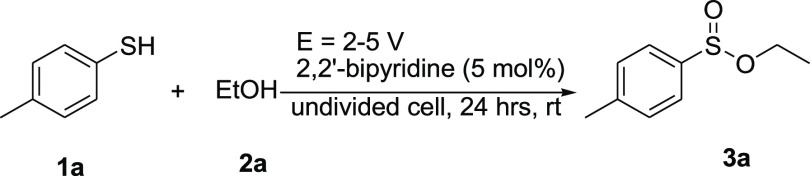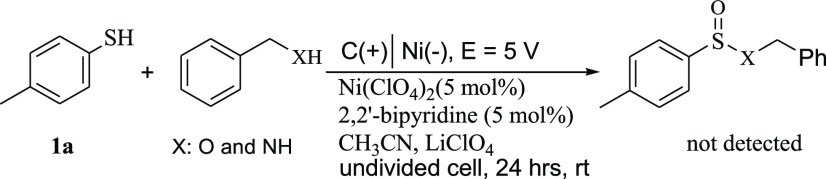Abstract
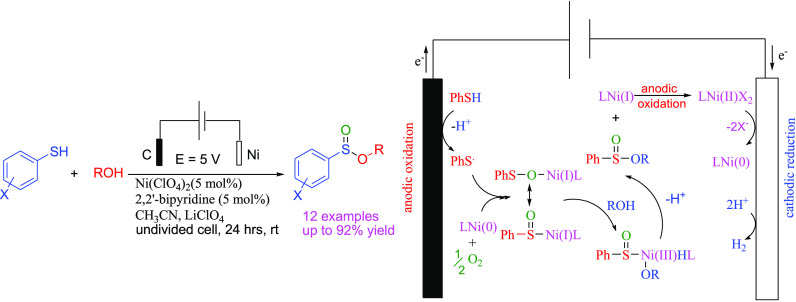
In this study, nickel-catalyzed electrochemical oxidative esterification of thiols with alcohols for the synthesis of sulfinate esters has been reported. The electrochemical oxidative esterification proceeded through a nickel-catalyzed oxidation of thiols using an undivided cell of graphite/nickel electrodes, where the nickel oxidation was studied by cyclic voltammetry. The method was conveniently and directly used for the one-pot synthesis of sulfinate esters of thiols.
Introduction
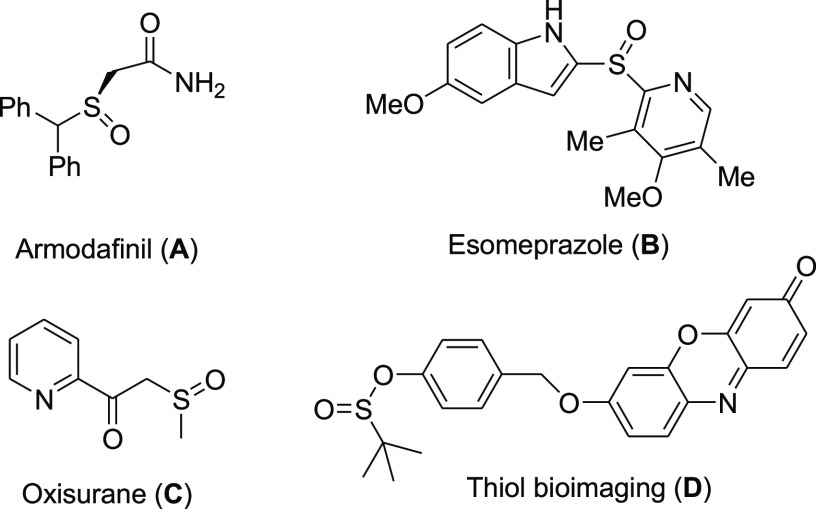
Organosulfur compounds are important with broad application in the manufacturing of many biologically active molecules, pharmaceuticals, and polymers.1 Among the various organosulfur compounds, sulfoxides and sulfinate esters with a S=O bond are of interest as they are effective in medicinal chemistry, synthesis of synthetic intermediates, and their application in biochemical studies.2−5 A number of well-known commercial sulfoxides and sulfinate esters with medicinal and biochemical applications are shown in Scheme 1.
Scheme 1. Structures of Commercial Sulfoxides and a Thiol Bioimaging Compound.
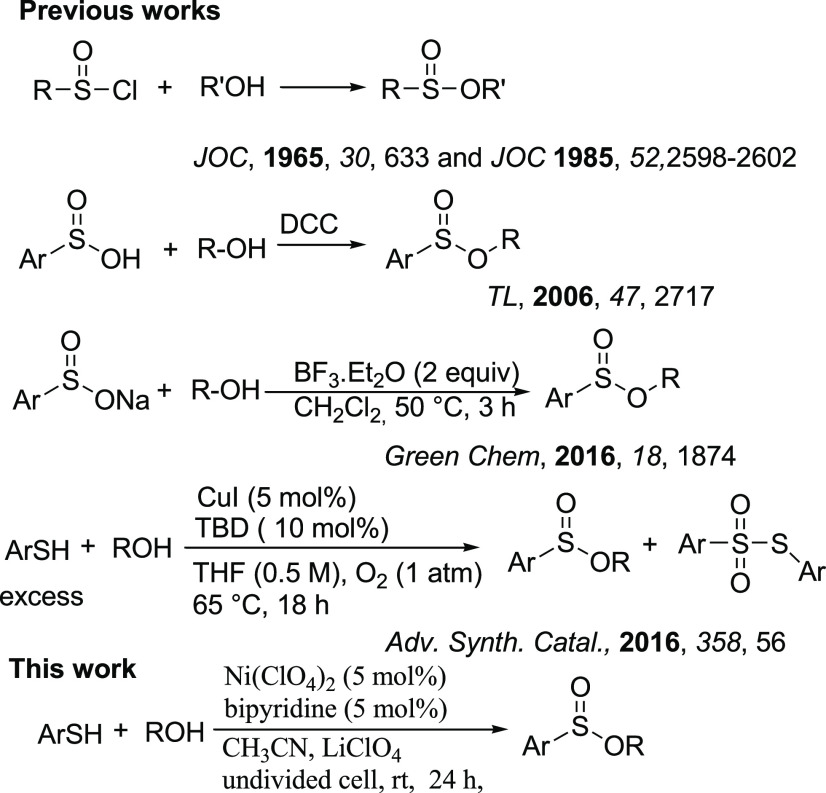
For example, compounds A, B, and C are commercial drugs and compound D was developed as a chemical probe for live-cell imaging. The O–S(O) structure of sulfinate esters serves as an important role in chiral sulfur reagents.6 On the other hand, sulfinate esters have been used as significant intermediates for the preparation of a variety of organic molecules.7 Sulfoxides are easily and widely prepared by the selective oxidation of thiols.8 However, the methods for the preparation of sulfinate esters are rare. These compounds are traditionally prepared by nucleophilic substitution of RS(O)Cl with alcohols.9,10 The method has one or more drawbacks such as a long reaction time, harsh reaction conditions, low yields, and use of hazardous materials. A more straightforward and common process for the synthesis of sulfinate esters is the reaction of sodium sulfinate or sulfinic acids with alcohols in the presence of Lewis acids or a coupling reagent.11−16 However, the preparation of sodium sulfonates or sulfinic acids is troublous and needs harsh conditions such as the reaction between an organometallic reagent and sulfur dioxide (SO2) that requires careful handling and specialized equipment because SO2 is toxic and corrosive. Recently, Jang and co-workers have reported a direct method for the preparation of sulfinate esters from the reaction of thiols (excess) with alcohols in the presence of a copper catalyst under aerobic conditions.17 The method was not selective for the preparation of sulfinate esters and an undesirable thiosulfonate side product was also obtained, and the reactions were uncontrollable (Scheme 2).
Scheme 2. Previously Reported Works and Our Work.
Electrosynthesis in an undivided cell is an attractive and important research area for the preparation of valuable intermediates and organic compounds.18 The selectivity of electrosynthesis is the main advantage over a routine oxidation–reduction reaction. Recently, Zhong and co-workers have reported an electrochemical method for the preparation of sulfinate esters from the reaction of thiols with alcohols (methanol, primary alcohols, and only one example of cyclohexanol) in an undivided electrochemical cell equipped with a platinum anode and a platinum cathode.19 According to their report, using graphite as an anode electrode has resulted in corresponding sulfinate esters in a much poorer yield, while nickel turned out to be an unsuccessful cathode in the sulfinate ester synthesis. On the other hand, excess of alcohol (near to 10-fold) has been used for the electrochemical synthesis of sulfinate esters. Recently, nickel compounds have excellent electrochemical properties due to the presence of the couple Ni(III)/Ni(I).20 Therefore, considering the increasing attention of the merger of metal catalysis and electrochemistry, the feasibility of the electrochemical method for the direct oxidative esterification of thiols with alcohols for the synthesis of sulfinate esters in an undivided cell in the presence of a Ni(II) catalyst is investigated.
Results and Discussion
Jamison and Fang reported an electrochemically nickel-catalyzed coupling of N-hydroxyphthalimide esters with aryl halides via a reversible one-electron redox process of the couple Ni(III)/Ni(II).21 Therefore, the study of a nickel-catalyzed direct oxidative esterification of thiols with alcohols for the synthesis of sulfinate esters in an undivided cell could be promising.
Initially, the oxidative esterification of 4-methylbenzenethiol (1a) with ethanol (2a) was chosen as the model reaction, and the initial screening results are listed in Table 1. As shown in Table 1, no reaction was observed when NiSO4 was used as a catalyst (entry 1) in the presence of 2,2′-bipyridine as a ligand in acetonitrile by the use of nickel foam as a cathode and modified graphite as an anode with LiClO4 as an electrolyte, at 5.0 V cell potential at room temperature for 24 h. Upon using NiCl2.6H2O, compound 3a was obtained in 56% yield after 12 h with the above conditions (entry 2). To find the optimal conditions, Ni(ClO4)2 as a convenient nickel salt that has been reported for many organic transformations was selected. Interestingly, when the reaction was conducted with Ni(ClO4)2 (5 mol %) in acetonitrile in the presence of 2,2′-bipyridine for 24 h, compound 3a was obtained in 95% yield (entry 3). Several types of electrodes, such as aluminum, graphite, nickel, cobalt, and copper, were examined for this reaction (entries 4–6). The best result was obtained when the graphite electrode was used for the anode and the nickel foam was used for the cathode (95% yield, entry 3). A trace amount of the desired product 3a was obtained using dichloromethane and dimethylformamide (DMF) (entries 7 and 8) as a solvent. Other electrolytes, such as LiBr, n-Bu4NBr, and n-Bu4NCl, were also examined and only the desired product 3a was obtained in the presence of n-Bu4NBr and n-Bu4NCl in 30 and 23% yields, respectively (entries 9–11). The reaction failed to give compound 3a at lower voltages of 2 and 3 V (entries 12 and 13).
Table 1. Screening of Various Reaction Conditions of the Oxidative Esterification of 4-Methylbenzenethiol.
| entry | anode/cathode (voltage) | solvent | electrolyte | catalyst | yield 3a (%)a |
|---|---|---|---|---|---|
| 1 | C/Ni(5) | MeCN | LiClO4 | NiSO4 | |
| 2 | C/Ni(5) | MeCN | LiClO4 | NiCl2·6H2O | 56 |
| 3 | C/Ni(5) | MeCN | LiClO4 | Ni(ClO4)2 | 95(92) |
| 4 | C/Al(5) | MeCN | LiClO4 | Ni(ClO4)2 | 20 |
| 5 | C/Cu(5) | MeCN | LiClO4 | Ni(ClO4)2 | |
| 6 | Ni/Co(5) | MeCN | LiClO4 | Ni(ClO4)2 | |
| 7 | C/Ni(5) | DMF | LiClO4 | Ni(ClO4)2 | trace |
| 8 | C/Ni(5) | CH2Cl2 | LiClO4 | Ni(ClO4)2 | trace |
| 9 | C/Ni(5) | MeCN | LiBr | Ni(ClO4)2 | |
| 10 | C/Ni(5) | MeCN | n-Bu4NBr | Ni(ClO4)2 | 30 |
| 11 | C/Ni(5) | MeCN | n-Bu4NCl | Ni(ClO4)2 | 23 |
| 12 | C/Ni(2) | MeCN | LiClO4 | Ni(ClO4)2 | |
| 13 | C/Ni(3) | MeCN | LiClO4 | Ni(ClO4)2 | |
| 14 | C/Ni(5) | MeCN | LiClO4 | Ni(II)-salen | 88b |
| 15 | C/Ni(5) | MeCN | LiClO4 | Ni(II)Cyclam | 35b |
| 16 | C/Ni(5) | MeCN | LiClO4 | ||
| 17 | C/Ni(5) | MeCN | LiClO4 | Ni(ClO4)2 | 30c |
| 18 | C/Ni(5) | MeCN | LiClO4 | Ni(ClO4)2 | 95d |
| 19 | C/Ni(5) | MeCN | LiClO4 | Ni(ClO4)2 | e |
| 20 | MeCN | LiClO4 | Ni(ClO4)2 | f |
Mixture of nickel salts (5 mol %) with 2,2′-bipyridine (5 mol %) was stirred (or other ligands; see experimental details in SI) in DMF for 1 h before adding to 4-methylbenzenethiol (1 mmol) and EtOH (1.2 equiv) in the solvent (1.5 mL, 0.66 M). All conversions (%) were determined by 1H NMR spectroscopy with an internal standard (yield in parentheses is isolated yield).
Without 2,2′-bipyridine.
Direct addition of all materials without 1 h stirring of the mixture of Ni(ClO4)2 with 2,2′-bipyridine in DMF.
Ni(ClO4)2 (10 mol %).
2,2′-bipyridine (10 mol %).
Under thermal conditions at 70 °C.
Other ligands, such as salen [N,N′-bis(salicylidene) ethylenediamine] and cyclam (1,4,8,11-tetraazacyclotetradecane), were also investigated. The reaction proceeded smoothly with Ni(II)-salen (entry 14) or Ni(II)Cyclam (entry 15) as a catalyst, and the desired product 3a was obtained in 88 and 35% yields, respectively. The reaction stopped without any catalyst (entry 16), and 3a was obtained in 30% yield by direct addition of all materials (entry 17). Further optimization indicated that an increased amount of Ni(ClO4)2 to 10 mol % gave a similar yield (entry 18). The reaction failed to give 3a with an increased amount of 2,2′-bipyridine (10 mol %) and in usual thermal conditions (entries 19 and 20).
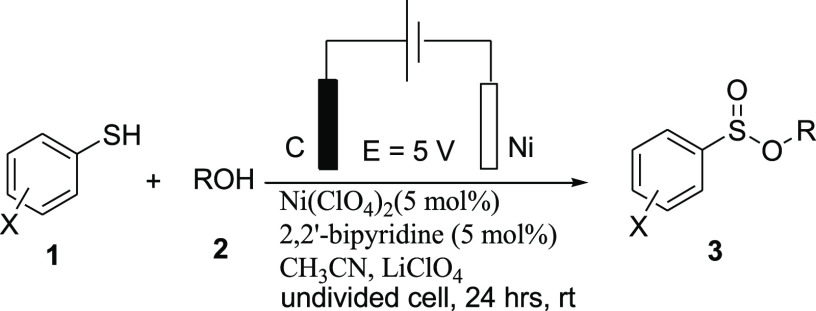
Therefore, the best reaction condition is the use of Ni(ClO4)2 for the electrochemical oxidative esterification of thiols with alcohols in the presence of 2,2′-bipyridine as a ligand in acetonitrile by the use of nickel foam as a cathode and modified graphite as an anode with LiClO4 as an electrolyte, at 5.0 V cell potential (or at a constant current of 10 mA) at room temperature for 24 h.
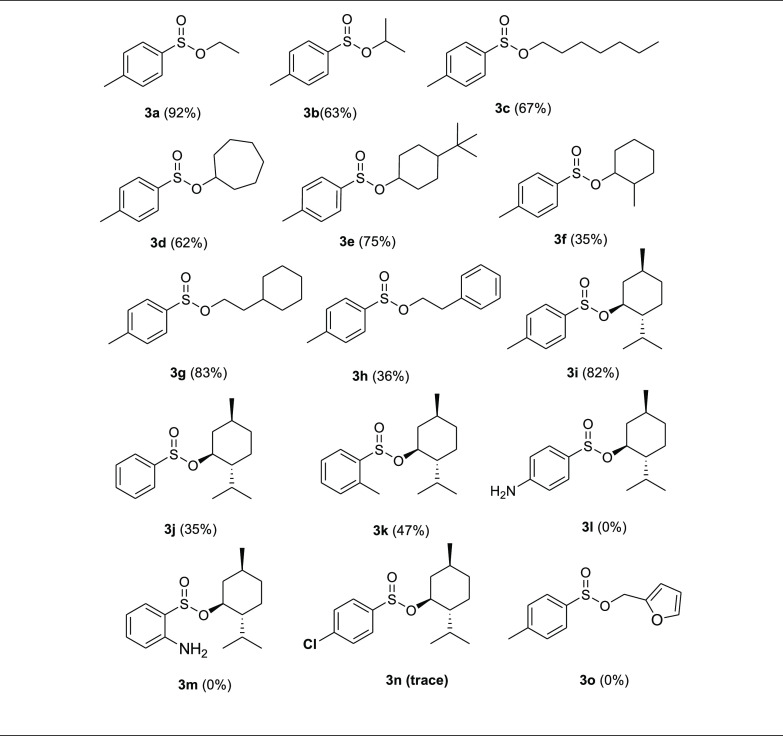
The substrate generality of this oxidative esterification reaction of thiols with alcohols was also investigated. Under the optimized conditions, a wide array of alcohols was employed in the electrochemical reaction with benzenethiols for the synthesis of sulfinate esters in good to moderate yields. The obtained results are summarized in Table 2.
Table 2. Oxidative Esterification of Benzenethiols with Alcohols in an Undivided Cella.
Thiol (1 mmol) with alcohol (1.2 mmol) in CH3CN (1.5 mL, 0.66 M). All reported yields are isolated yields.
The electrochemical oxidative esterification of 4-methylbenzenethiol with alcohols gave the corresponding sulfinate ester 3 in good to excellent yields (3a–3i, Table 2). The use of sterically hindered alcohol, l-menthol, also afforded the corresponding sulfinate ester in good yield (3i). The oxidative esterification of benzenethiol and 2-methylbenzenethiol with l-menthol gave the corresponding sulfinate esters in moderate to good yields (3j and 3k). The reaction of 2- or 4-aminobenzenethiol with l-menthol failed to give the desired products. The electrochemical oxidative esterification of 4-methylbenzenethiol with furfuryl alcohol failed to give the corresponding sulfinate ester 3o. The phenol compound easily oxidized in the reaction conditions and gave an unknown mixture of products.
The electrochemical reaction of benzyl alcohol or benzyl amine with 4-methylbenzenethiol failed to give the desired sulfinate ester or amide (Scheme 3) and only benzaldehyde (from electrochemical oxidation of benzyl alcohol) was detected under the reaction conditions. The corresponding bisulfide byproduct was also detected in the reaction mixture after further investigation.
Scheme 3. Reaction of 4-Methylbenzenethiol with Benzyl Alcohol or Benzyl Amine.
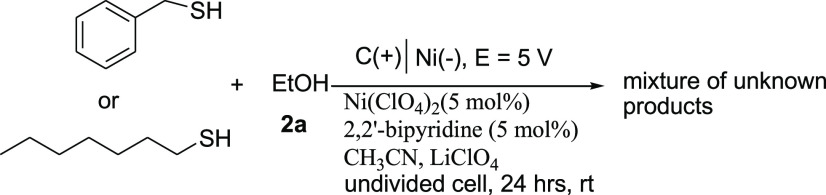
Unfortunately, the reaction with aliphatic thiols, benzylthiol, and heptanethiol gave a mixture of unknown products. It seems that the reaction intermediates resulted from alkyl thiols do not have enough stability to yield the corresponding sulfinate esters (Scheme 4). One of the suggestions for the formation of unknown products is that the intermediates of aliphatic thiols led to a Pummerer-type rearrangement.
Scheme 4. Reaction of Aliphatic Thiols with Ethanol.
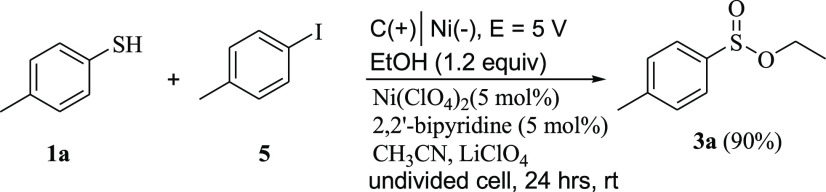
To demonstrate the high level of chemoselectivity obtained under this catalytic system for the S–O over S–C bond formation, we conducted a competition experiment. Under the optimized conditions, the electrochemical oxidative esterification reaction of 4-methylbenzenethiol with ethanol in the presence of 4-iodotoluene was examined. Results showed that no cross-coupling reaction occurred between 4-methylbenzenethiol and 4-iodotoluene (Scheme 5), and the corresponding sulfinate ester 3a was obtained chemoselectively in 90% yield.
Scheme 5. Chemoselectivity of the Oxidative Esterification of 4-Methylbenzenethiol with Ethanol in the Presence of 4-Iodotoluene.
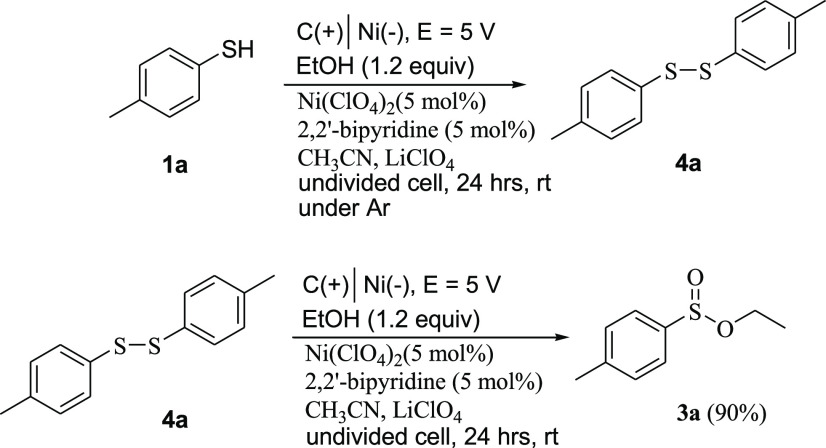
In further investigation, the electrochemical reaction of 4-methylbenzenethiol under Ar in the presence of Ni(ClO4)2 and ethanol gave the corresponding homocoupling bisulfide 4a in 78% yield (Scheme 6). A similar result was obtained by the electrochemical reaction of thiol 1a under Ar in the absence of Ni(ClO4)2 and ethanol. Further investigation showed that the oxidative esterification of bisulfide 4a with ethanol under optimized conditions gave the corresponding sulfinate ester 3a in 90% yield (Scheme 6). On the other hand, thin-layer chromatography (TLC) monitoring of the reaction mixture for 4–24 h showed no detectable intermediate. This data showed that the mechanism for this conversion is different than that reported by Zhong et al.19a
Scheme 6. Investigation of the Thiol Radical Homocoupling Reaction.
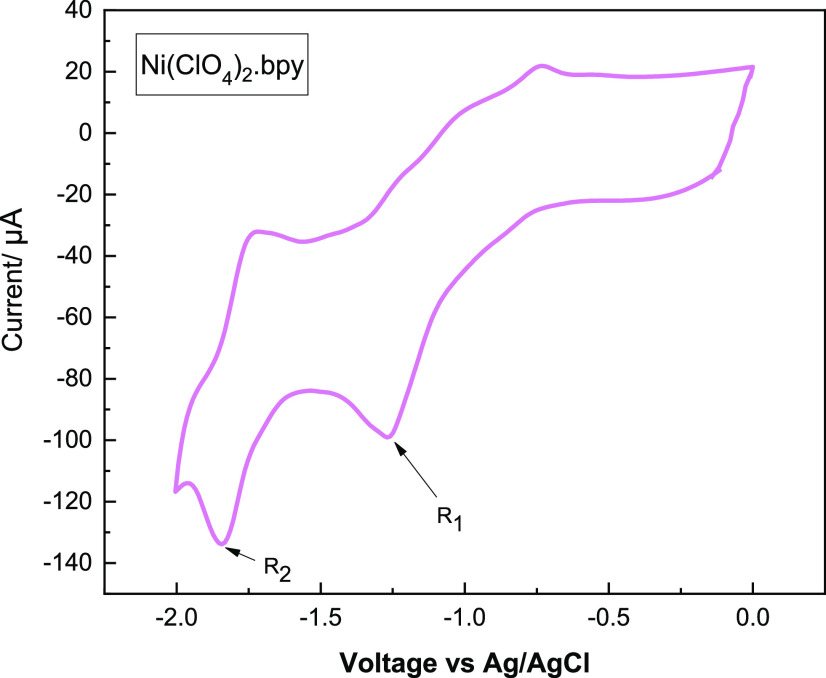
The electrochemical behavior of Ni(II)bipyridine present in a mixture of Ni(ClO4)2 and 2,2′-bipyridine was studied using the cyclic voltammetry (CV) technique (Figure 1).
Figure 1.
Cyclic voltammograms of Ni(ClO4)2.bpy (0.01 M) with a glassy carbon electrode, nickel foam as a counter electrode, and Ag/AgCl as a reference electrode in the presence of n-Bu4NPF6 (0.05 M) with a scan rate of 0.1 V s–1 in CH3CN.
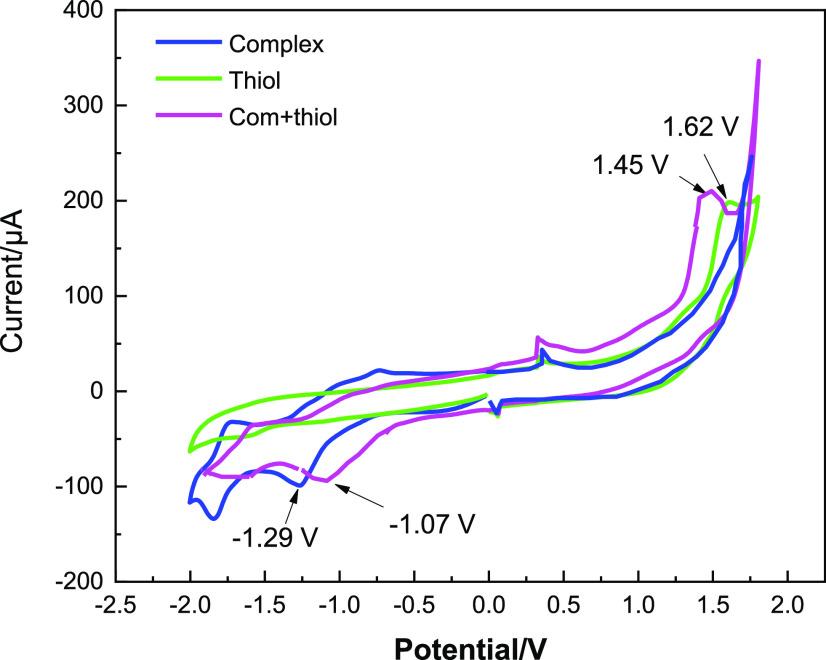
Figure 1 shows two redox peaks at −1.26 (R1) and −1.82 V (R2) corresponding to an irreversible two-electron reduction peak (Nill/Ni0)22 and a quasi-reversible reduction peak (Ni0/Ni0·–),23 respectively. In another voltammetric study, the CV analysis of thiol 1a and a mixture of 1a with Ni(II)bipyridine was studied (Figure 2). Thiol 1a exhibited almost high oxidative potential at 1.62 V vs Ag/AgCl. The oxidative wave of thiol 1a was shifted to 1.47 V, and the intensity of the peak was also increased in the presence of Ni(II)bipyridine. The results proved that the Ni(II)bipyridine complex as a catalyst could accelerate the oxidation process of thiol 1a (Figure 2).
Figure 2.
Cyclic voltammograms of Ni(ClO4)2.bpy (0.001 M) (blue), thiol 1a (0.01 M) (green), and the mixture of complex and thiol 1a (purple) with a glassy carbon electrode as a working electrode, nickel foam as a counter electrode, and Ag/AgCl as a reference electrode in the presence of n-Bu4NPF6 (0.05 M) with a scan rate of 0.1 V s–1 in CH3CN.
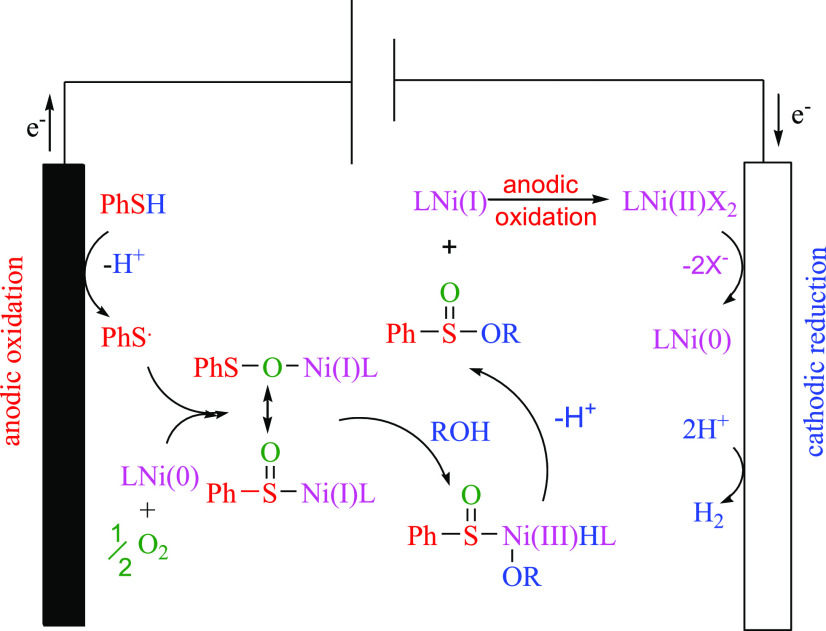
In accordance with the cyclic voltammetry results and literature reports, a proposed reaction mechanism is outlined in Scheme 7. The reaction begins with an anodic oxidation of benzenethiol to a thiyl radical and a cathodic reduction of the nickel complex to Ni(0). The interaction of the thiyl radical with Ni(0) may form a thiyloxy-Ni(I) complex in the presence of oxygen. The thiyloxy-Ni(I) complex (or its isomer) undergoes an oxidative addition with alcohols to form a sulfinate-Ni(III) complex.21 Finally, the sulfinate ester was obtained by a reductive elimination and Ni(I) was converted to Ni(II) at the anode.
Scheme 7. Proposed Mechanism for the Oxidative Esterification of Thiols with Alcohols in an Undivided Cell.
Conclusions
In conclusion, the electrochemical oxidative esterification of thiols with alcohols in the presence of Ni(ClO4)2 and 2,2′-bipyridine is reported. Under mild reaction conditions, various sulfinate esters could be synthesized in good to excellent yields. The electrochemical method showed more selectivity than the reported method for the preparation of sulfinate esters from the reaction of thiols (excess) with alcohols in the presence of a copper catalyst under aerobic conditions (uncontrollable thiosulfonate side product).17 On the other hand, there was no need for excess of thiol or alcohol for the synthesis of sulfinate esters in the presence of Ni(ClO4)2 by the use of nickel foam as a cathode and modified graphite as an anode. A great advantage of using a simple power supply system with two electrodes is a very simple and inexpensive system for the synthesis of sulfinate esters.24,25 Therefore, the presented method is highly efficient, easy to handle, air- and moisture-insensitive, and a simple method with readily available starting materials and reagents, compared with previously reported methods.
Experimental Section
General
All chemical materials and solvents were used without purification. Thin-layer chromatography was performed using silica gel 60 F254 (0.063–0.200 mm). Preparative thin-layer chromatography (PLC) was performed using silica gel (60, particle size 0.043–0.063 mm) and used for purification of products. 1H NMR (400 MHz) and 13C NMR (101 MHz) spectra were recorded on a 400 spectrometer. High-resolution mass spectrometry (HRMS) spectra were obtained on a time-of-flight liquid chromatography–mass spectrometry (TOF LC–MS) instrument. Electrochemical studies were reported utilizing PGSTAT 30 with a common three-electrode setup consisting of a glassy carbon working electrode, a nickel foam counter electrode, and Ag/AgCl reference electrodes.
General Procedure for Complex Preparation
The [Ni(bpy)(ClO4)2] complex was synthesized by a previously reported method.26 Ni(ClO4)2·6H2O (0.4 mmol, 147.87 mg) was added to 2,2′-bipyridine (0.4 mmol, 62.4 mg) in DMF (5 mL). The mixture was stirred at room temperature until a blue homogeneous solution was obtained (the method was applied for the synthesis of other complexes as shown in Table 1).
General Procedure for the Electrochemical Synthesis of Sulfinate Ester 3
Thiol (1.0 mmol) was added to a mixture of LiClO4 (53 mg, 0.5 mmol) and alcohol (1.2 mmol) in CH3CN (1.5 mL) and the mixture was stirred for 10 min at room temperature. The prepared [Ni(bpy)(ClO4)2] complex (625 μL, 5 mol %) was added to the reaction mixture in a test tube equipped with a modified graphite electrode27 as the anode and a nickel foam electrode as the cathode. The reaction mixture was stirred at room temperature for 24 h at a constant voltage of 5 V. After completion of the reaction, EtOAc (10 mL) was added to the reaction mixture, which was then washed with H2O (2 × 5 mL). The organic phase was dried over Na2SO4 and evaporated under vacuum. The pure sulfinate ester 3 was purified by flash column chromatography on silica (EtOAc:n-hexane, 1:99) or by PLC. Yields reported in the following are isolated yields after column chromatography.
Ethyl 4-Methylbenzenesulfinate (3a)
Yellow oil (137 mg, 92%);281H NMR (400 MHz, dimethyl sulfoxide (DMSO)): δ 7.62 (d, J = 7.4 Hz, 2H), 7.45 (d, J = 7.8 Hz, 2H), 4.10–3.96 (m, 1H), 3.79–3.65 (m, 1H), 2.41 (s, 3H), 1.20 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, DMSO): δ 143.0, 142.2, 130.1, 125.4, 61.4, 21.6, 15.9.
Isopropyl 4-Methylbenzenesulfinate (3b)
Colorless oil (125 mg, 63%);281H NMR (400 MHz, DMSO): δ 7.61 (d, J = 7.3 Hz, 2H), 7.44 (d, J = 7.8 Hz, 2H), 4.52–4.58 (m, 1H), 2.41 (s, 3H), 1.31 (d, J = 6.1 Hz, 3H), 1.17 (d, J = 6.2 Hz, 3H); 13C NMR (100 MHz, DMSO): δ 143.0, 142.8, 130.1, 125.3, 73.0, 24.1, 23.9, 21.5.
n-Heptyl 4-Methylbenzenesulfinate (3c)
Yellow oil (175 mg, 67%);291H NMR (400 MHz, DMSO): δ 7.61 (d, J = 7.1 Hz, 2H), 7.45 (d, J = 7.6 Hz, 2H), 4.01–4.04 (m, 1H), 3.65–3.59 (m, 1H), 2.41 (s, 3H), 1.57–1.53 (m, 2H), 1.18–1.25 (m, 8H), 0.85 (t, J = 6.7 Hz, 3H); 13C NMR (101 MHz, DMSO): δ 143.1, 142.8, 130.1, 125.3, 79.9, 35.7, 35.6, 28.5, 28.1, 28.1, 22.4, 21.5.
Cycloheptyl 4-Methylbenzenesulfinate (3d)
Colorless oil (157 mg, 62%);301H NMR (400 MHz, DMSO): δ 7.60 (d, J = 7.5 Hz, 2H), 7.43 (d, J = 7.7 Hz, 2H), 4.53–4.45 (m, 1H), 2.41 (s, 3H), 2.04–1.93 (m, 2H), 1.82–1.72 (m, 2H), 1.67–1.55 (m, 2H), 1.59–1.50 (m, 4H), 1.30–1.37 (m, 2H).13C NMR (101 MHz, DMSO): δ 143.1, 142.8, 130.1, 125.2, 80.1, 35.8, 28.1, 22.4, 21.3.
4-(Tert-butyl)cyclohexyl 4-Methylbenzenesulfinate (3e)
Colorless oil (221 mg, 75%); 1H NMR (400 MHz, DMSO): δ 7.60 (d, J = 8.0 Hz, 2H), 7.43 (d, J = 7.4 Hz, 2H), 4.19 (tt, J = 13.8, 5.8 Hz, 1H), 2.41 (s, 3H), 2.11 (d, J = 10.0 Hz, 1H), 1.89–1.64 (m, 2H), 1.59–1.21 (m, 3H), 1.05 (dt, J = 27.5, 12.0 Hz, 3H), 0.83 (s, 9H); 13C NMR (101 MHz, DMSO): δ 142.68, 136.53, 129.95, 125.68, 78.95, 46.87, 34.66, 34.38, 33.28, 28.06, 27.75, 25.63, 21.73.
HRMS (electrospray ionization (ESI)) m/z: calcd for C17H26O2NaS [M + Na]+: 317.1551, found: 317.1552.
2-Phenylethyl-4-Methylbenzenesulfinate (3f)
Colorless oil (91 mg, 35%);281H NMR (400 MHz, DMSO): δ 7.53 (d, J = 7.8 Hz, 2H), 7.33–7.23 (m, 5H), 7.18 (t, J = 7.1 Hz, 2H), 4.26 (dd, J = 16.5, 7.6 Hz, 1H), 3.84 (dd, J = 16.6, 7.5 Hz, 1H), 2.96 (t, J = 7.0 Hz, 2H), 2.44 (s, 3H); 13C NMR (100 MHz, DMSO): δ 142.5, 141.4, 137.2, 129.5, 128.8, 128.3, 126.5, 125.1, 64.6, 36.1, 21.4.
2-Cyclohexylethyl-4-Methylbenzenesulfinate (3g)
Colorless viscous oil (221 mg, 83%); 1H NMR (400 MHz, DMSO): δ 7.61 (d, J = 8.1 Hz, 2H), 7.44 (d, J = 8.0 Hz, 2H), 4.27 (dt, J = 12.4, 6.1 Hz, 1H), 4.19 (dt, J = 12.3, 6.2 Hz, 1H), 2.41 (s, 3H), 1.82–1.47 (m, 11H), 1.30 (dt, J = 11.5, J = 6.4 Hz, 2H); 13C NMR (101 MHz, DMSO): δ 142.7, 136.5, 129.9, 125.7, 78.9, 46.9, 34.7, 34.38, 33.3, 28.1, 27.8, 25.6, 21.7. HRMS (ESI) m/z: calcd for C15H22O2NaS [M + Na]+: 289.1238, found: 289.1237.
2-Methylcyclohexyl-4-Methylbenzenesulfinate (3h)
Colorless oil (90 mg, 36%); 1H NMR (400 MHz, DMSO): δ 7.62 (d, J = 8.1 Hz, 2H), 7.44 (d, J = 8.0 Hz, 2H), 3.81–3.97 (m, 1H), 2.41 (s, 3H), 1.78–1.17 (m, 9H), 0.90 (d, J = 6.4 Hz, 3H); 13C NMR (101 MHz, DMSO): δ 143.1, 142.8, 130.1, 125.3, 80.1, 35.8, 35.6, 25.6, 28.1, 28.1, 22.4, 21.5. HRMS (ESI) m/z: calcd for C14H20O2NaS [M + Na]+: 275.1082, found: 275.1079.
(1S,2R,5S)-2-Isopropyl-5-Methylcyclohexyl 4-Methylbenzenesulfinate (3i)
Yellow viscous oil (243 mg, 82%);281H NMR (400 MHz, DMSO): δ 7.59 (d, J = 7.6 Hz, 2H), 7.44 (d, J = 7.5 Hz, 2H), 4.06–4.23 (m, 1H), 2.41 (s, 3H), 2.04–1.94 (m, 2H), 1.62–1.64 (m, 2H), 1.28–1.14 (m, 2H), 0.94–0.92 (m, 3H), 0.88–0.80 (m, 9H).13C NMR (101 MHz, DMSO): δ 143.0, 142.8, 130.2, 124.7, 81.3, 80.0, 48.1, 43.6, 33.9, 31.4, 25.4, 22.4, 21.5, 21.1, 16.0.
(1S,2R,5S)-2-Isopropyl-5-Methylcyclohexybenzenesulfinate (3j)
Colorless oil (98 mg, 35%); 1H NMR (400 MHz, DMSO): δ 7.75–7.60 (m, 5H), 4.08–4.25 (m, 1H), 2.10–1.77 (m, 2H), 1.63 (d, J = 12.4 Hz, 2H), 1.46–1.26 (m, 2H), 1.25–0.98 (m, 3H), 0.95–0.79 (m, 9H); 13C NMR (101 MHz, DMSO): δ 132.6, 131.7, 127.0, 123.5, 79.5, 79.0, 48.1, 34.7, 30.8, 25.7, 23.3, 22.0, 21.9, 18.6.
HRMS (ESI) m/z: calcd for C16H24O2NaS [M + Na]+: 303.1395, found: 303.1394.
(1S,2R,5S)-2-Isopropyl-5-Methylcyclohexyl 2-Methylbenzenesulfinate (3k)
Colorless viscous oil (137 mg, 47%); 1H NMR (400 MHz, DMSO): δ 7.82 (d, J = 6.9 Hz, 1H), 7.57–7.46 (m, 2H), 7.36 (d, J = 7.3 Hz, 1H), 4.01–4.17 (m, 1H), 2.48 (s, 3H), 2.04–1.92 (m, 1H), 1.94–1.96 (m, 2H), 1.60–1.62 (m, 2H), 1.24–1.26 (m, 3H), 0.93–0.76 (m, 9H). 13C NMR (101 MHz, DMSO): δ 144.1, 143.6, 132.6, 131.7, 127.0, 123.5, 79.9, 78.5, 48.4, 34.7, 30.8, 25.7, 23.3, 22.4, 21.9, 18.6, 15.3. HRMS (ESI) m/z: calcd for C17H26O2NaS [M + Na]+: 317.1551, found: 317.1541.
p-Tolyl Disulfide (4a)
Colorless oil; 1H NMR (400 MHz, DMSO): δ 7.43 (d, J = 7.4 Hz, 2H), 7.24 (d, J = 7.6 Hz, 2H), 2.29 (s, 3H); 13C NMR (101 MHz, DMSO): δ 138.8, 133.2, 130.9, 129.3, 21.6.
MHz, CDCl3: δ 19.19; HRMS (ESI): calcd for C21H31NO6NaP2 [M + Na]+: 478.1523, found: 478.1523.
Acknowledgments
We thank the Institute for Advanced Studies in Basic Sciences for supporting this work. The authors thank Dr. F. Varmaghani, Institute for Advanced Studies in Basic Sciences, for her help with carrying out the CV analysis. The authors also thank Haruhiko Fukaya, Tokyo University of Pharmacy and Life Sciences, for his help with carrying out the HRMS measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00953.
General experimental procedures, characterization data, and copies of 1H NMR and 13C NMR of all products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cremlyn R. J.An Introduction to Organosulfur Chemistry, 1st ed.; John Wiley and Sons: Chichester, 1996. [Google Scholar]
- Bentley R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–614. 10.1039/b418284g. [DOI] [PubMed] [Google Scholar]
- Evans J. W.; Fierman M. B.; Miller S. J.; Ellman J. A. Catalytic Enantioselective Synthesis of Sulfinate Esters through the Dynamic Resolution of tert-Butanesulfinyl Chloride. J. Am. Chem. Soc. 2004, 126, 8134–8135. 10.1021/ja047845l. [DOI] [PubMed] [Google Scholar]
- Aziz J.; Messaoudin S.; Alami M.; Hamze A. Sulfinate derivatives: dual and versatile partners in organic synthesis. Org. Biomol. Chem. 2014, 12, 9743–9759. 10.1039/C4OB01727G. [DOI] [PubMed] [Google Scholar]
- Malwal S.; Andhalkar A. S.; Sengupta K.; Chakrapani H.; Harinath C. A highly selective sulfinate ester probe for thiol bioimaging. Chem Commun. 2014, 50, 11533–11535. 10.1039/C4CC05462H. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M.; Drabowicz J.; Kielbasinski P.. Chiral Sulfur Reagents: Applications in Asymmetric and Stereoselective Synthesis, 1st ed.; CRC Press, 1997. [Google Scholar]
- Gafur S. H.; Waggoner S. L.; Jacobsen E. Efficient Synthesis of Sulfinate Esters and Sulfinamides via Activated Esters of p-Toluenesulfinic Acid. Synthesis 2018, 50, 4855–4866. 10.1055/s-0037-1610254. [DOI] [Google Scholar]
- Drago C.; Caggiano L.; Jackson R. F. W. Vanadium-Catalyzed Sulfur Oxidation/Kinetic Resolution in the Synthesis of Enantiomerically Pure Alkyl Aryl Sulfoxides. Angew. Chem., Int. Ed. 2005, 44, 7221–7223. 10.1002/anie.200503046. [DOI] [PubMed] [Google Scholar]
- Douglass I. B. Sulfinate Esters I. Their Preparation and Some Properties. J. Org. Chem. 1965, 30, 633–635. 10.1021/jo01013a081. [DOI] [Google Scholar]
- Klunder J. M.; Sharpless K. B. Convenient synthesis of sulfinate esters from sulfonyl chlorides. J. Org. Chem. 1987, 52, 2598–2602. 10.1021/jo00388a051. [DOI] [Google Scholar]
- Hajipour A. R.; Falahati A. R.; Ruohu A. E. An efficient and novel method for the synthesis of sulfinate esters under solvent-free conditions. Tetrahedron Lett. 2006, 47, 2717–2719. 10.1016/j.tetlet.2006.02.080. [DOI] [Google Scholar]
- Drabowicz J.; Kwiatkowska M.; Kielbasinski P. The First Effective Procedure for the Direct Esterification and Thiolysis of Sulfinic Acids. Synthesis 2008, 3563–3564. 10.1055/s-0028-1083205. [DOI] [Google Scholar]
- Huang M.; Hu L.; Shen H.; Liu Q.; Hussain M. L.; Pan J.; Xiong Y. Sulfination of alcohols with sodium sulfinates promoted by BF3·OEt2: an unexpected access. Green Chem. 2016, 18, 1874–1879. 10.1039/C5GC02846A. [DOI] [Google Scholar]
- Li H.-J.; Wang R.; Gao J.; Wang Y.-Y.; Luo D.-H.; Wua Y.-C. Bismuth(III) Bromide-Catalysed Substitution of Benzyl Alcohols with Arylsulfonylmethyl Isocyanides: An Unexpected Access to Sulfinates. Adv. Synth. Catal. 2015, 357, 1393–1397. 10.1002/adsc.201401173. [DOI] [Google Scholar]
- Jacobsen E.; Chavda M. K.; Zikpi K. M.; Waggoner S. L.; Passini D. J.; Wolfe J. A.; Larson R.; Beckley C.; Hamaker C. C.; Hitchock S. R. A mixed anhydride approach to the preparation of sulfinate esters and allylic sulfones: Trimethylacetic p-toluenesulfinic anhydride. Tetrahedron Lett. 2017, 58, 3073–3077. 10.1016/j.tetlet.2017.06.074. [DOI] [Google Scholar]
- Du B.; Li Z.; Qian P.; Han J.; Pan Y. Copper-Catalyzed Aerobic Oxidative Reaction of Sulfonyl Hydrazides with Alcohols: An Easy Access to Sulfinates. Chem. Asian J. 2016, 11, 478–481. 10.1002/asia.201501262. [DOI] [PubMed] [Google Scholar]
- Shyam P. K.; Kim Y. K.; Lee C.; Jang H.-Y. Copper-Catalyzed Aerobic Formation of Unstable Sulfinyl Radicals for the Synthesis of Sulfinates and Thiosulfonates. Adv. Synth. Catal. 2016, 358, 56–61. 10.1002/adsc.201500785. [DOI] [Google Scholar]
- Sathisha V.; Swamy B. E. K.; Mahanthesha K. R.; Sathisha A.; Anvekar T. S.; Eswarappa B. Electrochemical Investigation of Ni(II) Ions in Nickel Chloride and Nickel Sulfate at Carbon Paste Electrode: A Cyclic Voltammetric Study. Anal. Bioanal. Electrochem. 2013, 5, 729–739. [Google Scholar]
- a Ai C.; Shen H.; Song D.; Li Y.; Yi X.; Wang Z.; Ling F.; Zhong W. Metal- and oxidant-free electrochemical synthesis of sulfinic esters from thiols and alcohols. Green Chem. 2019, 21, 5528–5531. 10.1039/C9GC02125F. [DOI] [Google Scholar]; b He Y.; Zhang J.; Xu L.; Wei Y. Electrochemical synthesis of sulfinic esters from alcohols and thiophenols. Tetrahedron Lett. 2020, 61, 151631 10.1016/j.tetlet.2020.151631. [DOI] [Google Scholar]
- Pang S.; Yang X.; Cao Z.-H.; Zhang Y.-L.; Zhao Y.; Huang Y.-Y. Intermolecular [2 + 2] Cycloaddition/Isomerization of Allenyl Imides and Unactivated Imines for the Synthesis of 1-Azadienes Catalyzed by a Ni(ClO4)2·6H2O Lewis Acid. ACS Catal. 2018, 8, 5193–5199. 10.1021/acscatal.8b01454. [DOI] [Google Scholar]
- a Li H.; Breen C. P.; Seo H.; Jamison T. F.; Fang Y.-Q.; Bio M. M. Ni-Catalyzed Electrochemical Decarboxylative C–C Couplings in Batch and Continuous Flow. Org. Lett. 2018, 20, 1338–1341. 10.1021/acs.orglett.8b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Vellakkaran M.; Das J.; Bera S.; Banerjee D. Nickel-catalysed alkylation of C(sp3)-H bonds with alcohols: direct access to functionalised N-heteroaromatics. Chem. Commun. 2018, 54, 12369–12372. 10.1039/C8CC06370B. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Luo Y.-W.; Xue T.; Zhang A.-J.; Lu J.-X. Nickel-catalyzed Electrochemical Coupling of Phenyl Halide and Study of Mechanism. Chin. J. Chem. 2006, 24, 877–880. 10.1002/cjoc.200690167. [DOI] [Google Scholar]
- Lowry M. S.; Goldsmith J. I.; Slinker J. D.; Rohl R.; Pascal J. R. A.; Malliaras G. G.; Bernhard S. Single-Layer Electroluminescent Devices and Photoinduced Hydrogen Production from an Ionic Iridium(III) Complex. Chem. Mater. 2005, 17, 5712–5719. 10.1021/cm051312+. [DOI] [Google Scholar]
- Siu J. C.; Parry J. B.; Lin S. Aminoxyl-Catalyzed Electrochemical Diazidation of Alkenes Mediated by a Metastable Charge-Transfer Complex. J. Am. Chem. Soc. 2019, 141, 2825–2831. 10.1021/jacs.8b13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z.; Zhang W.; Wang Y.; Kong L.; Karotsis G.; Wang Y.; Pan Y. Electrochemically Promoted Fluoroalkylation-Distal Functionalization of Unactivated Alkenes. Org. Lett. 2019, 21, 1857–1862. 10.1021/acs.orglett.9b00444. [DOI] [PubMed] [Google Scholar]
- Kawamata C.; Li Y.; Nakamura H.; Vantourout J. C.; Liu Z.; Hou Q.; Bao D.; Starr J. T.; Chen J.; Yan M. Electrochemically Enabled, Nickel-Catalyzed Amination. Angew. Chem., Int. Ed. 2017, 56, 13088–13093. 10.1002/anie.201707906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrouzi L.; Bagheri R.; Song Z.; Kazemi F.; Kaboudin B.; Najafpour M. M. Oxidation of alkylarenes by modified graphite. Mater. Res. Express 2019, 6, 125607 10.1088/2053-1591/ab54db. [DOI] [Google Scholar]
- Ji Y. Z.; Li H. J.; Zhang J. Y.; Wu Y. C. Sodium Arenesulfinates-Involved Sulfinate Synthesis Revisited: Improved Synthesis and Revised Reaction Mechanism. Eur. J. Org.Chem. 2019, 2019, 1846–1855. 10.1002/ejoc.201900097. [DOI] [Google Scholar]
- Pogaku N.; Krishna P. R.; Prapurna Y. L. Substrate- and temperature-controlled divergence in reactions of alcohols with TosMIC catalyzed by BF3 · Et2O: Facile access to sulfinates and sulfones. Synth. Commun. 2017, 47, 1239–1249. 10.1080/00397911.2017.1321128. [DOI] [Google Scholar]
- Huang M.; Hu L.; Shen H.; Liu Q.; Hussain M. I.; Pan J.; Xiong Y. Sulfination of alcohols with sodium sulfinates promoted by BF3·OEt2: an unexpected access. Green Chem. 2016, 18, 1874–1879. 10.1039/C5GC02846A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.