg.
Conclusions and Relevance
Transcatheter MViV using the SAPIEN 3 transcatheter heart valve is associated with high technical success, low 30-day and 1-year mortality, significant improvement of heart failure symptoms, and sustained valve performance. Transseptal MViV should be considered an option for most patients with failed surgical bioprosthetic valves and favorable anatomy.
This cohort study assesses contemporary outcomes of mitral valve-in-valve replacement with the SAPIEN 3 transcatheter heart valve.
Introduction
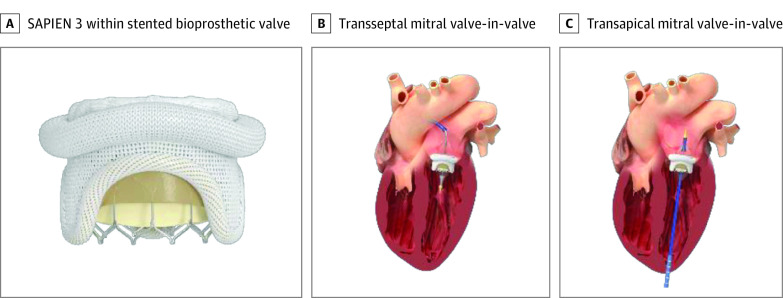
More than 20 000 mitral valve replacements are performed each year in the US, most of which are bioprosthetic with limited durability.1,2 Reoperation of failed bioprosthetic mitral valves is associated with significant morbidity and mortality.3,4,5 Surgical mitral bioprosthetic valves are radiopaque, stented, and circular and therefore ideal receptacles for balloon expandable transcatheter heart valves (THVs) (Figure 1). Transcatheter mitral valve-in-valve (MViV) replacement was explored early in the development of THVs with transseptal (TS) and transapical (TA) approaches.6,7,8,9,10,11 Transseptal MViV involves transesophageal echo-guided TS puncture and over-the-wire delivery of the THV through an expandable 14F or 16F sheath in the femoral vein.12 Transapical access includes surgical exposure, access, and closure of the left ventricular apex.
Figure 1. SAPIEN 3 Transcatheter Heart Valve, Transseptal, and Transapical Access Approaches.
A, SAPIEN 3 (Edwards Lifesciences) within the stented bioprosthetic valve. B, Transseptal mitral valve-in-valve. C, Transapical mitral valve-in-valve.
The US Centers for Medicare & Medicaid Services (CMS) provides reimbursement for patients undergoing transcatheter aortic valve replacement receiving a US Food and Drug Administration (FDA)–approved THV provided that hospitals participate in a prospective, nationally audited registry that enrolls consecutive patients and tracks outcomes. The Society of Thoracic Surgeons (STS) and the American College of Cardiology (ACC) developed the STS/ACC Transcatheter Valve Therapy (TVT) Registry with input from the FDA, CMS, and THV manufacturers. The TVT Registry began collecting MViV, mitral valve-in-ring (MViR), and valve in mitral annular calcification (ViMAC) procedures in July 2014. The 2016 and 2020 MViV/MViR/ViMAC TVT Registry published reports demonstrated high rates of procedure success and favorable mortality compared with the STS predicted risk of operative mortality (PROM) but were limited by high representation of the early clinical experience with several iterative generations of transcatheter valves and frequent TA access.13,14
The SAPIEN 3 valve (Edwards Lifesciences) is a low-profile, balloon-expandable, bovine pericardial valve mounted in a chromium cobalt frame and was approved by the FDA for commercial sale in June 2015. With availability of the low-profile SAPIEN 3 valve and growing physician experience with TS procedures, TS SAPIEN 3 MViV has become the predominant MViV procedure and is associated with high procedural success and lower 30-day mortality than predicted by the STS score.15 This current TVT Registry–based study was designed to explore contemporary MViV outcomes with the SAPIEN 3 valve and the evolution of TA to TS access.
Methods
Participating centers used standardized definitions to collect clinical information, including patient demographic characteristics, comorbidities, functional status, quality of life indexes, procedural details, and patient outcomes from consecutive MViV cases using commercially approved devices. The registry protocol was granted a waiver of informed consent by Advarra and the Duke University institutional review boards. Data were obtained from the registry for all 2144 patients undergoing transcatheter mitral valve replacement with third-generation balloon-expandable SAPIEN 3 THVs between commercial approval for transcatheter aortic valve replacement in June 2015 and July 2019. After excluding patients undergoing MViR, ViMAC, and transatrial MViV implant and those with insufficient data for classification, a cohort of 1529 patients (1326 undergoing TS [86.7%] and 203 undergoing TA [13.3%]) was available for the primary analysis (eFigure 1 in the Supplement).
Not all patients have reached the 1-year end point as of this article’s publication. In addition, clinical follow-up is not always available through the TVT registry for patients who were not followed up at the index hospital performing the MViV procedure. To overcome these limitations and ensure comprehensive collection of adverse events, patient survival and stroke rates were determined with linked CMS administrative claims data irrespective of patients following up at the hospital where the MViV procedure was performed (eFigure 2 in the Supplement). The TVT Registry follow-up data collected through mid-July 2019 and CMS claims data available through December 31, 2018, were used in the analysis. Univariate and multivariable analyses were conducted to determine predictors of procedural and 1-year mortality. The analyses were performed by Edwards Lifesciences on data from the TVT Registry and the authors had control of data analysis as well as the contents of this article.
Objectives and End Points
The primary objective of the study was to assess contemporary outcomes of MViV using the SAPIEN 3 THV. The primary efficacy end point was all-cause mortality at 1 year. The primary safety end point was procedural technical success defined per Mitral Valve Academic Research Consortium (MVARC) criteria at exit from the hybrid suite as patient alive with successful access, delivery, and retrieval of the device delivery system, successful deployment and correct position of the first intended device, and freedom from emergency surgery or reintervention associated with the device or access procedure.16 Secondary end points included procedural and in-hospital outcomes, New York Heart Association (NYHA) class, quality of life as defined by the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ), adverse events at 30 days and 1 year, and predictors of 1-year mortality. All adverse outcomes were defined using MVARC definitions.16
Statistical Analysis
Continuous variables were presented as mean (SD) or median (interquartile range [IQR]) and were compared between groups using the 2-sample t test or Wilcoxon rank sum test. Categorical variables were given as frequencies and percentages and were compared using the χ2 or Fisher exact test. The 30-day and 1-year adverse event rates were based on Kaplan-Meier estimates and all comparisons were made using the log-rank test. A multivariable analysis was also performed on TS and TA groups to determine predictors of 1-year all-cause mortality. Stepwise selection method of significant predictors was performed using enter and exit criterion of P = .10. The candidate covariates were endocarditis, permanent pacemaker, prior percutaneous coronary intervention, prior coronary artery bypass grafting, peripheral artery disease, diabetes, currently receiving dialysis, chronic obstructive pulmonary disease, immunocompromise present, prior myocardial infarction, heart failure within 2 weeks, NYHA class within 2 weeks, cardiogenic shock within 24 hours, atrial fibrillation/flutter, left ventricular ejection fraction, mitral insufficiency, tricuspid insufficiency, cardiopulmonary bypass, procedure duration, fluoroscopy time, device success, perforation with or without tamponade, conversion to open heart surgery, baseline KCCQ overall summary score, baseline glomerular filtration rate, creatinine levels, hemoglobin levels, and TS vs TA was forced into the model. Missing baseline characteristic values are also provided in eTable 1 in the Supplement. All statistical analyses were performed using SAS, version 9.4 (SAS Institute), and statistical significance was set at a 2-sided P < .05 without multiplicity adjustment.
Results
Registry Population and Baseline Characteristics
A total of 2144 patients underwent SAPIEN 3 TMVR procedures between June 2015 and July 2019 at 308 institutions in the US. After excluding those with MViR, ViMAC, and transatrial MViV implant and patients with insufficient data for classification, 1529 patients (1326 undergoing TS [86.7%] and 203 undergoing TA [13.3%]) underwent SAPIEN 3 MViV at 295 sites and were included in the current analysis (eFigure 1 in Supplement). A total of 26 SAPIEN 3 MViV implants were implanted at 14 sites during the second half of 2015. Participation has increased steadily with 630 cases (558 TS [88.6%], 61 TA [9.7%], 11 other cases [1.7%]) performed at 225 sites in 2018.
Baseline demographic and echocardiographic characteristics are presented in Table 1. The mean (SD) patient age was 73.3 (11.8) years, and the mean (SD) STS PROM for surgical reoperation was 11.1% (8.7%). Mitral stenosis (784 [55.4%]) was more common than mitral regurgitation (351 [24.8%]) and mixed disease (280 [19.8%]). The presence of paravalvular mitral regurgitation is not captured in the TVT registry. Moderate to severe tricuspid regurgitation was present in 848 patients (55.7%). Prior coronary artery bypass surgery was more common among patients undergoing TA than TS (41.4% vs 33.4%; P = .03). Otherwise, there were no significant demographic or echocardiographic differences between patients undergoing TS and TA.
Table 1. Baseline Clinical and Echocardiography Characteristics.
| Characteristic | No./total No. (%) of patients | P value | ||
|---|---|---|---|---|
| Transseptal (n = 1326) | Transapical (n = 203) | Combined (N = 1529) | ||
| Clinical characteristic | ||||
| Age, mean (SD), y | 73.4 (11.86) | 72.6 (11.66) | 73.3 (11.84) | .36 |
| Women | 785/1326 (59.2) | 119/203 (58.6) | 904/1529 (59.1) | .88 |
| NYHA class | ||||
| I | 18/1308 (1.4) | 2/202 (1.0) | 20/1510 (1.3) | >.99 |
| II | 159/1308 (12.2) | 16/202 (7.9) | 175/1510 (11.6) | .08 |
| III | 735/1308 (56.2) | 114/202 (56.4) | 849/1510 (56.2) | .95 |
| IV | 396/1308 (30.3) | 70/202 (34.7) | 466/1510 (30.9) | .21 |
| Atrial fibrillation | 952/1325 (71.8) | 130/203 (64) | 1082/1528 (70.8) | .02 |
| Prior stroke | 232/1325 (17.5) | 31/202 (15.3) | 263/1527 (17.2) | .45 |
| COPD | 607/1314 (46.2) | 95/202 (47) | 702/1516 (46.3) | .82 |
| Currently receiving dialysis | 70/1325 (5.3) | 12/203 (5.9) | 82/1528 (5.4) | .71 |
| Prior CABG | 442/1322 (33.4) | 84/203 (41.4) | 526/1525 (34.5) | .03 |
| Prior AVP | 315/1325 (23.8) | 49/203 (24.1) | 364/1528 (23.8) | .91 |
| Hostile chest | 223/1326 (16.8) | 45/203 (22.2) | 268/1529 (17.5) | .06 |
| STS score, mean (SD) | 11.0 (8.58) | 11.7 (9.46) | 11.1 (8.70) | .30 |
| STS, ≤4% | 157/1256 (12.5) | 30/192 (15.6) | 187/1448 (12.9) | .25 |
| 4% < STS ≤ 8% | 407/1256 (32.4) | 49/192 (25.5) | 456/1448 (31.5) | .07 |
| STS, >8% | 69/1256 (55.1) | 113/192 (58.9) | 805/1448 (55.6) | .35 |
| Echocardiography characteristic | ||||
| LV ejection fraction, mean (SD), % | 54.9 (12.14) | 54.1 (11.51) | 54.8 (12.06) | .36 |
| Mean MVG, mean (SD), mm Hg | 12.6 (5.48) | 13.3 (5.35) | 12.7 (5.47) | .08 |
| Tricuspid insufficiency (moderate-severe) | 734/1320 (55.6) | 114/203 (56.2) | 848/1523 (55.7) | .88 |
| Primary MV pathology | ||||
| Stenosis | 682/1226 (55.6) | 102/189 (54.0) | 784/1415 (55.4) | .65 |
| Regurgitation | 306/1226 (25.0) | 45/189 (23.8) | 351/1415 (24.8) | .79 |
| MS and MR | 238/1226 (19.4) | 42/189 (22.2) | 280/1415 (19.8) | .38 |
Abbreviations: AVP, aortic valve procedure; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LV, left ventricular; MR, mitral regurgitation; MS, mitral stenosis; MV, mitral valve; MVG, mitral valve gradient; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
Procedure and In-Hospital Outcomes
Commonly implanted SAPIEN 3 valves included the 29-mm (774 [50.6%]) and 26-mm (633 [41.4%]) sizes, while 23-mm (119 [7.8%]) and 20-mm (3 [0.2%]) valves were implanted infrequently. Iatrogenic atrial septal defects were closed in 101 of 1326 TS procedures (7.6%) and the mean (SD) procedure duration was 127 (65.8) minutes.
The primary safety end point of technical success defined per MVARC criteria at exit from the hybrid suite was achieved for 1480 patients (96.8%; 97.1% TS vs 94.6% TA; P = .08). Procedural complications were rare; included stroke (10 [0.7%]), device embolization (0.3%), LVOT obstruction (0.9%), and cardiac perforation (1.1%); and were similar with TS and TA access. In-hospital all-cause mortality was observed in 61 of 1529 patients (4%; 3.6% TS, 6.4% TA; P = .06). In-hospital cardiovascular mortality was observed in 33 of 529 patients (2.2%) and was lower with TS access compared with TA access (1.8% vs 4.4%; P = .03). Length of stay (median [IQR] 2 [1-5] vs 6 [3-9] days, P < .001) and discharge to home (82.5% vs 59.1%; P < .001) favored the TS approach. Most patients (1161 [80.8%]) were discharged with oral anticoagulants. The median number of TS SAPIEN 3 MViV procedures performed per site during the analysis period was 3 (IQR, 1-6) and the median number of TA procedures was 1 (IQR, 1-3) (Table 2; eFigure 3 in Supplement).
Table 2. Procedural and In-Hospital Outcomes.
| Outcomes | No./total No. (%) of patients | P value | ||
|---|---|---|---|---|
| Transseptal (n = 1326) | Transapical (n = 203) | Combined (N = 1529) | ||
| Procedural outcomes | ||||
| Implant volume per site, median (IQR) | 3 (1-6) | 1 (1-3) | 3 (1-7) | NA |
| MVARC technical successa | 1288/1326 (97.1) | 192/203 (94.6) | 1480/1529 (96.8) | .08 |
| SAPIEN 3 implanted | ||||
| 20-mm | 3/1326 (0.2) | 0/203 (0) | 3/1529 (0.2) | >.99 |
| 23-mm | 101/1326 (7.6) | 18/203 (8.9) | 119/1529 (7.8) | .54 |
| 26-mm | 553/1326 (41.7) | 80/203 (39.4) | 633/1529 (41.4) | .54 |
| 29-mm | 669/1326 (50.5) | 105/203 (51.7) | 774/1529 (50.6) | .74 |
| Procedural time, mean (SD), min | 125.8 (64.3) | 138.4 (73.9) | 127.4 (65.8) | .02 |
| Fluoroscopy time, mean (SD), min | 37 (25.7) | 18.2 (13.0) | 34.6 (25.2) | <.001 |
| Procedure aborted | 7/1326 (0.5) | 1/203 (0.5) | 8/1529 (0.5) | >.99 |
| Device embolization | 3/1326 (0.2) | 1/203 (0.5) | 4/1529 (0.3) | .43 |
| Cardiac perforation | 14/1326 (1.1) | 3/203 (1.5) | 17/1529 (1.1) | .48 |
| Conversion to open surgery | 9/1326 (0.7) | 5/203 (2.5) | 14/1529 (0.9) | .03 |
| Need for second valve | 0/1326 (0) | 0/203 (0) | 0/1529 (0) | NA |
| ASD closure | 101/1326 (7.6) | 0/203 (0) | 101/1529 (6.6) | NA |
| Anticoagulation at discharge | 1027/1255 (81.8) | 134/182 (73.6) | 1161/1437 (80.8) | .01 |
| In-hospital outcomes | ||||
| All-cause mortality | 48/1326 (3.6) | 13/203 (6.4) | 61/1529 (4.0) | .06 |
| Cardiovascular death | 24/1326 (1.8) | 9/203 (4.4) | 33/1529 (2.2) | .03 |
| Stroke | 9/1326 (0.7) | 1/203 (0.5) | 10/1529 (0.7) | >.99 |
| Mitral valve reintervention | 4/1326 (0.3) | 1/203 (0.5) | 5/1529 (0.3) | .51 |
| LVOT obstruction | 10/1326 (0.8) | 4/203 (2) | 14/1529 (0.9) | .10 |
| New pacemaker | 15/1326 (1.1) | 4/203 (2.0) | 19/1529 (1.2) | .30 |
| Periprocedural MI | 4/1326 (0.3) | 1/203 (0.5) | 5/1529 (0.3) | .51 |
| Device thrombosis | 2/1326 (0.2) | 1/203 (0.5) | 3/1529 (0.2) | .35 |
| Major vascular complications | 16/1326 (1.2) | 5/203 (2.5) | 21/1529 (1.4) | .18 |
| Length of stay, median (IQR) | 2 (1-5) | 6 (3-9) | 3.0 (2.0-6.0) | <.001 |
| Discharged home | 1094/1326 (82.5) | 120/203 (59.1) | 1214/1529 (79.4) | <.001 |
Abbreviations: ASD, atrial septal defect; IQR, interquartile range; LVOT, left ventricular outflow tract; MI, myocardial infarction; MVARC, mitral valve academic research consortium; NA, not applicable.
MVARC technical success was defined as at exit from the hybrid suite, patient is alive with successful access, delivery, and retrieval of the device delivery system, successful deployment and correct position of the first intended device, and freedom from emergency surgery or reintervention associated with the device or access procedure.
Thirty-Day and 1-Year Outcomes
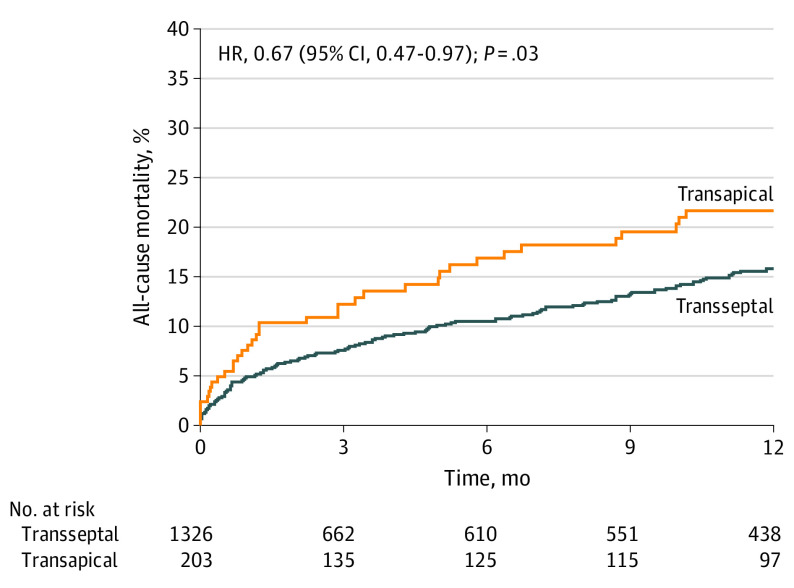
Outcomes at 30 days and 1 year are shown in Table 3 and Kaplan-Meier survival curves for the TA and TS cohorts are shown in Figure 2. The primary efficacy end point, 1-year all-cause mortality, was 16.7%. At 30 days, all-cause mortality was 5.4%, which represents an observed to expected ratio of 0.49 compared with surgical reoperation. Cardiovascular mortality at 30 days and 1 year was 2.5% and 3.9%, respectively. Transseptal access was associated with lower 1-year all-cause mortality than TA access (15.8% vs 21.7%; P = .03) and a trend toward lower mortality at 30 days (TS, 5.0% vs TA, 8.1%; P = .07). Transseptal and TA access demonstrated favorable observed to expected ratios (TS, 0.45; TA, 0.69).
Table 3. Thirty-Day and 1-Year Outcomesa.
| Outcome | No./total No. (%) of patients | P value | ||
|---|---|---|---|---|
| Transseptal (n = 1326) | Transapical (n = 203) | Combined (N = 1529) | ||
| 30-d Outcomes | ||||
| All-cause mortality | 62 (5.0) | 16 (8.1) | 78 (5.4) | .07 |
| Cardiovascular death | 26 (2.1) | 10 (5.1) | 36 (2.5) | .01 |
| Observed:expected ratio | 0.45 | 0.69 | 0.49 | NA |
| Stroke | 14 (1.1) | 2 (1) | 16 (1.1) | .91 |
| Mitral valve reintervention | 5 (0.4) | 1 (0.5) | 6 (0.4) | .82 |
| New dialysis requirement | 18 (1.5) | 6 (3.1) | 24 (1.7) | .10 |
| New pacemaker | 17 (1.4) | 4 (2.0) | 21 (1.4) | .44 |
| Device thrombosis | 3 (0.2) | 1 (0.5) | 4 (0.3) | .49 |
| LV ejection fraction, mean (SD), % | 54.2 (11.73) | 52.7 (12.55) | 54.0 (11.84) | .17 |
| Mean MVG, mean (SD), mm Hg | 7.4 (2.74) | 7.2 (2.69) | 7.3 (2.73) | .50 |
| 30-d KCCQ improvement, mean (SD) | 35.3 (27.13) | 37.4 (25.17) | 35.5 (26.90) | .47 |
| 30-d NYHA class | ||||
| I | 385/863 (44.6) | 58/131 (44.3) | 443/994 (44.6) | .94 |
| II | 356/863 (41.3) | 55/131 (42.0) | 411/994 (41.3) | .87 |
| III | 106/863 (12.3) | 13/131 (9.9) | 119/994 (12.0) | .44 |
| IV | 16/863 (1.9) | 5/131 (3.8) | 21/994 (2.1) | .18 |
| 1-y Outcomes | ||||
| All-cause mortality | 138 (15.8) | 37 (21.7) | 175 (16.7) | .03 |
| All-cause mortality No. at risk | 438 | 97 | 535 | NA |
| Cardiovascular death | 36 (3.7) | 11 (5.7) | 47 (3.9) | .07 |
| Stroke | 27 (3.3) | 5 (3.5) | 32 (3.3) | .95 |
| Mitral valve reintervention | 8 (0.8) | 1 (0.5) | 9 (0.8) | .78 |
| New dialysis requirement | 19 (1.6) | 6 (3.1) | 25 (1.8) | .13 |
| New pacemaker | 21 (2.0) | 5 (2.8) | 26 (2.1) | .44 |
| Device thrombosis | 4 (0.3) | 2 (1.2) | 6 (0.5) | .17 |
| LV ejection fraction, mean (SD), % | 53.3 (11.52) | 52.8 (13.11) | 53.2 (11.76) | .77 |
| Mean MVG, mean (SD), mm Hg | 7.0 (2.94) | 7.0 (2.61) | 7.0 (2.89) | .99 |
| 1-y KCCQ Improvement, mean (SD) | 40.2 (27.26) | 35.3 (26.37) | 39.4 (27.14) | .27 |
| 1-y NYHA class | ||||
| I | 143/290 (49.3) | 30/62 (48.4) | 173/352 (49.1) | .89 |
| II | 119/290 (41.0) | 26/62 (41.9) | 145/352 (41.2) | .90 |
| III | 23/290 (7.9) | 5/62 (8.1) | 28/352 (8.0) | >.99 |
| IV | 5/290 (1.7) | 1/62 (1.6) | 6/352 (1.7) | >.99 |
Abbreviations: LV, left ventricular; KCCQ, Kansas City Cardiomyopathy Questionnaire; MVG, mitral valve gradient; NYHA, New York Heart Association.
Event rates were calculated by Kaplan-Meier methods.
Figure 2. Time-to-Event Curves for All-Cause Mortality.
Kaplan-Meier estimates of the rate of all-cause mortality to 1 year in patients who underwent mitral valve-in-valve via a transseptal or transapical approach. HR indicates hazard ratio.
Transcatheter MViV led to early, sustained, and clinically meaningful improvements in heart failure and quality of life (Table 3; eFigure 4 in the Supplement). While 1315 patients (87.1%) demonstrated NYHA class III or IV heart failure at baseline, 318 patients (90.3%) demonstrated class I or II NYHA heart failure at 1 year without significant differences between TS and TA access. The KCCQ-defined quality of life improved markedly from baseline to 30 days (35.5 points), with continued improvement to 1 year (39.4 points), which was similar in both groups. Predictors of 1-year all-cause mortality are shown in eTable 2 in the Supplement. Predictors of 1-year mortality by multivariable analysis include TS vs TA approach (hazard ratio [HR], 0.58; 95% CI, 0.37-0.90; P = .01), cardiogenic shock within 24 hours (HR, 2.28; 95% CI, 1.14-4.57; P = .02), and moderate or severe tricuspid regurgitation (HR, 1.81; 95% CI, 1.16-2.84; P = .01). Left ventricular perforation with or without tamponade was associated with an HR of 70.6 (95% CI, 28.51-174.7; P < .001) of 1-year mortality in multivariable analysis. In patients with an STS PROM of less than 4%, the observed 1-year mortality was 4.0%. In patients with an STS PROM between 4% and 8%, the 1-year mortality was 9.5%; in patients with an STS PROM of greater than 8, it was 22.3% (eFigure 5 in Supplement).
The mean (SD) mitral valve gradient was 7.3 (2.73) mm Hg at 30 days and 7.0 (2.89) mm Hg at 1 year (Table 3). At 1 year, patients who received 26-mm or 29-mm SAPIEN 3 THVs had a mean (SD) gradient of 6.9 mm Hg while patients who received 20-mm or 23-mm SAPIEN 3 THVs had a mean gradient of 8.7 mm Hg (P = .003). One-year mortality was lower among patients receiving 26-mm or 29-mm valves than 20-mm or 23-mm valves (15.6% vs 28.9%; P < .001) (eFigure 6 in the Supplement). Device thrombosis at 1 year was rare (0.5%; Table 3). Among the 14 patients who developed in-hospital LVOT obstruction, 3 died within 30 days and 4 within 1 year.
Discussion
Transcatheter MViV using the SAPIEN 3 THV was associated with high technical success, few complications, and 30-day mortality rates markedly lower than predicted by the STS score. Most patients experienced clinically important improvement in heart failure symptoms and quality of life by 30 days that were maintained at 1 year. Transseptal access was associated with lower mortality compared with TA access and was an independent predictor of lower mortality at 1 year. All-cause and cardiovascular mortality at all intervals were lower in this analysis than prior registries, possibly because of the preponderance of TS access in this contemporary analysis. To our knowledge, this is the first registry to demonstrate the superiority of TS compared with TA access, perhaps because of a smaller TS sample size in prior studies.17
With the 1326 transseptal procedures divided between 268 centers yielding a median (IQR) implant volume of only 3 (1-6) implants per enrolling site, outstanding procedural success (97.1%) and low in-hospital cardiovascular mortality (1.8%) demonstrate the safety of TS MViV even early in the procedure’s learning curve. Nevertheless, a longitudinal analysis has demonstrated lower 30-day mortality with each successive year since 2015 (eFigure 7 in the Supplement). A major inflection point roughly coincides with the FDA approval of the SAPIEN 3 valve and a transition from TA to TS procedures, followed by continually improving outcomes after 2015 consistent with increased MViV experience and increasing conversion to TS access.
The observed mean mitral valve gradients were modestly higher than historical observations of newly implanted surgical mitral bioprosthetic valves but lower than thresholds of prosthesis patient mismatch18; they were also similar to other registries of transcatheter MViV.15,17 Smaller (20-mm and 23-mm) SAPIEN 3 THVs were associated with increased gradients and increased mortality; however, patients receiving 20-mm or 23-mm valves had more comorbidities at baseline (eTable 3 in the Supplement). Valve performance was maintained at 1 year and valve thrombosis was rare. As oral anticoagulation has been recommended based on observations of MViV associated thrombosis,15,19 and many patients were already receiving anticoagulation because of underlying atrial fibrillation, most patients in this registry (80.8%) were discharged with oral anticoagulants. The choice of oral anticoagulant, targeted or achieved level of anticoagulation, or use of anticoagulation during follow-up are not captured in the TVT Registry. In the absence of MViV anticoagulation guidelines, physicians may consider the guidelines for surgical bioprosthetic valves, which suggest anticoagulation with a vitamin K antagonist to achieve an International Normalized Ratio of 2.5 is reasonable for at least 3 months and for as long as 6 months after surgical bioprosthetic mitral valve repair in patients at low risk of bleeding.20 Additional studies are indicated to define the long-term durability of transcatheter mitral valves and the role of anticoagulants, including direct oral anticoagulants, to prevent MViV thrombosis. Anticoagulation should be discussed with patients considering MViV replacement. Additional studies are also indicated to define the risks of late MViV thrombosis, the association of mitral THV gradients with longevity, and the potential benefit of mitral bioprosthesis fracture to optimize THV gradients.21
Ventricular perforation is a largely preventable procedure-associated adverse event associated with mortality. Ventricular perforation has been observed when wires with an abrupt stiff-to-floppy transition kink, resulting in a sharp leading edge. With 17 perforation events, this was a rare event that decreased over time consistent with improved wire selection and training.
Left ventricular outflow tract (LVOT) obstruction was observed in 14 MViV procedures (0.9%); 3 individuals died at 30 days. The use and implications of preprocedure computed tomographic (CT) planning is not reported in the TVT Registry while the definition and implications of LVOT obstruction are loosely defined. As LVOT obstruction is a preventable and potentially lethal complication, preprocedure CT imaging is emphasized.22 The treatment for patients at risk for LVOT obstruction based on cardiac CT analysis should be individualized based on operative risk, anatomy, and operator experience, with consideration for surgical replacement or LVOT obstruction risk-reduction strategies, such as alcohol septal ablation and radiofrequency laceration of the anterior prosthetic valve leaflet.23,24
Mitral valve-in-valve is currently indicated for patients with degenerated bioprosthetic mitral valves and a high risk for cardiovascular surgery. Consistent with this label, the mean (SD) age of patients treated with MViV (73.3 [11.6] years) is older and the predicted operative risk (STS PROM, 11.1; Table 1) is higher than patients undergoing reoperation with isolated mitral valve replacement in the STS database (age 64 years; STS PROM, 8).4 Despite treating an older and higher-risk population, 30-day mortality was lower with MViV than surgical reoperation.
Younger patients with degenerated mitral bioprosthetic valves can potentially undergo reoperation with a low risk of morbidity and mortality.25 However, each successive surgery is associated with markedly escalating morbidity and mortality.26 The choice of surgery vs MViV should anticipate future interventions with limited durability of bioprosthetic valves. As such, there is a clinical rationale to avoid reoperation in even younger patients with a low operative risk. Mechanical mitral valves may be appropriate for some patients. This study suggests that MViV is an appropriate option for most patients with degenerated mitral valves and favorable anatomy for whom a second bioprosthetic valve is being considered regardless of age and surgical risk.
Limitations
The study has several limitations, including the inherent limitations of an observational study with limited independent adjudication of adverse events and potential underreporting of adverse events. There was no independent echocardiographic core laboratory and the STS/ACC TVT Registry database does not include a standard definition of LVOT obstruction. The true incidence of prosthesis dysfunction could be underestimated. Relative few TA procedures (n = 203) were compared with more TS procedures (n = 1326), which were performed more often once the technique and technology matured. This observational registry study includes complete quarterly data for all sites extracted through May 2019 and full quarterly data after May 2019 may not include all sites.
Conclusions
Transcatheter MViV using the SAPIEN 3 is associated with high technical success, a low complication rate, and 30-day mortality rates lower than predicted by the STS score. Most patients experienced significant improvement of symptoms and quality of life that were maintained at 1 year. Valve performance was maintained at 1 year. Transseptal access was associated with lower mortality compared with TA access and was an independent predictor of lower mortality at 1 year. Transseptal MViV should be considered an option for most patients with failed surgical bioprosthesis who have favorable anatomy electing bioprosthetic valve replacement.
eTable 1. Missing baseline characteristic values
eTable 2. Predictors of 1-year all-cause mortality
eTable 3. Baseline characteristics of patients who received 20mm/23mm valves and 26mm/29mm valves
eFigure 1. Study flowchart of patients who underwent SAPIEN 2 TMViV procedures
eFigure 2. Patient status
eFigure 3. Number of SAPIEN 3 MViV cases and sites by year
eFigure 4. Quality of life outcomes
eFigure 5. Cumulative event rate of all-cause mortality at 1 year in patients with MViV stratified by STS score
eFigure 6. Mitral mean gradient and all-cause mortality by valve size to 1 year
eFigure 7. 30-day mortality by year
References
- 1.Vemulapalli S, Grau-Sepulveda M, Habib R, Thourani V, Bavaria J, Badhwar V. Patient and hospital characteristics of mitral valve surgery in the United States. JAMA Cardiol. 2019;4(11):1149-1155. doi: 10.1001/jamacardio.2019.3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thourani VH, Weintraub WS, Guyton RA, et al. Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement: effect of age and concomitant coronary artery bypass grafting. Circulation. 2003;108(3):298-304. doi: 10.1161/01.CIR.0000079169.15862.13 [DOI] [PubMed] [Google Scholar]
- 3.Mehaffey HJ, Hawkins RB, Schubert S, et al. Contemporary outcomes in reoperative mitral valve surgery. Heart. 2018;104(8):652-656. doi: 10.1136/heartjnl-2017-312047 [DOI] [PubMed] [Google Scholar]
- 4.Kilic A, Acker MA, Gleason TG, et al. Clinical outcomes of mitral valve reoperations in the United States: an analysis of the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2019;107(3):754-759. doi: 10.1016/j.athoracsur.2018.08.083 [DOI] [PubMed] [Google Scholar]
- 5.Jamieson WR, Burr LH, Miyagishima RT, et al. Reoperation for bioprosthetic mitral structural failure: risk assessment. Circulation. 2003;108(suppl 1):II98-II102. doi: 10.1161/01.cir.0000089184.46999.f4 [DOI] [PubMed] [Google Scholar]
- 6.Walther T, Falk V, Dewey T, et al. Valve-in-a-valve concept for transcatheter minimally invasive repeat xenograft implantation. J Am Coll Cardiol. 2007;50(1):56-60. doi: 10.1016/j.jacc.2007.03.030 [DOI] [PubMed] [Google Scholar]
- 7.Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121(16):1848-1857. doi: 10.1161/CIRCULATIONAHA.109.924613 [DOI] [PubMed] [Google Scholar]
- 8.Cheung A, Webb JG, Barbanti M, et al. 5-year experience with transcatheter transapical mitral valve-in-valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol. 2013;61(17):1759-1766. doi: 10.1016/j.jacc.2013.01.058 [DOI] [PubMed] [Google Scholar]
- 9.Yoon SH, Whisenant BK, Bleiziffer S, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol. 2017;70(9):1121-1131. doi: 10.1016/j.jacc.2017.07.714 [DOI] [PubMed] [Google Scholar]
- 10.Michelena HI, Alli O, Cabalka AK, Rihal CS. Successful percutaneous transvenous antegrade mitral valve-in-valve implantation. Catheter Cardiovasc Interv. 2013;81(5):E219-E224. doi: 10.1002/ccd.24423 [DOI] [PubMed] [Google Scholar]
- 11.Cullen MW, Cabalka AK, Alli OO, et al. Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: a case series in children and adults. JACC Cardiovasc Interv. 2013;6(6):598-605. doi: 10.1016/j.jcin.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 12.Guerrero M, Salinger M, Pursnani A, et al. Transseptal transcatheter mitral valve-in-valve: a step by step guide from preprocedural planning to postprocedural care. Catheter Cardiovasc Interv. 2018;92(3):E185-E196. doi: 10.1002/ccd.27128 [DOI] [PubMed] [Google Scholar]
- 13.Grover FL, Vemulapalli S, Carroll JD, et al. ; STS/ACC TVT Registry . 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69(10):1215-1230. doi: 10.1016/j.jacc.2016.11.033 [DOI] [PubMed] [Google Scholar]
- 14.Guerrero M, Vemulapalli S, Xiang Q, et al. Thirty-day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (valve-in-valve), failed surgical rings (valve-in-ring), and native valve with severe mitral annular calcification (valve-in-mitral annular calcification) in the United States: data from the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13(3):e008425. doi: 10.1161/CIRCINTERVENTIONS.119.008425 [DOI] [PubMed] [Google Scholar]
- 15.Eleid MF, Whisenant BK, Cabalka AK, et al. Early outcomes of percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2017;10(19):1932-1942. doi: 10.1016/j.jcin.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 16.Stone GW, Adams DH, Abraham WT, et al. ; Mitral Valve Academic Research Consortium (MVARC) . Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. J Am Coll Cardiol. 2015;66(3):308-321. doi: 10.1016/j.jacc.2015.05.049 [DOI] [PubMed] [Google Scholar]
- 17.Yoon SH, Whisenant BK, Bleiziffer S, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40(5):441-451. doi: 10.1093/eurheartj/ehy590 [DOI] [PubMed] [Google Scholar]
- 18.Blauwet LA, Malouf JF, Connolly HM, et al. Comprehensive echocardiographic assessment of normal mitral Medtronic Hancock II, Medtronic Mosaic, and Carpentier-Edwards Perimount bioprostheses early after implantation. J Am Soc Echocardiogr. 2010;23(6):656-666. doi: 10.1016/j.echo.2010.03.031 [DOI] [PubMed] [Google Scholar]
- 19.Whisenant B, Jones K, Miller D, Horton S, Miner E. Thrombosis following mitral and tricuspid valve-in-valve replacement. J Thorac Cardiovasc Surg. 2015;149(3):e26-e29. doi: 10.1016/j.jtcvs.2014.10.075 [DOI] [PubMed] [Google Scholar]
- 20.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252-289. doi: 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 21.Kamioka N, Corrigan F, Iturbe JM, et al. Mitral bioprosthetic valve fracture: bailout procedure for undersized bioprosthesis during mitral valve-in-valve procedure with paravalvular leak closure. JACC Cardiovasc Interv. 2018;11(3):e21-e22. doi: 10.1016/j.jcin.2017.10.047 [DOI] [PubMed] [Google Scholar]
- 22.Wang DD, Eng MH, Greenbaum AB, et al. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv. 2018;92(2):379-387. doi: 10.1002/ccd.27447 [DOI] [PubMed] [Google Scholar]
- 23.Wang DD, Guerrero M, Eng MH, et al. Alcohol septal ablation to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: first-in-man study. JACC Cardiovasc Interv. 2019;12(13):1268-1279. doi: 10.1016/j.jcin.2019.02.034 [DOI] [PubMed] [Google Scholar]
- 24.Khan JM, Babaliaros VC, Greenbaum AB, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73(20):2521-2534. doi: 10.1016/j.jacc.2019.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter DD, Sundt TM III, Zehr KJ, et al. Risk of repeat mitral valve replacement for failed mitral valve prostheses. Ann Thorac Surg. 2004;78(1):67-72. doi: 10.1016/j.athoracsur.2004.02.014 [DOI] [PubMed] [Google Scholar]
- 26.Expósito V, García-Camarero T, Bernal JM, et al. Repeat mitral valve replacement: 30-years’ experience. Rev Esp Cardiol. 2009;62(8):929-932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Missing baseline characteristic values
eTable 2. Predictors of 1-year all-cause mortality
eTable 3. Baseline characteristics of patients who received 20mm/23mm valves and 26mm/29mm valves
eFigure 1. Study flowchart of patients who underwent SAPIEN 2 TMViV procedures
eFigure 2. Patient status
eFigure 3. Number of SAPIEN 3 MViV cases and sites by year
eFigure 4. Quality of life outcomes
eFigure 5. Cumulative event rate of all-cause mortality at 1 year in patients with MViV stratified by STS score
eFigure 6. Mitral mean gradient and all-cause mortality by valve size to 1 year
eFigure 7. 30-day mortality by year