Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing has lagged in many countries because of test kit shortages and analytical process bottlenecks. This study investigated the feasibility and accuracy of a sample pooling approach for wide-scale population screening for coronavirus disease 2019. A total of 940 nasopharyngeal swab samples (934 negative and 6 positive) previously tested for SARS-CoV-2 were deidentified and assigned random numbers for analysis, and 94 pools of 10 samples each were generated. Automated RNA extraction, followed by RT-PCR, was performed in a 96-well plate. Positive pools were identified, and the individual samples were reanalyzed. Of the 94 pools/wells, four were positive [Ct values: N (22.7 to 28.3), ORF1ab (23.3 to 27.2), and internal control (34.4 to 35.4)]. The 40 samples comprising the four pools were identified and reanalyzed individually; six samples were positive, with Ct values of N gene, ORF1ab, and internal control comparable to their respective wells. Additional experiments were performed on samples with high Ct values, and overall results showed 91.6% positive and 100% negative agreement compared with individual testing approach. Thus, 940 samples were tested in 148 reactions compared with 940 reactions in routine screening. The sample pooling strategy may help catch up with testing needs and minimal turnaround times and facilitate enormous savings on laboratory supplies, extraction, and PCR kits currently in short supply.
The outbreak of coronavirus disease 2019 [COVID-19; caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] is now a pandemic that has caused mass disruption of the world order, impacting public health care systems, social lifestyle, governance, and economics. Since its identification in the region of Wuhan, China, >6.24 million confirmed cases with >374,452 COVID-19–related deaths have been reported globally (https://coronavirus.jhu.edu/map.html, last accessed June 1, 2020). The incidence of disease is highly varied across the globe, with the incidence rates ranging from 5618 per million in United States, 617 per million in Spain, and approximately 50 per million in Africa, compared with the global incidence rate of 816 per million (https://www.worldometers.info/coronavirus/#countries, last accessed June 1, 2020).
To contain the spread of disease, multidisciplinary strategies have been launched in different regions of the world, including implementing social distancing, maintaining personal hygiene, contact tracing, and implementing quarantine, travel restrictions, and lockdowns.1 A widely accepted method, although not effectively implemented as a measure to control its spread, is testing for SARS-CoV-2, typically utilizing nasopharyngeal swab specimens. Patients tested positive require appropriate clinical management by either effective isolation or quarantine at home for mild symptoms or within health care facilities for moderate to severe symptoms. Wide-scale testing approaches, such as those implemented in the Republic of Korea, have resulted in great success at reducing community spread and lowering mortality rates.2 In addition, wide-scale testing provides more informative epidemiologic data for drafting policies on disease monitoring and control. Currently, at least 85 manufacturers of diagnostic assays have received Emergency Use Authorization from the Federal Drug and Food Administration for COVID-19 testing (https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#COVID19ivd, last accessed June 1, 2020).
However, testing has lagged behind in many countries because of various factors, most significant being supply chain issues with lack of reagents and adequate test kits. Therefore, many patients (both symptomatic and asymptomatic) remain untested and hence are potentially contributing to community spread of the virus. Furthermore, many countries, including high-income countries, have logically resorted to prioritize testing for the hospitalized, symptomatic, and high-risk population. With this approach, absence of testing or long turnaround times among exposed but asymptomatic individuals and patients exhibiting mild symptoms have been observed, a factor that likely contributes to exponential community spread.
This article proposes a mass population screening approach, based on sample pooling strategy for rapid and wide-scale population screening that may be adopted by laboratories currently using RT-PCR–based methods to test for SARS-CoV-2. The strategy proposed leverages existing high-throughput systems that employ high analytically sensitive [limit of detection (LOD), 5 to 20 copies/mL] real-time PCR chemistries, coupled with pooling of samples based on current COVID-19 incidence rates. Pooling of samples compared with individual testing has been investigated previously, such as in screening blood donations and infectious and genetic diseases.3, 4, 5 Pooling, when carefully executed, has been found to be useful and more cost-effective for estimating incidence rates in specific cases.6 The advantages of this approach include the potential to catch up with huge testing deficits, reducing turnaround times, and, most important, ensuring enormous savings through the most efficient use of RNA extraction and/or testing kits, which even today are in significant short supply.
Routine Screening versus Mass Population Screening Approach for COVID-19
The RT-PCR–based methods have two primary components: RNA extraction from clinical specimens and RT-PCR–based detection of SARS-CoV-2 nucleic acid region(s). The nucleic acid targets used in most commercial kits are based on primer/probe sequences published by either the US or China CDC. In a routine screening approach, the number of samples that could be tested in a 96-well RT-PCR plate varies from 30 (CDC; https://www.fda.gov/media/134919/download, last accessed June 1, 2020) to 94 (PerkinElmer, Inc.; https://www.fda.gov/media/136407/download, last accessed June 1, 2020). However, as these are sensitive methods with LOD as low as 5 to 20 copies/mL, an alternate, more effective approach may be adopted for mass population screening for COVID-19. In this approach, an equal aliquot from 10 samples can be pooled together after collection, and processed for nucleic acid extraction and RT-PCR analysis. The number of reactions/runs required to test 940 patients in routine screening versus mass population screening approach based on the incidence of COVID-19 in the respective countries (Table 1 ). Thus, the mass screening approach in United States has a potential to test 940 patients in 118 reactions/2 runs compared with 940 reactions/10 runs using PerkinElmer assay. Similarly, 940 patients can be tested in 133 reactions/7 runs compared with 940 reactions/32 runs using CDC assay. To demonstrate the feasibility of the mass population screening approach, a laboratory-based blinded study with 940 samples was performed using PerkinElmer assay.
Table 1.
Comparison of Individual Sample Screening versus Mass Population Screening Approach in the United States for COVID-19
| SARS-CoV-2 detection kit | Screening approach | Patients tested in one run/plate, N | LOD, copies/mL | Probability of number of samples positive in US population screening | Additional reactions to be performed, N | 940 Patients tested in following number of reactions | Runs to screen 940 patients, N |
|---|---|---|---|---|---|---|---|
| PerkinElmer Inc. | Individual sample screening | 94 | 20 | 0.51 | 0 | 940 | 10 |
| Mass population screening | 940 | 200 | 5.16 | 20 | 118 | 2 | |
| US CDC | Individual sample screening | 30 | 5–10 | 0.033 | 0 | 940 | 32 |
| Mass population screening | 300 | 50–100 | 0.33 | 10 | 133 | 7 |
COVID-19, coronavirus disease 2019; LOD, limit of detection; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Materials and Methods
Assay for the Detection of SARS-CoV-2
The assay is based on RNA extraction, followed by TaqMan-based RT-PCR assay, to conduct in vitro transcription of SARS-CoV-2 RNA, DNA amplification, and fluorescence detection (PerkinElmer Inc., Waltham, MA). The assay targets specific genomic regions of SARS-CoV-2: nucleocapsid (N) gene and ORF1ab. The TaqMan probes for the two amplicons are labeled with 6-carboxyfluorescein and 6-carboxy-X-rhodamine fluorescent dyes, respectively, to generate target-specific signals. The assay includes an RNA internal control (bacteriophage MS2) that serves as assay control from nucleic acid extraction to fluorescence detection. The internal control probe is labeled with Victoria fluorescent dye to differentiate its fluorescent signal from SARS-CoV-2 targets.
Routine Screening Approach
In routine screening, a batch of 94 samples were processed for the detection of SARS-CoV-2. In brief, an aliquot of 300 μL from each sample, including positive and negative controls, was added to respective wells in a 96-well plate. To each well, 5 μL internal control, 4 μL poly(A) RNA, 10 μL proteinase K, and 300 μL lysis buffer 1 were added. The plate was placed on a semiautomated instrument (chemagic 360 instrument; PerkinElmer, Inc.) following manufacturer's protocol. The nucleic acid was extracted in a 96-well plate, with an elution volume of 60 μL. From the extraction plate, 40 μL of extracted nucleic acid and 20 μL of RT-PCR master mix were added to the respective wells in a 96-well PCR plate. The PCR method was set up as per manufacturer's protocol on Quantstudio3 (Thermo Fisher Scientific, Waltham, MA). The samples resulted as positive or negative based on the Ct values specified by the manufacturer (Supplemental Table S1).
Mass Population Screening Approach
Sample Selection, Blinding, and Pooling of Samples: Step 1
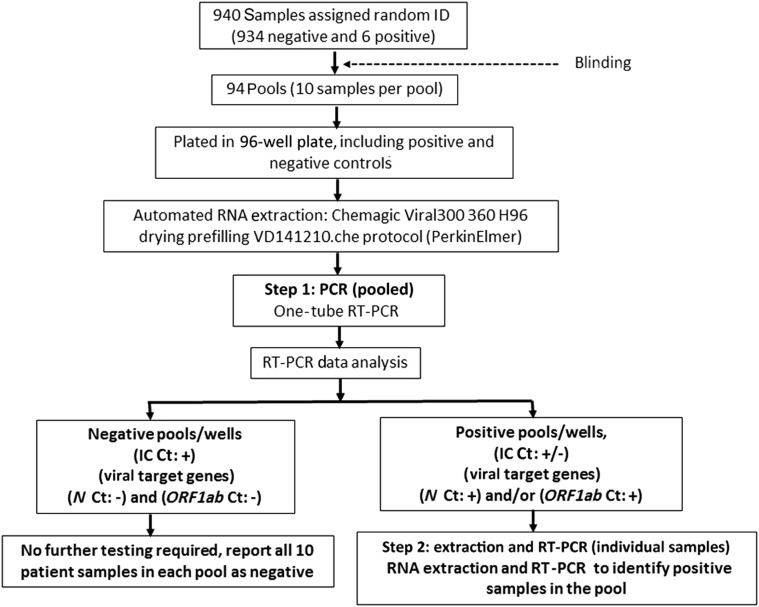
In mass population screening (Figure 1 ), under the institutional review board–approved protocol, 940 nasopharyngeal swab samples previously tested for SARS-CoV-2 were de-identified and assigned random numbers (performed by N.S.S.). The 940 samples contained 934 negative and 6 positive samples. From this, 94 pools of 10 samples each were generated and given a unique number (performed S.A., who was blinded to the initial sample preparation).7 , 8 To pool 10 samples, 35 μL of each sample was aliquoted into a vial (350 μL). The vial was vortex mixed and briefly spun, and 300 μL of pooled sample was transferred to the sample deep-well plate. The samples were processed for downstream extraction and RT-PCR, as stated in the routine screening approach (Supplemental Table S2).
Figure 1.
Study flowchart. IC, internal control; ID, identifier.
Identifying the Positive Sample: Step 2
Pool(s) that resulted positive were identified, and the 10 samples comprising each positive pool were retrieved and processed for downstream extraction and RT-PCR analysis for the identification of the positive sample(s). The positive sample(s) were identified based on the Ct value specified by the manufacturer. In addition, one negative pool was selected randomly, and each sample was re-analyzed individually as a quality control measure.
Results
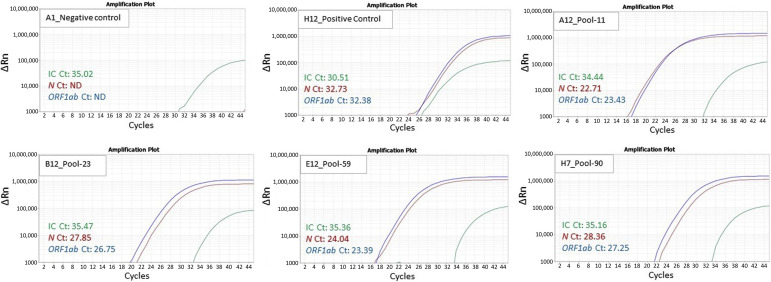
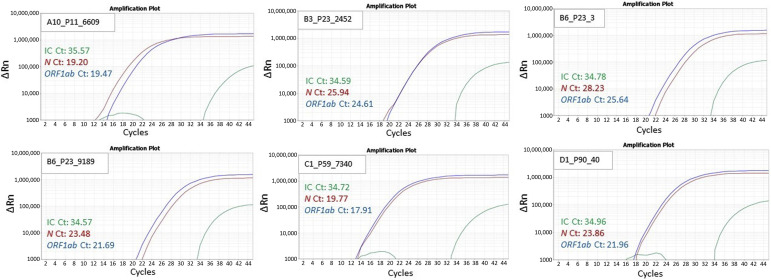
In step 1, the quality control of negative and positive sample was observed to be within the range recommended by the manufacturer. Of the 94 pools/well, 4 resulted positive, with Ct values for the two target genes within the range as follows: N (22.7 to 28.3), ORF1ab (23.3 to 27.2), and internal control (34.4 to 35.4) (Figure 2 ). The remaining 90 wells resulted as negative, with undetermined Ct values for N and ORF1ab gene. In step 2, 40 samples comprising the four pools were identified and re-analyzed individually, with 6 samples resulting positive (3 samples in pool 23 and 1 sample each in pools 11, 59, and 90), with the Ct values of N gene, ORF1ab, and internal control comparable to their respective pools/well (Figure 3 , Table 2 ). All samples in the negative pool were confirmed as negative. Thus, the 6 positive samples were correctly identified, as the 940 samples selected contained 934 negative and 6 positive samples. The data files from steps 1 and 2 are provided as Supplemental Tables S3 and S4, respectively. A supplement study was performed on six pools, each containing nine negative samples and one positive sample with high Ct values (N: 30.9 to 39.6; ORF1ab: 30.7 to 38.5). Each pool was processed for RNA extraction and RT-PCR analysis. All pools, except the pool containing the sample with very high Ct (N: 39.6; ORF1ab: 38.5) was not detected (Supplemental Table S5). Therefore, 1000 samples resulted in an overall 91.6% positive percentage agreement and 100% negative percentage agreement compared with individual testing approach.
Figure 2.
Step 1: 94 pools, each composed of 10 samples, were screened on an RT-PCR–based method. Of these 94 pools, 4 (ie, pools 11, 23, 59, and 90) showed amplification of severe acute respiratory syndrome coronavirus 2 and N and ORF1ab genes. Representative amplification curves for the negative, positive control, and four positive pools are shown. IC, internal control.
Figure 3.
Step 2: The four positive pools were individually tested; of these 40 samples, 6 showed amplification of severe acute respiratory syndrome coronavirus 2 and N and ORF1ab genes. Representative amplification curves for the individual positive samples (three samples in pool 23 and one sample each in pools 11, 59, and 90) from the respective positive pools are shown. IC, internal control.
Table 2.
The Ct Values from the Pools Resulting Positive in Step 1, and the Individual Positive Samples Identified in Step 2, with the Mass Population Screening Approach
| Pool ID | Pooled sample Ct (step 1) |
Individual sample ID | Individual sample Ct (step 2) |
||||
|---|---|---|---|---|---|---|---|
| N gene (Ct) | ORF1ab (Ct) | IC (Ct) | N gene (Ct) | ORF1ab (Ct) | IC (Ct) | ||
| 11 | 22.7 | 23.4 | 34.4 | 6609 | 19.2 | 19.4 | 36.5 |
| 23 | 27.8 | 26.7 | 35.4 | 2452 | 25.9 | 24.6 | 34.5 |
| 3 | 28.2 | 25.6 | 34.7 | ||||
| 9189 | 23.4 | 21.6 | 34.5 | ||||
| 59 | 24.0 | 23.3 | 35.3 | 7340 | 19.7 | 17.9 | 34.7 |
| 90 | 28.3 | 27.2 | 35.1 | 40 | 23.8 | 21.9 | 34.9 |
IC, internal control; ID, identifier.
Discussion
The COVID-19 pandemic has resulted in an overwhelming number of infected patients, leading to a tremendous burden on health care resources to the extent of outstripping the current production capabilities for supplies. Innovative ideas in specimen collection, isolation, respiratory support, and other patient care plans have been implemented. Scaling up testing has been identified as a key component to manage the pandemic.9 Laboratories and manufacturers of test kits across the world have also responded by ramping up testing. However, more innovative approaches are needed for wide-scale population testing and to side step the foreseeable shortages in test kits. This study demonstrates that pooling patient samples and testing them on high-sensitivity, high-throughput systems are both practical and accurate.
In this proof-of-concept study with 40 samples, four pools were formed with 10 samples (nine negative and one positive sample) in each pool, and processed for extraction and RT-PCR. In all four pools, a positive result was obtained. To investigate the effect of dilution, samples were selected with both high and low viral load (based on Ct values). On analysis, a positive result was obtained from all four pools, and the Ct value of approximately 3 points higher was observed for pools compared with individually tested samples. Furthermore, in validation studies, contrived samples (spiking 1 μL positive sample into 299 μL negative sample) were observed to result in positive identification by RT-PCR. These pilot studies indicated that patient samples probably contain viral loads that are sufficiently high as to be above the LOD, even with a 1:300 dilution (N.S.S., A.K.M., S.A., A.N., and R.K., unpublished data). However, as the viral load varies with respect to the day of infection and among individuals, pooling 10 samples (1:10 dilution) seems ideal as the LOD in pooling experiment will remain from 50 to 200 copies/mL for CDC or PerkinElmer assays, respectively.
In the primary study using 940 pooled samples, four wells representing the four pools with positive samples were accurately identified. In the second step, all 40 samples in these pools were retrieved and tested individually. The strategy was able to identify the pools with positive sample(s), and the one-step dissection accurately established the positive samples with comparable Ct values in the pool and individually tested samples. As a quality control measure, one pool identified as negative was randomly selected and the 10 samples were tested individually, which resulted as negative for each individual sample. A comparison of the pooling and individual testing strategies shows that it would take at least 10 extraction runs, 10 PCR runs (96-well plate format), and at least 30 hours to process all of the samples. The pooling strategy, on the other hand, required only two extraction runs, two PCR runs, and 6 hours for all 940 samples. Results for all the samples in the negative wells can potentially be released after the first run, whereas those in the positive wells would proceed for a second round of extraction and PCR in the typical individual testing scheme. A laboratory already conducting high-throughput testing for COVID-19 can effectively multiply its output by a factor proportional to the number of pooled samples. The sample pooling strategy can have high impact in acquisition of wide-scale epidemiologic data on the spread of SAR-CoV-2 across the globe.
An important consideration in the pooling strategy is the potential for false-negative results because of dilution of samples with low viral loads (LOD < 200 copies/mL). To highlight this point, a supplement study was performed on six samples with high Ct values (N: 30.9 to 39.6; ORF1ab: 30.7 to 38.5). Each positive sample was pooled with nine negative samples forming six pools. Each pool was processed for RNA extraction and RT-PCR analysis. All pools, except the pool containing the sample with high Ct (N: 39.6; ORF1ab: 38.5), were not detected (Supplemental Table S5). Another challenge to this approach is inaccurate designation of individual and pooled samples within the 96-well plate, resulting in sample mix-up. To minimize this, use of a minimum of two identifiers and well-documented workflows to ensure traceability of all pooled samples is essential. In addition, use of bar code readers and automated sample processing would minimize the chances of such a mix-up. Furthermore, selected samples from randomly selected negative pool(s) should also be analyzed individually as a quality control monitor. In the current study, all samples in one negative result pool/well were retested and were found to be in agreement with individually tested samples.
In terms of cost analysis, 1 million individuals can be tested for $9.1 million with the proposed mass population screening approach compared with $58 million with routine screening. More important, however, is the potential to massively increase the number of individuals tested using the same quantity of reagents/test kits. This is a critical advantage given the short supply of test kits, a fact with the disparity that ensues especially in low- and middle-income countries. The direct savings on reagent and test kits are complemented by indirect savings on laboratory supplies, including personal protective equipment, that are needed to perform testing on these infectious clinical samples. These savings will enhance sustainable laboratory operations throughout the pandemic or can be deployed to laboratories that are facing dire constraints in supplies.
In conclusion, this unprecedented crisis requires innovative solutions at all levels. The strategy proposed herein leverages existing high-throughput systems, which employ analytically high-sensitive RT-PCR chemistries, coupled with pooling of samples based on current COVID-19 incidence rates. This study analyzed 1000 samples in a pooled approach using only two extraction and PCR runs and achieved 91.6% positive percentage agreement and 100% negative percentage agreement. To optimize the number of pooled samples, real-time region-specific data in websites, such that hosted by the Center for Systems Science and Engineering at Johns Hopkins University (Baltimore, MD), are helpful. In addition, robust validation and knowledge of the analytical performance of the assay to be adopted as well as regional/laboratory positivity rates are critical in this approach. The number of samples pooled is inversely proportional to the analytical sensitivity of the assay and the local positivity rate.10 , 11 The advantages of this innovative approach include potential of catching up with testing, clearing backlogged samples, reducing turnaround times, and ensuring enormous savings on RNA extraction and/or testing kits and laboratory supplies that are in short supply. This would relieve the pressure mounting on laboratories for increased testing, hopefully making a significant contribution to control of this pandemic. In addition, this strategy may come in handy for effective and consistent disease surveillance as many states and countries begin to reopen businesses, airports, public gatherings, and work environments. Monitoring spikes in the number of cases in groups of individuals in the same environment will facilitate rapid and early containment.
Acknowledgment
We thank Lisa Middleton for help with manuscript review.
Footnotes
Disclosures: R.K. has received honoraria, travel funding, and research support from Illumina, Asuragen, Qiagen, and Bristol Myers Squibb. A.C. and M.H. hold stock options at PerkinElmer Inc.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2020.07.001.
Author Contributions
N.S.S. developed the idea, designed the study, collected, analyzed, and interpreted the data, and wrote the manuscript (first draft); A.K.M. designed the study, collected, analyzed, and interpreted the data, and created the figures; A.N. designed the study, analyzed and interpreted the data, and wrote the manuscript (first draft); S.A. designed the study and collected, analyzed, and interpreted the data; K.J., P.K.A., M.A., and Y.J. collected the data; A.C., M.H., V.K., and A.R. edited and reviewed the manuscript; and R.K. developed the idea, designed the study, collected, analyzed, and interpreted the data, and edited and reviewed the manuscript.
Supplemental Data
References
- 1.Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., Lane H.C., Memish Z., Oh M.D., Schuchat A., Ungchusak K. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi S.C., Ki M. Estimating the reproductive number and the outbreak size of novel coronavirus disease (COVID-19) using mathematical model in Republic of Korea. Epidemiol Health. 2020;42:e2020011. doi: 10.4178/epih.e2020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novack L., Sarov B., Goldman-Levi R., Yahalom V., Safi J., Soliman H., Orgel M., Yaari A., Galai N., Pliskin J.S., Shinar E. Impact of pooling on accuracy of hepatitis B virus surface antigen screening of blood donations. Trans R Soc Trop Med Hyg. 2008;102:787–792. doi: 10.1016/j.trstmh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Gomes J.P., Viegas S., Paulino A., Catry M.A. Sensitivity evaluation of the Gen-Probe AMP-CT assay by pooling urine samples for the screening of Chlamydia trachomatis urogenital infection. Int J STD AIDS. 2002;13:540–542. doi: 10.1258/095646202760159648. [DOI] [PubMed] [Google Scholar]
- 5.Gille C., Grade K., Coutelle C. A pooling strategy for heterozygote screening of the delta F508 cystic fibrosis mutation. Hum Genet. 1991;86:289–291. doi: 10.1007/BF00202411. [DOI] [PubMed] [Google Scholar]
- 6.Quinn T.C., Brookmeyer R., Kline R., Shepherd M., Paranjape R., Mehendale S., Gadkari D.A., Bollinger R. Feasibility of pooling sera for HIV-1 viral RNA to diagnose acute primary HIV-1 infection and estimate HIV incidence. AIDS. 2000;14:2751–2757. doi: 10.1097/00002030-200012010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Hogan C.A., Sahoo M.K., Pinsky B.A. Sample pooling as a strategy to detect community transmission of SARS-CoV-2. JAMA. 2020;323:1967–1969. doi: 10.1001/jama.2020.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdalhamid B., Bilder C.R., McCutchen E.L., Hinrichs S.H., Koepsell S.A., Iwen P.C. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153:715–718. doi: 10.1093/ajcp/aqaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guest J.L., Del Rio C., Sanchez T. The 3 steps needed to end the COVID-19 pandemic: bold public health leadership, rapid innovations, and courageous political will. JMIR Public Health Surveill. 2020;6:e19043. doi: 10.2196/19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aragón-Caqueo D., Fernández-Salinas J., Laroze D. Optimization of group size in pool testing strategy for SARS-CoV-2: a simple mathematical model. J Med Virol. 2020 doi: 10.1002/jmv.25929. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yelin I., Aharony N., Shaer-Tamar E., Argoetti A., Messer E., Berenbaum D., Shafran E., Kuzli A., Gandali N., Hashimshony T., Mandel-Gutfreund Y. Evaluation of COVID-19 RT-qPCR test in multi-sample pools. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa531. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.