Abstract

A new class of benzothiazole-appended quinoline derivatives (6–8) was synthesized via one-pot TPGS micellar-mediated acid-catalyzed nucleophilic addition, followed by aerobic oxidative cyclization of 3-formylquinoline-2-one (2), 3-formylquinoline-2-thione (3), and 2-azidoquinoline-3-carbaldehyde (4) individually with 2-amino thiophenol (5). The structures of the prepared compounds were confirmed using suitable spectroscopic methods complemented with single-crystal X-ray diffraction analysis. Time-dependent density functional theory-based optimization of molecular structures, bond lengths, bond angles, HOMO–LUMO energy gaps, and molecular electrostatic potential maps was theoretically computed at the B3LYP/6-311++g(d) level. The molecular docking studies recommended that 6–8 bound to the active site cavity of CD81 effectively with the binding energies of −6.9, −6.3, and −6.5 kcal mol–1, respectively. Further, MD simulation studies of compound 6 suggested that the binding resulted in the stabilization of the CD81 molecule. Thus, all theoretical predictions associated with the experimental verifications motivated to discover novel approaches for cancer therapy.
1. Introduction
Exosomes are ingenuous bionanoparticles secreted by most of the cell types and emerged recently as suitable vehicles for the local and distant intercellular communications. They are believed to be potential contributors to diagnosis and therapy through their crucial implications in a varied number of diseases. Exosomes often contain miRNA, proteins, lipids, and metabolites and were associated with the transportation of the biomolecules from one cell to another cell via membrane vesicle trafficking.1 Tetraspanins, a family of membrane proteins, are found in all exosomes alongside other cellular proteins (annexins and integrins).2 The tetraspanin-28 protein, also known as CD81 (cluster of differentiation 81), belongs to the tetraspanin family encoded by the CD81 gene. This CD81 protein has significant functions in cellular growth, activation, and development, thereby mediating the signal transduction process.3 Currently, exosomes have been investigated for their prospective role in the delivery of drugs, biomarkers and drugs per se. However, the foremost challenge for their applicability in therapy is to design and develop reproducible, scalable, and optimizable isolation/purification methods to harvest suitable exosomes.4 Differential ultracentrifugation is the frequently employed technique for the isolation of exosomes using small molecules as cell surface protein binders.5,6 The crucial roles of exosomes in the progression of cancer depend on the genetic factors, stage of cancer, and dynamics of specific cancer types. Exosomes of pancreatic cancer cells can initiate cell transformation, while exosomes generated from prostate and breast cancer cells instigate neoplasia by virtue of transferring their respective miRNA cargo.7 Notably, human cancer cell-derived exosomes have highly abundant heat shock proteins (Hsp70 and Hsp90) and their role in the innate immune response has been documented.8 Literature has also reported that micro amounts of DNA of exosomes can be used as a biomarker to identify the cancer-associated mutations in serum exosomes.9 Recently, natural product cannabidiol has been identified as a novel exosome inhibitor, which showed potential inhibition of three distinctive cancer cell lines such as hepatocellular carcinoma (HEPG2), prostate cancer (PC3), and breast adenocarcinoma (MDA-MB-231).10 Hence, the abovementioned literature demonstrated the novel approach of inhibiting exosomes for the effective treatment liver, prostate, and breast cancers.
Quinoline is one of the bioactive scaffolds identified in many natural products and synthetic medicinal compounds.11 Similarly, sulfur-containing quinolines have been used as anticancer, bacteriostatic, and enzyme modulators.12 Many of the functionalized planar quinolone derivatives are used in the diagnosis of various diseases and as DNA intercalators in cancer chemotherapy.13 Quinoline-based compounds have been documented for their significant and wider applications in cancer chemotherapy.14 Many of the quinolone derivatives have been reported to inhibit various key enzyme targets such as MEK1 kinase,15 cyclin-dependent kinase,16 c-Jun N-terminal kinase,17 and topoisomerase 1,18 exhibiting potential anticancer activity against various cancers of blood, liver, breast, and prostate. Earlier, we have reported novel quinoline bearing dihydropyridines for their inhibition against the lung cancer cell lines and obtained potential inhibition profiles.19 Compounds SYBR Green I and SYBR Safe (Figure 1) are mainly used as dyes for the quantification of double-stranded DNA (quantitative PCR) and to visualize DNA (gel electrophoresis).20,21 Similarly, thiazole orange (Figure 1) and a few benzothiazole derivatives have been reported for their applications in organic and medicinal chemistry.22
Figure 1.
Literature reported quinolone and benzothiazole compounds.
Understanding the therapeutic significance of the quinoline-based anticancer molecules, we are interested to synthesize and characterize novel quinoline analogues and determine their binding capability toward cell surface proteins. Further, this study envisaged to synthesizing novel planar benzoquinoline derivatives by aerobic oxidation to assess their reactive sites and electronic properties. Moreover, molecular electrostatic potential calculations of all the compounds assisted in the analysis of their inhibitory potential against exosomes. Bioinformatic studies such as molecular docking and molecular dynamics simulation of the synthesized compounds were conducted, which demonstrated the nature of the interactions in the compounds and their potential as a part of exosome–nanoparticle interfaces. The coplanarity of the prepared compounds helped further to propose their applicability as suitable candidates for pharmaceuticals.
2. Results and Discussion
2.1. Chemistry
Novel compounds 6 and 7 were synthesized via a simple one-step reaction of 2-thio-1,2-dihydroquinoline-3-carbaldehyde (2) or its thione derivative (3) with 2-amino thiophenol (5). Similarly, 2-azidoquinoline-3-carbaldehyde (4) was reacted with 2-amino thiophenol (5) and afforded compound 8. Scheme 1 presents the synthetic route for obtaining novel compounds 6–8. The mechanism of this reaction involves initial acid-catalyzed condensation with 5, followed by in situ aerobic oxidative cyclization. The products were characterized based on FT-IR, 1H-NMR, and 13C-NMR spectroscopic data. In the FT-IR spectra, the carbonyl (—C=O; 1685 cm–1) and secondary amine (−N–H; 3358 cm–1) stretching frequencies were observed, while no stretching vibration of the functional group S–H (2600–2540 cm–1) appeared. Incorporation of the benzothiazole moiety was confirmed by the absence of signals corresponding to the carbaldehyde and the presence of an increased number of signals in the aromatic region of 1H-NMR and 13C-NMR of 6–8. The 1H-NMR spectrum of 6 (Supporting information) displayed a broad singlet at δ 12.15 ppm due to the NH proton, and in the 13C-NMR, a signal at 161.37 ppm was assigned to the carbonyl carbon. Further, the molecular weight of 6 was confirmed by GC–MS (M·+ (m/z) 278.11 amu). All the abovementioned spectral data substantially confirmed the anticipated structures of the synthesized compounds, accordingly.
Scheme 1. Synthetic Route for Compounds 6–8.

2.2. Crystal Structure Determination of 2-(Benzylthio)quinoline-3-carbaldehyde (6)
The single-crystal X-ray crystallography was employed for the determination of the structure of compound 6. Needle-shaped, yellowish-green colored crystals were obtained by slow evaporation utilizing methanol as a suitable solvent. The data were deposited in the Cambridge Crystallography Data Center (CCDC) and obtained the reference number 1959426. The information of crystal and structural refinement data are shown in Table 1.
Table 1. Crystallographic Data and Structural Refinement of Compound 6 (CCDC no. 1959426).
| formula | C16H10N2OS | radiation type | MoKα |
| Dx (mg m–3) | 1.493 | T | 293(2) K |
| μ (mm–1) | 0.257 | S | 1.138 |
| formula weight | 278.32 | measured refl. | 15,350 |
| size (mm3) | 0.23 × 0.17 × 0.12 | independent refl. | 2500 |
| crystal system | monoclinic | reflections used | 2500 |
| space group | P21/c | Rint | 0.039 |
| a (Å) | 13.9176(11) | parameters | 221 |
| b (Å) | 3.9783(3) | restraints | 0 |
| c (Å) | 22.532(2) | largest peak | 0.22 |
| α (°) | 90.00 | deepest hole | –0.23 |
| β (°) | 97.142(7) | Z | 4 |
| γ (°) | 90.00 | R[F2 > 2σ(F2)] | 0.052 |
| V (Å3) | 1237.87(19) | wR(F2) | 0.126 |
| wavelength (Å) | 0.71073 |
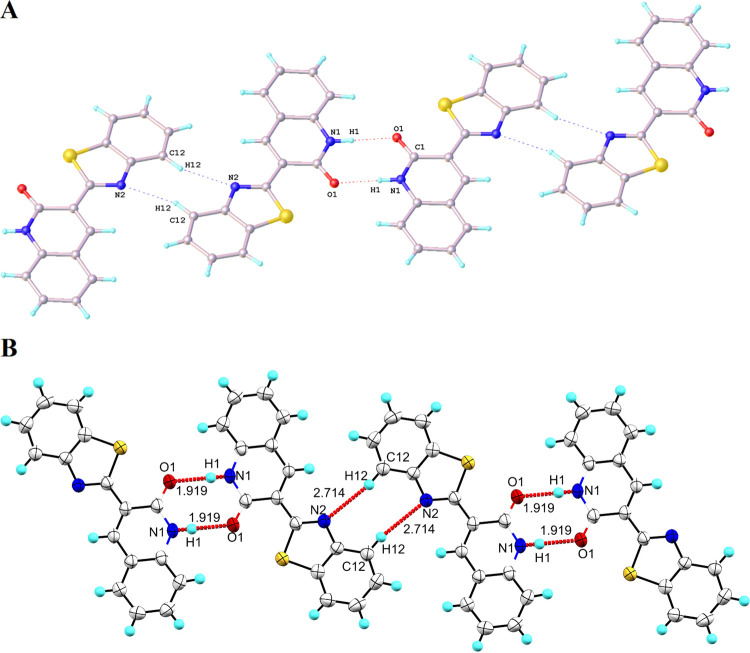
Compound 6 crystallized in the monoclinic cell with the P21/c space group. The ORTEP view of compound 6 along with the atom numbering scheme is shown in Figure 2. XRD results revealed a coplanarity between the quinolone framework and the benzothiazole moiety (Figure 2). The C(1)–N(1) (1.355 Å), C(10)=N(2) (1.308 Å), and C(1)=O(1) (1.245) bond distances found in compound 6 corroborated well with other benzothiazole quinolones containing C—N/C=N and C=O bonds. The bond length of C(10)–S(1) (1.753 Å) is found to be closer to the bond length of C=S (1.60 Å). An inspection of the packing diagram of 6 revealed that the structure exhibits interaction with an adjacent molecule by N–H···O and C–H···N intermolecular hydrogen bonding interactions (Figure 3A). This interaction is observed between the aryl NH proton (H1) and the quinolone oxygen (O1), thereby producing a one-dimensional chain, specifically, N1–H1–O1 (1.919) and N2–H12–C12 (2.714) along the b axis (Figure 3B).
Figure 2.

ORTEP diagram of compound 6 drawn at the 50% thermal ellipsoid probability level for non-H atoms.
Figure 3.
(A, B) Packing arrangement of 6, viewed down the b axis. (A) Dashed lines indicate hydrogen bonds (50% thermal ellipsoid probability level for non-H atoms). (B) Red dashed lines indicate weak intermolecular hydrogen bonding interactions N1–H1–O1 (1.919) and N2–H12–C12 (2.714) along the b axis (30% thermal ellipsoid probability level for non-H atoms).
2.3. Molecular Electrostatic Potential Surface
The molecular electrostatic potential surface represents the area around the molecule and gives an indication of the net electrostatic effect that is produced by the total charge distribution (electron + nuclei). It correlates the dipole moments, electronegativity, partial charges, and chemical reactivity of the molecule.23 This is a visual representation that allows us to recognize the relative polarity of the molecule. Using the electrostatic potential surface, the electron density is surface-mapped, thus depicting the size, charge density, shape, and reactive sites of the molecule.24 The use of different colors helps in specifying the electrostatic potential values. Red describes the regions of the most negative electrostatic potential, blue designates the most positive electrostatic potential regions, and green illustrates the region of zero potential. The region of the negative electrostatic potential indicates that there is an attractiveness of the proton by the concentrated electron density. The area of the positive electrostatic potential is also an indication of low electron density, which means that there is a repulsion of the proton in that region. For molecules 6–8, the electrostatic potential map (MEP) was calculated using the same functional and basis set as the FREQ, time-dependent density functional theory (TDDFT), and NMR but with the keyword pop = full. Figure 4 represents the MEP surface of compounds 6–8.
Figure 4.
(A–C) Molecular electrostatic potential surface of compounds 6–8.
2.4. Frontier Molecular Orbitals
The molecular orbital theory used to describe HOMO–LUMO interactions is known as the frontier molecular orbital (FMO) theory.25 The FMO of a compound refers to the energy of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). HOMO is the electron-donating (nucleophilic) outer orbital and LUMO is the electron acceptor (electrophilic) inner orbital. FMO theory utilizes these concepts to explain the structure and reactivity of a molecule.26 The energy gap (E-gap) (Figure 5) is the energy difference between the HOMO and LUMO orbitals. The magnitude of the HOMO–LUMO energy gap has considerable chemical effects.27 A large gap implies thermodynamic stability of the compound, while a small gap signifies easy electronic transition. HOMO energy (E-HOMO) is directly related to the ionization potential, and LUMO energy (E-LUMO) is linked to electron affinity. Hence, the HOMO–LUMO energy calculations aid in describing the chemical reactivity and kinetic stability of a molecule.28
Figure 5.

(A, B, D, E) Contour surfaces of frontier molecular orbitals of 6 and 7. (C, F) HOMO–LUMO energy gap of structures 6 and 7. (G, H) Contour surfaces of frontier molecular orbitals of 8. (I) HOMO–LUMO energy gap of structure 8.
2.5. Bioinformatic Studies
2.5.1. Molecular Docking
Computational molecular docking studies are executed to identify the ligands’ (6, 7, and 8) biochemical interaction with preferred pose on the binding site of the target protein CD81. The results revealed that the potential selective ligands interacted perfectly and occupied the binding site cavity of CD81, preferably. The best interactive pose of each ligand was confirmed based on the binding energy values and biochemical interactions. The conformations of all the docked complexes (6, 7, and 8 with CD81) are shown in Figure 6. The computational molecular binding energies were predicted to be −6.9, −6.3, and −6.5 kcal mol–1 for compounds 6, 7, and 8, respectively. The binding affinity and the amino acid residues of CD81 that participated in the interactions with ligands 6, 7, and 8 are given in Table 2.
Figure 6.
(A–C) Binding interactions of compounds 6–8 represented by a 3D ball-stick model and 2D plots. The residues shown in the 2D plot represent the name of amino acid, chain, and number. Green dashed lines indicate hydrogen bonds; brown and pink colors represent π and amide interactions, respectively.
Table 2. Binding Affinity and Crucial Amino Acid Residues of CD81 Interacting with Compounds 6, 7, and 8.
| S. no. | complex | binding affinity (kcal mol–1) | residual interactions |
|---|---|---|---|
| 1 | CD81-compound-6 | – 6.9 | Glu152, Asp155, Ser179, and Lys187 |
| 2 | CD81-compound-7 | –6.3 | Glu152, Asp155, Pro176, and Ser177 |
| 3 | CD81-compound-8 | –6.5 | His151, Glu152, Asp155, Cys156, Cys175, and Ser179 |
2.5.2. Molecular Dynamics Simulation
To characterize the influence of molecular structural stability on the complexed coordinates of compound 6 and CD81, it was considered to measure root mean square deviations (RMSD) against the timescale. As an essential property, RMSD proves to confirm the protein structural stability in proximity to the experimentally established protein structure. The molecular dynamics simulated RMSD values (avarege) of CD81-compound-6 and CD81 were determined to be 0.25 and 0.27 nm, respectively (Figure 7A). The binding of ligand 6 to CD81 resulted in lower RMSD deviations. It can be presumed that 6 binds tightly with the active pocket of CD81 and stabilizes the protein molecule. To improve the protein structural stability on the active biomolecular system, the radius of gyration (Rg) is generally integrated with protein’s tertiary structural volume. When a protein has a higher Rg, it may have packed loosely on the binding cavity. The average Rg for the complex of CD81-compound-6 and CD81 were determined to be 1.97 and 1.98 nm, respectively. The Rg trajectory of CD81-compound-6 is equilibrated as compared to CD81 alone. The Rg plot demonstrated the better binding capability of compound 6 on the CD81 molecule (Figure 7B). The solvent-accessible surface area (SASA) is a term specifically used to describe the protein’s surface area interacting with the solvent molecules. For the CD81-compound-6 complex and CD81, the average values of SASA were identified to be 164.77 and 170.67 nm2, respectively (Figure 7C). The plot exhibited a low value of SASA for the CD81 complexed with compound 6. This may be due to the average residues that are exposed to solvent molecules that were previously occupied by compound 6. This further evidenced dynamic binding interactions that occurred between compound 6 and the CD81 molecule. Additionally, the free energy of solvation of the CD81-compound-6 complex and CD81 alone were analyzed and determined as 304.51 and 314.23 kJ/mol/nm2, respectively (Figure 7D). The free energy of solvation is the lowest for CD81 upon binding of the compound 6.
Figure 7.
Dynamics of CD81 and CD81-compound-6 at 300 K. (A) RMSD plot as a function of time. (B) Time evolution of Rg. (C) SASA as a function of time. (D) Free energy of solvation as a function of time. Red and green colors represent values obtained for CD81 and CD81-compound-6, respectively.
2.5.3. Principal Component Analysis (PCA) and Gibbs Free Energy Landscape
For a protein molecule, essential dynamics (ED) or PCA demonstrates the overall inflations of the molecular structural stability during molecular dynamics simulations. The PCA for CD81 and the complex of CD81-compound-6 were calculated at 300 K. Based on the protein amide-bonded backbone, the dynamics of CD81 and CD81-compound-6 were computationally characterized by employing the gmx covar module. The ED perceives the crucial average atomic dynamic movements in a protein leading to variations of the structural elements due to atomic fluctuations. The overall mobility of a molecular system can be expressed in terms of the total eigenvalues, which can be employed to describe the elasticity of the macromolecule surrounded by various environmental conditions. Eigenvalues and the trace of the covariance matrix were determined as 377.37 and 251.46 nm2 and they were the specifically observed maximum for CD81 in comparison to the CD81-compound-6 complex. The binding of 6 led to a decrease in average random fluctuations in the CD81 molecule. Higher eigenvalues indicated the greater expansion of CD81. Figure 8A presents 2D structural projections of trajectories on eigenvectors of CD81 and CD81-compound-6, and a major difference in both cases was detected. The plausible reason for this difference in the positions of elemental atoms could be attributable to the impact of binding of compound 6 to CD81.
Figure 8.
PCA and Gibbs energy landscape. (A) 2D projections of trajectories on eigenvectors displayed various projections of CD81 (red) and the CD81-compound-6 complex. The graph presents a large-scale average motion in CD81 and the CD81-compound-6 complex (green), respectively. The Gibbs energy landscape plots acquired during 100 ns MD simulations for the (B) CD81 protein alone and (C) CD81-compound-6.
The Gibbs free energy landscape was computationally quantified by employing gmx covar, gmx anaeig, and gmx sham modules using projections of their own underlying initial (PC1) and next (PC2) eigenvectors. The gmx anaeig module discloses the sets of eigenvectors and eigenvalues that are to be considered as input files and reverts to project the molecular dynamics trajectory with the specific eigenvector value. The color-coded energy landscape displayed different forms of CD81 and CD81-compound-6, respectively (Figure 8B,C). The Gibbs free energy landscape analyzes the different divergences of the atomic orientations into two systems for all of the Cα atoms of the target protein CD81 from the 100 ns trajectory. The corresponding free energy contour map with blue color demonstrates the lower energy state. The main free energy in the global free energy minimum region of CD81 is different when it is bound to 6. The comparative analysis between the complete characterization of Gibbs free energy values of complex of CD81-compound-6 and CD81 protein alone recommended that CD81 showed a maximum rugged or irregular free energy surface when it binds to ligand 6.
3. Conclusions
We have synthesized novel benzothiazole-quinoline hybrid compounds 6–8 and characterized them by suitable spectroscopic techniques. Single-crystal XRD analysis of compound 6 revealed the geometry, conformation, and intermolecular interactions such as hydrogen bonding within the molecular framework. The chemical structures of 6–8 were further studied using density functional theory calculations. The NMR chemical shift values were computed theoretically and compared with experimentally obtained results. Frontier molecular orbitals (HOMO and LUMO) and molecular electrostatic potential were computed to determine the distribution of charge density and possible binding sites. Molecular docking studies revealed that these compounds bind tightly to the active pocket of CD81. The strength of the binding of 6 was further analyzed by MD simulations, and the obtained data suggested that ligand binding led to the stabilization of CD81. Hence, these chemical scaffolds act as small molecular probes for the binding of the exosomes with high affinity and specificity and thereby paved a way to discover novel chemotherapeutics.
4. Materials and Methods
4.1. General
All chemicals employed in this research work were purchased from Sigma-Aldrich (South Africa). Melting points of all the synthesized compounds were recorded using a Stuart SMP10 melting point apparatus. IR spectra were determined with a Varian 800 FTIR instrument using the KBr pellet technique, and the absorption frequencies are expressed in cm–1. 1H and 13C NMR spectra were taken from Bruker AVANCE 400 MHz and 600 MHz spectrometers, respectively, using TMS as an internal reference. The chemical shift values were expressed in parts per million (ppm). Compounds 2–4 were prepared from 2-chloro-3-quinoline-carboxaldehyde (1) according to the literature-reported method.29
4.2. General Procedure for the Synthesis of Compounds 6–8
To a constantly stirred suspension of 2-substituted-quinolone-3-carbaldehyde (2, 3, or 4; 6.0 mmol) in d-α-tocopheryl polyethylene glycol succinate (TPGS 2% with water; 50 mL) were added 2-amino thiophenol (5; 6.0 mmol) and few drops of glacial acetic acid at room temperature.30 The mixture was heated under reflux for 6 h, followed by cooling to yield the solid product, which was isolated by vaccum filtration. The obtained crude solid product was recrystallized using DMSO and dried to afford pure product 6,317, or 8 in excellent yields.
4.2.1. Characterization Data of Compounds 6–8
4.2.1.1. 3-(Benzo[d]thiazol-2-yl)quinolin-2(1H)-one (6)31
Pale yellow solid, yield 94%, mp. 247 °C; IR (KBr, cm–1): 3358, 2874, 1685, 1577, 1332; 1H-NMR (600 MHz, DMSO-d6): δ 12.41 (brs, 1H, NH), 9.1 (s, 1H, H3), 8.14 (d, J = 7.90 Hz, 1H, H12), 8.08 (d, J = 8.16 Hz, 1H, H15), 8.01 (d, JHH = 7.74 Hz, 1H, H5), 7.65 (t, J = 7.65 Hz, 1H, H13), 7.57 (t, J = 7.77 Hz, 1H, H14), 7.47 (t, 1H, J = 9.84 Hz, H6), 7.45 (d, J = 7.38 Hz, 1H, H8), 7.31 (t, J = 7.53 Hz, 1H, H7) ppm; 13C-NMR (600 MHz, DMSO-d6): 139.5, 139.0, 136.7, 132.6, 130.0, 126.7, 125.4, 123.6, 123.1, 122.7, 122.4, 119.4, 115.8 ppm.
4.2.1.2. 3-(Benzo[d]Thiazol-2-yl)quinoline-2(1H)-thione (7)
Yellow solid, yield 84%, mp. 254 °C; IR (KBr, cm–1): 3358, 2345, 1685, 1577, 1332, 1242, 1158, 1058, 931, 751; 1H-NMR (400 MHz, DMSO-d6): 9.40 (brs, 1H, NH), 8.8 (s, 1H), 7.4–7.8 (m, 1H), 7.39–7.11 (m, 2H), 7.15–7.10 (m, 1H), 7.09–6.92 (m, 2H), 6.8–6.6 (m, 2H) ppm; 13C-NMR (400 MHz, DMSO-d6): 181.1, 156.8, 151.4, 137.7, 134.2, 133.2, 132.6, 129.4, 127.9, 124.8, 122.3, 119.6, 119.3, 116.2 ppm.
4.2.1.3. 2-(2-Azidoquinolin-3-yl)benzo[d]thiazole (8)
Yellow solid, yield 90%, mp. 268 °C; IR (KBr, cm–1): 3489, 2848, 1668, 1557, 1384, 1320, 1151, 979, 750; 1H-NMR (400 MHz, DMSO-d6): 9.20 (s, 1H), 8.16 (s, 1H), 8.08–7.90 (m, 2H), 7.89–7.80 (m, 1H), 7.78–7.68 (m, 2H), 7.52–7.47 (m, 1H), 7.34–7.29 (m, 1H) ppm; 13C-NMR (400 MHz, DMSO-d6): 199.4, 161.4, 160.7, 152.2, 139.7, 138.7, 136.7, 132.6, 130.1, 126.7, 125.4, 123.2, 122.8, 115.9 ppm.
4.3. Crystal Structure Determination
Needle-shaped, monoclinic, and yellowish-green colored crystals of compound 6 were collected by the slow evaporation method employing methanol as an appropriate solvent. An Oxford Diffraction Xcalibur Sapphire3 diffractometer with a Sapphire CCD detector was employed for the study. Empirical absorption correction was applied using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. For data collection, CrysAlis PRO was used, and for cell refinement and data reduction, CrysAlis RED was used. SHELXS9732 within the WinGX program package was employed to solve and refine the structure. PLATON33 software was used for the documentation.
4.4. Density Functional Theory (DFT) Studies
The Gaussian 0934 package was employed for the DFT calculations. The structures of the three molecules (6–8) were optimized at the B3LYP/6-311++g(d)35−38 level of theory, and frequency calculations were also undertaken at the same level to identify whether the optimized geometries are true minima or not. Cartesian coordinates of the optimized geometries, total energies, zero-point vibrational energies, and the number of imaginary frequencies are given in the Supporting Information for brevity. A FREQ calculation was conducted to extract the IR spectra, and TDDFT39 calculation at the B3LYP/6-311++g(d) level was performed to obtain the UV–vis spectrum. The NMR keyword was specified to compute shielding tensors with the gauge-independent atomic orbital method.40 Spin–spin coupling constants were not calculated due to the higher computational cost. HOMO and LUMO orbitals in the gas phase were also obtained from the TDDFT calculation. The relevant spectral images are presented in the Supporting Information (Figures S11–S15).
4.5. Bioinformatic Studies
4.5.1. Molecular Docking
The three-dimensional (3D) X-ray crystal structure of CD81 was retrieved from the Protein Data Bank (PDB code: 5M3T).41 The chemical structures of 6, 7, and 8 were drawn using sketch molecules module of BIOVIA Discovery Studio 2017R2.42 Initially, hydrogen atoms were incorporated to optimize the geometry of ligands via molecular mechanics (MM) employing universal force fields and steepest descent algorithm implemented in Avogadro tools.43 The docking study was conducted to know the bioactive conformation and the binding affinity of compounds 6, 7, and 8 toward CD81. The compounds 6–8 were docked by defining the grid box with a 1 Å spacing and size of 30 × 30 × 30 pointing in x, y, and z directions around the binding site of CD81 following the standard protocols of AutoDock44 and AutoDock Vina45 tools. The Lamarckian genetic algorithm was employed as the search algorithm with default values.46 The most appropriate conformation of the docked complex was taken for further computational analysis. The complete protocol of the docking study was explained in our earlier communication.47 PyMol,48 Discovery Studio Visualizer,42 and LigPlot+49 were deployed for the 3D visualization and subsequent inspection of the docked complexes.
4.5.2. MD Simulations
To gain an insight into the impact of the best inhibitory compound 6 on CD81, MD simulations are performed at the MM level integrated in GROMACS 5.1.2 employing the GROMOS 96 43a1 force field at 300 K. The topologies of CD81 (gmx pdb2gmx modules of GROMACS) and compound 6 (PRODRG server) were tethered. CD81 and the complex of CD81-compound-6 were submerged into a cubic box composed of water molecules employing the gmx editconf module to generate suitable boundary conditions. We have accomplished 100 ns MD simulations of CD81 and the CD81-compound-6 complex at 300 K. The module gmx solvate was used to solvate the system. To solvate the system, the simple point charge (spc216) water model was utilized. The charges on the CD81 protein and CD81-compound-6 complex were counter-balanced by Na+ (sodium) and Cl– (chloride) ions using the gmx genion module to achieve neutrality. Further, the system minimization and two phases of equilibration were conducted based on the standard protocols described in the literature.50,51 While allowing for the free movement of most of the atoms, the backbone atoms of the initial model were specifically restrained in both steps. Further, particle-mesh Ewald method was deployed and the production phases (100 ns) were accomplished at 300 K. The final trajectories were obtained via gmx rms, gmx energy, gmx rmsf, gmx confirms, make_ndx, gmx hbond, gmx gyrate, gmx covar, gmx do_dssp, gmx sham, gmx anaeig, and gmx sasa functions of GROMACS. PyMol and visual molecular dynamics were employed for the pictorial representations.52
4.5.3. Principal Component Analysis (PCA) and Gibbs Free Energy Landscape
PCA or ED was performed to establish the atomic motions in CD81 after binding of compound 6 by following the reported method.53 On the other hand, the Gibbs free energy landscape is usually studied as post-MD analysis for the observation of structural and conformational changes of a protein.54 We have estimated the Gibbs free energy landscape for CD81 and the CD81-compound-6 complex at 300 K according to our earlier reported protocol.53
Acknowledgments
Authors gratefully acknowledges the University of KwaZulu-Natal and University of the Free State, South Africa (SA) for the financial support and infrastructural facilities for this project. K.A. is grateful to National Research Foundation (NRF), SA for the research funding in the form of NRF/DSI-Innovation Post-Doctoral Research Fellowship (grant no. 120677). All computational tasks were carried out using the software resources of the Centre for High Performance Computing, Cape Town, South Africa. C.B. is thankful to the Faculty of Pharmacy-Philadelphia University, Jordan for the university research funding (no. 467/34/100 PU).
Appendix
CCDC number 1959426 contain the supplementary crystallographic data for this paper. The data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01166.
Spectral images of the compounds 6–8, X-ray crystallography data of 6, and DFT data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Steven Conlan R.; Pisano S.; Oliveira M. I.; Ferrari M.; Pinto I. M. Exosomes as reconfigurable therapeutic systems. Trends Mol. Med. 2017, 23, 636–650. 10.1016/j.molmed.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey K. J.; Powell K. L.; Ashton A. W.; Morris J. M.; McCracken S. A. Exosomes: Mechanisms of uptake. J. Circ. Biomarkers 2015, 4, 7. 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G.; Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Corbett A. L.; Taatizadeh E.; Tasnim N.; Little J. P.; Garnis C.; Daugaard M.; Guns E.; Hoorfar M.; Li I. T. S. Challenges and opportunities in exosome research—perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503 10.1063/1.5087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarovni N.; Corrado A.; Guazzi P.; Zocco D.; Lari E.; Radano G.; Muhhina J.; Fondelli C.; Gavrilova J.; Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58. 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- Livshits M. A.; Khomyakova E.; Evtushenko E. G.; Lazarev V. N.; Kulemin N. A.; Semina S. E.; Generozov E. V.; Govorun V. M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319. 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosgodage U. S.; Trindade R. P.; Thompson P. R.; Inal J. M.; Lange S. Chloramidine/bisindolylmaleimide-I-mediated inhibition of exosome and microvesicle release and enhanced efficacy of cancer chemotherapy. Int. J. Mol. Sci. 2017, 18, 1007. 10.3390/ijms18051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Liu Z.; Ding H.; Zhou Y.; Doan H. A.; Sin K. W. T.; Zhu Z. J.; Flores R.; Wen Y.; Gong X.; Liu Q.; Li Y.-P. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. 10.1038/s41467-017-00726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Rai A. J.; DeCastro G. J.; Zeringer E.; Barta T.; Magdaleno S.; Setterquist R.; Vlassov A. V. An optimized procedure for exosome isolation and analysis using serum samples: Application to cancer biomarker discovery. Methods 2015, 87, 26–30. 10.1016/j.ymeth.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Kosgodage U. S.; Mould R.; Henley A. B.; Nunn A. V.; Guy G. W.; Thomas E. L.; Inal J. M.; Bell J. D.; Lange S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 2018, 9, 889. 10.3389/fphar.2018.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung P.-Y.; Bian Z.-X.; Pun H.-Y.; Chan D.; Chan A. S.-C.; Chui C.-H.; Tang J. C.-O.; Lam K.-H. Recent advances in research of natural and synthetic bioactive quinolines. Future Med. Chem. 2015, 7, 947–967. 10.4155/fmc.15.34. [DOI] [PubMed] [Google Scholar]
- Marella A.; Tanwar O. P.; Saha R.; Ali M. R.; Srivastava S.; Akhter M.; Shaquiquzzaman M.; Alam M. M. Quinoline: A versatile heterocyclic. Saudi Pharm. J. 2013, 21, 1–12. 10.1016/j.jsps.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasirajan G.; Krishnaswamy V.; Raju N.; Mahalingam M.; Sadasivam M.; Subramaniam M. P.; Ramasamy S. New pyrazolo-quinoline scaffold as a reversible colorimetric fluorescent probe for selective detection of Zn2+ ions and its imaging in live cells. J. Photochem. Photobiol., A 2017, 341, 136–145. 10.1016/j.jphotochem.2017.03.035. [DOI] [Google Scholar]
- Jain S.; Chandra V.; Jain P. K.; Pathak K.; Pathak D.; Vaidya A. Comprehensive review on current developments of quinoline-based anticancer agents. Arabian J. Chem. 2019, 12, 4920–4946. 10.1016/j.arabjc.2016.10.009. [DOI] [Google Scholar]
- Jin X.-Y.; Chen H.; Li D.-D.; Li A.-L.; Wang W.-Y.; Gu W. Design, synthesis, and anticancer evaluation of novel quinoline derivatives of ursolic acid with hydrazide, oxadiazole, and thiadiazole moieties as potent MEK inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 955–972. 10.1080/14756366.2019.1605364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchis-Reyes M. A.; Zentella A.; Martínez-Urbina M. A.; Guzmán Á.; Vargas O.; Apan M. T. R.; Gallegos J. L. V.; Díaz E. Synthesis and cytotoxic activity of 2-methylimidazo[1,2-a]pyridine- and quinoline-substituted 2-aminopyrimidine derivatives. Eur. J. Med. Chem. 2010, 45, 379–386. 10.1016/j.ejmech.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Jiang R.; Duckett D.; Chen W.; Habel J.; Ling Y. Y.; LoGrasso P.; Kamenecka T. M. 3,5-Disubstituted quinolines as novel c-Jun N-terminal kinase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6378–6382. 10.1016/j.bmcl.2007.08.054. [DOI] [PubMed] [Google Scholar]
- Antony S.; Jayaraman M.; Laco G.; Kohlhagen G.; Kohn K. W.; Cushman M.; Pommier Y. Differential induction of topoisomerase I-DNA cleavage complexes by the indenoisoquinoline MJ-III-65 (NSC 706744) and camptothecin: Base sequence analysis and activity against camptothecin-resistant topoisomerases I. Cancer Res. 2003, 63, 7428–7435. [PubMed] [Google Scholar]
- Nkosi S. M.; Anand K.; Anandakumar S.; Singh S.; Chuturgoon A. A.; Gengan R. M. Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of novel quinoline bearing dihydropyridines. J. Photochem. Photobiol., B 2016, 165, 266–276. 10.1016/j.jphotobiol.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Evenson W. E.; Boden L. M.; Muzikar K. A.; O’Leary D. J. 1H and 13C NMR assignments for the cyanine dyes SYBR Safe and thiazole orange. J. Org. Chem. 2012, 77, 10967–10971. 10.1021/jo3021659. [DOI] [PubMed] [Google Scholar]
- Zipper H.; Brunner H.; Bernhagen J.; Vitzthum F. Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications. Nucleic Acids Res. 2004, 32, e103 10.1093/nar/gnh101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar C.; Kishore D. P.; Rao K. V.; Narayana B. L.; Rajwinder K.; Rajkumar V.; Rao A. R. Design, microwave-assisted synthesis and in silico docking studies of new 4H-pyrimido[2,1-b]benzothiazole-2-arylamino-3-cyano-4-ones as possible adenosine A2B receptor antagonists. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2012, 51, 1105–1113. [Google Scholar]
- Pèpe G.; Siri D.; Reboul J.-P. The molecular electrostatic potential and drug design. J. Mol. Struct.: THEOCHEM 1992, 256, 175–185. 10.1016/0166-1280(92)87166-W. [DOI] [Google Scholar]
- Drissi M.; Benhalima N.; Megrouss Y.; Rachida R.; Chouaih A.; Hamzaoui F. Theoretical and experimental electrostatic potential around the m-nitrophenol molecule. Molecules 2015, 4042–4054. 10.3390/molecules20034042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L.-G.; Liao W.; Yu Z.-X. A Frontier molecular orbital theory approach to understanding the Mayr equation and to quantifying nucleophilicity and electrophilicity by using HOMO and LUMO energies. Asian J. Org. Chem. 2012, 1, 336–345. 10.1002/ajoc.201200103. [DOI] [Google Scholar]
- Dewar M. J. S. A Critique of frontier orbital theory. J. Mol. Struct.: THEOCHEM 1989, 200, 301–323. 10.1016/0166-1280(89)85062-6. [DOI] [Google Scholar]
- Abdou W. M.; Khidre R. E.; Shaddy A. A. Synthesis of tetrazoloquinoline-based mono- and bisphosphonate esters as potent anti-inflammatory agents. J. Heterocycl. Chem. 2013, 50, 33–41. 10.1002/jhet.968. [DOI] [Google Scholar]
- Aihara J.-i. Reduced HOMO–LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J. Phys. Chem. A 1999, 103, 7487–7495. 10.1021/jp990092i. [DOI] [Google Scholar]
- Srivastava A.; Singh R. M. Vilsmeier-Haack Reagent : A facile synthesis of 2-chloro-3-formylquinolines from N-arylacetamides and transformation into different functionalities. Indian J. Chem., Sect. B: Org. Chem. Incl/ Med. Chem. 2005, 44, 1868–1875. [Google Scholar]
- Anand K.; Naicker T.; Baijnath S.; Mphahlele M. J.; Katari N. K.; Zamisa S. J.; Balakumar C.; Vijayakumar K.; Palanisamy S.; Saravanan M.; Boomi P.; Chuturgoon A. TPGS-mediated one-pot synthesis, XRD structural analysis, antimicrobial evaluation and molecular docking of novel heterocycles as potential inhibitors of p53-MDM2 protein. J. Mol. Struct. 2020, 1202, 127252. 10.1016/j.molstruc.2019.127252. [DOI] [Google Scholar]
- Nawwar G. A. M.; Shafik N. A. Synthesis of 2-substituted benzothiazoles containing amino acid, imino or heteroaryl moieties with anticipated fungicidal activity. Collect. Czech. Chem. Commun. 1995, 60, 2200–2208. 10.1135/cccc19952200. [DOI] [Google Scholar]
- Oxford Diffraction (2006); Oxford Diffraction Ltd: Abingdon, Oxfordshire, England, 2006. [Google Scholar]
- Spek A. L. Structure validation in chemical crystallography. Acta Crystallogr., Sect. D: Struct. Biol. 2009, 65, 148–155. 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Peralta J. E. Jr.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09 Revision D.01,; Gaussian, Inc.: Wallingford CT, 2009.
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Becke A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Krishnan R.; Binkley J. S.; Seeger R.; Pople J. A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. 10.1063/1.438955. [DOI] [Google Scholar]
- Clark T.; Chandrasekhar J.; Spitznagel G. W.; Schleyer P. V. R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. 10.1002/jcc.540040303. [DOI] [Google Scholar]
- Trani F.; Scalmani G.; Zheng G.; Carnimeo I.; Frisch M. J.; Barone V. Time-dependent density functional tight binding: New formulation and benchmark of excited states. J. Chem. Theory Comput. 2011, 7, 3304–3313. 10.1021/ct200461y. [DOI] [PubMed] [Google Scholar]
- Wolinski K.; Hinton J. F.; Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. 10.1021/ja00179a005. [DOI] [Google Scholar]
- Cunha E. S.; Sfriso P.; Rojas A. L.; Roversi P.; Hospital A.; Orozco M.; Abrescia N. G. A. Mechanism of structural tuning of the hepatitis C virus human cellular receptor CD81 large extracellular loop. Structure 2017, 25, 53–65. 10.1016/j.str.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Dassault Systèmes Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment, Release 2017, Dassault Systèmes: San Diego, 2017. [Google Scholar]
- Hanwell M. D.; Curtis D. E.; Lonie D. C.; Vandermeersch T.; Zurek E.; Hutchison G. R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. Aust. J. Chem. 2012, 4, 17. 10.1186/1758-2946-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosconati S.; Forli S.; Perryman A. L.; Harris R.; Goodsell D. S.; Olson A. J. Virtual screening with AutoDock: Theory and practice. Expert Opin. Drug Discovery 2010, 5, 597–607. 10.1517/17460441.2010.484460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. M.; Goodsell D. S.; Halliday R. S.; Huey R.; Hart W. E.; Belew R. K.; Olson A. J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. . [DOI] [Google Scholar]
- Khan S.; Khan F. I.; Mohammad T.; Khan P.; Hasan G. M.; Lobb K. A.; Islam A.; Ahmad F.; Hassan M. I. Exploring molecular insights into the interaction mechanism of cholesterol derivatives with the Mce4A: A combined spectroscopic and molecular dynamic simulation studies. Int. J. Biol. Macromol. 2018, 111, 548–560. 10.1016/j.ijbiomac.2017.12.160. [DOI] [PubMed] [Google Scholar]
- Rigsby R. E.; Parker A. B. Using the PyMOL application to reinforce visual understanding of protein structure. Biochem. Mol. Biol. Educ. 2016, 44, 433–437. 10.1002/bmb.20966. [DOI] [PubMed] [Google Scholar]
- Laskowski R. A.; Swindells M. B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Khan F. I.; Govender A.; Permaul K.; Singh S.; Bisetty K. Thermostable chitinase II from thermomyces lanuginosus SSBP: Cloning, structure prediction and molecular dynamics simulations. J. Theor. Biol. 2015, 374, 107–114. 10.1016/j.jtbi.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Khan F. I.; Wei D.-Q.; Gu K.-R.; Hassan M. I.; Tabrez S. Current updates on computer aided protein modeling and designing. Int. J. Biol. Macromol. 2016, 85, 48–62. 10.1016/j.ijbiomac.2015.12.072. [DOI] [PubMed] [Google Scholar]
- Humphrey W.; Dalke A.; Schulten K. VMD: Visual molecular dynamics. J. Mol. Graphics 1996, 14, 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Khan F. I.; Hassan F.; Ali H.; Lai D. Mechanism of pH-induced conformational changes in MurE ligase obtained from Salmonella enterica Serovar Typhi. J. Biomol. Struct. Dyn. 2020, 10.1080/07391102.2020.1739560. [DOI] [PubMed] [Google Scholar]
- Papaleo E.; Mereghetti P.; Fantucci P.; Grandori R.; De Gioia L. Free-energy landscape, principal component analysis, and structural clustering to identify representative conformations from molecular dynamics simulations: The myoglobin case. J. Mol. Graphics Modell. 2009, 27, 889–899. 10.1016/j.jmgm.2009.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.