Abstract
Background
Long-term, real-world data are required to support the use of CT-P13 in chronic conditions such as ankylosing spondylitis. However, real-world evidence may be influenced by selection bias, which can confound outcomes. The aim of the current analysis was to confirm the long-term comparability of CT-P13 and reference infliximab treatment in patients with ankylosing spondylitis, using propensity score matching to adjust for baseline differences between groups.
Methods
A propensity score–matching analysis was conducted on data from patients with ankylosing spondylitis in the Korean College of Rheumatology Biologics registry who received CT-P13 or reference infliximab. Drug retention, reasons for biologic therapy changes or discontinuation, and efficacy parameters were analyzed overall and by treatment line with up to 4 years of follow-up. Adverse events were recorded for each treatment group.
Results
Propensity score matching was effective in matching 124 CT-P13–treated and 124 reference infliximab-treated patients. Median treatment duration and drug retention were similar between CT-P13 and reference infliximab. Three-year retention rates (95% confidence interval [CI]) were 64.2% (53.5–73.0) for CT-P13 and 55.6% (42.9–66.6) for reference infliximab. Overall, 17.1% (CT-P13) and 29.3% (reference infliximab) of patients discontinued biologic therapy, and 20.0% (CT-P13) and 15.2% (reference infliximab) changed biologic therapy. Efficacy assessments were generally similar between groups; both treatments were well tolerated.
Conclusions
Propensity score–matching analysis confirmed that CT-P13 treatment was not associated with significant differences in drug retention, treatment duration, most efficacy parameters, or safety versus reference infliximab in Korean patients with ankylosing spondylitis, building evidence for the long-term comparability of these treatments.
Trial registration
ClinicalTrials.gov identifier: NCT01965132.
Electronic supplementary material
The online version of this article (10.1007/s40259-020-00432-z) contains supplementary material, which is available to authorized users.
Key Points
| Propensity score matching was used to adjust for baseline differences between 124 CT-P13–treated and 124 reference infliximab-treated patients with ankylosing spondylitis who were included in the Korean College of Rheumatology Biologics registry. |
| Over a follow-up period of up to 4 years, treatment duration and retention rate did not significantly differ between CT-P13 and reference infliximab, efficacy assessments were generally similar between groups, and both treatments were well tolerated. |
| These real-world, long-term findings provide robust evidence of the comparability of CT-P13 and reference infliximab, supporting the routine clinical use of CT-P13 in patients with ankylosing spondylitis. |
Background
Ankylosing spondylitis (AS) is an inflammatory rheumatic disease characterized by back pain and structural and functional systemic features that can impair quality of life [1]. Being a long-term disease, the cost burden of AS is substantial. Annual healthcare expenditures for patients with AS in the Republic of Korea are high, particularly in cases with systemic manifestations and comorbidities that increase disability [2]. While the advent of biologic therapy has improved the quality of life of patients with AS, its use is constrained by high costs [3]. In Korea, for example, the cost of illness with biologic therapies is approximately double that of non-biologics [3], and overall expenditure has grown significantly year on year since the approval of the first biologic agents in 2006 [2]. Biosimilars have the potential to help alleviate the cost burden of biologic therapies and improve patient access, without compromising efficacy or quality of life [4, 5].
CT-P13 is a biosimilar of reference infliximab approved by the Korean Ministry of Food and Drug Safety [6], the European Medicines Agency, and the US Food and Drug Administration for the treatment of patients in all indications held by reference infliximab, including those with AS [7–12]. A wealth of evidence from clinical trials and observational studies supports the comparability of CT-P13 and reference infliximab in terms of pharmacokinetics, efficacy, and safety across indications [13–16]. However, limited data are available regarding long-term treatment with CT-P13. The PLANETAS study and its open-label extension phase demonstrated that up to 2 years of CT-P13 treatment was well tolerated and effective [17, 18], while a single-center study reported a higher 2-year retention rate for CT-P13 compared with reference infliximab [19]. In addition, the NOR-SWITCH extension study (in which 18% of the patients had spondyloarthritis) reported no difference in safety and efficacy between patients maintaining CT-P13 treatment for 78 weeks versus those who switched from reference infliximab at week 52 [20]. Additional long-term data are needed to confirm the utility, efficacy, and safety of CT-P13 therapy and its equivalence to reference infliximab throughout the course of this chronic inflammatory disease.
The Korean College of Rheumatology Biologics (KOBIO) registry (ClinicalTrials.gov identifier NCT01965132) is an ongoing, multicenter, prospective, observational study being conducted nationwide in the Republic of Korea [21]. This real-world registry was established in December 2012 as an inception cohort to gather and monitor data on disease activity, treatment patterns, efficacy, and safety in adult patients with AS, rheumatoid arthritis, or psoriatic arthritis who were eligible to initiate, restart, or change biologic therapies. Previous analyses of patients from the KOBIO registry have reported encouraging drug retention rates, efficacy, and safety for CT-P13 treatment in patients with AS, after 4 years of follow-up [22]. In addition, each of these outcomes was shown to be comparable for CT-P13 and reference infliximab in patients with rheumatoid arthritis [23].
While registries provide valuable, real-world data, selection bias is an inherent limitation of these non-randomized, observational studies, and this can confound outcome data. Propensity score matching (PSM) is a useful tool to adjust for baseline differences between treatment groups in these contexts [24]. The present analysis aimed to address this limitation of the KOBIO registry by implementing PSM, seeking to confirm the comparability of CT-P13 and reference infliximab in the AS patient population.
Methods
Study Population
The present analysis included data from patients aged ≥ 18 years with AS who were enrolled in the KOBIO registry. Patients had received treatment with CT-P13 between December 2012 and December 2017, either as first-line treatment or following failure of another tumor necrosis factor (TNF) inhibitor. PSM was used to match the group of CT-P13–treated patients with an equal number of AS patients who received reference infliximab between December 2012 and December 2017. All patients were treated at the discretion of the treating physician, including the selection of biologic, dosing, and treatment duration. This analysis was conducted according to the principles of the Declaration of Helsinki, and the study protocol and data collection forms were approved by institutional review boards or local ethics committees at each participating center. All patients provided informed written consent.
Data Collection and Outcomes
Data were collected at baseline and annually thereafter from the 44 participating hospitals in the Republic of Korea, using standardized case report forms. The primary outcome of this analysis was drug retention, defined as the time to treatment discontinuation or change of biologic therapy. Efficacy assessments were included as secondary outcomes of this analysis. Efficacy was assessed by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, Ankylosing Spondylitis Disease Activity Score (ASDAS)–erythrocyte sedimentation rate (ESR) score, ASDAS–C-reactive protein (CRP) score, and patient global assessment (GA). Data were also collected on treatment dates and line of therapy, treatment changes and reasons for changing, treatment discontinuations (defined as permanent discontinuation of biologic agents), and reasons for discontinuation. Patients who did not have a documented time to discontinuation were included in the analyses of baseline demographics, efficacy, and safety, but were excluded from the analysis of drug retention. Overall safety data, in terms of adverse events (AEs), were also collected.
Statistical Analyses
As appropriate, continuous variables for baseline characteristics were compared using the paired t test or the Wilcoxon signed-rank test, and categorical variables were compared using McNemar’s test. PSM was used to match patients receiving CT-P13 or reference infliximab. A 1:1 ratio between the CT-P13 and reference infliximab group was selected to increase the statistical power of the analysis and to maximize the number of patients included in the database. Propensity scores were calculated using a logistic regression model to predict the probability of being treated with CT-P13 given the baseline characteristics. The baseline characteristics included in the logistic regression model were age, sex, and baseline BASDAI score.
Drug retention was analyzed by treatment; and within treatment groups, drug retention was analyzed according to treatment line (first vs subsequent line). To account for the varied follow-up periods for different patients, due to the prospective, observational design of the KOBIO registry, we analyzed drug survival using the Hall–Wellner method to calculate 95% confidence intervals (CIs) for the Kaplan–Meier drug survival curve [25]. Treatment duration was compared between treatment groups and analyzed within treatment groups according to treatment line.
Efficacy was assessed as ‘major’ improvement in ASDAS scores [26, 27]. Major improvement was defined as improvement of at least 2 points between two consecutive ASDAS scores. Assessment in Ankylosing Spondylitis Response Criteria (ASAS20) improvement was defined as an improvement of at least 20% and an absolute improvement of at least 10 units on a scale of 0–100 in at least three of the following domains: patient GA, pain assessment, function (BASDAI), and inflammation (based on the last two questions of the BASDAI). ASAS40 was defined as for ASAS20, but required improvements of at least 40% in at least three of the constituent domains. Descriptive statistics were derived for baseline and follow-up scores for BASDAI, ASDAS-ESR, ASDAS-CRP, and patient GA.
All statistical analyses were two-sided and were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA), and p values < 0.05 were considered statistically significant.
Results
Patient Characteristics
Of the 3424 patients included in the KOBIO registry between December 2012 and December 2017, 1658 had been diagnosed with AS [22]. Among AS patients included in the registry, 256 received treatment with CT-P13 and 143 received treatment with reference infliximab. Of these patients, 124 CT-P13–treated patients were propensity score matched with 124 patients who received treatment with reference infliximab; these 248 patients comprise the overall study population reported herein.
Following PSM, there were no significant differences overall in baseline patient characteristics between the CT-P13 group and the matched reference infliximab group (Table 1). Table S1 in the electronic supplementary material shows the baseline characteristics for the 124 patients included in the analysis and the 132 patients who were not included after PSM. Ninety-eight of 124 patients (79.0%) in the CT-P13 group and 81 of 124 patients (65.3%) in the reference infliximab group were treated in the first-line setting (Table S2 in the electronic supplementary material). There were no significant differences in baseline characteristics after PSM between the CT-P13 and reference infliximab groups for the 179 patients receiving first-line biologic therapy, with the exception of mean disease duration, which was longer in the CT-P13 group (3.9 ± 5.2 vs 2.5 ± 3.8 years, respectively; p = 0.01). For patients receiving treatment in the subsequent-line setting (n = 69), baseline characteristics were generally comparable between groups after PSM (Table S3 in the electronic supplementary material). However, the CT-P13 group had a significantly shorter mean disease duration, higher mean ASDAS-CRP score, and higher CRP levels compared with the reference infliximab group. Among patients receiving subsequent-line treatment, prior TNF inhibitors were most commonly etanercept (n = 9), adalimumab (n = 8), or reference infliximab (n = 6) in the CT-P13 group, while stopping and restarting previous reference infliximab treatment (n = 16) was the single most common occurrence in the reference infliximab group (Table S4 in the electronic supplementary material).
Table 1.
Baseline patient characteristics after propensity score matching
| Characteristics | Overall (N = 248) | CT-P13 (n = 124) | Reference infliximab (n = 124) | p value |
|---|---|---|---|---|
| Age at start, years | 39.2 ± 13.4 | 38.6 ± 13.3 | 39.7 ± 13.5 | 0.50 |
| Disease duration, years | 4.3 ± 5.3 | 4.2 ± 5.2 | 4.3 ± 5.4 | 0.94 |
| Male, n (%) | 184 (74) | 94 (76) | 90 (73) | 0.60 |
| Smoking status, n (%) | 0.74 | |||
| Ex-smoker | 51 (21) | 26 (21) | 25 (20) | |
| Current smoker | 71 (29) | 33 (27) | 38 (31) | |
| Never | 126 (51) | 65 (52) | 61 (49) | |
| BMI, kg/m2 | 23.6 ± 3.2 | 23.8 ± 3.2 | 23.3 ± 3.2 | 0.17 |
| Swollen joint counts | 0.6 ± 1.9 | 0.8 ± 2.4 | 0.4 ± 1.0 | 0.11 |
| Tender joint counts | 0.8 ± 2.4 | 1.1 ± 3.1 | 0.5 ± 1.2 | 0.07 |
| BASDAI | 5.5 ± 1.9 | 5.5 ± 1.9 | 5.5 ± 2.0 | 0.50 |
| Patient’s GA | 5.8 ± 2.2 | 5.8 ± 2.1 | 5.8 ± 2.2 | 0.76 |
| ASDAS-ESR | 3.5 ± 1.1 | 3.4 ± 1.0 | 3.5 ± 1.1 | 0.51 |
| ASDAS-CRP | 3.3 ± 1.1 | 3.4 ± 1.0 | 3.3 ± 1.2 | 0.21 |
| ESR, mm/h, n (%) | ||||
| Normal | 84 (34) | 44 (36) | 40 (33) | 0.59 |
| ESR | 35.7 ± 30.8 | 34.9 ± 31.9 | 36.4 ± 29.9 | 0.71 |
| CRP, mg/dL, n (%) | ||||
| Normal | 90 (37) | 39 (32) | 51 (41) | 0.16 |
| CRP | 2.2 ± 3.2 | 2.3 ± 3.2 | 2.1 ± 3.3 | 0.47 |
| HLA-B27 status, n (%) | 0.32 | |||
| Not determined | 17 (7) | 11 (9) | 6 (5) | |
| Positive | 206 (83) | 98 (79) | 108 (87) | |
| Negative | 25 (10) | 15 (12) | 10 (8) | |
Data presented are mean (standard deviation), unless otherwise indicated
ASDAS Ankylosing Spondylitis Disease Activity Score, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BMI body mass index, CRP C-reactive protein, ESR erythrocyte sedimentation rate, GA global assessment, HLA-B27 human leukocyte antigen-B27
Treatment Duration
After 4 years of follow-up, there was no significant difference in median treatment duration between the CT-P13 and reference infliximab groups (Table S5 in the electronic supplementary material). The median (interquartile range [IQR]) treatment duration was 2.06 (0.99–3.05) years for the CT-P13 group and 1.75 (0.97–2.24) years for the reference infliximab group (p = 0.11). There were also no significant differences in median treatment duration between the CT-P13 and reference infliximab groups when considering the subgroups of patients receiving first-line or subsequent-line treatment. However, there was a slightly longer median treatment duration in the CT-P13 versus the reference infliximab group in the first-line setting (2.13 [IQR 1.02–3.07] years vs 1.64 [IQR 0.99–2.18] years; p = 0.06). Corresponding median treatment durations in the subsequent-line subgroup were 1.11 (IQR 0.41–2.99) years and 1.85 (IQR 0.95–2.58) years (p = 0.75).
Drug Retention
Overall, the primary outcome, drug retention, was similar between the CT-P13 and reference infliximab groups (p = 0.41; Fig. 1a). Drug retention was also similar between the CT-P13 and reference infliximab groups in patients who received first-line therapy (p = 0.15; Fig. 1b) and subsequent-line therapy (p = 0.45; Fig. 1c). The 3-year retention rate (95% CI) was 64.2% (53.5–73.0) for CT-P13 and 55.6% (42.9–66.6) for reference infliximab in the overall patient population. Among patients receiving first-line therapy, the retention rate (95% CI) was 66.3% (54.3–75.8) for CT-P13 and 50.6% (34.7–64.6) for reference infliximab after 3 years of follow-up, while corresponding retention rates (95% CI) in patients receiving subsequent-line therapy were 55.6% (30.4–74.9) and 64.6% (41.7–80.4).
Fig. 1.
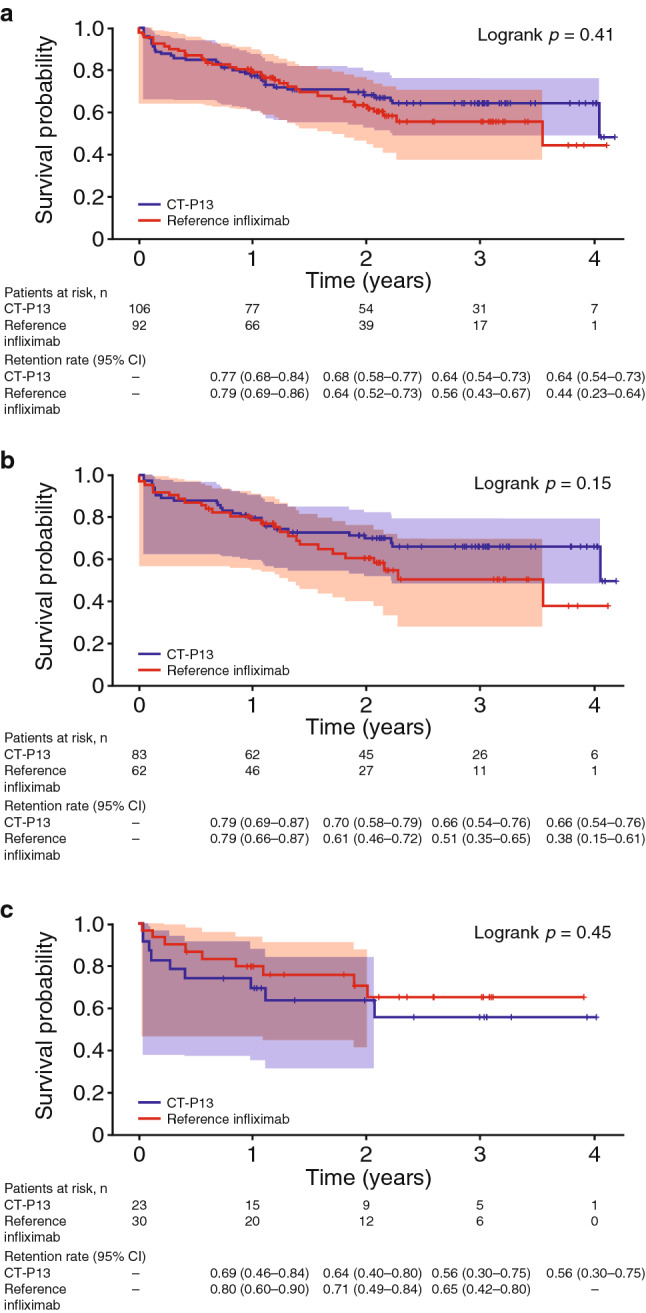
Drug retentiona up to 4 years in all patients (a) and those receiving first-line (b) and subsequent-line treatment (c) analyzed by Kaplan–Meier and Hall–Wellner methods to account for the varied follow-up periods of different patients due to the prospective, observational design of the KOBIO registry. Shading indicates 95% Hall–Wellner bands; + indicates censored patients. KOBIO Korean College of Rheumatology Biologics. aDefined as time to discontinuation or change of biologic therapy
The retention rates for CT-P13 and reference infliximab by treatment line are shown in Fig. S1a in the electronic supplementary material. Among patients receiving CT-P13, drug retention was not significantly different for patients receiving first-line or subsequent-line treatment (p = 0.30; Fig. S1b in the electronic supplementary material). In the reference infliximab group, drug retention was similar between patients receiving first- and subsequent-line treatment (p = 0.40; Fig. S1c in the electronic supplementary material).
Treatment Changes and Discontinuations
A total of 21 patients (of 105 evaluable; 20.0%) in the CT-P13 cohort and 14 patients (of 92 evaluable; 15.2%) in the reference infliximab group changed to another biologic, while 18 (17.1%) and 27 patients (29.3%), respectively, discontinued biologic therapy (Table 2).
Table 2.
Reasons for changing to another biologic or discontinuation of therapy
| CT-P13 (N = 105) | Reference infliximab (N = 92) | |||||
|---|---|---|---|---|---|---|
| Overall (N = 105) | First line (n = 82) | Subsequent line (n = 23) | Overall (N = 92) | First line (n = 62) | Subsequent line (n = 30) | |
| Patients changing biologic or discontinuing therapy, n | 39 | 30 | 9 | 41 | 29 | 12 |
| Patients changing biologic, n | 21 | 15 | 6 | 14 | 10 | 4 |
| Inefficacy | 13 | 10 | 3 | 9 | 6 | 3 |
| AE | 8 | 5 | 3 | 5 | 4 | 1 |
| Patients discontinuing therapy, n | 18 | 15 | 3 | 27 | 19 | 8 |
| Clinical remission | 4 | 4 | 3 | 2 | 1 | |
| Inefficacy | 2 | 2 | 1 | 1 | ||
| AE | 5 | 4 | 1 | 12 | 9 | 3 |
| Other reasons | 7 | 5 | 2 | 11 | 7 | 4 |
| Loss to follow-up | 2 | 2 | 2 | 1 | 1 | |
| Optionally stopped | 1 | 1 | 1 | 1 | ||
| Patient’s will | 2 | 1 | 1 | 5 | 3 | 2 |
| Plan for pregnancy | 1 | 1 | 1 | 1 | ||
| Insurance costs | 2 | 2 | ||||
| Unknown | 1 | 1 | ||||
AE adverse event
Changes in treatment to other biologics due to inefficacy or AEs occurred at comparable rates in both treatment groups. Of the 21 patients in the CT-P13 group who changed treatment, 13 did so due to lack of efficacy and eight due to AEs, while corresponding numbers of patients in the reference infliximab group were nine and five (14 patients changed biologic in total). Infusion or injection site reactions were the most frequent AE leading to a change in treatment in both the CT-P13 and reference infliximab groups (Table 3). Skin rash accounted for three patients and uveitis accounted for one patient changing biologic in the CT-P13 group, while skin rash accounted for the one remaining patient changing treatment due to an AE in the reference infliximab group. The drugs administered following a change in biologic treatment are presented in Table S6 in the electronic supplementary material. Golimumab was the most frequently administered biologic for the CT-P13 group, regardless of treatment line, while adalimumab was most frequently administered for the reference infliximab group, again regardless of treatment line. Two patients changed from CT-P13 to reference infliximab, due to AE (n = 1) and inefficacy (n = 1), while three patients changed from reference infliximab to CT-P13, due to inefficacy (n = 2) and AE (n = 1).
Table 3.
Changes and discontinuations due to AEs, overall and by treatment line
| CT-P13 (N = 105) | Reference infliximab (N = 92) | |||||
|---|---|---|---|---|---|---|
| Overall (N = 105) | First line (n = 82) | Subsequent line (n = 23) | Overall (N = 92) | First line (n = 62) | Subsequent line (n = 30) | |
| Patients changing biologic or discontinuing therapy due to an AE, n | ||||||
| Total | 13 | 9 | 4 | 17 | 13 | 4 |
| Changed biologic | 8 | 5 | 3 | 5 | 4 | 1 |
| Discontinued therapy | 5 | 4 | 1 | 12 | 9 | 3 |
| AEs leading to changed biologic or discontinued therapy, n | ||||||
| Changed biologic | ||||||
| Infusion/injection reaction | 5 | 2 | 3 | 5 | 4 | 1 |
| Uveitis | 1 | 1 | ||||
| Skin rash | 3 | 3 | 1 | 1 | ||
| Discontinued therapy | ||||||
| Infusion/injection reaction | 2 | 1 | 1 | 2 | 1 | 1 |
| Mycobacterium tuberculosis infection pulmonary | 1 | 1 | 4 | 4 | ||
| Headache | 2 | 2 | ||||
| Other infection | 1 | 1 | ||||
| Transaminitis only | 1 | 1 | ||||
| Psoriasis | 1 | 1 | ||||
| Uveitis | 1 | 1 | ||||
| Gastric ulcer | 1 | 1 | ||||
| Acute kidney injury | 1 | 1 | ||||
| Unknown | 1 | 1 | ||||
AE adverse event
Discontinuation of CT-P13 occurred due to AEs, clinical remission, inefficacy, and other reasons in five, four, two, and seven patients, respectively; corresponding numbers of patients discontinuing treatment in the reference infliximab group were 12, three, one, and 11 (Table 2). In the CT-P13 group, AEs leading to treatment discontinuation comprised infusion or injection site reactions (n = 2), headache (n = 2) and Mycobacterium tuberculosis pulmonary infection (n = 1) (Table 3). In the reference infliximab group, discontinuations due to AEs occurred most frequently as a result of M. tuberculosis pulmonary infection (n = 4) and infusion or injection site reactions (n = 2).
Efficacy
After both 1 and 2 years of follow-up, there were no statistically significant differences between the CT-P13 and reference infliximab groups with regard to the proportion of patients who achieved major (≥ 2-point) improvement in ASDAS scores from baseline (Table 4). This finding was consistent in the subgroup of patients receiving first-line treatment. In terms of ASAS20 and ASAS40, there were no significant differences in the proportion of patients achieving these responses between treatment groups at either follow-up time point.
Table 4.
Major improvement and clinically important improvements in ASDAS and improvements in ASAS at first and second follow-up: CT-P13 versus reference infliximab*
| Overall patient population | Year 1 | Year 2 | ||||
|---|---|---|---|---|---|---|
| CT-P13 (n = 64) | Reference infliximab (n = 38) | P value† | CT-P13 (n = 48) | Reference infliximab (n = 31) | p value† | |
| ASAS20a | 46 (71.9) | 28 (73.7) | 0.07 | 36 (75.0) | 23 (74.2) | 0.23 |
| ASAS40b | 34 (53.1) | 21 (55.3) | 0.17 | 28 (58.3) | 15 (48.4) | 0.11 |
| ASDAS improvement criteria | ||||||
| Major improvement | 34 (34.3) | 26 (31.0) | 0.13 | 28 (43.1) | 18 (34.6) | 0.98 |
| Patients receiving first-line treatment | CT-P13 (n = 52) | Reference infliximab (n = 31) | P value† | CT-P13 (n = 39) | Reference infliximab (n = 22) | p value† |
|---|---|---|---|---|---|---|
| ASAS20a | 36 (69.2) | 23 (74.2) | 0.51 | 29 (55.8) | 17 (77.3) | 0.51 |
| ASAS40b | 25 (48.1) | 17 (54.8) | 0.80 | 23 (44.2) | 12 (54.5) | 0.35 |
| ASDAS improvement criteria | ||||||
| Major improvement | 30 (39.0) | 23 (41.1) | 0.13 | 23 (44.2) | 14 (40.0) | 0.72 |
Data presented are n (%)
ASAS Assessment in Ankylosing Spondylitis Response Criteria, ASDAS Ankylosing Spondylitis Disease Activity Score, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, GA global assessment
*Significant p value (p < 0.05)
†P value based on Chi-square test
aASAS Response Criteria (ASAS20) is defined as an improvement of at least 20% and an absolute improvement of at least 10 units on a 0–100 scale in at least three of the following domains: patient GA, pain assessment, function (BASFI), and inflammation (last two questions of BASDAI)
bASAS40 is defined as for ASAS20 above, but with improvements of at least 40%
In general, efficacy assessments were similar between the CT-P13 and reference infliximab groups during the follow-up period of up to 4 years (Table S7 in the electronic supplementary material). Median CRP levels were the major exception to this finding, as they were significantly lower in the reference infliximab group compared with the CT-P13 group at 2, 3, and 4 years of follow-up. At the year 1 follow-up, ASDAS-ESR and patient’s GA were significantly higher for the reference infliximab group compared with the CT-P13 group, but these differences did not persist beyond this time point.
Safety
CT-P13 and reference infliximab were well tolerated during long-term treatment. Overall, 65 patients (52.4%) reported 214 AEs in the CT-P13 group, while 52 patients (41.9%) reported 151 AEs in the reference infliximab group (Table S8 in the electronic supplementary material). In the CT-P13 group, 23 patients (18.5%) experienced a total of 60 AEs that were considered by the investigators to be study drug related; 19 patients (15.3%) in the reference infliximab group experienced 42 AEs that were considered by the investigators to be study drug related (Table S9 in the electronic supplementary material). Three grade 3 CT-P13–related AEs were reported: uveitis, infusion or injection site reaction, and M. tuberculosis infection, which occurred in one patient each. A total of three grade 3 reference infliximab-related AEs were reported, all due to M. tuberculosis infection.
Discussion
In this analysis of the real-world, prospective, observational KOBIO registry, our data showed no significant differences in treatment duration between CT-P13 and reference infliximab in patients with AS, regardless of treatment line. Treatment changes due to inefficacy and AEs occurred at comparable rates between the two study groups. Drug retention (time to treatment discontinuation or change) was not significantly different between CT-P13 and reference infliximab. With regard to efficacy and disease activity, in general, we did not observe significant differences between CT-P13 and reference infliximab, and both treatments were well tolerated. These data contribute to the evidence base for CT-P13 treatment, providing further support for the equivalence of CT-P13 and reference infliximab in patients with AS over the long term.
In our analysis, the 3-year retention rate (95% CI) was 64.2% (53.5–73.0) for CT-P13 and 55.6% (42.9–66.6) for reference infliximab in the overall patient population. Therefore, the CT-P13 retention rate based on the 124 patients included in this analysis was similar to the 4-year retention rate of 66% reported in a previous analysis of 244 patients with AS enrolled in the KOBIO registry [22]. In addition, CT-P13 retention did not differ significantly between patients receiving first-line or subsequent-line therapy, in keeping with previous findings from this registry [22]. The 77% and 79% 1-year retention rates for CT-P13 and reference infliximab, respectively, in our analysis were slightly lower than those reported for the DANBIO registry of patients with rheumatic diseases, where overall crude retention rates (95% CI) were 84.1% (81.3–86.5) and 86.2% (84.0–88.0) for CT-P13 and reference infliximab, respectively [15]. In patients with axial spondyloarthritis alone, the corresponding rate for CT-P13 was 87% (83–91) [15]. The 1- and 2-year retention rates in our analysis were similar to the 83% and 76%, respectively, reported in a pooled analysis of 12 registries in the EuroSpA collaboration, including 2935 patients with AS [28]. On the other hand, the drug survival rates identified in our analysis were slightly lower than the 1-, 2-, and 3-year retention rates reported for the 657 patients with AS treated with any TNF inhibitor who were included in the Spanish BIOBADASER registry [29] and the 310 patients with AS treated with anti-TNF therapy who were included in the Czech national registry, ATTRA [30]. In addition, a single-center study enrolling patients with rheumatic diseases (including 109 patients [27.6%] with AS or spondyloarthritis) found that drug retention after 2 years was significantly better for patients who initiated CT-P13 rather than reference infliximab, in the combined patient population [19], contrasting with the findings of our analysis.
In this analysis, 20.0% and 17.1% of patients receiving CT-P13 changed to another biologic and permanently discontinued biologic treatments, respectively. This is fairly similar to the rates reported in a previous analysis of 244 patients with AS in the KOBIO registry (15.6% and 13.1%, correspondingly) [22]. While proportions of patients changing to another biologic were similar between groups, a greater proportion of patients receiving reference infliximab discontinued biologic therapy (29.3% vs 17.1% in the CT-P13 group), in contrast to what might be expected with biosimilar-related nocebo effects [31]. This may be related to an increased readiness of physicians to recommend discontinuation of the reference product, perceiving that reinstating treatment later with a first biosimilar option might provide similar treatment outcomes, in contrast with possible preferences of physicians already prescribing a biosimilar to maintain treatment with that agent. In this analysis, two patients switched from CT-P13 to reference infliximab, which could reflect patients experiencing nocebo effects with the biosimilar in this observational setting [31], although three patients underwent the reverse switch. AEs and lack of efficacy were the most common reasons for patients changing biologic or discontinuing treatment, while clinical remission was also a frequent reason for treatment discontinuation. These findings are in keeping with the previous KOBIO registry analysis [22]. Similarly, AEs were the most common reason for reference infliximab discontinuation for patients with AS in the ATTRA registry [30] and for patients with spondyloarthritis in the BIOBADASER registry [29], and lack of efficacy was the most common reason for treatment discontinuation for patients with AS in the Swedish ARTIS registry who were treated with reference infliximab, adalimumab, or etanercept [32].
In the current analysis, major improvement in ASDAS criteria was achieved by 34.3% and 43.1% of CT-P13–treated patients after 1 and 2 years of treatment, respectively. This is somewhat lower than the corresponding 56.5% and 56.9% of patients reported in a previous analysis of patients with AS included in the KOBIO registry [22], which may be a result of the differences in baseline characteristics between the studies. Nonetheless, efficacy was similar between CT-P13 and reference infliximab in both analyses. This is in keeping with findings from the multicenter, phase I, randomized, double-blind PLANETAS study, which reported highly similar efficacy across endpoints between CT-P13 and reference infliximab [13, 17], as well as comparable efficacy for long-term CT-P13 treatment and switching from reference infliximab to CT-P13 in the open-label extension [33]. Real-world efficacy data for CT-P13 treatment in patients with AS have recently been reported. In an observational study that enrolled patients with AS with inadequate response to conventional therapy, 80.0% of patients responded to CT-P13 treatment per BASDAI criteria, which was deemed in keeping with the PLANETAS study results [34]. A prospective observational study also compared continued reference infliximab treatment with switching to CT-P13 in patients with AS in clinical remission [35]. After 18 months of follow-up, efficacy was comparable between groups and all patients remained in clinical remission, in keeping with the comparable efficacy identified between groups in our study. In addition, two small studies evaluating switching from reference infliximab to CT-P13 in patients with rheumatologic diagnoses including AS found that, overall, responses were similar between treatments [36, 37].
Overall, long-term treatment with both CT-P13 and reference infliximab was well tolerated. A greater number of AEs and a greater proportion of patients experiencing AEs were reported for the CT-P13 group compared with the reference infliximab group. However, similar proportions of patients in each group (CT-P13 18.5%; reference infliximab 15.3%) experienced AEs that were considered by the investigators to be study drug related. For the CT-P13 group, the proportion of patients reporting any or study drug-related AEs was in line with findings from a previous analysis of AS patients from the KOBIO registry [22].
The KOBIO registry prospectively collects real-world patient data, without protocolized patient selection or mandated treatment as typically employed in clinical trials, and thus should be more representative of everyday clinical practice. Furthermore, the implementation of PSM can provide more robust data than conventional observational studies, through avoiding any selection bias associated with differing baseline patient characteristics [24]. This means that our results should reflect findings for patients with AS who are treated with CT-P13 or reference infliximab in the real-world clinical setting. However, only 69 patients (27.8%) included in this analysis received either CT-P13 or reference infliximab in the subsequent-line setting, which could have skewed the drug retention data in this subgroup. In addition, due to the prospective, observational design of the KOBIO registry, only eight out of 248 patients treated with CT-P13 or reference infliximab were followed up for 4 years, limiting the conclusions that could be drawn about the retention rate at this time point; therefore, we have described 3-year retention rates for our analysis. Our analysis is also limited by its retrospective, observational nature, which means that some information could not be obtained; for example, efficacy data were not collected for all patients and some reasons for treatment discontinuation were recorded as ‘unknown.’ A further limitation to the interpretation of the efficacy data is that for some parameters, such as CRP, measurement methods were not standardized between the participating centers. A key strength of the present analysis is the long duration of follow-up. As previously discussed, few studies to date have reported on the long-term drug retention, efficacy, or safety of CT-P13 in patients with AS, with the duration of follow-up limited to up to 2 years in contexts other than the KOBIO registry [19, 20, 33]. The 4 years of follow-up, to date, in the KOBIO registry provides unique insights into long-term treatment with CT-P13 and its comparability with reference infliximab. The availability of such data may contribute to alleviating any physician concerns regarding initiating, or switching patients to, a biosimilar drug such as potential loss of efficacy, unanticipated AEs or other safety considerations, or changes in immunogenicity [38]. Since biologic therapies contribute significantly to the overall treatment cost of AS in countries, including the Republic of Korea [2], overcoming such concerns may substantially reduce the cost burden of biologic therapies in rheumatologic conditions, and increase patient access to these therapies [4, 39, 40].
Conclusions
CT-P13 treatment was not associated with significant differences in drug retention, treatment duration, or most efficacy parameters when compared with reference infliximab treatment in Korean patients with AS. These real-world, long-term findings add to our increasing understanding of the comparability of CT-P13 and reference infliximab, and support the routine clinical use of CT-P13 in patients with AS.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing) was provided by Beatrice Tyrrell, DPhil at Aspire Scientific (Bollington, UK), and funded by Celltrion Healthcare Co., Ltd. (Incheon, Republic of Korea). Drafts of the manuscript were reviewed by Dasom Choi at Celltrion Healthcare Co., Ltd.
Authors Contributions
H-AK and KS made a substantial contribution to the conception or design of the study, and all authors made a substantial contribution to the acquisition, analysis, and interpretation of the data and to manuscript development, and gave final approval of the manuscript for submission. KS is the guarantor for the overall content of the article.
Data Availability
All data generated or analyzed during this study are included in this published article and its accompanying supplementary information file.
Compliance with Ethical Standards
Conflict of interest
The authors, Hyoun-Ah Kim, Eunyoung Lee, Sun-Kyung Lee, Yong-Beom Park, and Kichul Shin, declare that they have no competing interests.
Funding
Medical writing assistance was funded by Celltrion Healthcare Co., Ltd. (Incheon, Republic of Korea).
Research involving human participants
The analysis was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study protocol and data collection forms were approved by institutional review boards or local ethics committees at each participating center.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 2.Lee JS, Oh BL, Lee HY, Song YW, Lee EY. Comorbidity, disability, and healthcare expenditure of ankylosing spondylitis in Korea: a population-based study. PLoS One. 2018;13(2):e0192524. doi: 10.1371/journal.pone.0192524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee TJ, Park BH, Kim JW, Shin K, Lee EB, Song YW. Cost-of-illness and quality of life in patients with ankylosing spondylitis at a tertiary hospital in Korea. J Korean Med Sci. 2014;29(2):190–197. doi: 10.3346/jkms.2014.29.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha A, Upton A, Dunlop WC, Akehurst R. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther. 2015;32(8):742–756. doi: 10.1007/s12325-015-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kay J, Schoels MM, Dorner T, Emery P, Kvien TK, Smolen JS, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis. 2018;77(2):165–174. doi: 10.1136/annrheumdis-2017-211937. [DOI] [PubMed] [Google Scholar]
- 6.Generics and Biosimilars Initiative. Biosimilar monoclonal antibody approved in Korea. 2012. http://www.gabionline.net/Biosimilars/News/Biosimilar-monoclonal-antibody-approved-in-Korea. Accessed 12 Sept 2019.
- 7.European Medicines Agency. Inflectra Summary of Product Characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/inflectra-epar-product-information_en.pdf. Accessed 30 Sept 2019.
- 8.European Medicines Agency. Remsima Summary of Product Characteristics. 2019. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002576/WC500150871.pdf. Accessed 30 September 2019.
- 9.European Medicines Agency. Remicade Summary of Product Characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf. Accessed 30 Sept 2019.
- 10.US Food & Drug Administration. Inflectra Prescribing Information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125544s009lbl.pdf. Accessed 30 Sept 2019.
- 11.US Food & Drug Administration. Remicade Prescribing Information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103772s5385lbl.pdf. Accessed 30 Sept 2019.
- 12.Korean Ministry of Food and Drug Safety. Remsima. https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=201206204 [in Korean]. Accessed 17 Oct 2019.
- 13.Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–1612. doi: 10.1136/annrheumdis-2012-203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–2316. doi: 10.1016/S0140-6736(17)30068-5. [DOI] [PubMed] [Google Scholar]
- 15.Glintborg B, Sorensen IJ, Loft AG, Lindegaard H, Linauskas A, Hendricks O, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76(8):1426–1431. doi: 10.1136/annrheumdis-2016-210742. [DOI] [PubMed] [Google Scholar]
- 16.Baji P, Pentek M, Szanto S, Geher P, Gulacsi L, Balogh O, et al. Comparative efficacy and safety of biosimilar infliximab and other biological treatments in ankylosing spondylitis: systematic literature review and meta-analysis. Eur J Health Econ. 2014;15(Suppl 1):S45–S52. doi: 10.1007/s10198-014-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park W, Yoo DH, Jaworski J, Brzezicki J, Gnylorybov A, Kadinov V, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25. doi: 10.1186/s13075-016-0930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park DJ, Choi SJ, Shin K, Kim HA, Park YB, Kang SW, et al. Switching profiles in a population-based cohort of rheumatoid arthritis receiving biologic therapy: results from the KOBIO registry. Clin Rheumatol. 2017;36(5):1013–1022. doi: 10.1007/s10067-017-3584-y. [DOI] [PubMed] [Google Scholar]
- 19.Nikiphorou E, Hannonen P, Asikainen J, Borodina J, Kokko A, Paalanen K, et al. Survival and safety of infliximab bio-original and infliximab biosimilar (CT-P13) in usual rheumatology care. Clin Exp Rheumatol. 2018;37(1):55–59. [PubMed] [Google Scholar]
- 20.Goll GL, Jorgensen KK, Sexton J, Olsen IC, Bolstad N, Haavardsholm EA, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med. 2019;285(6):653–669. doi: 10.1111/joim.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov. NCT01965132: Korean College of Rheumatology Biologics registry (KOBIO).https://clinicaltrials.gov/ct2/show/NCT01965132. Accessed 30 Sept 2019.
- 22.Kim HA, Lee E, Lee SK, Park YB, Lee YN, Kang HJ, et al. Retention rate and long-term safety of biosimilar CT-P13 in patients with ankylosing spondylitis: data from the Korean College of Rheumatology Biologics registry. Clin Exp Rheumatol. 2020;38(2):267–274. [PubMed] [Google Scholar]
- 23.Kim H-A, Lee E-Y, Lee SK, Park Y-B, Lee YN, Kang H, et al. Retention rate and safety of biosimilar CT-P13 in rheumatoid arthritis: data from the Korean College of Rheumatology Biologics registry. BioDrugs. 2020;34(1):89–98. doi: 10.1007/s40259-019-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okoli GN, Sanders RD, Myles P. Demystifying propensity scores. Br J Anaesth. 2014;112(1):13–15. doi: 10.1093/bja/aet290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall WJ, Wellner JA. Confidence bands for a survival curve from censored data. Biometrika. 1980;67:133–143. doi: 10.1093/biomet/67.1.133. [DOI] [Google Scholar]
- 26.Machado P, Landewe R, Lie E, Kvien TK, Braun J, Baker D, et al. Ankylosing spondylitis disease activity score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis. 2011;70(1):47–53. doi: 10.1136/ard.2010.138594. [DOI] [PubMed] [Google Scholar]
- 27.Machado PM, Landewe RB, van der Heijde DM. Endorsement of definitions of disease activity states and improvement scores for the Ankylosing Spondylitis Disease Activity Score: results from OMERACT 10. J Rheumatol. 2011;38(7):1502–1506. doi: 10.3899/jrheum.110279. [DOI] [PubMed] [Google Scholar]
- 28.Ornbjerg LM, Brahe CH, Askling J, Ciurea A, Mann H, Onen F, et al. Treatment response and drug retention rates in 24 195 biologic-naive patients with axial spondyloarthritis initiating TNFi treatment: routine care data from 12 registries in the EuroSpA collaboration. Ann Rheum Dis. 2019;78(11):1536–1544. doi: 10.1136/annrheumdis-2019-215427. [DOI] [PubMed] [Google Scholar]
- 29.Carmona L, Gomez-Reino JJ, Group B Survival of TNF antagonists in spondylarthritis is better than in rheumatoid arthritis. Data from the Spanish registry BIOBADASER. Arthritis Res Ther. 2006;8(3):R72. doi: 10.1186/ar1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavelka K, Forejtova S, Stolfa J, Chroust K, Buresova L, Mann H, et al. Anti-TNF therapy of ankylosing spondylitis in clinical practice. Results from the Czech national registry ATTRA. Clin Exp Rheumatol. 2009;27(6):958–963. [PubMed] [Google Scholar]
- 31.Kravvariti E, Kitas GD, Mitsikostas DD, Sfikakis PP. Nocebos in rheumatology emerging concepts and their implications for clinical practice. Nat Rev Rheumatol. 2018;14(12):727–740. doi: 10.1038/s41584-018-0110-9. [DOI] [PubMed] [Google Scholar]
- 32.Lie E, Kristensen LE, Forsblad-d’Elia H, Zverkova-Sandstrom T, Askling J, Jacobsson LT, et al. The effect of comedication with conventional synthetic disease modifying antirheumatic drugs on TNF inhibitor drug survival in patients with ankylosing spondylitis and undifferentiated spondyloarthritis: results from a nationwide prospective study. Ann Rheum Dis. 2015;74(6):970–978. doi: 10.1136/annrheumdis-2014-206616. [DOI] [PubMed] [Google Scholar]
- 33.Park W, Yoo DH, Miranda P, Brzosko M, Wiland P, Gutierrez-Urena S, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2017;76(2):346–354. doi: 10.1136/annrheumdis-2015-208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Codreanu C, Sirova K, Jarosova K, Batalov A. Assessment of effectiveness and safety of biosimilar infliximab (CT-P13) in a real-life setting for treatment of patients with active rheumatoid arthritis or ankylosing spondylitis. Curr Med Res Opin. 2018;34(10):1763–1769. doi: 10.1080/03007995.2018.1441144. [DOI] [PubMed] [Google Scholar]
- 35.Kaltsonoudis E, Pelechas E, Voulgari PV, Drosos AA. Maintained clinical remission in ankylosing spondylitis patients switched from reference infliximab to its biosimilar: an 18-month comparative open-label study. J Clin Med. 2019;8(7):E956. doi: 10.3390/jcm8070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benucci M, Gobbi FL, Bandinelli F, Damiani A, Infantino M, Grossi V, et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6-month real-life observational study. Immunol Res. 2017;65(1):419–422. doi: 10.1007/s12026-016-8843-5. [DOI] [PubMed] [Google Scholar]
- 37.Malaiya RMZ, Kiely P. Infliximab biosimilars—switching Remicade to Remsima in routine care: patient acceptability and early outcome data. Rheumatology. 2016;55:i125–i126. [Google Scholar]
- 38.Azevedo V, Dörner T, Strohal R, Isaacs J, Castañeda-Hernández G, Gonçalves J, et al. Biosimilars: considerations for clinical practice. Consid Med. 2017;1:13–18. doi: 10.1136/conmed-2017-100005. [DOI] [Google Scholar]
- 39.Gulacsi L, Brodszky V, Baji P, Kim H, Kim SY, Cho YY, et al. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol. 2015;11(Suppl 1):S43–S52. doi: 10.1586/1744666X.2015.1090313. [DOI] [PubMed] [Google Scholar]
- 40.Brodszky V, Baji P, Balogh O, Pentek M. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ. 2014;15(Suppl 1):S65–S71. doi: 10.1007/s10198-014-0595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its accompanying supplementary information file.


