Abstract
Mitochondria act as a prime therapeutic target for the treatment of various diseases and dysfunctional states. In this study, we have demonstrated the proof of concept of surface functionalization for achieving two-photon active mitochondriotropic lanthanide nanorods for higher resolution fluorescence imaging, and thereby, the spectral profile of mitochondria. The presence of Gd in the nanorods also enabled us to utilize this material as a T1-T2 dual-mode contrast reagent for recording magnetic resonance images of the mouse brain.
Mitochondria, aptly called as the “powerhouses of cell”, regulate various biological processes within a cell that are crucial for its survival and functioning. Besides producing adenosine triphosphate (ATP), the “energy currency” of the cell through oxidative phosphorylation, they play a critical role in controlling the electron transport chain, cell cycle regulation, intracellular calcium signaling, cell proliferation and apoptosis.1 Mitochondrial dysfunction has been identified as the root cause of several diseases including type 2 diabetes, cancer, diabetes and a range of age-related neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease, muscular dystrophy, Lou Gehrig’s disease, Huntington’s disease and amyotrophic lateral sclerosis.2–4 To develop a better understanding on the role of mitochondrial dysfunction in various diseases, as well as to control them, recent years have seen a phenomenal impetus in the development of mitochondria-targeted probes.
A myriad of imaging techniques are used in current clinical practice which includes but not limited to optical imaging, magnetic resonance imaging (MRI), ultrasound, single-photon emission computed tomography (SPECT) and X-ray computed tomography (CT).5, 6 With the advent of higher resolution luminescence-based imaging techniques, there has been a recent surge in reports on various mitochondria-specific fluorescent markers.7–10 However, one single imaging modality may not be able to provide all the information required from a single experiment. To address this limitation, multimodal imaging strategies are being developed. Using two or more imaging modalities simultaneously synergizes the strengths of individual modalities and enhances accuracy of diagnosis through cross-modality validation and direct, inter-modality comparison.11, 12 Traditionally, each imaging modality employs a different probe, which possesses a distinctive chemical composition, unique physicochemical properties and pharmacokinetic profile. For in vivo applications, it is difficult to implement a “cocktail approach” where a mixture of multiple probes is administered through a single dose in order to achieve spatiotemporal homogeneity between the individual imaging modalities. Therefore, a single probe that integrates the contrasting properties of two or more imaging probes into an all-in-one system is actively being sought for dedicated multimodal imaging.
Among the various imaging modalities that have become an indispensable part of modern clinical diagnostics, MRI has gained substantial importance due to its noninvasiveness, high spatial resolution, deep penetration and absence of radiation hazards. Despite these promising attributes, a major limitation of MRI is its low sensitivity.13, 14 This limitation can be circumvented by using suitable contrast agents that can enhance the image contrast between normal and diseased tissues by altering the longitudinal (T1) and transverse (T2) relaxation rates of water protons surrounding the target region of interest. While MRI serves as an important diagnostic tool for high resolution anatomical and functional imaging, fluorescence-based optical imaging provides highly sensitive detection, quantification and real-time-tracking of bio-molecules of interest at the tissue, cellular and sub-cellular level.15, 16 Subsequently, bimodal contrast agents, integrating the synergistic advantages of both MRI and fluorescence-based optical imaging, have gained substantial attention in preclinical research. In this context, lanthanide-doped up-converting nanoparticles (UCNP) deserves special mention because of their unique ability to convert near-infrared excitation into visible and ultraviolet emission, endowing greater tissue penetration, lower autofluorescence and reduced toxicity.17, 18 With an additional dopant like the gadolinium (Gd3+) ion, which possesses intrinsic paramagnetism due to the presence of 7 unpaired electrons in its 4f shell, UCNPs can be used for simultaneous MRI and optical imaging. Furthermore, when conjugated with appropriate functional molecules (e.g., antibodies, peptides, drugs or photosensitizer), such UCNPs can serve as prospective candidates for targeted multimodal imaging and therapy.19
Herein, we report the development of a multifunctional nanoplatform based on NaYF4:Yb,Gd,Eu upconverting nanorods (UCNR) that can be used for concurrent mitochondria-targeted fluorescence imaging and preclinical MRI. The presence of Eu3+ ions in the core of this nanosystem facilitates robust upconversion luminescence whereas Gd3+ endows MRI visibility. To further facilitate mitochondrial targeting, the as-prepared UCNRs were functionalized with triphenylphosphonium cations, known mitochondria targeting agent,8 through covalent bonding. Our results demonstrated that UCNRs developed in this study can be used as a safe and effective bimodal probe for mitochondrial-targeted optical imaging and dual T1- and T2-weighted MRI. This nanoprobe demonstrated robust T1 contrast enhancement in vivo for high-resolution cerebral microangiography, corroborating that the UCNRs stay in blood circulation post-injection to demonstrate measurable contrast.
NaYF4:Eu,Gd,Eu UCNRs with uniform size were synthesized by a hydrothermal reaction (ESI†).20 The crystallinity, phase purity and composition of the synthesized UCNRs were determined by powder XRD. The powder-XRD pattern of the synthesized UCNRs (Fig. S1, ESI†) exhibited sharp diffraction peaks that are indexed to pure hexagonal-phase β-NaYF4 nanocrystals (JCPDS No. 0281192; space group: P63/m).21 Mitochondriotropic UCNRs were produced by chemical coupling of triphenylphosphine (TPP) with amine-functionalized, silica-coated UCNRs, prepared using typical Stöber-based surface modification.21 The ζ potentials of pristine UCNRs and amine-functionalized silica-coated-UCNR were measured as −26.7 and +34.07 mV, respectively. After reaction with TPP, the ζ potential of the UCNRs further increased to +36.4 mV. The presence of positive charges indicated that TPP-coated UCNRs possess a suitable targeting moiety for the negatively charged mitochondrial membrane. As evident from transmission electron microscopy (TEM) image, the synthesized nanoparticles were rod-shaped, had good monodispersity and possessed uniform sizes with an overall diameter of 18 ± 3 nm (Fig. 1a and Fig. S2, ESI†). The selected area electron diffraction pattern (SAED) and high-resolution TEM (HRTEM) image showed the lattice fringes with a d spacing of 0.30 nm, which was in good agreement with the lattice spacing of the (111) planes of hexagonal NaGdF4 (Fig. 1 b–c).
Figure 1.

TEM images of (a) β-NaYF4:Yb,Gd,Eu NRs; (b) and (c) are corresponding SAED patterns and HRTEM images of β-NaYF4:Yb,Gd,Eu NRs. (d) Up conversion fluorescence spectra of solid phosphine appended NaYF4:Yb,Gd,Eu NPs. (e) The FT-IR spectra of bare UCNRs (black); silica coated β-NaYF4:Yb,Gd,Eu NRs (red) and phosphine conjugated UCNRs (blue).
The upconversion photoluminescence properties of the UCNRs were studied by excitation with a 980 nm laser beam. As depicted in Fig. 1d, typical Eu3+ emissions coming from 5Dn→7F1 and 5D0→7F2 were cantered at 590.3 and 613.3 nm, respectively.22–24 Fourier transform infrared (FT-IR) spectroscopy was used to recognize the functional groups on the surface of the nanocomposites and provide evidence for successful modification. The FT-IR spectra of bare-UCNRs, amine-functionalized Si-coated UCNRs, and TPP functionalized UCNRs are presented in Fig. 1e. The transmission bands at 2841 and 2940 cm−1 were assigned to the asymmetric and symmetric stretching vibrations of methylene (−CH2) groups, associated with a thin layer of hypermer B246 on the surface of UCNRs. B246 is a polyhydroxystearic acid/polyethylene oxide/polyhydroxystearic acid, ABA block copolymer, used as a surfactant during the synthesis of nanocomposite. The FTIR spectra also reveal a broad band at around 3431 cm−1 originating from the O−H stretching vibration of the polyhydroxystearic acid block of B246. After the Stöber-based surface modification, new bands were observed at 3150 cm−1 (very broad) and 1596 cm−1, emanating from N–H stretching and N–H bending vibrations, respectively. In addition, typical Si−O−Si deformation (632 and 451 cm−1) and asymmetric stretching vibration of Si–O bond appeared at 1421 cm−1. After TPP conjugation, asymmetrical deformation of P-C bonds was observed at 1445–1322 cm−1, confirming the successful anchorage of phosphonium ions on the surface of UCNRs.
As NaYF4:Yb,Gd,Eu NRs are well-established optical upconversion systems, we evaluated the suitability of this new probe for two-photon microscopy (TPM) (Fig. 2). The intracellular uptake and subcellular trafficking of UCNRs were studied in a murine macrophage cell line viz. RAW 264.7. TPM images of RAW 264.7 cells revealed strong intracellular emission signals within the cytosol, confirming the cellular internalization of UCNRs Fig. 2a. As the concentration of UCNRs was increased, an associated increase in intracellular luminescence was detected Fig. 2c. This trend was also evident in the intensity maps generated from these data Fig. 2b and 2d. The punctated nature of the images, which is detected in the intensity maps, also suggests that UCNRs localize in specific regions within the cytoplasm.
Figure 2.

Two-photon emission microscopy images showing uptake of UCNRs in RAW cells: Effect of increasing concentration of UCNRs treatment: 50 μg (a), 100 μg (c). (b) and (d) are the intensity maps for the same concentrations shown in (a) and (c) respectively. λExt = 980 nm.
To further verify whether the TPP-functionalized UCNRs developed in this study have mitochondrial targeting property, cells were stained with Mito Tracker green (MTG), a mitochondria-selective dye whose spectral properties are complementary to those of the UCNRs. Subsequently, their subcellular trafficking and compartmentalization was examined in-depth using the super-resolution structured illumination microscopy. In these experiments, the nanorods were one-photon excited at 488 nm and their emission collected at 600 to 650 nm, whereas MTG was excited at 488 nm and observed to emit at 516 nm (Fig. 3).
Figure 3.
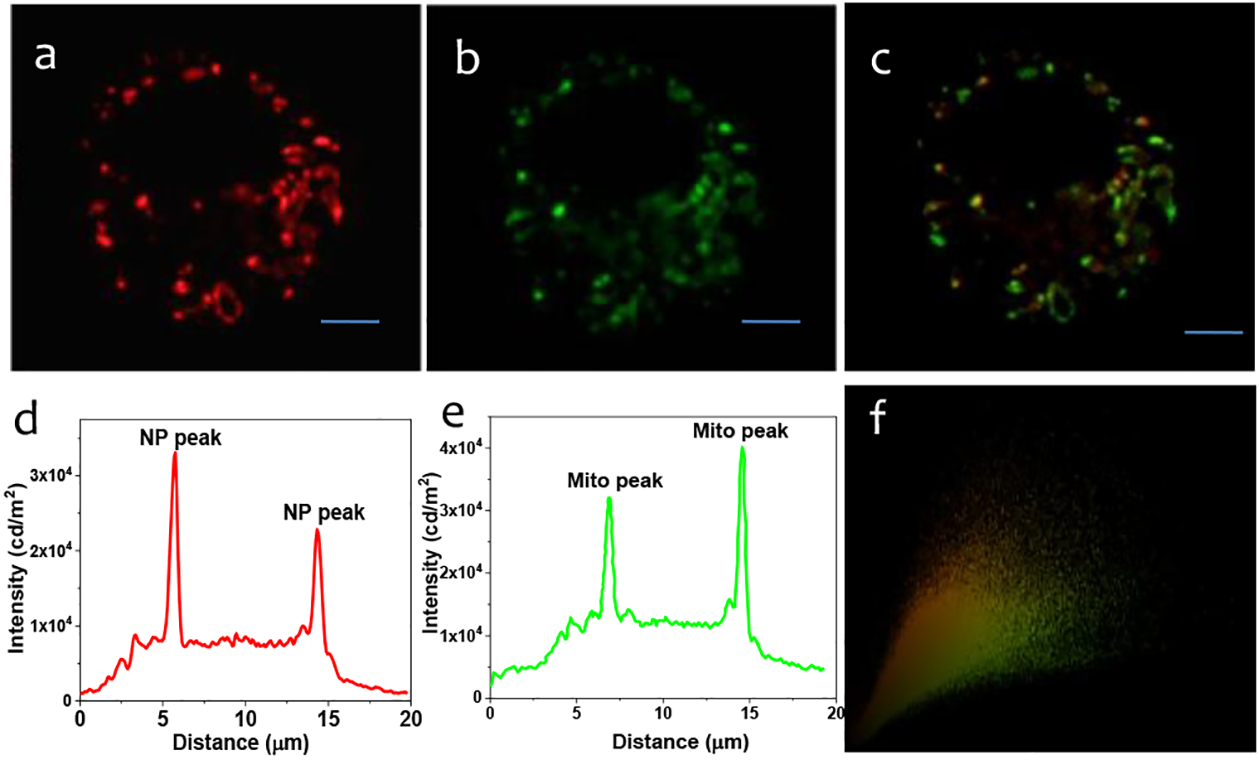
Co-localization experiments (SIM Microscopy) of intracellular localization of UCNRs using mitotraker probes: Widefield microscopy images of in cellulo-emission of UCNRs (panel a) with intensity along the traced line (panel d) shown underneath. Emission from mitotraker green (panel b) and intensity along the same line (panel e) shown below. The overlap of the intensity is shown in panel c. panel c shows the overlap of the green and red fluorescence, indicating mitochondrial localization of the UCNRs. Panel f shows the Pearson co-efficient = 0.92. Emissions from UCNRs and MTG fluorescence were recorded in the wavelength ranges of 600–650 nm and 500–550 nm. The scale bar is 10 μm.
The intensity profile of the Widefield images revealed that 80 percent of the signals of MTG matches with that of the TPP-UCNRs.This observation along with the high calculated Pearson’s coefficient (91%), suggests that TPP-UCNRs localize in the mitochondria of RAW cells (Fig. 3). For further validation of mitochondrial localization, similar co-localization experiments were performed with a lysosome-selective stain, LysoTracker Green (LTG) (Fig. S3 ESI†) and nuclear stain, DAPI (Fig. S4). Furthermore the bare UCNRs (without TPP functionalization) localize over lysosome (Fig. S5 and S6). Therefore our results confirmed that TPP-UCNRs do not localize over lysosome or nuclei and are specifically targeted towards mitochondria.
To further examine if the synthesized UCNRs can be used as a contrast agent for MRI applications, in vitro relaxometry studies with TPP-UCNRs was performed using a custom-designed holder and the well-established, spin-echo based T1-T2 mapping protocol. The T1- and T2-weighted MRI phantom images of UCNRs in PBS acquired at different repetition time (TR) and echo time (TE) are presented in Fig. 4a. As evident from these images, the exact relationship between MR signal intensity and Gd3+ concentration was nonlinear and dependent on the pulse sequence parameters used during image acquisition. At lower concentrations (0–8 mM Gd3+), T1 weighting was predominant and UCNRs exhibited concentration-dependent positive contrast-enhancement when compared to saline control. In this regime, the T1 relaxation rate increases linearly with increasing Gd3+ concentration (Fig. 4b) until the positive contrast reaches its maximum enhancement point, the value of which depends on the TR used as well as T1 and T2relaxivity of the contrast material (Fig 4c). Beyond this maximal contrast enhancement (maxCE) point, the initially observed linear relationship between T1 relaxation rate and Gd3+ concentration (Fig. 4b) becomes non-linear and the MR signal starts to diminish owing to T2* related signal loss. The R1 and R2 relaxivity of UCNRs were determined to be 3.4 mM−1s−1 and 23.5 mM−1s−1 respectively (Fig. 4c). The R1 relaxivity of UCNRs were comparable to that of literature-reported values for Gd-DOTA and Gd-DTPA at 9.4 T25. Although the UCNRs exhibited distinct T2-effects at higher Gd3+ concentrations and longer TR and TE, its R2/R1 ratio (6.91) was almost 14.8 times lower than the measured R2/R1 value (102.6) for the well-known ultrasmall iron-oxide nanoparticle-based MRI contrast agent, Ferumoxytol (Feraheme, AMAG Pharmaceuticals) measured using exactly same experimental conditions but in a separate session (R1 = 1.412 mM−1s−1; R2 = 144.9 mM−1s−1 (Fig S8 ESI†). These results indicate that TPP-UCNRs are more suited for T1-weighted MRI; however, depending on the magnetic field strength, pulse-sequence, imaging parameters (e.g., TR, TE, flip angle etc.) and concentration of Gd3+ chosen, this nanoprobe can be used to achieve enhanced T1 as well as tunable T1-T2 dual-modal contrasts for MRI applications. These observations are in agreement with earlier reports.26, 27
Figure 4.

In-vitro relaxometric analysis of UCNRs performed using a Bruker 9.4 T/30-cm bore small animal MRI system: (a) Representative T1- and T2-weighted MR phantom images of UCNRs in PBS acquired at different TR and TE; (b) Quantification of R1 and R2 from T1-recovery and T2-decay curves. (c) Effect of Gd3+ concentration on MRI signal intensity.
To further explore if the synthesized UCNRs can be used as a contrast agent for in vivo MRI applications, we performed cerebro microangiography in healthy C57BL/6 mice using UCNRs as the contrast agent. Of note, our in vivo studies were not designed for large group statistics tackling a biological question and were rather intended to establish a proof-of-concept that the nanoprobe developed in this study can stay in blood circulation to offer robust T1 contrast enhancement in vivo.Fig. 5a presents the raw pre-and post-contrast MR images of a mouse brain acquired at 200μm3 spatial resolution using a T1-weighted FLASH-3D sequence. The post-contrast image showed an improvement in signal-to-noise ratio in selected regions of interest (ROI) shown in Fig. S7 and revealed the presence of several small cerebral vessels that were not visible prior to the injection of UCNRs. Fig. 5b shows the representative 3D cerebrovasculature map of the mouse brain, presented as an overlay of the pre-contrast (white) and post-contrast (orange) images, constructed using the volume-rendering tools in Amira. We succeeded in identifying several key arteries and veins from this dataset including the superior sagittal sinus, transverse sinus, rostral rhinal vein, middle cerebral artery, internal carotid artery, anterior, posterior and superior cerebral artery and circle of Willis. These results indicate that UCNRs developed in this study can be used as a T1-agent for micro-magnetic resonance angiography (MRA)in vivo, allowing improved visualization and delineation of vessels. This feature is certainly beneficial for the diagnosis of vascular malformations associated with a wide range of medical conditions including vasculopathies such as athelosclerosis, neurogenerative disorders such as Alzheimer’s diseases, and neoplastic diseases e.g., glioblastoma multiferrome. Although in-depth pharmacokinetic evaluation is necessary to quantify the circulation half-life of these UCNRs, their MRI detectability in the vessels suggests that following intravenous injection, UCNRs are not immediately sequestered by the mononuclear phagocytic system and remains in the blood pool throughout the period of data acquisition (~12 min) to demonstrate robust T1 contrast enhancement for cerebral microangiography.
Figure 5.
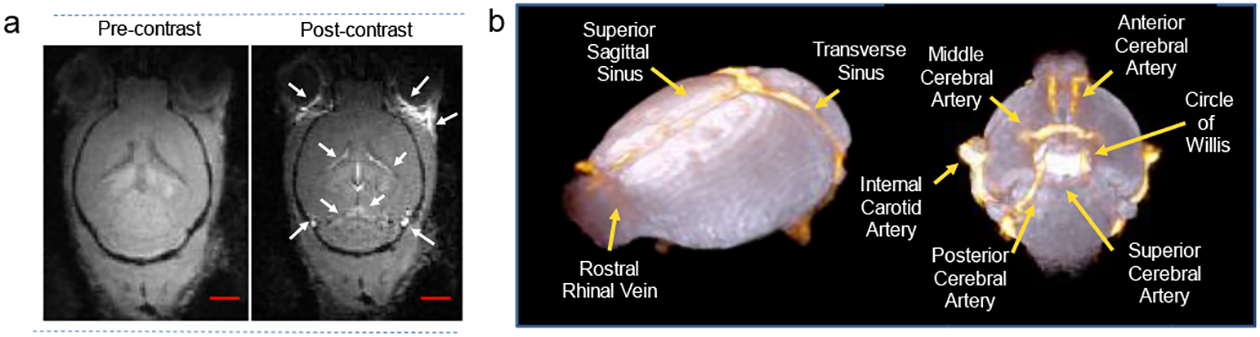
(a) FLASH-3D MR images of mouse brain (axial orientation) captured at 200 μm3 spatial resolution before and after injection of UCNRs. (b) Representative 3D cerebrovasculature map of the mouse brain generated using the volume rendering tools in Amira by overlapping the pre-contrast (in white) and post-contrast (in orange) images. Scale bar 5mm.
To further ensure the safety of TPP-UCNRs for biologically relevant applications, in vitro cell viability studies were performed using the MTT assay. Our results indicated that the cell viability remained unaltered as compared to that of control group. Certainly, no decrease below 98% was detected even after exposures to different concentrations of phosphine conjugated UCNRs up to 48 h (Fig. S8, ESI†). These results indicate that the UCNRs are biocompatible, making them excellent candidates for biomedical applications.
In conclusion, we have developed a novel, lanthanide-based upconversion nanoplatform for simultaneous mitochondria-targeted optical imaging and T1-T2 dual-modal MRI. Judicious integration of paramagnetic and upconversion luminescent properties on to the same platform results in a multifunctional, mitochondriotropic nano-system that opens up new avenues for mitochondrial-targeted theranostics. Our future studies will concentrate on 1) optimizing the pharmacokinetic properties of UCNRs for various preclinical imaging applications; 2) quantitative evaluation of contrast enhancement in vivo using aand 3)combining the current method with appropriate blood-brain-barrier opening technologies, which is crucial for UCR-mediated MR imaging of mitochondrial function in vivo.
Supplementary Material
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: [synthesis of the UCNRS, characterization data and cell viability data]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Porporato PE, Filigheddu N, Pedro JMB-S, Kroemer G and Galluzzi L, Cell Res., 2017, 28, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowell BB and Shulman GI, Science, 2005, 307, 384–387. [DOI] [PubMed] [Google Scholar]
- 3.Thornton B, Cohen B, Copeland W and Maria BL, J. Child Neurol, 2014, 29, 1179–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abou-Sleiman PM, Muqit MMK and Wood NW, Nat. Rev. Neurosci, 2006, 7, 207–219. [DOI] [PubMed] [Google Scholar]
- 5.Huang W-Y and Davis JJ, Dalton Trans., 2011, 40, 6087–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M and Shu J, Contrast Media Mol Imaging, 2018, 1382183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Hu F, Zhao R, Zhang G, Yang H and Zhang D, Chem. Eur. J, 2014, 20, 158–164. [DOI] [PubMed] [Google Scholar]
- 8.Pramanik SK, Sreedharan S, Singh H, Khan M, Tiwari K, Shiras A, Smythe C, Thomas JA and Das A, Bioconjugate Chem., 2018, 29, 3532–3543. [DOI] [PubMed] [Google Scholar]
- 9.Leung CWT, Hong Y, Chen S, Zhao E, Lam JWY and Tang BZ, J. Am. Chem. Soc, 2013, 135, 62–65. [DOI] [PubMed] [Google Scholar]
- 10.Zhao N, Chen S, Hong Y and Tang BZ, ChemComm, 2015, 51, 13599–13602. [DOI] [PubMed] [Google Scholar]
- 11.Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Venkatraman V, Roddey JC, Erhart M, McCabe C, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Darst BF, Schork NJ, Casey BJ, Chang L, Ernst TM, Gruen JR, Kaufmann WE, Kenet T, Frazier J, Murray SS, Sowell ER, van Zijl P, Mostofsky S, Jernigan TL and Dale AM, Proc. Natl. Acad. Sci. U.S.A, 2012, 109, 19620–19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D, Dai Y, Liu J, Zhou Y, Chen Y, Li C, Ma P. a. and Lin J, Biomaterials, 2014, 35, 2011–2023. [DOI] [PubMed] [Google Scholar]
- 13.Colombo M, Carregal-Romero S, Casula MF, Gutiérrez L, Morales MP, Böhm IB, Heverhagen JT, Prosperi D and Parak WJ, Chem. Soc. Rev, 2012, 41, 4306–4334. [DOI] [PubMed] [Google Scholar]
- 14.Peng E, Wang F and Xue JM, J. Mater. Chem. B, 2015, 3, 2241–2276. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Fan J, Du J and Peng X, Acc. Chem. Res, 2016, 49, 2115–2126. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z and Xu L, ChemComm, 2016, 52, 1094–1119. [DOI] [PubMed] [Google Scholar]
- 17.Prodi L, Rampazzo E, Rastrelli F, Speghini A and Zaccheroni N, Chem. Soc. Rev, 2015, 44, 4922–4952. [DOI] [PubMed] [Google Scholar]
- 18.Cheng X, Ge H, Wei Y, Zhang K, Su W, Zhou J, Yin L, Zhan Q, Jing S and Huang L, ACS Nano, 2018, 12, 10992–10999. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabortty S, Agrawalla BK, Stumper A, Vegi NM, Fischer S, Reichardt C, Kögler M, Dietzek B, Feuring-Buske M, Buske C, Rau S and Weil T, J. Am. Chem. Soc, 2017, 139, 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Han Y, Lim CS, Lu Y, Wang J, Xu J, Chen H, Zhang C, Hong M and Liu X, Nature, 2010, 463, 1061. [DOI] [PubMed] [Google Scholar]
- 21.Pramanik SK, Sreedharan S, Singh H, Green NH, Smythe C, Thomas JA and Das A, ChemComm, 2017, 53, 12672–12675. [DOI] [PubMed] [Google Scholar]
- 22.Shelton AH, Sazanovich IV, Weinstein JA and Ward MD, ChemComm, 2012, 48, 2749–2751. [DOI] [PubMed] [Google Scholar]
- 23.Jana A, Crowston BJ, Shewring JR, McKenzie LK, Bryant HE, Botchway SW, Ward AD, Amoroso AJ, Baggaley E and Ward MD, Inorg. Chem, 2016, 55, 5623–5633. [DOI] [PubMed] [Google Scholar]
- 24.Sykes D, Cankut AJ, Ali NM, Stephenson A, Spall SJP, Parker SC, Weinstein JA and Ward MD, Dalton Trans, 2014, 43, 6414–6428. [DOI] [PubMed] [Google Scholar]
- 25.De Sousa PL, Livramento JB, Helm L, Merbach AE, Même W, Doan BT, Beloeil JC, Prata MI, Santos AC and Geraldes CFC, Contrast Media Mol Imaging, 2008, 3, 78–85. [DOI] [PubMed] [Google Scholar]
- 26.Hagberg GE and Scheffler K, Contrast Media Mol Imaging, 2013, 8, 456–465. [DOI] [PubMed] [Google Scholar]
- 27.Lee M-J, Kim M-J, Yoon C-S, Song SY, Park K and Kim WS, Korean J Radiol, 2011, 12, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


