Abstract
Objective:
To evaluate associations between a readily available composite measurement of neighborhood socioeconomic disadvantage (the Area Deprivation Index (ADI)) and 30-day readmissions for patients who were previously hospitalized with sepsis.
Design:
A retrospective study.
Setting:
An urban, academic medical institution.
Patients:
The authors conducted a manual audit for adult patients (18 years of age or older) discharged with an ICD-10 code of sepsis during the 2017 fiscal year to confirm that they met SEP-3 criteria.
Measurements:
The ADI is a publicly available composite score constructed from socioeconomic components (e.g. income, poverty, education, housing characteristics) based on census block level, where higher scores are associated with more disadvantaged areas (range 1 through 100). Using discharge data from the hospital population health database, residential addresses were geocoded and linked to their respective ADI. Patient characteristics, contextual-level variables, and readmissions were compared by t-tests for continuous variables and Fisher’s exact test for categorical variables. The associations between readmissions and ADI were explored using logistic regression models.
Main Results:
A total of 647 patients had an ICD-10 diagnosis code of sepsis. Of these 647, 116 (17.9%) either died in hospital or were discharged to hospice and were excluded from our analysis. Of the remaining 531 patients, the mean age was 61.0 (± 17.6 years), 281 were females (52.9%), and 164 (30.9%) were active smokers. The mean length of stay was 6.9 (± 5.6 days) with the mean sequential organ failure assessment (SOFA) score 4.9 (± 2.5). The mean ADI was 54.2 (± 23.8). The mean ADI of patients who were readmitted was 62.5 (± 27.4), which was significantly larger than the ADI of patients not readmitted (51.8 (± 22.2)) (p<0.001). In adjusted logistic regression models, a greater ADI was significantly associated with readmissions (beta 0.03, p<0.001).
Conclusion:
Patients who reside in more disadvantaged neighborhoods have a significantly higher risk for 30-day readmission following a hospitalization for sepsis. The insight provided by neighborhood disadvantage scores, such as the ADI, may help to better understand how contextual-level socioeconomic status affects the burden of sepsis-related morbidity.
Keywords: health disparities, sepsis, area deprivation index
INTRODUCTION
Sepsis is a life-threatening, acute organ dysfunction caused by an infection.1 While millions of people are affected by sepsis every year2,3, in-hospital mortality from this condition has declined significantly over the last two decades4,5. With the fall in mortality, a large cohort of sepsis survivors has emerged. However, survivors of sepsis face significant healthcare consequences due to exacerbation of their chronic co-morbidities6 or to emergence of new symptoms and disabilities4,7. Further, these consequences have resulted in costly and disjointed healthcare utilization at a time where there is limited understanding of efficient post-sepsis recovery care and management.8,9
One healthcare consequence of patients who survived sepsis is higher likelihood of rehospitalizations10. Rehospitalizations after sepsis are common, with more than a third of sepsis survivors readmitted to the hospital within 30-days of initial discharge10, and up to 40% within 90-days of discharge11. Many factors are associated with likelihood of rehospitalization after sepsis, from antibiotic exposure12 to individual socioeconomic factors, such as minority status13, low income13, and male gender14. One challenge involved in using individual socioeconomic variables for assessment of rehospitalization risk for sepsis survivors is that many of these variables are not comprehensively captured in clinical practice, limiting patient-centered approaches to sepsis recovery management. Additionally, contextual-level (specifically, neighborhood) socioeconomic risk factors can influence health outcomes independently of commonly measured individual-level factors, through pathways such as higher exposure to unsanitary conditions or lack of healthy food availability, which may exacerbate existing conditions and increase the likelihood of readmission.15,16 However, the effect of neighborhood disadvantage on sepsis recovery and sepsis rehospitalizations in the United States is unknown. Understanding this relationship may aid in design and implementation of strategies to help specific populations discharged from the hospital after an admission with sepsis.
We investigated whether neighborhood disadvantage is associated with rehospitalizations for patients who survived sepsis. We hypothesized that patients discharged from a hospitalization with a diagnosis of sepsis would experience greater odds of 30-day rehospitalizations if they were from disadvantaged neighborhoods as compared to patients from more affluent areas.
METHODS
Study Population
Eligible patients included those aged 18 years and older who were discharged with an International Classification of Disease, 10th edition, Clinical Modification (ICD-10) code of sepsis (sepsis, severe sepsis, septic shock) during the 2017 fiscal year. These patients underwent a manual audit to confirm that they met the criteria of sepsis or septic shock, as defined by the International Sepsis Definitions Conference (Sepsis-3).1 In order to study only patients who had potential for readmissions, we excluded all patients who did not survive their hospitalization or were transferred to hospice. All patients reviewed were from a single-center, academic, urban hospital. An Institutional Review Board waiver was obtained before conducting this study in accordance with institutional regulations.
Measures
Area Deprivation Index (ADI).
Neighborhood disadvantage was measured by the Area Deprivation Index (ADI), a validated, publicly available geospatial index of socioeconomic disadvantage constructed from U.S. Census data and updated to incorporate 2013 American Community Survey data.17,18 A well-established composite measure of socioeconomic disadvantage for all areas of the United States, the ADI reliably drills down to the smallest geographic units (block groups) and has been associated with several chronic health outcomes.15,17,19–24
Using discharge data from the Johns Hopkins Bayview Medical Center Population Health Database, patients’ addresses were used to identify their respective U.S. Census block group, and block groups were matched to their respective ADI ranking. The ADI reports a value from 1 (least disadvantaged) to 100 (most disadvantaged) and is freely available at www.neighborhoodatlas.medicine.wisc.edu. The ADI is a composite score constructed from 17 indicators in the domains of income, education, housing, employment, home and vehicle ownership, and family structure weighted by factor score coefficients for each indicator.17,19
Individual Variables.
Gender, race (Caucasian/white or other), ethnicity (Hispanic or not?), and insurance status (Medicare, Medicaid, Other) are captured within the population health database and were included in the analyses. Additional exposures included smoking status (“active/former” versus “never” smoker). Former smokers were defined as individuals who have smoked more than 100 cigarettes in their lifetime and quit smoking within the last 5 years, and were categorized together with active smokers.25
To assess baseline severity of chronic co-morbidities, the Charlson co-morbidity index was calculated for each patient.26 To capture organ dysfunction brought on by the acute episode of sepsis or septic shock, sequential assessment of organ dysfunction (SOFA) was calculated on each patient, with the worst value recorded in the first 24-hours of the hospitalization reported.1,27,28 Finally, length of hospitalization and place of discharge were also collected.
Outcome Measures.
The primary outcome was a 30-day readmission, as defined by the Centers for Medicare and Medicaid Services (CMS) hospital-wide all-cause unplanned 30-day readmission measure.29
Statistical Analysis
Descriptive analyses examined the variable distribution and obtained means and proportions. To evaluate patients who were not readmitted within 30-days of their initial hospital discharge versus those patients who were, we dichotomized patients into “readmitted” and “not readmitted”. Patient characteristics, ADI value, and 30-day readmissions were compared by t-tests for continuous variables and Fisher’s exact test for categorical variables.
To assess the independent associations between ADI score and 30-day readmissions for patients who were discharged from the hospital after surviving sepsis, we employed a multivariable logistic regression with robust standard error estimator, employing link functions for continuous and count outcomes as appropriate. For continuous ADI, we estimated the predicted difference in outcome for one standard deviation increase in ADI adjusting for covariates that included age, sex, race, exposures (smoking status), insurance type, Charlson co-morbidity index, SOFA score, length of intensive care unit stay, and length of hospitalization.
Standard logistic regression diagnostics were performed including Hosmer-Lemeshow goodness of fit test and McFadden’s R squared test. Covariates were selected as they were identified to be clinically relevant. All analyses were completed using R Studio version 1.1.442. Statistical significance was defined as P<0.05.
RESULTS
Patient Characteristics
There were 1007 patients discharged with an ICD-10 code of sepsis. After a manual audit of the data, 647 (64.3%) patients met criteria for sepsis or septic shock per the Sepsis-3 definition. Of the 647 patients, 116 (17.9%) either died in hospital or were discharged to hospice. These patients were excluded from further analysis, resulting in 531 patients remaining in the study cohort.
Of the 531 patients who survived sepsis, mean age was 61.0 (± 17.6 years) and 281 were females (52.9%) (See Table 1). The mean Charlson co-morbidity index was 4.2 (± 2.1) and 164 (30.9%) were active smokers. Their mean ADI was 54.2 (± 23.8) (range went from as low as 2 to as high as 100). The mean SOFA score during their sepsis related hospitalization was 4.9 (± 2.5) and the mean length of stay for their hospitalization was 6.9 (± 5.6 days). The majority of patients, 358 (67.3%), were discharged home. Of the 531 patients, 117 (22.0%) had a readmission within 30 days. Of those 117 patients with a readmission, 39 patients had a re-infection, 68 had an exacerbation of their chronic conditions, and 10 were admitted for “concerning symptoms” without a primary admitting diagnosis.
Table 1.
Patient characteristics. Results are presented as mean ± standard deviation or median (interquartile range).
| Variable | Total (N=531) | Was Not Readmitted (N=414) | Was Readmitted (N=117) | p-value |
|---|---|---|---|---|
| Age (years) | 61.0 ± 17.6 | 60.8 ± 17.9 | 61.9 ± 16.3 | 0.53 |
| Female | 281 (52.9%) | 212 (51.2%) | 69 (59.0%) | 0.17 |
| Caucasian/White | 368 (69.3%) | 286 (69.1%) | 82 (70.1%) | 0.92 |
| Active Smoker | 164 (30.9%) | 122 (29.5%) | 42 (35.9%) | 0.22 |
| Charlson Co-Morbidity Index | 6.0 (5.0, 8.0) | 5.5 (4.0,7.0) | 5.0 (3.9, 8.0) | 0.58 |
| Insurance | ||||
| Other | 99 (18.6%) | 83 (20.0%) | 16 (13.7%) | 0.15 |
| Length of ICU admission (days) | 2.3 ± 1.9 | 2.1 ± 0.9 | 2.8 ± 1.4 | 0.04 |
| Length of Hospitalization (days) | 6.9 ± 5.6 | 6.4 ± 5.1 | 8.7 ± 6.9 | <0.01 |
| SOFA (first 24 hours) | 7.0 (4.0–10.0) | 6.5 (4.8–9.0) | 7.0 (4.0, 11.0) | 0.09 |
| ADI | 54.2 ± 23.8 | 51.8 ± 22.2 | 62.5 ± 27.4 | <0.01 |
| Discharge | ||||
| SNF | 105 (19.7%) | 68 (16.4%) | 37 (31.6%) | <0.01 |
ADI = Area Deprivation Index; SOFA = Sequential Organ Failure Assessment; SNF = Skilled Nursing Facility
Factors Associated with Readmissions
Patients who were readmitted had a greater mean ADI (62.5 (± 27.4)) as compared to those who were not readmitted within 30-days of initial hospital discharge (mean ADI 51.8 (± 22.2), p<0.01). Further, patients who were readmitted had a significantly longer index hospitalization (mean of 8.7 (± 6.9 days)) versus patients who were not readmitted (mean length of hospitalization 6.4 (± 5.1 days), p<0.01). In regards to discharge status, patients who were discharged to skilled nursing facilities, 37 (31.6%), were more likely to be readmitted as compared to those discharged to home?, 68 (16.4%) (p<0.01). There were no statistically significant differences between severity of baseline co-morbidities and acute organ dysfunction for patients who survived sepsis and were rehospitalized versus those who survived and were not rehospitalized at 30-days (Table 1). In-hospital variables were also compared (Table 2).
Table 2.
In-hospital variables and outcomes. Results are presented as mean ± standard deviation or median (interquartile range).
| Variable | Total (N=531) | Was Not Readmitted (N=414) | Was Readmitted (N=117) | p-value |
|---|---|---|---|---|
| Body Mass Index | 30.0 (22.9, 35.1) | 29.6 (24.9, 37.5) | 28.2 (24.2, 35.3) | 0.61 |
| History of Chronic Disease | ||||
| ESRD on HD | 19 (3.6) | 15 (3.6) | 4 (3.4) | 0.01 |
| Source of Sepsis | ||||
| Abdominal | 96 (18.1) | 76 (18.4) | 20 (17.1) | 0.11 |
| Other/Unknown | 14 (2.6) | 10 (2.4) | 4 (3.4) | 0.27 |
| Vasopressor (%) | 337 (63.5) | 257 (62.1) | 75 (64.1) | 0.07 |
| Mechanical Ventilation (%) | 101 (19.0) | 77 (18.6) | 24 (20.5) | 0.14 |
| Continuous Renal Replacement Therapy (%) | 39 (7.3) | 28 (6.8) | 9 (7.7) | 0.22 |
COPD = chronic obstructive pulmonary disease; ESRD = End Stage Renal Disease; HD = Hemodialysis
In adjusted logistic regression models that included ADI as a continuous exposure, neighborhood disadvantage remained significantly associated with 30-day rehospitalization in patients who were discharged with sepsis. Specifically, one standard deviation increase in ADI score was associated with greater odds of 30-day rehospitalization (β=0.03, P<0.001) (Figure 1; Table 3). Length of hospitalization in the index visit also remained statistically significant in the adjusted regression model, where longer lengths of stay were associated with greater odds of a subsequent 30-day rehospitalization (Table 3).
Figure 1.
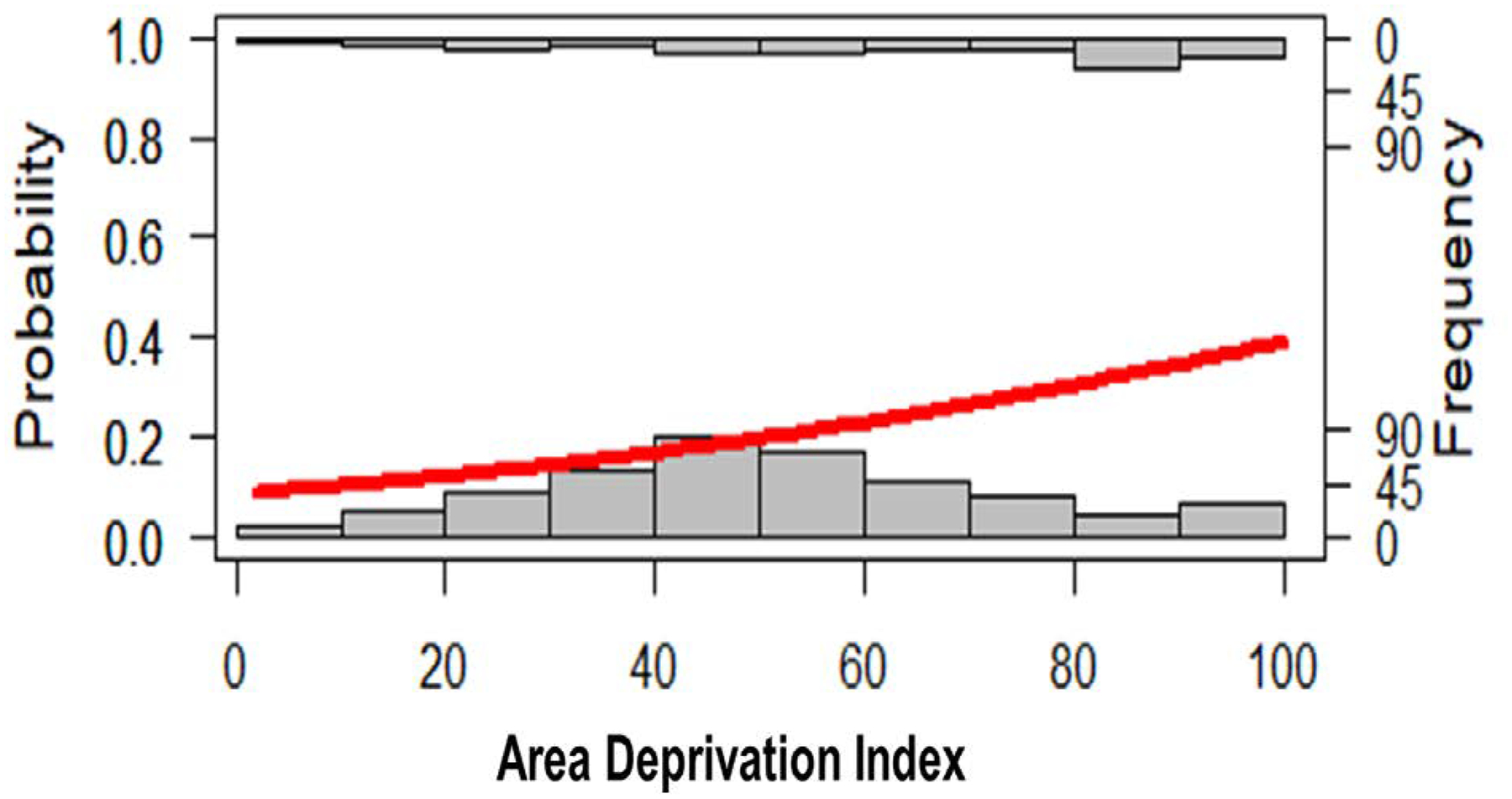
Logistic regression (red line) with readmissions as an outcome graphed against area deprivation index (ADI) frequency distribution for both readmissions (top x-axis) and non-readmissions (bottom x-axis).
Table 3.
Unadjusted and adjusted logistic regression models for 30-day readmissions in patients who were initially hospitalized due to sepsis.
| Variable | Unadjusted Model | Adjusted Model | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
| Age | 1.01 (0.99, 1.03) | 0.60 | 1.00 (−0.02, 1.02) | 0.88 |
| Female | 1.70 (0.84, 3.46) | 0.14 | 1.45 (0.69, 1.11) | 0.33 |
| Caucasian/White | 1.08 (0.51, 2.34) | 0.84 | 1.30 (0.56, 3.03) | 0.54 |
| Active Smoker | 2.25 (1.60, 4.95) | 0.04 | 2.14 (0.98, 4.71) | 0.06 |
| Charlson Co-Morbidity Index | 3.06 (0.64, 5.58) | 0.09 | 2.77 (0.80, 4.85) | 0.17 |
| Insurance | ||||
| Other | 2.12 (0.36, 3.13) | 0.45 | 2.25 (0.40, 2.77) | 0.78 |
| Length of ICU Admission | 1.12 (1.02, 1.20) | 0.03 | 1.09 (1.03, 1.16) | 0.03 |
| Length of Hospitalization | 1.14 (1.07, 1.21) | <0.001 | 1.08 (1.01, 1.16) | 0.04 |
| SOFA (first 24 hours) | 2.51 (0.52, 4.14) | 0.11 | 2.32 (0.59, 3.63) | 0.37 |
| ADI | 1.03 (1.02, 1.05) | <0.001 | 1.03 (1.01, 1.05) | <0.001 |
| Discharge | ||||
| SNF | 1.17 (1.08, 1.28) | <0.01 | 1.13 (0.99, 1.28) | 0.08 |
ADI = Area Deprivation Index; SOFA = Sequential Organ Failure Assessment; SNF = Skilled Nursing Facility
DISCUSSION
Living in a disadvantaged neighborhood, as measured by the Area Deprivation Index (ADI), is associated with increased odds of rehospitalizations within 30-days after discharge from hospital for patients who survived sepsis. This effect is retained after adjusting for individual demographic variables, active tobacco use, length of index hospitalization, severity of acute and chronic morbidity, and place of initial discharge. Our findings suggest the need for interventions that emphasize neighborhood-level socioeconomic variables in addition to individual-level efforts in an effort to promote and achieve health equity for patients who survive a hospitalization due to sepsis.
Our readmission rate (22.0%) is on par with other reported sepsis readmission rates, ranging from 19.9%30 to 29.0%10. Norman et al reported that many hospitals with high burden of sepsis readmissions served more socioeconomically disadvantaged patients as compared to hospitals with lower sepsis readmissions.10 Norman et al used CMS Hospital Compare Data from 2008 to 2010 and the American Hospital Association Database from 2008 to 2010 to obtain data on hospital and contextual-level socioeconomic status. The authors’ used ZIP codes as the area-based indicator used to define a region’s socioeconomic status. While ZIP codes are often used in large population studies in order to define a contextual-socioeconomic variable (e.g. median income), a drawback of using ZIP codes is that they often mask significant heterogeneity in the defined population region as compared to other area-based indicators, such as the census block group.31 Census block groups are small, relatively homogenous areas of approximately 1000 persons.31 Further, other factors of a region beyond income (e.g. employment32,33) impact health outcomes and warrant concomitant investigations. Therefore, our use of the Area Deprivation Index, a composite score of various census block socioeconomic and demographic variables, may provide a better understanding of the relationship between deprivation and health outcomes, specifically, 30-day readmissions for sepsis survivors.
The link between higher odds of rehospitalizations for survivors of sepsis and neighborhood disadvantage warrants exploration. Previous research has shown that neighborhood disadvantage has been linked with susceptibility to infections overall, from pneumonia to bacteremia.34–36 Since a third of our cohort was readmitted with an infection, it is possible that more disadvantaged neighborhoods created more challenges for a person’s immune system, which may be compromised after recovering from sepsis. The severity of immune suppression after treatment of sepsis is complex and influenced by many factors: patient’s pre-sepsis health status, pathogen load and virulence, host response, and quality of sepsis treatment.9 However, the immunomodulation in a survivor of sepsis in conjunction with neighborhood disadvantage may contribute to reinfections and rehospitalizations. With a third of our cohort rehospitalized with infections, and other studies emphasizing that the most common readmission diagnosis was infection11, attention towards both anticipating and attenuating the risk of infection in sepsis survivors, especially among those who live in higher risk neighborhoods, must be a priority for the prevention of readmissions.
The majority of the patients in our cohort who survived sepsis and were readmitted within 30-days of hospital discharge had worsening of their chronic co-morbidities. Common non-communicable diseases such as chronic obstructive lung disease (COPD) and heart failure (diseases captured as well in the Charlson co-morbidity index) are impacted by contextual-level socioeconomic variables37,38, often resulting in baseline disparities due to factors such as lack of resources to manage conditions as well as living conditions which can exacerbate severity of these conditions. Prescott et al showed that readmission diagnosis after hospitalization for severe sepsis often was due to worsening of chronic co-morbidities, such as COPD and heart failure.11 Therefore, for patients who survive sepsis, have a common non-communicable disease, and live in disadvantaged neighborhoods as identified by the ADI, these patients may warrant aggressive allocation of healthcare and social resources for a brief time after hospital discharge to assure co-morbidities are stable.
We sought to investigate what variables are likely to be associated with readmissions for patients discharged with sepsis that could be feasible to use for clinicians and persons planning patient discharges. We chose the ADI for this analysis as it is a well-established measure using US census-based measures for small, homogenous geographic regions.17 The effects of neighborhood disadvantage may result from individual-level socioeconomic factors for which the ADI (a community data variable) is a proxy. While the relative importance of both personal and community disadvantage cannot be determined from this data, clarifying these associations for patients discharged with sepsis deserves further attention. For now, with our findings, we believe the ADI should be used to identify at-risk populations for rehospitalizations being discharged from the hospital after surviving sepsis, where resources such as in-home nursing monitoring or telemedicine may be allocated.
Several limitations must be taken into account. First, this was a cross-sectional review of data from a single academic urban institution. While the findings are significant, they warrant larger investigations across more diverse regions and hospitals. Second, we did not capture specific interventions during the hospitalization that have been shown to result in frailty for patients surviving critical illnesses. For instance, prolonged sedation, immobilization, and certain medications have been linked with the complex syndrome of chronic critical illness.39 However, it is unclear if such resulting sequelae impact rehospitalizations,9 warranting future studies to evaluate which in-hospital interventions are associated with in rehospitalizations for survivors of sepsis. Third, we do not have information on the time spent in non-home discharge sites (e.g. skilled nursing facilities or acute rehabilitation centers), and whether they were readmitted to the hospital directly from such discharge sites. Finally, we cannot determine if length of time in a specific ADI impacts a person’s outcome, and whether a relocation to a different ADI ranking impacts rehospitalizations for patients who survive a hospitalization due to sepsis. Further, if a patient’s neighborhood’s ADI changed over time (as the ADI is composed at the same frequency as US Census Data is available) is unclear. Health benefits have been noted when persons move from disadvantaged to more affluent neighborhoods.40–42 Given that the ADI is a composite score,17 we cannot identify which component is the predominant driver of rehospitalizations for patients who survive sepsis. However, all components that make up the index are intertwined, and policy efforts targeting one (i.e. unemployment) will likely impact others (i.e. housing).
CONCLUSION
We report that persons who survived sepsis and reside in disadvantaged neighborhoods have a higher risk of rehospitalization, independent of certain demographic factors and morbidities. Our results underscore the relevance of neighborhood socioeconomic status—as identified by the ADI—in the role of patients who survive sepsis and are discharged from the hospital. While further research is warranted to understand the relationship between neighborhood socioeconomic status and sepsis-related outcomes (such as readmissions), area-level measures such as the ADI may be clinically useful to help create health equitable strategies around rehospitalizations of high-risk patients who survived sepsis.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. [DOI] [PubMed] [Google Scholar]
- 3.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Cooke CR, Wunsch H, et al. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–1316. [DOI] [PubMed] [Google Scholar]
- 6.Mayr FB, Talisa VB, Balakumar V, et al. Proportion and Cost of Unplanned 30-Day Readmissions After Sepsis Compared With Other Medical Conditions. JAMA. 2017;317(5):530–531. [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, et al. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadre SK, Shah M, Mireles-Cabodevila E, et al. Epidemiology and Predictors of 30-Day Readmission in Patients With Sepsis. Chest. 2019;155(3):483–490. [DOI] [PubMed] [Google Scholar]
- 9.Prescott HC, Angus DC. Enhancing Recovery From Sepsis: A Review. JAMA. 2018;319(1):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman BC, Cooke CR, Ely EW, et al. Sepsis-Associated 30-Day Risk-Standardized Readmissions: Analysis of a Nationwide Medicare Sample. Crit Care Med. 2017;45(7):1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313(10):1055–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baggs J, Jernigan JA, Halpin AL, et al. Risk of Subsequent Sepsis Within 90 Days After a Hospital Stay by Type of Antibiotic Exposure. Clin Infect Dis. 2018;66(7):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin AJ, Ford DW. Readmissions Among Sepsis Survivors: Risk Factors and Prevention. Clin Pulm Med. 2018;25(3):79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang DW, Tseng CH, Shapiro MF. Rehospitalizations Following Sepsis: Common and Costly. Crit Care Med. 2015;43(10):2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durfey SNM, Kind AJH, Buckingham WR, et al. Neighborhood disadvantage and chronic disease management. Health Serv Res. 2019;54 Suppl 1:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung D, Kind A, Robert S, et al. Linking Neighborhood Context and Health in Community-Dwelling Older Adults in the Medicare Advantage Program. J Am Geriatr Soc. 2018;66(6):1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med. 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh GK, Azuine RE, Siahpush M, et al. All-cause and cause-specific mortality among US youth: socioeconomic and rural-urban disparities and international patterns. J Urban Health. 2013;90(3):388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh GK, Miller BA, Hankey BF. Changing area socioeconomic patterns in U.S. cancer mortality, 1950–1998: Part II--Lung and colorectal cancers. J Natl Cancer Inst. 2002;94(12):916–925. [DOI] [PubMed] [Google Scholar]
- 22.Singh GK, Miller BA, Hankey BF, et al. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101(5):1051–1057. [DOI] [PubMed] [Google Scholar]
- 23.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25–64 years by area socioeconomic status, 1969–1998. Int J Epidemiol. 2002;31(3):600–613. [DOI] [PubMed] [Google Scholar]
- 24.Singh GK, Siahpush M. Widening socioeconomic inequalities in US life expectancy, 1980–2000. Int J Epidemiol. 2006;35(4):969–979. [DOI] [PubMed] [Google Scholar]
- 25.Parascandola M, Augustson E, Rose A. Characteristics of current and recent former smokers associated with the use of new potential reduced-exposure tobacco products. Nicotine Tob Res. 2009;11(12):1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. [DOI] [PubMed] [Google Scholar]
- 29.CMS. Hospital-Wide All-Cause Unplanned Readmission Measure (NQF #1789). 2019.
- 30.Donnelly JP, Hohmann SF, Wang HE. Unplanned Readmissions After Hospitalization for Severe Sepsis at Academic Medical Center-Affiliated Hospitals. Crit Care Med. 2015;43(9):1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger N, Chen JT, Waterman PD, et al. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brydsten A, Hammarstrom A, San Sebastian M. Health inequalities between employed and unemployed in northern Sweden: a decomposition analysis of social determinants for mental health. Int J Equity Health. 2018;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLeod CB, Hall PA, Siddiqi A, et al. How society shapes the health gradient: work-related health inequalities in a comparative perspective. Annu Rev Public Health. 2012;33:59–73. [DOI] [PubMed] [Google Scholar]
- 34.Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch K, Sogaard M, Norgaard M, et al. Socioeconomic inequalities in risk of hospitalization for community-acquired bacteremia: a Danish population-based case-control study. Am J Epidemiol. 2014;179(9):1096–1106. [DOI] [PubMed] [Google Scholar]
- 36.Rudd KE, Kissoon N, Limmathurotsakul D, et al. The global burden of sepsis: barriers and potential solutions. Crit Care. 2018;22(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raju S, Keet CA, Paulin LM, et al. Rural Residence and Poverty Are Independent Risk Factors for Chronic Obstructive Pulmonary Disease in the United States. Am J Respir Crit Care Med. 2019;199(8):961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akwo EA, Kabagambe EK, Harrell FE Jr., et al. Neighborhood Deprivation Predicts Heart Failure Risk in a Low-Income Population of Blacks and Whites in the Southeastern United States. Circ Cardiovasc Qual Outcomes. 2018;11(1):e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson JE, Cox CE, Hope AA, et al. Chronic critical illness. Am J Respir Crit Care Med. 2010;182(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leventhal T, Dupere V. Moving to Opportunity: does long-term exposure to ‘low-poverty’ neighborhoods make a difference for adolescents? Soc Sci Med. 2011;73(5):737–743. [DOI] [PubMed] [Google Scholar]
- 41.Orr L, Feins JD, Jacob R, et al. Moving to opportunity for fair housing demonstration program: interim impacts evaluation. Washington, DC: 2003. [Google Scholar]
- 42.Sciandra M, Sanbonmatsu L, Duncan GJ, et al. Long-term effects of the Moving to Opportunity residential mobility experiment on crime and delinquency. J Exp Criminol. 2013;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]


