Abstract
Background:
Endoscopic treatment for malignant biliary obstruction (MBO) in patients bearing surgically altered anatomy (SAA) is not well-established. Although endoscopic ultrasound-guided biliary drainage (EUS-BD) has emerged as a new treatment option for MBO, limited data are available regarding the efficacy and safety of EUS-BD in patients with SAA. We conducted a multicenter prospective registration study to evaluate the efficacy and safety of EUS-BD in this population.
Methods:
This study involved 10 referral centers in Japan. Patients with SAA who were scheduled to receive EUS-BD for unresectable MBO between May 2016 and September 2018 were prospectively registered. The primary endpoint was technical success and the secondary outcomes were clinical success, procedure time, procedure-related adverse events (AEs), stent patency, and overall survival.
Results:
In total, 40 patients were prospectively enrolled. The surgical reconstruction methods were gastrectomy with Roux-en-Y reconstruction (47.5%), gastrectomy with Billroth-II reconstruction (15%), pancreaticoduodenectomy (27.5%), and hepaticojejunostomy with Roux-en-Y reconstruction (10%). EUS-BD was performed for primary biliary drainage in 31 patients and for rescue biliary drainage in nine patients. Transmural stenting alone (60%), antegrade stenting alone (5%), and a combination of the two techniques (35%) were selected for patients treated with EUS-BD. Technical and clinical success rates were 100% (95% confidence interval, 91.2–100.0%) and 95% (95% confidence interval, 83.1–99.4%), respectively. Mean procedure time was 36.5 min. Early AEs were noted in six patients (15%): three self-limited bile leak, one bile peritonitis, and two pneumoperitonea. Late AEs occurred in six patients (15%): one jejunal ulcer and five stent occlusions. Stent patency rate after 3 months of survival was 95.7% (22/23). Median overall survival was 96 days.
Conclusion:
EUS-BD for MBO in patients with SAA appears to be effective and safe not only as a rescue drainage technique after failed endoscopic retrograde cholangiography but also as a primary drainage technique.
Clinical Trial Registration:
UMIN000022101
Keywords: endoscopic ultrasound, EUS, EUS-guided biliary drainage, interventional EUS, surgically altered anatomy
Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is widely performed as the current gold standard for patients exhibiting malignant biliary obstruction (MBO).1,2 However, ERCP can sometimes be challenging due to difficulties accessing or cannulating the papilla, which is located at abnormal positions in patients with surgically altered anatomy (SAA), including those with Billroth-II anastomosis and Roux-en-Y anastomosis. Significant progress has been made in improving the procedural success rates for MBO in SAA patients by using enteroscopy-assisted ERCP (e-ERCP), a technique combining balloon enterscopes with ERCP.3 However, even with e-ERCP guidance, several challenges remain. First, endoscopists sometimes encounter difficulties obtaining an en face view of the papilla using forward-viewing endoscopes. Second, the devices available for e-ERCP have limited variability. Third, accessing the papilla or anastomotic site with e-ERCP is often time-consuming. Thus, endoscopic management for MBO in SAA patients has emerged as a growing problem facing endoscopists despite the development of e-ERCP.
Since its introduction in 2001,4 endoscopic ultrasound-guided biliary drainage (EUS-BD) has attracted significant attention as an alternative for patients with MBO in whom conventional ERCP has failed. Indeed, various endoscopic approaches have been developed for EUS-BD within the last two decades.5–8 EUS-BD has several advantages over e-ERCP. The procedure time for the former is generally shorter due to shorter distance from the endoscope insertion to the biliary tree. Furthermore, the risk of procedure-related pancreatitis may be lower because EUS-BD does not make direct contact with the papilla.
Given these advantages, SAA patients exhibiting MBO may be good candidates for EUS-BD rather than e-ERCP. However, limited data are available regarding the efficacy and safety of this procedure for this population.5,9 To date, only one retrospective study addressing the clinical efficacy of EUS-BD for SAA patients compared with e-ERCP10 and one single-center pilot study evaluating the feasibility of antegrade stenting for SAA patients11 have been reported. More importantly, few studies have investigated the feasibility and safety of EUS-BD as a first-line drainage modality for SAA patients because it is usually performed as rescue drainage after unsuccessful e-ERCP. Therefore, we conducted a multicenter prospective registration study evaluating the efficacy and safety of EUS-BD as primary or rescue drainage modality for SAA patients bearing unresectable MBO.
Materials and methods
Study design and eligibility criteria
The present study was conducted at 10 referral centers that are part of the Therapeutic Endoscopic Ultrasound (TEUS) group to evaluate the efficacy and safety of EUS-BD for MBO in SAA patients. Patients visiting any of the 10 participating centers between May 2016 and September 2018 were enrolled and followed up till December 2018. Unresectable MBO patients were enrolled in this study. Unresectability was judged using one or more imaging modalities, including computed tomography (CT) and/or magnetic resonance imaging. Performing EUS-BD after the failure of another biliary drainage procedure such as e-ERCP (defined as a rescue EUS-BD) or as the first-choice for biliary drainage (defined as the primary EUS-BD) was at the discretion of the endoscopists at each facility. The inclusion criteria were: (a) presence of unresectable MBO and (b) surgically altered gastrointestinal or biliary tract anatomies, except for Billroth I reconstruction. The exclusion criteria were: age <20 years, Eastern Cooperative Oncology Group performance status of 4, predicted survival <4 weeks, bleeding tendency (prothrombin time international normalized ratio >1.5, platelet count <50,000), or refusal for study participation. Patients who fulfilled the eligibility criteria were prospectively registered on the web and thus this study was performed on the prospectively registered database. All patients provided written informed consent prior to study participation. The study protocol was approved by the Institutional Review Board at each participating facility and the trial was registered at the University Hospital Medical Information Network (UMIN000022101).
EUS-BD procedures for MBO in SAA patients
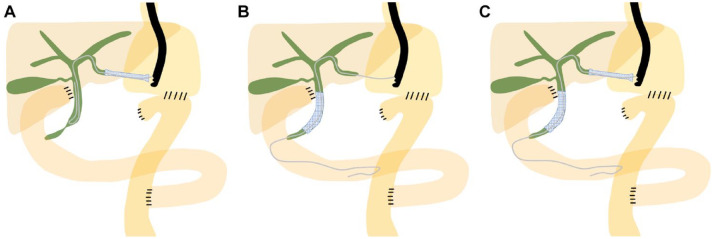
Schematic images for EUS-BD in the present study are shown in Figure 1. Although EUS-guided transmural stenting was employed as the first-line EUS-BD technique, EUS-guided antegrade stenting or a combination of the two techniques could also be utilized to achieve successful biliary drainage (Figure 1). For transmural stenting, the dilated left intrahepatic bile duct (IHBD) was punctured using a 19-gauge fine aspiration needle from the stomach or jejunum under EUS guidance. On injecting contrast medium to obtain a cholangiogram through the needle, a 0.025-inch guidewire was inserted into the bile duct and the fistulous tract was dilated if necessary. Finally, a stent was deployed between the IHBD and the stomach or small intestine (Figure 1A). A braided-type covered metal stent (Niti-S S-type Stent; Taewoong Medical, Seoul, South Korea) or a dedicated plastic stent (TYPE-IT Stent; Gadelius Medical, Tokyo, Japan)12 was used for transmural stenting. When antegrade stenting was performed, IHBD puncture was followed by guidewire insertion and passing the guidewire through the MBO site and toward the papilla into the gastrointestinal lumen. Thereafter, a stent was placed to cover the MBO site (Figure 1B). A laser-cut type uncovered metal stent (BileRush Selective; Piolax Medical Devices, Yokohama, Japan) was used for antegrade stenting. On combining transmural and antegrade stentings, transmural stenting was performed following antegrade stenting as previously described (Figure 1C).13
Figure 1.
Schematic images of endoscopic ultrasound (EUS)-guided biliary drainage for distal biliary obstruction in patients with surgically altered anatomy (gastrectomy with Roux-en-Y reconstruction). A. EUS-guided transmural biliary stenting. B. EUS-guided biliary antegrade stenting. C. EUS-guided transmural biliary stenting combined with antegrade biliary stenting.
Study endpoints and definitions
The primary endpoint of the present study was technical success, defined as successful stent placement between the left IHBD and gastrointestinal lumen for transmural stenting and a successful stent placement over the MBO site for antegrade stenting. The secondary endpoints were clinical success, procedure time, adverse events (AEs), stent patency, and overall survival time. Clinical success was defined as the improvement of cholangitis or a decrease in the serum bilirubin level either to a normal level or a reduction rate of more than 50% within 2 weeks following EUS-BD. AE severity was classified according to the American Society for Gastrointestinal Endoscopy (ASGE) lexicon.14 Stent dysfunction was defined as the recurrence of symptoms of biliary obstruction, including obstructive jaundice and cholangitis (leukocytosis, fever, and increased serum bilirubin levels) and biliary dilatation on imaging studies. Thus, stent dysfunction includes both occlusion and migration. The stent patency time was defined as the interval between stent placement and stent dysfunction. Patients who died without stent dysfunction were censored. The stent patency rate 3 and 6 months after stent placement was also evaluated. The overall survival was defined as the interval between stent placement and death. As a subgroup analysis, clinical success and stent patency time were evaluated by dividing patients into two groups (with and without native papilla) in terms of the types of reconstruction surgery.
Abdominal CT was performed within 2 days of the EUS-BD procedure and during the follow-up period if necessary. Laboratory examinations and clinical symptoms were assessed every month for up to 3 months after the procedure on an outpatient basis.
Statistical analyses
Previous reports regarding EUS-BD for SAA patients showed that the procedural technical success rate was 89–98%.10,11,15 Given this study’s prospective nature, we set the anticipated technical success rate as 90%. The number of cases needed was determined based on the precision of estimation so that the width from the estimate to the lower limit of the 95% confidence interval (CI) was within 15%. The number of cases necessary for this prospective study was determined to be 38 because adequate CI variation was obtained (75.2–97.1%) with a success rate of 89.5% (34/38). Based on this minimum number, the target number of cases was formally set to 40. Registration would be terminated on reaching the target number of cases. If the registration period ended with less than the target number of cases, the study would be terminated. The data were summarized as numbers and percentages for categorical variables or means (with standard deviation) and medians (with ranges) for continuous variables. The technical and clinical success rates were presented with a 95% CI. Stent patency and overall survival were evaluated using the Kaplan–Meier method. All statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Patients
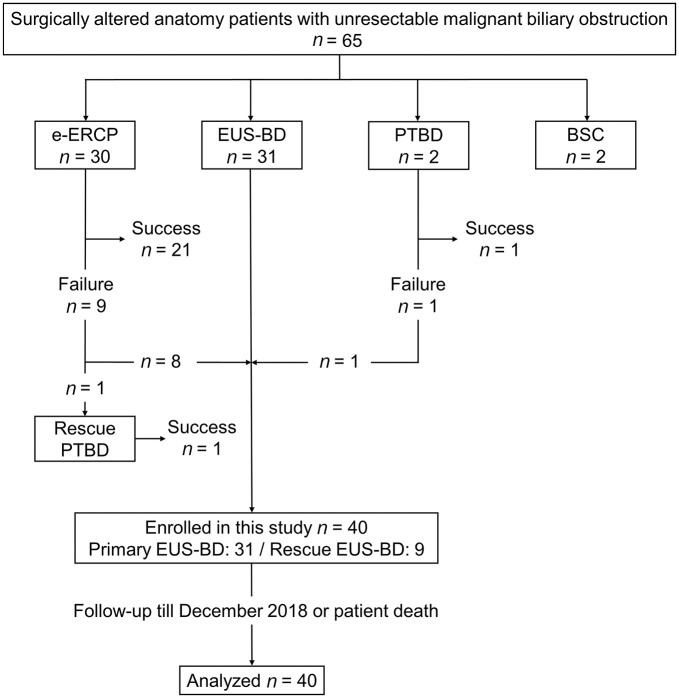
Figure 2 shows the patient flow chart for this study. During the study registration period, 65 SAA patients with unresectable MBO were treated at the 10 participating facilities. e-ERCP, EUS-BD, and percutaneous transhepatic biliary drainage (PTBD) were performed as the first-choice biliary drainage procedure for 30, 31, and two patients, respectively. In two patients who underwent primary PTBD, PTBD via the right hepatic lobe was considered to be safer because the right intrahepatic bile duct was predominantly dilated. Nine patients underwent EUS-BD as a rescue biliary drainage from a total of 10 patients for whom biliary drainage by e-ERCP or PTBD was unsuccessful. One patient with unsuccessful e-ERCP refused to participate in this study and was treated with PTBD. Primary e-ERCP was unsuccessful for nine patients because of difficulties in accessing the papilla or anastomotic site endoscopically in five patients and difficulties in biliary cannulation in four patients. In one case of primary PTBD failure, the guidewire deviated from the bile duct after successful biliary puncture. Finally, a total of 40 patients (primary EUS-BD, 31; rescue EUS-BD, nine) who met the inclusion and exclusion criteria were recruited.
Figure 2.
Flow diagram demonstrating enrollment of the study patients.
BSC, best supportive care; e-ERCP, enteroscopy-assisted ERCP; EUS-BD, endoscopic ultrasound-guided biliary drainage; PTBD, percutaneous transhepatic biliary drainage.
Table 1 shows the patient characteristics and diseases enrolled in this study. In total, 25 (62.5%) patients had a native papilla, whereas the remaining 15 (37.5%) had an enteric-biliary anastomosis. No patients were excluded after enrollment or were lost to follow-up.
Table 1.
Patient demographic and clinical characteristics.
| Variable | Patient no. = 40 |
|---|---|
| Median age, years (range) | 72.5 (40–92) |
| Sex, men/women, n (%) | 31 (77.5)/9 (22.5) |
| ECOG performance status, median (range) | 1 (0–2) |
| Underlying malignancy, n (%) | |
| Pancreatobiliary cancer | 20 (50) |
| Gastric cancer | 16 (40) |
| Duodenal cancer | 3 (7.5) |
| Ascending colon cancer | 1 (2.5) |
| Surgically altered anatomy, n (%) | |
| Gastrectomy with Roux-en-Y | 19 (47.5) |
| Gastrectomy with Billroth-II | 6 (15) |
| Pancreaticoduodenectomy | 11 (27.5) |
| Hepaticojejunostomy with Roux-en-Y | 4 (10) |
| Presence of ascites, n (%) | 7 (17.5) |
| Attempt for biliary drainage prior to EUS-BD | |
| Yes/No, n (%) | 9 (22.5)/31 (77.5) |
| Laboratory values before drainage (mean ± SD) | |
| Hemoglobin (g/dl) | 10.0 ± 1.4 |
| WBC (× 102/μl) | 67.4 ± 24.8 |
| Platelet (× 104/μl) | 23.0 ± 7.7 |
| Aspartate aminotransferase (IU/L) | 130 ± 82 |
| Alanine aminotransferase (IU/L) | 115 ± 84 |
| Alkaline phosphatase (IU/L) | 1832 ± 852 |
| Total bilirubin (mg/dl) | 6.5 ± 4.2 |
| C-reactive protein (mg/dl) | 4.7 ± 3.9 |
ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell.
Study outcomes
Technical success was achieved in 40 (100%) patients (95% CI, 91.2–100.0%). Transmural stenting alone (60%), antegrade stenting alone (5%), and a combination of the two techniques (35%) were selected for patients treated with EUS-BD. The median procedure time was 36.5 min (range 10–100 min). The puncture site was the biliary branch ducts of B3 and B2 in 33 (82.5%) and 7 (17.5%) patients, respectively. The mean IHBD diameter measured on EUS at the time of puncture was 4.9 ± 1.5 mm. The types of stents used are shown in Table 2. Clinical success was achieved for 38 (95%) patients (95% CI, 83.1–99.4%). Clinical success rates in cases with native papilla and enteric-biliary anastomosis were 96% (95% CI, 88.3–99.9%) and 93.3% (95% CI, 68.1–99.8%), respectively. Thus, surgical reconstruction methods did not affect the clinical success of EUS-BD.
Table 2.
Study outcomes of endoscopic ultrasound-guided biliary drainage in patients with surgically altered anatomy.
| †Technical Success, n (%) | 40 (100) |
| EUS-BD technique, n (%) | |
| Transmural stenting alone | 24 (60) |
| Antegrade stenting alone | 2 (5) |
| Combination of transmural and antegrade stenting | 14 (35) |
| Accessed biliary branch duct, n (%) | |
| Left B3: B2 | 33 (82.5): 7 (17.5) |
| Mean biliary diameter of the puncture site, mm ± SD | 4.9 ± 1.5 |
| Puncture site, n (%) | |
| Stomach: jejunum | 35 (87.5): 5 (12.5) |
| Mean procedure time, min ± SD | 36.5 ± 18.6 |
| Type of stent | |
| Transmural stent, n | 38 |
| Covered metal stent, n (%) | 35 (92.1) |
| Stent diameter, n (%) 8 mm: 10 mm | 25 (65.8): 10 (26.3) |
| Stent length, n (%) 80 mm: 100 mm: 120 mm | 3 (7.9): 26 (68.4): 6 (15.8) |
| Plastic stent, n (%) | 3 (7.9) |
| Stent diameter/length, n (%) 7Fr/140 mm | 3 (7.9) |
| Antegrade stent, n | 16 |
| Uncovered metal stent, n (%) | 16 (100) |
| Stent diameter, n (%) 8 mm: 10 mm | 13 (81.3): 3 (18.7) |
| Stent length, n (%) 60 mm: 80 mm | 10 (62.5): 6 (37.5) |
| ‡Clinical success, n [%, (95% CI)] | 38 [95, (83.1–99.4)] |
| Clinical success with native papilla, n [%, (95% CI)] | 24 [96, (88.3–99.9)] |
| Clinical success without native papilla, n [%, (95% CI)] | 14 [93.3 (68.1–99.8)] |
| Early adverse events (⩽14 days), n (%) | 6 (15) |
| Bile leak: bile peritonitis: pneumoperitoneum | 3 (7.5): 1 (2.5): 2 (5) |
| Late adverse events (>14 days), n (%) | 6 (15) |
| Jejunal ulcer: stent dysfunction | 1 (2.5): 5 (12.5) |
| Median stent patency time, days (range) | Not reached |
| Median overall survival, days (range) | 96 (17–539) |
CI, confidence interval; EUS-BD, endoscopic ultrasound-guided biliary drainage.
Technical success was defined as successful deployment of the stent between the bile duct and gastrointestinal lumen.
Clinical success was defined as the improvement of cholangitis or a decrease in the serum bilirubin level either to a normal level or a reduction rate of more than 50% within 2 weeks following EUS-BD.
Early and late AEs were noted in six (15%) and six (15%) patients, respectively (Table 2). These six early AEs cases were self-limited and classified as mild according to the ASGE lexicon. One jejunal ulcer case and five stent dysfunction cases were recorded as late AEs. All five cases of stent dysfunction were stent occlusions, and no stent migration was observed. The five patients with stent occlusion were treated as follows: one patient received PTBD and the other four received endoscopic re-intervention using a stent exchange or additional stent placement.
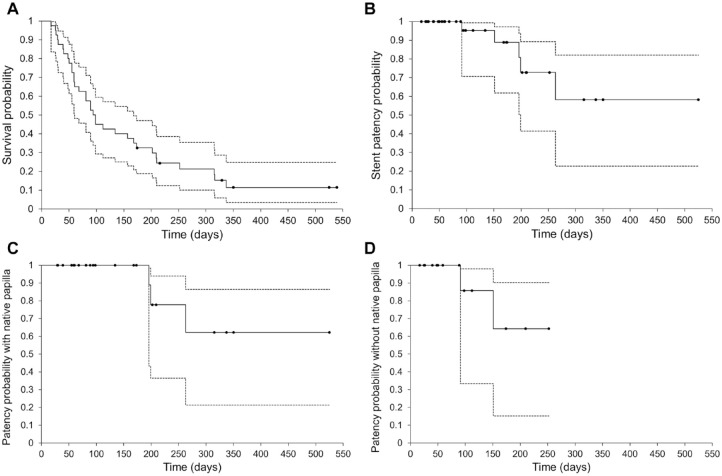
The average follow-up duration was 150 ± 103 days. The median overall survival time was 96 days (95% CI, 60–168 days) (Figure 3A). The median stent patency time was not reached (Figure 3B) and the stent patency rate after 3 and 6 months of survival was 95.7% (22/23) and 85.7% (12/14), respectively. Kaplan–Meier curves of stent patency in patients with native papilla and enteric-biliary anastomosis are shown in Figure 3C and D.
Figure 3.
Kaplan–Meier plot of overall survival (A) and stent patency (B). In the analysis of all 40 patients, the median overall survival time was 96 days and the median stent patency time was not reached. Kaplan–Meier plot of stent patency in patients with native papilla (C) and without native papilla (with enteric-biliary anastomosis) (D). The dashed line indicates the 95% confidence interval.
Discussion
In this study involving 10 referral centers, EUS-BD for unresectable MBO with SAA patients was technically and clinically successful in 100% and 95% of enrolled patients, respectively. Previous large-scale studies reported the efficacy of EUS-BD for MBO;5,9,13,16–25 however, these studies included a limited number of patients bearing SAA (Table 3). Compared with these studies, this study aimed to determine the efficacy of EUS-BD specifically for SAA patients. To the best of our knowledge, this is the largest study to prospectively evaluate the efficacy of EUS-BD in patients with SAA. Notably, in this study, EUS-BD was selected as primary biliary drainage for more than three-quarters of patients and most of these cases were successfully treated with EUS-BD alone. Taken together, the results of our study strongly suggest the utility of EUS-BD as a primary drainage technique for MBO patients bearing SAA.
Table 3.
Summary of published studies on EUS-guided biliary drainage, including patients with surgically altered anatomy (n ⩾ 40).
| Author | Study design | No. of cases | No. (%) of SAA cases | Type of Access | Technical success, n (%) | Clinical success, n (%) | Total AEs, n (%) |
|---|---|---|---|---|---|---|---|
| Maranki et al.16 | Retrospective Single arm |
49 | 7 (14) | Extrahepatic: 14 Intrahepatic 35 |
41 (84) | 36 (73) | 8 (16%) |
| Vila et al.17 | Retrospective Single arm |
106 | 18 (17) | Intrahepatic: 34 Extrahepatic: 26 Rendezvous: 46 |
73 (69) | N/A | 24 (23) |
| Kawakubo et al.5 | Retrospective Single arm |
64 | 4 (6) | Extrahepatic: 44 Intrahepatic: 20 |
61 (95) | N/A | 12 (19) |
| Poincloux et al.9 | Retrospective Single arm |
101 | 7 (7) | Intrahepatic: 71 Extrahepatic: 30 |
99 (98) | 93 (92) | 12 (12) |
| Khashab et al.10 | Retrospective Comparative |
49 | 49 (100) | Intrahepatic: 49 | 48 (98) | 43 (88) | 10 (20) |
| Tyberg et al.18 | Prospective Single arm |
52 | 11 (21) | Intrahepatic: 35 Extrahepatic: 17 |
50 (96) | 40 (77) | 5 (10) |
| Khashab et al.19 | Prospective Single arm |
96 | 10 (10) | Intrahepatic: 36 Extrahepatic: 56 |
92 (96) | 86 (90) | 10 (11) |
| Nakai et al.20 | Retrospective Comparative | 56 | 10 (18) | Rendezvous: 13 Extrahepatic: 4 Intrahepatic: 23 Gallbladder: 16 |
53 (95) | N/A | 12 (21) |
| Cho et al.21 | Prospective Single arm |
54 | 1 (2) | Intrahepatic: 21 Extrahepatic: 33 |
54 (100) | 51 (94) | 9 (17) |
| Paik et al.22 | Randomized trial | 64 | 4 (6) | Intrahepatic: 32 Extrahepatic: 32 |
60 (94) | 54 (90) | 7 (11) |
| Ogura et al.13 | Prospective Single arm |
49 | 19 (39) | Intrahepatic: 49 | 47 (96) | N/A | 5 (10) |
| Uchida et al.23 | Retrospective Comparative |
43 | 14 (33) | Intrahepatic: 43 | 41 (95) | 38 (88) | 3 (7) |
| Kanno et al.24 | Retrospective Single arm | 99 | 15 (15) | Intrahepatic: 53 Extrahepatic: 46 |
97 (98) | 90 (93) | 10 (10) |
| Nakai et al.25 | Retrospective Single arm |
110 | 16 (15) | Intrahepatic: 110 | 110 (100) | 103 (94) | 27 (25) |
| Current study | Prospective Single arm |
40 | 40 (100) | Intrahepatic: 40 | 40 (100) | 38 (95) | 6 (15) |
AE, adverse event; EUS, endoscopic ultrasound; N/A, not applicable; SAA, surgical altered anatomy.
One major question arising from this study is regarding the optimal endoscopic drainage procedure for biliary drainage in SAA patients exhibiting MBO. According to recent meta-analyses on e-ERCP, the cumulative technical success rate was reported to range from 63% to 69.4% for such patients,26,27 which is much lower than that of EUS-BD.28–31 Furthermore, a recent retrospective comparative study comparing EUS-BD and e-ERCP showed that e-ERCP requires a significantly longer procedure time than EUS-BD.10 Thus, EUS-BD appears to be superior to e-ERCP for SAA patients exhibiting MBO in terms of the success rate and procedure time. The indications for EUS-BD have been expanding due to the high success rate and it has been utilized as a rescue biliary drainage after ERCP failure and as the primary biliary drainage.32,33 In line with these previous meta-analyses, a very high success rate was achieved in SAA patients treated with primary EUS-BD in this study. In addition, a recent pilot study found that EUS-BD was technically feasible and clinically effective and safe even in patients with ascites.34 In this study, the presence of a small amount of perihepatic ascites was observed in seven patients (17.5%) on CT obtained prior to EUS-BD. However, similar to the previous report, EUS-BD could be safely performed without severe AEs, including bile peritonitis. We summarize that the risk of bile leakage or peritonitis might be lower in patients with SAA due to organ adhesion caused by the previous surgery.
Two randomized clinical trials were published in 2018 comparing EUS-BD and ERCP as the primary biliary drainage.35,36 Both studies demonstrated comparable technical and clinical success between EUS-BD and ERCP. However, these studies included only the extrahepatic bile duct (EHBD) approach in normal anatomic patients for whom conventional ERCP could be performed. There are two main puncture access routes for EUS-BD: EHBD and IHBD. Although no universal consensus has been reached on the optimal access route or drainage method, several treatment algorithms for EUS-BD have been described based on patient anatomy, underlying disease, and location of the biliary stricture.18,37,38 However, for patients with SAA, the access route is anatomically restricted to the IHBD only. Regarding the two access routes, recent studies provide evidence that EUS-BD via EHBD and IHBD approaches have similar efficacy and safety for MBO patients after unsuccessful ERCP.39,40 Although evidence supporting the safety and efficacy of EUS-BD continues to accumulate, data on EUS-BD via the IHBD in comparison with ERCP for SAA patients exhibiting MBO are lacking. Given the very high technical and clinical success rates of EUS-BD as the first-line treatment for SAA patients, the procedure has potential as a first-line endoscopic treatment for SAA patients with MBO in the near future. Confirmation of this idea requires future prospective studies directly addressing the efficacy and safety of EUS-BD via the IHBD route and e-ERCP.
Three major techniques are available for drainage via the IHBD: transmural biliary stenting, antegrade biliary stenting, and the rendezvous technique.7,41 Because the rendezvous technique requires endoscopic access to the papilla or anastomosis, transmural or antegrade biliary stenting is preferentially performed for SAA patients. Although little information exists on whether transmural or antegrade stenting is more appropriate, our previous study showed that transmural stenting combined with antegrade stenting contributed to long stent patency.13 In this study, transmural stenting alone and transmural stenting combined with antegrade stenting were performed in 60% and 35% of cases, respectively. In line with our previous report, the stent patency rate was higher in SAA patients treated with the combined procedure compared with those treated with transmural stenting alone. Thus, the combined procedure is recommended for SAA patients exhibiting MBO, despite the drawbacks in terms of procedure time and cost. A recent pilot study involving 20 SAA cases demonstrated technical and clinical success rates of 95% with antegrade stenting,11 which was performed in only 5% of cases in this study. One concern regarding the treatment of SAA patients with antegrade stenting alone is that endoscopic re-intervention in the event of stent dysfunction cannot be performed via the previously deployed transmural stent. In fact, all six patients with stent dysfunction in this study had undergone transmural stenting, and endoscopic re-intervention via the transmural stent was successful in five patients. A comparative study with a larger cohort is needed to determine which EUS-BD drainage method is the best option for MBO in SAA patients.
Our study has several limitations. First, the study cohort was limited in size due to relative scarcity of the study population. The small sample size limited the ability to perform predictive statistical analyses, such as logistic regression or meaningful subgroup analyses. Second, because this study was not a randomized trial, EUS-BD may not have been selected in cases where the IHBD was barely dilated. Therefore, selection bias might affect the results of this study. Third, it is difficult to exclude the effects of performance bias. Although 10 institutions participated in this study, the decision for the selection of EUS-BD or e-ERCP was left to each institution. During the study period, there were four institutions that used EUS-BD as the first-choice treatment for unresectable MBO in SAA patients and three institutions with e-ERCP as the first-choice treatment. Among the remaining three institutions, either treatment was administered as the first-choice treatment following informed consent between physicians and patients depending on the case. In addition, details of the EUS-BD technique, including the drainage method and type of stents, were not standardized, although our TEUS group regularly met every 4 months to share and refine our knowledge of the EUS-BD procedure. Fourth, 42.5% and 65% of patients died within 3 and 6 months of stent placement, respectively; therefore, we could not accurately evaluate long-term stent patency. Fifth and finally, this study was conducted at specialized facilities familiar with the EUS-BD procedure. Previous studies have indicated that a learning curve period was likely required to improve the technical skills of endoscopists.9,16 Therefore, the outcomes in this study are not applicable to facilities where appropriate expertise in EUS-BD is unavailable.
In conclusion, despite the aforementioned limitations, EUS-BD for MBO in SAA patients appears to be effective and safe as not only rescue biliary drainage after ERCP failure but also as the primary biliary drainage. Although ERCP is presently considered the standard treatment even for SAA patients, the data presented here prompt us to recognize that EUS-BD has the potential to be the first-line treatment for MBO in SAA patients. Further studies comparing EUS-BD and e-ERCP with a larger cohort are necessary to confirm the suitability and utility of EUS-BD for MBO in SAA patients.
Footnotes
Funding: The present study was supported by grants from the Japan Society for Promotion of Science (grant no. 16K09410).
Conflict of interest: The authors declare that there is no conflict of interest.
ORCID iDs: Takeshi Ogura  https://orcid.org/0000-0003-2916-6568
https://orcid.org/0000-0003-2916-6568
Masayuki Kitano  https://orcid.org/0000-0001-6885-9223
https://orcid.org/0000-0001-6885-9223
Contributor Information
Kosuke Minaga, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Japan.
Mamoru Takenaka, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Japan.
Takeshi Ogura, Second Department of Internal Medicine, Osaka Medical College, Takatsuki, Japan.
Takashi Tamura, Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan.
Taira Kuroda, Department of Gastroenterology and Metabology, Ehime University Graduate School of Medicine, To-on, Japan.
Toyoma Kaku, Department of Gastroenterology, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan.
Yoshito Uenoyama, Department of Gastroenterology, Japanese Red Cross Wakayama Medical Center, Wakayama, Japan.
Chishio Noguchi, Department of Gastroenterology, Shinbeppu Hospital, Beppu, Japan.
Hidefumi Nishikiori, Department of Gastroenterology, Oita San-ai Medical Center, Oita, Japan.
Hajime Imai, Department of Gastroenterology, Minami Wakayama Medical Center, Tanabe, Japan.
Ryota Sagami, Department of Gastroenterology, New Tokyo Hospital, Matsudo, Chiba.
Nao Fujimori, Department of Gastroenterology, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan.
Kazuhide Higuchi, Second Department of Internal Medicine, Osaka Medical College, Takatsuki, Japan.
Masatoshi Kudo, Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Japan.
Yasutaka Chiba, Clinical Research Center, Kindai University Hospital, Osaka-Sayama, Japan.
Masayuki Kitano, Second Department of Internal Medicine, Wakayama Medical University, 811-1 Kimiidera, Wakayama, Wakayama 641-8509, Japan.
References
- 1. Fogel EL, McHenry L, Sherman S, et al. Therapeutic biliary endoscopy. Endoscopy 2005; 37: 139–145. [DOI] [PubMed] [Google Scholar]
- 2. Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European society of gastrointestinal endoscopy (ESGE) clinical guideline - updated October 2017. Endoscopy 2018; 50: 910–930. [DOI] [PubMed] [Google Scholar]
- 3. Shimatani M, Matsushita M, Takaoka M, et al. Effective “short” double-balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy 2009; 41: 849–854. [DOI] [PubMed] [Google Scholar]
- 4. Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy 2001; 33: 898–900. [DOI] [PubMed] [Google Scholar]
- 5. Kawakubo K, Isayama H, Kato H, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci 2014; 21: 328–334. [DOI] [PubMed] [Google Scholar]
- 6. Teoh AYB, Dhir V, Kida M, et al. Consensus guidelines on the optimal management in interventional EUS procedures: results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018; 67: 1209–1228. [DOI] [PubMed] [Google Scholar]
- 7. Minaga K, Kitano M. Recent advances in endoscopic ultrasound-guided biliary drainage. Dig Endosc 2018; 30: 38–47. [DOI] [PubMed] [Google Scholar]
- 8. Isayama H, Nakai Y, Itoi T, et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J Hepatobiliary Pancreat Sci 2019; 26: 249–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poincloux L, Rouquette O, Buc E, et al. Endoscopic ultrasound-guided biliary drainage after failed ERCP: cumulative experience of 101 procedures at a single center. Endoscopy 2015: 47; 794–801. [DOI] [PubMed] [Google Scholar]
- 10. Khashab MA, El Zein MH, Sharzehi K, et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: an international comparative study. Endosc Int Open 2016; 4: E1322–E1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwashita T, Yasuda I, Mukai T, et al. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: single-center prospective pilot study. Dig Endosc 2017; 29: 362–368. [DOI] [PubMed] [Google Scholar]
- 12. Umeda J, Itoi T, Tsuchiya T, et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: a prospective preliminary feasibility study (with videos). Gastrointest Endosc 2015: 82; 390–396. [DOI] [PubMed] [Google Scholar]
- 13. Ogura T, Kitano M, Takenaka M, et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video). Dig Endosc 2018; 30: 252–259. [DOI] [PubMed] [Google Scholar]
- 14. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 2010; 71: 446–454. [DOI] [PubMed] [Google Scholar]
- 15. Siripun A, Sripongpun P, Ovartlarnporn B. Endoscopic ultrasound-guided biliary intervention in patients with surgically altered anatomy. World J Gastrointest Endosc 2015: 7; 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maranki J, Hernandez AJ, Arslan B, et al. Interventional endoscopic ultrasound-guided cholangiography: long-term experience of an emerging alternative to percutaneous transhepatic cholangiography. Endoscopy 2009; 41: 532–538. [DOI] [PubMed] [Google Scholar]
- 17. Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc 2012: 76; 1133–1141. [DOI] [PubMed] [Google Scholar]
- 18. Tyberg A, Desai AP, Kumta NA, et al. EUS-guided biliary drainage after failed ERCP: a novel algorithm individualized based on patient anatomy. Gastrointest Endosc 2016; 84: 941–946. [DOI] [PubMed] [Google Scholar]
- 19. Khashab MA, Van der Merwe S, Kunda R, et al. Prospective international multicenter study on endoscopic ultrasound-guided biliary drainage for patients with malignant distal biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Endosc Int Open 2016; 4: E487–E496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakai Y, Isayama H, Yamamoto N, et al. Indications for endoscopic ultrasonography (EUS)-guided biliary intervention: does EUS always come after failed endoscopic retrograde cholangiopancreatography? Dig Endosc 2017; 29: 218–225. [DOI] [PubMed] [Google Scholar]
- 21. Cho DH, Lee SS, Oh D, et al. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos). Gastrointest Endosc 2017; 85: 1067–1075. [DOI] [PubMed] [Google Scholar]
- 22. Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: a multicenter randomized clinical trial. Am J Gastroenterol. 2018; 113: 987–997. [DOI] [PubMed] [Google Scholar]
- 23. Uchida D, Kawamoto H, Kato H, et al. The intra-conduit release method is useful for avoiding migration of metallic stents during EUS-guided hepaticogastrostomy (with video). J Med Ultrason (2001). 2018; 45: 399–403. [DOI] [PubMed] [Google Scholar]
- 24. Kanno Y, Koshita S, Ogawa T, et al. EUS-guided biliary drainage for unresectable malignant biliary obstruction: 10-year experience of 99 cases at a single center. J Gastrointest Cancer 2019; 50: 469–477. [DOI] [PubMed] [Google Scholar]
- 25. Nakai Y, Sato T, Hakuta R, et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video). Gastrointest Endosc. Epub ahead of print 9 April 2020. DOI: 10.1016/j.gie.2020.03.3856. [DOI] [PubMed] [Google Scholar]
- 26. Inamdar S, Slattery E, Sejpal DV, et al. Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc 2015; 82: 9–19. [DOI] [PubMed] [Google Scholar]
- 27. Shao XD, Qi XS, Guo XZ. Endoscopic retrograde cholangiopancreatography with double balloon enteroscope in patients with altered gastrointestinal anatomy: a meta-analysis. Saudi J Gastroenterol 2017; 23: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: a systematic review and meta-analysis. Dig Dis Sci 2016; 61: 684–703. [DOI] [PubMed] [Google Scholar]
- 29. Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: a systematic review. Gastrointest Endosc 2016; 83: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 30. Moole H, Bechtold ML, Forcione D, et al. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine 2017; 96: e5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Logiudice FP, Bernardo WM, Galetti F, et al. Endoscopic ultrasound-guided vs endoscopic retrograde cholangiopancreatography biliary drainage for obstructed distal malignant biliary strictures: a systematic review and meta-analysis. World J Gastrointest Endosc 2019; 11: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bishay K, Boyne D, Yaghoobi M, et al. Endoscopic ultrasound-guided transmural approach versus ERCP-guided transpapillary approach for primary decompression of malignant biliary obstruction: a meta-analysis. Endoscopy 2019; 51: 950–960. [DOI] [PubMed] [Google Scholar]
- 33. Han SY, Kim SO, So H, et al. EUS-guided biliary drainage versus ERCP for first-line palliation of malignant distal biliary obstruction: a systematic review and meta-analysis. Sci Rep 2019; 9: 16551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alvarez-Sánchez MV, Luna OB, Oria I, et al. Feasibility and safety of endoscopic ultrasound-guided biliary drainage (EUS-BD) for malignant biliary obstruction associated with ascites: results of a pilot study. J Gastrointest Surg 2018; 22: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 35. Park JK, Woo YS, Noh DH, et al. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: prospective randomized controlled study. Gastrointest Endosc 2018; 88: 277–282. [DOI] [PubMed] [Google Scholar]
- 36. Bang JY, Navaneethan U, Hasan M, et al. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc 2018; 88: 9–17. [DOI] [PubMed] [Google Scholar]
- 37. Park DH, Jeong SU, Lee BU, et al. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest Endosc 2013; 78: 91–101. [DOI] [PubMed] [Google Scholar]
- 38. Minaga K, Takenaka M, Yamao K, et al. Clinical utility of treatment method conversion during single-session endoscopic ultrasound-guided biliary drainage. World J Gastroenterol 2020; 26: 947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uemura RS, Khan MA, Kahaleh M, et al. EUS-guided choledochoduodenostomy versus hepaticogastrostomy: a systematic review and meta-analysis. J Clin Gastroenterol 2018; 52: 123–130. [DOI] [PubMed] [Google Scholar]
- 40. Minaga K, Ogura T, Shiomi H, et al. Comparison of the efficacy and safety of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for malignant distal biliary obstruction: multicenter, randomized, clinical trial. Dig Endosc 2019; 31: 575–582. [DOI] [PubMed] [Google Scholar]
- 41. Shah RM, Tarnasky P, Kedia P. A review of endoscopic ultrasound guided endoscopic retrograde cholangiopancreatography techniques in patients with surgically altered anatomy. Transl Gastroenterol Hepatol 2018; 3: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]