Abstract
Background:
Trials reported there are beneficial effects of the addition of bevacizumab to chemotherapy in advanced cervical cancer but might have adverse effects. The purposes of the study were to evaluate the treatment response and safety of the addition of bevacizumab to paclitaxel plus carboplatin in advanced cervical cancer women.
Methods:
Data on treatment response, adverse effects, and overall survival of women who received paclitaxel plus carboplatin every 3 weeks (ACT cohort, n = 161) or paclitaxel, carboplatin, and bevacizumab every 3 weeks (PCB cohort, n = 127) until disease progression or severe adverse events were collected and analyzed.
Results:
The treatment response of paclitaxel plus carboplatin increased on addition of bevacizumab (P = .037). Neutropenia (grade ≥3, P = .001), leukopenia (grade 4, P = .041), anemia (grade ≥3, P = .031), hypertension (grade ≥2, P = .002), and gastrointestinal fistula (grade ≥2, P = 0.006) are reported in the PCB cohort. Women of ACT and PCB cohorts reported an overall survival of 20.11 ± 3.15 months and 24.52 ± 4.05 months, respectively.
Conclusions:
Addition of bevacizumab increases the treatment response of paclitaxel and carboplatin chemotherapy and overall survival of women with advanced cervical cancers, but it is not well tolerated.
Keywords: bevacizumab, carboplatin, cervical cancer, hypertension, gastrointestinal fistula, paclitaxel
Introduction
Cervical cancer ranks fourth for incidence and mortality for women worldwide.1 The incidence and mortality in Chinese women due to cervical cancer is increased.2 Early- and advanced-stage cervical cancers of women can be cured by chemotherapy and/or radiotherapy, but those with recurrent cervical cancer after chemotherapy and/or radiotherapy have limited options.3 In advanced cervical cancer, there is a need for chemotherapeutic treatment that improves overall survival.4
Investigations focused on chemotherapeutic agents for cervical cancer. Cisplatin–paclitaxel is a global standard for metastatic or recurrent cervical cancer.5,6 A phase III trial showed noninferiority of carboplatin–paclitaxel compared to cisplatin–paclitaxel.5 Bevacizumab is a monoclonal type antibody that inhibits the vascular endothelial growth factor, which is the key parameter of tumor angiogenesis.7 Paclitaxel and topotecan or paclitaxel and cisplatin is approved by the United States Food and Drug Administration for advanced cervical cancer.8 A randomized trial reported that the addition of bevacizumab to present chemotherapy in recurrent, persistent, or metastatic cervical cancer women improved overall survival but has emergent adverse effects such as gastrointestinal fistula, hypertension, and thromboembolism.9 However, the phase II trial reported that the addition of bevacizumab to carboplatin–paclitaxel chemotherapy is effective for advanced or recurrent cervical cancer women with hematologic adverse effects.3
Randomized clinical trials are generally accepted for predicting the efficacy and the safety of a new drug entity,10 but they are conducted in highly selective patients. Therefore, there are gaps between clinical trials and clinical practice.11 There is a need for a retrospective study on clinical practice to check the treatment response and safety of the addition of bevacizumab with paclitaxel and carboplatin for advanced-stage cervical cancer. The purposes of the study were to evaluate the treatment response, safety, and overall survival of women with advanced cervical cancer when treated with paclitaxel, carboplatin, and bevacizumab.
Materials and Methods
Ethics Approval and Consent to Participate
The designed study was approved by the oncological review board of the institute and registered in the Information Network of the University of Electronic Science and Technology of China (Protocol No.: UESTC1420 dated April 10, 2020). The study adheres to the Law of China and the V2008 Declarations of Helsinki. An informed consent form was signed by all the enrolled patients regarding pathology, chemotherapy, radiology, and publication of study irrespective of time and language during hospitalization.
Patient Population
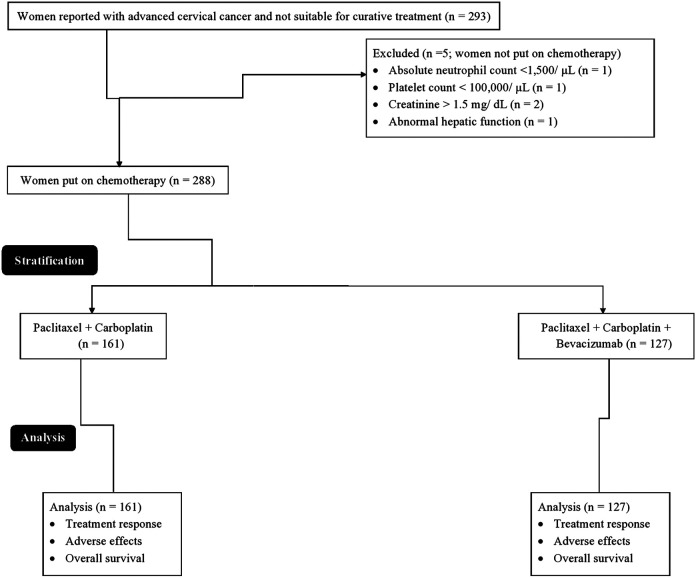
From January 12, 2016 to October 15, 2017, a total of 293 women (age >18 years) reported with advanced cervical cancer (The International Federation of Gynecology and Obstetrics stage IVB or recurrent stage) and measurable with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria12 and not suitable for surgery or radiation therapy (curative treatment) at the Chengdu Women’s and Children’s Central Hospital, Chengdu, China, and the Sichuan Cancer Hospital and Institute, Chengdu, China, were included. Among them, 1 woman had absolute neutrophil count less than 1500/µL and 1 woman had a platelet count of less than 100 000/μL. Two females had a creatinine level of more than 1.5 mg/dL and 1 woman had an abnormal hepatic function. Therefore, those women were not put on chemotherapy. A total of 288 women were put on chemotherapy. Data on treatment response, adverse effects, and overall survival of women were collected and analyzed in the study (Figure 1).
Figure 1.
Flow diagram of the study.
Cohorts
Women received intravenous 175 mg/m2 paclitaxel (Taxol; Bristol-Myers Squibb) and intravenous 6 mg/mL/min area under the curve carboplatin (Paraplatin; Bristol-Myers Squibb) every 3 weeks (ACT cohort, n = 161) or intravenous 175 mg/m2 paclitaxel, intravenous 6 mg/mL/min area under the curve carboplatin, and intravenous 15 mg/m2 bevacizumab (Roche, Holding AG) every 3 weeks (PCB cohort, n = 127). Treatment was run until severe adverse events or disease progression in both cohorts. The chemotherapeutic treatment was discontinued (3-4 weeks) if patients experienced either tumor remission or tumor progression and it was withdrawn if patients developed unacceptable toxicity. Calvert formula with the creatinine clearance was used for calculation of the dose of carboplatin, and the Cockcroft-Gault formula was used for calculation of the creatinine clearance.3
Treatment Response
Cancer tumor was assessed by clinical examinations and the computed tomography before treatment and after every 2 cycles.3 RECIST version 1.112 was used for evaluation of treatment response. Oncologists (a minimum of 3 years of experience) of institutes were involved in the evaluation of treatment efficiency.
Adverse Effects
Toxicities were monitored by history, physical examinations, and laboratory assessments. Treatment-emergent adverse effects were defined as per Common Terminology Criteria for Adverse Events (CTCAE) v5.0.13
Overall Survival
The follow-up was performed every 3 months for 3.5 years after treatment. The computed tomography was performed every 6 months.
Statistical Analysis
The sample size was calculated at 80% power and a 5% level of confidence. SPSS v25.0 (IBM Corporation) was used for statistical analysis. The Fisher exact test was performed for statistical analysis of constant data. Univariate analysis following multivariate analysis was performed to find the association of demographical parameters and clinical conditions of the enrolled women with treatment response.14 All results were considered significant at a 95% level of confidence.
Results
Women Characteristics
At the time of enrollment, the demographical and clinical conditions of the enrolled women had no significant difference (P > .05 for all) among cohorts. The detailed demographical parameters and clinical conditions of the enrolled women are reported in Table 1.
Table 1.
Demographical and Clinical Conditions of the Enrolled Women.a
| Characteristics | Cohorts | Comparisons between cohorts, P value | ||
|---|---|---|---|---|
| ACT | PCB | |||
| Treatment | Paclitaxel + carboplatin | Paclitaxel + carboplatin + bevacizumab | ||
| Women enrolled | 161 | 127 | ||
| Age (years) | Minimum | 30 | 30 | .055 |
| Maximum | 70 | 70 | ||
| Mean ± SD | 55.41 ± 15.17 | 52.31 ± 11.15 | ||
| Gynecologic Oncology Group performance score | 0 | 142 (88) | 113 (89) | .967 |
| 1 | 19 (12) | 14 (11) | ||
| Histologic type | Squamous cell carcinoma | 110 (68) | 88 (69) | .959 |
| Adenocarcinoma | 39 (24) | 29 (23) | ||
| Adenosquamous cell carcinoma | 12 (8) | 10 (8) | ||
| Disease status | Persistent or FIGO stage IVB | 45 (28) | 32 (25) | .651 |
| First recurrence | 103 (64) | 81 (64) | ||
| Second recurrence | 13 (8) | 14 (11) | ||
| Prior hysterectomy | Yes | 92 (57) | 75 (59) | .809 |
| No | 69 (43) | 52 (41) | ||
| Menopausal status | Pre | 105 (65) | 81 (64) | .805 |
| Post | 56 (35) | 46 (36) | ||
| Body mass index (kg/m2) | 24.15 ± 2.14 | 24.52 ± 2.27 | .157 | |
| Ethnicity | Han Chinese | 147 (92) | 115 (91) | .511 |
| Mongolian | 10 (6) | 11 (8) | ||
| Tibetan | 2 (1) | 1 (1) | ||
| Uighur | 2 (1) | 0 (0) | ||
Abbreviation: FIGO, The International Federation of Gynecology and Obstetrics.
a Constant and ordinal data demonstrate frequency (percentage), and numerical data are presented mean ± SD. Fisher exact test performed for constant/ordinal data and the Mann-Whitney U test was performed for continuous data. A P < .05 was considered significant.
Treatment Response
There was no difference in the average length of treatment between both cohorts. The treatment response was increased with the addition of bevacizumab when compared with the present paclitaxel plus carboplatin treatment (P = .037; Table 2).
Table 2.
Treatment Response.a
| Response for treatment | Cohorts | Comparisons between cohorts, P value | |
|---|---|---|---|
| ACT | PCB | ||
| Treatment | Paclitaxel + carboplatin | Paclitaxel + carboplatin + bevacizumab | |
| Women enrolled | 161 | 127 | |
| Complete response | 82 (51) | 69 (54) | .037 |
| Partial response | 45 (28) | 46 (36) | |
| Stable disease | 26 (16) | 11 (9) | |
| Progressive disease | 8 (5) | 1 (1) | |
a Data are presented as frequency (percentage). The Fisher exact test was performed for statistical analysis. A P < .05 was considered significant. Clinical examinations and the computed tomography were used for treatment response evaluation. RECIST version 1.1 was used for treatment response evaluation.
Univariate analysis reported significant difference in age (P = .022), Gynecologic Oncology Group performance score (P < .0001), histologic type (P < .0001), disease status (P < .0001), hysterectomy status (P < .0001), and menopausal status (P = .048) of women between those who received complete response and those who received partial response or those with stable disease or progressive disease (Table 3). Gynecologic Oncology Group performance score 1 (P = .029) and recurrent status of disease (P = .021) were associated with response failure or partial response (Table 4).
Table 3.
Univariate Analysis to Find the Association of Demographical Parameters and Clinical Conditions of the Enrolled Women With a Treatment Response.a
| Characteristics | Groups | Comparisons between groups, P value | ||
|---|---|---|---|---|
| Complete response | Partial response or stable disease or progressive disease | |||
| Women enrolled | 151 | 137 | ||
| bAge (years) | Minimum | 30 | 30 | .022 |
| Maximum | 70 | 70 | ||
| Mean ± SD | 51.41 ± 11.17 | 54.31 ± 10.15 | ||
| bGynecologic Oncology Group performance score | 0 | 149 (99) | 106 (77) | <.0001 |
| 1 | 2 (1) | 31 (23) | ||
| bHistologic type | Squamous cell carcinoma | 139 (92) | 59 (43) | <.0001 |
| Adenocarcinoma | 10 (7) | 58 (42) | ||
| Adenosquamous cell carcinoma | 2 (1) | 20 (15) | ||
| bDisease status | Persistent or FIGO stage IVB | 51 (34) | 25 (18) | <.0001 |
| First recurrence | 97 (64) | 87 (64) | ||
| Second recurrence | 3 (2) | 25 (18) | ||
| bPrior hysterectomy | Yes | 106 (70) | 60 (44) | <.0001 |
| No | 45 (30) | 77 (56) | ||
| bMenopausal status | Pre | 106 (70) | 80 (58) | .048 |
| Post | 45 (30) | 57 (42) | ||
| Body mass index (kg/m2) | 24.05 ± 2.03 | 24.41 ± 2.35 | .164 | |
| Ethnicity | Han Chinese | 132 (87) | 130 (95) | .083 |
| Mongolian | 14 (10) | 7 (5) | ||
| Tibetan | 3 (2) | 0 (0) | ||
| Uighur | 2 (1) | 0 (0) | ||
Abbreviation: FIGO, The International Federation of Gynecology and Obstetrics.
a Constant and ordinal data demonstrate frequency (percentage), and numerical data are presented mean ± SD. Fisher exact test was performed for constant/ordinal data and the Mann-Whitney U test performed for continuous data. A P < .05 was considered significant.
b Significant difference.
Table 4.
Association of Parameters for Response Failure of Treatment.a
| Women enrolled | 137 | ||
|---|---|---|---|
| Characteristics | Odd ratio | 95% Confidence limit | P value |
| Age (years) | 0.62 | 0.41-0.87 | .055 |
| Gynecologic Oncology Group performance score (1b vs 0) | 0.59 | 0.35-0.94 | .029 |
| Histologic type (adenocarcinoma and adenosquamous cell carcinoma vs squamous cell carcinoma) | 0.64 | 0.45-0.86 | .052 |
| Disease status (recurrenceb vs persistent) | 0.55 | 0.32-0.96 | .021 |
| Prior hysterectomy (no vs Yes) | 0.65 | 0.45-0.85 | .082 |
| Menopausal status (post vs pre) | 0.67 | 0.46-0.81 | .102 |
a Multivariate analysis. Data of women with complete responses were considered as the reference standard. Odd ratio >0.5 and P < .05 were considered significant.
b Significant parameter for response failure or partial response.
Adverse Effects
Hematological adverse effects such as thrombocytopenia, neutropenia, leukopenia, and anemia and nonhematological adverse effects such as headache, emesis, seizures, proteinuria, visual loss, hypertension, thromboembolic event, peripheral neuropathy, gastrointestinal fistula, gastrointestinal perforation, and bronchopulmonary hemorrhage were reported in women during the course of chemotherapy.
Neutropenia (grade ≥3, P = .001), neutropenia (grade 4, P = .012), leukopenia (grade 4, P = .041), and anemia (grade ≥3, P = .031) were reported in the PCB cohort. Grade ≥2 (P < .0001), grade ≥3 (P = .017), and grade 4 (P = .023) total hematological adverse effects per woman were higher in the PCB cohort than the ACT cohort. Hypertension (grade ≥1, P = .001), hypertension (grade ≥2, P = .002), and gastrointestinal fistula (grade ≥2, P = .006) were reported in the PCB cohort. Grade ≥2 (P = .0001) and grade ≥3 (P = .029) total nonhematological adverse effects per woman were higher in the PCB cohort than the ACT cohort. The other detailed hematological and nonhematological adverse effects during the course of chemotherapy are reported in Tables 5 and 6.
Table 5.
Hematological Adverse Effects.
| Adverse effects | Cohorts | Comparisons between cohorts, P value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACT | PCB | |||||||||||||
| Treatment | Paclitaxel + carboplatin | Paclitaxel + carboplatin + bevacizumab | ||||||||||||
| Women enrolled | 161 | 127 | ||||||||||||
| Grade | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | ≥1 | ≥2 | ≥3 | 4 |
| Thrombocytopenia | 36 (22) | 49 (30) | 35 (22) | 21 (13) | 20 (13) | 22 (17) | 35 (28) | 31 (24) | 25 (20) | 14 (11) | 0.305 | 0.193 | 0.355 | 0.854 |
| Neutropenia | 41 (26) | 37 (23) | 35 (22) | 28 (17) | 20 (12) | 30 (24) | 17 (13) | 17 (13) | 32 (25) | 31 (25) | 0.784 | 0.056 | 0.001 | 0.012 |
| Leukopenia | 9 (6) | 8 (5) | 68 (42) | 55 (34) | 21 (13) | 5 (4) | 4 (3) | 51 (40) | 38 (30) | 29 (23) | 0.591 | 0.479 | 0.406 | 0.041 |
| Anemia | 5 (3) | 65 (40) | 57 (35) | 20 (13) | 14 (9) | 1 (1) | 40 (31) | 43 (34) | 25 (20) | 18 (14) | 0.234 | 0.067 | 0.031 | 0.186 |
| Total hematological events | 91 | 159 | 126 | 124 | 75 | 58 | 96 | 142 | 120 | 92 | 0.042 | <0.0001 | 0.017 | 0.023 |
| Hematological event(s) per woman | 0.57 | 0.99 | 0.78 | 0.77 | 0.47 | 0.46 | 0.76 | 1.12 | 0.95 | 0.72 | ||||
Data presented as frequency.
The Fischer exact was performed for statistical analysis.
A p < 0.05 considered as significant.
Adverse effects defined as per CTCAE v5.0.
Table 6.
Non-Hematological Adverse Effects.
| Adverse effects | Cohorts | Comparisons between cohorts | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACT | PCB | |||||||||||||
| Treatment | Paclitaxel + Carboplatin | Paclitaxel + Carboplatin + Bevacizumab | ||||||||||||
| Women enrolled | 161 | 127 | p-value | |||||||||||
| Grade | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | ≥1 | ≥2 | ≥3 | 4 |
| Proteinuria | 154 (96) | 3 (2) | 2 (1) | 2 (1) | 0 (0) | 122 (96) | 2 (1) | 1 (1) | 1 (1) | 1 (1) | 0.999 | 0.999 | 0.999 | 0.441 |
| Hypertension | 145 (90) | 7 (4) | 4 (3) | 3 (2) | 2 (1) | 95 (75) | 10 (8) | 11 (9) | 6 (5) | 5 (3) | 0.001 | 0.002 | 0.067 | 0.245 |
| Thromboembolic event | 150 (94) | 5 (3) | 2 (1) | 2 (1) | 2 (1) | 111 (87) | 5 (4) | 5 (4) | 4 (3) | 2 (2) | 0.107 | 0.085 | 0.345 | 0.998 |
| Peripheral neuropathy | 147 (91) | 7 (5) | 5 (3) | 2 (1) | 0 (0) | 120 (94) | 5 (4) | 1 (1) | 1 (1) | 0 (0) | 0.365 | 0.307 | 0.997 | N/A |
| Gastrointestinal fistula | 137 (86) | 20 (12) | 2 (1) | 2 (1) | 0 (0) | 106 (83) | 7 (6) | 9 (7) | 3 (2) | 2 (2) | 0.745 | 0.006 | 0.247 | 0.194 |
| Gastrointestinal perforation | 156 (97) | 3 (2) | 2 (1) | 0 (0) | 0 (0) | 124 (97) | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 0.998 | 0.997 | N/A | N/A |
| Bronchopulmonary hemorrhage | 152 (94) | 5 (3) | 2 (1) | 1 (1) | 1 (1) | 123 (96) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0.399 | 0.997 | 0.994 | 0.992 |
| Total non-hematological events | 1,041 | 50 | 19 | 12 | 5 | 801 | 32 | 29 | 16 | 11 | 0.085 | 0.001 | 0.029 | 0.082 |
| Nonhematological events per woman | 6.47 | 0.31 | 0.12 | 0.07 | 0.03 | 6.31 | 0.25 | 0.23 | 0.13 | 0.09 | ||||
Abbreviation: N/A, not applicable.
a Data are presented as frequency. The Fisher exact was performed for statistical analysis. A P < .05 was considered as significant. Adverse effects defined as per CTCAE v5.0.
Overall Survival
Women of ACT cohort had an overall survival of 2 to 29 months (20.11 ± 3.15 months), whereas women of PCB cohort had an overall survival of 2 to 31 months (24.52 ± 4.05 months) after the start of treatment. Women of the PCB cohort had 20% (about 5 months) higher overall survival than those of the ACT cohort (P = 0.038; Figure 2).
Figure 2.
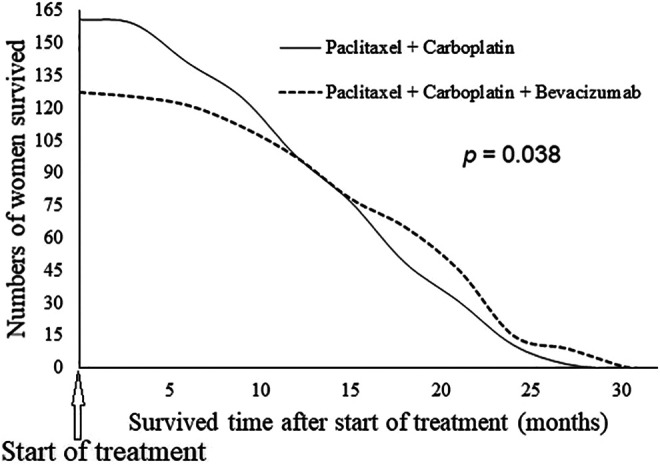
Overall survival of women after treatment. Data presented as frequency. The Fisher exact was performed for statistical analysis. A P < .05 was considered as significant.
Discussion
The treatment response was reported higher in the PCB cohort than the ACT cohort. The results of the current study agreed with a randomized phase III trial,9 phase II trial,3 and phase III trial.15 Bevacizumab has efficacy against solid tumors of cervical cancer.3 Also, increased response is attributed to factor that striking difference,9 women (89%; 255 out of 288 women) enrolled in the study had 0 Gynecologic Oncology Group performance score and enrollment of treatment-naive women.3 The addition of bevacizumab with the current chemotherapeutic regimen of paclitaxel plus carboplatin has beneficial effects over treatment response.
Gynecologic Oncology Group performance score 1 and recurrent status of disease were associated with response failure or partial response of chemotherapeutic treatment irrespective of bevacizumab. The results of the current study agreed with the phase III trial on cisplatin-based chemotherapy14 and randomized phase III trials on bevacizumab plus chemotherapy.9,10 Before women were put on chemotherapy, all parameters should be considered for the prediction of chemotherapeutic response.
Grade ≥2, grade ≥3, and grade 4 hematological adverse effects including neutropenia, leukopenia, and anemia and grade ≥2 and grade ≥3 nonhematological adverse effects including hypertension and gastrointestinal fistula were reported due to bevacizumab. The results of the current study were in parallel with the results of randomized trials,9 phase II study,3 a retrospective study,10 phase III trials,5,15 and CECILIA study.16 Bevacizumab inhibits nitric oxide synthase, which leads to nonhematological adverse effects such as hypertension.7 Hematological adverse effects were attributed to treatment-naive women.3 The other nonhematological adverse effects, that is, gastrointestinal fistula, were due to recurrence status10,16 of women (73%; 211/288). The addition of bevacizumab with the current chemotherapeutic regimen of paclitaxel plus carboplatin is not a well-tolerated treatment and increased vascular complications in women.
The addition of bevacizumab increased 20% of overall survival irrespective of treatment-emergent adverse effects. The results of the current study agreed with the results of a randomized phase III trial,9 a retrospective study,10 and a phase III trial.15 Increased treatment response, younger age of women, and the other demographical and clinical conditions of the enrolled women were responsible for increased overall survival.10 These data support further research of bevacizumab in cervical cancers.
The limitation of the study is that survival without disease-free condition was not evaluated. Although bevacizumab treatment is cost-effective,17 cost analysis was not performed. A rebound effect was not observed in the study. The multicenter study exclusively consisted of Chinese women. However, the geographical regions have effects over the response and tolerability of bevacizumab.16 Subjective quality of life of women could have over the response and tolerability of bevacizumab,3 but the current study did not evaluate the same. The chemotherapies were discontinued for 3 to 4 weeks when needed, which may affect the overall treatment response.18
Conclusions
The addition of bevacizumab with the current chemotherapeutic regimen of paclitaxel plus carboplatin at every 3 weeks has beneficial effects over treatment response and increased overall survival of women with advanced cervical cancer but is not a well-tolerated treatment and has hypertension, neutropenia, and gastrointestinal fistula as adverse effects. Before we put women on chemotherapy, all parameters should be considered for the prediction of chemotherapeutic response. The research data of the current study support further research of bevacizumab in the cervical cancers.
Acknowledgments
The authors are thankful to the medical and pharmacy staff of the Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China, and the Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Medicine School of University of Electronic Science and Technology, Chengdu, People’s Republic of China.
Authors’ Note: The authors read and approved the manuscript for publication. W.T. was project administrator and contributed to visualization, resources, supervision, and the literature review of the study. J.Y. contributed to resources, the literature review, formal analysis, data curation, and validation of the study. Y.J. contributed to the literature review, supervision, methodology, resources, and data curation of the study. W.C. contributed to the literature review, investigation, validation, formal analysis, and resources of the study. Y.W. contributed to resources, software, validation, supervision, and the literature review of the study, draft, review, and edited the manuscript for intellectual content. The authors agree to be accountable for all aspects of work ensuring integrity and accuracy. The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yixin Wang  https://orcid.org/0000-0002-4792-1849
https://orcid.org/0000-0002-4792-1849
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Tuoheti Z, Han L, Husaiyin S, Liu X, Ma C, Niyazi M. RIPK1 polymorphisms alter the susceptibility to cervical cancer among the Uyghur population in China. BMC Cancer. 2020;20(1):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki K, Nagao S, Shibutani T, et al. Phase II trial of paclitaxel, carboplatin, and bevacizumab for advanced or recurrent cervical cancer. Gynecol Oncol. 2019;154(3):554–557. [DOI] [PubMed] [Google Scholar]
- 4. Malik L, Mejia AV. Cervical cancer: is the wall crumbling? Cancer. 2014;120(22):3585–3586. [DOI] [PubMed] [Google Scholar]
- 5. Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol. 2015;33(19):2129–2135. [DOI] [PubMed] [Google Scholar]
- 6. Nogueira-Rodrigues A, Moralez G, Grazziotin R, et al. Phase 2 trial of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced cervical cancer. Cancer. 2014;120(8):1187–1193. [DOI] [PubMed] [Google Scholar]
- 7. Minion LE, Tewari KS. The safety and efficacy of bevacizumab in the treatment of patients with recurrent or metastatic cervical cancer. Expert Rev Anticancer Ther. 2017;17(13):191–198. [DOI] [PubMed] [Google Scholar]
- 8. Richardson DL. New and novel therapies for gynecologic cancers. Semin Oncol Nurs. 2019;35(2):217–219. [DOI] [PubMed] [Google Scholar]
- 9. Tewari KS, Sill MW, Long HJ, III, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godoy-Ortiz A, Plata Y, Alcaide J, et al. Bevacizumab for recurrent, persistent or advanced cervical cancer: reproducibility of GOG 240 study results in “real world” patients. Clin Transl Oncol. 2018;20(7):922–927. [DOI] [PubMed] [Google Scholar]
- 11. Parkinson B, Viney R, Haas M, Goodall S, Srasuebkul P, Pearson SA. Real-world evidence: a comparison of the Australian herceptin program and clinical trials of trastuzumab for HER2-positive metastatic breast cancer. Pharmacoeconomics. 2016;34(10):1039–1050. [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 13. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Accessed February 1, 2019 https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50
- 14. Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2010;116(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390(10103):1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Redondo A, Colombo N, Mary LD, et al. Preliminary results from CECILIA, an open-label global safety study of bevacizumab (BEV), carboplatin (C) and paclitaxel (P) therapy for metastatic, recurrent or persistent cervical cancer (CC). J Clin Oncol. 2018;36(15_suppl):5528 doi:10.1200/JCO.2018.36.15_suppl.5528 [Google Scholar]
- 17. Minion LE, Bai J, Monk BJ, et al. A Markov model to evaluate cost-effectiveness of antiangiogenesis therapy using bevacizumab in advanced cervical cancer. Gynecol Oncol. 2015;137(3):490–496. [DOI] [PubMed] [Google Scholar]
- 18. Haemmerle M, Bottsford-Miller J, Pradeep S, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest. 2016;126(5):1885–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]