Abstract
The release of negative regulators of immune activation (immune checkpoints) that limit antitumor responses has resulted in unprecedented rates of long-lasting tumor responses in patients with a variety of cancers. This can be achieved by antibodies blocking the cytotoxic T lymphocyte antigen-4 (CTLA-4) or the programmed death-1 (PD-1) pathway, either alone or in combination. The main premise for inducing an immune response is the pre-existence of antitumor T cells that were limited by specific immune checkpoints. Most patients who have tumor responses maintain long lasting disease control, yet one third of patients relapse. Mechanisms of acquired resistance are currently poorly understood, but evidence points to alterations that converge on the antigen presentation and interferon gamma signaling pathways. New generation combinatorial therapies may overcome resistance mechanisms to immune checkpoint therapy.
Introduction:
In 2013, Science named cancer immunotherapy it’s Breakthrough of the Year, based on therapeutic gains being made in two fields: chimeric antigen receptor (CAR)-modified T cells and immune modulation using antibodies which block immune regulatory checkpoints. It is critical to note that the apparent rapid clinical progress reported in the last few years was the result of decades of investment in basic science in numerous fields. Without basic mechanistic knowledge in molecular biology, virology, immunology, cell biology and structural biology, clinical advances in cancer immunotherapy never would have been realized. It is also important to consider the long history of efforts to employ the potency of the immune system as a therapeutic modality for cancer. The field traces its earliest efforts to the observations of William Coley, a surgeon in New York, who correlated the occurrence of post-operative infection with improved clinical outcomes in cancer patients. After a series of fits and starts throughout the ensuing century, several immunotherapeutics were approved for use in cancer, including Bacillus Calmette-Guerin, interferon-alpha and interleukin-2 (IL-2). The latter is particularly important in that it demonstrated for the first time that advanced metastatic cancer, specifically melanoma and renal cell carcinoma, could be durably controlled in a small subset of patients using a cytokine expanding T cells. The activity of IL-2 substantiated the importance of adaptive immunity in controlling tumors and provided a solid foundation for the incorporation of basic science knowledge of T cell regulation in the development of new immunotherapy strategies.
CTLA-4 as a non-redundant immune checkpoint and clinical activity
A pivotal moment occurred when a protein known as cytotoxic T lymphocyte antigen-4 (CTLA-4) was demonstrated to have a potent inhibitory role in regulating T cell responses by two groups, one led by James Allison and the other by Jeffrey Bluestone (1, 2). In resting T cells, CTLA-4 is an intracellular protein; however, after T cell receptor engagement and a co-stimulatory signal through CD28, CTLA-4 translocates to the cell surface where it outcompetes CD28 for binding to critical costimulatory molecules (CD80, CD86) and mediates inhibitory signaling into the T cell, resulting in arrest of both proliferation and activation (Fig. 1) (1). Generation of mouse models lacking CTLA-4 provided additional support of CTLA-4 as a non-redundant co-inhibitory pathway as those animals died of fulminant lymphocytic infiltration of almost all organs (1). While Bluestone went on to apply this critical knowledge to control autoimmune diseases, Allison theorized that if this molecular ‘brake’ could be transiently blocked with an antibody, that might allow for the T cell repertoire to proliferate and become activated to a higher point than normal physiology would allow (1). After initial preclinical proof-of-principle studies conclusively showed that checkpoint blockade with a CTLA-4 blocking antibody could lead to durable regression of established tumors in syngeneic animal models (1, 2), the strategy moved toward clinical evaluation.
Fig. 1.
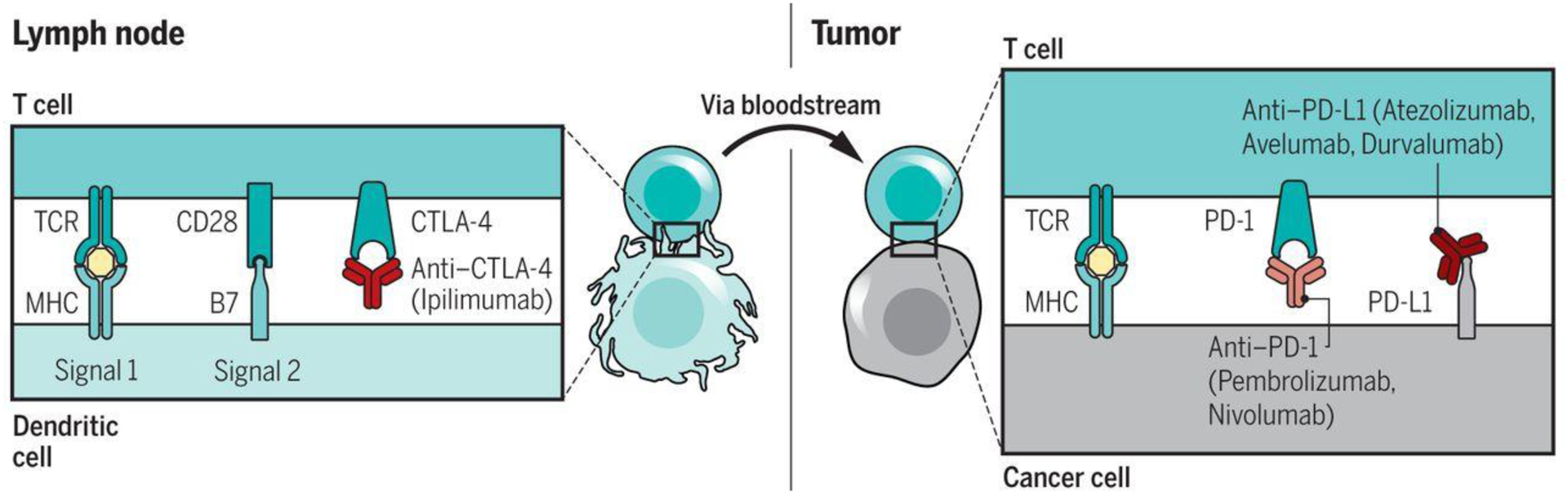
Blockade of CTLA-4 and PD-1/L1 to induce antitumor responses. Left) CTLA-4 is a negative regulator of costimulation that is required for initially activating an antitumor T cell in a lymph node upon recognition of its specific tumor antigen presented by an antigen-presenting cell. The activation immune checkpoint CTLA-4 can be blocked with anti-CTLA-4 antibodies. Right) Once the T cells are activated, they circulate through the body to find their cognate antigen presented by cancer cells. Upon their recognition, the triggering of the T cell receptor (TCR) leads to the expression of the negative regulatory receptor PD-1, and the production of interferon-gamma results in the reactive expression of PD-L1, turning off the antitumor T cell responses. This negative interaction can be blocked by anti-PD-1 or anti-PD-L1 antibodies.
Initially, two fully-human CTLA-4 blocking antibodies (ipilimumab and tremelimumab) entered clinical trials in patients with advanced cancer in 2000 (Fig. 2). It quickly became apparent that durable tumor regressions could occur, although these were relatively infrequent and accompanied by a set of mechanism-related toxicities resulting from tissue-specific inflammation (3, 4). The most common of these toxicities included enterocolitis, inflammatory hepatitis and dermatitis. Algorithmic use of corticosteroids or other forms of immune suppression readily controlled these symptoms without any apparent loss of anti-tumor activity (5). However, less frequent adverse events also included inflammation of the thyroid, pituitary and adrenal glands with the need for lifelong hormone replacement. Clinical activity of CTLA-4 blockade was most apparent in patients with advanced metastatic melanoma, with a 15% rate of objective radiographic response that has been durable in some patients for >10 years since stopping therapy (6, 7). The patterns of clinical response shown by radiographic imaging after ipilimumab were sometimes distinct from those associated with therapies that have more direct anti-proliferative mechanisms of action (8). Patients treated with ipilimumab sometimes showed delayed response after initial progression or new tumors appearing and then regressing while baseline tumors decreased in size. This led to challenges in securing regulatory approval based on the commonly used surrogate metrics of objective response rate, or progression free survival. Instead, it necessitated assessment of overall survival, a much longer-term outcome, as the primary endpoint registration trials. Eventually, two large phase 3 trials showed that ipilimumab was the first treatment to significantly extend survival in metastatic melanoma when compared with a peptide vaccine (9), or with standard dacarbazine chemotherapy (10). FDA approval was granted in 2011. Tremelimumab is still under investigation in clinical trials and additional CTLA-4 blocking antibodies have recently entered clinical trials (NCT02694822).
Fig. 2.
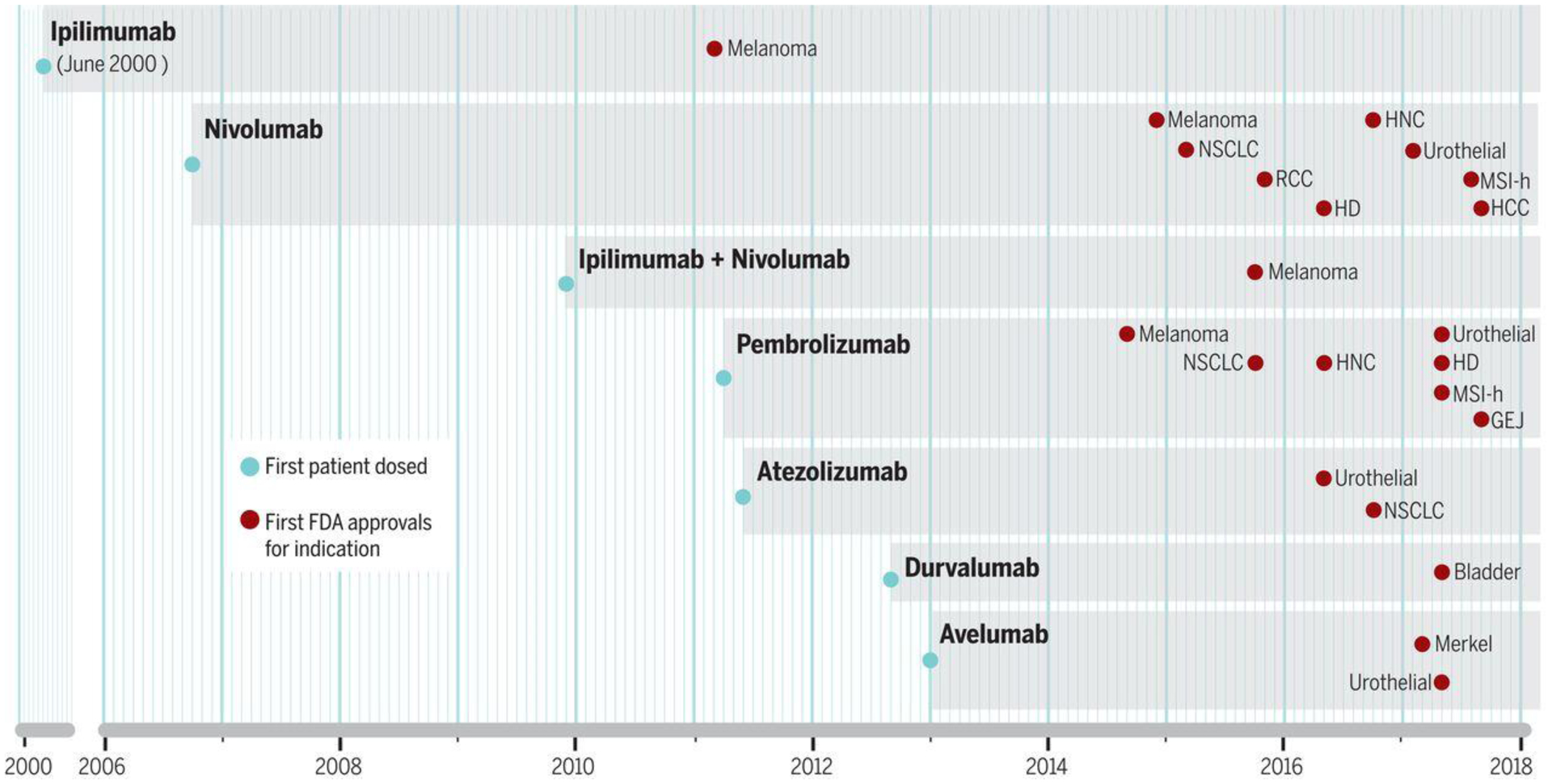
Timing of clinical development of anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies from first administration to humans to FDA approval. Thus far, there has been drug regulatory approval for six antibodies blocking immune checkpoints and one combination of two immune checkpoints. The gray shading represents the period of clinical development of each of these antibodies from the dosing of the first patient until their regulatory approval (red checkmarks) in different indications.
Given the relatively low response rate and frequent toxicity associated with CTLA-4 blockade, identification of predictive and pharmacodynamic biomarkers emerged as research priorities. Analysis of tumors from patients with or without a response to anti-CTLA-4 therapy support that a higher tumor mutational burden is associated with higher likelihood of response (11, 12). On-treatment increases in peripheral blood absolute lymphocyte counts and induction of the inducible costimulator ICOS both correlate with eventual treatment response (13). Despite numerous pre-clinical mouse studies showing that CTLA-4 blocking antibodies with appropriate Fc domains could mechanistically deplete regulatory T cells (Tregs) in regressing tumors, data remains scarce in humans associating this with clinical response. A recently initiated clinical trial (NCT03110307) is investigating a version of ipilimumab with enhanced depleting capability via a non-fucosylated Fc domain to test this hypothesis further.
PD-1 as a non-redundant immune checkpoint
The programmed death-1 (PD-1) receptor has emerged as a dominant negative regulator of anti-tumor T cell effector function when engaged by its ligand PD-L1, expressed on the surface of cells within a tumor. PD-1 bears its name from its initial description as a receptor inducing cell death of an activated T cell hybridoma (14). However, further work demonstrated that it is instead an immune checkpoint, with its inhibitory function mediated by the tyrosine phosphatase SHP-2 that de-phosphorylates signaling molecules downstream of the T cell receptor (TCR) signaling molecules (15). PD-1 has two ligands, programmed death-ligand 1 (PD-L1; also known as CD274 or B7-H1), which is broadly expressed by many somatic cells mainly upon exposure to pro-inflammatory cytokines (15), and programmed death-ligand 2 (PD-L2, also known as CD273 or B7-DC), which has more restricted expression in antigen-presenting cells (15). Inflammation-induced PD-L1 expression in the tumor microenvironment results in PD-1-mediated T cell exhaustion, inhibiting the antitumor cytotoxic T cell response (15–17) (Fig. 1 and Fig. 3).
Fig. 3.
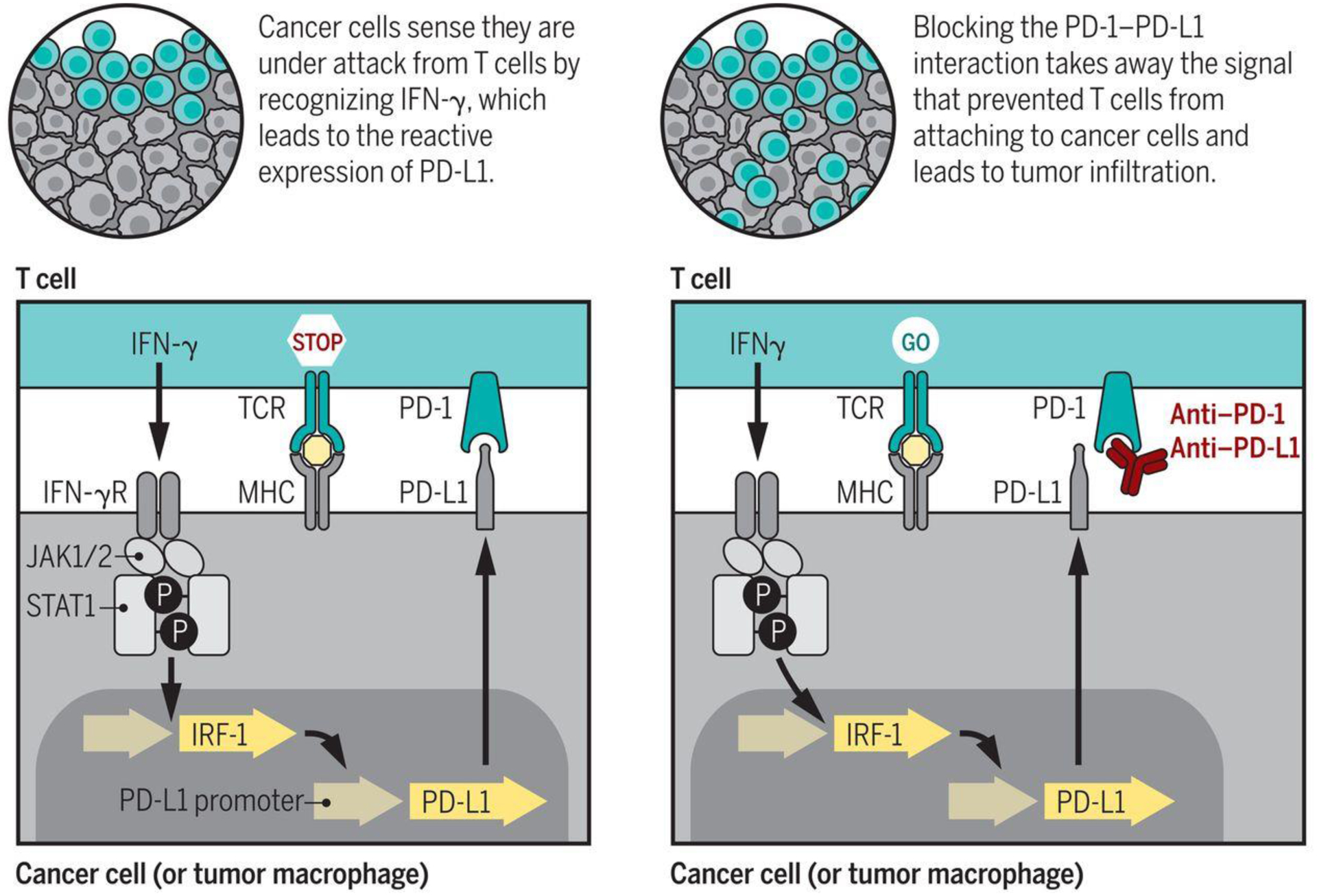
Mechanism of action of PD-1 blockade therapy. Left) the T cell receptor (TCR) recognition of the cognate antigen presented by MHC molecules on the surface of cancer cells results in T cell activation. T cells then produce interferon-gamma and other cytokines. Cancer cells and other cells in the tumor microenviroment have interferon gamma receptors that signal through the Janus kinases 1 and 2 (JAK1 and JAK2), which phosphorylate and activate signal transducers and activators of transcription (STAT) proteins that dimerize and turn on a series of interferon-response genes, including the interferon regulatory factor 1 (IRF-1), which binds to the promoter of PD-L1 leading to its surface expression. The reactive expression of PD-L1 turns off the T cells that are trying to attack the tumor, and these T cells remain in the margin of the cancer. Right) Blockade of the PD-1:PD-L1 interaction with therapeutic antibodies results in T cell proliferation and infiltration into the tumor, inducing a cytotoxic T cell response that leads to an objective tumor response.
Antitumor T cells repeatedly recognize cognate tumor antigen as the cancer advances from a primary to metastatic lesions over time. Triggering of the TCR results in production of pro-inflammatory cytokines, including IFN-γ, which is the strongest stimulator of reactive PD-L1 expression (15, 18). Chronic exposure of T cells to cognate antigen results in reactive PD-L1 expression by target cells and continuous PD-1 signaling in T cells induces an epigenetic program of T cell exhaustion (19, 20). Several other interactions in the PD-1 pathway have a less clear functional meaning. PD-L1 has been shown to bind the costimulatory molecule CD80 (B71) expressed on T cells, delivering an inhibitory signal (15). RGMb (repulsive guidance molecule b) binds to PD-L2 but not PD-L1, and seems to be relevant for pulmonary tolerance (15).
PD-1 is therefore a negative regulator of pre-existing immune responses, which becomes relevant to cancer as its blockade results in preferential stimulation of anti-tumor T cells (Fig. 3). The restricted effect of PD-1 is highlighted by the limited phenotype of PD-1 compared to CTLA-4 deficient mice, as PD-1 deficient mice are mostly devoid of autoimmune diseases unless these are induced by other means (15). Consequently, PD-1 pathway blockade has a more specific effect on anti-tumor T cells, perhaps due to their chronically stimulated state, resulting in increased therapeutic activity and more limited toxicity compared to CTLA-4 blockade (21, 22).
Clinical effects of PD-1/PD-L1 blockade therapy
The unique biology and durable response rates in patients with multiple types of cancer indicate that therapeutic blockade of the PD-1 pathway is arguably one of the most important advances in the history of cancer treatment. There are currently five anti-PD-1 or anti-PD-L1 antibodies approved by the FDA in 11 cancer indications (Table 1 and Fig. 2). The first evidence of the anti-tumor activity of PD-1 blockade was with the fully human monoclonal antibody nivolumab (previously known as MDX-1106/BMS936558). Nivolumab was first administered to a patient in October, 2006 in a phase 1 single infusion dose escalation trial, and represents the first instance of PD-1 blockade in humans (Fig. 2). Among the 16 initial patients who received nivolumab every 2 weeks, six (37.5%) had objective tumor responses, including patients with melanoma, renal cell carcinoma and non-small cell lung cancer (NSCLC) (23). The remarkable early evidence of antitumor activity in this phase 1 trial was accompanied by limited toxicity, although the rare development of pneumonitis was an indicator of occasional serious toxicities (23, 24). The presentation of the phase 1 data with nivolumab triggered rapid acceleration of clinical trial plans with this and other anti-PD-1 and anti-PD-L1 antibodies (Fig. 2). The anti-PD-1 antibody pembrolizumab entered clinical testing in April, 2011. With the encouraging clinical data from nivolumab, pembrolizumab’s clinical development focused on patients with metastatic melanoma and NSCLC, resulting in the largest phase 1 trial ever conducted in oncology, eventually enrolling 1,235 patients (25, 26).
Table 1.
Major indications approved for the use of anti–PD-1 and anti–PD-L1 therapies and the suspected mechanism of action of the antitumor response.
| Group | Indication | Objective response rate (%) | Agents approved* | Main driver of response |
|---|---|---|---|---|
| High response rate | Hodgkin’s disease | 87 | nivolumab pembrolizumab | PDJ amplicon |
| Desmoplastic melanoma | 70 | nivolumab pembrolizumab | Mutations from chronic sun exposure | |
| Merkel cell | 56 | avelumab pembrolizumab | Merkel cell virus | |
| MSI-h cancers | 53 | nivolumab pembrolizumab | Mutations from mismatch repair deficiency | |
| Intermediate response rate | Skin melanoma | 35 to 40 | nivolumab pembrolizumab | Mutations from intermittent sun exposure |
| NSCLC | 20 | atezolizumab nivolumab pembrolizumab | Mutations from cigarette smoking | |
| Head and neck | 15 | nivolumab pembrolizumab | Mutations from cigarette smoking | |
| Gastroesophageal | 15 | pembrolizumab | Mutations from cigarette smoking | |
| Bladder and urinary tract | 15 | atezolizumab avelumab durvalumab nivolumab pembrolizumab | Mutations from cigarette smoking | |
| Renal cell carcinoma | 25 | nivolumab pembrolizumab | Insertions and deletions (indels) | |
| Hepatocellular carcinoma | 20 | nivolumab | Hepatitis virus |
in alphabetical order
The first FDA approvals of PD-1 blocking antibodies were through accelerated and breakthrough filing pathways, with pembrolizumab and nivolumab approved for the treatment of patients with refractory melanoma in 2014, and in 2015 for patients with advanced NSCLC (Figure 2). The first anti-PD-L1 antibody approved was atezolizumab for urothelial cancers in 2016, followed by avelumab for Merkel cell carcinoma in 2017 (Fig. 2). This class of agents were the first to be granted FDA approval based on a genetic characteristic as opposed to the site of origin of the cancer, with the approval of pembrolizumab and nivolumab for the treatment of microsatellite-unstable cancers of any origin in 2017 (27). This rapid drug development and broad range of approvals is based on a series of characteristics of the clinical activity of PD-1 pathway blocking antibodies, and are outlined below.
There is antitumor activity in a subset of patients within a broad range of cancers, in particular in carcinogen-induced cancers or cancers driven by viral infections (Table 1). The highest antitumor activity of single agent PD-1 blockade therapy is in Hodgkin’s lymphoma, where there is constitutive expression of PD-L1 through a common amplification of the PD-L1 locus together with PD-L2 and JAK2 (termed PDJ amplicon (28), the virally-induced Merkel cell carcinoma of the skin (29), microsatellite-instability cancers with high mutational load from mismatch repair deficiency leading to high frequency of insertions/deletions (indels) (27), and in desmoplastic melanoma, a rare subtype of melanoma that has very high mutational load arising from chronic ultraviolet light-induced point mutations (30). In these cases, response rates currently are 50–90%. A second group of cancers with relatively high response rates are carcinogen-induced cancers, such as the more common variants of melanoma arising from intermittently-exposed skin where upfront response rates are presently in the range of 35–40%, and a series of cancers associated to the carcinogenic effects of cigarette smoking such as NSCLC, head and neck, gastro-esophageal and bladder/urothelial cancers, with response rates in the range of 25–15% (25, 31–33). The other two approvals of single agent anti-PD-1 therapies are in hepatocellular carcinoma, with its known relationship to hepatitis virus infection (34), and renal cell carcinoma (35), which has a low single nucleotide mutational load but instead has a higher frequency of indels than other common cancers, resulting in increased immunogenicity (36).
Once an objective tumor response has been achieved, the majority remain durable. As opposed to targeted oncogene therapies, where the majority of tumor responses last until the cancer develops a way to reactivate the pathway or alternate oncogene signaling bypassing the blocked oncogene, in cancer immunotherapies the rate of relapse is lower. It was hoped that immunotherapy could induce long lasting responses, due to the ability of T cells to maintain memory to their target, and induce a polyclonal response that the cancer should have trouble escaping. However, primary refractoriness and acquired resistance after a period of response are major problems with checkpoint blockade therapy (reviewed in ref. (37)).
Single agent PD-1 pathway blockade has a relatively favorable toxicity profile, with toxicities requiring medical intervention (grade 3–4) in the range of 10–15% in the majority of series (21, 25, 26, 32, 38). Most patients treated with single agent anti-PD-1/PD-L1 have no toxicities over what would be expected from placebo, and treatment-related deaths are very uncommon. Very few patients (~5%) discontinue therapy due to toxicities. The most common treatment-related adverse events of any grade are fatigue, diarrhea, rash and pruritus in 20–15% of patients (21, 25, 26, 32, 38). In a smaller percentage, toxicities are more serious and include several endocrinopathies, where the immune system infiltrates a hormone-producing gland and leads to permanent dysfunction requiring life-long substitutive hormonal therapy, such as thyroid disorders (10–15%), hypophysiitis, adrenal gland disorders (1–3%) and type I diabetes (1%). Serious visceral organ inflammatory toxicities are uncommon (~1%) but can affect any organ including the brain (encephalopathy), meninges (meningitis), lung (pneumonitis), heart (myocarditis), gastrointestinal tract (esophagitis, colitis), liver (hepatitis), kidney (nephritis), muscles (myositis) and joints (arthritis). These can be life-threatening. The cornerstone of treatment for clinically-relevant toxicities with both PD-1 and CTLA-4 blockade therapy is immune suppressive therapy, with high doses of corticosteroids, and sometimes tumor necrosis factor (TNF) antagonists (which are counter-indicated in patients with hepatitis) and mycophenolate mofetil (5).
Mechanisms of response and resistance to single agent PD-1 therapy
The majority of data supports a model in which patients respond to single agent anti-PD-1/L1 therapy because of a pre-existing anti-tumor T cell response. Such a response retains therapeutic potential until the infiltrating T cells engage their TCR by recognition of a tumor antigen, triggering expression of PD-1 on T cells and release of IFN-γ, resulting in reactive expression of PD-L1 by cancer-resident cells (15–17, 30, 39, 40) (Fig. 3). This process, termed adaptive immune resistance, occurs when tumor cells disarm specific T cells through PD-L1 expression (16, 17). It results in a specific state of immune privilege that does not require a systemic immune deficiency and is reversible simply by blocking the PD-1:PD-L1 interaction (40) (Fig. 3).
The first step in this mechanism is the immune system recognizing cancer cells differentially from normal cells, where the cancer cells had auto-vaccinated the patient to induce a specific T cell response. The most common mechanism for this differential recognition is related to the increased mutational load in cancers (40, 41). However, not all mutations seem to have the necessary qualities to give rise to robust targets of an anti-tumor immune response. Mutations that appear in the founder cancer cell and are carried on by the majority of progeny cells (clonal mutations) are favorable, while mutations that appear later in the course of the cancer and may vary among different cancer cells (subclonal mutations)and not sensitize to PD-1 blockade (42). The processing and presentation by MHC molecules of neoepitopes resulting from mutations further shapes the landscape of neoantigens recognized by antitumor T cells (43, 44).
The most common reason why a cancer would not have pre-existing T cell infiltration is likely a state of low immunogenicity resulting from lack of mutations that become recognized neoantigens (41), or an active means of T cell exclusion (37). Certain cancer phenotypes resulting from expression of specific transcriptomic programs may contribute to the lack of T cell recognition, such as expression of genes of the WNT pathway (45), or a series of partially overlapping gene sets that are related to stemness, mesenchymal transition and wound healing, collectively termed IPRES (for innate anti-PD-1 resistance) as they are enriched in biopsies of patients with melanoma not responding to anti-PD-1 therapy (46). It is also possible that anti-tumor T cells are impaired by earlier checkpoints such as CTLA-4, or immune suppressive cells in the tumor microenviroment,such as myeloid lineage cells or Tregs (37). Recognition of these processes suggest combinations approaches that may synergize with PD-1 blockade.
The expression of PD-L1 by cells within the cancer was explored as a biomarker to enrich for patients who may be more likely to respond to PD-1 blockade therapies (24, 25, 47). PD-L1 is most frequently expressed reactively upon T cell infiltration and sensing of IFN-γ production, in which case it could be considered as a “canary in a coal mine”, where its presence is a surrogate for a pre-existing T cell response (Fig. 3). In this setting, co-localized PD-L1, PD-1 and CD8+ T cells in an area of the tumor termed the invasive margin is associated with response to PD-1 blockade (30, 48). PD-L1 can also be expressed constitutively through a series of processes, and it is currently unclear if the mere presence of PD-L1 without detecting a T cell infiltrate is a favorable or detrimental event for PD-1 blockade therapy. Therefore, tumors that may be strongly positive for PD-L1 but do not contain a pre-existing cytotoxic CD8+ T cell response would be unlikely to respond to therapy. The notable exception is Hodgkin’s lymphoma, where the Reed-Stenberg cells have the PDJ amplicon resulting in constitutive PD-L1 expression (28). Of note, this is a cancer that is notorious for a reactive T cell infiltrate mostly comprised of CD4 T helper cells while the Reed-Stenberg cells are frequently deficient in β−2 microglobulin (B2M), the required subunit for surface expression of MHC class I (49). These facts are at odds with the notion that PD-1 blockade therapy mainly reactivates pre-existing intratumoral MHC class I-restricted CD8+ T cells.
Once a tumor is immunogenic enough to trigger a specific T cell response, the cancer cells may undergo a series of genetic and non-genetic processes to avoid being eliminated by the immune system, termed cancer immunoediting (50). Cancer immunoediting may result in loss of mutations that are most immunogenic, or the mutation or decreased expression of genes involved in the antigen presentation pathway. Any of these events would be expected to result in primary resistance to PD-1 blockade, or leading to acquired resistance if it developed during therapy. Strong immune selective pressure can lead to shaping the mutational landscape of cancer (43, 44, 51), specific deletion of HLA class I alleles that putatively present strong neoantigens (44) or loss of B2M (52–54). Genetic immunoediting events that can be found at baseline, and in particular B2M homozygous loss of function mutations, have been reported to be associated with both primary and acquired resistance to PD-1 blockade (52–54).
The process that leads to the reactive expression of PD-L1 upon T cell attack of cancer is mediated by IFN-γ pathway signaling (15, 18, 20) (Fig. 3). If the cancer cell is unable to sense IFN-γ and signal through the pathway, then PD-L1 will not be reactively expressed. In this setting, it could be futile to give antibodies blocking the PD-1:PD-L1 interaction (18, 20, 55). Within the IFN-γ receptor pathway, the bottleneck for signaling seems to be the janus kinases 1 and 2 (JAK1 and JAK2), as absence of either one results in complete lack of signaling (18, 20). Homozygous loss of function mutations in JAK1/2 are rare baseline events but are more frequent than would be expected randomly, suggesting an active immunoediting process to delete them (20, 56). In the setting of fully inactivating JAK1/2 mutations, patients do not respond to anti-PD-1 therapy (20, 56). Mutating JAK1/2 provides an advantage to the cancer cells as it limits favorable effects of IFN-γ, such as increased expression of antigen presenting machinery molecules, production of chemokines that potently attract other T cells to that area and amplify the immune response, or avoiding the direct anti-proliferative effects of interferon (55). In some cases of acquired resistance to anti-PD-1 therapy, homozygous loss of JAK1 or JAK2 has been documented (53, 56). These are rare genetic events that would explain a minority of cases with primary or acquired resistance to PD-1 blockade, but highlight the ability to mechanistically understand these processes. This body of data suggests that molecular mechanisms of resistance to anti-PD-1 therapy converges in alterations in the antigen presentation machinery and the IFN-γ receptor pathway, an observation recently confirmed in unbiased CRISPR/Cas9 screens in preclinical models (57, 58).
The current understanding of response and resistance to PD-1 blockade therapy suggests that there cannot be a single biomarker to select patients. Therefore, selection of patients who are highly likely to respond to single agent anti-PD-1 therapy (as opposed to being exposed to the greater toxicity and expense of combined therapy) would require a combination of studies in baseline tumor biopsies with sufficient tissue that included: i) DNA analyses for tumor mutational load and absence of deleterious mutations in key immune signaling pathways, ii) RNA analyses to detect presence or absence of IFN-γ signaling and a favorable tumor phenotype, iii) and morphological analyses documenting the co-localization of CD8+ T cells expressing PD-1 and interacting with reactively expressed PD-L1 in the tumor microenvironment. However, such extensive testing is currently not done routinely and in a timely enough manner to inform therapeutic decisions in patients with advanced cancer.
Combination CTLA-4 and PD-1 blockade therapy
In December, 2009, the first patient was treated with combination checkpoint blockade using ipilimumab to block CTLA-4 and concurrent nivolumab to block PD-1 (Fig. 2). This was designed based on the non-redundant co-inhibitory roles of the two pathways, after pre-clinical studies showed evidence of synergy in syngeneic mouse models (59). Further, the distinct immune microenvironments in which CTLA-4 and PD-1 pathway blockade could act provided additional mechanistic rationale (Fig. 1). CTLA-4 is mainly associated with affecting inhibitory cross-talk in the draining lymph node. While PD-1 blockade may also have activity in that immunologic space, the presence of PD-L1 on tumor and immune cells in the immediate tumor microenvironment provides an additional anatomic venue for activity (Fig. 1). Most recently, the work of the Allison lab has shown using CyTOF that CTLA-4 and PD-1 blockade result in distinct phenotypic signatures in T cell subsets (60). The initial phase 1 dose-ranging trial of ipilimumab plus nivolumab was conducted in patients with metastatic melanoma and demonstrated >50% objective response rate in the dose level chosen to move to phase 2 and 3 trials (59). Importantly, this was associated with a significantly higher frequency of high-grade immune related toxicities (up to 60%), in comparison to data from monotherapy trials. Phase 2 and 3 studies of the combination of ipilimumab plus nivolumab confirmed a response rate of approximately 60% and the most recent analysis showed that patients initially randomized to the combination had a slightly higher 3-year survival than patients initially receiving nivolumab alone (58% vs 52%), yet with higher frequency of toxicity (22). Initial attempts to identify which patients require the combination have focused on tumor expression of PD-L1 and do suggest that patients tumors with little or no PD-L1 expression (<1% tumor cells with surface staining) have improved survival with combination therapy compared with nivolumab alone. Ongoing trials are examining an adaptive dosing regimen with early assessment for response in an attempt to dose-spare the combination and reduce toxicity (NCT03122522).
Other Combination Therapies and Conclusions
Immune checkpoint blocking antibodies are actively being investigated in combination with an ever-widening spectrum of agents. While the goal of such investigations is laudable, that being to increase the number of patients who may benefit from this type of therapy, the sometimes empiric manner of how agents are brought together is leading to an unrealistic number of trials and expected volunteers, making it unlikely that all of the hypotheses will be robustly answered. Yet, there are some combination strategies which are in late-stage development and are mechanism-based. The description of cellular and molecular mechanisms of primary and acquired resistance to checkpoint blockade therapy allows designing combination immunotherapy approaches to overcome these resistance mechanisms. In the setting of low pre-existing levels of T cells in the tumor, besides the combination of anti-CTLA-4 and anti-PD-1, other potential approaches include changing the tumor microenviroment by direct injection of interferon-inducing molecules such as toll-like receptor agonists or oncolytic viruses, blocking T cell-excluding proteins like IDO or arginase, or inhibiting immune suppressive cells like Treg or macrophages (reviewed in (59)). Furthermore, other modes of cancer therapy, such as radiotherapy, chemotherapy or oncogene-targeted therapies, have been shown to change the immune suppressive tumor microenviroment and potentially synergize with immune checkpoint blockade therapy (reviewed in (59)). Building on recent success in this field is important, but continuing to incorporate the emerging knowledge from mechanistic basic science studies is critical to achieve greater therapeutic success.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grants R35 CA197633, P01 CA168585 and P30 CA016042 (A.R.), R01 CA056821 and P30 CA008748 (J.D.W.), with additional support from the Ressler Family Fund, the Grimaldi Family Fund and the Garcia-Corsini Family Fund (A.R.), and Swim Across America, Ludwig Cancer Research, the Hazen-Polsky Family Fund, the Hogan Family Fund, Live4Life Foundation and the Etta Weinheim Fund (J.D.W.). A.R. and J.D.W. are members of the Parker Institute for Cancer Immunotherapy.
Acknowledgements of conflict of interests:
A.R. has received honoraria from consulting with Amgen, Bristol-Myers Squibb, Chugai, Genentech, Merck, Novartis and Roche, is a member of the scientific advisory board and holds stock in Advaxis, Arcus Biosciences, Bioncotech Therapeutics, Compugen, CytomX, Five Prime, FLX-Bio, ImaginAb, Isoplexis, Kite-Gilead, Lutris Pharma, Merus, PACT Pharma, Rgenix and Tango Therapeutics. J.D.W has received honoraria from consulting with Adaptive Biotech; Advaxis; Amgen; Apricity; Array BioPharma; Ascentage Pharma; Beigene; Bristol Myers Squibb; Celgene; Chugai; Elucida; Eli Lilly; F Star; Genentech; Imvaq; Kleo Pharma; MedImmune; Merck; Neon Therapuetics; Ono; Polaris Pharma; Polynoma; Psioxus; Puretech; Recepta; Trienza; Sellas Life Sciences; Serametrix; Surface Oncology; Syndax. J.D.W receives research support from Bristol Myers Squibb; Medimmune; Merck Pharmaceuticals; Genentech. Equity in Potenza Therapeutics; Tizona Pharmaceuticals; Adaptive Biotechnologies; Elucida; Imvaq; Beigene and Trienza.
References
- 1.Chambers CA, Kuhns MS, Egen JG, Allison JP, CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 19, 565–594 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Leach DR, Krummel MF, Allison JP, Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736. (1996). [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G, Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 100, 4712–4717 (2003); published online EpubApr 15 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J, Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol 23, 8968–8977 (2005); published online EpubDec 10 (JCO.2005.01.109 [pii] 10.1200/JCO.2005.01.109). [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Sidlow R, Hellmann MD, Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 378, 158–168 (2018); published online EpubJan 11 ( 10.1056/NEJMra1703481). [DOI] [PubMed] [Google Scholar]
- 6.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD, Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 33, 1889–1894 (2015); published online EpubJun 10 ( 10.1200/JCO.2014.56.2736). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eroglu Z, Kim DW, Wang X, Camacho LH, Chmielowski B, Seja E, Villanueva A, Ruchalski K, Glaspy JA, Kim KB, Hwu WJ, Ribas A, Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur J Cancer 51, 2689–2697 (2015); published online EpubNov ( 10.1016/j.ejca.2015.08.012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS, Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15, 7412–7420 (2009); published online EpubDec 1 (1078–0432.CCR-09–1624 [pii] 10.1158/1078-0432.CCR-09-1624). [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ, Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 363, 711–723 (2010); published online EpubAug 19 ( 10.1056/NEJMoa1003466). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, O’Day S, Garbe DJM,C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr., Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD, Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. The New England journal of medicine 364, 2517–2526 (2011); published online EpubJun 30 ( 10.1056/NEJMoa1104621). [DOI] [PubMed] [Google Scholar]
- 11.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA, Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371, 2189–2199 (2014); published online EpubDec 4 ( 10.1056/NEJMoa1406498). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA, Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015); published online EpubOct 9 ( 10.1126/science.aad0095). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP, Sharma P, Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 16, 2861–2871 (2010); published online EpubMay 15 ( 10.1158/1078-0432.CCR-10-0569). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida Y, Agata Y, Shibahara K, Honjo T, Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO journal 11, 3887–3895 (1992); published online EpubNov ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH, Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 34, 539–573 (2016); published online EpubMay 20 ( 10.1146/annurev-immunol-032414-112049). [DOI] [PubMed] [Google Scholar]
- 16.Pardoll DM, The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer 12, 252–264 (2012); published online EpubApr ( 10.1038/nrc3239). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas A, Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov 5, 915–919 (2015); published online EpubSep ( 10.1158/2159-8290.CD-15-0563). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A, Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell reports 19, 1189–1201 (2017); published online EpubMay 09 ( 10.1016/j.celrep.2017.04.031). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, Tonnerre P, Chung RT, Tully DC, Allen TM, Frahm N, Lauer GM, Wherry EJ, Yosef N, Haining WN, The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016); published online EpubDec 2 ( 10.1126/science.aae0491). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, Palaskas N, Rodriguez GA, Parisi G, Azhdam A, Chmielowski B, Cherry G, Seja E, Berent-Maoz B, Shintaku IP, Le DT, Pardoll DM, Diaz LA Jr., Tumeh PC, Graeber TG, Lo RS, Comin-Anduix B, Ribas A, Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov 7, 188–201 (2017); published online EpubFeb ( 10.1158/2159-8290.CD-16-1223). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, K.−. investigators, Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372, 2521–2532 (2015); published online EpubJun 25 ( 10.1056/NEJMoa1503093). [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J, Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 377, 1345–1356 (2017); published online EpubOct 5 ( 10.1056/NEJMoa1709684). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sznol M, Powderly JD, Smith DC, Brahmer JR, Drake CG, McDermott DF, Lawrence DP, Wolchok JD, Topalian SL, Lowy I, Safety and antitumor activity of biweekly MDX-1106 (Anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies. Journal of Clinical Oncology 28, 15s (2010). [Google Scholar]
- 24.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine 366, 2443–2454 (2012); published online EpubJun 28 ( 10.1056/NEJMoa1200690). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, K.−. Investigators, Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372, 2018–2028 (2015); published online EpubMay 21 ( 10.1056/NEJMoa1501824). [DOI] [PubMed] [Google Scholar]
- 26.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu WJ, Weber JS, Gangadhar TC, Hersey P, Dronca R, Joseph RW, Zarour H, Chmielowski B, Lawrence DP, Algazi A, Rizvi NA, Hoffner B, Mateus C, Gergich K, Lindia JA, Giannotti M, Li XN, Ebbinghaus S, Kang SP, Robert C, Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA 315, 1600–1609 (2016); published online EpubApr 19 ( 10.1001/jama.2016.4059). [DOI] [PubMed] [Google Scholar]
- 27.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr., Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017); published online EpubJul 28 ( 10.1126/science.aan6733). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P, PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372, 311–319 (2015); published online EpubJan 22 ( 10.1056/NEJMoa1411087). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA, PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 374, 2542–2552 (2016); published online EpubJun 30 ( 10.1056/NEJMoa1603702). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, Liniker E, Ben K, Munhoz R, Rapisuwon S, Gherardini PF, Chmielowski B, Wang X, Shintaku IP, Wei C, Sosman JA, Joseph RW, Postow MA, Carlino MS, Hwu WJ, Scolyer RA, Messina J, Cochran AJ, Long GV, Ribas A, High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553, 347–350 (2018); published online EpubJan 18 ( 10.1038/nature25187). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML, Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 375, 1856–1867 (2016); published online EpubNov 10 ( 10.1056/NEJMoa1602252). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R, Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016); published online EpubMay 7 ( 10.1016/S0140-6736(16)00561-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF, K.−. Investigators, Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 376, 1015–1026 (2017); published online EpubMar 16 ( 10.1056/NEJMoa1613683). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I, Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017); published online EpubJun 24 ( 10.1016/S0140-6736(17)31046-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I, Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 373, 1803–1813 (2015); published online EpubNov 5 ( 10.1056/NEJMoa1510665). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, Chambers T, Salgado R, Savas P, Loi S, Birkbak NJ, Sansregret L, Gore M, Larkin J, Quezada SA, Swanton C, Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol 18, 1009–1021 (2017); published online EpubAug ( 10.1016/S1470-2045(17)30516-8). [DOI] [PubMed] [Google Scholar]
- 37.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A, Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 168, 707–723 (2017); published online EpubFeb 09 ( 10.1016/j.cell.2017.01.017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA, Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372, 320–330 (2015); published online EpubJan 22 ( 10.1056/NEJMoa1412082). [DOI] [PubMed] [Google Scholar]
- 39.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L, Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4, 127ra137 (2012); published online EpubMar 28 ( 10.1126/scitranslmed.3003689). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blank CU, Haanen JB, Ribas A, Schumacher TN, CANCER IMMUNOLOGY. The “cancer immunogram”. Science 352, 658–660 (2016); published online EpubMay 6 ( 10.1126/science.aaf2834). [DOI] [PubMed] [Google Scholar]
- 41.Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015); published online EpubApr 3 ( 10.1126/science.aaa4971). [DOI] [PubMed] [Google Scholar]
- 42.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C, Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016); published online EpubMar 25 ( 10.1126/science.aaf1490). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, Rizvi NA, Merghoub T, Levine AJ, Chan TA, Wolchok JD, Greenbaum BD, A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520 (2017); published online EpubNov 23 ( 10.1038/nature24473). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P, Herrero J, Swanton C, Consortium TR, Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 171, 1259–1271 e1211 (2017); published online EpubNov 30 ( 10.1016/j.cell.2017.10.001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spranger S, Bao R, Gajewski TF, Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015); published online EpubJul 9 ( 10.1038/nature14404). [DOI] [PubMed] [Google Scholar]
- 46.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS, Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165, 35–44 (2016); published online EpubMar 24 ( 10.1016/j.cell.2016.02.065). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR Jr., Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA, CheckMate I, First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 376, 2415–2426 (2017); published online EpubJun 22 ( 10.1056/NEJMoa1613493). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A, PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014); published online EpubNov 27 ( 10.1038/nature13954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichel J, Chadburn A, Rubinstein PG, Giulino-Roth L, Tam W, Liu Y, Gaiolla R, Eng K, Brody J, Inghirami G, Carlo-Stella C, Santoro A, Rahal D, Totonchy J, Elemento O, Cesarman E, Roshal M, Flow sorting and exome sequencing reveal the oncogenome of primary Hodgkin and Reed-Sternberg cells. Blood 125, 1061–1072 (2015); published online EpubFeb 12 ( 10.1182/blood-2014-11-610436). [DOI] [PubMed] [Google Scholar]
- 50.Schreiber RD, Old LJ, Smyth MJ, Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011); published online EpubMar 25 ( 10.1126/science.1203486). [DOI] [PubMed] [Google Scholar]
- 51.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N, Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015); published online EpubJan 15 ( 10.1016/j.cell.2014.12.033). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum H, Blackmon SM, Frederick DT, Hazar-Rethinam M, Nadres BA, Van Seventer EE, Shukla SA, Yizhak K, Ray JP, Rosebrock D, Livitz D, Adalsteinsson V, Getz G, Duncan LM, Li B, Corcoran RB, Lawrence DP, Stemmer-Rachamimov A, Boland GM, Landau DA, Flaherty KT, Sullivan RJ, Hacohen N, Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun 8, 1136 (2017); published online EpubOct 26 ( 10.1038/s41467-017-01062-w). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A, Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 375, 819–829 (2016); published online EpubSep 1 ( 10.1056/NEJMoa1604958). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, Du VY, Schlessinger J, Goldberg SB, Chiang A, Sanmamed MF, Melero I, Agorreta J, Montuenga LM, Lifton R, Ferrone S, Kavathas P, Rimm DL, Kaech SM, Schalper K, Herbst RS, Politi K, Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov, (2017); published online EpubOct 12 ( 10.1158/2159-8290.CD-17-0593). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bach EA, Aguet M, Schreiber RD, The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol 15, 563–591 (1997) 10.1146/annurev.immunol.15.1.563). [DOI] [PubMed] [Google Scholar]
- 56.Sucker A, Zhao F, Pieper N, Heeke C, Maltaner R, Stadtler N, Real B, Bielefeld N, Howe S, Weide B, Gutzmer R, Utikal J, Loquai C, Gogas H, Klein-Hitpass L, Zeschnigk M, Westendorf AM, Trilling M, Horn S, Schilling B, Schadendorf D, Griewank KG, Paschen A, Acquired IFNgamma resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat Commun 8, 15440 (2017); published online EpubMay 31 ( 10.1038/ncomms15440). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, Weiss SA, Lo J, Fisher DE, Miao D, Van Allen E, Root DE, Sharpe AH, Doench JG, Haining WN, In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 547, 413–418 (2017); published online EpubJul 27 ( 10.1038/nature23270). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, Merchant AS, Mehta GU, Chichura A, Shalem O, Tran E, Eil R, Sukumar M, Guijarro EP, Day CP, Robbins P, Feldman S, Merlino G, Zhang F, Restifo NP, Identification of essential genes for cancer immunotherapy. Nature 548, 537–542 (2017); published online EpubAug 31 ( 10.1038/nature23477). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Postow MA, Callahan MK, Wolchok JD, Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol 33, 1974–1982 (2015); published online EpubJun 10 ( 10.1200/JCO.2014.59.4358). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe’er D, Allison JP, Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 170, 1120–1133 e1117 (2017); published online EpubSep 7 ( 10.1016/j.cell.2017.07.024). [DOI] [PMC free article] [PubMed] [Google Scholar]


