Abstract
The mechanistic target of the rapamycin (mTOR) pathway plays a role in features common to both excess salt/aldosterone and cardiovascular/renal diseases. Dietary sodium can upregulate mTORC1 signaling in cardiac and renal tissue, and the inhibition of mTOR can prevent aldosterone-associated, salt-induced hypertension. The impact of sex and age on mTOR’s role in volume homeostasis and the regulation of aldosterone secretion is largely unknown. We hypothesize that both age and sex modify mTOR’s interaction with volume homeostatic mechanisms. The activity of 3 volume homeostatic mechanisms—cardiovascular, renal, and hormonal (aldosterone [sodium retaining] and brain natriuretic peptide [BNP; sodium losing])—were assessed in mTORC1 deficient (Raptor +/-) and wild-type male and female littermates at 2 different ages. The mice were volume stressed by being given a liberal salt (LibS) diet. Raptor +/-mice of both sexes when they aged: (1) reduced their blood pressure, (2) increased left ventricular internal diameter during diastole, (3) decreased renal blood flow, and (4) increased mineralocorticoid receptor expression. Aldosterone levels did not differ by sex in young Raptor +/- mice. However, as they aged, compared to their littermates, aldosterone decreased in males but increased in females. Finally, given the level of Na+ intake, BNP was inappropriately suppressed, but only in Raptor +/- males. These data indicate that Raptor +/- mice, when stressed with a LibS diet, display inappropriate volume homeostatic responses, particularly with aging, and the mechanisms altered, differing by sex.
Keywords: mTOR, Raptor, aldosterone, salt intake, cardiovascular disease
A large body of data has documented that an inappropriate increase in aldosterone levels for the level of sodium intake are often associated with cardiovascular (CV) and/or renal diseases (particularly those associated with hypertension). For example, the salt/aldosterone combination has been reported to increase the risk of the development of hypertension even in individuals who are otherwise healthy (1–3). These data raise the question as which signaling pathways are affected by salt/aldosterone thereby leading to an increased risk of CV diseases (CVD). One candidate is the mechanistic target of rapamycin (mTOR).
mTOR is a serine/threonine kinase belonging to the phosphoinositide 3-kinase family. mTOR exerts its main cellular functions by forming 2 distinct complexes—mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2)—through the assembly with the specific adaptor proteins: regulatory-associated protein of mTOR (Raptor) for mTORC1 and rapamycin-insensitive companion of mTOR (Rictor) for mTORC2 (4–11). Raptor is necessary for substrate recruitment and for subcellular localization of mTORC1, and it is required for its kinase activity (12–15). mTORC1 promotes protein synthesis, cell proliferation, ribosomal and mitochondrial biogenesis, autophagy, and metabolism. Nutrients, growth factors, cellular energy, and oxygen levels are all upstream regulators of mTORC1 signaling (16).
Recently, our laboratory demonstrated that the mTOR signaling pathways are affected by changes in dietary Na+ and levels of aldosterone (17). Furthermore, we (and others) have shown an association between mTORC1 signaling and aldosterone in various tissues (18–23). Finally, inhibition of mTOR has been reported to prevent aldosterone-associated, salt-induced hypertension in humans and rodents (24). These results are of interest because the mTORC1 pathway plays a key role in many of the features common to both excess salt/aldosterone and CVD, such as the development of cardiac hypertrophy (25), endothelial and smooth muscle cell proliferation, altered nitric oxide synthesis, inflammation, and altered renal Na+ handling (26–29). These data suggest that mTOR, or other proteins involved in its pathway, may be potential therapeutic targets to treat hypertension, renal diseases, and/or CVD.
However, there are gaps in our knowledge concerning these relationships. For example, most studies noted above have only been performed in 1 sex (usually males) or the sex has not been specified (17, 24, 30–33). This is a critical issue since there are emerging reports of sex differences in mTOR signaling (21, 34). Furthermore, there are discrepancies in the literature concerning whether the observed decreased mTOR signaling with age is beneficial or not (35). Finally, some studies have suggested that the relationship between the level of mTOR signaling and its effect on an organism is tissue, substrate, age, or sex specific (34). This makes the interpretation of earlier mTOR reports difficult when considering mTOR as a potential therapeutic target for various CVD and renal diseases, especially for long-term treatment.
For these reasons, we addressed the hypothesis that both age and sex modify mTOR’s interaction with volume homeostatic mechanisms with a focus on the mTORC1 pathway. Therefore, we employed a mTORC1 deficiency model, Raptor +/- mice (as described in (36)). Both sexes were studied on a LibS (1.6% Na+) diet at 2 different ages. The activity of 3 volume homeostatic mechanisms were assessed: cardiovascular (cardiac and blood pressure), renal (renal blood flow), and hormonal (aldosterone [sodium retaining] and BNP [sodium losing]). Based on our previous data in male C57BL/6 mice (8), we hypothesized that a reduction in mTORC1 signaling on a LibS diet will be beneficial for the mice.
Methods
Study approval
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the guidelines of the Institutional Animal Care and Use Committee at Brigham and Women’s Hospital.
Animals
The mice were purchased from The Jackson Laboratory (B6.129S5-Rptortm1Lex/J Stock No: 013191). The mice were originally created by Guertin et al (36), and in this strain the allele replaced alternate exon 1 and common exon 2 of the Rptor gene with an internal ribosome entry site (IRES)-lacZ (β-galactosidase) and a neomycin resistance cassette, abolishing the gene function. Heterozygous Raptor male and female mice and wild-type (Wt) littermates were given an LibS diet (Purina 1.6% high Na+) for 7 days. Two groups of mice, differing by age, were studied: Study 1 was at ~12 weeks of age (n = 8/group) and Study 2 was at ~31 weeks of age (n = 9/group). The mice were housed in the animal facility in a 12-hour light/dark cycle at 22 ± 1°C ambient temperature and were allowed unlimited access to water.
Tissue collection at euthanasia
All mice were euthanized under isoflurane anesthesia (37) and hearts and kidneys were immediately excised. Blood was collected via submandibular puncture and hearts were excised and placed in liquid nitrogen in preparation for further analyses (37).
mRNA expression analysis
mRNA was extracted from heart tissue using the RNeasy mini kit (Qiagen, Germantown, Maryland). cDNA was synthesized from 1.5 μg RNA with the first-strand cDNA synthesis kit (GE Healthcare, Piscataway, New Jersey). Polymerase Chain Reaction (PCR) amplification reactions to detect Raptor, MR, BNP, ANP, and the housekeeping 18S ribosomal RNA were performed in duplicate using TaqMan gene expression assays (proprietary primers and probes designed and synthesized by Applied Biosystems, Foster City, California) using the ABI Prism 7000 sequence detection system (Applied Biosystems). The mRNA expression levels were normalized to 18S ribosomal RNA levels and were determined using the ΔΔCT method. The data were presented as a fold increase relative to the measurements in LibS Wt mice.
Western blot analysis
Protein from the heart tissue was extracted using RIPA buffer (Boston BioProduct, Ashland, Massachusetts) and protein concentration was determined using the BCA assay (Thermoscientific, Waltham, Massachusetts). Proteins were run on 4–12% acrylamide mini-protein TGX stain-free gels (BioRad, California) and then transferred to nitrocellulose membranes, followed by incubating the membranes in 5% nonfat dried milk in Tris-buffered saline Tween for 1 hour at room temperature. The membranes were then incubated with phosphorylated p70S6K1 primary antibody from Cell Signaling Technology (Danvers, Massachusetts) (38) at 4°C overnight followed by 1-hour of incubation in horseradish peroxidase-conjugated secondary antibody (39) from Santa Cruz Biotechnology (Dallas, Texas). The proteins were detected using Clarity Western ECL (BioRad, California). Following this the phosphorylated antibody was stripped off the membrane and the membrane was incubated overnight with an antibody for p70S6K1 (Cell Signaling) (40) followed by a secondary antibody incubation as described above. The data were analyzed using Image J.
Imaging protocol for echocardiogram
All animals were imaged at the Cardiovascular Physiology Rodent Core at Brigham and Women’s Hospital as previously described (41, 42). In brief, a MS550D probe with a center frequency of 40 and 21 MHz was used to capture images of the heart. Images were acquired using the Vevo3100 system (Fujifilm/Visualsonics, Toronto, Ontario, Canada) and the data were analyzed using the Vevo Lab software (Fujifilm/Visualsonics). The mice were studied after 7 days on 1.6% sodium diet and acclimatized overnight prior to obtaining the echocardiogram. During the echocardiogram, the mice were conscious and kept warm on a prewarmed platform to ensure that the body temperature was maintained at physiologic levels. The probe was positioned using the rail system to obtain left ventricular internal diameter diastole and systole (LVID[d]; LVID[s]). The ejection fraction (EF) was calculated by the formula (stroke volume / end-diastolic volume). All wall measurements were performed in M mode (short axis) and volume measurements in B mode (long axis).
Renal blood flow measurement with Doppler ultrasound
To obtain renal blood flow, pulse-wave doppler ultrasound was used (as previously described (42–44). Briefly, the mice were studied after 7 days on 1.6% sodium, acclimatized overnight, and in the morning lightly anesthetized (inhaled isoflurane 2–3% to induce and 1.0% to maintain) that allowed us to obtain consistent measurements as previously reported (43, 44). The renovascular function was assessed by measuring peak systolic velocity (mm/s) and end diastolic velocities (mm/s) to estimate the resistive index (RI) and pulsatility index (PI). The left renal artery (LRA) RI was calculated by the formula (peak systolic velocity – end diastolic velocity/ peak systolic velocity). The LRA PI was calculated by the formula (peak systolic velocity – end diastolic velocity/ time averaged velocity). Images were acquired using Vevo3100 system (Fujifilm/Visualsonics) and the data was analyzed using the Vevo Lab software (Fujifilm/Visualsonics).
Blood pressure measurement
Blood pressure measurement was performed as previously reported by us (45, 46). Systolic and diastolic blood pressure (SBP; DBP) was assessed by tail-cuff plethysmography (CODA tail-cuff blood pressure system, Kent Scientific Corporation, Torrington, Connecticut). All measurements were taken in the morning after 3 rounds of acclimation over a 3-week period. Values from 30 to 35 cycles were used to calculate the average SBP, DBP, and standard deviation (SD) for each mouse. Any readings greater than two SDs from the mean were excluded. The final mean value of SBP and DBP for each cohort was calculated from average SBPs and DBPs of individual mice and presented as mean ± SEM. The mice were not restrained longer than 15 minutes; this is the approach we have been using for past 15 years (45, 46).
Plasma aldosterone measurement
Blood was collected in BD Microtainer tubes with ethylenediamine tetraacetic acid (EDTA). The plasma was separated by centrifugation for 10 minutes at 1300 RCF. Plasma aldosterone levels were measured using an aldosterone ELISA kit (IBL International GMBH, Hamburg, Germany) (47).
Statistics
The statistical significance of a difference between groups was determined by an unpaired t-test (two-tailed). Calculated P values less than or equal to 0.05 were considered statistically significant except for those analyses with multiple comparisons where the P value was adjusted to 0.025. Values are presented as mean ± SEM.
Results
Rptor gene expression
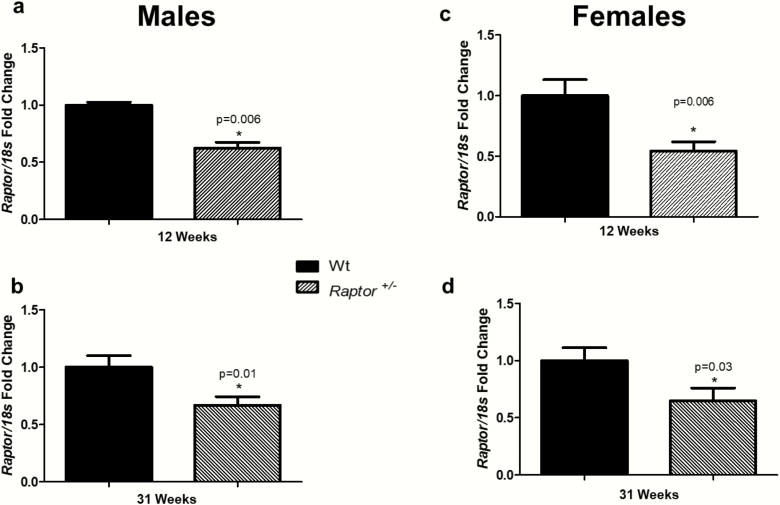
We validated that heterozygous male and female mice had similar decreases in the expression level of Raptor in cardiac tissue at 12 and 31 weeks of age (Fig. 1). To confirm, the observed effects in our study were due to the partial reduction in Rptor gene expression and we assessed the expression of S6K1, a second messenger in this pathway. Raptor +/- mice had lower expression of S6K1 than Wt mice. All supplementary material and figures are located in a digital research materials repository (48). We studied 2 different ages, in both sexes, due to the wide range of literature investigating mTORs’ role in aging (5, 49–51).
Figure 1.
Cardiac Raptor expressions were decreased in Raptor+/- males and females. *Levels in Wt mice were significantly different (P < 0.05) from corresponding levels in Raptor+/- mice at 12 weeks of age and 31 weeks of age: males (A and B) and females (C and D); (n = 7–9 per group). Data represented as means ± SEM. Student t-test for unpaired data was used to compare groups.
Blood pressure responses to salt intake
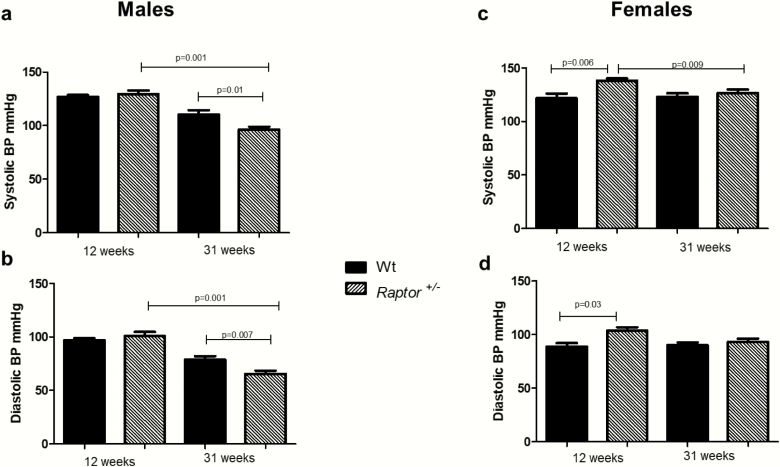
Data are emerging linking mTOR signaling to salt-induced hypertension (24, 32). However, the data obtained came from studies using inhibitors of the pathway, making it difficult to determine the impact of mTORC1 versus mTORC2 signaling. Therefore, the impact of a LibS diet on blood pressure was assessed in a model of genetic disruption of mTORC1 signaling. Twelve-week-old Raptor +/- males had no significant changes in SBP and DBP on a LibS diet when compared to Wt littermates (Fig. 2A and 2B). Interestingly, at 31 weeks, Raptor +/- males displayed a decrease in both SBP and DBP compared to Wt littermates or 12-week-old Raptor +/- males (Fig. 2A and 2B).
Figure 2.
Effect of LibS diet on blood pressure in male and female Raptor +/- mice. Systolic blood pressure levels in Wt mice were significantly different (P < 0.05) from corresponding levels in Raptor +/- males at 31 weeks of age (A). Systolic blood pressure levels (A) were significantly different between Raptor+/- males at 12 and 31 weeks of age. Diastolic blood pressure levels in Wt mice were significantly different (P < 0.05) from corresponding levels in Raptor +/- males (B) at 31 weeks of age, and Raptor+/- males at 31 weeks of age were significantly different from Raptor+/- males at 12 weeks of age (A). Systolic blood pressure levels (C) were significantly different between Wt and Raptor+/- females at 12 weeks of age, and significantly different at 12 and 31 weeks of age in Raptor+/- females. Diastolic blood pressure levels were significantly different between Wt and Raptor+/- females at 12 weeks of age (D). Data represent means ± SEM; (n = 7–9 per group). Student t-test for unpaired data was used to compare groups. Significance was set at 0.05, except for those analyses with multiple comparisons where the P value was adjusted to 0.025.
At 12 weeks of age, Raptor +/- females had a significant increase in SBP and DBP on a LibS diet when compared to Wt littermates (Fig. 2C and 2D). However, compared to 12-week-old Raptor +/- female mice, 31-week-old mice had a significant reduction in SBP (Fig. 2C and 2D). Thus, in general, a lower SBP was observed in Raptor +/- males and females as they age (Fig. 2).
Cardiac responses
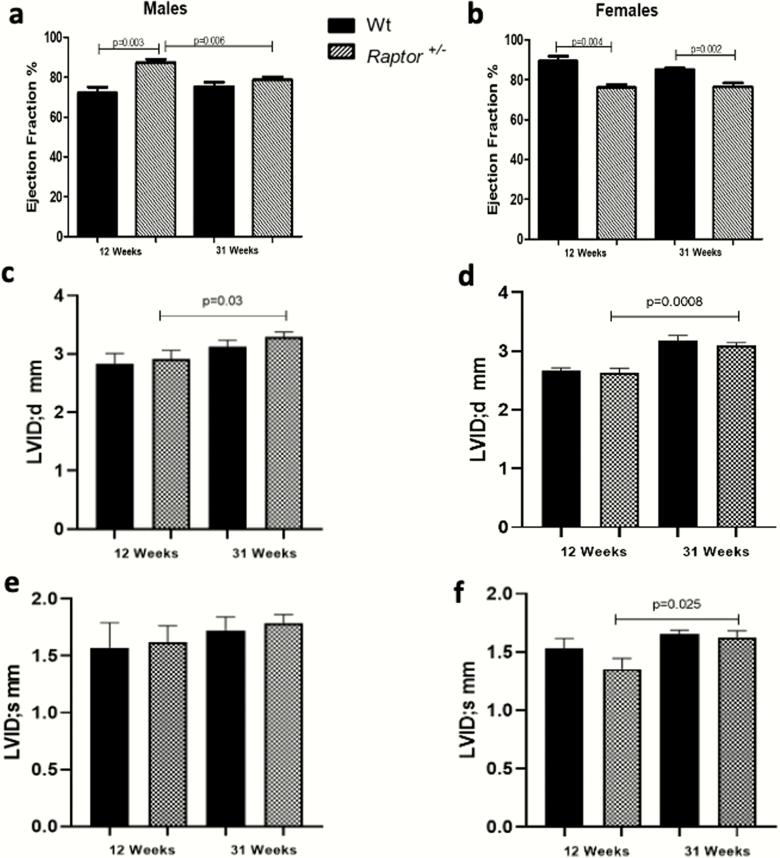
It is well documented that mTORC1 signaling is important for maintaining cardiac homeostasis (31, 36); therefore, we performed a cardiac ultrasound on Raptor +/- mice fed a LibS diet to assess echocardiographic parameters due to the differences we observed in blood pressure. Young heterozygous males showed an increased EF compared to Wt littermates (Fig. 3A). As they age their EF significantly decreased, a phenomenon not observed in the Wt littermates. Thus, the EFs of 31-week-old Raptor +/- and Wt mice are similar. (Fig. 3A). The 31-week-old Raptor +/- males also had an increased LVIDd as they aged (Fig. 3C). While there were increasing trends in LVIDs in both genotypes at both ages, they were not significant (Fig. 3E).
Figure 3.
Echocardiographic parameters of Raptor +/- mice on LibS diet. Ejection fraction in Raptor+/- males was significantly different from Wt at 12 weeks of age and significantly different between 12 and 31 weeks of age in Raptor+/- males, a phenomenon not observed in the Wt littermates. The 31-week-old Raptor +/- males (A) also had an increased left ventricular internal diameter diastole (C). While there were increasing trends in LVIDs in both genotypes at both ages, they were not significant (E). On the other hand, Raptor +/- females consistently had decreased ejection fraction compared to Wt, regardless of age (B). The LVIDd levels in females, Wt, and Raptor +/- were similar to what was seen in the males. In contrast to the males, Raptor +/- females significantly increased LVIDs with age (D and F). Thus, the cardiac results did not support a sex difference related to aging but did suggest that the Raptor +/- mice differed from Wt. Data represent means ± SEM; (n = 4–9 per group). Student t-test for unpaired data was used to compare groups. Significance was set at 0.05, except for those analyses with multiple comparisons where the P value was adjusted to 0.025.
On the other hand, Raptor +/- females consistently had a decreased EF compared to Wt, regardless of age (Fig. 3B). The LVIDd levels in female Wt and Raptor+/- were similar to what was seen in males. In contrast to the males, Raptor +/- females also significantly increased LVIDs with age. (Fig. 3D and 3F). Thus, the cardiac results do not support a sex difference related to aging, but do suggest that the Raptor +/- mice differ from Wt. Hence, it is possible that this phenotype is consistent with the beginning of dilated cardiomyopathy, which has previously been reported in other studies that have constitutive cardiac mTOR gene deletion at the early postnatal stage or inducible cardiac mTOR gene deletion during adulthood (31, 52, 53). Finally, these cardiac ultrasound findings were concordant with the reduction in blood pressure observed in both sexes at 31 weeks.
Aldosterone responses
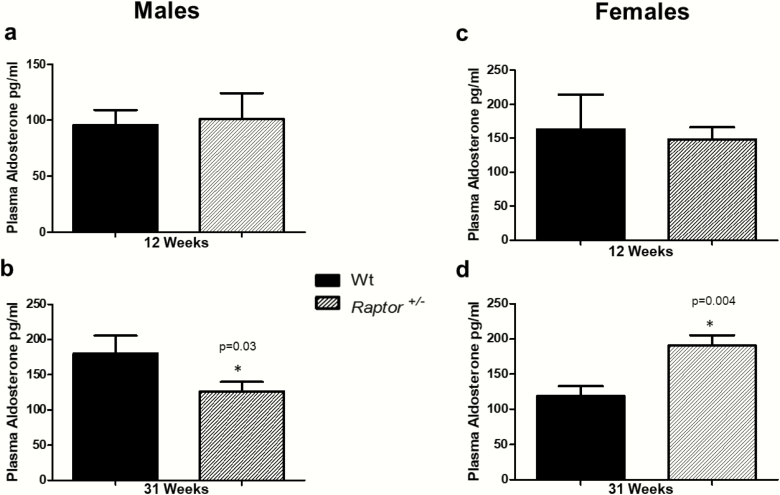
To determine if the differences in blood pressure on a LibS diet in the Raptor +/- mice could be due to dysregulated renin-angiotensin-aldosterone system (RAAS) signaling we measured the level of plasma aldosterone. We observed no significant change in aldosterone levels at 12 weeks of age in Raptor +/- of either sex (Fig. 4A and 4C). However, compared to Wt mice, in heterozygous mice at 31 weeks of age differences emerged: males had significantly lower aldosterone levels, while females had significantly higher levels (Fig. 4B and 4D). To determine if changes in aldosterone were driven by changes in renin, we measured plasma renin activity. There were no significant changes in plasma renin activity in either sex or age group when compared to Wt littermates. There were no significant changes in food intake, body weight, water intake, urine output, and fluid balance in either sex or age group when compared to Wt littermates (Supplement Table S1) (48). Taken together, these data indicated that at 31 weeks of age Raptor +/- animals had a decrease in blood pressure in response to a LibS diet. In males, aldosterone levels also were reduced, but in females they were increased, highlighting sex differences in mTORC1 signaling and regulation of aldosterone on a LibS diet.
Figure 4.
Plasma aldosterone levels in Raptor +/- compared to Wt mice on a LibS diet. *Aldosterone levels in wildtype (Wt) mice were significantly different (P < 0.05) from corresponding levels in Raptor +/-. No significant differences were detected in 12-week-old males (A) or females (C). At 31 weeks there was a significant (P < 0.05) decrease in aldosterone levels in Raptor+/- males (B), but a significant (P < 0.05) increase in Raptor+/- females (D). Data represent means ± SEM. Student t-test for unpaired data was used to compare groups (n = 7–9 per group).
Renal responses
In addition to performing a cardiac ultrasound (Fig. 3), we also assessed left kidney function in Raptor +/- and Wt mice at 31 weeks of age. We chose this age to study because 31 weeks is the timepoint where we observed a sex difference in plasma aldosterone levels. On a LibS diet it is normal for an organism to increase renal blood flow to excrete salt. Knowing this, we assessed how mTORC1 deficiency affects indicators of renal blood flow on a LibS diet.
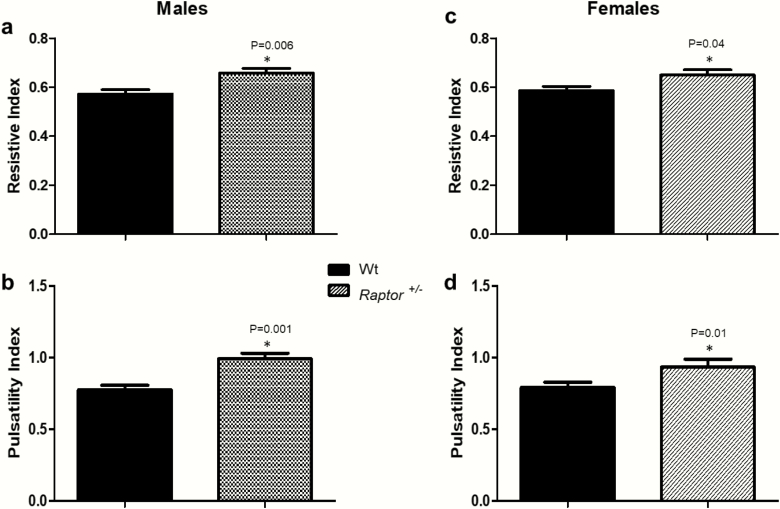
At 31 weeks of age, compared to Wt littermates, both Raptor +/- sexes had increased resistive and pulsatility indices on a LibS diet (Fig. 5). Increased resistive and pulsatility indices suggest a decrease in renal blood flow (54), the opposite of what would be expected physiologically with a LibS diet.
Figure 5.
Resistive and pulsatility indices in 31 weeks old Raptor +/- compared to Wt mice on a LibS diet. *Levels in Wt littermates were significantly different (P < 0.05) from corresponding levels in Raptor +/- mice: resistive index in males (A) and females (C) (n = 6–9) and pulsatility index in males (B) and females (D) (n = 6–9). Data represent means ± SEM. Student t-test for unpaired data was used to compare groups.
Natriuretic peptide responses
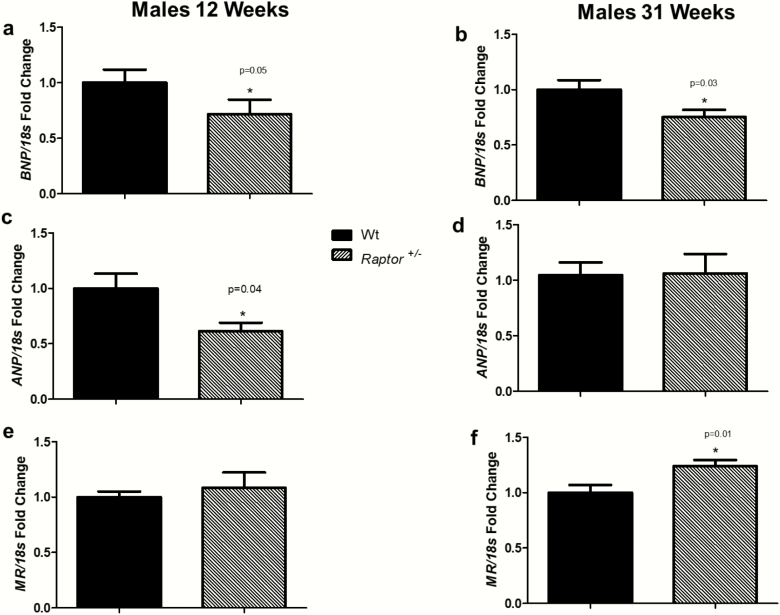
The observations of decreased blood pressure, dysregulation of aldosterone, and abnormalities in cardiac and renal ultrasound led us to assess proteins involved with volume homeostasis. On a LibS diet, the normal response of an organism is to increase BNP and atrial natriuretic peptide (ANP) in order to increase renal blood flow, reduce aldosterone levels, and excrete the excess sodium. Compared to Wt littermates, Raptor +/- males had decreased expression of BNP on a LibS diet at 12 weeks and 31 weeks of age (Fig. 6A and 6B). Atrial natriuretic peptide was decreased in Raptor +/- males at 12 weeks of age (Fig. 6C), with no change at 31 weeks of age (Fig. 6D). Mineralocorticoid receptor (MR) plays a role in volume homeostasis by binding aldosterone and translocating to the nucleus, thereby facilitating expression of specific genes involved in sodium reabsorption. On a LibS diet, one might assume expression of MR would decrease because sodium does not need to be retained. However, compared to Wt littermates, no significant differences in MR expression was detected at 12 weeks of age (Fig. 6E), but the Raptor +/- males did increased expression of MR on a LibS diet at 31 weeks of age (Fig. 6F).
Figure 6.
RT-PCR analysis of cardiac BNP, ANP, and MR expression in response to LibS in Wt and Raptor +/- male mice. *Levels in Wt mice were significantly different (P < 0.05) from corresponding levels in Raptor +/- mice: expression of BNP at 12 weeks old (n = 7–9 per group) (A) and at 31 weeks old (n = 9 per group) (B); expression of ANP at 12 weeks old (n = 7–9 per group) (C) and at 31 weeks old (n = 9 per group) (D); expression of MR at 12 weeks old (n = 7–9 per group) (E) and at 31 weeks old (n = 9 per group) (F). Data represent means ± SEM, and relative to 18S rRNA. Student t-test for unpaired data was used to compare groups.
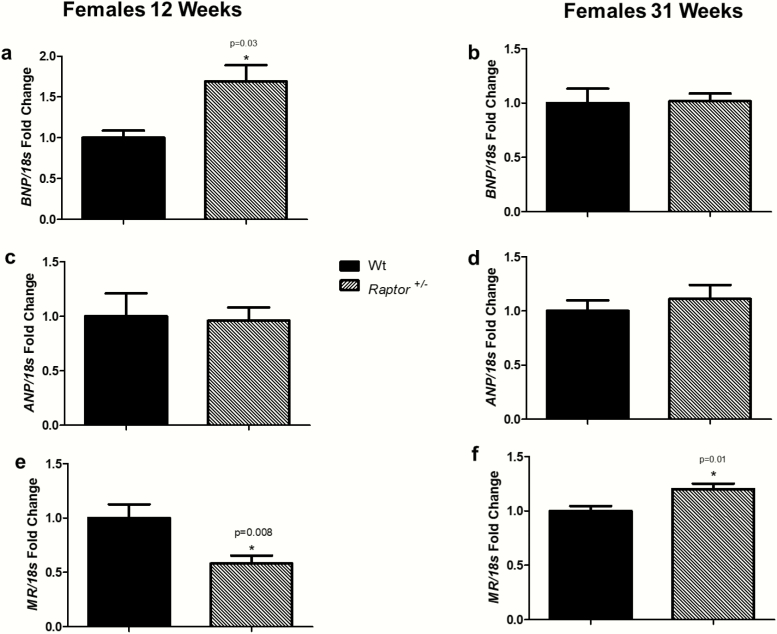
In contrast to the males, Raptor +/- females at 12 weeks increased their BNP levels (Fig. 7A); however, there was no significant change from Wt littermates at 31 weeks of age (Fig. 7B). Similarly, ANP expression levels did not differ between Raptor +/- and Wt females at either age. (Fig. 7C and 7D). Changes in MR expression were observed in Raptor+/- females as they aged. Compared to Wt littermates, at 12 weeks of age, expression of MR was decreased (Fig. 7E); however, at 31 weeks of age it was increased (Fig. 7F).
Figure 7.
RT-PCR analysis of cardiac BNP, ANP, and MR expression in response to LibS in Wt and Raptor +/- female mice. *Levels in Wt mice were significantly different (P < 0.05) from corresponding levels in Raptor +/- mice: expression of BNP at 12 weeks old (n = 7–9 per group) (A) and at 31 weeks old (n = 9 per group) (B); expression of ANP at 12 weeks old (n = 7–9 per group) (C) and at 31 weeks old (n = 9 per group) (D); expression of MR at 12 weeks old (n = 7–9 per group) (E) and at 31 weeks old (n = 9 per group) (F). Data represent means ± SEM, and relative to 18S rRNA. Student t-test for unpaired data was used to compare groups.
Discussion
Our results supported our overall hypothesis that both age and sex modify mTORC1’s interaction with volume homeostatic mechanisms. However, in contrast to what we had anticipated, mTORC1 deficiency secondary to raptor deficiency was not advantageous in response to a liberal salt intake. Specifically, regarding cardiac mechanisms, in both Raptor +/- sexes, blood pressure was reduced as the mice aged and the mice had decreased EF and increased LVIDd as they aged. Regarding renal mechanism, compared to Wt mice, both 31-week-old Raptor +/- sexes had decreased renal blood flow in contrast to the usual adaptive increased renal flow with a liberal salt diet. Finally, regarding hormonal mechanisms, there were sex- and age-associated differences. Thirty-one-week-old females had increased plasma aldosterone, while 31-week-old males had decreased plasma aldosterone levels on the LibS diet. These differences extended to BNP levels where, given the LibS diet, the males, but not females, had inappropriately suppressed BNP levels. Finally, compared to the Wt mice, both sexes had increased MR expression—an inappropriate response given the level of salt intake. Thus, mTORC1 deficiency on a LibS diet is associated with inappropriate responses in several volume homeostatic mechanisms both in young and older mice that differed by sex.
The mTOR pathway regulates both physiological and pathological processes in the cardiovascular system. It is necessary for proper cardiovascular development and for maintaining cardiac homeostasis in postnatal life (55). For example, the development of adaptive cardiac hypertrophy in response to mechanical overload requires mTOR, as complete loss-of-function prevents this adaptive response (31, 56). On the other hand, partial genetic or pharmacological inhibition of mTORC1 reduces cardiac remodeling and heart failure in response to pressure overload and chronic myocardial infarction (57, 58). Furthermore, mTORC1 blockade reduces cardiac abnormalities induced by metabolic disorders and extends the life span in mice (59, 60). Taken together, these data suggest that pharmacological targeting of mTORC1 may be a potential therapeutic approach for treating various cardiovascular diseases; however, clinical evidence in support of this posit remains insufficient.
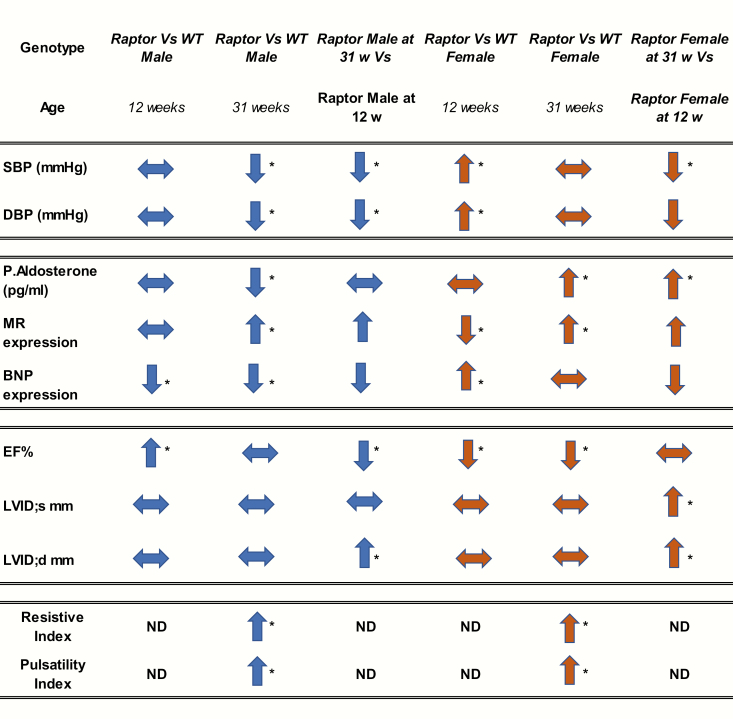
Compared to Wt mice, Raptor +/- males, as they aged, displayed a dramatic decrease in blood pressure (Fig. 2, Fig. 8). Given this observation, one might anticipate a compensatory increase in aldosterone levels. However, paradoxically, a decrease in the level of circulating aldosterone was observed as the animals aged (Fig. 4, Fig. 8), suggesting that the inappropriate aldosterone response could be the cause of the reduction in blood pressure.
Figure 8.
Summary of results. Trends are depicted by arrows: ↑ increase; ↓ decrease; → no change. If a trend is significant (P < 0.05, it is *). Abbreviations: BNP, brain natriuretic peptide; DBP, diastolic blood pressure; EF, ejection fraction; LVIDd, left ventricular internal diameter, diastole millimeter; LVIDs mm, left ventricular internal diameter, systole millimeter; MR, mineralocorticoid receptor; ND, not done; P. Aldosterone, plasma aldosterone; SBP, systolic blood pressure.
Intriguingly, cardiac MR expression increased with age in Raptor +/- males (Fig. 6, Fig. 8). These results were not anticipated given that the animals were on an LibS diet and had decreased aldosterone levels. However, it has been shown in mouse models exposed to chronic severe pressure overload that cell-specific deletion of MR in cardiomyocytes prevented left ventricular dilatation and increased LVIDd (61–63). Taken together, the finding of increased cardiac MR expression in Raptor deficient animals may contribute to the decreased EF and the increased LVIDd observed in 31-week-old male mice (Fig. 3, Fig. 8).
These data shed light on a possible interaction between MR and mTORC1 signaling in the heart that needs to be further explored. Mineralocorticoid receptor activity and mTOR signaling have already been linked in the intercalated cells of the kidney (33). The authors demonstrate that ULK1, a substrate for mTOR signaling, plays a role in the regulation of MR activity in intercalated cells and is an important determinant of how the kidney mounts distinct responses to aldosterone with volume depletion versus hyperkalemia (33). This could explain the decreased renal blood flow (Fig. 5, Fig. 8) and inappropriate response to changes in blood pressure, thereby effecting volume homeostasis in the Raptor +/- males. ULK1 also has been shown to inhibit cell proliferation by phosphorylating Raptor (64). It would be of interest to further investigate the interaction between MR and mTORC1 signaling in cardiac aging given that both play a role in fibrosis and inflammation (62, 65–68).
Raptor +/- females, like their male counterparts, exhibited a reduced blood pressure at 31 weeks when compared to blood pressure measurements at 12 weeks of age. However, in contrast to males, for females there was no difference in blood pressure levels when compared to age matched Wt littermates (Fig. 2, Fig. 8). Females had decreased EF at both timepoints, but in contrast to males, LVIDs did not increase until 31 weeks of age (Fig. 3). However, the increase was only apparent when compared to 12-week-old females and not age matched Wt littermates (Fig. 8).
In contrast to Raptor +/- males, females had appropriate hormonal responses to blood pressure and salt intake levels as they aged. Thus, plasma aldosterone levels increased at 31 weeks of age as the blood pressure decreased (Fig. 4, Fig. 8) and compared to the Wt, BNP expression was increased at 12 weeks of age and, similar to Wt littermates, at 31 weeks (Fig. 7, Fig. 8), indicating they appropriately responded to excess sodium intake. However, like the males, Raptor +/- females did not show the anticipated increased renal blood flow on the LibS diet (Fig. 5, Fig. 8). Interestingly, MR expression decreased on the LibS Diet at 12 weeks of age in Raptor +/- females compared to Wt littermates, but expression significantly increased by 31 weeks of age (Fig. 7, Fig. 8). Typically, in rodent models, estrogen and/or the activity of its receptors modulate aldosterone and/or MR levels (69, 70). Thus, a reduction in estrogen levels as the mice aged could explain the differences in aldosterone and MR expression in older Raptor +/- females. However, mice do not experience irregular estrous cycles until around 9 to 12 months of age. These irregular cycles, termed estropause, may be prolonged, and then animals may transition into an anestrous state, where ovulatory cycles halt and low levels of gonadal steroids are present (71, 72). The oldest time point we studied was approximately 7.5 months of age, suggesting that the age-related differences observed is more likely a direct effect of reduced mTORC1 signaling.
In support of this possibility are the responses of rodents treated with the mTOR inhibitor, rapamycin. Rapamycin treatment has been shown to downregulate the estrogen receptor beta (ERβ) in female mice. Further, treating DOCA-salt mice with rapamycin decreased mTORC1 and mTORC2 signaling to a greater extent in female than male mice with an associated greater degree of cardiovascular damage in the females (21). Finally, maintenance of both mTORC1 and mTORC2 signaling seems to be important for adaptive cardiac remodeling in females. Given the above, it is paradoxical that rapamycin treatment extended the lifespan in females to a greater extent than in males (73, 74). However, our results support this sexual dimorphic effect: 31-week-old female Raptor +/- mice (the genetic equivalent of long-term rapamycin treatment) had less derangements of volume homeostatic systems than males.
The difference in circulating aldosterone levels observed in the Raptor +/- mice raises the question as to the role mTORC1 signaling plays in the adrenal glands. Thus far, very few studies have investigated this question. However, it has been reported that the mTOR pathway is upregulated in patients with primary aldosteronism (18). Another study recently reported that mTORC1 inhibition may be a novel treatment for primary aldosteronism (75). The authors demonstrated that treatment of mice with rapamycin significantly decreased plasma aldosterone levels. In patients with primary aldosteronism treated with everolimus, plasma renin activity significantly increased and blood pressure was significantly reduced. However, the significant reduction of plasma aldosterone levels in the everolimus-treated group was observed in only 4 of 12 patients. Thus, the interpretation of these intriguing results is limited by the study design.
What are the clinical implications from our study? mTORC1 inhibitors are commonly used as immunosuppressants in solid-organ transplantation and as antiproliferative agents in various cancers. It may be advantageous to repurpose these drugs for the treatment of salt-sensitive hypertension. Even though data linking mTOR’s role in hypertension and volume homeostasis (24, 26, 33) is emerging, very little is known about the impact of inhibiting mTORC1 to treat hypertension. Some challenges with studying mTORC1 signaling are that not all downstream targets are equally inhibited by rapamycin, with potency varying for weak versus strong substrates (76, 77). Finally, it has proved challenging to develop a molecule that specifically inhibits mTORC1 (and not mTORC2) signaling, further complicating interpretation of the available data. Currently, rapamycin is FDA approved as an immunosuppressant, and its analogs temsirolimus and everolimus have been approved for the treatment of renal cell carcinoma and renal allograft rejection (8, 78). Interestingly, male patients who received rapamycin in post-transplantation and cancer therapies have a high prevalence of infertility (79), and women may have a worse outcome as compared with men when treated with an mTOR inhibitor eluting stent implantation (80). These data highlight the importance of studying the impact of sex when studying mTORC1 signaling.
Our study had several limitations. First, while there were derangements in volume homeostatic mechanisms in our 31-week-old mice, none appeared to be life threatening; longer-term studies will be required to assess the overall health consequences of these findings, eg, studies in 12- to 18-month-old mice. Second, detailed molecular and cellular studies were not performed. Thus, a more precise understanding of the mechanisms underlying each of these derangements is required. However, it does seem likely that the derangements are associated with a reduction in mTORC1 signaling as a downstream second messenger, S6K1, was also reduced (Supplement Fig.S1) (48). Third, it is unclear if the sex-associated differences observed are related to differences in hormone levels, the estrogen receptor type that is important, and/or some other factors associated with the female genetic status. Additional studies using estrogen receptor antagonists would be required to resolve these questions. Fourth, we used a global Raptor deficiency model. Tissue specific knock-out of Raptor may provide more specific target organ effects. Finally, to resolve the effect of salt intake on our findings, similar studies will need to be performed on mice maintained on a sodium-restricted diet. In general, sodium restriction tends to ameliorate adverse CV and renal effects.
In conclusion, Raptor +/- mice, when stressed with a LibS diet, displayed inappropriate responses to the activity of 3 volume homeostatic mechanisms—cardiovascular (cardiac and blood pressure), renal (renal blood flow), and hormonal (aldosterone [sodium retaining] and BNP [sodium losing]). While the inappropriate responses were observed in both males and females and became more distinctive with aging, males tended to have more severe defects than females. Thus, the altered mechanisms in response to a salt challenge associated with mTORC1 deficiency differ by sex. These sex-based findings will likely be relevant for the use of mTORC1 inhibitors in treating cardiovascular, renal, and other diseases.
Acknowledgments
We thank Paul Loutraris and Tham Yao for their expert assistance with the animal protocols and preparing all experimental materials. We also thank the associate director, Sudeshna Fisch, at the Cardiovascular Physiology Core at Brigham and Women’s Hospital.
Financial Support: This work was supported by the National Institute of Health National Heart, Lung and Blood Institute Grants R01HL11476 (G.H.W.), R01HL104032 (L.H.P.), K24HL103845 (G.K.A.), R01HL096518 (J.R.R.); American Heart Association Grant 14GRNT20500000 (L.H.P.); American Heart Association Grant 18POST33990336 (D.L.B.); and NIH T32 training grant T32HL007609-27(S.R., S.M.).
Author Contribution: DL. Brooks, and GH. Williams conceived and designed the experiments. DL. Brooks, and AE. Garza and SK Gholami performed the experiments. DL. Brooks, and GH. Williams analyzed the data. LH. Pojoga, AK. Adler, and GH. Williams contributed the reagents, materials, funding and analysis tools. DL. Brooks, GH. Williams, E. Caliskan Guzelce and SK Gholami wrote the manuscript. All authors read and approved the final manuscript.
Glossary
Non-standard Abbreviations and Acronyms
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- EF
ejection fraction
- LibS Diet
1.6% sodium
- LVID
left ventricular internal diameter
- MR
mineralocorticoid receptor
- mTOR
mechanistic target of rapamycin
- mTORC1
mechanistic target of rapamycin complex 1
- mTORC2
mechanistic target of rapamycin complex 2
- Na+
sodium
- PI
pulsatility index
- RAAS
renin–angiotensin–aldosterone system
- Raptor
regulatory-associated protein of mTOR
- RI
resistive index
- SBP
systolic blood pressure
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Baudrand R, Guarda FJ, Fardella C, et al. Continuum of renin-independent aldosteronism in normotension. Hypertension. 2017;69(5):950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension. 2018;71(2):218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. [DOI] [PubMed] [Google Scholar]
- 5. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kapahi P, Chen D, Rogers AN, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11(6):453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126(Pt 8):1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. [DOI] [PubMed] [Google Scholar]
- 10. Sabers CJ, Martin MM, Brunn GJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270(2):815–822. [DOI] [PubMed] [Google Scholar]
- 11. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. [DOI] [PubMed] [Google Scholar]
- 12. Nojima H, Tokunaga C, Eguchi S, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278(18):15461–15464. [DOI] [PubMed] [Google Scholar]
- 13. Sancak Y, Peterson TR, Shaul YD, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol. 2003;13(10):797–806. [DOI] [PubMed] [Google Scholar]
- 15. Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110(2):177–189. [DOI] [PubMed] [Google Scholar]
- 16. Sciarretta S, Forte M, Frati G, Sadoshima J. New Insights Into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122(3):489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brooks DL, Garza AE, Katayama IA, et al. Aldosterone modulates the mechanistic target of rapamycin signaling in male mice. Endocrinology. 2019;160(4):716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Su H, Gu Y, Li F, et al. The PI3K/AKT/mTOR signaling pathway is overactivated in primary aldosteronism. Plos One. 2013;8(4):e62399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aghamohammadzadeh R, Zhang YY, Stephens TE, et al. Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension. Faseb J. 2016;30(7):2511–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110(2):163–175. [DOI] [PubMed] [Google Scholar]
- 21. Gürgen D, Kusch A, Klewitz R, et al. Sex-specific mTOR signaling determines sexual dimorphism in myocardial adaptation in normotensive DOCA-salt model. Hypertension. 2013;61(3):730–736. [DOI] [PubMed] [Google Scholar]
- 22. Whaley-Connell AT, Habibi J, Nistala R, et al. Mineralocorticoid receptor-dependent proximal tubule injury is mediated by a redox-sensitive mTOR/S6K1 pathway. Am J Nephrol. 2012;35(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang B, Ding W, Zhang M, Li H, Gu Y. Rapamycin attenuates aldosterone-induced tubulointerstitial inflammation and fibrosis. Cell Physiol Biochem. 2015;35(1):116–125. [DOI] [PubMed] [Google Scholar]
- 24. Kumar V, Evans LC, Kurth T, et al. Therapeutic suppression of mTOR (mammalian target of rapamycin) signaling prevents and reverses salt-induced hypertension and kidney injury in dahl salt-sensitive rats. Hypertension. 2019;73(3):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu L, Brink M. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim Biophys Acta. 2016;1863(7 Pt B):1894–1903. [DOI] [PubMed] [Google Scholar]
- 26. Gleason CE, Frindt G, Cheng CJ, et al. mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J Clin Invest. 2015;125(1):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lang F, Pearce D. Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway. Nephrol Dial Transplant. 2016;31(2):200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169(2):361–371. [DOI] [PubMed] [Google Scholar]
- 29. Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114(3):549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16(1):46–51. [DOI] [PubMed] [Google Scholar]
- 31. Shende P, Plaisance I, Morandi C, et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123(10):1073–1082. [DOI] [PubMed] [Google Scholar]
- 32. Kumar V, Wollner C, Kurth T, Bukowy JD, Cowley AW Jr. Inhibition of mammalian target of rapamycin complex 1 attenuates salt-induced hypertension and kidney injury in dahl salt-sensitive rats. Hypertension. 2017;70(4):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shibata S, Ishizawa K, Wang Q, et al. ULK1 Phosphorylates and regulates mineralocorticoid receptor. Cell Rep. 2018;24(3):569–576. [DOI] [PubMed] [Google Scholar]
- 34. Baar EL, Carbajal KA, Ong IM, Lamming DW. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell. 2016;15(1):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle. 2009;8(24):4055–4059. [DOI] [PubMed] [Google Scholar]
- 36. Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11(6):859–871. [DOI] [PubMed] [Google Scholar]
- 37. Whelton PK, Appel LJ, Sacco RL, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–2889. [DOI] [PubMed] [Google Scholar]
- 38. RRID:AB_2269803. https://antibodyregistry.org/search.php?q=AB_2269803
- 39. RRID:AB_628497. https://antibodyregistry.org/search.php?q=AB_628497
- 40. RRID:AB_331676. https://antibodyregistry.org/search.php?q=AB_331676
- 41. Chang WT, Fisch S, Chen M, Qiu Y, Cheng S, Liao R. Ultrasound based assessment of coronary artery flow and coronary flow reserve using the pressure overload model in mice. J Vis Exp. 2015;13(98):e52598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fisch S, Liao R, Hsiao LL, Lu T. Early detection of drug-induced renal hemodynamic dysfunction using sonographic technology in rats. J Vis Exp. 2016;(109). doi: 10.3791/52409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bjornerheim R, Grogaard HK, Kjekshus H, Attramadal H, Smiseth OA. High frame rate Doppler echocardiography in the rat: an evaluation of the method. Eur J Echocardiogr. 2001;2(2):78–87. [DOI] [PubMed] [Google Scholar]
- 44. Zhang L, Xu X, Hu C, et al. A high-frequency, high frame rate duplex ultrasound linear array imaging system for small animal imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2010;57(7):1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pojoga LH, Romero JR, Yao TM, et al. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-L-arginine methyl ester and angiotensin II. Endocrinology. 2010;151(3):1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chuengsamarn S, Garza AE, Krug AW, et al. Direct renin inhibition modulates insulin resistance in caveolin-1-deficient mice. Metabolism. 2013;62(2):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baudrand R, Gupta N, Garza AE, et al. Caveolin 1 Modulates Aldosterone-Mediated Pathways of Glucose and Lipid Homeostasis. J Am. Heart Assoc. 2016;5(10):e003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brooks DL, Garza AE, Caliskan Guzelce E, et al. mTORC1 deficiency modifies volume homeostatic responses to dietary sodium in a sex-specific manner. Deposited 13 February 2020. ed. zenodo; 2020.DOI: 10.5281/zenodo.3666819. [DOI] [PMC free article] [PubMed]
- 49. Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. [DOI] [PubMed] [Google Scholar]
- 50. Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. [DOI] [PubMed] [Google Scholar]
- 51. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mazelin L, Panthu B, Nicot AS, et al. mTOR inactivation in myocardium from infant mice rapidly leads to dilated cardiomyopathy due to translation defects and p53/JNK-mediated apoptosis. J Mol Cell Cardiol. 2016;97:213–225. [DOI] [PubMed] [Google Scholar]
- 53. Zhang D, Contu R, Latronico MV, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120(8):2805–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Terry JD, Granger SH, Chen BC, et al. Adjusted resistive index: a method to estimate rapidly renal blood flow: preliminary validation in hypertensives. J Ultrasound Med. 1993;12(12):751–756. [DOI] [PubMed] [Google Scholar]
- 55. Zhu Y, Pires KM, Whitehead KJ, et al. Mechanistic target of rapamycin (Mtor) is essential for murine embryonic heart development and growth. Plos One. 2013;8(1):e54221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tamai T, Yamaguchi O, Hikoso S, et al. Rheb (Ras homologue enriched in brain)-dependent mammalian target of rapamycin complex 1 (mTORC1) activation becomes indispensable for cardiac hypertrophic growth after early postnatal period. J Biol Chem. 2013;288(14):10176–10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu X, Cao Y, Nie J, et al. Genetic and pharmacological inhibition of Rheb1-mTORC1 signaling exerts cardioprotection against adverse cardiac remodeling in mice. Am J Pathol. 2013;182(6):2005–2014. [DOI] [PubMed] [Google Scholar]
- 58. Buss SJ, Muenz S, Riffel JH, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol. 2009;54(25):2435–2446. [DOI] [PubMed] [Google Scholar]
- 59. Düvel K, Yecies JL, Menon S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Flynn JM, O’Leary MN, Zambataro CA, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12(5):851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lother A, Berger S, Gilsbach R, et al. Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension. 2011;57(4):746–754. [DOI] [PubMed] [Google Scholar]
- 62. Jaisser F, Swynghedauw B, Delcayre C. The mineralocorticoid receptor in heart: different effects in different cells. Hypertension. 2011;57(4):679–680. [DOI] [PubMed] [Google Scholar]
- 63. Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J. 2009;30(4):469–477. [DOI] [PubMed] [Google Scholar]
- 64. Jung CH, Seo M, Otto NM, Kim DH. ULK1 inhibits the kinase activity of mTORC1 and cell proliferation. Autophagy. 2011;7(10):1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gorini S, Kim SK, Infante M, et al. Role of aldosterone and mineralocorticoid receptor in cardiovascular aging. Front Endocrinol (Lausanne). 2019;10:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54(3):537–543. [DOI] [PubMed] [Google Scholar]
- 67. Buerger C, Shirsath N, Lang V, et al. Inflammation dependent mTORC1 signaling interferes with the switch from keratinocyte proliferation to differentiation. Plos One. 2017;12(7):e0180853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15(10):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Macova M, Armando I, Zhou J, et al. Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats. Neuroendocrinology. 2008;88(4):276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oelkers WK. Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids. 1996;61(4):166–171. [DOI] [PubMed] [Google Scholar]
- 71. Huang HH, Steger RW, Bruni JF, Meites J. Patterns of sex steroid and gonadotropin secretion in aging female rats. Endocrinology. 1978;103(5):1855–1859. [DOI] [PubMed] [Google Scholar]
- 72. Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21(1):193–203. [DOI] [PubMed] [Google Scholar]
- 73. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y, Bokov A, Gelfond J, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69(2):119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Trinh B, Hepprich M, Betz MJ, et al. Treatment of primary aldosteronism with mTORC1 inhibitors. J Clin Endocrinol Metab. 2019;104(10):4703–4714. [DOI] [PubMed] [Google Scholar]
- 76. Viana SD, Reis F, Alves R. Therapeutic use of mTOR inhibitors in renal diseases: advances, drawbacks, and challenges. Oxid Med Cell Longev. 2018;2018:3693625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. [DOI] [PubMed] [Google Scholar]
- 78. Nguyen LS, Vautier M, Allenbach Y, et al. Sirolimus and mTOR inhibitors: a review of side effects and specific management in solid organ transplantation. Drug Saf. 2019;42(7):813–825. [DOI] [PubMed] [Google Scholar]
- 79. Huyghe E, Zairi A, Nohra J, Kamar N, Plante P, Rostaing L. Gonadal impact of target of rapamycin inhibitors (sirolimus and everolimus) in male patients: an overview. Transpl Int. 2007;20(4):305–311. [DOI] [PubMed] [Google Scholar]
- 80. Niccoli G, Sgueglia GA, Cosentino N, et al. Impact of gender on clinical outcomes after mTOR-inhibitor drug-eluting stent implantation in patients with first manifestation of ischaemic heart disease. Eur J Prev Cardiol. 2012;19(5):914–926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.