Abstract
We tested the hypothesis that the entopeduncular (EP) nucleus (feline equivalent of the primate GPi) and the globus pallidus (GPe) contribute to both the planning and execution of locomotion and voluntary gait modifications in the cat. We recorded from 414 cells distributed throughout these two nuclei (referred to together as the pallidum) while cats walked on a treadmill and stepped over an obstacle that advanced towards them. Neuronal activity in many cells in both structures was modulated on a step-by-step basis during unobstructed locomotion and was modified in the step over the obstacle. On a population basis, the most frequently observed change, in both the EP and the GPe, was an increase in activity prior to and/or during the swing phase of the step over the obstacle by the contralateral forelimb, when it was the first limb to pass over the obstacle. Our results support a contribution of the pallidum, in concert with cortical structures, to the control of both the planning and the execution of the gait modifications. We discuss the results in the context of current models of pallidal action on thalamic activity, including the possibility that cells in the EP with increased activity may sculpt thalamo-cortical activity.
Keywords: basal ganglia, entopeduncular nucleus, globus pallidus, pallidum, visually guided gait modification
Introduction
The ability to step over an obstacle in one’s path involves a series of planning processes that include object localization, step-by-step gait adjustments, and the selection of which limb will be the first to step over the obstacle. This process culminates with one leg (the plant limb) being appropriately placed in front of the obstacle while the other (the lead forelimb) steps over that obstacle without touching it.
The neural structures that are involved in these processes include a number of cortical areas including the posterior parietal cortex (PPC, Beloozerova and Sirota 2003; Lajoie and Drew 2007; Andujar et al. 2010; Lajoie et al. 2010; Drew and Marigold 2015; Marigold and Drew 2017), the premotor cortex (PMC, Nakajima et al. 2019) and, for the execution of the movement, the motor cortex (Armstrong 1988; Amos et al. 1990; Beloozerova and Sirota 1993; Drew 1993; Drew et al. 2008; Drew and Marigold 2015). Many neurons in both the PPC and the PMC modify their activity several steps (2–4) before the step over the obstacle and can potentially contribute to the localization of the obstacle with respect to the body (Marigold and Drew 2017) and to the transformation of these signals to provide the precise information required to step over the obstacle (Nakajima et al. 2019).
While each of these different cortical areas may preferentially contribute to specific aspects of the planning and execution of voluntary gait modifications, it is likely that many of these planning processes are distributed through multiple cortical areas, as well as subcortical structures, including the basal ganglia and the cerebellum (Allen and Tsukuhara 1974; Middleton and Strick 2000; Bostan and Strick 2010; Cisek and Kalaska 2010; Rizzolatti et al. 2014; Drew and Marigold 2015). However, while the contribution of the cerebellum to locomotion, including visually-guided gait modifications, has received some study (Armstrong and Edgley 1984a, 1984b; Armstrong and Marple-Horvat 1996; Marple-Horvat et al. 1998; Marple-Horvat and Criado 1999; Aoki et al. 2012; Aoki et al. 2013), information on the contribution of the basal ganglia to the control of locomotion is more fragmentary.
Nonetheless, a basal ganglia contribution to the control of locomotion is suggested by the clear locomotor deficits observed in patients with Parkinson’s disease (Knutsson 1972; Murray et al. 1978; Giladi 2001a; Nutt et al. 2011). That these deficits reflect a true contribution of the basal ganglia to the control of locomotion has been shown from a variety of studies. Takakusaki, for example, has shown that stimulation of the substantia nigra, pars reticulata (SNpr) has a powerful modulatory effect on locomotion induced by stimulation of the mesencephalic locomotor region (MLR) (Takakusaki et al. 2003; Takakusaki et al. 2004). In a similar manner, experiments using optogenetic stimulation in mice have shown that manipulation of the direct and indirect pathways in the striatum modulates locomotion and modifies the discharge activity of cells in the MLR (Kravitz et al. 2010; Roseberry et al. 2016). Moreover, single unit recordings have shown the presence of neuronal activity modulated at the rhythm of the step cycle in different parts of the basal ganglia (Schwarz et al. 1984; Shi et al. 2004; Robbe 2018; Sales-Carbonell et al. 2018). However, with specific respect to single unit recordings in the pallidum, there is only evidence from the rat (Shi et al. 2004) to show that cells are rhythmically active during locomotion; the specific relationship of this activity to different parts of the step cycle is only poorly described.
In addition, most studies have concentrated on the regulation of unobstructed locomotion over a flat surface, although studies in patients with Parkinson’s disease show that deficits are amplified in situations in which gait has to be modified (Burleigh-Jacobs et al. 1997; Giladi 2001a; Boonstra et al. 2008; Galna et al. 2010; Nutt et al. 2011). In animals, it has equally been shown that lesion of the striatum impairs the ability of rats to step over an obstacle attached to a treadmill belt (Perrot et al. 2009) in much the same manner that work from this laboratory previously demonstrated for lesions to the PPC (Lajoie and Drew 2007). Indeed, a contribution of the basal ganglia to modifications of gait is only to be expected given that the major inputs to the basal ganglia come from cortical areas that have been demonstrated to be critically important for the control of gait modifications made on the basis of visual information (Drew and Marigold 2015). For example, there are projections from the motor cortex, the PPC and the PMC to the striatum and subthalamic nucleus (Kunzle 1975; Ragsdale and Graybiel 1981; Selemon and Goldman-Rakic 1985; Yeterian and Pandya 1993; Nambu 2004; Nambu 2011), the major inputs to the pallidum. One would therefore predict that these signals providing, as they do, information on both the planning and the execution of gait modifications (references above) will modify the activity of cells in both the entopeduncular nucleus (correlate of the internal division of the primate globus pallidus, GPi) and the globus pallidus (correlate of the external division of the primate globus pallidus, GPe) in the same way as for the control of voluntary reaching movements (DeLong 1971; Georgopoulos et al. 1983; Anderson and Horak 1985; Hamada et al. 1990; Anderson and Turner 1991; Turner et al. 1995; Turner and Anderson 1997, 2005).
However, the signal observed in the pallidum is unlikely to simply reflect the signal observed in the cortical projection areas. There is widespread convergence of cortical inputs onto striatal neurons, including inputs from different cortical areas (Malach and Am 1986; Flaherty and Graybiel 1991, 1993; Rosell and Giménez-Amaya 1999) and further convergence of input from the striatum to the pallidum. It is therefore to be expected that pallidal cells will not simply mimic the pattern of activity observed in the cortical input areas but should reflect an integration of these inputs. In the case of unobstructed locomotion, any such integration of activity is likely to be subtle. However, during voluntary gait modifications, one might expect more complex functions to be expressed. For example, our conceptual model of limb selection during gait modification (Marigold and Drew 2017) suggests that determination of the leading forelimb results from an integration of information concerning the location of an obstacle (resulting from information in the PPC, and perhaps the PMC) together with information about limb state carried in signals from motor (and perhaps somatosensory) cortex. Although such integration might be expected to occur primarily in the striatum, it should also be reflected as the presence of a limb-dependent signal in the pallidum, defining the lead limb. The basal ganglia might also be implicated in determining the timing and the onset of the gait modification in which case one might expect to see sharp changes in the discharge activity of neurons in the pallidum just before and during the time of the gait modifications, especially when the contralateral forelimb is the first to step over the obstacle. Moreover, based on current concepts of the basal ganglia contribution to motor control (Mink 1996; see also Ozaki et al. 2017) and from work on basal ganglia control of saccades (Hikosaka and Wurtz 1983) one would expect most of these changes in the EP to be expressed as decreases of the background activity, thus releasing the thalamic nuclei from inhibition and activating the cortical regions implicated in the execution of the modified limb activity.
To test these hypotheses, we recorded from both the EP and the GPe in cats trained to walk on a treadmill and to step over moving obstacles attached to that treadmill. The results show populations of cells in both nuclei that modify their discharge activity both during unobstructed locomotion and with respect to different events during the gait modification.
Methods
Training
These experiments were performed on four male cats (5 hemispheres) (weight: 4–6 kg), which were first trained to walk steadily on a treadmill at a comfortable pace (0.45 m/s). They were subsequently trained over a period of several months to step over obstacles that were attached to the moving belt and which advanced towards them (Drew 1993; Andujar et al. 2010). Two cats (recordings from 3 hemispheres, BG1-BG3) were trained on a treadmill (TM1) on which the obstacles were spaced 3 m apart, allowing for 12–14 steps between the steps over each obstacle (Andujar et al. 2010). The design of this treadmill allowed the cat to see the obstacle 10–12 steps before it was required to modify its gait to step over it. The obstacles used on this treadmill were cylindrical in shape with one obstacle having a cross-section of 10 cm and the other 6 cm. The other 2 cats (BG5–6) were trained on a shorter treadmill (TM2) on which the obstacles were only visible for 2 step cycles (4 steps) before the step over the obstacle and the cats made only 3 step cycles (six steps) between each obstacle. One obstacle was cylindrical in cross-section (10 cm) and the other rectangular (7.5 cm high and 5 cm wide). In all experiments the position of the obstacles was fixed on the treadmill belts so that the distance between obstacles was invariant. However, cats did not use a constant sequence of steps between obstacles as the lead limb over a given obstacle changed from one limb to the other and back throughout the recording session. Cats BG5-6 were also trained in a reaching task (Schepens et al. 2008; Yakovenko and Drew 2009) that will not be discussed further in this manuscript.
Surgical Procedures
After several months of training, the cats were prepared for the implantation of a recording chamber as well as for the recording of electromyographic (EMG) activity. Cats received an injection of ketamine (11 mg/kg) together with Acepromazine maleate (Atravet, 0.05 mg/kg) and Glycopyrrolate (0.01 mg/kg). An i.v. catheter was installed, and the animals were intubated. General anesthesia was induced and maintained during surgery with 2–3% isofluorane with oxygen. The cats were then placed in a stereotaxic apparatus (David Kopf) with the use of atraumatic ear bars coated with lidocaine ointment. An eye lubricant (Optixcare) was used to prevent drying of the cornea, and the cats were administered buprenorphine (5 μg/kg). All surgical and experimental procedures followed the guidelines of the Canadian Council for the Protection of Animals and were approved by the Institutional Animal Care Committee.
During the surgery, a craniotomy was made in the parietal bone and a stainless-steel plate (external measurements 17 × 25 mm with an internal opening of 12 × 15 mm) was stereotaxically positioned to allow access to the globus pallidus and/or the entopeduncular nucleus. In cats BG1,2,5 and 6 the chamber was positioned over the right side of the brain; in BG3 the chamber from BG2 was subsequently displaced to the left side. A small socket was also attached to the skull to serve as a receptor for a unity-gain FET during the recording session. Pairs of Teflon-insulated, braided stainless-steel wires (Cooner AS 633; Cooner wire, Chatsworth, CA) for EMG recording were implanted in the muscle bellies of selected flexor and extensor muscles in all four limbs as well as in nuchal muscles. These wires were led subcutaneously to a connector cemented to the skull. Dental acrylic was used to finish the head implant and the skin was sutured around this implant. Buprenorphine (5 μg/kg) was administered, and the cat was placed in an incubator to recover. Analgesia (buprenorphine; 5 μg/kg) was continued as necessary postoperatively and appropriate veterinary medical follow-up was provided to the cat until the completion of the experiment.
Protocol
Recordings were made using glass-coated tungsten microelectrodes (impedance, 0.5–1.5 MΩ) held in a custom-made micromanipulator attached to the base plate. This was used to drive the electrode through the intact dura mater and into the pallidum. The micromanipulator could be adjusted in both the anteroposterior and mediolateral planes to allow exploration of the entire chamber, as necessary. Experiments were performed three to five times a week, and each session was comprised of one electrode penetration that lasted 2–3 h. In each session, the cat rested quietly on the treadmill while the microelectrode was advanced slowly to the level of the EP or GPe. The GPe was identified based on its depth and on the presence of a gap between cell recordings from the striatum and the presence of cells with a high-frequency discharge. The EP was identified on the basis of its depth, of a gap following the large action potentials recorded from the ventral parts of the thalamus, the presence of cells with a high discharge frequency and, below the region from which we recorded, the presence of the optic tract, characterized by axonal recordings and increased discharge to a light stimulus. Recording sites were positively identified following the recordings based on histological reconstruction (see below).
All isolated neurons were recorded during a period of locomotion while the cat walked on the treadmill at a speed of 0.45 m/s and stepped over the obstacles attached to that belt. Neuronal activity was generally recorded for 2–5 min to allow us to record cell activity during 10 steps over each obstacle with both the contralateral and ipsilateral limb leading. However, we discontinued recording after only a brief period for cells that clearly showed no relationship between discharge activity and locomotor activity (generally an irregular or intermittent discharge with no evidence of rhythmicity or change in activity during the steps over the obstacle). Following recording of a cell the electrode was advanced to record other neurons. Small electrolytic lesions (25–35 μA) were made in selected penetrations to aid histological reconstruction. Video recordings (60 frames/s) were made for all experiments and synchronized to the EMG and unit data by the implementation of a digital time code on both the video and the data file. Data from EMG recordings were filtered at 100–450 Hz and digitized at 1 Khz. Cell activity was digitized at 50 or 100 KHz for off-line discrimination.
Histology
At the end of the series of experiments, cats were premedicated with ketamine and then deeply anesthetized with an intravenous injection of pentobarbital sodium (Somnotol, 30 mg/kg) before being perfused per cardium with a formaldehyde solution. The brain was removed and sectioned at 40 μm in the transverse (cats BG1-BG3) or sagittal (BG5-6) plane. Sections were stained with cresyl violet. The location of the different electrode penetrations was determined based on the marking lesions performed in selected penetrations during the experiments, together with the depth of the recorded cells. The calculated locations of the recording sites were transposed to standard sections of the brain stem and diencephalon taken from the atlas of Berman (Berman and Jones 1982). Each penetration was associated with the AP section (for the transverse sections) or the appropriate laterality (for the sagittal sections) that was closest to the calculated position of the penetration based on the surrounding structures visible in each section.
To synthesize the location of the cells recorded from each cat, we collapsed the location of these cells onto the horizontal plane. The borders of the EP and GPe were interpolated by plotting onto the horizontal plane the most rostral and caudal extent of each nucleus as determined from the sagittal plots in the atlas of Berman. These borders therefore represent the maximum extent of each nucleus. These nuclei in each cat were then scaled appropriately to fit the standard outlines calculated from the Berman atlas.
Data Analysis
Neurons for which our off-line inspection showed the presence of well isolated action potentials throughout the recording period were selected for further analysis. Neurons were isolated and discriminated using an off-line spike sorter (Plexon, TX). Cells were discriminated using principal components and the resulting cluster examined to ensure that there was no overlap with other clusters. The stability of the discrimination was also validated by ensuring that the pattern of cell discharge, as demonstrated by the rasters that we compiled, was constant throughout the recording period. Generally, only one neuron was isolated from each recording, although occasionally 2 cells could be discriminated at the same time.
We used the video recordings to identify sections of locomotion in which the cat was walking stably. We then used a custom program to identify different parts of the locomotor sequence. These included the step over the obstacle, which was classified according to whether the limb contralateral or ipsilateral to the recording site was the first to step over, together with the preceding 2 (TM2) or 3 (TM1) step cycles before the step over the obstacle. Our previous experiments (Drew 1993; Andujar et al. 2010; Nakajima et al. 2019) have shown that cell activity in the earliest of these step cycles is indistinguishable from that occurring in locomotion with no obstacles attached to the treadmill belt. Indeed, as detailed later, few cells in the pallidum discharged earlier than 1 step cycle (2 steps) before the step over the obstacle. Therefore, step cycle N-3 for TM1 and N-2 for TM2 (with respect to the step over the obstacle) were defined as the control step cycles for the purposes of comparing the activity changes that occurred during the step over the obstacle. In some cases, we also recorded cell data without an obstacle attached to the treadmill belt and in these cases cell activity in those steps was not significantly different from the designated control steps occurring prior to the step over the obstacle.
To analyze activity during unobstructed locomotion, we selected the control cycles identified in the previous process and synchronized cell and EMG activity to the onset of a flexor muscle of the contralateral forelimb, either the cleidobrachialis (ClB) or the brachialis (Br). Both muscles become active almost coincidentally with the onset of the swing phase, and the activity of the ClB continues throughout the swing period (Drew 1993). We converted cell activity in each selected control cycle to its instantaneous frequency (1000/interspike interval in ms) before low pass filtering the trace at 25 Hz. The data were then normalized by interpolating each step cycle into 512 bins (binwidth ~ 2 ms) by using the routine interpft from Matlab (Mathworks). The frequency in each bin was then averaged across the number of trials. EMG data were similarly normalized into 512 bins and low pass filtered at 25 Hz.
To quantify and compare cell discharge across cells during unobstructed locomotion, we applied two series of calculations. First, to determine if a cell was modulated during locomotion, we formed a circular representation of the average discharge activity of the cell (Fig. 1A–C), and we used the Rayleigh test for directionality (Batschelet 1981; Drew and Doucet 1991; Drew 1993) to determine if cell activity was uniform (unmodulated) throughout the step cycle, or not (modulated). We applied a level of P < 0.05 to define modulated cells. For cells that were modulated, we also measured the dispersion of the discharge (indicated by the variable, r). A cell that discharged only in a single phase would have a value of 1.0; one that was completely uniform would have a value of 0.0 (Fig. 1C). Second, as in previous studies (Lavoie and Drew 2002), for cells that were modulated, we determined the phase during which the cell showed increased and decreased activity relative to its period of mean activity (Fig. 1A). In brief, the period in which cell activity surpassed the mean level for more than 20 bins (~40 ms) was classified as a transition (either a peak or trough). Note that some cells showed more than 1 transition during a step cycle. For both modulated and unmodulated cells, the maximum and minimum discharge frequency was determined from the histograms of the averaged activity (Fig. 1). Maximum and minimum values were only measured if a given transition endured for 50 bins (~100 ms).
Figure 1.
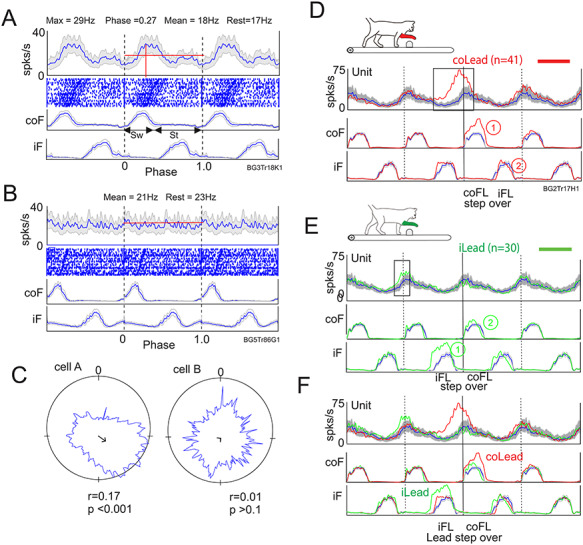
Quantification of cell activity. A, B, example of a modulated (A) and unmodulated (B) cell. For each cell we show a perievent histogram (PEH) of the averaged activity of the cell during unobstructed locomotion, together with a raster display of cell activity and the averaged activity of the contralateral and ipsilateral forelimb flexors (coF, iF). The activity during the cycle is repeated 3 times to emphasize the nature of the rhythmical activity. Data are synchronized to the onset of activity in the coF. The horizontal red line indicates the mean activity during the step cycle (mean), and the red vertical line indicates the maximum discharge frequency (max) and the phase of that activity (phase). Sw and St in A indicate the swing and stance phases, respectively, of the step cycle. Rest indicates the resting rate discharge frequency measured just before locomotion. C: the cell discharge is displayed in a circular format with the onset of the step cycle beginning at 0 and moving clockwise. The arrow gives an indication of the dispersion of the discharge (r) with the maximum value for a cell discharging always in the same place being 1. The value of p indicates the probability that the discharge is directional. D, E: Averaged unit and EMG activity from a different cell when the contralateral (D) or the ipsilateral (E) limb is the first to step over the obstacle. F: contralateral and ipsilateral lead superimposed. Activity in D–F is synchronized to the onset of activity in the coF. Blue traces indicate activity during unobstructed locomotion and the shaded gray area indicates the interval of confidence (P = 0.01) of the standard error (SE) of the mean. Red traces indicate activity when the contralateral limb is the first to step over the obstacle and green traces the activity when the ipsilateral limb leads. coFL and iFL beneath the displays in D, E indicate when the contralateral and ipsilateral limb, respectively passed over the obstacle. “Lead step over” in F indicates when the lead limb passed over the obstacle in the contralateral and ipsilateral lead condition. Rectangles on the cell trace in D, E indicate cell activity during the gait modification that is considered to be significantly different from that in unobstructed locomotion. Numbers within circles on D, E indicate the sequence of activation of the coF and iF.
To analyze cell activity during the steps over the obstacle, we first categorized the data according to whether the forelimb contralateral or ipsilateral to the cell recordings was the first to step over the obstacle. For each condition, we normalized and averaged the cell and EMG activity as described above for the unobstructed data. In producing displays of these data, we typically present the data for the step over the obstacle, together with two step cycles before that step and one step cycle after. The data during the step over the obstacle are superimposed onto the cell and EMG activity during the unobstructed, control cycle, which is repeated four times (see Fig. 1D–F). We considered that changes in activity that differed from the 99% interval of confidence of the SE of the control activity for > 10% of a step cycle (~50 bins or ~100 ms) are both significant and meaningful (Drew 1993). In the example illustrated in Figure 1, the cell showed a large increase in activity preceding the step over the obstacle in the contralateral lead condition (Fig. 1D) and a much smaller change in the preceding step in the ipsilateral limb lead condition (Fig. 1E). These changes from the control level of activity (indicated by the black rectangles in Fig. 1D and E) were quantified both on the basis of their phase and of their magnitude. In the latter case, we integrated the modified activity (sum of each bin) and expressed the magnitude as the difference with respect to the integrated value of the control activity (at the same phase). Peak frequency was measured when it was well defined (as opposed to a plateau or a continual increase or decrease in frequency). Because of the relatively low number of steps recorded with each obstacle, we made no attempt to differentiate differences in activity between obstacles but instead combined the activity from the two.
It should be noted that in both the contralateral and the ipsilateral limb lead condition, we synchronized activity to the onset of activity in the coClB/Br. As such, the sequence of activity in the two forelimbs differs in the two conditions. In the contralateral lead condition, the ipsilateral limb follows the contralateral limb over the obstacle in our displays while in the ipsilateral limb leads condition, the ipsilateral limb precedes the contralateral limb (Fig. 1D–E, respectively). Consequently, when the data are superimposed (Fig. 1F), the step over the obstacle with the lead limb in the ipsilateral lead condition precedes that of the lead limb in the contralateral leads condition. Note that the difference in the sequence of the activity also extends to the hindlimbs. In the contralateral lead condition, the contralateral hindlimb follows the ipsilateral forelimb over the obstacle and precedes the ipsilateral hindlimb (see. Fig. 12). In the ipsilateral lead condition, the sequence is reversed. These gait modifications also involve changes in the timing and duration of the swing and stance phases of locomotion as can be seen by inspection of the EMG traces for the forelimbs in Figure 1 and for the hindlimbs in Figure 12.
Figure 12.
Cells discharging in relation to the hindlimb. A, B: Examples of cells in the EP (A) and the GPe (B) showing their major change in activity during the step over the obstacle by the contralateral hindlimb. Data organized as in Figure 6, with data aligned on the onset of activity in the coClB/Br. Numbers within circles indicate the sequence of activation of the contralateral fore- and hindlimb muscles in the contralateral (illustrated in C) and ipsilateral (illustrated in D) lead conditions. Eding step.
For both the control activity and for steps over the obstacles, we calculated heat maps to illustrate the change of the pattern of activity with respect to the mean activity. For the discharge activity during the unobstructed steps we calculated a Z score with respect to the mean discharge activity. To allow for the differences in the variations of activity in different cells we set a maximum and minimum value of 2 standard deviations (SD). Cells that had variations greater or less than 2SD were adjusted to this level (2SD). For the steps over the obstacle, we subtracted the averaged control activity from the activity during the step over the obstacle prior to calculating the Z score. We therefore illustrate only the changes in activity during the step over the obstacle. As for the control activity, we set a maximum of ±2SD.
Step-Advanced and Step-Related Cells
As in previous publications (Andujar et al. 2010; Nakajima et al. 2019), we identify two categories of cells, step-advanced and step-related. We identify step-advanced cells as those that begin to discharge >0.2 step cycles before the step over the obstacle and which continued to discharge at least until the onset of the flexor muscle activity when the limb stepped over the obstacle (Andujar et al. 2010). The cell in Figure 1D shows such a discharge. Cells were classified as limb-dependent if they maintained a similar relationship to a given limb in both the contralateral and ipsilateral lead conditions. They were classified as limb-independent if they maintained a similar relationship to the lead limb regardless of whether the contralateral or ipsilateral limb was the first to pass over the obstacle. All other changes in activity were defined as step-related, regardless of whether these changes in activity occurred during, or after the step over the obstacle or whether they occurred in the steps preceding the step over the obstacle (as in the example illustrated in Figure 1E).
Terminology
To avoid confusion in terminology and to facilitate comparison with the primate, we refer to the feline globus pallidus as the GPe throughout this report. Further, we refer to the entopeduncular nucleus and the GPe together as the pallidum, as is frequently the case in the primate (e.g., DeLong 1971; Hamada et al. 1990; Turner and Anderson 1997) as well as in the cat literature (e.g., Cheruel et al. 1994).
Results
Neuronal Database
A total of 414 neurons, including 267 cells in the entopeduncular (EP) nucleus and 147 cells in the globus pallidus (GPe) nucleus, were recorded during locomotion from 90 penetrations from four cats (Table 1, 1 cat recorded both sides). 114/414 of these neurons (74 EP and 40 GPe) showed no relationship to either locomotion or to the voluntary gait modification and were not analyzed quantitatively. The remaining 300 neurons (193 EP and 107 GPe) form the basis for our quantitative examination of the discharge characteristics of the GPe and EP cells during unobstructed locomotion. Most of these cells, 279/300 (181 EP and 98 GPe), were also recorded during at least four steps over the obstacles with each of the left and right limbs and form the database for our examination of the contribution of these two regions to the control of visually-guided gait modifications.
Table 1.
Cells recorded in the EP and GPe
| EP(tot) | EP(ana) | EP(mod) | GPe(tot) | GPe(ana) | GPe(mod) | TOTAL | |
|---|---|---|---|---|---|---|---|
| BG1 | 17 | 10 | 1 | 5 | 5 | 2 | 22 |
| BG2 | 41 | 41 | 22 | 41 | |||
| BG3 | 61 | 55 | 25 | 61 | |||
| BG5 | 100 | 74 | 29 | 25 | 10 | 5 | 125 |
| BG6 | 89 | 54 | 40 | 76 | 51 | 36 | 165 |
| Total | 267 (74) | 193 | 95 | 147 (40) | 107 | 65 | 414 |
Note: For each cat, we report the total (tot) number of cells recorded in each nucleus, the number that were quantitatively analyzed (ana) and the number that were modulated (mod). The values in parentheses in the bottom line indicate the number of cells that were recorded but that were not analyzed quantitatively. Note that in one cat, we recorded from both sides: to facilitate the description in the text we refer to these as two separate animals (BG2 and BG3).
Pallidal Cells Are Modulated During Unobstructed Locomotion (Control Task)
Of the 300 cells that were subject to quantitative analysis (see database and Table 1), 95/193 cells (49.2%) in EP and 65/107 cells (60.7%) in GPe, showed a nonuniform pattern of discharge activity during unobstructed locomotion, as indicated by the Rayleigh test for directionality (see Methods). Including those 114 cells that were not quantitatively analyzed because they showed no relationship to the task (see above), 95/267 EP cells (35.6%) and 65/147 GPe cells (44.2%) of the total population of isolated cells showed significant modulation during locomotion. The following sections describe the detailed pattern of activation during the step cycle as well as the relationship of these changes in activity to the resting rate (when data are available).
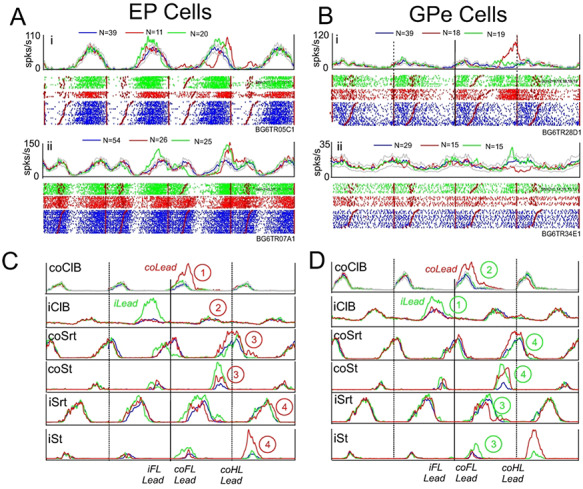
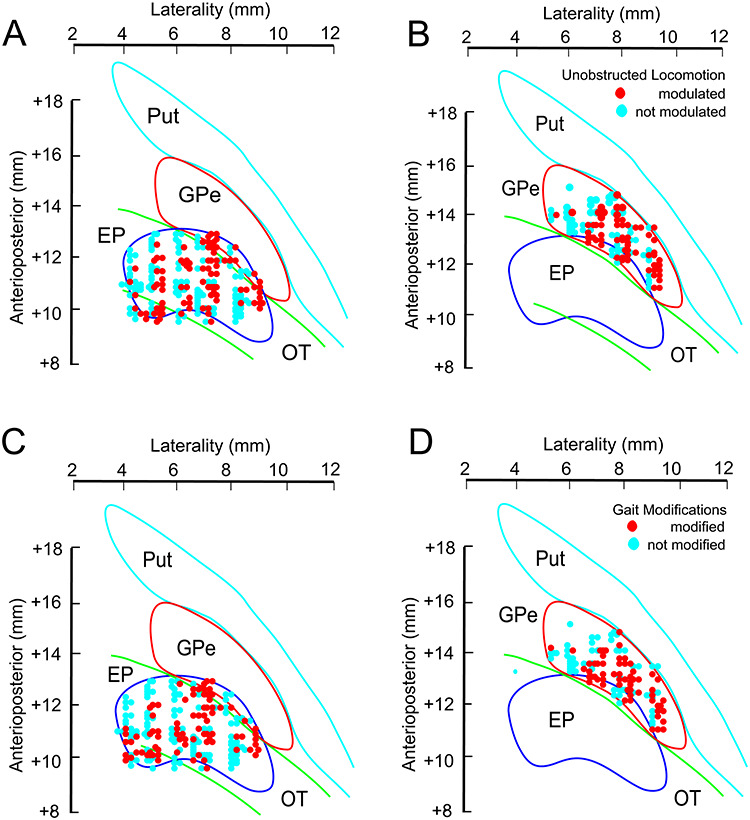
Cells in both the EP and the GPe showed a variety of patterns of activity, including many that were similar in the two nuclei, as indicated by the examples illustrated in Figure 2A and B. Modulated cells included those that showed increased levels of activity (with respect to the mean activity) during the swing phase of the contralateral forelimb, either throughout the period of activity of the coClB (Fig. 2Ai and Bi), or at the end of its period of activity (Fig. 2Aii and Bii); those that showed peak activity primarily in the reciprocal, stance, period of locomotion (Fig. 2Aiii, iv and Biii, iv); and those that showed more than one period of activity (Fig. 2Av, vi and Bv, vi). In many of these cells, activity was tightly linked to the onset and offset of muscle activity as can be observed from the rank-ordered rasters. The increase of activity in the cell in Fig. 2Ai, for example, is tightly related to the duration of the coClB activity while that in Figure 2Aii is phase-locked to the end of the period of activity in the coClB. Similar relationships to the onset and offset of coClB activity (including reciprocal activity) can be observed in Figure 2Aiii–v and Bi-iv. Note that although we use peak discharge to describe the general patterns of activity in these examples, we could as easily use the relative decreases. The former was chosen because, as will be shown later, the principal change during the gait modifications is increased activity superimposed on this background, control activity. Nonetheless, in quantifying these data, we describe both relative increases and relative decreases.
Figure 2.
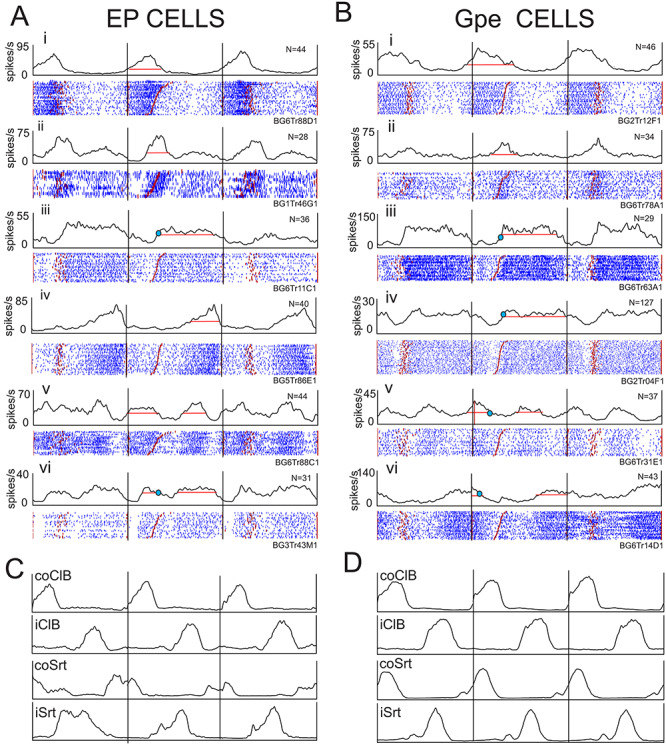
Examples of discharge activity during unobstructed locomotion. A, B: 6 examples (i–vi) of cells recorded from the EP (A) and the GPe (B). For each cell, we illustrate perievent histograms (PEHs) and raster displays triggered on the onset of activity of the contralateral Br/ClB. In each case the central step cycle represents the cycle 2 (TM2) or 3 (TM1) steps before the step over the obstacle, together with the preceding and the following cycle. The black vertical lines delimit the three step cycles. The horizontal red line(s) through the PEH in the central cycle indicates the mean level of activity (see Methods). Cell activity in this and all other figures is rank-ordered according to the duration of the period of ClB/Br activity (red staggered lines in the raster displays). Small blue circles, when present, indicate the discharge frequency of the cell in the absence of locomotion (resting rate: data were not available for all cells). C, D: averaged EMG activity from the coClB and iClB, together with that from the contralateral and ipsilateral sartorius muscles (coSrt and iSrt, hindlimb flexor muscles), recorded simultaneously with the cell illustrated in Aiii for part C and simultaneously with the cell illustrated in Biv for part D.
The population activity in the two nuclei is shown in Figure 3 by means of heat maps (Fig. 3A and B) and phase plots (Fig. 3C, D). These plots include only those modulated cells for which we were able to clearly identify 1 or 2 clear peaks (EP, 86/95 cells; GPe 63/65 cells). Cells that were classified as significantly modulated but which showed no clear pattern of activity were not included. Most modulated cells showed only one period of relative increase and reciprocal decrease of activity. The period of increased discharge (with respect to the mean activity) of the population therefore forms a continuum from the onset of the swing phase in the contralateral forelimb (phase = 0.0) until the time of the next contralateral forelimb onset (phase = 1.0). Altogether, there were 96 periods of significantly modified activity among the 86 EP cells included in the analysis and 73 periods of modified activity among the 63 GPe cells. For reference, the cells illustrated in Figure 2Ai–v and Bi–v are indicated on the phase plots of Figure 3C and D by the red, horizontal lines and accompanying Roman numbers. The distribution of relative increases of discharge in Figure 3C and D may be compared with the distribution of troughs in Supplementary Figure 1. As would be expected, the distribution of troughs equally forms a continuum with discharge being largely reciprocal to that observed in Figure 3.
Figure 3.
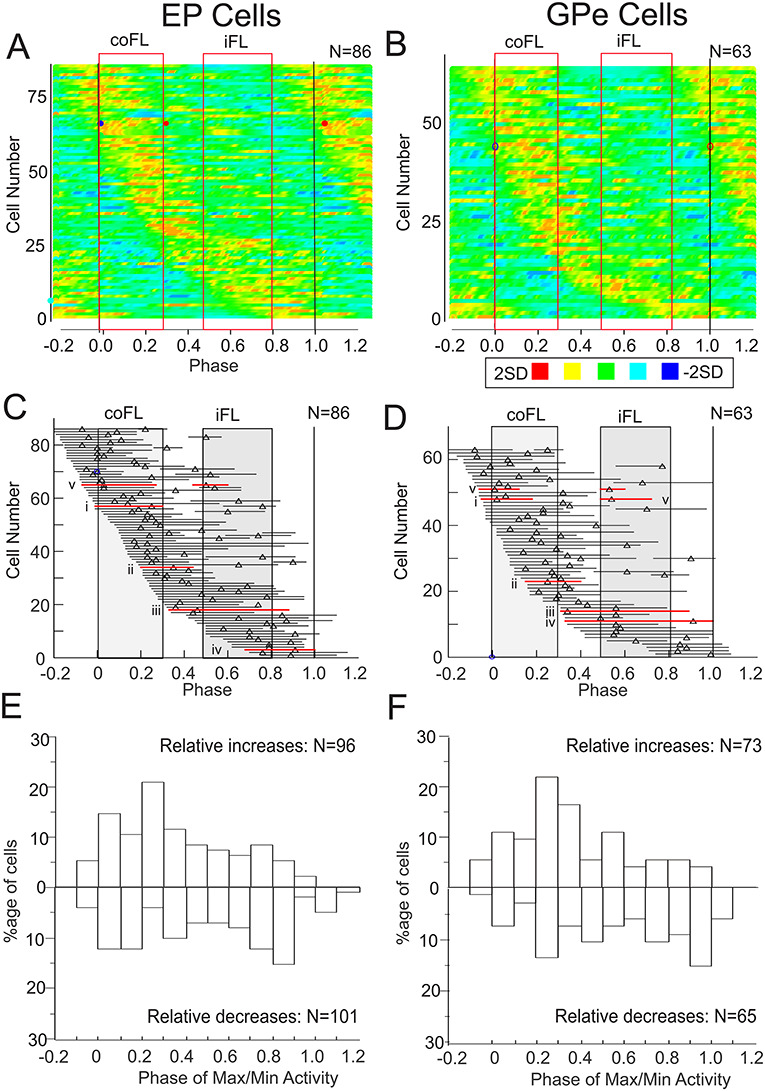
Population activity during unobstructed locomotion. A, B: heat maps compiled from all significantly modulated cells in the EP and GPe for which we could also visually determine clear periods of activity (see Methods). Cell activity is rank-ordered according to the phase of the onset of the period of increased activity (starting at −0.2), as determined from PEHs of the type illustrated in Figure 2. Each horizontal line represents periods of modified activity in one cell and the discharge activity of each cell has been transformed into a Z score (the standard deviation of the discharge in each bin with respect to the mean). Each cell is scaled to maximum and minimum values of ±2.0SD. In bins in which the values exceeded 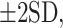 values were manually set to 2. Positive and negative Z scores are divided into 3 equal divisions represented by the colors in the key below Figure 3B, with red indicating maximum peak activity. C, D: phase plots indicating the period(s) of discharge activity exceeding the mean activity of the cell (see Methods and Fig. 2). Cells are rank-ordered as in parts A, B and triangles indicate the phase of the peak activity, when detectable. Red horizontal lines indicate the phase plots for cells i–v illustrated in Figure 2A and B. Red, vertically-oriented, rectangles in A–D indicate the averaged activity of the coClB and the iClB as measured from 22 cells included in the plots. E, F: Histograms showing the distribution of the phase of the maximum (for relative increases) and minimum (for relative decreases) discharge for the 86 cells in A and the 63 cells in B. Note that some cells had more than one peak during the step cycle (see C, D) so that the number of values in the histograms of E, F is greater than the number of cells. Further, maxima and minima were not valid for all cells (plateaus for example) so that the number of maxima and minima is not the same. Binwidth = 10% of the step cycle (phase difference of 0.1). Data in A–F are synchronized with respect to the onset of the coClB/Br (phase = 0.0).
values were manually set to 2. Positive and negative Z scores are divided into 3 equal divisions represented by the colors in the key below Figure 3B, with red indicating maximum peak activity. C, D: phase plots indicating the period(s) of discharge activity exceeding the mean activity of the cell (see Methods and Fig. 2). Cells are rank-ordered as in parts A, B and triangles indicate the phase of the peak activity, when detectable. Red horizontal lines indicate the phase plots for cells i–v illustrated in Figure 2A and B. Red, vertically-oriented, rectangles in A–D indicate the averaged activity of the coClB and the iClB as measured from 22 cells included in the plots. E, F: Histograms showing the distribution of the phase of the maximum (for relative increases) and minimum (for relative decreases) discharge for the 86 cells in A and the 63 cells in B. Note that some cells had more than one peak during the step cycle (see C, D) so that the number of values in the histograms of E, F is greater than the number of cells. Further, maxima and minima were not valid for all cells (plateaus for example) so that the number of maxima and minima is not the same. Binwidth = 10% of the step cycle (phase difference of 0.1). Data in A–F are synchronized with respect to the onset of the coClB/Br (phase = 0.0).
Despite the lack of any clear clustering of the periods of activity, the distribution was not uniform. A substantial proportion of the peaks of the relative increases (47/96, 49% for EP and 33/73, 45% for GPe) occurred at phases between −0.1 to 0.3, encompassing the major period of activity just before and during the swing phase of the contralateral forelimb (Fig. 3E and F, labeled as relative increases). Fewer cells showed their minimum discharge frequency during the same period (EP, 32/96, 33%; GPe, 17/73, 19%). In other words, there was a tendency for more cells to show increased activity during the swing phase and correspondingly to show more periods of decreased activity during stance. Moreover, the duration of the troughs was significantly longer (EP, P = 0.013; GPe, P = 0.002) than those of the peaks, corresponding to the increased preponderance of troughs during the longer stance period.
In cells with two periods of relative increases and in which the first burst occurred during the period of the swing phase of the contralateral forelimb, there was a tendency for the second burst to occur during the period of the ipsilateral forelimb swing (e.g., Fig. 2Av and Bv). Indeed, in 10/14 EP cells and 7/9 GPe cells with two periods of activity, the phase of peak activity of the first fell during the period of activity of the contralateral forelimb swing and the 2nd during the period of the ipsilateral forelimb swing.
Cells in both the EP and the GPe showed a range of discharge frequencies during unobstructed locomotion, ranging from 4 to 120 Hz (median = 37 Hz), in the EP and from 11–140 Hz (median = 36 Hz) in the GPe (Supplementary Figure 2).
While our analysis in Figure 3 shows the relative increase and decrease of activity during the step cycle with respect to the mean rate, ideally, we would like to know how this relates to the resting activity. Unfortunately, this information was available only for a subset of our database. Nonetheless, the data plotted in Figure 4 show that the mean resting discharge activity of cells in both the EP and GPe was significantly and closely related to the mean discharge observed during unobstructed locomotion, as indicated in Figure 1 and in Figure 2 (see red lines and blue circles in Fig. 2). Moreover, the majority of the sites lay close to the line of equivalence, albeit with some that showed larger variation.
Figure 4.
Discharge activity at rest. A: Scatterplots of the mean discharge frequency during unobstructed locomotion (as calculated in Fig. 1) as a function of the mean discharge frequency at rest (during either quiet sitting/lying or standing: data combined). B, C: plots of the maximum (B) and minimum (C) discharge frequency during locomotion as a function of the discharge at rest. Blue symbols = EP; red symbols = GPe. Blue and red lines in A indicate the linear regressions for the EP and GPe. Black diagonal line in A–C indicates the line of equivalence.
More importantly, Figure 4B shows that the maximum discharge frequency during locomotion was greater than the resting discharge frequency in all but 4/61 cells, while Figure 4C shows that the minimum discharge frequency was less than this rest rate in the majority of cells. In other words, as stated above, most cells for which data were available showed both increased and decreased discharge activity with respect to the mean resting rate.
Overall, the results from this analysis demonstrate the presence of a large proportion of cells in both pallidal nuclei that modulate their activity at the rhythm of the step cycle. Moreover, the majority of these neurons, for which data are available, exhibit both increased and decreased activity with respect to the mean resting rate. Increases and decreases in activity were observed throughout the step cycle but with a small tendency for more of the increased periods of activity to occur in the swing phase of locomotion.
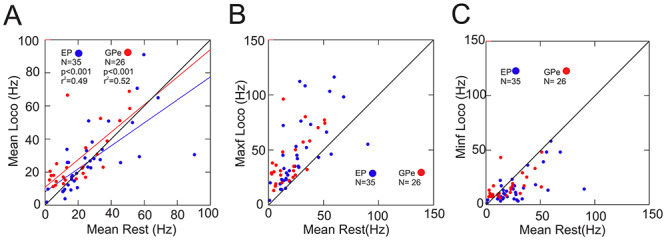
Localization
Cells were recorded throughout the extent of both the EP and the GPe as illustrated by the filled cyan symbols in Figure 5. In general, cells showing modulated activity during unobstructed locomotion were equally recorded throughout the extent of the EP (Fig. 5A) while there was a slight tendency for modulated cells to be located more laterally within the GPe (Fig. 5B). However, when considering only those cells with the strongest relative increases or decreases during the swing phase of the step cycle, there was some evidence of a more limited localization (Supplementary Fig. 3A and B). Strongly modulated cells in the EP were located from the rostral to the caudal border but within the middle of the nucleus in the mediolateral plane. Strongly modulated cells in the GPe tended to be located more laterally and caudally within the nucleus. The location of these strongly modulated cells overlapped closely with the location of those cells for which we identified receptive fields that included the contralateral forelimb (Supplementary Fig. 3C and D).
Figure 5.
Localization of the recordings. A, B: recording sites of all cells included in our database from the EP (A, 267 cells) and the GPe (B, 147 cells) plotted on standardized representations of each nucleus in the horizontal plane (see Methods). Nuclei are positioned within the stereotaxic framework of the atlas of Berman (Berman and Jones 1982). Cells recorded in the same penetration have been slightly displaced (jittered by a maximum of 0.2 mm) one from the other to represent the number of cells recorded, rather than simply the number of tracks. The plots differentiate cells that were significantly modulated during locomotion (red circles) from those that showed no modulation (blue circles). The position of the putamen (Put) and the optic tract (OT) are also indicated. C, D. the location of those cells whose activity was modified during the gait modification (red circles).
Pallidal Neurons Show Strong Changes In Discharge Activity During Gait Modifications
During the voluntary gait modifications, we analyzed data from 181 cells in the EP and 98 cells in the GPe (Table 2). These cells included all of those that showed modulated activity during unobstructed locomotion (95 EP and 65 GPe, Table 1).
Table 2.
Modification of cell activity during gait modifications
| EP cells | GPe cells | |
|---|---|---|
| Recorded with obstacles | 181/193 | 98/107 |
| Modification during gait modification | 88/181 | 62/98 |
| Modification in contralateral lead | 74/88 | 57/62 |
| Modification in ipsilateral lead | 55/88 | 39/62 |
| Modification in both contralateral and ipsilateral lead | 41/88 | 34/62 |
Note: We indicate the number of cells for which activity was modified in the contralateral and ipsilateral lead conditions.
Altogether, 88/181 (49%) cells in the EP and 62/98 cells in the GPe (63%) that were analyzed during voluntary gait modifications showed a change in their discharge activity during either the contralateral and/or the ipsilateral lead condition (Table 2). Most of these cells were also modulated during unobstructed locomotion (63/88 EP and 48/62 GPe, leaving 25/88 EP and 14/62 GPe cells in which discharge activity was modified during the gait modification but that were not modulated during unobstructed locomotion, see Supplementary Fig. 4A and B). Conversely, in both the GPe and the EP there were populations of cells that were modulated during unobstructed locomotion but that showed no modification of that activity during the gait modifications (32/95 cells EP and 17/65 GPe).
As we will detail in the following sections, several aspects of the modified cell discharge are important in considering the pallidal contribution to voluntary gait modifications. First, cells showed both increases and decreases of cell discharge with increases in activity predominating. Second, while some cells maintained a constant relationship to the activity of one limb in both the contralateral and ipsilateral lead conditions, in other cases the discharge varied according to which limb was the first to step over the obstacle. Third, as in the PPC and the PMC, we found cells that were step advanced and others that were step-related.
Contralateral Lead Condition
A substantial proportion of the cells with modified discharge activity, in each nucleus (74/88, 84% EP and 57/62, 92%, GPe), showed at least one significant change in their discharge frequency either before, during or after the step over the obstacle in the contralateral lead condition (Table 2). These included cells that were modulated during unobstructed locomotion as well as those that were not. In addition, other cells showed step by step modulation of their discharge during unobstructed locomotion but showed no additional changes to that activity during the gait modification (Supplementary Fig. 4C and D).
The most common response in both nuclei was for an increase in discharge frequency during the swing phase of the step over the obstacle (30/74, 41%, EP and 22/57, 39%, GPe). Examples of such cells are illustrated by the red traces in Figure 6Ai–iii and Bi, ii and included cells with increased activity restricted to the swing phase as well as those that equally discharged in advance of the swing phase (see below). Other cells showed a decrease in activity during the swing phase of the step over the obstacle in the contralateral lead condition (Fig. 6Aiv and Biii–v). As for the periods of activity during unobstructed locomotion, several of these bursts of activity were time-locked to events in the step cycle; this is particularly evident for the illustrated cells that discharged at the end of the period of activity in the coClB (e.g., Fig. 6Ai and Bi, ii). In some cells there were also multiple periods of modified discharge activity (e.g., Fig. 6Avi and Biii–v).
Figure 6.
Examples of cell discharge during gait modifications. We illustrate six examples of cell discharge (i–vi) recorded from the EP (A) and the GPe (B) during the voluntary gait modifications. For each cell, we illustrate both PEHs and rasters showing cell activity in control steps (blue traces); during steps over the obstacle in which the contralateral forelimb was the first to step over the obstacle (red traces) and trials in which the ipsilateral limb was the first to step over the obstacle (green traces). Shaded region around the control trials indicates the 0.01 interval of confidence of the standard error of the mean. Note that contralateral and ipsilateral lead trials were intermingled randomly with the control steps during the recording session. All data are synchronized to the onset of activity of the coClB/Br in the step over the obstacle by the contralateral forelimb (solid vertical lines). We display 2 step cycles before this step and 1 step cycle after (step cycles delimited by vertical lines). Note that because we always synchronize on the coClB, the step over the obstacle by the ipsilateral forelimb occurs following coClB in the contralateral lead condition but precedes it in the ipsilateral lead condition (see Fig. 1). C, D: averaged EMG activity. N, indicates number of trials for each condition.
The changes in activity for the population of cells in the EP and GPe showing significant changes in the contralateral lead condition are illustrated in the heat maps and phase plots of Figure 7A–D. Cells were first categorized into those showing an initial increase and those showing an initial decrease and then rank-ordered according to the phase of the onset of the activity within each category. As such we have 2 blocks of data in Figure 7A–D, the top block illustrating cells with an initial increase in activity and the lower block illustrating those with an initial decrease in activity. In each illustration we plot only the changes in activity with respect to those observed during unobstructed locomotion.
Figure 7.
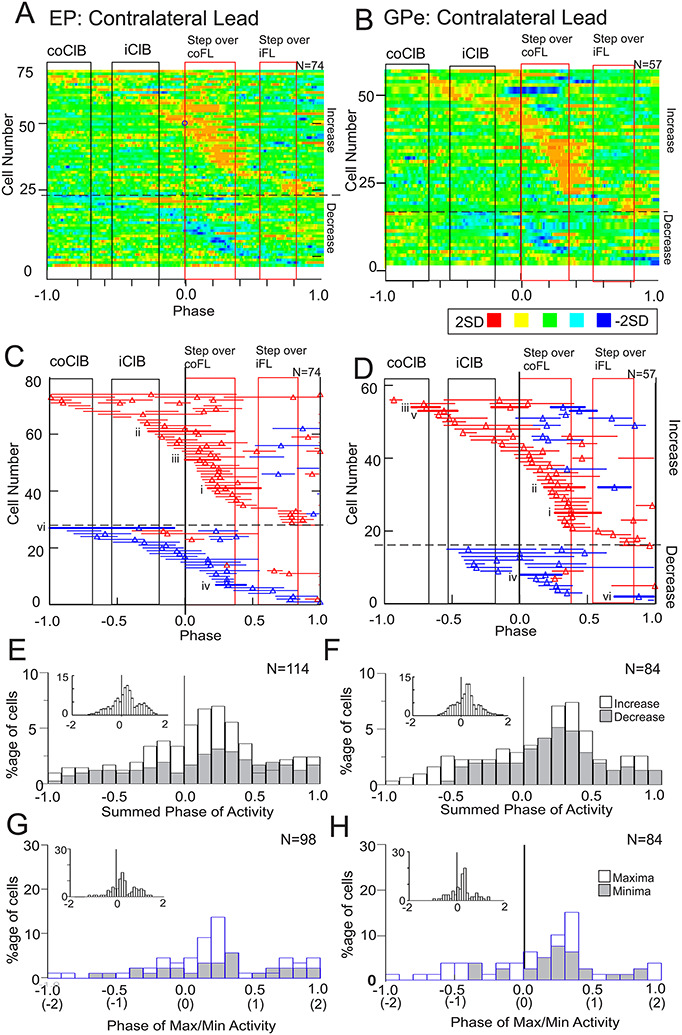
Population activity during the contralateral lead condition. A-B: Heat maps of the change in activity when the cat first steps over the obstacle with the contralateral limb. Data are initially treated by subtracting the control activity (blue traces in Fig. 6) from the discharge activity during the contralateral lead condition (red traces in Fig. 6). Subsequently, we calculate the Z-score and plot the data as in Figure 3. Data are organized so that we plot first all cells in which the initial significant change in activity was an increase (above the horizontal dotted line in A, B) and then subsequently those cells in which the initial change was a decrease (below the line). C, D: Phase plots indicating the periods of significantly modified activity (see Methods). Red horizontal lines indicate periods of significantly increased activity and blue lines indicate periods with a significant decrease. Thicker red or blue lines with associated Roman numerals indicate cells illustrated in Figure 6. Vertical rectangles in A-D indicate the periods of activity of the coClB and iClB in the step before and the step over the obstacle, with the red rectangles indicating the period of activity of the ClB during the step over the obstacle by the contralateral forelimb and ipsilateral forelimb. E, F: Summed changes in activity for the same period (−1.0 to 1.0) as illustrated in A–D. The histogram effectively sums the periods of activity illustrated in C, D in bins equal to 0.1 of the step cycle. Data are shown separately for cells with increased (open bars) and decreased (shaded bars) activity but are shown as a percentage of the total summed activity (increases and decreases combined). G, H: Phase of maxima (increased activity) and minima (decreased activity) of the changes in activity. Note that peaks were not identifiable for all periods of significantly modified activity. A–H indicate activity for 1 step cycle (2steps) before and after the onset of activity in the coClB (phase = 0, solid, black vertical line). Note that on the x-axis for G, H, we also indicate the number of steps before and after the step over the obstacle (in parentheses). Insets in E–H indicate data for all cells (increases and decreases combined) and extended to 2 step cycles before and after the step over the obstacle. N in A–D indicates the number of cells. N in E–F indicates the number of periods of significantly modified activity while N in G, H indicates the number of periods of modified activity for which the phase of peak activity could be defined.
The majority of cells showed increased activity only just before and during the step over the obstacle, as in the examples of Figure 6Ai, ii. Cells showing significant decreases in activity showed a similar pattern. A small population of cells showed their first and only change of activity at the end of the step cycle, corresponding to the time that the hindlimbs stepped over the obstacle (see later). Cells showing more than one period of modified activity frequently showed changes of opposite sign in these periods (e.g., both a period of increased activity and one of decreased activity). Cells with modified activity recorded from the GPe (Fig. 7B) showed similar patterns of activity.
The most sustained changes were observed during the period of activity in the coClB, associated with the swing phase as the cat stepped over the obstacle (represented by the rectangle aligned with phase = 0.0). This propensity for modified activity during the contralateral swing was observed both in cells showing increases and those showing decreases, as can be observed in Figure 7E and F which show the total change in activity (summed activity, see legend) for each population leading up to and following the step over the obstacle. In total, for the 74 EP cells showing modified activity, there were 77 periods of increased discharge and 37 periods of decreased discharge, providing a total of 114 periods of significantly modified activity (31/74 cells showed more than one burst of activity). For the GPe, there were 54 periods of increased discharge and 30 periods of decreased activity for a total of 84 periods of significantly modified activity (24/57 cells showed more than one burst of activity). Therefore, increased activity accounted for 77/114 (68%) of the periods of modified activity for the EP cells and for 54/84 (64%) of the GPe cells. The period with the highest percentage of active cells (both for increases and decreases) occurred at phases 0.0 to 0.5, corresponding to the swing phase of the contralateral forelimb as it stepped over the obstacle. A similar distribution was observed for the maxima and minima (Fig. 7G and H). Few cells discharged earlier than two steps (1 step cycle) before the step over the obstacle (4/114 EP cells and 0/84 GPe cells).
Ipsilateral Lead Condition
Many cells also showed changes in discharge activity in the ipsilateral lead condition (green traces in Fig. 6). As indicated in Table 2, 55/88 EP cells showed increased activity in the ipsilateral lead condition as did 39/62 GPe cells. Some cells were active only during ipsilateral lead and others in both (Table 2 and Supplementary Fig. 4H). Cells showing increases of activity in both the contralateral and ipsilateral lead conditions are illustrated in Figure 6Ai, ii and Bi) while others showing a reciprocal pattern of activity are illustrated in Figure 6Aiii and Biii. Cells showing changes of activity primarily, or only, in the ipsilateral lead condition are illustrated in Figure 6Av, vi and Bv, vi. As in the contralateral lead condition, many of these cells showed modulated activity during unobstructed locomotion (Supplementary Fig. 4E and F).
Changes during the ipsilateral lead condition, when the ipsilateral limb preceded the contralateral limb over the obstacle, were characterized by a relative displacement of the period of maximal activity which now occurred prior to the step over the obstacle by the contralateral forelimb (Fig. 8). Moreover, both the heat maps (Fig. 8A and B) and the phase plots (Fig. 8C and D) show the presence of 2 concentrations of increased activity, one before and during the passage of the lead, ipsilateral, forelimb and one later before and during the subsequent passage of the contralateral forelimb (some cells also discharged during the period of coClB activity in the preceding step). Two periods of activity can also be observed for those cells showing decreased activity with respect to the control activity. However, while many cells in both nuclei were clearly maximally active at the time that the lead, ipsilateral forelimb passed over the obstacle, the histograms of Figure 8E–H show that there was no clear increase in the population activity, or of the time of peak discharge, at this time and that this holds for cells with both increased and decreased activity. Rather, there was a recruitment of activity that built to a peak just before the contralateral forelimb passed over the obstacle and which then declined. As in the contralateral limb lead condition, relatively few cells showed any change in activity earlier that 2 steps before the step over the obstacle (6/78 EP cells and 6/59 GPe cells).
Figure 8.
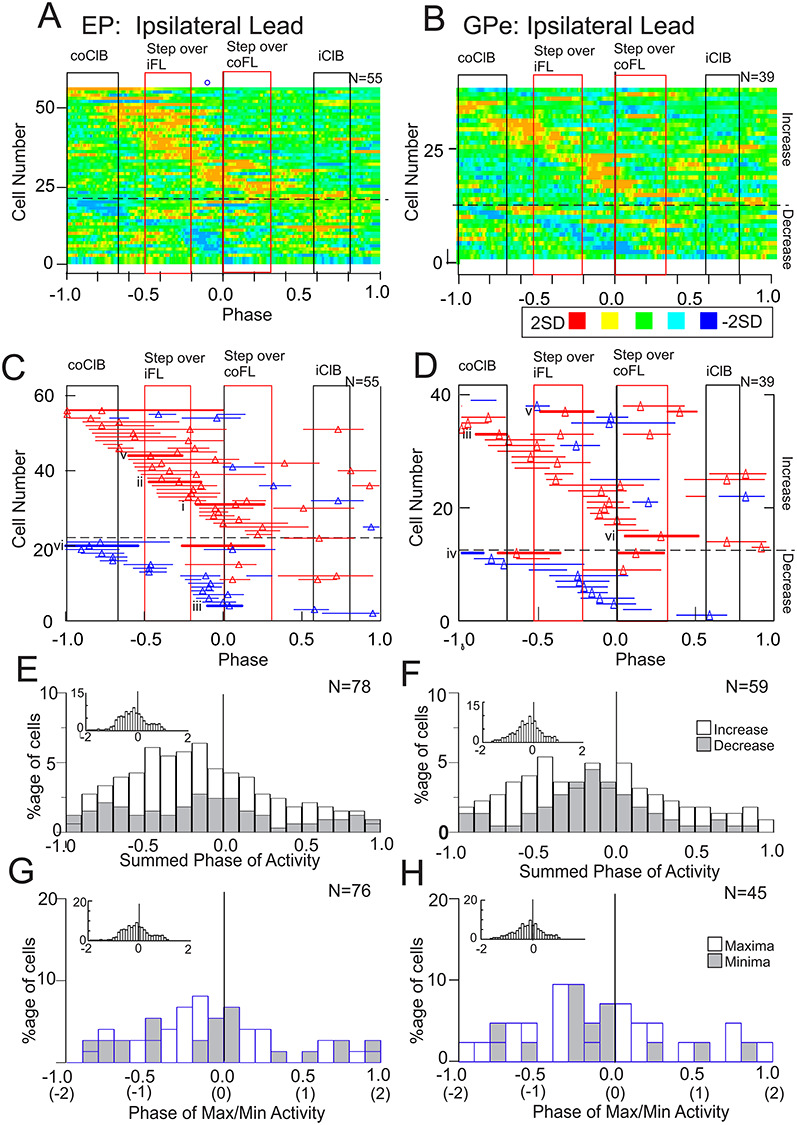
Population activity during the ipsilateral lead condition. A–H: Data are organized as in Figure 7, with the solid black vertical line in A–H at time = 0.0 indicating the onset of the period of activity in the coClB associated with the step over the obstacle by the contralateral forelimb. However, in this condition, the step over the obstacle by the ipsilateral limb precedes this step (see Fig. 1). As in Figure 7, the period of activity of the coClB and the iClB during the step over the obstacle, and in the preceding step cycle, is indicated by the rectangles and bars. Thicker red bars and Roman numerals indicate the activity during the ipsilateral lead condition of the cells illustrated in Figure 6.
Step-Advanced and Step-Related Cells
As in our recent publications examining the contributions of the PPC and the PMC to voluntary gait modifications (Andujar et al. 2010; Nakajima et al. 2019), we make a distinction between cells that showed a prolonged discharge in advance of the step over the obstacle and those cells that discharged more discretely (see Methods for definitions). We propose that cells that discharge in advance of the step over the obstacle can contribute to the planning of the step over the obstacle, while step-related cells are more likely to be primarily involved in the execution of that step.
Step-Advanced Cells
From the total of 88 cells in the EP showing modified activity in either the contralateral or the ipsilateral lead condition, 24/88 (27%) showed step-advanced activity (Table 3). In the GPe, 20/62 (32%) showed step-advanced activity. Three examples of step-advanced cells from each nucleus are illustrated in Figure 9A and B.
Table 3.
Diverse populations of modified cells
| EP cells | GPe cellss | |||||
|---|---|---|---|---|---|---|
| Contra | Ipsi | Contra | Ipsi | |||
| Modified Cells | (74) | (55) | (57) | (39) | ||
| (88) | (62) | |||||
| Step-Advanced Cells | 24 | 20 | ||||
| coLead | 15/24 | 12/20 | ||||
| coLead only | 5/15 | 7/12 | ||||
| iLead | 20/24 | 14/20 | ||||
| iLead only | 9/20 | 7/14 | ||||
| coLead and iLead | 10/24 | 6/20 | ||||
| Discharge >1step before obstacle in coLead | 4/15 | 2/12 | ||||
| Discharge >1step before Obstacle in iLead | 7/20 | 0/14 | ||||
| Step-Related Cells | ||||||
| (Not including step-advanced) | 59/74 | 37/55 | 44/57 | 25/39 | ||
| (Including step-advanced) | (66/74) | (44/55) | (52/57) | (30/39) | ||
| Increases restricted to coClB In coLead | 23/59 (30/74) | 18/44 (22/57) | ||||
| Increases restricted to coClB In iLead | 10/37 (13/55) | 4/25 (7/39) | ||||
| Decreases during coClB In coLead | 5/59 (7/74) | 4/44 (4/57) | ||||
| Decreases during coClB In iLead | 7/37 (8/55) | 1/25 (4/39) | ||||
| Increases during iClB in iLead | 4/37 | 4/25 | ||||
Note: Numbers of step-advanced and step-related cells discharging at different times in relation to the step over the obstacle in the contralateral lead (coLead) and/or the ipsilateral lead (iLead) condition. Numbers of cells for the step-advanced condition are provided with respect to the total number of cells showing modification of their activity during the gait modification. For the step-related cells, we use two denominators. One of these provides values as a function of those cells that showed only (purely) step-related activity. The second value (in parentheses) includes in the denominator those step-advanced cells that also showed step-related activity. For example, of the 74 cells in the EP that showed modified activity in the contralateral lead condition, 15 showed step-advanced activity in this condition, theoretically leading to 59 step-related cells. However, 7/15 step-advanced cells had additional step-related activity leading to a total of 66 EP cells that showed step-related activity. Similar calculations for the other three combinations (GPe, EP, contralateral and ipsilateral lead) underlie the values in parentheses in Table 3 (“including step-advanced”).
Figure 9.
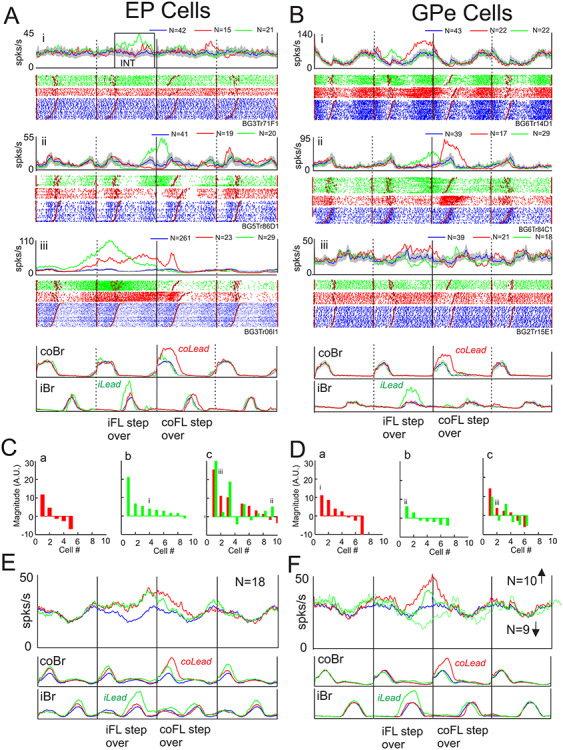
Step-advanced activity. A, B: Examples of step-advanced cells (i-iii) in the EP and GPe. Data are organized as for Figure 6. EMG activity in A was recorded simultaneously with cell Aiii, EMG activity in B was recorded simultaneously with Bi. C, D: magnitude of the change in activity for cells showing step-advanced activity only in the contralateral lead condition (a), only in the ipsilateral lead condition (b), or in both conditions (c). Values indicate the total integrated value of the averaged activity during the burst minus the control activity in the same period (see box in Fig. 9Ai [INT)]). Units are arbitrary but are comparable for each cell. Data are rank-ordered (left to right) according to the magnitude of the burst; in Figure 9Cc and Dc, the values in the contralateral lead condition are used to rank-order the data. Roman numerals indicate the cells illustrated in Figure 9A and B. E, F: population averages of the EP and GPe cells compiled from all cells that showed an increase in activity prior to the step over the obstacle, regardless of condition. In F we additionally display the average of those cells that showed a decrease in activity prior to the step over the obstacle in the ipsilateral lead condition.
A variety of patterns of activity were observed, including cells that were step-advanced only in either the contralateral or ipsilateral lead condition (e.g., Fig. 9Ai and Bii), cells with reciprocal activity in the two conditions (e.g., Fig. 9Aii and Biii) and cells that showed symmetrical changes (e.g., Fig. 9Aiii). Most of the cells in the EP showed increases in activity as can be observed in Figure 9C which illustrates the magnitude and the sign of the responses in all cells identified as step-advanced. Multiple cells showed responses only during contralateral lead (Fig. 9Ca), only during ipsilateral lead (Fig. 9Cb) or in both conditions (Fig. 9Cc). The phase of the activity of these cells is equally shown in Supplementary Figure 5, which shows that the discharge activity in most cells finished either just before, or during, the step over the obstacle with the contralateral forelimb. Moreover, this was equally true for most cells that showed step-advanced activity in the ipsilateral lead condition. Only two cells (dotted horizontal lines in Supplementary Fig. 5A) showed step-advanced activity related to the ipsilateral, lead limb.
Cells in the GPe had qualitatively similar effects, although with a slightly higher proportion of cells that showed decreases in activity than in the EP (Fig. 9D). As in the EP, the step-advanced activity in the GPe was almost exclusively related to the onset of the step over the obstacle by the contralateral forelimb (Supplementary Fig. 5B).
Only 1 cell discharged earlier than I step cycle (2 steps) before the step over the obstacle in the EP but 7 cells showed activity that began > 1 step before the step over the obstacle (Supplementary Fig. 5A). In the GPe, no cells began earlier than 1 step cycle before the step over the obstacle and only 2 cells discharged > 1 step before the step over the obstacle (Supplementary Fig. 5B).
This difference in the time of discharge of the cells in the EP and GPe can also be observed in the population averages of Figure 9E and F. In the EP (Fig. 9E), we illustrate the population average of the 18 EP cells that showed increased activity during either the contralateral or ipsilateral lead condition. Activity in both conditions deviated from control levels 1 step cycle before the step over the obstacle and continued in unison until the onset of the step over the obstacle. At that time discharge in the contralateral lead condition continued later than that in the ipsilateral lead condition, perhaps related to the difference in the duration of the swing phase as indicated by the duration of the period of activity in the coBr. In the GPe, because of the relatively large proportion of cells that showed decreases in activity, we averaged separately cells showing increased activity and those showing decreased activity. In both populations, the change in activity began later than in the EP (Fig. 9F). In the contralateral lead condition, cells with increased activity predominated while in the ipsilateral lead condition the major change was a decrease in activity.
Step-Related Cells
All other cells in which discharge activity was significantly modified during the gait modification were defined as step-related. This included cells that discharged only just before (<0.2 step cycle) or during the step over the obstacle as well as those cells that discharged discretely at different times before, or after, that step. As such, step-related and step-advanced cells are not necessarily exclusive as a cell defined as step-advanced might show an additional, brief, step-related discharge either before or after the step advanced activity (see legend for Table 3).
The most frequent response that we observed in the step-related cells was an increase in discharge frequency when the contralateral forelimb was the lead limb (e.g., Fig. 6Ai–iii and Bi, ii, See Table 3). Changes in activity restricted to the period just before (<0.2 step cycles) or during the period of activity of the coClB were observed in 23/59 purely step-related cells in the EP in this condition. Averages of the activity of this population are illustrated in Fig. 10A. These averages show that despite the clear increase in activity during the step over the obstacle by the contralateral forelimb in the contralateral lead condition (red traces), there was very little change in activity in the ipsilateral lead condition (green traces), either during the step over the obstacle by the contralateral forelimb or during the preceding step over the obstacle by the ipsilateral forelimb. In addition, in neither condition was there any noticeable change in activity following the swing phase of the contralateral forelimb that might be attributed to a contribution to the subsequent passage of the hindlimbs over the obstacle. In other words, this population appears to be almost exclusively active during the swing phase of the contralateral forelimb when it is the first to step over the obstacle.
Figure 10.
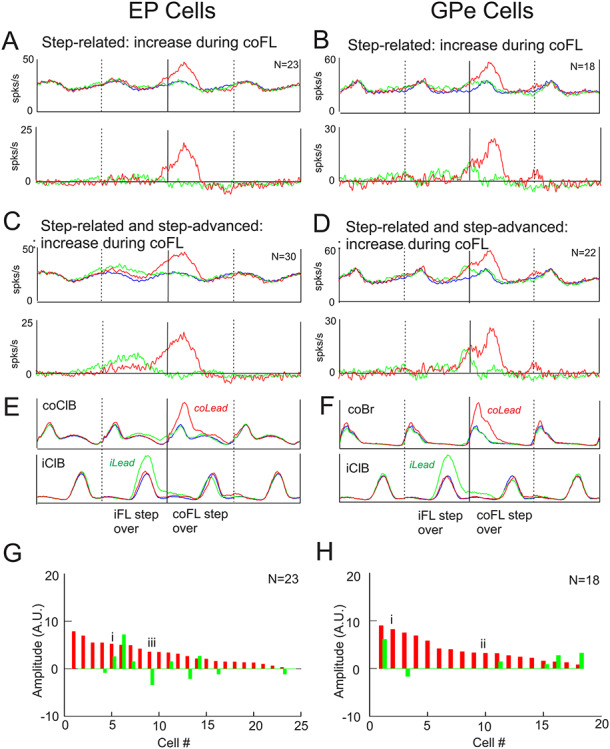
Example step-related population activity averages. A, B. population averages of step-related cells in the EP (A) and the GPe (B) that showed activity changes beginning just before (<0.2 step cycles) or during the step over the obstacle, regardless of any other changes in activity. Data are displayed superimposed on the control activity in the top row and after subtraction of the control activity (before averaging) in the bottom row. C, D: similar plots, but now including selected step-advanced cells (see text). E, F: averaged EMG activity taken from the averages of A, B. The color of the traces follows the conventions of Figure 6. N indicates the number of cells included in the averages. G, H: magnitude of the changes in activity of the step-related cells. The roman numerals identify cells illustrated in Figure 6. The magnitude of the responses is scaled identically to those of the step-advanced cells of Figure 9.
Expanding the population to include 7 step-advanced cells in the EP that continued to discharge during the passage of the contralateral forelimb over the obstacle had little effect on the averaged activity in the contralateral lead condition beyond advancing the time that the average deviated from the control activity (Fig. 10C). However, this expanded population (30/74 cells) now showed a clear period of activity in the ipsilateral lead condition that began prior to and during the passage of the ipsilateral forelimb over the obstacle, but that continued until the step over the obstacle by the contralateral limb (Fig. 10C, see Fig. 9E). A similar change in population activity was observed in the cells recorded from the GPe (Fig. 10B and D), with 18/44 purely step-related cells showing changes in activity restricted to the step over the obstacle by the contralateral forelimb (Fig. 10B) in the contralateral lead condition. Including the 4 step-advanced cells whose activity continued into the step over the obstacle, produced little change in the population activity and changes related to the ipsilateral forelimb were not expressed at a population level (Fig. 10D).
Figure 10G and H show the integrated magnitude of the responses in the individual step-related neurons used to make the averages of Figure 10A and B. It shows that the magnitude of the changes occurred on a continuum, with the cells illustrated in Figure 6Ai, iii and Bi, ii having responses that were at the larger and intermediate end of the spectrum. In agreement with the lack of response in the population averages during the ipsilateral lead condition (green traces), only 5 of the EP cells showed increased activity in this condition, with a further 5 cells showing a small decrease in activity. In only 1 EP cell was there a large response during the ipsilateral lead. Similarly, in the GPe only 5 cells showed an increase in activity during the ipsilateral lead with a further one showing a decrease. Lastly, the figure illustrates that the magnitude of the responses in the EP and GPe were comparable, but that all responses were smaller than those observed in the step-advanced cells (Fig. 9E and F), as would be excepted given the longer duration of the changes in activity in the step-advanced cells.
The averaged traces in Figure 10A and B show that the population discharge activity of the cells during unobstructed locomotion (blue traces) was modulated, with the maximum discharge activity occurring during the swing phase of the step over the obstacle; that is, in the same phase as the major changes in modified activity. Inspection of the discharge activity of the individual cells making up this population showed that 20/23 of the EP cells in this population showed relative increases of discharge during the swing phase of the contralateral limb during unobstructed locomotion. In the GPe, all 18 cells in the average discharged during the swing phase of the contralateral forelimb during unobstructed locomotion. In this major subpopulation of cells, it therefore appears as if the changes during the step over the obstacle represent, at least in general terms, an increase of their preferential period of activity in unobstructed locomotion.
Although increased activity during the step over the obstacle with the contralateral limb leading was the most frequently observed response, we also recorded some cells that showed decreased activity at this time (see Table 3), as would be expected on the basis of current models of basal ganglia function (see Introduction). In addition, we also found cells related to the ipsilateral limb, and others that discharged reciprocally in the contralateral and ipsilateral lead conditions, speaking to the complexity of the pallidal contribution to these gait modifications. Examples of these diverse patterns of activity are provided in Figure 11.
Figure 11.
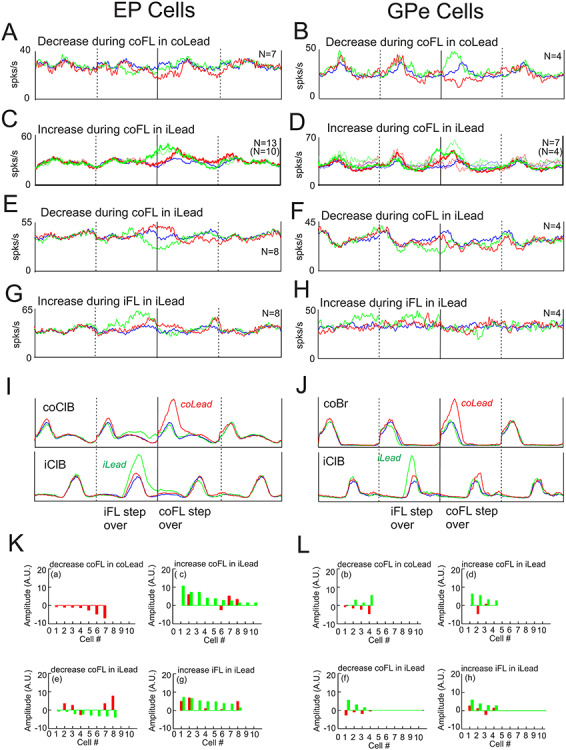
Additional step-related population averages. A–H: population averages created from cells that showed different patterns of activity. Convention as in Figure 10. I, J: Averaged EMG activity taken from the averages in A, B. K, L: magnitude of the responses of the changes in activity of the individual cells included in the population averages of A–H (indicated by a-h). Magnitudes are rank-ordered according to locomotor condition and are comparable with those in Figures 9 and 10.
First, while a relatively large number of cells increased their activity when the contralateral limb stepped over the obstacle (Fig. 10), substantially fewer showed decreases in activity, and these decreases were relatively weak (Fig. 11A, B, Ka and Lb). In the EP, the decrease in activity during the step over the obstacle by the contralateral forelimb (Fig. 11A) occurred without any accompanying changes during the ipsilateral lead in all 7 cells. In contrast, in the GPe, increases in activity during the ipsilateral lead were observed in 3/4 cells used in the average of Figure 11B (see Figs 6Biii and 11Lb), leading to the reciprocal pattern of activity in the average.
There were also a number of cells that modified their discharge with respect to the contralateral forelimb during the ipsilateral lead condition; these included both increases (Fig. 11C and D) and decreases (Fig. 11E and F) of activity. Cells that showed increases in activity in the ipsilateral lead condition in both the EP and GPe generally also showed increased activity during the contralateral lead condition. No differences were observed if including only cells that were step-related (thinner traces) or if also including cells that were step-advanced. Cells in the EP that showed decreased activity in the ipsilateral lead condition (Fig. 11E) showed reciprocal activity in the contralateral lead condition. No such reciprocal changes were observed in the GPe, although it must be noted that the number of cells in these populations is low. Last, some cells showed their major increase during the step over the obstacle by the ipsilateral forelimb in the ipsilateral lead condition (Fig. 11G and H). Some of these cells in the EP also showed increases during the contralateral lead condition, but this time during the step over the obstacle with the contralateral forelimb.
Taking together the data illustrated in Figures 10 and 11A–F, 43 EP cells showed increased activity during the step over the obstacle by the contralateral forelimb (including contralateral and ipsilateral lead) while only 15 showed decreased activity. The corresponding numbers for the GPe were 29 showing an increase and 8 showing a decrease in activity.
The magnitude of the changes in activity of the individual cells making up the population averages is illustrated in Figure 11K and L. These bar graphs plot the responses for each cell in both the contralateral and ipsilateral lead condition allowing insight into the pattern of activity in each of the cells included in the averages. For example, in the EP, none of the cells that were averaged together based on the criterion that they all showed a decrease in activity during the period of activity of the coClB in the contralateral lead condition (Fig. 11A), showed any changes of activity at all in the ipsilateral lead condition (Fig. 11Ka). Similarly, few of the EP cells showing increased activity during the period of activity of the coClB in the ipsilateral lead condition were activity during contralateral lead (Fig. 11Kc). Although numbers are small, it is noticeable that only 13/33 (39%) of the cells included in the population averages of Figure 11A, C, E, G showed changes in activity in both the contralateral and ipsilateral lead condition while 12/16 (75%) of the GPe cells showed such changes.
Inspection of the localization of all of those cells that modified their activity during the step over the obstacle did not reveal any evidence for selective localization (Fig. 5C and D), although as for the modulated cells (Fig. 5A and B), there was a tendency for the modified cells in the GPe to be located slightly more laterally within the total population. Restricting the analysis only to those cells that showed changes in activity during the step over the obstacle with the contralateral limb leading showed a more restricted localization, corresponding to the area from which cells with a receptive field that included the forelimb were localized (Supplementary Fig. 3C–F).
Cells Related to the Hindlimbs
In addition to cells that discharged during the passage of the forelimbs over the obstacle, we also recorded other cells that changed their activity solely or primarily during the passage of the hindlimbs over the obstacle. In total, 23/74 cells in the EP showed some change of activity just before or during the step over the obstacle by the contralateral hindlimb, while 12/57 cells in the GPe showed a similar pattern. In the EP, the most common pattern that was observed (14/23 cells), was a change in activity during the step over the obstacle by the hindlimbs together with a preceding change of activity either before or during the step over the obstacle by the contralateral forelimb. Two examples are illustrated in Figure 12Ai, ii. In both the contralateral and ipsilateral lead condition, discharge was increased prior to the step over the obstacle by the hindlimbs (label 3 in Fig. 12C). However, in both examples, there was also a small increase in activity prior to the step over the obstacle by the lead, contralateral forelimb. Whether this discharge is related to regulating activity in the fore- or hindlimbs is not clear from the current experiments. An additional 4/23 EP cells fired only in relation to the passage of the hindlimbs and 5/23 discharged between the passage of the forelimbs and the hindlimbs.
An example of a GPe cell discharging only to the passage of the contralateral hindlimb is shown in Figure 12Bi. This was one of 4/12 GPe cells that showed such a pattern. In this example, there was equally an increase in activity during the ipsilateral lead condition. In contrast, in the example illustrated in Figure 12Bii, which also discharged only during passage of the contralateral hindlimb, there was a decrease in activity in the contralateral lead condition and a reciprocal increase in the ipsilateral lead condition. A further 4/12 cells discharged to forelimbs and hindlimbs, as in Figure 12Aii; the other 4/212 cells discharged between the passage of the forelimbs and the hindlimbs. Thus, as for the cells discharging in relationship to the passage of the forelimbs, there was a varied pattern of activity.
Discussion
In the present study, we highlight the contribution of the pallidum to the control of locomotion. A large proportion of cells in both the EP (35%) and the GPe (44%) was strongly modulated at the frequency of the locomotor rhythm and, importantly, showed strong changes in activity when the cats modified their gait to step over obstacles in their path (EP, 49% and the GPe, 63%). The results further demonstrate that these changes in activity were tightly linked to changes in behavior required to perform this task.
A Pallidal Contribution to the Execution of Gait Modifications
During the gait modification the cat steps over the obstacle with each limb in turn. This task requirement allows us to readily determine whether a cell discharges only when a particular limb steps over an obstacle, whether it discharges in swing or stance, and whether its activity is dependent on whether that limb is the first or second to pass over the obstacle.
On this basis, our data support a preferential contribution of both EP and GPe step-related cells to the control of the swing phase of the contralateral forelimb, when it is the first limb to pass over the obstacle (Fig. 10 and Table 3). Cells in both the EP and the GPe showed both decreases and increases in their discharge activity during the gait modifications. Of those cells that showed changes in activity when the contralateral limb stepped over the obstacle, the change in the majority was one of increased discharge. Indeed, of 58 EP cells showing changes during the step over the obstacle with the contralateral forelimb, 43/58 (74%) showed increases in activity as did 29/37 (78%) of GPe cells. This relationship with activity related to the contralateral limb is in agreement with studies in primates and cats showing large changes in pallidal activity during reaching and grasping tasks, and equally the presence of a strong population of cells showing increased, rather than decreased, activity during such tasks (DeLong 1971; Georgopoulos et al. 1983; Hamada et al. 1990; Nambu et al. 1990; Mink and Thach 1991b; Cheruel et al. 1994; Turner et al. 1995; Dormont et al. 1997; Turner and Anderson 1997).
As illustrated in Figure 10, in many cases, this relationship with the swing phase of the contralateral forelimb was observed only when the contralateral forelimb was the lead limb led while in others it was observed only when the contralateral forelimb trailed. In only a relatively small number of cells did individual cells discharge with respect to the contralateral forelimb in both the contralateral lead and ipsilateral lead conditions. This is different from the situation in the motor cortex in which many cells (81% in Drew 1993) that increased their discharge activity when the contralateral forelimb leads also showed changes in activity when that limb is the second to pass over the obstacle (trails). This suggests a context-dependent discharge that might be related to the selection of the forelimb to pass over the obstacle (see also below). Moreover, although changes in discharge activity related to the contralateral limb were the most frequent relationship that we observed, we also found some cells related to the passage of the ipsilateral limb (primarily in the ipsilateral lead condition). This would also be in agreement with experiments in primates showing discharge related to movements of either arm, although with a strong bias for the contralateral limb (DeLong 1971; Iansek and Porter 1980). Other cells were related to the passage of the hindlimbs but these were less frequently observed than cells related to the forelimbs (see also Delong et al. 1985). Moreover, it is pertinent that some cells showed multiple periods of modified activity and that some cells with modified activity as the hindlimb passed over the obstacle also modified their activity when the preceding forelimb passed over the obstacle. This relationship to multiple limbs leads to speculation that some cells might be contributing to selecting or defining the sequence and temporal coordination of activity in the limbs. In this respect, it is pertinent to note that striatal lesions in in the rat lead to an inability to correctly place the contralateral forelimb (and hindlimb) when stepping over an obstacle (Perrot et al. 2009), a deficit that the authors link to a problem in limb selection.
A pallidal Contribution to the Planning of Gait Modifications
In addition to the step-related activity, we also recorded cells discharging prior to the step over the obstacle (step-advanced cells) in both the EP and the GPe. The presence of such cells has also been described by us in different cortical structures during an identical task (Andujar et al. 2010; Marigold and Drew 2017; Nakajima et al. 2019) and has been attributed to a contribution to the planning of the upcoming step over the obstacle. Such precocious activity has also been observed in primates in both the pallidum (Nambu et al. 1990; Jaeger et al. 1993; Arimura et al. 2013) and the striatum (Schultz and Romo 1992; Jaeger et al. 1993) in the preparatory period preceding a reach and speaks to a contribution to the planning of the movement. However, the step-advanced activity that we observed in the pallidum differed in several ways from that we observed in the cortical structures. In particular, step-advanced activity in the pallidum rarely began more than 1 step before the step over the obstacle whereas in the PPC (Marigold and Drew 2017), and especially in the PMC (Nakajima et al. 2019), it frequently started 2–4 steps prior to the step over the obstacle. This suggests that the contribution of the pallidum occurs relatively late in the planning processes involved in the gait modification. A similar conclusion has also been reached from studies in the primate (Nambu et al. 1990; Arimura et al. 2013; Thura and Cisek 2017).
It is also pertinent that in all except a few step-advanced cells in the pallidum, the discharge was limb-dependent, occurring almost exclusively with respect to the contralateral forelimb step over the obstacle, regardless of which limb led. In contrast, step-advanced activity in the PPC and the PMC occurred nearly always during both contralateral and ipsilateral lead conditions and was predominantly limb-independent, occurring with respect to the lead limb. However, some cells in the PMC did discharge asymmetrically in the contralateral and ipsilateral condition and were biased towards the contralateral limb (Nakajima et al. 2019). We suggested that such cells might play a role in limb selection. It is possible that the step-advanced cells in the pallidum are also involved in limb selection, although probably at a later stage in the processing as argued in the preceding paragraph. Moreover, the presence of a majority of cells only showing step-advanced activity related to the contralateral limb suggests a contribution to implementing a decision made elsewhere rather than a direct contribution to the limb selection itself.
A Contribution to Unobstructed Locomotion
During unobstructed locomotion, the sequence of activity in the limbs is largely under spinal and brainstem control (Grillner 1981; Armstrong 1986; Rossignol 1996). However, as shown previously for the motor cortex (Palmer et al. 1985; Armstrong and Drew 1984; Beloozerova and Sirota 1988), our results show the presence of neural activity in the pallidum related to limb movement during unobstructed locomotion as well as during the gait modifications. As such, this activity might contribute to the regulation of limb movement even during this stereotypical behavior. This would be similar to the situation in the lamprey in which output neurons in the pallidum act through projections to the brainstem to modulate the rhythmical act of swimming (Ménard et al. 2007; Stephenson-Jones et al. 2011; Grillner and Robertson 2015). During the gait modifications, in which the relative timing of the activity in the four limbs is modified, we suggested above that some cells in the pallidum may contribute to the regulation of the modified sequential timing. Given that several cells show activity both during the swing phase of the contralateral and the ipsilateral limb (Fig. 3), it is possible that the discharge patterns during unobstructed locomotion could equally contribute to regulate the timing and structure of the step cycle, perhaps via the pallidal projections to the pedunculopontine nucleus (see below). The presence of both relative increases and decreases of activity with respect to the resting discharge frequency would further suggest a contribution to both the facilitation and suppression of activity at different phases of the step cycle.
Segregate Populations for the Control of Unobstructed Locomotion and Gait Modifications
The major output from the EP (or GPi in primates) in the cat is primarily directed towards the thalamus (Filion and Harnois 1978) and forms part of a cortico-thalamo-cortical motor loop (Alexander et al. 1986). As such, it is likely that the discharge patterns of the cells that we recorded in the EP will act to influence cortical activity during the task. However, some of these cells also send collaterals to the pedunculopontine nucleus (PPN) and might equally act to modify details of the timing of the rhythmical activity. In this respect, we note that in addition to the large population of modified cells there was also a population of cells that were modulated during locomotion but that showed no change during the gait modifications (Supplementary Fig. 4). Possibly, cells active only in unobstructed locomotion might also project to the thalamus and might be activated in different contexts or locomotor tasks. However, it is also possible that such modulated cells might act primarily not through the thalamus but via the projection to the PPN (Filion and Harnois 1978; Garcia-Rill 1986; Parent et al. 2001; Caggiano et al. 2018), which has been suggested to be especially important for the initiation and control of locomotion and posture (Takakusaki et al. 2003; Roseberry et al. 2016). Indeed, recordings from cells in the pontomedullary reticular formation during the same task have equally shown multiple periods of modulated activity related to different limbs (Prentice and Drew 2001). It is possible that the pallidal output from the basal ganglia contributes to the coordination of this pattern, which we have suggested is related to regulating postural activity in the supporting limbs as the active limb steps over the obstacle.
It should also be considered, however, that the thalamus and the PPN are not the only target of cells in the EP. There are also projections to the habenula, although in the cat this projection seems to be weak and concentrated in the rostromedial region of the EP (Filion and Harnois 1978), from which we recorded few modulated cells.
Implications for the Control of Movement and Locomotion
One of the prevailing theories of basal ganglia function is that an inhibition of discharge activity in a small number of neurons in the GPi releases the focal movement required while an increase in activity in other neurons inhibits competing movement and/or postures (Mink 1996). It was our expectation (see Introduction), that in agreement with this theory, we would observe cells with clear decreases of activity related to the changes in muscle activity that are required during the step over the obstacle. Indeed, such cells were present in the EP and formed 26% of those cells discharging during the swing phase of the contralateral limb during the step over the obstacle. These cells are likely to lead to a disinhibition of thalamic cells and facilitate movement. However, as shown in the population averages of Figure 11, this contribution was relatively weak.
In contrast, the other 74% of EP cells showed increases in activity that were sometimes strong and tightly related to changes in EMG activity. Such a predominance of increased activity would be compatible with the proposed role for the GPi in inhibiting competing movement and postural activity, as proposed by Mink and Thach (Mink and Thach 1991b, 1991c). However, it is not clear whether the same mechanisms are required during locomotion in which the gait modification is superimposed on a background of rhythmical activity. Both biomechanical (Lavoie et al. 1995) and neurophysiological studies (Prentice and Drew 2001) suggest that the postural adjustments accompanying gait modifications involve compensatory increases in extensor muscle activity in the supporting limbs. Moreover, these postural responses are mostly enhanced when the limb steps over the obstacle rather than being suppressed. In addition, many of the neurons showing increased activity during the step over the obstacle became active in the latter part of the step rather than at its onset. This can be observed in the individual examples of Figure 6Ai and Bi, ii as well as in the phase plots of Figure 7C and D. Similar, relatively late increases in GPi activity have been reported during reaching movements (see e.g., Turner and Anderson 1997; Turner and Desmurget 2010).
An alternative possibility to that proposed by Mink is that the increased discharge is modulating the activity of the thalamic neurons responsible for the activation of the muscle groups with which the discharge is so tightly related, rather than inhibiting them and suppressing movement. Such a suggestion is compatible with the finding that blockade of GPi activity with muscimol does not abolish movement-related thalamic activity (Inase et al. 1996). More direct evidence has come from recent experiments by Turner (Schwab et al. BioRxiv) who has shown that simultaneously recorded populations of cells in the GPi and in the ventrolateral anterior nucleus of the thalamus (to which GPi projects) both overwhelmingly increase their activity during a reaching task. Such a discharge pattern is not compatible with the disinhibition hypothesis but is compatible with a role in modulating activity in the thalamus. Compelling evidence for this view also comes from experiments on the songbird where it has been explicitly demonstrated that GPi cells that project to a given thalamic cell increase their discharge activity in parallel with that thalamic cell (Person and Perkel 2007; Goldberg et al. 2013).
The other major pertinent finding is that, on a population basis, cells in the GPe discharge in a very qualitatively similar manner to those in the EP (GPi). This is particularly clear for those cells that increase their discharge during the step over the obstacle with the contralateral forelimb. Similarity in the discharge patterns has been observed in several studies (e.g., Mink and Thach 1991a, 1991b; Cui et al. 2013). Possibly, the discharge activity in the GPe is sculpting the activity of cells in the GPi (Mink 1996), either directly or via its connections through the subthalamic nucleus, in the same way that activity in the GPi may sculpt the activity of the thalamocortical cells. However, one must also take into consideration that in addition to the cells in the GPe that target downstream targets, such as the STN and the EP, there are other, arkypallidal, neurons that target the striatum (Mallet et al. 2012; Gittis et al. 2014). It is entirely possible that some of the cells that we recorded may include this class of cell and influence activity in their major target nucleus, the striatum.
Conclusions
Overall, the results show that the discharge patterns of neurons in the pallidum are well related to the step by step changes in muscle activity during unobstructed locomotion as well as to the changes in muscle activity that occur during visually-guided gait modifications. Such a contribution to the regulation of a basic motor activity is to be expected on the basis of evolutionary considerations in that in many vertebrates the basal ganglia is the higher level controller in the absence of the neocortex (Grillner and Robertson 2015, 2016). It is also compatible with the major locomotor deficits observed in patients with Parkinson’s disease (Knutsson 1972; Giladi 2001b), including problems with gait modifications (Galna et al. 2010; Nutt et al. 2011), as well as the large literature showing a contribution of the basal ganglia to the control of movement in primates (see Turner and Desmurget 2010 and references above). While some of these cells may make a specific contribution to locomotion regulation via their brainstem projections, the fact that ~ 40% of cells in the EP and GPe were modulated during locomotion suggests that many cells probably have a more general role in motor control, regardless of behavioral task, as do cells in the motor cortex (Yakovenko and Drew 2015; Miri et al. 2017). As for the nature of the contribution of the pallidal output from the basal ganglia to the control of locomotion, our results suggest a contribution late in the planning stage of the gait modifications and during its execution. The bias towards the contralateral forelimb, particularly when it is the first to step over the obstacle, suggests a possible function in the selection of that limb, although the late onset of discharge activity suggests that this contribution to selection occurs only in the final stages of this process.
Supplementary Material
Acknowledgements
We would like to thank T Ariel, M. Bourdeau, N. De Sylva, P. Drapeau, F. Lebel and J. Soucy for technical assistance in the performance and analysis of these experiments. We would like to thank Stéphane Ménard for veterinary care. We would also like to acknowledge the contribution of Julia Leonard who participated in some of these experiments. We thank Drs. Elaine Chapman and Paul Cisek for helpful comments on this manuscript. This work was supported by operating grants PJT-156281, MOP 130372 and MOP 53339 from the CIHR.
Major Subject Area: Neuroscience
Funding
Canadian Institutes of Health Research (CIHR).
References
- Alexander GE, Delong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 9:357–381. [DOI] [PubMed] [Google Scholar]
- Allen GI, Tsukuhara N. 1974. Cerebrocerebellar communication systems. Physiol Rev. 54:957–1006. [DOI] [PubMed] [Google Scholar]
- Amos A, Armstrong DM, Marple-Horvat DE. 1990. Changes in the discharge patterns of motor cortical neurones associated with volitional changes in stepping in the cat. Neurosci Lett. 109:107–112. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Horak FB. 1985. Influence of the globus pallidus on arm movements in monkeys. III. Timing of movement-related information. J Neurophysiol. 54:433–448. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Turner RS. 1991. A quantitative analysis of pallidal discharge during targeted reaching movement in the monkey. Exp Brain Res. 86:623–632. [DOI] [PubMed] [Google Scholar]
- Andujar J-E, Lajoie K, Drew T. 2010. A contribution of area 5 of the posterior parietal cortex to the planning of visually guided locomotion: limb-specific and limb-independent effects. J Neurophysiol. 103:986–1006. [DOI] [PubMed] [Google Scholar]
- Aoki S, Sato Y, Yanagihara D. 2012. Characteristics of leading forelimb movements for obstacle avoidance during locomotion in rats. Neurosci Res. 74:129–137. [DOI] [PubMed] [Google Scholar]
- Aoki S, Sato Y, Yanagihara D. 2013. Lesion in the lateral cerebellum specifically produces overshooting of the toe trajectory in leading forelimb during obstacle avoidance in the rat. J Neurophysiology. 110:1511–1524. [DOI] [PubMed] [Google Scholar]
- Arimura N, Nakayama Y, Yamagata T, Tanji J, Hoshi E. 2013. Involvement of the Globus Pallidus in Behavioral goal determination and action specification. J Neurosci. 33:13639–13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM. 1986. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 26:273–361. [DOI] [PubMed] [Google Scholar]
- Armstrong DM. 1988. The supraspinal control of mammalian locomotion. JPhysiol. 405:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Drew T. 1984. Discharges of pyramidal tract and other motor cortical neurones during locomotion in the cat. J Physiol. 346:471–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Edgley S. 1984a. Discharges of nucleus interpositus neurones during locomotion in the cat. J Physiol. 351:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Edgley S. 1984b. Discharges of purkinje cells in the paravermal part of the cerebellar anterior lobe during locomotion in the cat. J Physiol. 352:403–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Marple-Horvat DE. 1996. Role of the cerebellum and motor cortex in the regulation of visually controlled locomotion. Can J Physiol Pharmacol. 74:443–455. [PubMed] [Google Scholar]
- Batschelet E. 1981. Circular statistics in biology. New York: Academic Press. [Google Scholar]
- Beloozerova IN, Sirota MG. 1988. Role of motor cortex in control of locomotion In: Gurfinkel VS, Ioffe ME, Massion J, Roll JP, editors. Stance and motion: facts and concepts. New York: Plenum Press, pp. 163–176. [Google Scholar]
- Beloozerova IN, Sirota MG. 1993. The role of the motor cortex in the control of accuracy of locomotor movements in the cat. J Physiol. 461:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG. 2003. Integration of motor and visual information in the parietal area 5 during locomotion. J Neurophysiol. 90:961–971. [DOI] [PubMed] [Google Scholar]
- Berman AL, Jones EG. 1982. The thalamus and basal telencephalon of the cat A cytoarchitectonic atlas with stereotaxic coordinates. Madison: University of Wisconsin Press. [Google Scholar]
- Boonstra TA, Kooij H, Munneke M, Bloem BR. 2008. Gait disorders and balance disturbances in Parkinson's disease: clinical update and pathophysiology. Curr Opin Neurobiol. 21:461–471. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. 2010. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 20:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. 1997. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 12:206–215. [DOI] [PubMed] [Google Scholar]
- Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, Kiehn O. 2018. Midbrain circuits that set locomotor speed and gait selection. Nature. 553:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheruel F, Dormont JF, Amalric M, Schmied A, Farin D. 1994. The role of putamen and pallidum in motor initiation in the cat. I. Timing of movement-related single-unit activity. Exp Brain Res. 100:250–266. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. 2010. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 33:269–298. [DOI] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. 2013. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 494:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. 1971. Activity of pallidal neurons during movement. J Neurophysiol. 34:414–427. [DOI] [PubMed] [Google Scholar]
- Delong MR, Crutcher MD, Georgopoulos AP. 1985. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 53:530–543. [DOI] [PubMed] [Google Scholar]
- Dormont JF, Condé H, Cheruel F, Farin D. 1997. Correlations between activity of pallidal neurons and motor parameters. Somatosens Mot Res. 14:281–294. [DOI] [PubMed] [Google Scholar]
- Drew T. 1993. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol. 70:179–199. [DOI] [PubMed] [Google Scholar]
- Drew T, Andujar J-E, Lajoie K, Yakovenko S. 2008. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res Rev. 57:199–211. [DOI] [PubMed] [Google Scholar]
- Drew T, Doucet S. 1991. Application of circular statistics to the study of neuronal discharge during locomotion. J Neurosci Methods. 38:171–181. [DOI] [PubMed] [Google Scholar]
- Drew T, Marigold DS. 2015. Taking the next step: cortical contributions to the control of locomotion. Curr Opin Neurobiol. 33:25–33. [DOI] [PubMed] [Google Scholar]
- Filion M, Harnois C. 1978. A comparison of projections of entopeduncular neurons to the thalamus, the midbrain and the habenula in the cat. J Comp Neurol. 181:763–780. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. 1991. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol. 66:1249–1263. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. 1993. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. JNeurosci. 13:1120–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galna B, Murphy AT, Morris ME. 2010. Obstacle crossing in people with Parkinson's disease: foot clearance and spatiotemporal deficits. Hum Mov Sci. 29:843–852. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. 1986. The basal ganglia and the locomotor regions. Brain Res. 396:47–63. [PubMed] [Google Scholar]
- Georgopoulos A, DeLong M, Crutcher M. 1983. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 3:1586–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N. 2001a. Freezing of gait: clinical overview. Adv Neurol. 87:191–197. [PubMed] [Google Scholar]
- Giladi N. 2001b. Gait disturbances in advanced stages of PArkinson's disease. Adv Neurol. 86:273–278. [PubMed] [Google Scholar]
- Gittis AH, Berke JD, Bevan MD, Chan CS, Mallet N, Morrow MM, Schmidt R. 2014. New roles for the external Globus Pallidus in basal ganglia circuits and behavior. J Neurosci. 34:15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Farries MA, Fee MS. 2013. Basal ganglia output to the thalamus: still a paradox. Trends Neurosci. 36:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. 1981. Control of locomotion in bipeds, tetrapods and fish In: Brooks VB, editor. Handbook of Physiology, Vol II, Part 2. Bethesda, Maryland: American Physiological Society, pp. 1179–1236. [Google Scholar]
- Grillner S, Robertson B. 2015. The basal ganglia downstream control of brainstem motor centres — an evolutionarily conserved strategy. Curr Opin Neurobiol. 33:47–52. [DOI] [PubMed] [Google Scholar]
- Grillner S, Robertson B. 2016. The basal ganglia over 500 million years. Curr Biol. 26:R1088–R1100. [DOI] [PubMed] [Google Scholar]
- Hamada I, DeLong MR, Mano N. 1990. Activity of identified wrist-related pallidal neurons during step and ramp wrist movements in the monkey. J Neurophysiol. 64:1892–1906. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. 1983. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 49:1285–1301. [DOI] [PubMed] [Google Scholar]
- Iansek R, Porter R. 1980. The monkey globus pallidus: neuronal discharge properties in relation to movement. J Physiol. 301:439–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inase M, Buford JA, Anderson ME. 1996. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey. J Neurophysiol. 75:1087–1104. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Gilman S, Aldridge JW. 1993. Primate basal ganglia activity in a precued reaching task: preparation for movement. Exp Brain Res. 95:51–64. [DOI] [PubMed] [Google Scholar]
- Knutsson E. 1972. An analysis of Parkinsonian gait. Brain. 95:475–486. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. 2010. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 466:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle H. 1975. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia: an autoradiographic study in Macaca fascicularis. Brain Res. 88:195–209. [DOI] [PubMed] [Google Scholar]
- Lajoie K, Andujar J-E, Pearson KG, Drew T. 2010. Neurons in area 5 of the posterior parietal cortex in the cat contribute to interlimb coordination during visually guided locomotion: a role in working memory. J Neurophysiol. 103:2234–2254. [DOI] [PubMed] [Google Scholar]
- Lajoie K, Drew T. 2007. Lesions of area 5 of the of the posterior parietal cortex in the cat produce errors in the accuracy of paw placement during visually guided locomotion. J Neurophysiol. 97:2339–2354. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Drew T. 2002. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 88:1791–1814. [DOI] [PubMed] [Google Scholar]
- Lavoie S, McFadyen B, Drew T. 1995. A kinematic and kinetic analysis of locomotion during voluntary gait modification in the cat. Exp Brain Res. 106:39–56. [DOI] [PubMed] [Google Scholar]
- Malach R, Am G. 1986. Mosaic architecture of the somatic sensory-recipient sector of the cat's striatum. J Neurosci. 6:3436–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ. 2012. Dichotomous organization of the external globus pallidus. Neuron. 74:1075–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigold DM, Drew T. 2017, 2017. Posterior parietal cortex estimates the relationship between object and body location during locomotion. Elife. 6:e28143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marple-Horvat DE, Criado JM. 1999. Rhythmic neuronal activity in the lateral cerebellum of the cat during visually guided stepping. J Physiol. 518:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marple-Horvat DE, Criado JM, Armstrong DM. 1998. Neuronal activity in the lateral cerebellum of the cat related to visual stimuli at rest, visually guided step modification, and saccadic eye movements. J Physiol. 506:489–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard A, Auclair F, Bourcier-Lucas C, Grillner S, Dubuc R. 2007. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. J Comp Neurol. 501:260–273. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. 2000. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 31:236–250. [DOI] [PubMed] [Google Scholar]
- Mink JW. 1996. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 50:381–425. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. 1991a. Basal ganglia motor control. I. Nonexclusive relation of pallidal discharge to five movement modes. J Neurophysiol. 65:273–300. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. 1991b. Basal ganglia motor control. II. Late pallidal timing relative to movement onset and inconsistent pallidal coding of movement parameters. J Neurophysiol. 65:301–329. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. 1991c. Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 65:330–351. [DOI] [PubMed] [Google Scholar]
- Miri A, Warriner CL, Seely JS, Elsayed GF, Cunningham JP, Churchland MM, Jessell TM. 2017. Behaviorally selective engagement of short-latency effector pathways by motor cortex. Neuron. 95:683–696.e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MP, Sepic S, Gm G, Wj D. 1978. Walking patterns of men with parkinsonism. Am J Phys Med. 57:294. [PubMed] [Google Scholar]
- Nakajima T, Fortier-Lebel N, Drew T. 2019. Premotor cortex provides a substrate for the temporal transformation of information during the planning of gait modifications. Cereb Cortex. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A. 2004. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 143:461–466. [DOI] [PubMed] [Google Scholar]
- Nambu A. 2011. Somatotopic organization of the primate basal ganglia. Front Neuroanat. 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Yoshida S, Jinnai K. 1990. Discharge patterns of pallidal neurons with input from various cortical areas during movement in the monkey. Brain Res. 519:183–191. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. 2011. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 10:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M, Sano H, Sato S, Ogura M, Mushiake H, Chiken S, Nakao N, Nambu A. 2017. Optogenetic activation of the sensorimotor cortex reveals “local inhibitory and global excitatory” inputs to the basal ganglia. Cereb Cortex. 27:5716–5726. [DOI] [PubMed] [Google Scholar]
- Palmer CI, Marks WB, Bak MJ. 1985. The responses of cat motor cortical units to electrical cutaneous stimulation during locomotion and during lifting, falling and landing. Exp Brain Res. 58:102–116. [DOI] [PubMed] [Google Scholar]
- Parent M, Lévesque M, Parent A. 2001. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol. 439:162–175. [DOI] [PubMed] [Google Scholar]
- Perrot O, Laroche D, Pozzo T, Marie C. 2009. Quantitative assessment of stereotyped and challenged locomotion after lesion of the striatum: a 3D kinematic study in rats. PloS One. 4:e7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Perkel DJ. 2007. Pallidal neuron activity increases during sensory relay through thalamus in a songbird circuit essential for learning. J Neurosci. 27:8687–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice SD, Drew T. 2001. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 85:679–698. [DOI] [PubMed] [Google Scholar]
- Ragsdale CW Jr, Graybiel A. 1981. The fronto-striatal projection in the cat and monkay and its relationship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Res. 208:259–266. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S. 2014. Cortical mechanisms underlying the Organization of Goal-Directed Actions and Mirror Neuron-Based Action Understanding. Physiol Rev. 94:655–706. [DOI] [PubMed] [Google Scholar]
- Robbe D. 2018. To move or to sense? Incorporating somatosensory representation into striatal functions. Curr Opin Neurobiol. 52:123–130. [DOI] [PubMed] [Google Scholar]
- Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC. 2016. Cell-type-specific control of brainstem Locomotor circuits by basal ganglia. Cell. 164:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Giménez-Amaya J-M. 1999. Anatomical re-evaluation of the corticostriatal projections to the caudata nucleus: a retrograde labeling study in the cat. Neurosci Res. 34:257–269. [DOI] [PubMed] [Google Scholar]
- Rossignol S. 1996. Neural control of stereotypic limb movements In: Rowell LB, Sheperd JT, editors. Handbook of physiology. Section 12. Regulation and integration of multiple systems. 5th ed. American Physiological society, pp. 173–216. [Google Scholar]
- Sales-Carbonell C, Taouali W, Khalki L, Pasquet MO, Petit LF, Moreau T, Rueda-Orozco PE, Robbe D. 2018. No discrete start/stop signals in the dorsal striatum of mice performing a learned action. Curr Biol. 28:3044–3055.e3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Stapley PJ, Drew T. 2008. Neurones in the pontomedullary reticular formation signal posture and movement both as an integrated behaviour and independently. J Neurophysiol. 100:2235–2253. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. 1992. Role of primate basal ganglia and frontal cortex in the internal generation of movements. I. Preparatory activity in the anterior striatum. Exp Brain Res. 91:363–384. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Sontag KH, Wand P. 1984. Sensory-motor processing in substantia nigra pars reticulata in conscious cats. J Physiol. 347:129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. 1985. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 5:776–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. 2004. Neural responses in multiple basal ganglia regions during spontaneous and treadmill locomotion in rats. Exp Brain Res. 157:314. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson.B., Grillner S.. 2011. Evolutionalry conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr Biol. 21:1081–1091. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. 2003. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neurosci. 119:293–308. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. 2004. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res. 50:137–151. [DOI] [PubMed] [Google Scholar]
- Thura D, Cisek P. 2017. The basal ganglia do not select reach targets but control the urgency of commitment. Neuron. 95:1160–1170.e1165. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. 1997. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 77:1051–1074. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. 2005. Context-dependent modulation of movement-related discharge in the primate Globus Pallidus. J Neurosci. 25:2965–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Desmurget M. 2010. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 20:704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Owens JWM Jr, Anderson ME. 1995. Directional variation of spatial and temporal characteristics of limb movements made by monkeys in a two-dimensional work space. J Neurophysiol. 74:684–697. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Drew T. 2009. A motor cortical contribution to the anticipatory postural adjustments that precede reaching in the cat. J Neurophysiol. 102:853–874. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Drew T. 2015. Similar motor cortical control mechanisms for precise limb control during reaching and locomotion. J Neurosci. 35:14476–14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian E, Pandya DN. 1993. Striatal connections of the parietal cortices in rhesus monkeys. J Comp Neurol. 332:175–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


