Abstract

Rare-earth-containing ultrastable Y zeolite (ReUSY) was modified by oxalic acid solution leaching treatment and applied in industrial units for catalytic olefin removal from aromatic hydrocarbons. The porous structure and amount of acidity of the modified ReUSY (denoted as ReUSY-x, where x indicated the amount of oxalic acid in solution) could be tuned by different concentrations of oxalic acid solution, and the ReUSY-x samples exhibited different catalytic performances. Based on the best catalytic performance of ReUSY-25, an industrial catalyst was prepared and applied in industrial units for catalytic olefin removal. The industrial catalyst exhibited excellent activity and regeneration stability with a long lifetime of about 2 years, which was about 13 times longer than that of activated clay.
Introduction
Aromatic hydrocarbons are primary organic raw materials in the petrochemical industry, which play great roles in fields ranging from synthesis of fine chemicals, military industries, to aerospace research.1,2 Aromatic hydrocarbons can be obtained through naphtha reforming and thermal cracking in petrochemical processes and refineries. Undesirable olefin impurities are very harmful to the further technological processing of aromatics, and it is very important to eliminate these olefins before the utilization of aromatics in petrochemical processes.
One tactic to remove trace olefins from aromatics is catalytic hydrogenation treatment.3−6 Nonetheless, the hydrogenation process operating at a high temperature is energy-consuming and would also cause the loss of aromatics. Another commonly applied method is catalytic olefin removal with activated clay. However, the clay treatment has some drawbacks such as quick deactivation and short lifetime; furthermore, the landfill of the used clay would cause severe environmental problems. Consequently, many efforts have been made to develop effective and environment-friendly catalytic materials for olefin removal. In recent years, modified clays,7,8 ionic liquids,9,10 nontoxic organic acids,11 sulfated zirconia,12−15 and mesoporous materials16 have been applied to remove olefins from aromatic streams.
Zeolites are widely used catalysts because of their high specific surface areas, high tolerance to coke deposition, and long lifetime. The ultrastable Y (USY), obtained by the steam treatment of Y zeolite, is a common catalyst in petroleum processes because of its high initial catalytic activity.17−20 Immense physical and chemical methods have been employed to obtain a highly active USY, such as thermal or hydrothermal treatment,21 doping metal oxides,22,23 and acid leaching.17 Herein, we report that by the oxalic acid solution leaching treatment, the modified rare-earth-containing USY zeolite (ReUSY) could be applied in industrial plants for catalytic olefin removal from aromatic hydrocarbons. An industrial-scale catalyst based on the oxalic acid-modified ReUSY was prepared and applied in industrial plants for practical olefin removal. Until now, the industrial catalyst has been employed successfully in dozens of industrial units, including Sinopec, PetroChina, CNOOC, and so forth,24−27 and the excellent catalytic activity and recyclability of the industrial catalyst brought enormous social and economic benefits.
Results and Discussion
In the modification process, ReUSY was treated with oxalic acid solution of different concentrations, and the obtained samples were denoted as ReUSY-x, in which x meant the grams of oxalic acid used in the treatment (see the Experimental Section). As listed in Table 1, after oxalic acid modification, the sodium content in ReUSY was greatly reduced because of the proton exchange. The specific surface area also gradually increased with the concentration of oxalic acid used, and this could be explained by the leaching of the extra-framework Al in ReUSY. ReUSY-25 has the highest surface area, and its X-ray diffraction (XRD) pattern remained almost the same as that of ReUSY (Figure 1). The specific surface area of ReUSY-35 became lower than that of ReUSY-25, and it could be explained by the dealumination and partial collapse of the zeolitic framework caused by the excess amount of oxalic acid.
Table 1. Pore Structure Parameters of Catalysts.
| sample | Na2O content (wt %) | BET surface area/m2 g–1 | average pore size/nm | total pore volumea/cm3 g–1 |
|---|---|---|---|---|
| ReUSY | 4.5 | 442 | 2.8 | 0.31 |
| ReUSY-15 | 1.2 | 548 | 2.8 | 0.38 |
| ReUSY-25 | 0.8 | 618 | 2.9 | 0.45 |
| ReUSY-35 | 0.7 | 458 | 3.2 | 0.40 |
| clay | 154 | 3.1 | 0.22 |
Calculated from P/P0 = 0.99 by the BET method.
Figure 1.
XRD patterns of original ReUSY and ReUSY-25.
The ReUSY-x samples were tested for catalytic olefin removal on a 10 mL scale reactor, and the results are shown in Figure 2. It could be seen that after oxalic acid treatment, the lifetime of the modified ReUSY-x gradually increased, and ReUSY-25 exhibited the longest lifetime. From the NH3-temperature-programmed desorption (TPD) profiles of the ReUSY-x samples (Figure 3), it could be observed that for ReUSY, there appeared one main desorption peak at about 300 °C, together with a small shoulder peak at about 400 °C, corresponding to the relatively weak and strong acid sites. Upon oxalic acid modification, for ReUSY-15, the acidity amount gradually increased because of the decrease of the sodium content by proton exchange. For ReUSY-25, the amount of acidity continuously increased; meanwhile, the desorption peak corresponding to the weak acid slightly shifted to about 320 °C. Generally, for zeolites, the acid sites corresponding to the NH3 desorption peak at 320 °C are moderately strong. The acidity amount of ReUSY-35 decreased drastically because of the partial collapse of the framework by the excess oxalic acid washing; meanwhile, the catalytic performance of ReUSY-35 was also greatly reduced. Among the ReUSY-x samples, the best performance of ReUSY-25 could be explained by its largest surface area and the highest amount of acidity, created by proper oxalic acid modification.
Figure 2.
Catalytic activity of ReUSY-x, running on a 10 mL scale reactor.
Figure 3.
NH3-TPD profiles of the ReUSY-x samples.
Based on its best catalytic performance, ReUSY-25 was selected as the main gradient to fabricate the industrial-scale catalyst. The catalytic olefin removal performances of activated clay and the industrial catalyst were first evaluated in a 10 mL-scale fixed-bed mircoreactor, with the deactivation criteria of bromine index (BI) of the outlet stream beyond 300 mgBr/100 g. The BI of the raw material was 1200–2000 mgBr/100 g, and when activated clay was employed as the catalyst, after 12 h reaction, the BI of the outlet material became 325 mgBr/100 g, exceeding the deactivation criteria. For the industrial catalyst, the lifetime reached 124 h, about 10 times longer than that of activated clay (Figure 4a). To simulate the industrial unit, a 100 mL scale fixed-bed reactor was also employed to perform the long-period activity test. The reaction conditions were the same as in the 10 mL scale microreactor, and the experiment results are displayed in Figure 4b. The effective running time for the industrial catalyst was as long as 58 days until the BI rose up to 300 mgBr/100 g, whereas the running time for activated clay was less than 5 days. More significantly, the BI of aromatics remained less than 20 mgBr/100 g at the 51st day.
Figure 4.

Catalytic activity of activated clay and the industrial catalyst for olefin removal: (a) running on a 10 mL scale reactor and (b) running on a 100 mL scale reactor.
Besides the porous structure, surface acidity also has a great impact on the catalytic activity. For both activated clay and the industrial catalyst, the type and quantity of surface acidic sites were investigated by the pyridine adsorption Fourier transform infrared (FTIR) spectra (Figure 5). In the spectra, vibration bands were observed in the wavenumber range of 1400–1600 cm–1, which were attributed to the interaction of pyridine with Lewis (L) and Brønsted (B) acid sites on the sample surface.8,13 The band at around 1450 cm–1 was attributed to pyridine adsorbed on the L acid sites. Another band at around 1490 cm–1 was ascribed to the contributions of both the L and B acid sites. The band at 1540 cm–1 was assigned to the formation of pyridinium ions on the B acid sites. It was obvious that the total amount of acid sites on the industrial catalyst was much higher than that on activated clay. The much higher activity of the industrial catalyst than activated clay could be attributed to the stable crystalline framework, high amount of acid sites, high specific surface area, as well as the hierarchically porous structure.
Figure 5.
Pyridine adsorption FTIR spectra of clay (a) and the industrial catalyst (b).
Activated clay and the industrial catalyst were applied in industrial plants for olefin removal, whereas the commercial adsorbent ADS-27 was employed to separate the para-xylene from other isomers. As olefins are irreversibly poisonous to the ADS-27 adsorbent, the BI of mixed xylene fed by the xylene distillation unit is required to be lower than 17 mgBr/100 g.
First, 85 tons activated clay were filled in the xylene refining tower for the nonhydrogenation olefin removal. During the 3 months test of activated clay (from May 17 to August 20), the average BI of the raw material was 1107 mgBr/100 g. The running result of activated clay is illustrated in Figure 6a. Four batches of activated clay were replaced in 3 months, and the longest lifetime of activated clay was less than 28 days on the industrial line. Because of the frequent replacement of activated clay, large amounts of labor and solid were wasted in this process, and more than 300 tons of solid waste were generated in 3 months.
Figure 6.
Running results of different refinement catalysts. (a) Four batches of activated clay were used in 94 days. (b) One batch of the industrial catalyst in 188 days. Black bars: BI of raw material (SN802); red bars: BI in the outlet of catalyst refining (SN804); blue bars: BI in the inlet before the adsorption separation process (SN809).
For the application of the industrial catalyst produced based on ReUSY-25, 63.7 tons of the industrial catalyst were filled in the same tower, with all the other conditions remaining the same. Here, it is to be noticed that the loading capacity of the refinement catalyst was different from that of activated clay because of the difference in density. The total online running time of the industrial catalyst in the first cycle is 188 days (Figure 6b). In the initial stage, the BI of the raw material fluctuated smoothly, which was at around 1400 mgBr/100 g. The BI in the outlet of the refinement catalyst remained at a low level. The BI of the feedstock for adsorption separation met the requirement of 17 mgBr/100 g. In the period of 120–150 days, the BI fluctuated, and the average value was roughly 1800 mgBr/100 g. Accordingly, there was some fluctuation in the BI of the feedstock for adsorption separation. After 150 days, the BI of the raw material decreased gradually to 1400 mgBr/100 g and then kept stable; accordingly, the BI of feedstock for adsorption separation decreased lower than 15 mgBr/100 g steadily.
Although the single-pass lifetime of the industrial catalyst was much longer than that of activated clay, it is well known that the cost of zeolite is much higher than that of clay. Thus, the reusability of the industrial catalyst is necessary to reduce the cost. The first-generated and the second-generated catalysts were employed in the second and third cycles, respectively. The working conditions in the three cycles were constant; the application sequence and the corresponding running time are illustrated in Table 2. In the second cycle, the first-regenerated industrial catalyst ran 183 days. During this process, the fluctuation of BI in the raw material was smooth, which was roughly steady at 1400 mgBr/100 g. The BI in the outlet of the first-regenerated industrial catalyst remained at a low level, from 60 mgBr/100g to 200 mgBr/100 g. The BI of the feedstock for adsorption separation was always lower than 10 mgBr/100 g, as shown in Figure 7a. In the third cycle (Figure 7b), the second-regenerated catalyst ran 199 days. In this cycle, the BI of the raw material was 1500–1100 mgBr/100 g. The average BI of the raw material was 1211 mgBr/100 g, which was slightly lower than that in the previous two cycles. The BI of the feedstock for adsorption separation increased from 5 to 15 mgBr/100 g. The total lifetime of the fresh and regenerated industrial catalyst was 13 times longer than that of activated clay.
Table 2. Application Overview of the Industrial Catalyst in Three Cycles.
| cycle | sample | starting time | end time | single-way lifetime (month) |
| 1 | industrial cat. | 2012.8.20 | 2013.2.29 | 6.3 |
| 2 | first-regenerated | 2013.9.18 | 2014.3.29 | 6.3 |
| 3 | second-regenerated | 2014.12.20 | 2015.7.15 | 6.8 |
Figure 7.
Running results of the industrial catalyst regenerated one time (a) and two times (b). Black bars: BI of raw material (SN802); red bars: BI in the outlet of catalyst refining (SN804); blue bars: BI in the inlet before the adsorption separation process (SN809).
The deactivated and regenerated industrial catalysts were characterized by XRD and N2 adsorption. Figure 8 showed a comparison between the diffraction patterns of the industrial catalyst before and after the olefin removal catalytic reaction. Figure 8a shows the XRD pattern of the fresh industrial catalyst, displaying the zeolite Y structure (JCPDS no. 88-2288). Figure 8b shows the XRD pattern of the deactivated catalyst, which had been used for more than 6 months in the olefin removal process. The color of the deactivated catalyst was black because of coke deposition. The positions of the XRD peaks were not shifted; however, the intensity of the peaks decreased obviously. The peak intensities of the (111) and (220) planes in the deactivated catalyst are much lower than those of the fresh catalyst, which could be caused by the microporous channel blockage by coke deposition.28 The deactivated catalyst was regenerated by calcination at 550 °C in air to remove the deposited carbon, and a recovery of the peaks in the XRD spectra was observed, as shown in Figure 8c. It can be deduced that the main reason for the catalyst deactivation was the coke deposition in the micropores of the zeolite, and these deposited carbons can be removed by a simple calcination step to obtain a regenerated catalyst.
Figure 8.

XRD patterns of (a) industrial catalyst, (b) deactivated industrial catalyst, and (c) second-regenerated catalyst.
The fresh, deactivated, and regenerated industrial catalysts were also characterized by N2 adsorption–desorption isotherms, and the results are summarized in Figure 9 and Table 3. It was clear that the fresh industrial catalyst contained both micropores and mesopores, as illustrated by the adsorption steps at very low P/P0 and ∼0.2–0.9 P/P0, respectively. For the deactivated catalyst, the adsorption step at very low P/P0 was reduced greatly, indicating the blockage in the micropores by the coke formation. Both the micropore surface area and micropore volume were greatly reduced (Table 3). After the second regeneration, both the micropores and mesopores were almost recovered. Considering the olefin removal performance of the regenerated industrial catalyst, it could be confirmed that the reason for the deactivation of the industrial catalyst was the coke formation mainly inside the micropores; however, the structure of the catalyst could be well recovered by burning the coke by calcination.
Figure 9.
Nitrogen adsorption–desorption isotherms and BJH pore size distribution of (a) fresh industrial catalyst, (b) deactivated catalyst, (c) second-regenerated catalyst.
Table 3. Pore Structure Parameters of the Regenerated Industrial Catalyst.
| sample | BET surface area/m2 g–1 | micropore surface area/m2 g–1 | micropore volume/cm3g–1 | mesopore volume/cm3g–1 |
|---|---|---|---|---|
| ind. cat. | 631 | 421 | 0.25 | 0.23 |
| deactivated | 228 | 152 | 0.06 | 0.17 |
| ind. cat.-R2 | 592 | 401 | 0.21 | 0.22 |
Conclusions
ReUSY was modified by the oxalic acid solution leaching treatment and was applied in industries for catalytic olefin removal from aromatic hydrocarbons. The porous structure and amount of acidity of the modified ReUSY could be tuned by different concentrations of oxalic acid solution, and the modified samples exhibited different catalytic performances. Based on the properly modified ReUSY, the industrial catalyst was fabricated and applied in industrial units. The industrial catalyst exhibited excellent activity and regeneration stability with a long lifetime of about 2 years, which was about 13 times longer than that of activated clay. The remarkable enhancement of activity and stability can be attributed to the hierarchical porous structure and more acid sites of the catalyst. Compared with activated clay, the application of the ReUSY-based industrial catalyst is cost-effective and can greatly decrease the generation of solid waste.
Experimental Section
Chemicals
ReUSY zeolite was obtained from CNOOC’s Tianjin Chemical Research and Design Institute, with a SiO2/Al2O3 molar ratio of 8.0, rare earth (mainly La3+) 5 wt %, numbered as CZ180. Pseudo-boehmite was purchased from CNOOC’s Tianjin Chemical Research and Design Institute, labeled as A101. Activated clay was purchased from Yongsheng Catalyst Company, China. Oxalic acid was purchased from Tianjin Chemical Reagent No. 3 Factory. HNO3 was purchased from Tianjin Gangfu Fine Chemical Research Institute. Experimental raw oil were aromatic intermediate products with a weight percent of 95.26%, which were obtained from the industrial reforming units of CNOOC Huizhou Petrochemicals Company Limited. The BI is about 1200–2000 mgBr/100 g.
Washing of ReUSY by Oxalic Acid
The commercial ReUSY was washed by oxalic acid solution. Typically, 100 g of ReUSY zeolite was dispersed in 1000 g of deionized water by gradually heating to 60–80 °C. Typically, 25 g oxalic acid was added into the slurry proportion wisely when the temperature reaches. Then, the slurry was kept stirring for 2 h, with the temperature kept constant. The modified zeolite was dried in air for 12 h after filtering and washing, which was labeled as ReUSY-25. The samples with different amounts of oxalic acid (x grams) in the above process were denoted as ReUSY-x.
Fabrication of the Industrial Catalyst
Industrial-scale catalyst was produced by a shape modification of ReUSY-25. Typically, 180 g ReUSY-25 mixed with pseudo-boehmite was blended homogeneously. Then, 195 mL of 3% HNO3 was added dropwise into the above mixture with kneading. After kneading, they were extruded by a 1.6 mm cylinder molding unit. The stick-shaped catalysts were dried at 110 °C for 12 h and then calcined at 550 °C for 4 h. After cooling to room temperature, they were crushed and screened to 20–40 mesh for use.
Characterization
The XRD patterns of the samples were obtained on a Bruker D8 Focus under Cu K irradiation (λ = 0.15418 nm) from 5 to 45°. N2 adsorption and desorption isotherms were recorded on a Micromeritics ASAP 2010 at a liquid nitrogen temperature (77 K). The specific surface areas and total pore volumes of the samples were calculated by the Brunauer–Emmett–Teller (BET) method. The pore size distribution was calculated from the adsorption branch, employing the Barrett–Joyner–Halenda (BJH) method. The surface acidity was investigated by the adsorption of pyridine on the solid surface of samples on IR TENSOR27. Prior to pyridine adsorption, the sample wafers (same amount) were evacuated at 480 °C under a high vacuum, followed by pyridine adsorption at room temperature. Finally, the adsorbed pyridine was desorbed at 200 °C.
Lab-Scale Catalytic Activity Test
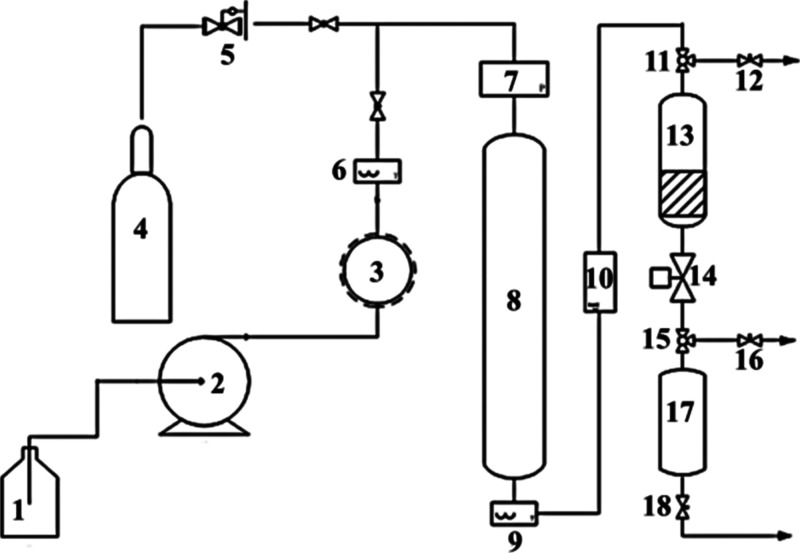
Scheme 1 shows the process of catalytic activity test in lab scale.
Scheme 1. Scheme of a Lab-Scale Catalytic Reactor Process.
(1) Tank for raw material, (2) transverse flow pump, (3) feeding streaming heating, (4) nitrogen cylinder, (5) pressure relief valve, (6) temperature index, (7) pressure gage, (8) fixed-bed reactor, (9) temperature index, (10) pressure gage, (11) three-way valve, (12) needle valve, (13) condenser, (14) pressure valve, (15) three-way valve, (16) needle valve, (17) tank for 500 mL product, (18) needle valve.
Condition I: the catalytic activity was first evaluated by a fixed-bed tubular reactor with a volume of 10 mL. 5 g of the catalyst (20–40 mesh) was packed into the middle of the reactor. The other space was filled up with quartz sand (20–40 mesh). The catalytic activity test was carried out under the following conditions: reaction temperature of 170 °C, reaction pressure of 1.2 MPa, and weight hourly space velocity (WHSV) of 10 h–1. The inlet and outlet liquids from the reactor were analyzed by a BI analyzer.
Condition II: A 100 mL fixed-bed tubular microreactor is employed to evaluate the activity of the catalyst. 50 g of the catalyst (20–40 mesh) is packed into the middle of the reactor. The rest of the space of the reactor is filled up with quartz sand (20–40 mesh). The catalytic activity test obeys the following conditions: temperature 170 °C, pressure 1.2 MPa, and WHSV 1 h–1. A BI analyzer is employed to determine the BI of the inlet and outlet material stream.
BI Analysis
The olefin content was quantified by the BI, which is defined as the number of milligrams of bromine consumed by 100 g of the sample. The BI is determined by a ZBR-2000 bromide BI analyzer. This method meets the criteria of SH/T 0630, ASTMD1492, and so forth.
Commercial Test Process and Regeneration of the Industrial Catalyst
The industrial test of activated clay and the industrial catalyst was performed in Sinopec Shanghai Petrochemical Company Limited. A simplified scheme of the commercial test process is summarized in Scheme 2. In the aromatic hydrocarbon department of Sinopec Shanghai Petrochemical Company Limited, the nonhydrogenation refining process was also conducted on a fixed-bed reactor, as shown in Scheme 2. The BIs of the inlet and outlet liquids from the reactor were detected and labeled as SN802 and SN804. The BI of the samples was detected before ADS-27 (commercial adsorbent from UOP) and labeled as SN809. Because ADS-27 is employed as an adsorbent to separate para-xylene from other isomers and olefins are irreversibly poisonous to the ADS-27 adsorbent, the BI of samples, before ADS-27, is required to decrease below 17 mgBr/100 g. The operating temperature and pressure were controlled by regulating the steam flow. The commercial reaction was conducted under the following conditions: 0.95 MPa, the reaction temperature of 170–200 °C, and WHSV of 1.05–1.35 h–1. The deactivated industrial catalyst was regenerated through a calcination procedure in air flow at 550 °C for 3 h.
Scheme 2. Simplified Scheme of the Production Process in Industrial Plant.

Acknowledgments
This work was supported by CNOOC of China.
The authors declare no competing financial interest.
References
- Marcilly C. R. Where and How Shape Selectivity of Molecular Sieves Operates in Refining and Petrochemistry Catalytic Processes. Top. Catal. 2000, 13, 357–366. 10.1023/a:1009007021975. [DOI] [Google Scholar]
- Corma A.; Huber G.; Sauvanaud L.; Oconnor P. Processing Biomass-Derived Oxygenates in the Oil Refinery: Catalytic Cracking (FCC) Reaction Pathways and Role of Catalyst. J. Catal. 2007, 247, 307–327. 10.1016/j.jcat.2007.01.023. [DOI] [Google Scholar]
- Brémaud M.; Vivier L.; Pérot G.; Harlé V.; Bouchy C. Hydrogenation of Olefins over Hydrotreating Catalysts - Promotion Effect on the Activity and on the Involvement of H2S in the Reaction. Appl. Catal., A 2005, 289, 44–50. 10.1016/j.apcata.2005.04.014. [DOI] [Google Scholar]
- Chakraborty S.; Blacque O.; Fox T.; Berke H. Trisphosphine-Chelate-Substituted Molybdenum and Tungsten Nitrosyl Hydrides as Highly Active Catalysts for Olefin Hydrogenations. Chem.—Eur. J. 2014, 20, 12641–12654. 10.1002/chem.201402736. [DOI] [PubMed] [Google Scholar]
- Mazuela J.; Pàmies O.; Diéguez M. A Phosphite-Pyridine/Iridium Complex Library as Highly Selective Catalysts for the Hydrogenation of Minimally Functionalized Olefins. Adv. Synth. Catal. 2013, 355, 2569–2583. 10.1002/adsc.201201017. [DOI] [Google Scholar]
- van den Berg M.; Minnaard A.; Haak R. M.; Leeman M. Monodentate Phosphoramidites: A Breakthrough in Rhodium-Catalysed Asymmetric Hydrogenation of Olefins. Adv. Synth. Catal. 2003, 345, 308–323. 10.1002/adsc.200390026. [DOI] [Google Scholar]
- Luan J. N.; Li G. L.; Shi L. Study of Modified Clay and Its Industrial Testing in Aromatic Refining. Ind. Eng. Chem. Res. 2011, 50, 7150–7154. 10.1021/ie200147x. [DOI] [Google Scholar]
- Pu X.; Liu N. W.; Jiang Z. H.; Shi L. Acidic and Catalytic Properties of Modified Clay for Removing Trace Olefin from Aromatics and Its Industrial Test. Ind. Eng. Chem. Res. 2012, 51, 13891–13896. 10.1021/ie301706s. [DOI] [Google Scholar]
- Hou J. Z.; Shi L. Removal of Trace Olefins from Aromatics by Using Ionic Liquid. J. Fuel Chem Technol. 2009, 37, 377–380. [Google Scholar]
- Sun Y.; Shi L. Removal of Trace Olefins from Aromatics at Room Temperature Using Pyridinium and Imidazolium Ionic Liquids. Ind. Eng. Chem. Res. 2011, 50, 9339–9343. 10.1021/ie200523f. [DOI] [Google Scholar]
- Tian Y.; Meng X.; Duan J. Y.; Shi L. A Novel Application of Methanesulfonic Acid as Catalyst for the Alkylation of Olefins with Aromatics. Ind. Eng. Chem. Res. 2012, 51, 13627–13631. 10.1021/ie302015v. [DOI] [Google Scholar]
- Ren K.; Kong D.; Meng X.; Wang X.; Shi L.; Liu N. The Effects of Ammonium Sulfate and Sulfamic Acid on the Surface Acidity of Sulfated Zirconia. J. Saudi Chem. Soc. 2019, 23, 198–204. 10.1016/j.jscs.2018.06.006. [DOI] [Google Scholar]
- Yao J.; Liu N.; Shi L.; Wang X. Sulfated Zirconia as a Novel and Recyclable Catalyst for Removal of Olefins from Aromatics. Catal. Commun. 2015, 66, 126–129. 10.1016/j.catcom.2015.03.025. [DOI] [Google Scholar]
- Zhao Q.; Yao J.; Shi L.; Wang X. Effect of Calcination Temperature on Structure, Composition and Properties of S2O82-/ZrO2 and Its Catalytic Performance for Removal of Trace Olefins from Aromatics. RSC Adv. 2016, 6, 84553–84561. 10.1039/c6ra15226k. [DOI] [Google Scholar]
- Liu J.; Liu N.; Ren K.; Shi L.; Meng X. Sulfated Zirconia Synthesized in a One Step Solvent-Free Method for Removal of Olefins from Aromatics. Ind. Eng. Chem. Res. 2017, 56, 7693–7699. 10.1021/acs.iecr.7b01660. [DOI] [Google Scholar]
- Chen C. W.; Wu W. J.; Zeng X. S.; Jiang Z. H.; Shi L. Study on Several Mesoporous Materials Catalysts Applied to the Removal of Trace Olefins from Aromatics and Commercial Sidestream Tests. Ind. Eng. Chem. Res. 2009, 48, 10359–10363. 10.1021/ie901062c. [DOI] [Google Scholar]
- Pu X.; Liu N. W.; Shi L. Acid Properties and Catalysis of USY Zeolite with Different Extra-Framework Aluminum Concentration. Microporous Mesoporous Mater. 2015, 201, 17–23. 10.1016/j.micromeso.2014.08.056. [DOI] [Google Scholar]
- Pu X.; Shi L. Commercial Test of the Catalyst for Removal of Trace Olefins from Aromatics and Its Mechanism. Catal. Today 2013, 212, 115–119. 10.1016/j.cattod.2012.09.010. [DOI] [Google Scholar]
- Pande A.; Niphadkar P.; Pandare K.; Bokade V. Acid Modified H-USY Zeolite for Efficient Catalytic Transformation of Fructose to 5-Hydroxymethyl Furfural (Biofuel Precursor) in Methyl lsobutyl Ketone-Water Biphasic System. Energy Fuels 2018, 32, 3783–3791. 10.1021/acs.energyfuels.7b03684. [DOI] [Google Scholar]
- Kassargy C.; Awad S.; Burnens G.; Upreti G.; Kahine K.; Tazerout M. Study of the Effects of Regeneration of USY Zeolite on the Catalytic Cracking of Polyethylene. Appl. Catal., B 2019, 244, 704–708. 10.1016/j.apcatb.2018.11.093. [DOI] [Google Scholar]
- Zhang W.; Guo P.; Xu H.; Zhao Z. Influence of Hydrothermal Treatment on Acid Property and Catalytic Performance of Nano-HZSM-5 Zeolite for Reducing Olefins in Gasoline. Chin. J. Catal. 2003, 24, 900–904. [Google Scholar]
- Escobar A. S.; Pinto F. V.; Cerqueira H. S.; Pereira M. M. Role of Nickel and Vanadium over USY and RE-USY Coke Formation. Appl. Catal., A 2006, 315, 68–73. 10.1016/j.apcata.2006.09.004. [DOI] [Google Scholar]
- Wang N. N.; Wang Y.; Cheng H. F.; Tao Z.; Wang J.; Wu W. Z. Impact of Cationic Lanthanum Species on Zeolite Y: an Infrared, Excess Infrared and Raman Spectroscopic Study. RSC Adv. 2013, 3, 20237. 10.1039/c3ra42634c. [DOI] [Google Scholar]
- Liu G.; Zang J.; Yu H.; Qin Z.; Chen X.; Huang S. Commercial Application of TCDTO-1 De-Olefin Catalyst in Aromatic Unit. Ind. Catal. (in Chinese) 2015, 2, 410–414. [Google Scholar]
- Du B.; Cheng J.; Yu H.; Zang J.; Wang S.; Liu G. Commercial Application of TCDTO-1 Refining Catalyst for Olefins Removal from Reformate. Ind. Catal. (in Chinese) 2014, 22, 874–877. [Google Scholar]
- Xiu Z. Application of TCDTO-1 Olefins Removal Refining Agent in 0.6 Mt/a Para-Xylene Unit. Inorg. Chem. Ind. (in Chinese) 2014, 46, 68–71. [Google Scholar]
- Gong C.; Lan X.; Hou Z.; Chen Z. Application of Aromatics Refining Catalyst TCDTO-1 in Huizhou Refinery. Petrochem. Technol. Appl. (in Chinese) 2014, 32, 39–41. [Google Scholar]
- de Lucas A.; Canizares P.; Durán A.; Carrero A. Coke formation, location, nature and regeneration on dealuminated HZSM-5 type zeolites. Appl. Catal., A 1997, 156, 299–317. 10.1016/s0926-860x(97)00045-8. [DOI] [Google Scholar]