Abstract
Purpose
To investigate serum vitamin D levels in patients newly diagnosed with non-Hodgkin lymphoma/diffuse large B-cell lymphoma (NHL-DLBCL), multiple myeloma (MM) and chronic lymphocytic leukemia (CLL).
Patients and methods
We measured serum levels of vitamin D by ELISA in 103 patients prior to initiation of treatment, of whom 37 were diagnosed with MM, 32 with CLL and 34 with NHL-DLBCL.
Results
Suboptimal serum vitamin D levels (<30 ng/mL) were observed in all 103 patients. In 14 patients, serum vitamin D levels were between 20 and 30 ng/mL, while all other patients had vitamin D deficiency (<20 ng/mL). Severe vitamin D deficiency (<10 ng/mL) was observed in 32.3% of NHL-DLBCL patients, 28.1% of CLL patients and 81% of MM patients.
Conclusion
We observed low serum vitamin D levels in the majority of patients newly diagnosed with NHL-DLBCL, CLL and MM.
Keywords: Vitamin D, non-Hodgkin lymphoma, diffuse large B-cell lymphoma, multiple myeloma, chronic lymphocytic leukemia, serum biomarkers
Introduction
Vitamin D began its evolutionary history more than a billion years ago as an inert molecule. Over time, it evolved into a hormone with multiple extracellular effects that regulate the expression of about 2000 genes.1,2 1,25-dihydroxyvitamin D or 1,25(OH)2D exerts its biological activity by regulating gene transcription (genomic effects) and by activating signal transduction pathways near plasma membranes (non-genomic or rapid effects).3 The mechanism of action involves binding to the vitamin D receptor, leading to activation or suppression of transcription.4–6 Vitamin D deficiency is a global health problem. Globally, 1 in 7 people (14%) are thought to suffer from vitamin D insufficiency or deficiency.2 In clinical practice, serum levels of 25(OH)D are used to evaluate vitamin D status in the body. Despite a lack of strong consensus on optimal levels of vitamin D, experts consider the lower limit of 30 ng/mL to be an adequate level (Table 1).7 The suggested limit of 1,25(OH)2D for optimal skeletal health is a level that minimizes parathyroid hormone and maximizes calcium absorption.8,9 Many studies have assessed potential links between vitamin D deficiency and the occurrence of neoplastic diseases as well as their outcomes. The purpose of the study was to investigate serum vitamin D levels in patients newly diagnosed with non-Hodgkin lymphoma/diffuse large B-cell lymphoma (NHL-DLBCL), multiple myeloma (MM), and chronic lymphocytic leukemia (CLL).
Table 1.
Diagnostic levels of vitamin D.
| 1,25(OH)2D level (ng/mL) | 1,25(OH)2D level (nmol/L) | Laboratory diagnosis |
|---|---|---|
| <10 | <25 | Severe deficiency |
| <20 | <50 | Deficiency |
| 20–30 | 50–75 | Insufficiency |
| 30–100 | 75–250 | Normal in sunny countries |
| >100 | >250 | Excess |
| >150 | >325 | Intoxication |
Patients and methods
Patients
Vitamin D levels were assessed in patients with NHL-DLBCL, CLL and MM prior to treatment initiation. The patients were diagnosed at the Clinical Hematology Clinic of UMHAT St. George, Medical University of Plovdiv, from November 2014 to April 2016. The study was approved by the Scientific Ethics Committee of the Medical University of Plovdiv and was conducted in accordance with the principles laid out in the Helsinki declaration. All patients participating in the study signed an informed consent form. Patient data are presented in Table 2.
Table 2.
Patient characteristics.
| Patients with MM (n = 32) | ||
| Gender | Female | 51.4% |
| Male | 48.6% | |
| Mean age | 68 years (38–86 years) | |
| Clinical stage (Durie & Salmon) | IA | 10.8% |
| IB | 0% | |
| IIA | 13.5% | |
| IIB | 5.4% | |
| IIIA | 18.9% | |
| Clinical stage (ISS) | I | 13.5% |
| II | 13.5% | |
| III | 70.0% | |
| Patients with NHL-DLBCL (n = 34) | ||
| Gender | Female | 47.1% |
| Male | 52.9% | |
| Mean age | 60.4 years (38–82 years) | |
| Clinical stage (Ann Arbor) | IA | 11.8% |
| IB | 2.9% | |
| IIA | 8.8% | |
| IIB | 11.8% | |
| IIBs | 2.9% | |
| IIIA | 8.8% | |
| IIIB | 23.5% | |
| IIIBs | 2.9% | |
| IVB | 14.7% | |
| Extranodal involvement | Yes | 44.1% |
| No | 55.9% | |
| “В” symptoms | Yes | 41.3% |
| No | 58.7% | |
| Patients with CLL (n = 32) | ||
| Gender | Female | 34.4% |
| Male | 65.6% | |
| Mean age | 68 years (45–90 years) | |
| Clinical stage (Rai) | 0 | 3.1% |
| I | 43.8% | |
| II | 37.5% | |
| III | 6.3% | |
| IV | 9.4% | |
| Clinical stage (Binet) | A | 62.5% |
| B | 25.0% | |
| C | 12.5% |
Statistical methods
Descriptive statistics and statistical inferences and conclusions were used to conduct the study. Data processing was carried out using IBM SPSS, version 25.0 (IBM Corp., Armonk, NY, USA) and MS Excel 2017 (Redmond, WA, USA). Statistical significance in testing the null hypothesis H0 was assumed for values of α = 0.05 (Z criterion = 1.96).
Measurement of vitamin D
Serum tests for vitamin D were performed at the first hospitalization of patients before starting treatment. Serum vitamin D levels were tested at the Central Clinical Laboratory at UMHAT St. George in Plovdiv, Bulgaria. An ELISA was used to quantify 1,25(OH)2D levels (Immundiagnostik, Bensheim, Germany; Catalog No. K2109).
Results
A total of 103 patients were enrolled in this study. Thirty-seven patients (19 women, 18 men) with a mean age of 68 years had a diagnosis of MM according to the criteria of the International Myeloma Working Group. Thirty-two patients (11 women, 21 men) with a mean age of 68 years had a diagnosis of CLL according to the International Workshop on Chronic Lymphocytic Leukemia criteria. Thirty-four patients (16 women, 18 men) with a mean age of 60 years had a histologically confirmed diagnosis of NHL-DLBCL.
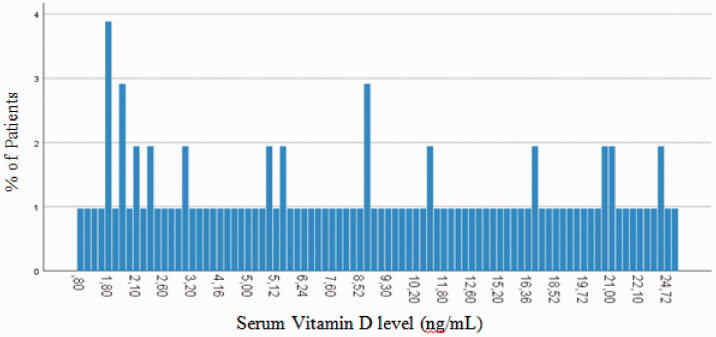
Serum levels of vitamin D below the optimum (<30 ng/mL) were observed in all 103 patients. The lowest measured level of vitamin D was 0.80 ng/mL and the highest measured level was 28.60 ng/mL. In 14.42% of patients, serum vitamin D levels were between 20 and 30 ng/mL, while all other patients had vitamin D deficiency (<20 ng/mL). These results are presented in Figure 1. Severe vitamin D deficiency (<10 ng/mL) was observed in 58.3% of patients and vitamin D levels between 10 and 20 ng/mL were observed in 27.28% of patients.
Figure 1.
Level of vitamin D in all patients.
Vitamin D and NHL-DLBCL
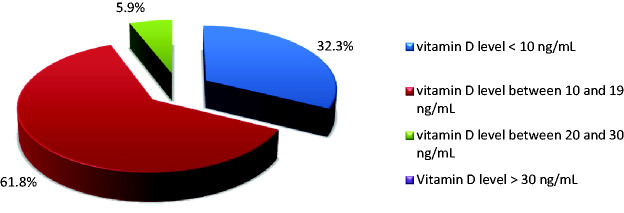
In all 34 patients (17 men, 17 women) with newly diagnosed NHL-DLBCL, serum levels of vitamin D below the optimum (<30 ng/mL) were observed. Two patients had vitamin D insufficiency (serum levels between 20–30 ng/mL), while all other patients had vitamin D deficiency (serum levels <20 ng/mL). Severe vitamin D deficiency (<10 ng/mL) was observed in 61.8% of patients. These results are presented in Figure 2. No statistically significant differences were observed between serum vitamin D levels in men (9.3±5.7 mg/mL) and women (10.1±7.1 mg/mL). There was also no significant difference in vitamin D level associated with age. No statistically significant association was identified between vitamin D levels and Ann Arbor clinical stage. Patients with “B” symptoms had lower mean vitamin D levels. The Revised International Prognostic Index (R-IPI) and National Comprehensive Cancer Network International Prognostic Index (NCCN IPI) were used to determine patient prognosis. A statistically significant association was identified between R-IPI and vitamin D levels (p = 0.038). Patients with lower levels of vitamin D had worse prognoses than patients with higher levels of vitamin D. No such correlation was identified between NCCN IPI and vitamin D levels. All patients were treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or CHOP-like regimens as a first line therapy. Patients with the lowest levels of vitamin D had the worst responses to treatment and progressed most quickly.
Figure 2.
Distribution of patients with NHL-DLBCL according vitamin D level.
Vitamin D and CLL
In all 32 (21 male, 11 female) patients with newly diagnosed CLL, suboptimal serum levels of vitamin D (<30 ng/mL) were observed. Eleven patients had vitamin D insufficiency (serum levels between 20–30 ng/mL), while the remaining 21 patients had vitamin D deficiency (levels <20 ng/mL). Severe vitamin D deficiency (<10 ng/mL) was observed in 28.1% of patients. These results are presented in Figure 3. No statistically significant difference was observed between serum vitamin D levels in men (15.4 ± 6.8 mg/mL) and women (13.6 ± 9.3 mg/mL). There was also no significant difference in vitamin D level associated with age. A statistically significant association was identified between vitamin D level and clinical stage according to the Rai and Binet systems. Patients with higher levels of vitamin D had lower clinical stages and less often required chemotherapy. Patients with higher leukocyte counts were found to have lower levels of vitamin D (p = 0.017). A similar relationship was found observed serum vitamin D levels and serum lactate dehydrogenase (LDH) levels. Patients with higher levels of vitamin D had lower LDH levels. Treatment with various immunochemotherapy regimens was started in 17 (53.1%) patients. No significant difference was identified between levels of vitamin D and response to treatment in patients with CLL.
Figure 3.
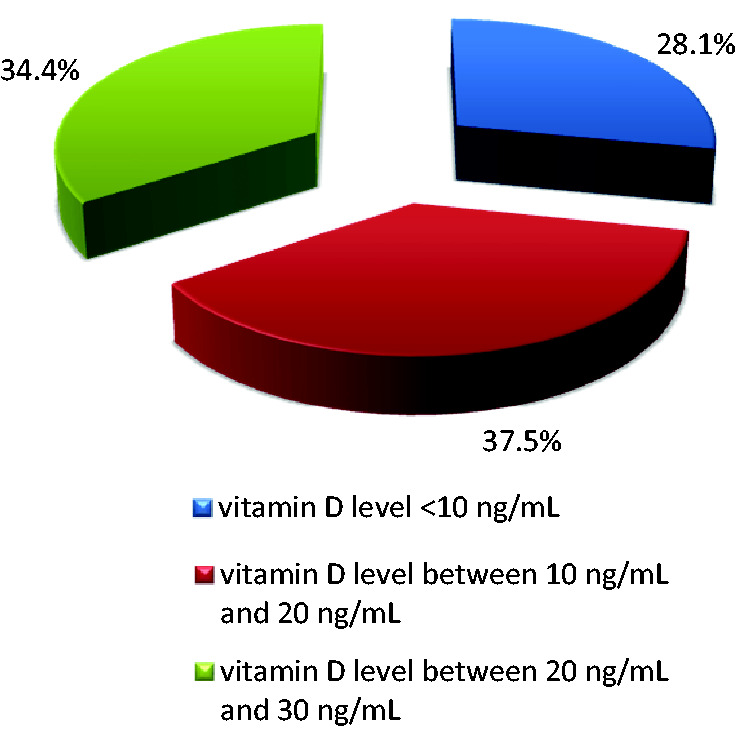
Distribution of patients with CLL according to vitamin D level.
Vitamin D and MM
In all 37 (18 male, 19 female) patients with newly diagnosed MM, suboptimal serum levels of vitamin D (<30 ng/mL) were observed. One patient had vitamin D insufficiency (serum levels between 20–30 ng/mL), while the remaining 36 patients had vitamin D deficiency (levels below 20 ng/mL). Severe vitamin D deficiency (<10 ng/mL) was observed in 81% of patients. These results are presented in Figure 4. No statistically significant difference was observed between serum vitamin D levels in men (6.2 ± 5.2 mg/mL) and women (6.7 ± 6.1 mg/mL). There was also no significant difference in vitamin D level associated with age. However, the mean vitamin D level in patients 69–78 years old (4.1 ± 3.4 mg/mL) was significantly lower than that in patients 59 to 68 years old (9.4 ± 7.2 mg/mL). According to the Durie & Salmon staging system, 19 (51.4%) patients were in stage IIIB. Using the international staging system (ISS), 27 (73.0%) patients were in stage III. No statistically significant association was observed between serum vitamin D levels and clinical stage using either staging system. No statistically significant association was observed between vitamin D levels and response to treatment. No statistically significant association was observed between vitamin D levels and response to first-line therapy in patients with MM.
Figure 4.
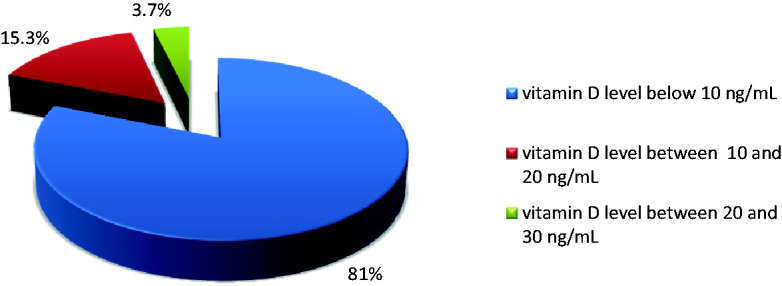
Distribution of patients with MM according to vitamin D level.
Discussion
Many data have been accumulated assessing the effects of vitamin D on cellular differentiation and proliferation, as well as angiogenesis and apoptosis.10 Low serum levels of vitamin D were associated with a higher risk of developing NHLs.11,12 Conversely, more frequent and longer sun exposure, which leads to higher serum levels of vitamin D, reduces the risk of developing NHLs.13 van der Rhee et al.14 stated that almost all epidemiological studies have documented an association between long-term sun exposure and reduced risks of colon, breast and prostate cancers as well as NHLs. In a prospective study of 155 patients with NHL-DLBCL, Hohaus et al.15 observed vitamin D deficiency (<20 mg/mL) in 105 patients (67%), insufficiency (20–30 ng/mL) in 32 patients (21%) and normal levels (>30 ng/mL) in 18 patients (12%). The results of this study were similar to our own: we observed vitamin D insufficiency in 5.9% of patients, deficiency in 61.8% of patients and severe deficiency in 32.3% of patients with NHL-DLBCL. Molica et al. studied 130 patients with B-cell CLL and observed a deficiency of vitamin D in 82.3% of patients. Severe vitamin D deficiency (<10 ng/mL) was observed in 41 patients (31.5%), moderate deficiency (10–24 ng/mL) was observed in 66 patients (50.24%) and optimal vitamin D levels (25–80 ng/mL) were observed in 23 patients. No association was identified between vitamin D levels and season.16 In our study, we observed vitamin D deficiency in 34.4% of patients, deficiency in 66.6% of patients, and severe deficiency in 28.1% of patients with CLL. In a study of 148 patients with newly diagnosed MM, vitamin D deficiency (<20 mg/mL) was observed in 24% of patients. According to the ISS, vitamin D deficiency was observed in 16% of patients at stage I, 20% of patients at stage II, and 37% of patients at stage III. Patients with low vitamin D levels had elevated C-reactive protein levels as well as high serum creatinine levels. In our study, no statistically significant associations were observed between levels of vitamin D and clinical stage, nor between levels of vitamin D and those of C-reactive protein and creatinine. Previous studies found no correlation between vitamin D levels and myeloma bone disease, in agreement with our results.17
In conclusion, we observed low serum vitamin D levels in the majority of patients newly diagnosed with NHL-DLBCL, CLL and MM. Despite these unidirectional results, the inclusion of three cohorts of patients was an advantage, enabling comparisons between the three groups. It was also a disadvantage because of the different nature of these three diseases. Our study was limited in power because of the low number of patients included in each group and the lack of a control group of healthy individuals. Our results warrant further evaluation in other prospective studies, ideally using a large number of patients and a matched healthy control group. Because the prevalence of vitamin D deficiency appears to be high in different hematological malignancies, further studies evaluating the impact of maintaining adequate vitamin D levels on treatment and survival as week as prevention of secondary neoplasms is needed.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by a grant from the Medical University of Plovdiv (project number ДП-02/2013).
ORCID iD
Vasko Graklanov https://orcid.org/0000-0002-7059-1411
References
- 1.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005; 26: 662–687. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 3.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 2004; 3: 27–41. [DOI] [PubMed] [Google Scholar]
- 4.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004; 80: 1689S–1696S. [DOI] [PubMed] [Google Scholar]
- 5.Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene 2000; 246: 9–21. [DOI] [PubMed] [Google Scholar]
- 6.Christakos S, Dhawan P, Liu Y, et al. New insights into the mechanisms of vitamin D action. J Cell Biochem 2003; 88: 695–705. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 8.Heaney RP, Dowell MS, Hale CA, et al. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003; 22: 142–146. [DOI] [PubMed] [Google Scholar]
- 9.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteopros Int 1997; 7: 439–443. [DOI] [PubMed] [Google Scholar]
- 10.Hossein-Nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc 2013; 88: 720–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006; 98: 451–459. [DOI] [PubMed] [Google Scholar]
- 12.Lim U, Freedman DM, Hollis BW, et al. A prospective investigation of serum 25- hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer 2009; 124: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kricker A, Armstrong BK, Hughes AM, et al. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the InterLymph Consortium. Int J Cancer 2008; 122: 144–154. [DOI] [PubMed] [Google Scholar]
- 14.Van Der Rhee H, Coebergh JW, De Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer 2013; 49: 1422–1436. [DOI] [PubMed] [Google Scholar]
- 15.Hohaus S, Tisi MC, Bellesi S, et al. Vitamin D deficiency and supplementation in patients with aggressive B-cell lymphomas treated with immunochemotherapy. Cancer Med 2018; 7: 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molica S, Digiesi G, Antenucci A, et al. Vitamin D insufficiency predicts time to first treatment (TFT) in early chronic lymphocytic leukemia (CLL). Leuk Res 2012; 36: 443–447. [DOI] [PubMed] [Google Scholar]
- 17.Ng AC, Kumar SK, Rajkumar SV, et al. Impact of vitamin D deficiency on the clinical presentation and prognosis of patients with newly diagnosed multiple myeloma. Am J Hematol 2009; 84: 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]