To the Editor:
CORONAVIRUS DISEASE 2019 (COVID-19) is a contagious infection precipitated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2. It is a novel virus of which transmissability, incidence and mortality rates have made it a global emergency. While the clinical manifestations of the virus may vary in severity, it is widely known that the cardiorespiratory system is the principle infection point of the virus, with acute respiratory distress syndrome (ARDS) and shock being possibilities.1
Although severe and critically ill patients account for 15-26% of patients, there are currently no targeted COVID-19 therapeutics.2 At present, supportive care forms the core of disease management, with emphasis on oxygen delivery in the early stage of the disease.3 In March 2020, the World Health Organization (WHO) published interim guidelines recommending the use of extracorporeal membrane oxygenation (ECMO) in ARDS patients unresponsive to mainstream therapies, in order to maintain cardiorespiratory function.4
In this letter, we present a systematic review of the literature to summarize the evidence behind using ECMO in COVID-19 patients, in accordance to the "Preferred Reporting Items for Systematic Reviews and Meta-Analysis: (PRISMA) Guidelines. We have performed a comprehensive electronic literature search using key words “COVID-19,” “SARS-CoV2,” “Coronavirus,” “ECMO,” “Extracorporeal membrane oxygenation,” “VA-ECMO,” “VV-ECMO,” “Outcomes,” “Respiratory support,” and “circulatory support,” either as MeSH terms or in the combined key word formats.
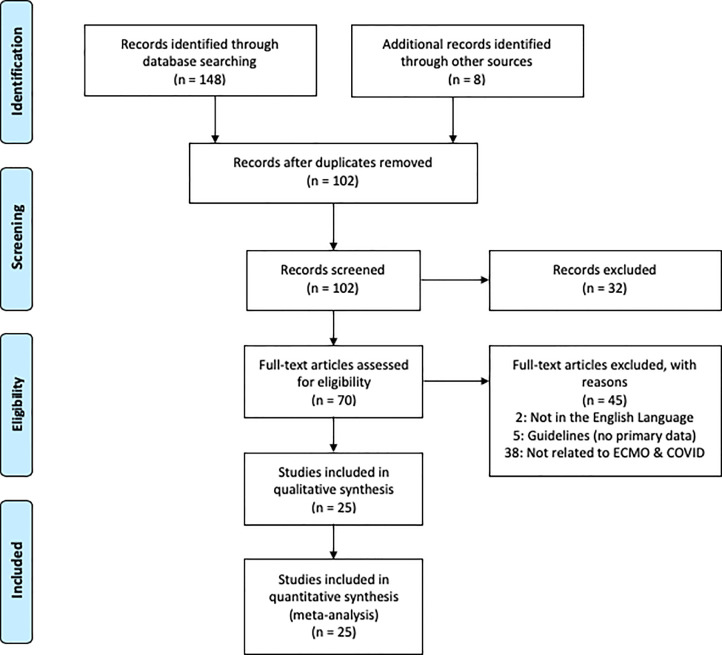
Our results showed a total of 102 articles that were collected from the database search and through snowballing. A total of 25 articles were selected to be included, after exclusion of duplication and subsequent screening (Fig 1 ). A summary of each of the chosen studies was conducted as shown in Table 1 . After combining the data from the studies, 3,428 patients were diagnosed with COVID-19 overall, 612 patients were diagnosed with ARDS, and 479 were placed on ECMO, with VV-ECMO being the most commonly used type.
Fig 1.
PRISMA chart of the literature search.
Table 1.
Summary of Included Articles.
| Author | Country | Study Type | Cohort Size (No. of Patients on ECMO) | ARDS | Time and Type of ECMO (VV or VA) | Overall Mortality (%) |
| Barrasa et al.5 | Spain | Retrospective | 48 (1) | 48 | N/A | 6 (15) |
| Bemtgen et al.20 | Germany | Case report | 1 (1) | 1 | VA-ECMO and then switched to VV-ECMO | N/A |
| Chen et al.24 | China | Retrospective | 99 (3) | 17 | N/A | 11 (11) |
| Firstenberg et al.21 | USA | Case report | 1 (1) | 1 | VV-ECMO (introduced on seventh day) | N/A |
| Guan et al.13 | China | Cross-sectional | 1,099 (5) | 37 | N/A | 14 (1.4) |
| Hartman et al.22 | USA | Case report | 1 (1) | N/A | VV-ECMO Started ECMO on hospital day 4 |
N/A |
| Huang et al.6 | China | Cross-sectional | 41 (2) | 12 | N/A | 6 (14.6) |
| Jacobs et al.31 | USA | Cross-sectional | 32 (32) | N/A | VV-ECMO used in 78.1% of cases | 10 (31.3) |
| Li et al.15 | China | Case series | 16 (8) | N/A | 7 on VV-ECMO 1 on VA-ECMO |
N/A |
| Loforte et al.17 | Italy | Observational | 59 (4) | 59 | VV-ECMO used in all patients | 1 (25) |
| Marullo et al.16 | Europe | Retrospective | 333 (333) | N/A | VV-ECMO used in 93.7% of cases | 57 (17.1) |
| Nakamura et al.23 | Japan | Case report | 1 (1) | 1 | VV-ECMO introduced on hospital day 2 | N/A |
| Ruan et al.9 | China | Retrospective | 150 (7) | 62 | N/A | 68 (48.3) |
| Shen et al.25 | China | Case series | 5 (1) | 5 | N/A | N/A |
| Sultan et al.29 | USA | Case series | 10 (10) | 10 | VV ECMO Median time from first symptom to ECMO: 11 days 7 and 10 days in total on ECMO |
1 (10) |
| Takeda30 | Japan | Letter to the Editor | 26 (26) | N/A | N/A | N/A |
| Tang et al.7 | China | Retrospective case-control | 179 (10) | 73 | N/A | 21 (28.3) |
| Taniguchi et al.26 | Japan | Case report | 1 (1) | 1 | VV-ECMO (introduced on sixth day, day 2 after intubation) 6 days on ECMO in total |
N/A |
| Wang et al.2 | China | Case series | 138 (4) | 22 | N/A | 6 (4.3) |
| Wu et al.10 | China | Retrospective | 201 (1) | 84 | N/A | 44 (22.9) |
| Yang et al.11 | China | Retrospective | 710 (6) | 35 | N/A | 32 (4.5) |
| Zangrillo et al.8 | Italy | Case series | 73 (5) | 73 | N/A | 17 (23.3) |
| Zeng et al.28 | China | Case series | 12 (12) | 12 | 1.3 mean days on ECMO | 5 (41.6) |
| Zhan et al.27 | China | Case report | 1 (1) | N/A | VV-ECMO 5 days on ECMO |
N/A |
| Zhou et al.12 | China | Retrospective | 191 (3) | 59 | N/A | 54 (28.3) |
Abbreviations: ARDS (Acute Respiratory Distress Syndrome) , VA-ECMO (Veno-arterial Extracorporeal Membrane Oxygenation), VV-ECMO (Veno-venous Extracorporeal Membrane Oxygenation).
Commonly used as a form of rescue therapy, ECMO was delivered to COVID-19 patients with induced ARDS and other complications demanding urgent care.5, 6, 7, 8 After data collation from the selected articles, an overall mortality rate of 19.83% was estimated. Nevertheless, the actual mortality rate may have been higher as some papers did not account for deaths of patients put on ECMO. The estimated figure, however, supported the claim that the use of ECMO does not exacerbate patient outcomes for COVID-19 patients in critical condition.
Few studies reported high mortality rates for patients with COVID-19. In this review, a total of three studies found a 100% mortality in patients with ARDS placed on ECMO.Yang et al. published similar findings ,with mortality rate of 83.33% (an overall total of 15 deaths).9, 10, 11, 12 Additionally, a study by Guan et al. reported that 5 patients receiving ECMO all faced the same composite primary endpoint (admission to the ICU, mechanical ventilation or death).13 However, with a number of deaths attributed to septic shock and multiple organ failure, the connection of VA-ECMO support and mortality outcomes could not be ascertained. Another consideration is whether the patients under observations experienced multiple organ failure while on ECMO, this is because ECMO is not advised in multiple organ failure (as highlighted in ELSO guidelines).14
Other reports showed conflicting data, with Li et al. depicting an ambivalent 50% mortality rate.15 Meanwhile, 75% of deaths occurred in patients older than age 75 with comorbidities. Marullo et al. had similar findings, with a marginal difference between the number of patients who weaned off ECMO (60) and the number of deaths after ECMO (57) and an elevated risk in patients older than 60 with comorbities.16
In a separate research study by Loforte et al., 75% weaning rate was reported; however,one- third of weaned patients died after subsequent VV-ECMO removal.17 Also, the remaining patient of the four had severe gastrointestinal bleeding while on ECMO, a severe complication linked to ECMO.18 Thus, with a final mortality rate of 50%, with 2 out of 4 patients eventually dying, these results were also inconclusive in terms of the benefit of ECMO in COVID-19.
Despite results in these studies being less-than- optimistic, several considerations should be noted. First, the small sample in the majority of these articles offered no reliable conclusions. Second, the patient's disease severity at the time of ECMO initiation was often not addressed; therefore, ECMO administration may have been delayed to an extent that its effect on patients was questionnable.
Even though the postive results of some studies did not suffice as concrete evidence for ECMO use in COVID-19, they may be useful to determine whether specific patient characteristics are more compatible with ECMO use. Such findings have been reported by 2 case series and six case reports and 2 case series, which all found positive endpoints for patients on ECMO (weaned off ECMO or discharged from the hospital).19, 20, 21, 22, 23, 24, 25, 26, 27
The included literature postulated that if given earlier, ECMO may improve patient outcomes. Both Zhan et al. and Taniguchi et al. favored early ECMO provision to improve recovery chances of their patients via protection of their organ oxygen supply and prevention of lung injury through mechanical damage (ventilators).26, 27 Firstenberg et al. reported similar findings, with emphasis on the impact of decision-making for ECMO initiation on patient discharge; the timing between reaching the threshold for indication and the decision to begin therapy were found to be of utmost importance21 Additionally, the role of ECMO in stabilizing oxygenation and supporting the lung have been deemed essential by Taniguchi et al. to improve outcomes.26 In patients with deteriorating lung function, determining and treating its cause are imperative. In this respect, aggravated oxygenation may be attributed to to worsening ARDS by COVID-19 pneumonia. In cases with stronger inflammatory results than pulmonary congestion, ECMO was initiated to assist the patient's recovery.28, 29, 30 The ELSO guidelines outlined the principal contraindications for the use of ECMO, which have been summarized in Table 2 .
Table 2.
Absolute Contraindications for ECMO in COVID-19 Patients With Cardiopulmonary Failure.
| 1. Advanced age | 2. Clinical frailty scale category ≥3 |
| 3. Severe multiple organ failure (renal failure is not an exclusion criterion) | 4. Severe acute neurological injury |
| 5. Significant underlying comorbidities | 6. Mechanical ventilation >10 days |
| 7. Uncontrolled bleeding | 8. Contraindications to anticoagulation |
| 9. Inability to accept blood products | 10. Ongoing CPR |
Abbreviations: CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation.
Adapted from ELSO.17
The authors searched other literature and found that these results were comparable with regard to the use of ECMO. A systematic review and meta-analysis, including studies such as the CESAR31 and EOLIA32 trials among othe, posited that the use of VV-ECMO in acute severe respiratory failure was linked to a 60-day reduced mortality (RR 0.73, 95% CI 0.58-0.92) in comparison to conventional mechanical ventilation.33 Despite many clinicians against the results in the CESAR study, the EOLIA study still did not reveal any significant difference in overall mortality. Furthermore, an estimate of 40%-predicted survival to discharge on VA has been reported by ELSO,34 compared with 58% on VV35; yet since no comparison has been made to conventional care, the survival advantage of VV is still to be determined.36 , 37
Supported by several guidelines,the use of VV-ECMO in COVID-19 ARDS,4 , 14 , 38 , 39 VV-ECMO has proven useful in offering respiratory support, particularly in severe respiratory failure. Hence, with acute respiratory failure and occasionally acute respiratory failure as distinctive features of SARS-COV-2 pneumonia,VV-ECMO use has been on the increase.
Meanwhile, 22% of COVID-19 patients experienced cardiovascular complications, such as heart failure, myocarditis and a hypercoagulability. The evidence in favor of VA-ECMO use for both respiratory and hemodynamic support is well-founded, with VA-ECMO recommended in an array of scanarios, such as myocarditis, acute myocardial infarction, decompensated cardiac failure, or even cardiogenic shock as complications of cardiac injury. This presents an opportunity for additonal use VA-ECMO in COVID-19 patients.40
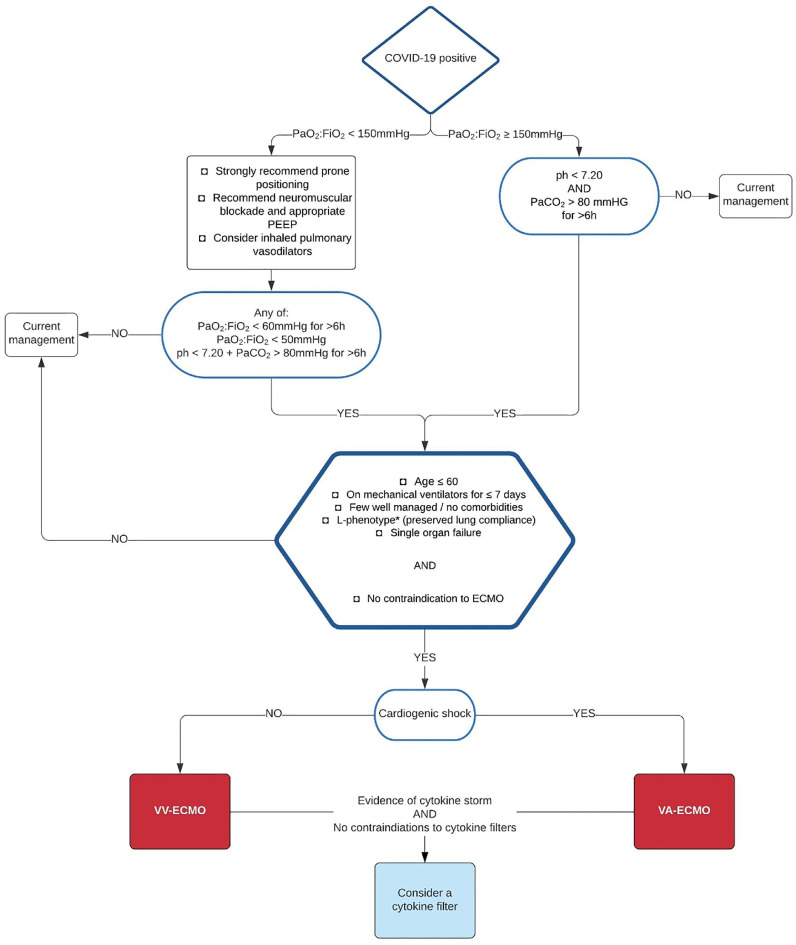
Through compilation of the published findings, the authors devised the systematic plan portayed in Figure 2 . Decisions about patient suitability for ECMO should be done in a timely manner to avoid overuse of ventilators and associated complications. For futher risk minimization, patient referrals to specialized tertiary centers are suggested for those in need of ECMO in order to ensure proper care provision and compliance to standardized ECMO guidelines.
Fig 2.
Algorithm for decision-making regarding ECMO provision in COVID-19 patients. Abbreviations: EMCO, extracorporeal membrane oxygenation; Pao2:Fio2, ratio of partial pressure of oxygen in arterial blood to the fractional concentration of oxygen in inspired air; PaCo2, partial pressure of carbon dioxide in arterial blood; PEEP, positive end-expiratory pressure. *L-phenotype has been associated with preserved lung compliance and shown to have favorable outcomes with ECMO.29 Adapted from the Extracorporeal Life Support Organization (ELSO).14
Furthermore, risk of thrombotic and hemorrhagic complications can increase due to disruption in coagulation pathways. This may be because of the use of anticoagulants when delivering ECMO and concurrent systemic inflammation. Hence, additional attention must be provided to coagulability levels in patients during ECMO.41
Apart from present guidelines, other tools have been produced for the purpose of decision-making around VV-ECMO use to optimize outcomes. Such prediction tool scores include PRESERVE (AUC 0.75, 95% CI 0.57-to-0.92, p = 0.01) and RESP scores (AUC 0.81, 95% CI 0.67-to-0.95, p = 0.035).42 Scores like the survival after veno-arterial-ECMO (SAVE) can help to discern patients best suited for VA-ECMO in order to maximize allocation of resources,43 They also have been salient in forecasting survival outcomes for patients with ARDS using VV-ECMO, but they do not come without limitation. With COVID-19 manifastations being very multifactorial, prediction tool scores often do not consider individual pathophysiologies; for example cytokine storms.
In conclusion, these authors recommend ECMO use in COVID-19 but with caution and in compliance with current guidelines. While evidence advocating ECMO use in COVID-19 is not substantial, ongoing studies may provide new insight in ECMO use in COVID-19 patients in critical cases. To ensure optimal patient care, a case-by-case approach should be implement, with risk-benefit analysis conducted for each patient.
Limitations
Due to the scarcity of data in this area, particularly for use of VA-ECMO, the suitability of ECMO use for COVID-19 is still to be determined. A plausible reason for this is that the prevalence of ARDS (utilizing VV-ECMO) among COVID-19 patients is higher with respect to those with shock (for which VA-ECMO is suggested).
As several studies did not report patient outcomes, we were unable to acertain the true extent to which ECMO affected patient health. The gaps in our outcomes data prevented us from performing a high-quality meta-analysis, as effect sizes could not be estimated for some studies. Therefore, the overall mortality rate that we reported, which included studies without reported outcomes, may be subject to change.
As the characteristics of the study participants in this review were not always mentioned, we were unable to make any assumptions on how ECMO may reduce mortality in particular patient groups. Since demographic factors and presence of comorbidities have shown to have an influence COVID-19 prognoses, these variables should be taken into consideration as potential determinants of patients’ outcomes, along with initiation of ECMO as salvage therapy.
Treatment in COVID-19 patients is often complex with ECMO as one of many therapy elements involved in primary treatment. For this reason, the extent to which ECMO use has contributed to patient recovery is still to be determined.
There is no single gold standard to decide initiation of ECMO use, resulting in inconsistent data. In studies with patients receiving ECMO at a more advanced stage, baseline risk of mortality would be elevated. Additionally, hospitals with low numbers of ECMO machines may have prioritized critical patients, due to their greater need of salvage therapy, which could have been reflected in the reported mortality rates (selection bias).
Although collation of data has produced an overall mortality rate, treatment variations, such as treatment technique and time of ECMO initiation, are not being considered. This stems from the fact that diversity of guidelines and degree of compliance to guidelines may have resulted in variable patient outcomes, which cannot be distilled into quantifiable data.
Conflict of Interest
None.
References
- 1.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment coronavirus (COVID-19) StatPearls. 2020 [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance.https://apps.who.int/iris/rest/bitstreams/1272156/retrieve Available at: Accessed 27 May, [Google Scholar]
- 5.Barrasa H, Rello J, Tejada S, et al. SARS-CoV-2 in Spanish intensive care units: Early experience with 15-day survival in Vitoria [E-pub ahead of print] Anaesth Crit Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.04.001. Accessed August 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X, Du R, Wang R, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy [E-pub ahead of print]. Crit Care Resusc [Accessed 1st June 2020] [DOI] [PMC free article] [PubMed]
- 9.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Extracorporeal Life Support Organization . 2020. COVID-19 interim guidelines.https://www.elso.org/Portals/0/Files/pdf/ELSO%20covid %20guidelines%20final.pdf Available at: Accessed May 25. [Google Scholar]
- 15.Li X, Guo Z, Li B, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J. 2020;66:475–481. doi: 10.1097/MAT.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marullo AG, Cavarretta E, Biondi-Zoccai G, et al. Extracorporeal membrane oxygenation for critically ill patients with coronavirus-associated disease 2019: An updated perspective of the European experience [E-pub ahead of print] Minerva Cardioangiol. 2020 doi: 10.23736/S0026-4725.20.05328-1. Accessed August 4. [DOI] [PubMed] [Google Scholar]
- 17.Loforte A, Dal Checco E, Gliozzi G, et al. Veno-venous extracorporeal membrane oxygenation support in COVID-19 respiratory distress syndrome. ASAIO J. 2020;66:734–738. doi: 10.1097/MAT.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6:1–10. doi: 10.1186/s13613-016-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg E, Balakrishna A, Habboushe J, et al. Calculated decisions: COVID-19 calculators during extreme resource-limited situations. Emerg Med Pract. 2020;22(suppl 4) CD1-5. [PubMed] [Google Scholar]
- 20.Bemtgen X, Krüger K, Supady A, et al. First successful treatment of coronavirus disease 2019 induced refractory cardiogenic plus vasoplegic shock by combination of percutaneous ventricular assist device and extracorporeal membrane oxygenation: A case report. ASAIO J. 2020;66:607–609. doi: 10.1097/MAT.0000000000001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firstenberg MS, Stahel PF, Hanna J, et al. Successful COVID-19 rescue therapy by extra-corporeal membrane oxygenation (ECMO) for respiratory failure: A case report. Patient Saf Surg. 2020;14:20. doi: 10.1186/s13037-020-00245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartman M, Hernandez R, Patel K, et al. COVID-19 respiratory failure: Targeting inflammation on VV-ECMO support. ASAIO J. 2020;66:603–606. doi: 10.1097/MAT.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K, Hikone M, Shimizu H, et al. A sporadic COVID-19 pneumonia treated with extracorporeal membrane oxygenation in Tokyo, Japan: A case report. J Infect Chemother. 2020;26:756–761. doi: 10.1016/j.jiac.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi H, Ogawa F, Honzawa H, et al. Veno-venous extracorporeal membrane oxygenation for severe pneumonia: COVID-19 case in Japan. Acute Med Surg. 2020;7 doi: 10.1002/ams2.509. e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan W, Li M, Xu M, et al. Successful treatment of COVID-19 using extracorporeal membrane oxygenation, a case report. Eur Rev Med Pharmacol Sci. 2020;24:3385–3389. doi: 10.26355/eurrev_202003_20705. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Y, Cai Z, Xianyu Y, et al. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Critical Care. 2020;24:1–48. doi: 10.1186/s13054-020-2840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sultan I, Habertheuer A, Usman AA, et al. The role of extracorporeal life support for patients with COVID-19: Preliminary results from a statewide experience. J Card Surg. 2020;35:1410–1413. doi: 10.1111/jocs.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda S. Nationwide system to centralize decisions around extracorporeal membranous oxygenation use for severe COVID‐19 pneumonia in Japan. Acute Med Surg. 2020;7:e510. doi: 10.1002/ams2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobs J, Stammers A, St.Louis J, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in COVID-19: Experience with 32 patients. ASAIO J. 2020;66:722–730. doi: 10.1097/MAT.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 33.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 34.Munshi L, Walkey A, Goligher E, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir Med. 2019;7:163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 35.Extracorporeal Life Support Organization (ELSO) 2020. Guidelines for adult cardiac failure.https://www.elso.org/Portals/0/IGD/Archive/FileManager/e76ef78eabcusersshyerdocumentselsoguidelinesforadultcardiacfailure1.3.pdf Available at: Accessed May 25. [Google Scholar]
- 36.Extracorporeal Life Support Organization (ELSO) 2020. Guidelines for adult respiratory failure.https://www.elso.org/Portals/0/ELSO %20Guidelines %20For%20Adult%20Respiratory%20Failure%201_4.pdf Available at: Accessed May 25. [DOI] [PubMed] [Google Scholar]
- 37.Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med. 2017;5:70. doi: 10.21037/atm.2016.11.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alhazzani W, Møller M, Arabi Y, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson KC, Chotirmall SH, Bai C, et al. 2020. COVID‐19: Interim guidance on management pending empirical evidence. From an American Thoracic Society‐led International Task Force.https://www.thoracic.org/covid/covid-19-guidance.pdf Available at: Accessed May 25. [Google Scholar]
- 41.Chow J, Alhussaini A, Calvillo-Argüelles O, et al. Cardiovascular collapse in COVID-19 infection: The role of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) CJC Open. 2020;2:273–277. doi: 10.1016/j.cjco.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowalewski M, Fina D, Słomka A, et al. COVID-19 and ECMO: The interplay between coagulation and inflammation—A narrative review. Crit Care. 2020;24:205. doi: 10.1186/s13054-020-02925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klinzing S, Wenger U, Steiger P, et al. External validation of scores proposed for estimation of survival probability of patients with severe adult respiratory distress syndrome undergoing extracorporeal membrane oxygenation therapy: A retrospective study. Crit Care. 2015;19:142. doi: 10.1186/s13054-015-0875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]