Abstract
Colorectal cancer (CRC) is one of the most common types of cancer in world and has a high rate of mortality. The majority of cases of CRC are sporadic; however, factors such as age, a family history of inflammatory diseases, diet, lifestyle and genetics increase the risk. HOX genes and lncRNAs are two classes of genes, and alterations in the expression levels of these genes are significantly associated with numerous different types of cancer. In the present study, the expression levels of HOXC10, HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS in tumor tissues were compared with normal healthy tissues in patients with CRC. Paired tumor and normal tissues were collected from 39 patients with CRC, and reverse transcription-quantitative PCR was used the expression of HOXC-AS3, HOXC13 and HOXC10 in the tumor tissues compared with the respective normal tissues. Expression of these genes were increased in the tumor tissues compared with normal tissues; however, the difference was only significant for HOXC10. Additionally, there was a strong and significant correlation between the expression of HOTAIR and HOXC13, a moderate and significant correlation between the expression of HOTAIR and HOXC13-AS, and between HOXC13 and HOXC13-AS genes. The expression of HOXC10 was significantly higher in tumor tissues compared with the normal tissues; whereas the upregulation of HOXC-AS3 and HOXC13 were not significant. Only the correlation between the expression of HOTAIR and HOXC13 was strong and significant. As HOXC10 expression was significantly upregulated in the tumor tissues relative to normal tissues, it may serve as a biomarker for the diagnosis of CRC and as a potential therapeutic target.
Keywords: colorectal cancer, HOXC10, HOXC-AS3, HOTAIR, HOXC13, HOXC13-AS
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer (1) and 880,792 CRC-associated deaths were reported in 2018(2). The prevalence of CRC is 9.2% in women and 10% in men, and it is the second most common type of cancer in women and third most common type of cancer in men (3). In ~90% of cases, CRC is sporadic, and patients do not have any family history of the disease (4). Risk factors for CRC include age (higher in individuals >50 years), and a family history of inflammatory bowel disease (3.7% risk for CRC) and Crohn's disease (2.5% risk for CRC) (5-7). Diet and lifestyle may increase the risk of CRC (8), such as smoking (9,10), consumption of red meat (11,12) and low levels of physical activity (13).
Mutations in various genes, alterations in DNA methylation and chromosomal instability have been identified as genetic causes of CRC. Analysis of gene expression profiles of cancer cells serves an important role in the diagnosis and treatment of patients with cancer and may result in improved insight into the dysregulated mechanisms associated with specific types of cancer. HOX genes are involved in the regulation of different cellular processes, including differentiation, angiogenesis, signaling, apoptosis, mobility and metastasis (14,15). HOXC genes (HOXC4, HOXC5, HOXC6, HOXC8, HOXC9, HOXC10, HOXC11, HOXC12 and HOXC13) are members of the HOX family of genes and are located on chromosome 12q13.3(16), and HOXC13 is involved in the growth and formation of hair and nails (17,18).
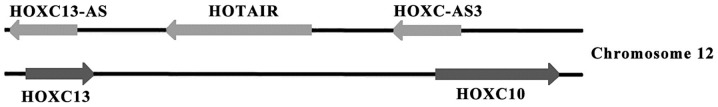
Three long non-coding RNAs (lncRNAs), HOTAIR, HOXC13-AS and HOXC-AS3 are located on the antisense strand of the HOXC gene cluster (Fig. 1). HOTAIR is a polyadenylated RNA that binds to certain protein complexes, such as PRC2, and regulates the conformation of chromatin (19-21). HOXC10, HOXC-AS3, HOXC13 and HOXC13-AS are located in close proximity to the oncogenic lncRNA, HOTAIR. Thus, it was hypothesized that there may be an association between these genes with HOTAIR and development of cancer. In the present study, the expression levels of these genes were assessed using reverse transcription-quantitative (RT-q) PCR in tumor tissues and matching healthy adjacent tissues in patients with CRC.
Figure 1.
HOXC10, HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS are located on the sense or antisense strands of chromosome 12.
Materials and methods
Patients and tissue samples
A total of 39 pairs of tumor tissue and healthy adjacent tissue was obtained from patients. The median age of patients was 54 years old (range, 30-79 years) and included 20 males and 19 females. Tissues were obtained from patients with CRC following surgery and immediately stored in liquid nitrogen. The present study was approved by the Ethics Committee of Shahid Chamran University of Ahvaz (Ahvaz, Iran) and written informed consent was obtained from all patients.
RT-qPCR
A total of 50 mg frozen tissue from each patient was crushed and homogenized. Total RNA was extracted using TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and RNA was dissolved in diethyl pyrocarbonate. The RNA concentration of each sample was measured using a NanoDrop™ 2000/c spectrophotometer (Thermo Fisher Scientific, Inc.) and stored at -80˚C. RNA integrity was assessed using electrophoresis on a 1% agarose gel containing SafeStain (CinnaGen), and the 28S, 18S and 5S bands were observed. Extracted RNAs were treated using DNaseI (Takara Bio, Inc.). Primescript™ RT reagent kit (Takara Bio, Inc.) was used for reverse transcription of RNA to cDNA. A total of 1 µl random hexamers (100 µM) and 1 µl oligo(dT) primer (50 µM) were added to 1 µg DNase treated RNA and RNase free water was added to a final volume of 5 µl and incubated at 65˚C for 5 min. Subsequently, 1 µl reverse transcriptase enzyme (1 U/µl) and 4 µl 5X buffer was added, and RNase free H2O was added to a final volume of 20 µl and incubated at 37˚C for 30 min for cDNA synthesis, followed by incubation at 80˚C for 5 sec to inactivate the enzyme and cDNA was stored at -20˚C. For qPCR, the following reagents were mixed as follows: 10 µl SYBR Premix Ex Taq II (2X) (Takara Bio, Inc.), 0.5 µl each forward and reverse primers (10 pmol each; Macrogen, Inc.), 2 µl cDNA and 7 µl DNase free water. The primer sequences used are presented in Table I. β-actin was used as the loading control. The thermocycling conditions were: 95˚C for 30 sec; 40 cycles of 95˚C for 5 sec and 60˚C for 32 sec; and a dissociation stage of 95˚C for 15 sec, 60˚C for 60 sec and 95˚C for 1 sec. Relative expression of HOXC10, HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS was measured using the 2-∆∆Cq method (22) for the 39 pairs of the tumor tissues and marginal normal tissues in patients with CRC. β-actin was used as the reference gene.
Table I.
Sequence of primers used in this study.
| Genes | Primer sequences (5'-3') |
|---|---|
| HOXC10 | F: CCAGACACCTCGGATAACG |
| HOXC10 | R: GGCACCTCTTCTTCCTTCC |
| HOXC13 | F: TCTCCCTTCCCAGACGTGGT |
| HOXC13 | R: CGCTCAGAGAGGTTCGTGGT |
| HOTAIR | F: GAAAGGTCCTGCTCCGCTTC |
| HOTAIR | R: TCCTCTCGCCGCCGTCTG |
| HOXC-AS3 | F: CGATAGGCGGCTTTGG |
| HOXC-AS3 | R: CGTCTTGTGTGCTGGTTTCC |
| HOXC13-AS | F: CGGACATCGGAGCACTATG |
| HOXC13-AS | R: CGGCTGGTCTTCTTGAGG |
| β-actin | F: ATTGGCAATGAGCGGTTC |
| β-actin | R: TGAAGGTAGTTTCGTGGATG |
F, forward; R, reverse.
Data analysis
All analysis was performed using GraphPad Prism version 5 (GraphPad Software, Inc.). Differences between two groups were compared using a Wilcoxon test. A Spearman's rank correlation coefficient was used for correlation analysis of relative gene expression with clinical parameters.
Results
Comparison of expression of HOX genes between tumor and normal tissues
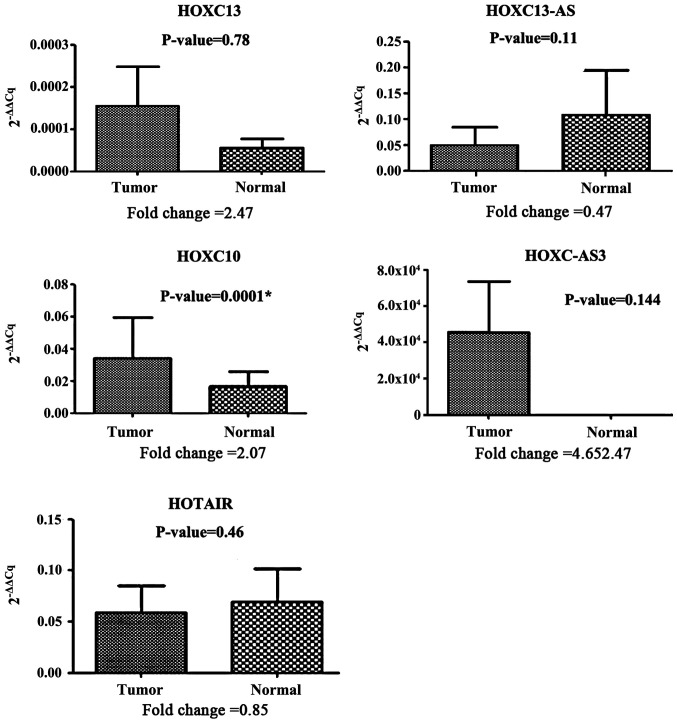
Expression of HOX expression of HOXC-AS3 and HOXC13 in the tumor tissues was upregulated compared with the marginal normal tissues with a fold change of 4.65 and 2.47, respectively. Although HOXC-AS3 and HOXC13 were upregulated in the tumor tissues, statistical analysis showed the difference was not significant (P=0.144 and P=0.78, respectively; Fig. 2). However, expression of HOXC10 was significantly upregulated in the tumor tissues compared with the marginal normal tissues with a fold change of 2.07 (P=0.0001; Fig. 2). Comparison of the expression of HOXC10, HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS in the tumor and normal tissues is shown in Fig. 2.
Figure 2.
Comparison of the expression of HOXC10, HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS in paired tumor and normal tissues. Only the expression levels of HOXC10 were significantly different between the two tissues. *P=0.0001.
Correlation between the expression of genes
To determine the correlation coefficients, the fold change ratio of each gene was used. There was a significant positive correlation between expression of HOTAIR and HOXC13 (r=0.75, strong correlation; P=0.00000004); between HOTAIR and HOXC13-AS (r=0.57, moderate correlation; P=0.001); and between HOXC13 and HOXC13-AS (r=0.43, moderate; P=0.006). There was positive correlation between HOTAIR and HOXC10 (r=0.306, moderate; P=0.058), and between HOTAIR and HOXC-AS3 (r=0.384, moderate; P=0.016). There was a significantly negative correlation between HOXC10 and HOXC-AS3 (r=-0.331, moderate; P=0.039).
Association between gene expression and clinicopathological features
The association between the expression of the five genes with clinicopathological characteristics of the patients was calculated. Statistical analysis showed that there was no significant association between sex, histological grade, tumor size (cm), Tumor-Node-Metastasis (TNM) stage (23), lymphatic invasion, vascular invasion and the expression of HOXC10, HOXC-AS3, HOTAIR, HOXC13 or HOXC13-AS in the patients with CRC (all P>0.05; Table II).
Table II.
Association between expression of the selected genes and clinicopathological characteristics.
| Characteristics | n | HOXC13 | HOXC13-AS | HOTAIR | HOXC10 | HOXC-AS3 |
|---|---|---|---|---|---|---|
| Sex | 0.66 | 0.37 | 0.56 | 0.42 | 0.83 | |
| Male | 20 | |||||
| Female | 19 | |||||
| Histological grade | 0.3 | 0.57 | 0.4 | 0.07 | 0.57 | |
| ≤2 | 33 | |||||
| >2 | 6 | |||||
| Tumor size, cm | 0.078 | 0.81 | 0.74 | 0.32 | 0.66 | |
| ≤5 | 20 | |||||
| >5 | 19 | |||||
| TNM stage | 0.16 | 0.28 | 0.22 | 0.85 | 0.06 | |
| ≤3 | 20 | |||||
| >3 | 19 | |||||
| Lymphatic invasion | 0.41 | 0.66 | 0.6 | 0.81 | 0.36 | |
| Yes | 20 | |||||
| No | 19 | |||||
| Vascular invasion | 0.53 | 0.42 | 0.83 | 0.6 | 0.15 | |
| Yes | 19 | |||||
| No | 20 |
TNM, Tumor-Node-Metastasis staging system.
Discussion
In 2012, ~1.4 million new cases of CRC were diagnosed. By 2035, it is estimated that there will be >2.4 million new cases of colorectal cancer (24). In the present study, the expression levels of HOXC10, HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS in matching normal and tumor tissues from 39 patients with CRC were assessed using RT-qPCR. HOXC10, HOXC-AS3, HOXC13, and HOXC13-AS are located in proximity to the oncogenic lncRNA HOTAIR. Thus, it was hypothesized that there may be an association between these genes and HOTAIR in carcinogenesis. To evaluate this hypothesis, the correlation between the expression of these five genes with HOTAIR, and the association between these genes and certain clinicopathological characteristics were calculated. The results showed that the expression of HOTAIR was not significantly altered in tumor tissues compared with marginal normal tissues, contrasting with previous studies which reported a significant increase in the expression of HOTAIR in CRC (25-29). Kogo et al (30) studied 100 pairs of tumor and normal CRC samples and reported that there was no significant increase in expression of HOTAIR. Svoboda et al (31) also reported that the relative expression of HOTAIR between the tumor and adjacent normal tissue was not significantly different in patients with CRC. These differences may be related to the ethnicity of the individuals studied, their lifestyle, diet or other lifestyle factors. Upregulation of HOXC13 has been reported in odontogenic tumors, liposarcoma, metastatic melanoma, esophageal squamous cell carcinoma, lung adenocarcinoma and ameloblastoma (32-37). However, the expression levels of HOXC13 in CRC was not significantly altered in the present study. Tatangelo et al (38) reported a significant upregulation in the expression of HOXC13 and HOTAIR in right (proximal) side of CRCs tissues.
Overexpression of HOXC13-AS has been reported in nasopharyngeal carcinoma (39). To the best of our knowledge, the present study is the first to evaluate the expression of HOXC13-AS in CRC, and the results showed that there was no significant difference in the expression of HOXC13-AS between normal and cancer tissues.
Studies have shown that the expression of HOXC10 is significantly increased in thyroid cancer, breast carcinoma and lung cancer (40-43). Kim et al (44) reported that expression of HOXC10 was upregulated in gastric cancer. They showed that upregulation of HOXC10 increases proliferation and migration of gastric cancer cells (44). In the present study the expression levels of HOXC10 in CRC were assessed and the results showed that the relative expression in the tumor tissues was significantly higher compared with marginal normal tissues in CRC (P=0.0001). Zhang et al (45) showed that expression of HOXC-AS3 was upregulated in gastric cancer tissues compared with normal tissues, but in the present study, expression of HOXC-AS3 was not changed significantly altered in tumor tissues of patients with CRC compared with normal tissues. To the best of our knowledge, the present study is the first to assess the expression of HOXC-AS3 in patients with CRC.
The association between the expression HOXC10, HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS with clinicopathological characteristics, including sex, histological grade, tumor size (cm), TNM stage, lymphatic invasion and vascular invasion were calculated. Although there was no significant relationship between any of the genes evaluated and any of the clinicopathological characteristics, the correlation coefficients were positive for all the clinical parameters and expression data, suggesting that, whilst an association may exist it is not strong and not statistically significant. Therefore, upregulation of the studied genes may have weak or moderate effects on these characteristics. For protein coding genes, it may be possible that the expression levels of the protein products may be directly correlated with these characteristics. Additionally, upregulated genes may affect the initiation and progression of tumorigenesis, including increasing proliferation and reducing apoptosis (46).
To determine the correlation coefficient, the fold change ratio of each gene was used. There was a strong and significant correlation between the expression of HOTAIR and HOXC13; and a moderate but significant correlation between the expression of HOTAIR and HOXC13-AS, and between HOXC13 and HOXC13-AS.
The correlation between the expression of HOTAIR and HOXC10 and between the expression of HOTAIR and HOXC-AS3 were moderate but not significant. There was a moderate negative correlation between the expression of HOXC-AS3 and HOXC10 and this difference was significant.
HOTAIR and HOXC13 appeared to exhibit similar expression pattern as there was a strong correlation between them. The results of the present study suggest that these genes may affect expression of each other expression in cis; however, additional functional studies are required to determine whether this is the case. Due to the significant upregulation of HOCX10 in the tumor tissues, it may be used as a biomarker for the diagnosis and treatment of CRC.
Future studies should use larger cohorts and evaluate the expression of the studied genes in the serum and blood of patients. Additionally, the expression of these genes in other types of cancer and the expression of other HOX family genes in CRC should be assessed. To determine whether HOTAIR influences expression of any of the HOX family genes, HOTAIR expression should be knocked down and the expression of surrounding genes assessed.
In conclusion the present study showed that HOXC10 expression was significantly higher in CRC samples compared with the normal adjacent tissues, but expression of HOXC-AS3, HOTAIR, HOXC13 and HOXC13-AS did not differ significantly. Based on these results, HOXC10 may be considered as a biomarker for diagnosis of CRC. Additionally the expression of HOTAIR and HOXC13 were strongly correlated and thus may share a regulatory mechanism of expression or one of these genes may regulate the expression of the other. Further functional studies are required to elucidate the mechanism underlying this correlation.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Shahid Chamran University of Ahvaz (Ahvaz, Iran).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MZ and HG conceived and designed the study; MG and ME acquired, analyzed and interpreted the data; ME, MG, MZ and HG participated in drafting the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethical Committee of Shahid Chamran University of Ahvaz (Ahvaz, Iran) and written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer WHO. Cancer today. IARC Web site. https://gco.iarc.fr/today/. Accessed Jan 21, 2019. [Google Scholar]
- 3. Stewart BW and Wild CP (eds): World Cancer Report 2014. IARC Nonserial Publication, Lyon, pp630, 2014. [Google Scholar]
- 4.Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol. 2014;27:9–14. [PMC free article] [PubMed] [Google Scholar]
- 5.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American cancer society, the US multi-society task force on colorectal cancer, and the American college of radiology. CA Cancer J Clin. 2008;58:130–16. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 6.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canavan C, Abrams KR, Mayberry J. Meta-analysis: Colorectal and small bowel cancer risk in patients with crohn's disease. Aliment Pharmacol Ther. 2006;23:1097–1104. doi: 10.1111/j.1365-2036.2006.02854.x. [DOI] [PubMed] [Google Scholar]
- 8.Robertson DJ. ABC of colorectal cancer. Gastroenterology. 2012;143:868–886. [Google Scholar]
- 9.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: A meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 10.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: Systematic review and meta-analysis. Int J Cancer. 2009;124:2406–2415. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 11.Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: A meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila) 2011;4:177–184. doi: 10.1158/1940-6207.CAPR-10-0113. [DOI] [PubMed] [Google Scholar]
- 12.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: A review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60:131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: Molecular features of adipose tissue. J Transl Med. 2016;14(21) doi: 10.1186/s12967-016-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grier DG, Thompson A, Kwasniewska A, McGonigle GJ, Halliday HL, Lappin TR. The pathophysiology of HOX genes and their role in cancer. J Pathol. 2005;205:154–171. doi: 10.1002/path.1710. [DOI] [PubMed] [Google Scholar]
- 15.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 16.Apiou F, Flagiello D, Cillo C, Malfoy B, Poupon MF, Dutrillaux B. Fine mapping of human HOX gene clusters. Cytogenet Cell Genet. 1996;73:114–115. doi: 10.1159/000134320. [DOI] [PubMed] [Google Scholar]
- 17.Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12:11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulessa H, Turk G, Hogan BL. Inhibitionof Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;129:1257–1259. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps oflong noncoding rna occupancy reveal principles of rna-chromatin interactions. Mol Cell. 2011;44:667–668. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Galli F, Ruspi L, Marzorati A, Lavazza M, Di Rocco G, Boni L, Dionigi G, Rausei S. N staging system: Tumor-node-metastasis and future perspectives. Transl Gastroenterol Hepatol. 2017;2(4) doi: 10.21037/tgh.2017.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pauler FM, Koerner MV, Barlow DP. Silencing by imprinted noncoding RNAs: Is transcription the answer? Trends Genet. 2007;23:284–292. doi: 10.1016/j.tig.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue Y, Ma G, Gu D, Zhu L, Hua Q, Du M, Chu H, Tong N, Chen J, Zhang Z, Wang M. Genome-wide analysis of long noncoding RNA signature in human colorectal cancer. Gene. 2015;556:227–234. doi: 10.1016/j.gene.2014.11.060. [DOI] [PubMed] [Google Scholar]
- 26.Yang XD, Xu HT, Xu XH, Ru G, Liu W, Zhu JJ, Wu YY, Zhao K, Wu Y, Xing CG, et al. Knockdown of long non-coding RNA HOTAIR inhibits proliferation and invasiveness and improves radiosensitivity in colorectal cancer. Oncol Rep1. 2016;35:479–487. doi: 10.3892/or.2015.4397. [DOI] [PubMed] [Google Scholar]
- 27.Dou J, Ni Y, He X, Wu D, Li M, Wu S, Zhang R, Guo M, Zhao F. Decreasing lncRNA HOTAIR expression inhibits human colorectal cancer stem cells. Am J Transl Res. 2016;8:98–108. [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X, Liu Z, Ning X, Huang L, Jiang B. The long noncoding RNA HOTAIR promotes colorectal cancer progression by sponging miR-197. Oncol Res. 2018;26:473–481. doi: 10.3727/096504017X15105708598531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X, Lu S. MicroR-545 mediates colorectal cancer cells proliferation through up-regulating epidermal growth factor receptor expression in HOTAIR long non-coding RNA dependent. Mol Cell Biochem. 2017;431:45–54. doi: 10.1007/s11010-017-2974-4. [DOI] [PubMed] [Google Scholar]
- 30.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 31.Svoboda M, Slyskova J, Schneiderova M, Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z, Protivankova M, et al. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;35:1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 32.Zhong M, Wang J, Gong YB, Li JC, Zhang B, Hou L. Expression of HOXC13 in ameloblastoma. Zhonghua Kou Qiang Yi Xue Za Zhi. 2007;42:43–46. (In Chinese) [PubMed] [Google Scholar]
- 33.Hong YS, Wang J, Liu J, Zhang B, Hou L, Zhong M. Expressionof HOX C13 in odontogenic tumors. Shanghai Kou Qiang Yi Xue. 2007;16:587–591. (In Chinese) [PubMed] [Google Scholar]
- 34.Cantile M, Scognamiglio G, Anniciello A, Farina M, Gentilcore G, Santonastaso C, Fulciniti F, Cillo C, Franco R, Ascierto PA, Botti G. Increased HOXC13 expression in metastatic melanoma progression. J Transl Med. 2012;10(91) doi: 10.1186/1479-5876-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantile M, Galletta F, Franco R, Aquino G, Scognamiglio G, Marra L, Cerrone M, Malzone G, Manna A, Apice G, et al. Hyperexpression of HOXC13, located in the 12q13 chromosomal region, in welldifferentiated and dedifferentiated human liposarcomas. Oncol Rep. 2013;30:2579–2586. doi: 10.3892/or.2013.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo J, Wang Z, Huang J, Yao Y, Sun Q, Wang J, Shen Y, Xu L, Ren B. HOXC13 promotes proliferation of esophageal squamous cell carcinoma via repressing transcription of CASP3. Cancer Sci. 2018;109:317–329. doi: 10.1111/cas.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Y, Luo J, Sun Q, Xu T, Sun S, Chen M, Lin X, Qian Q, Zhang Y, Cao L, et al. HOXC13 promotes proliferation oflung adenocarcinoma via modulation of CCND1 and CCNE1. Am J Cancer Res. 2017;7:1820–183. [PMC free article] [PubMed] [Google Scholar]
- 38.Tatangelo F, Di Mauro A, Scognamiglio G, Aquino G, Lettiero A, Delrio P, Avallone A, Cantile M, Botti G. Posterior HOX genesand HOTAIR expression in the proximal and distal colon cancer pathogenesis. J Transl Med. 2018;16(350) doi: 10.1186/s12967-018-1725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao C, Lu W, Lou W, Wang L, Xu Q. Long noncoding RNA HOXC13-AS positively affects cell proliferation and invasion in nasopharyngeal carcinoma via modulating miR-383-3p/HMGA2 axis. J Cell Physiol. 2019;234:12809–12820. doi: 10.1002/jcp.27915. [DOI] [PubMed] [Google Scholar]
- 40.Feng X, Li T, Liu Z, Shi Y, Peng Y. HOXC10 up-regulation contributes to human thyroid cancer and indicates poor survival outcome. Mol Biosyst. 2015;11:2946–2954. doi: 10.1039/c5mb00253b. [DOI] [PubMed] [Google Scholar]
- 41.Ansari KI, Hussain I, Kasiri S, Mandal SS. HOXC10 is overexpressed in breast cancer and transcriptionally regulated by estrogen via involvement of histone methylases MLL3 and MLL4. J Mol Endocrinol. 2012;48:61–75. doi: 10.1530/JME-11-0078. [DOI] [PubMed] [Google Scholar]
- 42.Sadik H, Korangath P, Nguyen NK, Gyorffy B, Kumar R, Hedayati M, Teo WW, Park S, Panday H, Munoz TG, et al. HOXC10 expression supports the development of chemotherapy resistance by fine tuning DNA repair in breast cancer cells. Cancer Res. 2016;76:4443–4456. doi: 10.1158/0008-5472.CAN-16-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang XL, Ding BX, Hua Y, Chen H, Wu T, Chen ZQ, Yuan CH. Hoxc10 promotes the metastasis of human lung adenocarcinoma and indicates poor survival outcome. Front Physiol. 2017;8(557) doi: 10.3389/fphys.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Kim J, Bae DH, Kim JH, Song KS, Kim YS, Kim SY. HOXC10 overexpression promotes cell proliferation and migration in gastric cancer. Oncol Rep. 2019;42:202–212. doi: 10.3892/or.2019.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang E, He X, Zhang C, Su J, Lu X, Si X, Chen J, Yin D Han L, De W. A novel long noncoding RNA HOXC-AS3 mediates tumorigenesis of gastric cancer by binding to YBX1. Genome Biol. 2018;19(154) doi: 10.1186/s13059-018-1523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. J Mol Med. 2014;92:811–823. doi: 10.1007/s00109-014-1181-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.