Abstract
The new type of pneumonia caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is endemic worldwide, and many countries cannot be spared, becoming a global health concern. The disease was named COVID-19 by the World Health Organization (WHO) on January 30, 2020, when the WHO declared the Chinese outbreak of COVID-19 to be a public health emergency of international concern. The clinical features of COVID-19 include dry cough, fever, diarrhea, vomiting, and myalgia. Similar to SARS-CoV and MERS-CoV, nearly 20% of patients experienced various fatal complications, including acute kidney injury and acute respiratory distress syndrome caused by cytokine storm. Furthermore, systemic cytokine storm induced vascular endothelial injury, which extensively mediates hypercoagulability in blood vessels and disseminated intravascular coagulation. The autopsy pathology of COVID-19 confirmed the above. This article briefly summarizes the mechanism of hypercoagulability and thrombotic complications of severe COVID-19 and proposes that blood hypercoagulability and intravascular microthrombosis are the development nodes of severe COVID-19. Therefore, anticoagulation and anti-inflammatory therapy can be used as important treatment strategies for severe COVID-19.
Keywords: COVID-19, SARS-CoV-2, Disseminated intravascular coagulation, inflammation, pulmonary embolism, Thrombosis, anticoagulants
SARS-CoV-2 Structure and Pathogenesis
Structure
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)1,2 belongs to the genera betacoronavirus containing single-stranded (positive-sense) RNA associated with a nucleoprotein within a capsid comprised of matrix protein. All the structural and accessory proteins are translated from the single guide RNAs of CoVs. The genetic and phenotypic structure of COVID-19 in the pathogenesis is important.3
The Role of Angiotensin-Converting Enzyme 2 in Pathogenicity
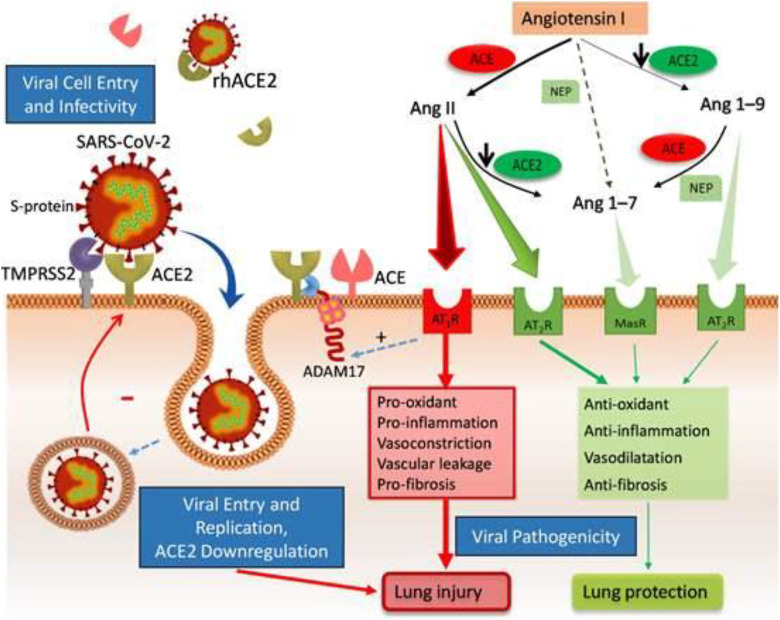
After being cleaved by serine protease protein, SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) receptor through its protuberances, enters and infects alveolar epithelial cells, macrophages, perivascular cells, cardiomyocytes, and further the virus is unshelled for gene transcription and translation.3,4 In addition to being SARS-CoV-2 receptor, ACE2 has physiological functions: the negative regulator of the renin–angiotensin system (RAS) and the promotion of amino acid transport.5 Angiotensin-converting enzyme 2 is widely expressed, including lung, cardiovascular system, intestine, kidney, central nervous system, and adipose tissue.6 A COVID-19 study found that cardiac pericytes express ACE2, which is related to many patients with COVID-19 accompanied by underlying cardiac lesions.7 Other studies also believed that by blocking the kinin–kallikrein system regulated by ACE2, it may reduce the occurrence of pulmonary edema (PE) and acute respiratory distress syndrome (ARDS).4,8,9
Angiotensin-Converting Enzyme 2 Mediates Hemostatic Disorders by Reducing Fibrinolytic Activity
The dynamic balance of the hemostasis and anticoagulation fibrinolysis system is the basis to ensure the flow state of blood fluid, neither bleeding out of the blood vessel nor forming thrombus in the blood vessel.10 This is a complex and huge system with many factors, but the regulation of the vascular endothelium and RAS plays an important role. Angiotensin-converting enzyme 2 is an important component of the RAS and is expressed in vascular endothelial cells. It is a key link that affects blood hypercoagulability and thrombosis. Research has shown that ACE2 alternatively converts angiotensin (Ang) II into Ang-(1-7) and Ang I into Ang-(1-9) (Figure 1). Ang-(1-9) enhanced thrombosis development, decreased plasma concentration of tissue plasminogen activator (t-PA), and increased the level of plasminogen activator inhibitor-1 (PAI-1).11 Therefore, the balance between t-PA and PAI-1 is broken and the production of plasmin is reduced, resulting in an increased risk of thrombosis in blood vessels.5,6
Figure 1.
The pathogenesis of COVID-19 binding to angiotensin-converting enzyme 2 (ACE2) receptor and the mechanism of ACE2 regulating blood hypercoagulability. Figure from the network: http://home.xue63.com/wendangku/z1s/f14g/j0c58a868dcv/k5022aaea998fcc22bcd127ff425fl.html
COVID-19—Related Hypercoagulant and Thrombotic Complications
Many literatures reported that the changes in the coagulation system of COVID-19 patients generally show enhanced coagulation and thrombosis.12,13 Complications of severe and critical COVID-19 include ARDS, embolism or pulmonary thrombosis, and hypercoagulable state.14–18 COVID-19 accompanied by hypercoagulable state and the occurrence of thrombotic diseases and microthrombus can be seen in cardiac vessels, hepatic portal area, and renal interstitium. The autopsy pathology of COVID-19 confirmed this.19,20 COVID-19-related hypercoagulant and thrombotic complications may be due to the following pathological mechanisms.
General Mechanism
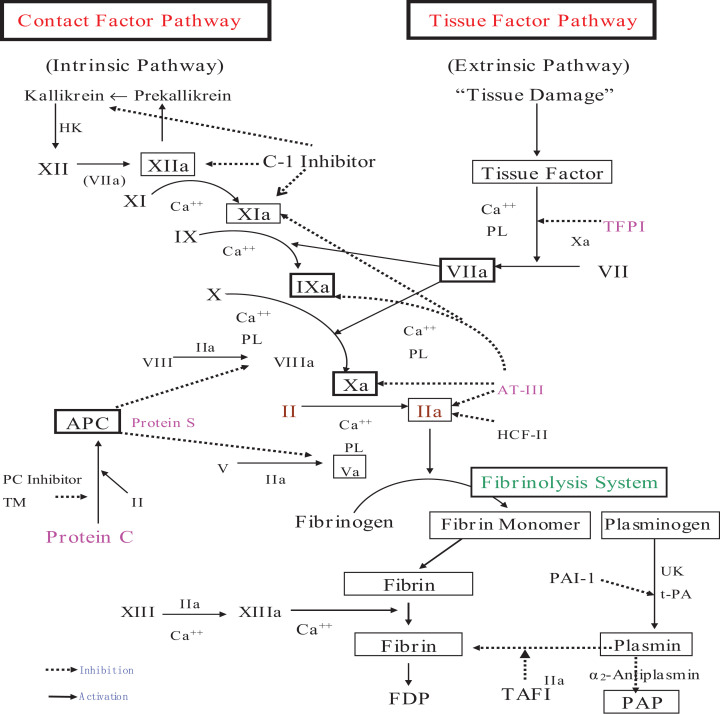
There are 2 aspects of factors for the formation of hypercoagulability and thrombotic diseases: one is the enhancement of coagulation, the other is the weakening of anticoagulation and fibrinolysis. From the coagulation cascade (Figure 2), we can see that the enhancement of coagulation can be initiated through an intrinsic or extrinsic pathway, causing the chain-level expansion of coagulation factors and thrombin formation. Many factors are activators of the coagulation system, and damage to the vascular endothelium and extrinsic tissue factors are the main promoters of coagulation. The core of the fibrinolytic system is plasmin, which is used to further dissolve the fibrin, fibrinogen, and fibrin monomer cross-linking. Plasmin is activated by plasminogen under the action of t-PA and then quickly combines with α2-antiplasmin to form plasmin–antiplasmin (PAP) complex. Vascular endothelial cells secrete t-PA, which is usually in the form of tPA-PAI-1 complex (tPAIC), and there is abundant thrombomodulin (TM) under vascular endothelial cells, which can be activated by thrombin, thereby activating the protein C pathway anticoagulant. Therefore, PAP, tPAIC, and TM can be used as molecular markers for early monitoring of hypercoagulability and microthrombosis in blood vessels.21 Vascular endothelial cells are an important link in regulating the coagulation, anticoagulation, and fibrinolysis systems and are closely related to the body’s hypercoagulable state and the occurrence of thrombotic diseases. This may lead to myocardial dysfunction and damage, endothelial dysfunction, microvascular dysfunction, plaque instability, and myocardial infarction (MI).4
Figure 2.
Coagulation, anticoagulation, and fibrinolysis.
Renin–Angiotensin System and Cytokine Storms Work Together to Extensively Form Microvascular Thrombosis
Inflammation and coagulation reactions interact through multiple links to form an automatically amplified cascade effect.22 Without intervention, it may lead to diffuse vascular endothelial damage, organ dysfunction, and eventually death. Ang II in the RAS system not only has the effect of raising blood pressure but also affects the function of the coagulation system, fibrinolysis system, and platelets and is involved in atherosclerosis, acute MI, and other diseases related to the occurrence and development of thrombosis.23,24
Thrombosis Directly Leads to Severe COVID-19 and/or Death
The SARS-CoV-2 binds to the ACE2 receptor and enters the cell, causing extensive tissue and organ endothelial cell damage. At the same time, the coagulation activity that mediates the regulation of the RAS is enhanced, the anticoagulation and fibrinolytic activity are weakened, and extensive thrombosis is present in the blood vessel, further leading to DIC and secondary fibrinolysis.25 Inflammatory factors mediate the increase of fibrinogen, which further promotes the conversion to severe COVID-19, such as ARDS and alveolar fibrin exudation and PE. Extensive hypercoagulability and the diffuse intravascular thrombosis are also the cause of direct fatal diseases such as pulmonary embolism.18
Treatment Strategies of COVID-19-Related Hypercoagulant and Thrombotic Complications
Monitoring the Development Nodes of Severe COVID-9
The treatment of severe COVID-19 is the key to reducing mortality and improving the cure rate. Based on the fact that severe COVID-19 is closely related to the hypercoagulable state of blood and thrombotic diseases, finding the node for the conversion of blood to hypercoagulable state is one of the indications for the general conversion of COVID-19 to severe COVID. Thrombosis markers and blood hypercoagulability markers, such as PAP, TM, and tPAIC, can be used as auxiliary indicators.
Anti-Inflammatory Therapy Treatment
Inflammatory factor storm is an important factor leading to blood hypercoagulability and thrombotic diseases, sepsis, ARDS, and DIC.22 Anti-inflammatory treatment can be used as a general program of COVID-19 and has universality.
Anticoagulant Therapy
Once COVID-19 has blood hypercoagulability and thrombotic disease complications, anticoagulation therapy should be performed.26 Through anticoagulation therapy, the activity of coagulation factors is reduced, the blood flow state is maintained, and the body’s tissues and organs are fully perfused and oxygen is supplied. It should be noted that, during anticoagulation therapy, the coagulation index should be closely monitored to prevent excessive anticoagulation from bleeding complications.
Blood Purification Systems
In COVID-19-related acutely severe patients, cytokine-storm-targeted therapy is recommended for the treatment of severe lung failure secondary to excessive inflammation.26 Some studies have shown that cytokine/chemokine clearance can be achieved through blood purification systems, such as plasma exchange, plasma absorption, and hemofiltration/plasma filtration. It has been confirmed in the treatment of patients with severe H7N9 influenza infection that blood purification systems remarkably reduced the levels of 17 cytokines/chemokines.27 Blood purification system has a good therapeutic effect in the treatment of heavy COVID-19, and the positive results proved to significantly reduce the level of cytokines/chemokines and increase coagulation factors, fibrinolysis, and anticoagulant activity, thereby reducing inflammatory storms and maintaining the stability of the body internal environment and stable blood flow.28–30
Conclusion
COVID-19 is often accompanied by thrombotic complications. Inflammatory factors and enhanced ACE2-mediated activity of the RAS expressed by vascular endothelial cells are associated with COVID-19-related thrombotic complications. The molecular markers of thrombosis such as PAP, TM, and tPAIC can be used for the diagnosis of severe COVID-19 conversion nodes. Anti-inflammatory and anticoagulation therapy can be used as the treatment strategies for preventing thrombotic complications and serious diseases such as ARDS and pulmonary embolism due to COVID-19.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Haimei MA  https://orcid.org/0000-0002-2715-7732
https://orcid.org/0000-0002-2715-7732
References
- 1. World Health Organization. WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. Published February 11, 2020 https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed May 7, 2020.
- 2. World Health Organization. Novel Coronavirus (2019-nCoV), Situation Report-12 (2020).
- 3. Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis [published online ahead of print March 31, 2020]. J Microbiol Immunol Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options [published online ahead of print April 30, 2020]. Cardiovasc Res. 2020;cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gheblawi M, Wang K, Viveiros A, et al. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system [published online ahead of print April 8, 2020]. Circ Res. 2020;126(10):1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target—a perspective [published online ahead of print April 27, 2020]. Endocr Metab Immune Disord Drug Targets. 2020 doi:10.2174/1871530320666200427112902 [DOI] [PubMed] [Google Scholar]
- 7. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van de Veerdonk FL, Netea MG, van Deuren M, et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome [published online ahead of print April 27, 2020]. Elife. 2020;9:e57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muciño-Bermejo J, Carrillo-Esper R, Uribe M, Méndez-Sánchez N. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol. 2013;12(5):713-724. [PubMed] [Google Scholar]
- 11. Mogielnicki A, Kramkowski K, Hermanowicz JM, Leszczynska A, Przyborowski K, Buczko W. Angiotensin-(1-9) enhances stasis-induced venous thrombosis in the rat because of the impairment of fibrinolysis. J Renin Angiotensin Aldosterone Syst. 2014;15(1):13-21. [DOI] [PubMed] [Google Scholar]
- 12. Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure [published online ahead of print April 21, 2020]. Thromb Haemost. 2020;120(6):998–1000. doi:10.1055/s-0040-1710018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines [published online ahead of print, April 21, 2020]. Thromb Haemost. 2020;120(6):937–948. doi:10.1055/s-0040-1710019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? [published online ahead of print April 29, 2020]. Thromb Haemost. 2020. doi:10.1055/s-0040-1712097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19 [published online ahead of print April 23, 2020]. J Thromb Haemost. 2020;18(7):1548–1555. doi:10.1111/jth.14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Idell S, James KK, Levin EG, et al. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84(2):695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online ahead of print April 10, 2020]. Thromb Res. 2020;191:145–147. S0049-3848(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. [DOI] [PubMed] [Google Scholar]
- 20. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng Y, Liu J, Su Y, et al. Clinical impact of coagulation and fibrinolysis markers for predicting postoperative venous thromboembolism in total joint arthroplasty patients. Clin Appl Thromb Hemost. 2019;25:1076029619877458 doi:10.1177/1076029619877458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allen KS, Sawheny E, Kinasewitz GT. Anticoagulant modulation of inflammation in severe sepsis. World J Crit Care Med. 2015;4(2):105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Groß S, Jahn C, Cushman S, Bär C, Thum T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: From basic science to clinical implications. J Mol Cell Cardiol. 2020;144:47–53. doi:10.1016/j.yjmcc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He M, He X, Xie Q, Chen F, He S. Angiotensin II induces the expression of tissue factor and its mechanism in human monocytes. Thromb Res. 2006;117(5):579-590. [DOI] [PubMed] [Google Scholar]
- 25. Lillicrap D. Disseminated intravascular coagulation inpatients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18(4):786-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge [published online ahead of print April 29, 2020]. Thromb Haemost. 2020;120(6):949–956. doi:10.1055/s-0040-1710317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu X, Zhang Y, Xu X, et al. Evaluation of plasma exchange and continuous veno-venous hemofiltration for the treatment of severe avian influenza a (H7N9): a cohort study. Ther Apheresis Dial. 2015;19(2):178–184. [DOI] [PubMed] [Google Scholar]
- 28. Xu K, Cai H, Shen Y, et al. Management of corona virus disease-19 (COVID-19): The Zhejiang Experience [in Chinese]. Zhejiang Da Xue Xue Bao Yi Xue Bao. 2020;49(1):0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Yu L, Tang L, et al. A Promising anti-cytokine-storm targeted therapy for COVID-19: the artificial-liver blood-purification system [published online ahead of print March 20, 2020]. Engineering (Beijing). 2020. doi:10.1016/j.eng.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang C, Huang S, Zheng F, Dai Y. Controversial treatments: an updated understanding of the coronavirus disease 2019 [published online ahead of print March 26, 2020]. J Med Virol. 2020. doi:10.1002/jmv.25788 [DOI] [PMC free article] [PubMed] [Google Scholar]