Abstract
Background:
Faecal microbiota transplantation (FMT) is a reasonable therapeutic option for the treatment of Clostridioides difficile infection (CDI) recurrent and refractory (RCDI) to therapy, but little evidence on the long-term impact of this therapy is currently available in the literature. The aim of this study was to evaluate the efficacy and safety of FMT in recurrent and refractory CDI and the modifications of the recipient’s gut microbiota in the medium–long term.
Methods:
This prospective study collects the clinical and laboratory data of RCDI patients treated with FMT by colonoscopy from February 2016 to October 2019. Stool samples for metagenomic analysis were collected pre-FMT at 1 week and at 6 and 12–24 months post-FMT.
Results:
In the study period, 20 FMT procedures were performed on 19 patients. Overall, FMT was effective in 85% of treated patients. No serious adverse event was recorded. In the medium- to long-term follow up, a newly diagnosed case of collagenous colitis was observed. Post-FMT, significant changes in microbiota were observed, characterised by the transition from a low- to a greater-diversity profile. Therefore, FMT restores eubiosis and maintains it over time.
Conclusion:
FMT is a safe and effective treatment option in RCDI patients. This procedure induces profound microbiota changes that explain its high clinical efficacy.
Keywords: Clostridioides difficile infection, faecal microbiota transplantation
Introduction
Faecal microbiota transplantation (FMT) consists of integrating the intestinal microbiota of healthy individuals into the gut of patients with dysbiosis in order to reconstitute a normal microbial composition.1 Because of that, this approach has been found particularly effective in patients with Clostridioides difficile infection (CDI).2 Another potential explanation of the success of FMT comes from the fact that bactericidal and bacteriostatic proteins (bacteriocins) produced by bacterial species introduced by FMT can also be highly effective against C. difficile.2 Furthermore, microbiota stimulates the mucosal immune system, which enforces gut barrier defences. Indeed, after FMT, an increased abundance of bacterial genera known to provide resistance to the germination and growth of C. difficile has been found.3 Thus, guidelines for the treatment of CDI recurrent and refractory (RCDI) to therapy indicates FMT as a reasonable therapeutic option,4 as a result of the significant success rate of this technique,4 its excellent cost–benefit ratio,5 and the low number of serious side effects related to the procedure.4
Beyond its clinical interest, this is a research field that arouses a great scientific interest, because very little evidence on the long-term consequences of this therapy is currently available in the literature.1 In their meta-analysis, Lai et al. evaluated the short-term side effects of the procedure in 135 studies, including a total of 4493 patients.6 The most common side effects were related to the gastrointestinal tract, specifically diarrhoea (13.0%), abdominal distension/flatulence (11.6%), nausea/vomiting (6.1%), abdominal pain (5.5%) and constipation (2.1%). Other common extraintestinal side effects included fever (2.7%), breathing difficulties (2.4%), headache (1.5%) and fatigue (1.4%). It is worth noting that, after FMT, 1.3% of patients experienced the onset of inflammatory bowel disease (IBD), its relapse if previously diagnosed, or the onset of IBD-like symptoms.
Despite the great potential that lies behind this technique for the treatment of CDI, it has not been possible to propose it systematically as standard of care due to the lack of clear and consistent regulation.4 For this purpose, on May 2018 the ‘National Program on Human Faecal Microbiota Transplantation (FMT)’ was launched in Italy – a post-experimental phase in collaboration with the National Transplantation Center for the application of FMT in the context of RCDI treatment (i.e. in case of recurrent infection or if it appears within 8 weeks of the last standard antibiotic therapy with vancomycin or fidaxomicin administered for 10 days).7
To note, this programme classifies FMT as tissue transplantation, in contrast with the current guidelines from the United States, France and the United Kingdom,8 where this procedure is considered as drug administration.
Our study reports the experience we gained in FMT at our Center (Padua Hospital), one of the few in Italy to perform FMT. The aim of our research was to evaluate the efficacy and safety of FMT in RCDI and to evaluate the modifications of the recipient’s gut microbiota in the medium–long term.
Materials and methods
In our prospective and monocentric study, we collected clinical and laboratory data of patients with RCDI treated with FMT, as administered by colonoscopy at the Gastroenterology Unit of ‘Azienda Ospedaliera di Padova’, from February 2016 to October 2019. The study was approved by the Ethical Committee for Clinical Practice (n. 34358, 24/06/2015). Written informed consent was obtained from all eligible participants or their legal representatives before participation. The purposes of our study were to evaluate: (a) the clinical recovery of the infection and its maintenance up to 24 months post-procedure; (b) the mean hospital stay of patients treated with FMT, as expenditure main indicator in the treatment of CDI; (c) the safety of FMT, with particular attention to long-term side effects, for which still few data are available in the literature; (d) the evolution of recipient’s microbiota, through metagenomic investigations on faeces at baseline (before transplant) and 7 days, 4–6 months and 12–24 months post-FMT.
Donor selection
The donors enrolled in our study were subjects of both sexes, age ⩾18 years, of Italian nationality, not family-related with recipients, and who did not present evidence of illness on the basis of the anamnestic data collected and the results of serum and faecal screening tests as indicated in the National Program.7 We setup a register of donors that included information of each donor as well as their medical records, the results of the screening tests, the results of the analysis of the faecal microbiota and consent to the donation. A doctor (BB) of the staff in charge of the paper records, updated the screening analyses that every donor underwent (every 6 months initially, then every 3 months according to the new guidelines), re-evaluating their suitability based on the microbiota screening and analysis reports.4
Recipient inclusion and exclusion criteria
Recipient inclusion criteria, according to 2017 consensus on the FMT,4 were as follows.
(1) Relapsing CDI: three recurrences of mild or moderate infection, two episodes of mild or complicated infection.
(2) Refractory CDI: absence of clinical improvement after a week of specific antibiotic treatment.
(3) Positive enzyme immunoassay for C. difficile enzyme immunoassays (EIA) toxin or molecular test for faecal C. difficile (CD) specific toxin gene (locus locus).
(4) Informed consent obtained.
Recipient exclusion criteria
Recipient exclusion criteria were4:
(1) under 18 years of age;
(2) active gastroenteritis due to pathogens other than CD;
(3) neutropenia ⩽0.5 × 109/l;
(4) radiological evidence of toxic megacolon or intestinal perforation [abdominal ultrasound, computed tomography (CT) scan or X-ray];
(5) presence of colostomy;
(6) contraindications to colonoscopy;
(7) any condition that, according to the attending physician, might endanger the patient’s health;
(8) pregnancy.
Follow up of the recipient post-FMT
After the procedure, the response was evaluated based on the clinic and the normalisation of laboratory parameters [white blood cell count (WBC), C-reactive protein (CRP), faecal calprotectin (FC)] at 1 week and 1, 2, 6 and 12 months following FMT. The presence of Clostridium toxin was not analysed because, as reported in the literature, it can remain positive up to 6 weeks post-treatment, even though the treatment is considered successful.9 It is worth noting that all patients who did not respond to treatment after 1 week could undergo the procedure again.
Preparation of the faecal material
The faecal material is diluted with sterile physiological solution and mixed with a spatula to homogenise it. A 30 g sample of faeces and 150 ml of physiological solution should be used. A sterile magnet is inserted into the preparation in order to shake it and homogenise it further with a stirrer. The material is then poured into a sterile container through a funnel on which sterile gauze or similar filtration system (e.g. sterilisable metal filter) has previously been placed, thus allowing filtration of the product and consequent separation of insoluble pieces. Then, 50 ml sterile Falcon tubes are prepared for storing the preparation, filled with glycerol in order to obtain a final concentration of 10%. The quantities of faeces–physiological solution–glycerol reflect the model proposed by Costello et al.,10 which verified the superiority of the addition of glycerol compared with dilution with physiological solution alone, using a faeces:glycerol:physiological solution ratio of 2:7:1. The product is sealed in a first bag with a label that contains information about the donor, with the maximum limit date, time of administration of the product and the storage temperature. The transport of the product, placed in a second bag, takes place in an external container to avoid both damage to the bag and temperature changes. At this point the material can be utilised immediately, stored at −20° for up to 1 month or stored at −80° for up to 7 months. Furthermore, as per guidelines,1 a 5 g sample is stored at −80° for possible future examinations.
FMT procedure
After taking the patient’s usual bowel preparation with Macrogol, the preparation is administered during colonoscopy, releasing it from the ileo-cecal valve to the transverse colon, using syringes (previously loaded) connected to the colonoscope through a catheter. Re-feeding was possible starting 6–8 h after transplanting, reintroducing fibre-poor foods. It was recommended not to take proton pump inhibitors (PPIs) or antibiotics after the procedure.11
Metagenomic analysis of the faecal microbiota
For our investigation we relied on the next generation sequencing (NGS) service provided by BMR Genomics (Padua, Italy) which uses Illumina MiSeq Sequencer technology, a single integrated tool able to automatically perform all the steps of the metagenomic analysis through the variable regions V3-V4 of the 16S-RNA. During follow up, it was not possible to collect faecal samples from all patients, so the metagenomic analysis was performed only on those patients whose samples were available at each of the follow up times (pre-FMT and 1 week, 6, 12 and 24 months post-FMT).
Computational analysis of metagenomic data
Data obtained from the metagenomics were processed through STAMP137, a software capable of analysing taxonomic and functional profiles and performing a set of statistical surveys on them. Samples were grouped by time of collection and these groups were compared, up to the taxonomic category of the genus, excluding species as, very often, these are not identified and therefore not informative. The analysis of possible significance was performed by comparing pairs of groups through a two-sided non-parametric White t test.
The Shannon alpha-diversity (α-diversity) index, which measures the complexity of a community within a sample, has been calculated.
Results
Between February 2016 to October 2019, 20 FMT procedures were performed on 19 patients at the Gastroenterology Unit of the Padua Hospital, 11 using fresh stools and 9 frozen stools. Table 1 shows the main characteristics of patients undergoing FMT. Of the 19 patients treated, 17 were women (90%) and 2 men (10%), with an average age of 68 years (range 22–87). Two patients expelled the infused faecal material in the first hours following the procedure, whereas in one patient the cecum was not reached at colonoscopy and the faecal material was infused in the left colon. Of our series, 72.2% (13/20) were over 65 years old, 33.3% (6/20) were immunocompromised and the same percentage were on proton pump inhibitors. Before resorting to FMT, all patients had been treated previously with several cycles of antibiotics (an average of 1.8 antibiotics): all patients were treated with at least one cycle of Vancomycin, 47.36% of the patients had received at least one cycle of metronidazole and 36.84% at least one course of therapy with Fidaxomicin.
Table 1.
Main characteristics of patients treated with FMT.
| Patient code | Age | Gender | Total number of relapse | Risk factor for relapse | Previous antibiotics |
Fresh or frozen stools | Full colonoscopy | Efficacy | Relapse post-FMT |
|---|---|---|---|---|---|---|---|---|---|
| 1c | 70 | F | 4 | 4 | V, M | Fresh | YES | YES | YESd |
| 2 | 46 | M | 4 | 0 | V, M | Fresh | YES | YES | NO |
| 3 | 87 | F | 3 | 3 | V, F | Fresh | YES | YES | NO |
| 4 | 69 | F | 3 | 2 | V, M | Fresh | YES | YES | NO |
| 5 | 52 | M | 2 | 2 | V, F | Fresh | YES | YES | NO |
| 6 | 77 | F | 5 | 2 | V, F | Fresh | YES | YES | NO |
| 7c | 70 | F | 5 | 0 | V, M | Fresh | YESa | NO | |
| 8 | 74 | F | 4 | 3 | V, F | Fresh | YES | YES | YESd |
| 9 | 51 | F | 4 | 2 | V, M | Fresh | YES | YES | NO |
| 10 | 81 | F | 3 | 3 | V, M, F | Fresh | YES | YES | NO |
| 11 | 22 | F | 3 | 1 | V | Fresh | YES | YES | NO |
| 12 | 86 | F | 3 | 3 | V | Frozen | YES | YES | YESd |
| 13 | 73 | F | 3 | 3 | V | Frozen | YES | YES | NO |
| 14 | 77 | F | 2 | 2 | V | Frozen | YES | YES | NO |
| 15 | 86 | F | 2 | 3 | V, F | Frozen | YES | YES | NO |
| 16 | 71 | F | 3 | 2 | V, M | Frozen | YESa | NO | NO |
| 17 | 63 | F | 2 | 2 | V | Frozen | YES | YES | NO |
| 18 | 79 | F | 3 | 2 | V, M, F | Frozen | YES | YES | NO |
| 19 | 78 | F | 3 | 2 | V, M | Frozen | NO | NO | −a |
| 20 | 52 | F | 2 | 2 | V | Frozen | YES | YES | NO |
F, Fidaxomicin; FMT, faecal microbiota transplantation; M, Metronidazole; V, Vancomycin.
Patients expelled the infused faecal material in the first hours following the procedure.
Patient without subsequent follow up, due to inefficacy of FMT.
1 and 7 were the same patient, treated with FMT twice.
Different relative abundance of g_Bacteroides in donor faeces: donor of P(1):51.51%; donor of P(8): 56.61%; donor of P(12):50.82% versus a mean value of 39.43%.
Overall, FMT was effective in 85% of treated patients. The mean hospital stay was 7.4 days (standard deviation 5.79 days). The longest hospitalisation time was 18 days (1 patient), whereas the shortest was 2 days (4 patients). Three patients experienced CDI relapse during follow up, the average number of risk factors (i.e. immunodepression; intake of proton pump inhibitors, age of over 65, female gender) in these patients being 3.3 compared with 2.2 in the group that did not experience disease recurrence (p = 0.1). One of the female patients who relapsed underwent FMT for recurrence again after 10 months from the first procedure with benefit.
Regarding side effects, we distinguished those occurring in the 5 days immediately following the procedure from those reported in the medium- to long-term follow up. In the first group we reported: fever (37.2°C) resolved the next day (1/20); abdominal pain (5/20); abdominal distension, bloating and flatulence (4/20); constipation (2/20); self-resolving diarrhoea for 2–3 days (3/20). In the medium- to long-term follow up we observed: frequent diffuse abdominal pains (4/20), urinary tract infections (4/20), recurrence of C. difficile infection (3/20), non-CDI-related and non-infectious diarrhoea (2/10), a newly diagnosed case of collagenous colitis. In the follow up, we observed only one death, 2 months after the procedure, due to cardiac causes.
For the metagenomic analysis, we used the faecal samples of 15 of the transplanted patients and their respective donors. Pre- and 1 week post-FMT samples were available for all 15 patients, 6 months follow up samples were available for 10 patients, and 1- to 2-year follow-up samples were available for 5 patients. First, we assessed the α-diversity indices within the different samples through the Shannon index. We found a statistically significant difference (p = 0.005) between the Shannon index of healthy donors (Shannon: 2.42) and that of pre-FMT patients (Shannon: 1.59). Likewise, the difference (p = 0.019) between patients before (Shannon: 1.59) and after (Shannon: 2.21) FMT was significant. On the other hand, the difference between the Shannon index between donors and patients faecal samples after FMT was not statistically significant (p = 0.084).
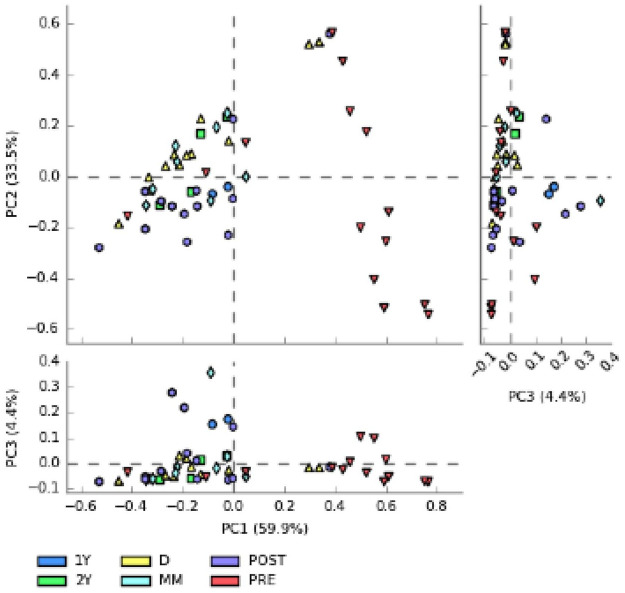
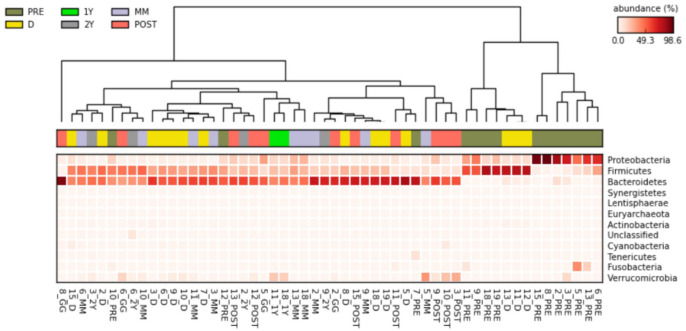
Subsequently, we assessed the difference between the different samples in the context of the principal component analysis (PCA) (Figure 1). As reported in the graph, PC1 represents the most informative axis, explaining most of the variability (59.9%). Interestingly, the cluster detached from the rest of data is the one representing samples from the pre-FMT patient group. Then, we obtained a heatmap plot (Figure 2), a graphic representation of the trend of the relative abundances at the phylum level, in which data are automatically sorted and clustered based on common characteristics. We identified two main clusters: one characterised by a greater relative abundance of Proteobacteria or Firmicutes and a low concentration of Bacterioidetes (Cluster 1), the other characterized by high concentration of Bacterioidetes and low concentration of Proteobacteria (Cluster 2). Cluster 1, and particularly the sub-cluster characterized by a high concentration of Proteobacteria, is populated mainly by samples of patients with CDI before treatment. Instead, the other sub-cluster is characterized by the abundance in Firmicutes compared with Bacterioidetes. In addition, this second sub-cluster is composed mainly of pre-FMT patient samples, although in a lower proportion. In the cladogram (Figure 3) we represented the phylogenetic tree of the bacterial species found in the pre- and post-FMT faecal samples. Taxa with the statistically significant differences highlighted were: Bacteroidetes phylum, more abundant in post-FMT; Proteobacteria phylum, more abundant in pre-FMT; Bacteroidaceae family, more abundant in post-FMT; Enterobacteriaceae family, more abundant in pre-FMT; Bacteroides and Faecalibacterium genus, more abundant in post-FMT; Proteus genus, more abundant in the pre-FMT.
Figure 1.
Principal component analysis. We evaluated the differences between the principal components and the PC1, that is the most informative with the 59.9% of the variability. There is a separate cluster from the cloud of data, mainly characterized by PRE samples.
1Y: stool samples collected at 1 year post-FMT; 2Y: stool samples collected at 2 year post-FMT; D, donor’s stool samples; FMT, faecal microbiota transplantation; PRE: all recipient samples pre-FMT; MM: stool samples collected at 4–6 months post-FMT; PCA, principle component analysis; POST: all recipient samples post-FMT.
Figure 2.
Heatmap plot of Phyla’s abundance distribution. In this type of representation, the data is automatically sorted and clustered on the basis of common characteristics.
1Y: stool samples collected at 1 year post-FMT; 2Y: stool samples collected at 2 year post-FMT; D, donor’s stool samples; FMT, faecal microbiota transplantation; PRE: all recipient samples pre-FMT; MM: stool samples collected at 4–6 months post-FMT; PCA, principle component analysis; POST: all recipient samples post-FMT.
Figure 3.
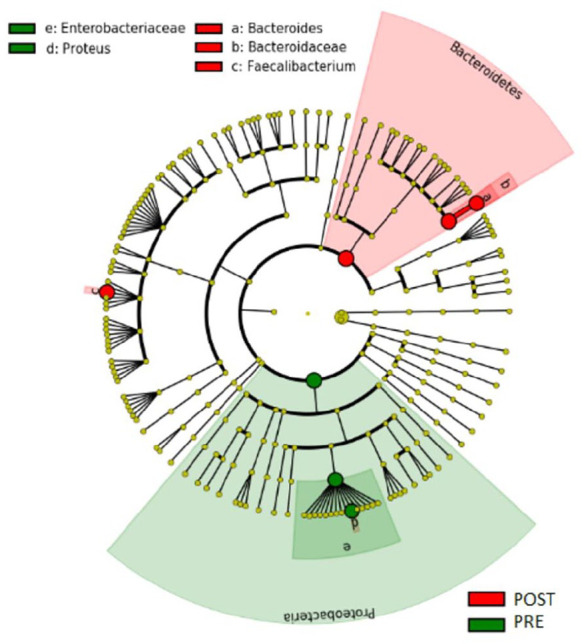
Cladogram with different taxa. This is a targeted analysis that takes into consideration pairs of groups of patient samples before and after the faecal transplantation. a, b and c were the taxa with statistically significant difference in the stool samples post-FMT. e and d were the taxa with statistically significant difference in the stool samples pre-FMT.
FMT, faecal microbiota transplantation; PRE: all recipient samples pre-FMT; POST: all recipient samples post-FMT.
We subsequently built a bar plot to check statistically significant trends of those taxa in the different patients (Figure 4). Interestingly, levels of the Faecalibacterium genus increased significantly in the post-FMT compared with the pre-FMT samples, though remaining always lower than 5% and different from the greater proportion present in donors (p < 0.001). During follow up, their concentration progressively increased over time in the various faecal samples analysed. Through a non-parametric White t test we compared the relative abundance of Faecalibacterium in the different groups under examination: we observed that it remains significantly reduced in FMT recipients compared with donors, even at 4–6 months follow up, and then increases after 1–2 years.
Figure 4.
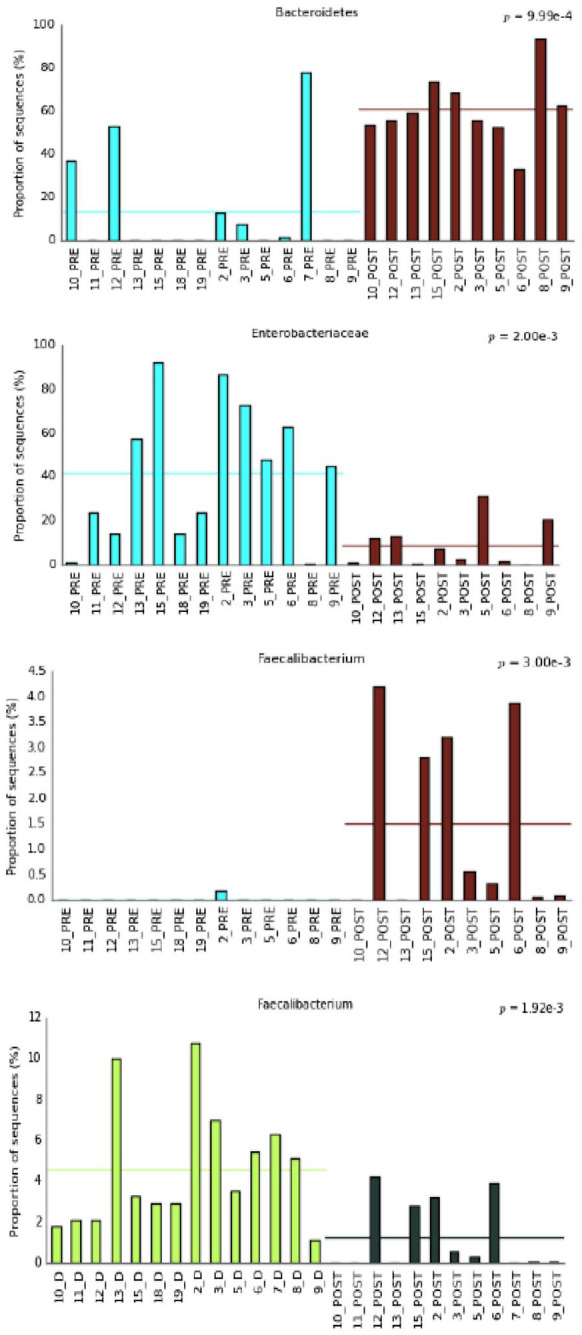
Bar plot showing statistically significant trends of taxa in the different patients.
D, donor’s stool samples; FMT, faecal microbiota transplantation; PRE: all recipient samples pre-FMT; POST: all recipient samples post-FMT.
To note, we observed that in the feces of donors associated to FMT-failure there was a high abundance of Bacteroides compared to the feces of donors associated to FMT-success (Table 1).
Discussion
FMT is the process of transplantation of faecal bacteria from a healthy individual into a recipient. Despite its great potential, in Italy the procedure was only recently approved in clinical practice and only in patients with CDI. Data about the feasibility, effectiveness and safety of this procedure are urgently required. Thus, we decided to analyse our experiences with this novel therapeutic option and report the data obtained in clinical practice. In our study population, the total success rate was 85%, in line with literature reports,12–15 which suggests that our hospital met the criteria of adequate preparation and conservation of the faecal emulsion and FMT procedure. Thus, our case study, one of the first in Italy, show that FMT is an effective and safe technique for the treatment of both recurrent and refractory C. difficile infections.
As previously mentioned, the total success rate of FMT in patients suffering from recurrent CDI unresponsive to antibiotic treatments was 85%, whereas the literature shows an 81–94% success rate of FMT.12–15 We have to mention, however, that we had a recurrence of infection in 3 out of 20 patients. Of these, two had taken antibiotic therapy for other infections (urinary tract and bronchopneumonia) whereas in the third full FMT was not possible due to technical difficulties, hence the faecal solution was infused distally to the cecum. The average hospitalisation time in our cases was lower than described in literature for patients treated with standard antibiotic therapy. Indeed, from the retrospective study of Petrosillo,16 conducted in five Italian hospitals from 2011 to 2014, in which 503 episodes of C. difficile infection were treated with conventional antibiotic therapy, the average length of hospitalisation was 14.6 days [±13, standard deviation (SD)] whereas we had a mean hospital stay of 7.4 days (SD 5.8 days). We reported a hospitalisation time lower than that reported in the literature for FMT patients who show, overall, a hospitalisation time of 13.3 days, compared with that found in patients treated with conventional therapy (29.7 days).17 Note that, within our population, although data did not allow reach statistical significance, likely due to the small sample size, the average hospital stay after FMT was reduced by 50% compared with that with traditional antibiotic therapy.
In general, the procedure proved to have a good safety profile. The most frequent side effects in the hours following the transplant were mild and in most cases they resolved spontaneously. Regarding side effects observed immediately following FMT, we recorded onset of mild fever in a patient (quickly self-resolved), onset of cramp-like abdominal pain and constipation. Moreover, we observed four cases of urinary tract infections, one case of bronchopneumonia and one of sepsis from Klebsiella pneumoniae at 6-month follow up, unlikely related to FMT, given the long time lapse. The cases of post-FMT urinary tract infections are also difficult to correlate with FMT, which, on the other hand, could be attributable primarily to risk factors related to gender – being most frequent in female patients – and age of the patients. The most frequently found etiological agent was Pseudomonas aeruginosa (3/4). We therefore searched the metagenomics of each patient’s stool samples for a trace of the causative agent prior to the infection. In none of the cases of Pseudomonas infection we found traces of 16S RNA attributable to Pseudomonadaceae; hence, we can reasonably exclude that it developed from the colonic microbiota. Of these six cases of infections treated with antibiotic therapy, two had a recurrence of CD. In a long-term analysis by Lee et al. conducted on 23 patients, 12/23 had taken antibiotic therapy for other types of infection during FMT follow-up (urinary tract, dental, appendicitis, pneumonia, sinusitis, cellulitis) and none developed a recurrence of CDI.18 It should be noted that, in the literature, there is evidence of rare severe adverse events subsequent to FMT that seriously compromised patients health. In some cases, they were preventable with adequate donor screening, such as the recent case of a multi-drug resistant (MDR) infection of two immunocompromised patients following transplantation of a donor’s colonised faeces that were not adequately screened.19 However, we did not experience any such effect in our cases.
The metagenomic study of patients stool samples, collected before and after FMT, showed a significant change in the microbiota, characterised by the transition from a low diversity profile (typical of clear dysbiosis) to a profile with greater α-diversity, comparable with that of healthy donors. Therefore, the procedure restores the eubiosis and maintains it over time. In particular, the element that suggests the immediate eubiotic effect of FMT is the abrupt drop in the relative abundance of Proteobacteria, a bacterial Phylum known to be related to dysbiosis and inflammatory status. Proteobacteria, which were significantly more abundant in the patients’ samples before performing the procedure, belong to a bacterial phylum whose expansion (especially in the context of the Enterobacteriacee family, also found significantly increased) is correlated with dysbiosis and impairment of the ability to maintain a balance within microbial communities.20 The fact that some patients presented an increase in Firmicutes compared with Bacteroidetes is another significant element. Firmicutes are one of the main phyla constituting the microbiota, whose excess, compared with the Bacterioidetes phylum, has been linked to pathological conditions of an inflammatory (e.g. IBD), metabolic (e.g. obesity) or dysbiosis nature. C. difficile itself belongs to the Firmicutes.20,21 It can be suspected that the inflammatory state resulting from the infection accounts for this altered ratio Bacterioides: Firmicutes, unbalanced towards the Firmicutes. Another striking element is the increase in Faecalibacterium prausnitzii, which, although decreased significantly in samples before the transplant, gradually normalises 1 year after FMT. This also suggests that, although FMT generates a major and radical change in the gut microbiota, the complete eubiosis process can take more than 4–6 months.
The fact that, despite the immediate evident change in microbiota composition already a week after FMT, the microbiota continues to remodel itself towards a eubiotic status during many months from the transplant, is extremely interesting. It is therefore crucial to try to maintain the health of the recipient’s microbiota at least during the first year after the procedure. This finding is in keeping with the scientific literature; in fact Jalanka et al. also showed recently that the intestinal microbiota composition of a C. difficile infection patient could be relatively permanently altered with FMT to resemble that of the donor, and that this shift in the microbial composition lasted throughout the 1-year follow-up period.22
In the follow up, we observed only one death, 2 months after the procedure, due to cardiac causes in a patient who had a history of decompensated diabetes mellitus and two previous revascularisation interventions for ischemic heart disease. One patient developed collagenous colitis 1 year after FMT. Only one case report of post-FMT collagenous colitis is reported in the literature; however, in that case the onset was earlier, 2 months post-FMT. The pathogenesis of microscopic colitis is complex, multifactorial and poorly understood. However, current concepts point to innate immunity or microbiome alterations as well as gut barrier dysfunction, all of which lead to the development of subtle inflammatory lesions in gut mucosa. The results of numerous basic and clinical studies involving molecular techniques as well as advanced endoscopic imaging revealed the important role of both intrinsic (e.g. hormonal) as well as extrinsic (e.g. nonsteroidal anti-inflammatory drugs and proton-pump inhibitors) factors in the modulation of gastrointestinal microbiome and microscopic colitis pathogenesis.23
Despite the satisfying results obtained, our study presents some limitations. First, the follow up was challenging because often it was impossible to reach the patient and collect samples for microbiome analysis. Moreover, the antibiotic treatment protocol prescribed prior to FMT was not standardized. Results could have also been affected by diversities in the patient population with variations in comorbidities. Further, our study is limited by the small sample size, single centre design and the lack a control group, which may have led to under/overestimation of some results, and limiting the generalisability of our results. The main strengths of our study include the long follow up and the use of universal faecal donors who were screened by consensus donor screening recommendations at the time.1,4,7
This follow-up study has demonstrated that FMT is an effective treatment option in our cohort of RCDI patients. The durability of the procedure was supported by a large post-FMT disease-free interval of 24 months, with low rates of FMT-related complications. In addition, FMT was also able to maintain patient eubiosis in the long run, similar to what is observed in healthy subjects.
Footnotes
Author contributions: BB, SF, EVS: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content.
BB, SF, EM, RD, GCS, FF, FZ, AQ, MG, DM, CC: acquisition of data, and critical revision of the manuscript.
BB, SF, AQ: statistical analysis.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Brigida Barberio  https://orcid.org/0000-0002-3164-8243
https://orcid.org/0000-0002-3164-8243
Fabiana Zingone  https://orcid.org/0000-0003-1133-1502
https://orcid.org/0000-0003-1133-1502
Edoardo Vincenzo Savarino  https://orcid.org/0000-0002-3187-2894
https://orcid.org/0000-0002-3187-2894
Contributor Information
Brigida Barberio, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Sonia Facchin, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Edoardo Mele, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Renata D’Incà, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Giacomo Carlo Sturniolo, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Fabio Farinati, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Fabiana Zingone, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Andrea Quagliariello, Department of Comparative Biomedicine and Food Science (BCA), University of Padova, Legnaro, Italy.
Matteo Ghisa, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Davide Massimi, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Cesare Casadei, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, Italy.
Edoardo Vincenzo Savarino, Division of Gastroenterology, Department of Surgery, Oncology and Gastroenterology, University of Padua, Via Giustiniani 2, Padua, 35128, Italy.
References
- 1. Cammarota G, Ianiro G, Gasbarrini A, et al. Fecal Microbiota Transplantation for the treatment of Clostridium difficile infection. J Clin Gastroenterol 2014; 48: 693–702. [DOI] [PubMed] [Google Scholar]
- 2. Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol 2016; 13: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown JRM, Flemer B, Joyce SA, et al. Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol 2018; 18: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017; 66: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dehlholm-Lambertsen E, Hall BK, Jørgensen SM, et al. Cost savings following faecal microbiota transplantation for recurrent Clostridium difficile infection. Therap Adv Gastroenterol 2019; 12: 1756284819843002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai CY, Sung J, Cheng F, et al. Systematic review with meta-analysis: review of donor features, procedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment Pharmacol Ther 2019; 49: 354–363. [DOI] [PubMed] [Google Scholar]
- 7. Ministero Della Salute. Programma Nazionale Trapianto di Microbiota Fecale: aspetti regolatori, clinici e organizzativi. 2018; 0–76. [Google Scholar]
- 8. Cani PD. Human gut microbiome: hopes, threats and promises. Gut 2018; 67: 1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults. Infect Control Hosp Epidemiol 2010; 31: 431–455. [DOI] [PubMed] [Google Scholar]
- 10. Costello SP, Conlon MA, Vuaran MS, et al. Faecal microbiota transplant for recurrent Clostridium difficile infection using long-term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther 2015; 42: 1011–1018. [DOI] [PubMed] [Google Scholar]
- 11. Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep 2013; 15: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mattila E, Uusitalo-Seppala R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012; 142: 490–496. [DOI] [PubMed] [Google Scholar]
- 13. Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- 14. Sha S, Liang J, Chen M, et al. Systematic review: faecal microbiota transplantation therapy for digestive and non-digestive disorders in adults and children. Aliment Pharmacol Ther 2014; 39: 1003–1032. [DOI] [PubMed] [Google Scholar]
- 15. Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017; 46: 479–493. [DOI] [PubMed] [Google Scholar]
- 16. Petrosillo N, Ravasio R. The cost of Clostridium difficile infection in hospital in Italy. Glob Reg Heal Technol Assess Ital North Eur Spanish 2017; 4: 5000257. [Google Scholar]
- 17. Ianiro G, Murri R, Sciumè GD, et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: a prospective cohort study. Ann Intern Med 2019; 171: 695–702. [DOI] [PubMed] [Google Scholar]
- 18. Lee CH, Chai J, Hammond K, et al. Long-term durability and safety of fecal microbiota transplantation for recurrent or refractory Clostridioides difficile infection with or without antibiotic exposure. Eur J Clin Microbiol Infect Dis. 2019; 38: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 19. FDA. Important Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Risk of Serious Adverse Reactions Due to Transmission of Multi-Drug Resistant Organisms 2019. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse.
- 20. Shin NR, Whon TW, Bae JW, et al. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015; 33: 496–503. [DOI] [PubMed] [Google Scholar]
- 21. Kho ZY, Lal SK. The human gut microbiome - a potential controller of wellness and disease. Front Microbiol 2018; 9: 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jalanka J, Mattila E, Jouhten H, et al. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med 2016; 14: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Hemert S, Skonieczna-Żydecka K, Loniewski I, et al. Microscopic colitis-microbiome, barrier function and associated diseases. Ann Transl Med 2018; 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]