Abstract
The physiological colonization resistance exerted by the murine gut microbiota prevents conventional mice from Campylobacter jejuni infection. In the present study we addressed whether this also held true for Campylobacter coli. Following peroral application, C. coli as opposed to C. jejuni could stably establish within the gastrointestinal tract of conventionally colonized mice until 3 weeks post-challenge. Neither before nor after either Campylobacter application any changes in the gut microbiota composition could be observed. C. coli, but not C. jejuni challenge was associated with pronounced regenerative, but not apoptotic responses in colonic epithelia. At day 21 following C. coli versus C. jejuni application mice exhibited higher numbers of adaptive immune cells including T-lymphocytes and regulatory T-cells in the colonic mucosa and lamina propria that were accompanied by higher large intestinal interferon-γ (IFN-γ) concentrations in the former versus the latter but comparable to naive levels. Campylobacter application resulted in decreased splenic IFN-γ, tumor necrosis factor-α (TNF-α), and IL-6 concentrations, whereas IL-12p70 secretion was increased in the spleens at day 21 following C. coli application only. In either Campylobacter cohort decreased IL-10 concentrations could be measured in splenic and serum samples. In conclusion, the commensal gut microbiota prevents mice from C. jejuni, but not C. coli infection.
KEYWORDS: colonization resistance, murine gut microbiota, Campylobacter coli, Campylobacter jejuni, pro-inflammatory immune responses, host-pathogen-interaction
INTRODUCTION
Campylobacter have been recognized as one of the leading causes of bacterial gastroenteritis worldwide [1–3]. The Campylobacter genus consists of a large and diverse group of Gram-negative bacteria comprising more than 30 species and subspecies [3]. Among these Campylobacter jejuni and Campylobacter coli are the most prevalent ones causing human morbidities. Campylobacter are widely distributed in the environment and can be found in a multitude of warm-blooded domestic and wild animals as commensal gastrointestinal inhabitants [4]. The pathogens are mostly transmitted via the food chain upon ingestion of undercooked or raw meat derived from farm animals – mostly poultry – or of milk and contaminated surface water [5–7]. Whereas many infected hosts do not display any clinical signs upon Campylobacter acquisition, others exhibit a broad variety of symptoms ranging from mild disease to abdominal cramps, fever, myalgia, and watery to bloody diarrhea [3, 8–11]. In most cases, the disease is self-limited and requires, if at all, symptomatic treatment such as replacement of fluids and electrolytes. Infected multi-morbid, immunocompromized patients, however, are subjected to antimicrobial therapy [3, 10, 12]. Rather rarely post-infectious sequelae such as the Guillain-Barré syndrome, Miller Fisher syndrome, reactive arthritis, and chronic inflammatory conditions of the gastrointestinal tract might arise with a latency of weeks to months post-infection [3, 12, 13].
The host-specific composition of the gut microbiota determines whether the vertebrate host is susceptible to or resistant against Campylobacter infections [14–16]. Adult wildtype mice harboring a conventional gut microbiota, for instance, are protected from stable C. jejuni colonization even following peroral infection with high bacterial loads [17]. This physiological colonization resistance provided by the intact complex murine gut microbiota is abrogated upon broad-spectrum antibiotic treatment, however, rendering mice susceptible to intestinal C. jejuni colonization following peroral pathogenic challenge [18–20]. This also holds true for conditions that are accompanied by gut microbiota shifts towards elevated intestinal loads of commensal enterobacteria, including Escherichia coli [16, 21]. Furthermore, secondary abiotic mice that had been reassociated with a complex gut microbiota from human as opposed to murine donors by fecal microbiota transplantation before C. jejuni infection harbored the pathogen in their gastrointestinal tract at high loads, but did not exhibit typical clinical signs of human campylobacteriosis such as wasting or bloody diarrhea [18]. However, C. jejuni induced apoptotic cell and proinflammatory immune cell responses in the large intestines by Toll-like receptor (TLR) -4 dependent signaling of C. jejuni lipooligosaccharide (LOS) [18].
C. coli strains are divided into three distinct clades. Clade 1 isolates refer to microorganisms which dominate clinical and agricultural samples, while clade 2 and 3 microorganisms are more abundantly found in waterfowl and riparian environments. All disease causing genotypes are believed to belong to clade 1, although the 3-clade structure and its relationship to disease is not fully understood [22, 23]. Previous investigations of C. coli in a mouse model pointed towards the existence of several subgroups of C. coli with varying ability to colonize the murine gut microbiota and resist displacement by competition [24]. However, data regarding C. coli-host interactions are rather scarce. In the present study we therefore performed a comprehensive survey comparing C. coli and C. jejuni regarding their i.) gastrointestinal colonization properties, ii.) induced commensal gut microbiota changes, iii.) macroscopic and microscopic inflammatory sequelae, as well as iv.) intestinal and systemic immune responses upon high dose infection of conventional adult wildtype mice.
MATERIAL AND METHODS
Mice, Campylobacter infection
Conventional C57BL/6J wildtype mice were purchased from Charles River by the age of 6 weeks and kept in the Forschungsinstitute für Experimentelle Medizin (FEM, Charité – University Medicine Berlin) until the experiment. Three-month old female and male mice were perorally infected with 109 colony forming units (CFU) of either the C. jejuni strain 81-176 or a C. coli strain that had been isolated from a patient with bloody diarrhea (kindly provided by Dr. Torsten Semmler, Robert-Koch-Institute Berlin, Germany) in a volume of 0.3 mL phosphate buffered saline (PBS; Gibco, life technologies, UK) on two consecutive days (days 0 and 1) by gavage as reported previously [18].
Cultural analyses
C. jejuni and C. coli loads were surveyed in fecal samples over time post-infection (p.i.), and upon necropsy in luminal samples taken from the stomach, duodenum, ileum and colon, as well as in homogenates of ex vivo biopsies derived from mesenteric lymph nodes (MLNs), spleen, liver, kidneys, lungs, and in cardiac blood samples by culture as described previously [18, 25]. In brief, intraluminal gastrointestinal samples and respective ex vivo biopsies were homogenized in sterile PBS with a pistil and serial dilutions plated onto karmali agar (Oxoid, Wesel, Germany) and incubated in a microaerophilic atmosphere for at least 48 h at 37 °C. Cardiac blood (0.2 mL) was immediately streaked onto karmali agar plates. For quantification of commensal E. coli in respective samples, Mac Conkey agar plates (Oxoid) were inoculated and incubated for 48 hours at 37 °C. The detection limit of viable pathogens was approximately 100 CFU per g.
Molecular analysis of gut microbiota composition
DNA was extracted from fecal samples as described previously [26, 27]. In brief, bacterial DNA was quantified by Real-time PCR using Quant-iT PicoGreen reagent (Invitrogen, UK) adjusted to 1 ng per μL. Then, total eubacterial loads (TL), as well as the main bacterial groups abundant in the murine intestinal microbiota including enterobacteria (EB), enterococci (EC), lactobacilli (LB), bifidobacteria (BB), Bacteroides/Prevotella species (BP), Mouse Intestinal Bacteroides (MIB), Clostridium leptum group (CL), and Clostridium coccoides (CC) group were assessed by quantitative real-time polymerase chain reaction (qRT-PCR) with species-, genera- or group-specific 16S rRNA gene primers (Tib MolBiol, Germany) as described previously [18, 28, 29] and numbers of 16S rRNA gene copies per ng DNA of each sample were determined.
Clinical conditions
Before and after C. coli and C. jejuni infection the clinical conditions of mice were assessed on a daily basis by using a standardized cumulative clinical score (maximum 12 points), addressing the clinical aspect/wasting (0: normal; 1: ruffled fur; 2: less locomotion; 3: isolation; 4: severely compromised locomotion, pre-final aspect), the abundance of blood in feces (0: no blood; 2: microscopic detection of blood by the Guajac method using Haemoccult, Beckman Coulter/PCD, Germany; 4: macroscopic blood visible), and diarrhea (0: formed feces; 2: pasty feces; 4: liquid feces) as described earlier [30].
Sampling procedures
At day 21 p.i., mice were sacrificed by isofluran inhalation (Abbott, Germany). Luminal gastrointestinal samples (from stomach, duodenum, ileum and colon) and ex vivo biopsies from MLN, spleen, liver, kidneys, lungs and respective gastrointestinal compartments were taken under sterile conditions from each mouse in parallel for microbiological, immunohistopathological and immunological analyses. For serum cytokine measurements cardiac blood (approximately 1 mL) was taken. The absolute large intestinal lengths were measured with a ruler (in cm).
Immunohistochemistry
In situ immunohistochemical analyses were performed in colonic ex vivo biopsies that had been immediately fixed in 5% formalin and embedded in paraffin as described earlier [28, 31–33]. In brief, in order to detect apoptotic epithelial cells, proliferating epithelial cells, T lymphocytes, regulatory T cells (Tregs), and B lymphocytes, 5 μm thin paraffin sections of ex vivo biopsies were stained with primary antibodies directed against cleaved caspase 3 (Asp175, Cell Signaling, Beverly, MA, USA, 1:200), Ki67 (TEC3, Dako, Denmark, 1:100), CD3 (#N1580, Dako, 1:10), FOXP3 (clone FJK-165, #14-5773, eBioscience, 1:100), and B220 (No. 14-0452-81, eBioscience; 1:200), respectively. Positively stained cells were then examined by light microscopy (magnification 100 x and 400 x), and for each mouse the average number of respective positively stained cells was determined within at least six high power fields (HPF, 0.287 mm2, 400 x magnification) by a blinded independent investigator.
Pro- and anti-inflammatory mediator measurements
Colonic ex vivo biopsies were cut longitudinally, washed in phosphate buffered saline (PBS; Gibco, Life Technologies, UK), and strips of approximately 1 cm2 tissue and ex vivo biopsies derived from the spleen (one half) were placed in 24-flat-bottom well culture plates (Nunc, Germany) containing 500 μL serum-free RPMI 1640 medium (Gibco, life technologies, UK) supplemented with penicillin (100 U/mL) and streptomycin (100 μg/mL; PAA Laboratories, Germany). After 18 h at 37 °C, respective culture supernatants, as well as serum samples were tested for interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein 1 (MCP-1), and interleukin (IL) -6, IL-12p70 and IL-10 by the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences, Germany) on a BD FACSCanto II flow cytometer (BD Biosciences).
Statistical analysis
Medians and levels of significance were determined using the Mann-Whitney test (GraphPad Prism v7, USA) for pairwise comparisons of not normally distributed data, and using the Kruskal-Wallis test with Dunn’s post-correction for multiple comparisons as indicated. Two-sided probability (P) values ≤ 0.05 were considered significant. Experiments were reproduced three times.
Ethical statement
All animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin; registration numbers G0172/16). Animal welfare was monitored daily by assessment of clinical conditions.
RESULTS
Fecal C. coli and C. jejuni loads over time following peroral infection of conventional mice
Mice with a conventional gut microbiota were perorally challenged either with 109 viable C. jejuni or C. coli cells on two consecutive days (namely, d0 and d1) by gavage and the fecal loads surveyed thereafter by culture. As early as 24 and 48 hours after the first bacterial application C. jejuni could be isolated at relatively low cell numbers from 26.3% to 42.1% of mice, respectively (Fig. 1A). At the end of the observation period (i.e., at d21 p.i.) only 2 out of 19 mice (i.e., 10.5%) harbored C. jejuni in their intestinal tract (Fig. 1A). In case of C. coli application, however, the pathogen could be detected in all mice 24 and 48 hours after the first application (Fig. 1B). From d3 until d21 p.i. except for one out of 22 animals C. coli could be cultured at median loads between 106 and 107 CFU per g feces. Hence, C. coli as opposed to C. jejuni could stably establish within the intestinal tract of conventionally colonized mice until 3 weeks post challenge.
Fig. 1.
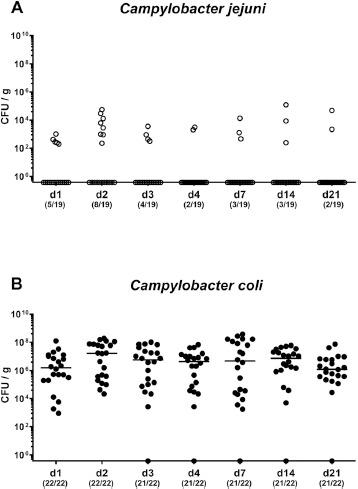
Fecal Campylobacter loads over time following peroral infection of conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with (A) C. jejuni (open circles) or (B) C. coli (closed circles) on day (d) 0 and d1 by gavage. Fecal Campylobacter loads were quantitatively assessed from each mouse post-infection over time by culture and expressed in colony forming units per g (CFU/g). Medians (black bars) and numbers of culture-positive mice out of the total number of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
Fecal E. coli loads over time before and after peroral Campylobacter infection of conventional mice
Given that elevated intestinal E. coli loads have been shown to abrogate colonization resistance in mice and hence, facilitate C. jejuni infection [16, 21], we compared commensal intestinal E. coli loads following either Campylobacter spp. challenge over time. In fact, commensal E. coli loads were comparable at either time point post C. jejuni versus C. coli application (n.s.; Fig. 2).
Fig. 2.
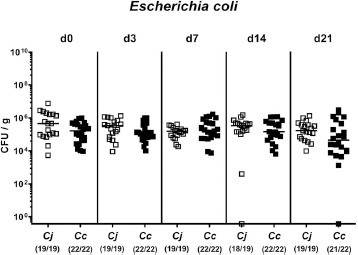
Fecal Escherichia coli loads over time before and after peroral Campylobacter infection of conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (Cj, open squares) or C. coli (Cc, closed squares) on day (d) 0 and d1 by gavage. Fecal E. coli loads were quantitatively assessed from each mouse pre- and and at defined time points post-infection by culture and expressed in colony forming units per g (CFU/g). Medians (black bars) and numbers of culture-positive mice out of the total number of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
Gastrointestinal Campylobacter and E. coli loads in Campylobacter infected conventional mice
We next determined if respective Campylobacter species coli and jejuni and commensal E. coli loads in distinct parts of the gastrointestinal tract at the end of the observation period differed significantly. Irrespective of the gastrointestinal compartment, until day 21 p.i., mice harbored not only more frequently, but also higher loads of C. coli as compared to C. jejuni (P < 0.05–0.001; Fig. 3A). The commensal E. coli numbers in stomach, duodenum, ileum and colon, however, did not differ between the C. coli and C. jejuni cohorts (n.s.; Fig. 3B).
Fig. 3.
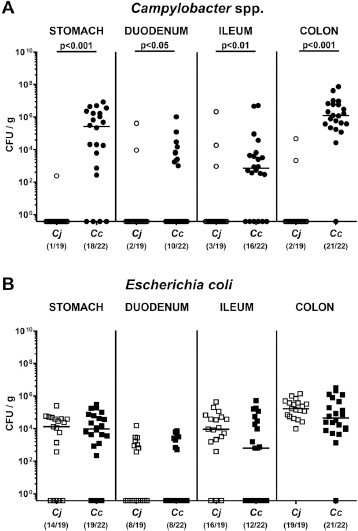
Gastrointestinal Campylobacter and Escherichia coli loads in Campylobacter infected conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (Cj; open symbols) or C. coli (Cc; closed symbols) on day (d) 0 and d1 by gavage as indicated. On day 21 post-infection, (A) respective Campylobacter (circles) and (B) E. coli (squares) loads were assessed in luminal samples taken from distinct parts of the gastrointestinal tract by culture and expressed as colony forming units per g (CFU/g). Medians (black bars), significance levels (P-values) as determined by the Mann Whitney U test and numbers of culture-positive mice out of the total number of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
Changes in intestinal microbiota composition following peroral Campylobacter infection of conventional mice
We further addressed in more detail whether either Campylobacter challenge was associated with changes in the composition of the commensal gut microbiota. Our quantitative culture independent (i.e., molecular) analyses revealed no changes in the gene numbers of the most abundant commensal gut bacterial groups and genera before (i.e., d0) and 21 days following peroral C. jejuni or C. coli challenge (n.s.; Fig. 4).
Fig. 4.
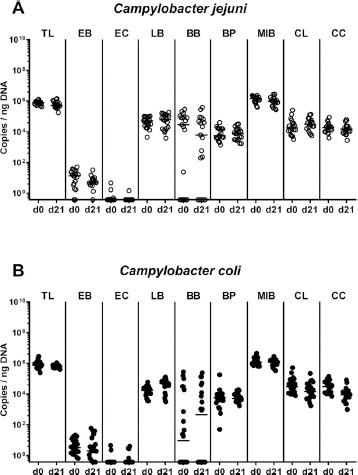
Changes in intestinal microbiota composition following peroral Campylobacter infection of conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with (A) C. jejuni (open circles, n = 19) or (B) C. coli (closed circles, n = 22) on day (d) 0 and d1 by gavage. Immediately before infection (i.e., d0) and upon necropsy (i.e., d21 post-infection) the fecal commensal microbiota composition was assessed applying culture-independent 16S rRNA methods quantitating the total eubacterial load (TL) and main commensal bacterial groups such as enterobacteria (EB), enterococci (EC), lactobacilli (LB), bifidobacteria (BB), Bacteroides/Prevotella species (BP), Mouse Intestinal Bacteroides (MIB), Clostridium leptum group (CL), and Clostridium coccoides group (CC), and expressed as gene copies per ng DNA. Medians (black bars) are indicated. Data were pooled from four independent experiments
Macroscopic and microscopic sequelae upon Campylobacter infection of conventional mice
We next assessed potential macroscopic Campylobacter induced sequelae over time. Mice were virtually completely clinically uncompromised following either Campylobacter species application, as indicated by median clinical score of “0” (Fig. 5A). Of note, in only one single mouse from the C. coli cohort occult blood could be detected microscopically at the end of the observation period (Fig. 5A). Given that intestinal inflammation is accompanied by the shortening of the inflamed intestinal tissue [19, 26], we measured large intestinal lengths upon necropsy. Interestingly, C. coli infected mice displayed slightly shorter colons as compared to C. jejuni infected counterparts (P < 0.01; Fig. 5B).
Fig. 5.
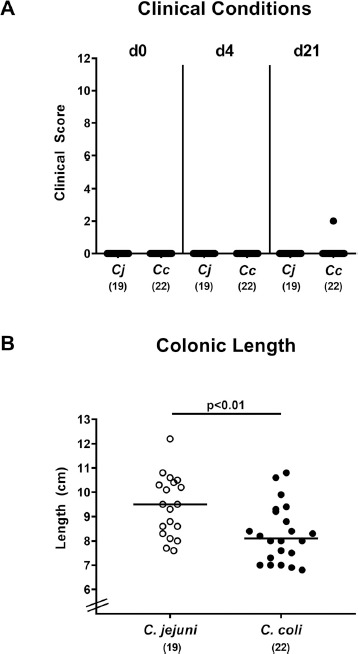
Macroscopic sequelae upon Campylobacter infection of conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (Cj; open circles) or C. coli (Cc; closed circles) on day (d) 0 and d1 by gavage. (A) Immediately before and at defined time points after Campylobacter infection the clinical conditions of mice were quantitatively assessed applying a standardized clinical scoring system (see methods). (B) Upon necrospy (i.e., d21 post-infection) the colonic lengths were measured (in cm). Medians (black bars), levels of significance (P-values) assessed by the Mann Whitney U test and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
We further surveyed Campylobacter associated microscopic changes in the large intestines. Since apoptosis is regarded as a reliable marker of intestinal inflammatory grading [18], we assessed numbers of caspase3+ cells in colonic epithelia applying in situ immunohistochemistry and detected comparable numbers in mice from either Campylobacter cohort and from uninfected counterparts (n.s.; Fig. 6A). Peroral C. coli, but not C. jejuni challenge, however, was associated with increases in Ki67 positive colonic epithelia cells indicative for induced cell proliferation and regeneration at day 21 p.i. (P < 0.01; Fig. 6B). Hence, C. coli, but not C. jejuni application, was associated with pronounced regenerative, but not apoptotic responses in colonic epithelia cells.
Fig. 6.
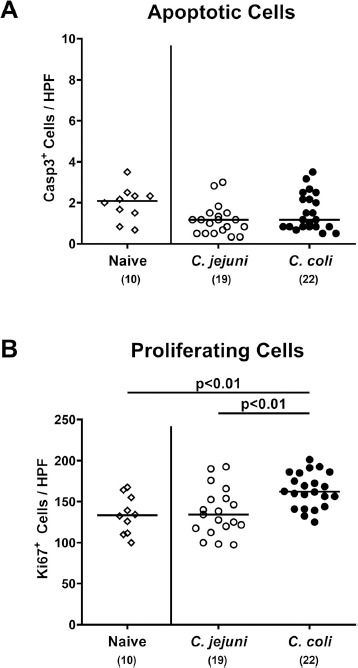
Colonic epithelial cell apoptosis and cell proliferation/regeneration in Campylobacter infected conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (open circles) or C. coli (closed circles) on day (d) 0 and d1 by gavage. On day 21 post-infection, the average numbers of colonic epithelial (A) apoptotic (Casp3+) and (B) proliferating (Ki67+) cells were assessed microscopically from six high power fields (HPF, 400 x magnification) per mouse in immunohistochemically stained colonic paraffin sections. Naive mice served as negative control animals (open diamonds). Medians (black bars), levels of significance (P-values) assessed by the Kruskal-Wallis test and Dunn’s post-correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
Colonic immune responses in Campylobacter infected conventional mice
We next addressed whether infection of conventional mice with either Campylobacter species was associated with intestinal immune cell responses. At day 21 following C. coli and C. jejuni application mice displayed higher numbers of adaptive immune cell subsets such as CD3+ T lymphocytes and FOXP3+ regulatory T cells in the colonic mucosa and lamina propria of the former as compared to the latter (P < 0.01; Fig. 7A and B), whereas B220+ B lymphocyte numbers were comparable (n.s.; Fig. 7C). Increased colonic adaptive immune cell counts were accompanied by increased colonic IFN-γ secretion upon C. coli versus C. jejuni challenge (P < 0.01; Fig. 8A), whereas TNF-α, MCP-1, IL-6, IL-12p70 and anti-inflammatory IL-10 concentrations did not differ between Campylobacter infected and uninfected control mice (n.s.; Fig. 8B–F). Of note, both adaptive immune cell counts and IFN-γ concentrations in the large intestines of infected mice were only detected in C. coli infected animals, whereas C. jejuni infected mice displayed inflammatory cell loads comparable to those obtained from uninfected controls.
Fig. 7.
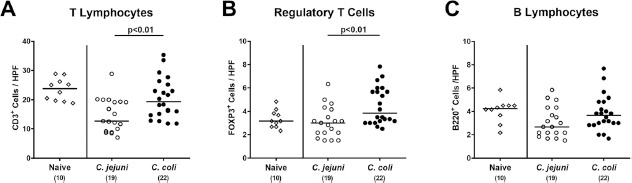
Colonic immune cell responses in Campylobacter infected conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (open circles) or C. coli (closed circles) on day (d) 0 and d1 by gavage. On day 21 post-infection, the average numbers of (A) T lymphocytes (CD3+), (B) regulatory T cells (FOXP3+) and (C) B lymphocytes (B220+) were assessed microscopically from six high power fields (HPF, 400 x magnification) per mouse in immunohistochemically stained colonic paraffin sections. Naive mice served as negative control animals (open diamonds). Medians (black bars), levels of significance (P-values) assessed by the Kruskal-Wallis test and Dunn’s post-correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
Fig. 8.
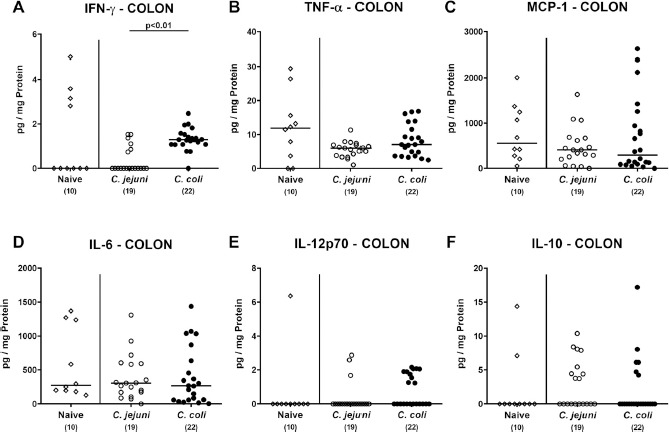
Colonic secretion of pro- and anti-inflammatory mediators in Campylobacter infected conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (open circles) or C. coli (closed circles) on day (d) 0 and d1 by gavage. On day 21 post-infection, (A) IFN-γ, (B) TNF-α, (C) MCP-1, (D) IL-6, (E) IL-12p70 and (F) IL-10 concentrations were determined in supernatants derived from colonic ex vivo biopsies. Naive mice served as negative control animals (open diamonds). Medians (black bars), levels of significance (P-values) assessed by the Kruskal-Wallis test and Dunn’s post-correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
Pro- and anti-inflammatory mediators in the spleens of C. coli versus C. jejuni infected conventional mice
We next addressed whether the observed C. coli induced immune responses were restricted to the intestinal tract or could also be observed beyond. We therefore measured pro-and anti-inflammatory mediators in splenic ex vivo biopsies taken at day 21 p.i. Campylobacter application of either species resulted in decreased concentrations of pro-inflammatory mediators, such as IFN-γ, TNF-α, and IL-6, in the spleen (P < 0.01–0.001; Fig. 9A, B and D), which also held true for splenic MCP-1 concentrations in C. jejuni infected mice (P < 0.001; Fig. 9C). Conversely, C. coli, but not C. jejuni challenged mice displayed even increased splenic IL-12p70 concentrations at day 21 p.i. (P < 0.001 vs naive; Fig. 9E). Furthermore, mice from either infection cohort exhibited decreased anti-inflammatory IL-10 concentrations in their spleen (P < 0.001; Fig. 9F).
Fig. 9.
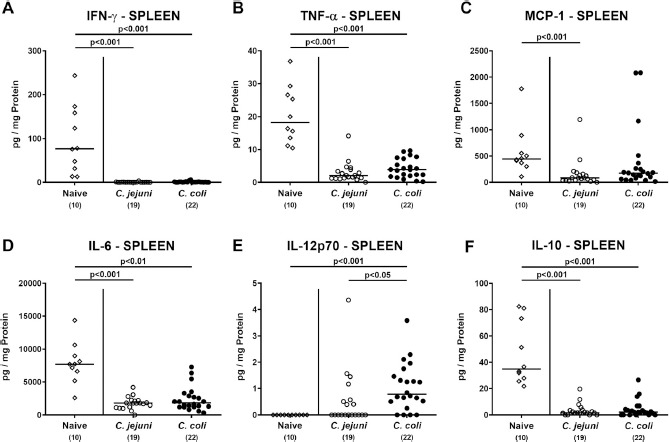
Splenic secretion of pro- and anti-inflammatory mediators in Campylobacter infected conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (open circles) or C. coli (closed circles) on day (d) 0 and d1 by gavage. On day 21 post-infection, (A) IFN-γ, (B) TNF-α, (C) MCP-1, (D) IL-6, (E) IL-12p70 and (F) IL-10 concentrations were determined in supernatants derived from splenic ex vivo biopsies. Naive mice served as negative control animals (open diamonds). Medians (black bars), levels of significance (P-values) assessed by the Kruskal-Wallis test and Dunn’s post-correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
Pro- and anti-inflammatory serum mediators in Campylobacter infected conventional mice
We finally addressed whether Campylobacter challenge of conventional mice resulted in systemic inflammatory mediator responses. In fact, neither C. jejuni nor C. coli application resulted in increased serum concentration of pro-inflammatory mediators, such as IFN-γ, TNF-α, MCP-1, IL-6 and IL-12p70 (n.s.; Fig. 10A–E). At day 21 p.i. with either Campylobacter species, however, lower anti-inflammatory IL-10 concentrations could be measured in the serum samples as compared to naïve counterparts (P <0.01–0.001; Fig. 10F).
Fig. 10.
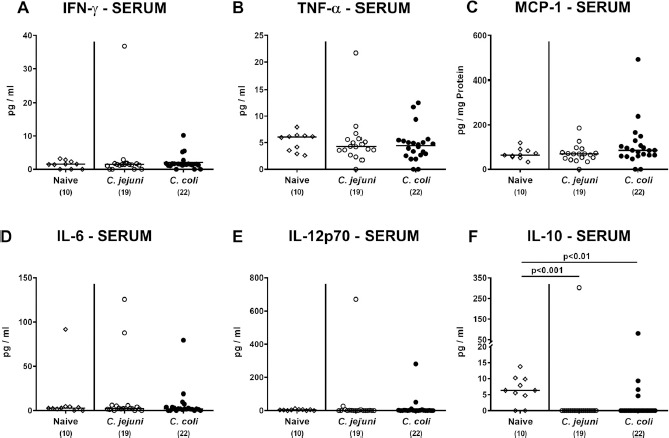
Pro- and anti-inflammatory serum mediators in Campylobacter infected conventional mice. Conventionally colonized C57BL/6 mice were perorally infected with C. jejuni (open circles) or C. coli (closed circles) on day (d) 0 and d1 by gavage. On day 21 post-infection, (A) IFN-γ, (B) TNF-α, (C) MCP-1, (D) IL-6, (E) IL-12p70 and (F) IL-10 concentrations were determined in serum samples. Naive mice served as negative control animals (open diamonds). Medians (black bars), levels of significance (P-values) assessed by the Kruskal-Wallis test and Dunn’s post-correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments
DISCUSSION
Given that solid data regarding C. coli-host interactions are virtually lacking, we here performed a comprehensive comparative survey regarding i.) bacterial colonization properties within the gastrointestinal tract, ii.) subsequent gut microbiota changes, and iii.) intestinal, as well as iv) systemic pro-inflammatory immune responses following peroral challenge of conventionally colonized adult wildtype mice with either C. coli or C. jejuni.
Both Campylobacter species are closely related evolutionarily sharing many features including growth conditions and expression of distinct virulence factors [4]. Thus, one could have expected that alike C. jejuni, also C. coli might not be able to colonize the murine gastrointestinal tract due to the host specific gut microbiota composition mediating physiological colonization resistance [14, 18]. Rather unexpectedly, however, more than 95% of mice harbored C. coli at relatively high median loads of more than 106 bacterial cells per g for three weeks in their gastrointestinal tract upon peroral challenge, whereas applied C. jejuni had been expelled within only a few days thereafter. A previous study addressed C. coli colonization in the intestinal tract of weanling mice (21–24 days of age). The authors reported strain dependent differences in C. coli colonization capacities and categorized the applied C. coli strains into three phenotypic groups according to their colonization abilities. The results obtained upon peroral application of the C. coli Group I by Ciftici and coworkers coincides with the colonization properties of the C. coli patients isolate used in our study, given that all weanling mice showed immediate bacterial colonization within 24 hours post-challenge and prolonged excretion until 21 days thereafter [24]. Whereas bacterial factors such as pili, flagella, chemotaxis, and adhesins are known prerequisites for successful intestinal colonization of C. jejuni in the vertebrate host [34, 35], not much, however, is known so far about the molecular mechanisms underlying C. coli colonization and invasion and thus it awaits further investigations. In addition, it is tempting to speculate that distinct features of C. coli that are missing in C. jejuni, such as capabilities in metabolizing and utilizing nutrients derived from the gastrointestinal lumen [36, 37] are responsible for stable pathogenic establishment of C. coli within the gastrointestinal ecosystem, as opposed to C. jejuni.
We have already shown that elevated intestinal loads of commensal E. coli can override colonization resistance and subsequently facilitate C. jejuni colonization [21]. In our present survey, however, the commensal E. coli loads were comparable before and after C. coli and C. jejuni peroral application, as assessed by both culture and culture-independent (molecular) analyses of intestinal samples. We further excluded potential differences in the complex gut microbiota composition of respective cohorts before peroral Campylobacter challenge by quantitative 16S rRNA based analyses of the main gut bacterial groups. Furthermore, neither C. coli nor C. jejuni application induced gut microbiota shifts within 21 days.
Despite the high gastrointestinal colonization densities, C. coli neither induced any typical signs of campylobacteriosis, such as wasting or bloody diarrhea, nor microscopic inflammatory sequelae, such as apoptosis of colonic epithelial cells, that could be observed upon C. jejuni infection of microbiota depleted mice [18, 19]. Interestingly, upon C. coli as compared to C. jejuni challenge, mice displayed shorter colonic lengths at necropsy, which might be indicative of intestinal inflammation that is commonly accompanied with shrinkage of the affected intestinal part [26]. Notably, C. coli, but not C. jejuni colonized mice displayed higher numbers of Ki67+ proliferating colonic epithelial cells as compared to naive counterparts, which might point towards cell regenerative measures counteracting potential cell damage. Despite the virtual lack of overt clinical or microscopic sign of C. coli induced inflammatory sequelae, C. coli did in fact induce pro-inflammatory immune responses. As compared to C. jejuni challenged mice, C. coli challenged mice exhibited higher numbers of adaptive immune cells such as CD3+ T lymphocytes and FOXP3+ regulatory T cells in their colonic mucosa and lamina, that were accompanied by higher pro-inflammatory IFN-γ concentrations in the large intestines of the former versus the latter. Interestingly, upon either Campylobacter application, lower splenic concentrations of pro-inflammatory mediators such as IFN-γ, TNF-α, and IL-6 could be measured as compared to naive counterparts, which also held true for anti-inflammatory IL-10 levels. This might be explained by recruitment of leukocytes from the spleen to the intestinal tract following peroral Campylobacter application. In line with the results obtained from spleens, peroral challenge of mice with either C. coli or C. jejuni resulted in decreased IL-10 concentrations measured in serum samples upon necropsy, whereas pro-inflammatory mediators did not differ from naive serum levels.
In order to further unravel potential inflammatory sequelae of C. coli infection, one would need to change from the wildtype mouse model which is rather considered a Campylobacter colonization model (given the lack of overt clinical signs and immunopathological features [14, 16, 30]) to an infection model. Our group has recently shown that secondary abiotic IL-10-/- mice, in which the gut microbiota had been depleted by broad-spectrum antibiotic treatment, can not only be effectively colonized by the pathogen upon peroral infection, but also develop key features of acute campylobacteriosis such as wasting and bloody diarrhea within one week [19]. One major reason for these severe immunopathological responses mounting in acute ulcerative enterocolitis is the absence of colonization resistance and the lack of IL-10 providing murine resistance to C. jejuni LOS [38, 39]. In consequence, C. jejuni infected IL-10-/- mice display pronounced LOS induced and TLR-4-dependent innate and adaptive immune responses that are not restricted to the intestinal tract, but can also be observed in extra-intestinal including systemic compartments [19, 30, 40–46]. Based on the results of this study, we therefore plan to include more C. coli strains from different sources (environmental, animal, human) in our future in vivo infection studies.
ACKNOWLEDGEMENTS
We thank Alexandra Bittroff-Leben, Ines Puschendorf, Ulrike Escher, Ulrike Fiebiger, Gernot Reifenberger, and the staff of the animal research facility at Charité – University Medicine Berlin for excellent technical assistance and animal breeding.
Footnotes
Funding: This work was supported from the German Federal Ministries of Education and Research (BMBF) in frame of the zoonoses research consortium PAC-Campylobacter to SB and MMH (IP7/01KI1725D) and from the Federal Ministry for Economic Affairs and Energy following a resolution of the German National Parliament, Deutscher Bundestag to SB and MMH (ZIM, ZF4117908 AJ8).
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Authors contributions:
CG: Performed experiments, analyzed data, co-wrote the paper.
SK: Performed experiments, analyzed data.
SM: Performed experiments, analyzed data.
SB: Provided advice in experimental design, critically discussed results, co-edited the paper.
MMH: Designed and performed experiments, analyzed data, wrote the paper.
Competing financial interests: Stefan Bereswill and Markus M. Heimesaat are members of the Editorial Board.
REFERENCES
- 1.Nachamkin I, Shadomy SV, Moran AP, Cox N, Fitzgerald C, Ung H, et al. Anti-ganglioside antibody induction by swine (A/NJ/1976/H1N1) and other influenza vaccines: insights into vaccine-associated Guillain-Barre syndrome. J Infect Dis. 2008;198(2):226–33. [DOI] [PubMed] [Google Scholar]
- 2.Poly F, Guerry P. Pathogenesis of Campylobacter. Curr Opin Gastroenterol. 2008;24(1):27–31. [DOI] [PubMed] [Google Scholar]
- 3.Backert S, Tegtmeyer N, Cróinín T, Boehm M, Heimesaat M. Human campylobacteriosis. 2017. pp. 1–25. [Google Scholar]
- 4.Alter T, Bereswill S, Glunder G, Haag LM, Hanel I, Heimesaat MM, et al. [Campylobacteriosis of man : livestock as reservoir for Campylobacter species]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2011;54(6):728–34. [DOI] [PubMed] [Google Scholar]
- 5.Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008;16(9):428–35. [DOI] [PubMed] [Google Scholar]
- 6.Lane JA, Mehra RK, Carrington SD, Hickey RM. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int J Food Microbiol. 2010;142(1-2):1–13. [DOI] [PubMed] [Google Scholar]
- 7.Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, et al. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012;12(2): 89–98. [DOI] [PubMed] [Google Scholar]
- 8.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5(9):665–79. [DOI] [PubMed] [Google Scholar]
- 9.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008;21(3):505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JP, Gross U, et al. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit Rev Microbiol. 2009;35(1):1–22. [DOI] [PubMed] [Google Scholar]
- 11.O Cróinín T, Backert S. Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism? Front Cell Infect Microbiol. 2012;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001; 8:150–65. [DOI] [PubMed] [Google Scholar]
- 13.Allos BM. Association between Campylobacter infection and Guillain-Barré syndrome. J Infect Dis. 1997;176 Suppl 2:S125–8. [DOI] [PubMed] [Google Scholar]
- 14.Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Gross U, et al. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol. 2013;2013:526860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimesaat MM, Bereswill S. Murine infection models for the investigation of Campylobacter jejuni–host interactions and path-ogenicity. Berl Munch Tierarztl Wochenschr. 2015;128(3-4): 98–103. [PubMed] [Google Scholar]
- 16.Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol (Bp). 2016;6(4):253–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takata T, Fujimoto S, Amako K. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect Immun. 1992;60(9):3596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, Gobel UB, et al. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling. PLoS One. 2012;7(7):e40761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimesaat MM, Mrazek K, Bereswill S. Murine fecal microbiota transplantation lowers gastrointestinal pathogen loads and dampens pro-inflammatory immune responses in Campylobacter jejuni infected secondary abiotic mice. Sci Rep. 2019;9(1):19797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haag LM, Fischer A, Otto B, Plickert R, Kuhl AA, Gobel UB, et al. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One. 2012;7(5):e35988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheppard SK, McCarthy ND, Falush D, Maiden MC. Convergence of Campylobacter species: implications for bacterial evolution. Science. 2008;320(5873):237–9. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard S, Dallas J, Wilson D, Strachan N, McCarthy N, Jolley K, et al. Evolution of an agriculture-associated disease causing Campylobacter coli clade: evidence from national surveillance data in Scotland. PloS one. 2010;5:e15708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciftci A, Savasan S, Ica T, Diker S. Mouse intestine colonization ability of Campylobacter coli strains. DTW Deutsche tierärztliche Wochenschrift. 2009;116:255–9. [PubMed] [Google Scholar]
- 25.Heimesaat MM, Haag LM, Fischer A, Otto B, Kuhl AA, Gobel UB, et al. Survey of extra-intestinal immune responses in asymptomatic long-term Campylobacter jejuni-infected mice. Eur J Microbiol Immunol (Bp). 2013;3(3):174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177(12):8785–95. [DOI] [PubMed] [Google Scholar]
- 27.Bereswill S, Kuhl AA, Alutis M, Fischer A, Mohle L, Struck D, et al. The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog. 2014;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59(8):1079–87. [DOI] [PubMed] [Google Scholar]
- 29.Rausch S, Held J, Fischer A, Heimesaat MM, Kuhl AA, Bereswill S, et al. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS One. 2013;8(9):e74026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimesaat MM, Alutis M, Grundmann U, Fischer A, Tegtmeyer N, Böhm M, et al. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol. 2014;4(77). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heimesaat MM, Dunay IR, Schulze S, Fischer A, Grundmann U, Alutis M, et al. Pituitary adenylate cyclase-activating polypeptide ameliorates experimental acute ileitis and extra-intestinal sequelae. PLoS One. 2014;9(9):e108389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alutis ME, Grundmann U, Fischer A, Hagen U, Kuhl AA, Gobel UB, et al. The Role of Gelatinases in Campylobacter Jejuni Infection of Gnotobiotic Mice. Eur J Microbiol Immunol (Bp). 2015;5(4):256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alutis ME, Grundmann U, Hagen U, Fischer A, Kuhl AA, Gobel UB, et al. Matrix Metalloproteinase-2 Mediates Intestinal Immunopathogenesis in Campylobacter Jejuni-Infected Infant Mice. Eur J Microbiol Immunol (Bp). 2015;5(3):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backert S, Hofreuter D. Molecular methods to investigate adhesion, transmigration, invasion and intracellular survival of the foodborne pathogen Campylobacter jejuni. J Microbiol Methods. 2013;95(1):8–23. [DOI] [PubMed] [Google Scholar]
- 35.Burnham PM, Hendrixson DR. Campylobacter jejuni: collective components promoting a successful enteric lifestyle. Nat Rev Microbiol. 2018;16(9):551–65. [DOI] [PubMed] [Google Scholar]
- 36.Gao B, Vorwerk H, Huber C, Lara-Tejero M, Mohr J, Goodman AL, et al. Metabolic and fitness determinants for in vitro growth and intestinal colonization of the bacterial pathogen Campylobacter jejuni. PLoS Biol. 2017;15(5):e2001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorwerk H, Huber C, Mohr J, Bunk B, Bhuju S, Wensel O, et al. A transferable plasticity region in Campylobacter coli allows isolates of an otherwise non-glycolytic food-borne pathogen to catabolize glucose. Mol Microbiol. 2015;98(5):809–30. [DOI] [PubMed] [Google Scholar]
- 38.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010;201(2): 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod. 2007;76(5):738–48. [DOI] [PubMed] [Google Scholar]
- 40.Alutis ME, Grundmann U, Fischer A, Kuhl AA, Bereswill S, Heimesaat MM. Selective gelatinase inhibition reduces apoptosis and pro-inflammatory immune cell responses in Campylobacter jejuni-infected gnotobiotic IL-10 deficient mice. Eur J Microbiol Immunol (Bp). 2014;4(4):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heimesaat MM, Grundmann U, Alutis ME, Fischer A, Bereswill S. Absence of Nucleotide-Oligomerization-Domain-2 Is Associated with Less Distinct Disease in Campylobacter jejuni Infected Secondary Abiotic IL-10 Deficient Mice. Front Cell Infect Microbiol. 2017;7:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bereswill S, Grundmann U, Alutis ME, Fischer A, Heimesaat MM. Campylobacter jejuni infection of conventionally colonized mice lacking nucleotide-oligomerization-domain-2. Gut Pathog. 2017;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bereswill S, Ekmekciu I, Escher U, Fiebiger U, Stingl K, Heimesaat MM. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci Rep. 2017; 7(1):2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekmekciu I, Fiebiger U, Stingl K, Bereswill S, Heimesaat MM. Amelioration of intestinal and systemic sequelae of murine Campylobacter jejuni infection by probiotic VSL#3 treatment. Gut Pathog. 2017;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt AM, Escher U, Mousavi S, Boehm M, Backert S, Bereswill S, et al. Protease activity of Campylobacter jejuni HtrA modulates distinct intestinal and systemic immune responses in infected secondary abiotic IL-10 deficient mice. Front Cell Infect Microbiol. 2019;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heimesaat MM, Grundmann U, Alutis ME, Fischer A, Bereswill S. Microbiota Composition and Immune Responses During Campylobacter Jejuni Infection in Conventionally Colonized IL-10(-/-) Mice Lacking Nucleotide Oligomerization Domain 2. Eur J Microbiol Immunol (Bp). 2017;7(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


