Abstract
Advanced and recurrent gynecological cancers are associated with poor prognosis and lack of effective treatment. The developments of the molecular mechanisms on cancer progression provide insight into novel targeted therapies, which are emerging as groundbreaking and promising cancer treatment strategies. In gynecologic malignancies, potential therapeutic targeted agents include antiangiogenic agents, poly (ADP-ribose) polymerase (PARP) inhibitors, tumor-intrinsic signaling pathway inhibitors, selective estrogen receptor downregulators, and immune checkpoint inhibitors. In this article, we provide a comprehensive review of the clinical evidence of targeted agents in gynecological cancers and discuss the future implication.
Subject terms: Gynaecological cancer, Drug development
Introduction
Gynecological malignancies, mainly including ovarian, cervical, and endometrial cancer, seriously affect the health of women worldwide, contributing considerably to the global cancer burden. Epithelial ovarian cancer (OC) comprises ~90% of the malignant ovarian neoplasms, which is one of the leading causes of death in women.1,2 The 5-year overall survival (OS) rate of OC is ~47% for all stages, and >70% of patients are diagnosed at the advanced stage with an even lower 5-year OS rate.3,4 The standard-of-care fist-line treatments for OC are debulking surgery and perioperative platinum-based chemotherapy.5,6 Although the response rate of the first-line treatment is high, most of the patients will eventually experience relapses within the subsequent 3 years.7 At first relapse, ~20–25% of patients have platinum-resistant (disease recurs ≤6 months from the last platinum-based chemotherapy) or platinum-refractory (disease progress during or within 4 weeks of platinum-based chemotherapy) disease, with poor prognosis.8,9 In the platinum-resistant disease, single non-platinum agent is used, such as paclitaxel, docetaxel, pegylated liposomal doxorubicin (PLD), gemcitabine and topotecan. However, the response rates and outcomes are disappointing. Cervical cancer (CC), as the fourth most common female cancer globally, is also a major health problem especially for women in developing countries.10 High-risk human papilloma virus (HPV) infection is considered to be responsible for more than 90% of CC development.11 HPV overexpresses E6 and E7 oncoproteins which inhibit TP53 and RB1 proteins from altering cell cycle, apoptosis, and DNA repair.12,13 Thus, HPV testing is an important part of CC screening, and immunization against HPV (e.g., vaccines) has been designed to prevent CC.14,15 With early screening and effective treatments such as radical surgery or concurrent chemoradiation (a combination of radiation and chemotherapy), the cure rate of CC can reach 80% in the early-stage disease (FIGO stage I–II). The 5-year OS rate for all stages is ~66%. However, treatment options are limited and the survival rate is low for patients who present with distant metastatic disease, as well as those with unresectable recurrent disease and those who recur at distant. Endometrial cancer (EC), also known as uterine cancer, is the sixth most common female cancer.10,16 Elevated estrogen levels and increasing age are well-known risk factors of EC.17,18 Thus, the incidence of EC is increasing due to the increased life expectancy and obesity (causing elevated estrogen level). The standard treatment consists of surgery with or without adjuvant radiotherapy and/or chemotherapy, which is based on the risk of disease recurrence.19 Traditionally, EC has been classified in two types mainly according to histology and estrogen dependence. Furthermore, the Cancer Genome Atlas (TCGA) identified EC into four molecular subgroups: polymerase epsilon (POLE) ultramutated, microsatellite instability hypermutated, copy-number low, and copy-number high, each with a distinct prognosis.20 Most low-risk patients with early-stage disease can be cured by surgery and have good prognoses. However, the prognosis for advanced EC is poor with 5-year OS rate of 40–65% in stage III and 15–17% in stage IV disease, respectively.21 All those malignancies, when progressed to the advanced stage, have very poor prognoses under conventional treatment. Due to the lack of effective treatment for advanced-stage, refractory, recurrent, and drug-resistance disease, we are facing very tough challenges. However, based on the improved understanding of the mechanisms on cancer progression, targeted therapies are emerging as groundbreaking and promising treatment strategies.
In targeted therapies, individual patients are treated by agents targeting the changes in tumor cells that help them grow, divide, and spread. Currently in gynecological malignancies, potential therapeutic targets include tumor-intrinsic signaling pathways, angiogenesis, homologous-recombination deficiency (HDR), hormone receptors, and immunologic factors. The corresponding targeted agents include signaling pathway inhibitors, antiangiogenic agents, poly (ADP-ribose) polymerase (PARP) inhibitors, selective estrogen receptor downregulators, and Immune checkpoint inhibitors. For gynecological cancers, bevacizumab, olaparib, rucaparib, niraparib, and pembrolizumab have been approved by the US Food and Drug Administration (FDA) for selected patients with recurrent, metastatic, or high-risk diseases (Table 1). The clinical uses of these and other targeted agents are being actively and extensively investigated.
Table 1.
FDA-approved targeted drugs for gynecological cancers
| Target | Drug | Approval year | Indication | Administration | |
|---|---|---|---|---|---|
| VEGFi | Bevacizumab (Avastin, Genentech) | 2014 | CC | Persistent, recurrent, or metastatic disease | 15 mg/kg IV every 3 weeks with chemotherapy |
| 2014 | OC | Platinum-resistant recurrent, and received no more than 2 prior chemotherapy regimens | 10 mg/kg IV every 2 weeks with chemotherapy | ||
| 2016 | Platinum-sensitive recurrent | 15 mg/kg IV every 3 weeks with chemotherapy, and in maintenance | |||
| 2018 | Advanced (FIGO stage III–IV) | ||||
| PARPi | Olaparib (Lynparza, AstraZeneca) | 2014 | OC | Advanced, with BRCAm, and have received three or more prior lines of chemotherapy | 300 mg orally twice daily, until disease progression or unacceptable toxicity |
| 2017 | Recurrent, and in complete or partial response to platinum-based chemotherapy | ||||
| 2018 | Advanced, with BRCAm, and in complete or partial response to platinum-based chemotherapy | ||||
| Rucaparib (Rubraca, Clovis) | 2016 | OC | Recurrent, with BRCAm, and have received two or more chemotherapies | 600 mg orally twice daily, until disease progression or unacceptable toxicity | |
| 2018 | Recurrent and in a complete or partial response to platinum-based chemotherapy | ||||
| Niraparib (Zejula, Tesaro) | 2017 | OC | Recurrent and in a complete or partial response to platinum-based chemotherapy | 300 mg orally once daily, until disease progression or unacceptable toxicity | |
| Anti-PD-1 | Pembrolizumab (Keytruda, Merck) | 2017 | EC | Unresectable or metastatic, with a biomarker as MSI-H or dMMR | 200 mg IV over 30 min every 3 weeks |
| 2018 | CC | Recurrent or metastatic, with disease progression on or after chemotherapy, and expressing PD-L1 | |||
| Anti-PD-1 + VEGFi | Pembrolizumab (Keytruda, Merck) + lenvatinib (Lenvima, Eisai) | 2019* | EC | Advanced disease without MSI-H/dMMR who have disease progression following prior systemic therapy, but are not candidates for surgery or radiation | Lenvatinib 20 mg orally once daily with pembrolizumab 200 mg IV over 30 min every 3 weeks |
CC cervical cancer, OC epithelial ovarian, fallopian tube, or primary peritoneal cancer, EC endometrial cancer, VEGFi VEGF inhibitor, PARPi PARP inhibitor, IV intravenous infusion, BRCAm deleterious or suspected deleterious BRCA mutation, MSI-H microsatellite instability high, dMMR mismatch repair-deficient. *Accelerated approval
In this paper, we review the clinical efficacy and safety of the targeted therapies in gynecological cancers, by summarizing the results of previous clinical trials. We further describe the ongoing phase II/III clinical trials and expound future directions.
Methods
A comprehensive literature review was performed on PubMed, including systematic reviews, review articles, clinical trials, and observation studies published in English. ClinicalTrials.gov was queried to collect the data of completed and ongoing clinical trials. For each approved targeted drug, the FDA website was searched for indication, usage and references as the basis for approval. Search terms included “gynecological cancers”, “ovarian cancer”, “cervical cancer”, “endometrial cancer”, “targeted therapy”, “antiangiogenic agents”, “PARP inhibitor”, “signaling pathway inhibitors”, “immune checkpoint inhibitors”, and each name of the targeted agent (e.g., “bevacizumab”, “olaparib”). We also used the ESMO and ASCO websites for preliminary results reported from ongoing trials.
Antiangiogenic agents
Neovasculature is considered as a crucial process for tumor growth and progression.22 In decades, efforts have been made to develop vascular-targeted therapies for cancer treatment. Depending on the distinctly different mechanisms, vascular-targeted therapies include antiangiogenic agents and vascular-disrupting agents.23 Here, we focus on the action of antiangiogenic agents in this review.
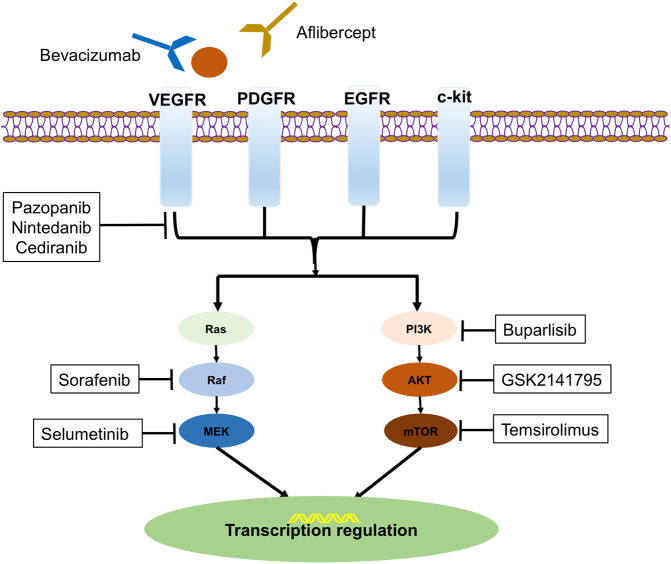
Angiogenesis is a complex process regulated by various pro-angiogenic and antiangiogenic factors.24 Vascular endothelial growth factor (VEGF), a major driver of angiogenesis in solid tumors, binds to the VEGF receptors (VEGFR, including VEGFR-1/2/3) on target cells and initiates the signaling pathway through intracellular tyrosine kinases.25 It can initiate several endothelial cell signaling pathways and promote endothelial cell precursors from bone marrow.24 The VEGF pathway also interacts with the PI3K/AKT/mTOR pathway.26,27 Moreover, the process of angiogenesis is further modulated by the platelet-derived growth factor (PDGF) pathway, the fibroblast growth factor (FGF) pathway, the epidermal growth factor (EGF) pathway, and the angiopoietin family and their receptor tyrosine kinase (Tie2) pathways.28 There are complicated interplays of these pro-angiogenic pathways (Fig. 1).29 In addition, the VEGF expression can be induced by hypoxia-associated transcription factors, such as hypoxia inducible factors (HIF1A and HIF2A). It is also associated with other genetic alterations such as TP53, RAS, and EGFR.30
Fig. 1.
The VEGF, PI3K/AKT/mTOR, and Ras/Raf/MEK signal transduction pathway and therapeutic interventions. After ligand binding, the receptors initiate the signaling cascade reaction, which is overactive in cancer cells. The figure shows the main elements in those pathways and the therapeutic agents
In tumor cells, the expression levels of the pro-angiogenic factors, especially VEGF, are upregulated to develop tumor’s own endogenous blood vessels, which is associated with the poor prognosis.22,31 Therefore, antiangiogenic therapies are developed by inhibiting target signaling pathways at different points. The main classes of antiangiogenic agents are anti-VEGF monoclonal antibodies (e.g., bevacizumab), soluble VEGFRs (e.g., aflibercept), inhibitors of angiopoietin-Tie2 receptor (e.g., trebananib), and tyrosine kinase inhibitors (e.g., cediranib).24,32 Tyrosine kinases are enzymes that catalyze the transfer of phosphate from adenosine triphosphate (ATP) onto target proteins to elicit a response.33 Tyrosine kinase inhibitors (TKIs) are small molecules which can block intracellular tyrosine kinases in multiple signaling pathways (e.g., VEGF, EGF).
A number of antiangiogenic agents, such as bevacizumab, pazopanib, sunitinib, sorafenib, vandetanib, aflibercept, axitinib, regorafenib, ramucirumab, and lenvatinib are FDA-approved for cancer treatment (e.g., colorectal cancer, lung cancer, renal cell carcinoma, and thyroid cancer). For gynecological cancers, bevacizumab was the first and only FDA-approved anti-VEGF drug. As of January 2020, there are a dozen of completed phase III trials assessing the efficacy and safety of antiangiogenetic agents for gynecological cancers, especially in OC. The main data from completed Phase II/III clinical trials are summarized in Tables 2 and 3.
Table 2.
Completed phase III trials of antiangiogenic agents in gynecological cancers
| ID | Cancer/condition | No. | Intervention | mPFS (mon.) | mOS (mon.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|---|
| NCT00483782 ICON7 | OC/high-risk stage I–IIa, IIb–IV | 1528 | (1) PC | 17.5 | 58.6 | – | 37 |
| (2) PC + bevacizumab | 19.9, P = 0.25 | 58.0, P = 0.85 | – | ||||
| NCT00976911 AURELIA | OC/platinum-resistant recurrent | 361 | (1) Single-agent chemotherapy | 3.4 | 13.3 | 27.1 | 42 |
| (2) Chemotherapy + bevacizumab | 6.7, P < 0.001 | 16.6, P = 0.174 | 31.28 | ||||
| NCT00434642 OCEANS | OC/platinum-sensitive recurrent | 484 | (1) GC + placebo | 8.4 | 32.9 | 25.32 | 40 |
| (2) GC + bevacizumab | 12.4, P < 0.0001 | 33.6, P = 0.65 | 36.44 | ||||
| NCT00262847 GOG-0218 | OC/stage III–IV | 1873 | (1) PC + placebo | 10.3 | 41.1 | 38.49 | 35 |
| (2) PC + bevacizumab throughout | 14.1, P < 0.001 | 40.8, P = 0.34 | 41.19 | ||||
| (3) PC + bevacizumab combination only | 11.2, P = 0.16 | 43.4, P = 0.53 | 46.37 | ||||
| NCT00565851 GOG-0213 | OC/platinum-sensitive recurrent | 674 | (1) PC | 10.4 | 37.3 | 86 | 41 |
| (2) PC + bevacizumab | 13.8, P < 0·0001 | 42.2, P = 0.045 | 96 | ||||
| NCT00803062 GOG-0240 | CC/metastatic, persistent, or recurrent | 452 | (1) PC | 6 | 13.3 | 37.5 | 42,43 |
| (2) PT | 34.58 | ||||||
| (3) PC + bevacizumab | 47.75 | ||||||
| (4) PT + bevacizumab | 8.2, P = 0.002 | 16.8, P = 0.007 | 55.96 | ||||
| NCT00532194 ICON6 | OC/platinum-sensitive recurrent | 486 | (1) Chemotherapy + placebo | 8.7 | – | – | 73 |
| (2) Chemotherapy + cediranib throughout | 9.9 | – | |||||
| (3) Chemotherapy + cediranib combination only | 11, P < 0.0001 | – | |||||
| NCT01015118 AGO-OVAR12 | OC/stage IIb–IV | 1503 | (1) PC + placebo | 16.6 | 62.8 | 34.89 | 67 |
| (2) PC + nintedanib | 17.2, P = 0.24 | 62, P = 0.087 | 42.02 | ||||
| NCT00866697 AGO-OVR16 | OC/stage II–IV, after first-line chemotherapy | 940 | (1) Placebo | 12.3 | 64.0 | 11.06 | 63 |
| (2) Pazopanib | 17.9, P = 0.0021 | 59.1, P = 0.64 | 25.37 | ||||
| NCT01204749 TRINOVA-1 | OC/recurrent | 919 | (1) Paclitaxel + placebo | 5.4 | 17.3 | 52 | 78 |
| (2) Paclitaxel + trebananib | 7.2, P < 0.0001 | 19.0, P = 0.19 | 53 | ||||
| NCT01281254 TRINOVA-2 | OC/recurrent | 223 | (1) PLD + placebo | 7.2 | 17.0 | 72 | 81 |
| (2) PLD + trebananib | 7.6, P = 0.57 | 19.4, P = 0.76 | 73 | ||||
| NCT01493505 TRINOVA-3 | OC/stage III–IV | 1164 | (1) PC + placebo | 15.0 | – | 66 | 80 |
| (2) PC + trebananib | 15.9, P = 0.36 | 73 |
ID identifier, No. enrollment number, mPFS median progression-free survival, mOS median overall survival, Mon. months, SAEs serious adverse events, Refs references, Stage FIGO stage, PC paclitaxel + carboplatin, GC gemcitabine + carboplatin, PT topotecan + paclitaxel, PLD pegylated liposomal doxorubicin
Table 3.
Completed phase II trials of antiangiogenic agents in gynecological cancers
| ID | Cancer/condition | No. | Intervention | ORR (%) | mPFS (mon.) | mOS (mon.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|---|---|
| NCT00025233 | CC/persistent or recurrent | 46 | Bevacizumab | 10.9 | 3.4 | 7.29 | 58.7 | 45 |
| NCT00548418 GSK107278 | CC/persistent or recurrent | 27 | Bevacizumab + topotecan + cisplatin | 59 | 7.1 | 13.2 | 44.44 | 46 |
| NCT00369122 RTOG0417 | CC/stage Ib–IIIb | 60 | Bevacizumab + cisplatin + radiotherapy | 68.7 | – | – | 22.03 | 49 |
| – | CC/advanced or recurrent | 34 | Bevacizumab + PC | 88 | 9 | 26 | – | 47 |
| NCT00937560 OCTAVIA | OC/stage IIb–IV | 189 | Bevacizumab + PC | 84.6 | 23.7 | – | 22.8 | 396 |
| NCT01010126 | EC/stage III–IV | 26 | Bevacizumab + temsirolimus | 25.1 | 6.0 | 11.5 | 61.5 | 60,339 |
| OC/stage III–IV | 58 | 6.4 | 5.6 | 16.3 | 58.6 | |||
| NCT01305213 GOG-0186I | OC/recurrent | 107 | (1) Bevacizumab | 28.2 | 4.8 | – | 16.98 | 397 |
| (2) Bevacizumab + fosbretabulin | 35.7 | 7.3, P = 0.05 | 29.6 | |||||
| NCT00696670 | OC/resistant | 39 | Bevacizumab + erlotinib | 23.1 | 4 | – | 30 | 398 |
| NCT00945139 | OC/platinum-resistant recurrent | 46 | Bevacizumab + PLD | 30.2 | 6.6 | 33.2 | 6.52 | 399 |
| NCT01091259 | OC/recurrent | 29 | Bevacizumab + irinotecan | 27.6 | 6.8 | 15.4 | 31 | 400 |
| NCT00886691 GOG-0186G | OC/recurrent | 150 | (1) Bevacizumab | 12.1 | 4.5 | 17.3 | 32 | 401 |
| (2) Bevacizumab + temsirolimus | 22.2 | 5.9, P = 0.39 | 16.6, P = 0.55 | 46.7 | ||||
| NCT00407563 ACORN | OC/platinum-resistant recurrent | 48 | Bevacizumab + abraxane | 50 | 8.08 | 17.15 | 27.1 | 402 |
| NCT00267696 OSU-05070 | OC/platinum-resistant recurrent | 45 | Bevacizumab + GC | 69 | 13.3 | 36.1 | 8.9 | 403 |
| NCT00977574 GOG-0086P | EC/stage III–IV | 339 | (1) Bevacizumab + PC | 60 | – | 34 | 42.9 | 404 |
| (2) Temsirolimus + PC | 55 | 25 | 50.4 | |||||
| (3) Bevacizumab + carboplatin | 53 | 25.2 | 46.5 | |||||
| NCT01770171 MITO END-2 | EC/advanced or recurrent | 108 | (1) PC | 53.1 | 10.5 | 29.7 | – | 54 |
| (2) PC + bevacizumab | 74.4 | 13.7, P = 0.43 | 40.0, P = 0.24 | |||||
| NCT01005329 RTOG 0921 | EC/high risk | 34 | Bevacizumab + cisplatin + radiotherapy | The 2-year estimate of OS was 96.7% | 26.7 | 53 | ||
| NCT00879359 | EC/advanced or recurrent | 15 | Bevacizumab + PC | 73 | 18 | 58 | 73.3 | 52 |
| NCT00723255 GOG-0229G | EC/recurrent | 43 | Bevacizumab + temsirolimus | 24.5 | 5.6 | 16.9 | 63.3 | 405 |
| NCT00301964 GOG-0229E | EC/persistent or recurrent | 56 | Bevacizumab | 13.5 | 4.2 | 10.5 | 34.6 | 51 |
| - | EC/persistent or recurrent | 46 | Bevacizumab + pemetrexed | 41 | 7.9 | 25.7 | 52 | 406 |
| NCT01468909 | OC/recurrent | 106 | (1) Paclitaxel | 31.8 | 7.5 | 23.3 | 30.00 | 407 |
| (2) Pazopanib + paclitaxel | 22.7 | 6.2, P = 0.20 | 20.7, P = 0.90 | 42.31 | ||||
| NCT01644825 MITO-11 | OC/stage Ic–IV | 74 | (1) Paclitaxel | 25 | 6.5 | – | 34 | 408 |
| (2) Pazopanib + paclitaxel | 56 | 16.1, P <0.01 | 46 | |||||
| NCT00430781 | CC/stage IVb, persistent, or recurrent | 230 | (1) Pazopanib | 9 | 4.22 | – | 37.84 | 257 |
| (2) Lapatinib | 5 | 3.99, P = 0.013 | 29 | |||||
| NCT02055690 | OC/recurrent | 21 | (1) Pazopanib | 22 | 3.7 | – | – | 45 |
| (2) Pazopanib + fosbretabulin | 18 | 7.6, P = 0.08 | ||||||
| NCT01669798 | OC/recurrent, bevacizumab-resistant | 27 | Nintedanib | 7.4 | 1.8 | 16 | 22.2 | 68 |
| NCT01225887 GOG-0229K | EC/recurrent | 37 | Nintedanib | 9.4 | 3.3 | 10.1 | 43.8 | 69 |
| NCT01210222 GOG-0229L | EC/recurrent | 35 | Trebananib | 3.1 | 1.7 | 6.6 | 43 | 82 |
| NCT01253681 | OC/recurrent | 61 | (1) Placebo | 27 | 4.6 | – | 64 | 409 |
| (2) Trebananib | 19 | 5.7 | 55 | |||||
| (3) Trebananib + paclitaxel | 37 | 7.2 | 65 | |||||
| NCT01111461 | EC/recurrent | 133 | Lenvatinib | 14.3 | 5.4 | 10.6 | 46.62 | 410 |
| NCT00278343 | OC/recurrent | 74 | Cediranib | 26 | 4.9 | 18.9 | 6.8 | 72 |
| NCT01132820 GOG-0229J | EC/recurrent | 48 | Cediranib | 12.5 | 3.65 | 12.5 | 41.7 | 74 |
| NCT00888173 GOG-0229I | EC/recurrent | 43 | Brivanib | 7 | 3.3 | 10.7 | 41.86 | 95 |
| NCT01267253 GOG-0227G | CC/recurrent | 28 | Brivanib | 8 | 3.2 | 7.9 | 50 | 94 |
| NCT02867956 | OC/platinum-refractory | 35 | Apatinib + etoposide | 54 | – | – | 5.7 | 87 |
| NCT02867956 | OC/recurrent | 29 | Apatinib | 41.4 | 5.1 | 14.5 | 31 | 86 |
| NCT00979992 GOG-0254 | OC/clear cell, recurrent or persistent | 30 | Sunitinib | 6.7 | 2.7 | 12.8 | – | 91 |
| NCT00388037 | OC/recurrent | 30 | Sunitinib | 3.3 | 4.1 | – | 50.00 | 90 |
| NCT00543049 AGO 2.11 | OC/platinum-resistant recurrent | 76 | Sunitinib (noncontinuous/continuous) | 16.7/5.4 | 4.8/4.9 | 13.6/13.7 | – | 89 |
| NCT00768144 | OC/recurrent, platinum-refractory | 35 | Sunitinib | 8.3 | 9.9 | – | 19.44 | 88 |
| NCT00478426 | EC/metastatic or recurrent | 33 | Sunitinib | 18.1 | 3 | 19.4 | 52 | 92 |
| NCT00389974 | CC/advance or metastatic | 19 | Sunitinib | 0 | 3.5 | – | 73.68 | 93 |
ORR objective response rate
Bevacizumab
Bevacizumab is a humanized anti-VEGF monoclonal antibody, which is the best-known antiangiogenetic agent. In gynecological cancers, bevacizumab is currently approved by FDA as combination treatment and/or maintenance treatment for selected patients with: (1) persistent, recurrent, or metastatic CC; (2) advanced or recurrent OC (including stage III/IV epithelial ovarian cancer, fallopian tube, or primary peritoneal cancer) (Table 1). The decisions of these indications are mainly grounded on findings from the following six Phase III clinical trials (five for OC and one for CC) (Table 2).
GOG-0218 trial (NCT00262847) evaluated the efficacy of bevacizumab (15 mg/kg intravenously every 3 weeks) in combination with chemotherapy plus/without bevacizumab maintenance for patients with newly diagnosed advanced OC following initial surgery. The median progression-free survival (PFS) was increased in the bevacizumab-concurrent plus maintenance arm when compared with control (chemotherapy alone) arm (3.8 months longer, P < 0.001). PFS was not significantly increased in the bevacizumab-concurrent arm (without bevacizumab maintenance).34 However, final results of this trial were updated in July, 2019. When compared with the control arm, there is no significant increase in the median OS either in the bevacizumab-concurrent plus maintenance arm or in the bevacizumab-concurrent arm. In a subset analysis stratified by stage, for patients with stage IV disease, the control and bevacizumab-concurrent arms were associated with a median OS of 32.6 and 34.5 months, respectively. The median OS was increased in patients with stage IV disease who received bevacizumab-concurrent plus maintenance (42.8 months, HR, 0.75; 95% CI, 0.59–0.95).35 Another phase III trial, ICON7 (NCT00483782) found a modest increase in the median PFS (2.4 months longer, P = 0.25) with no OS benefit in chemotherapy plus bevacizumab (both concurrence and maintenance) arm in the updated analyses.36 However, in a subset analysis of patients at high risk of progression, a significant difference in the median OS was noted between patients in chemotherapy plus bevacizumab arm and those in chemotherapy alone arm (39.3 vs. 34.5 months, P = 0·03).37 Data from these two trials did not show a statistically different quality of life (QOL) in the whole study population.38 Owing to the above trials, the FDA approved bevacizumab in combination with chemotherapy and followed as maintenance therapy for newly diagnosed advanced OC patients after initial surgical resection.
For patients with platinum-sensitive recurrent OC, OCEANS trial (NCT00434642) showed that the median PFS was significantly increased (4 months longer, P < 0.0001) in chemotherapy plus bevacizumab arm compared with chemotherapy alone.39 However, no significant difference in OS was observed at the final analysis.40 On the other hand, another phase III trial GOG-0213 (NCT00565851) showed that the addition of bevacizumab to chemotherapy led to a significant difference in both median PFS (3.4 months longer, P < 0.0001) and OS (4.9 months longer, adjusted P = 0.0447) in patients with platinum-sensitive recurrent OC.41 The FDA approved bevacizumab in combination with first-line chemotherapy and followed as maintenance therapy for platinum-sensitive recurrent OC patients in 2016.
For patients with platinum-resistant recurrent OC, an open-label phase III trial, AURELIA (NCT00976911), found that the addition of bevacizumab to chemotherapy improved the median PFS (3.3 months longer, P < 0.001), but with no benefit in OS at the final analysis.42,43 Based on this trail, the FDA approved bevacizumab in combination with chemotherapy for platinum-resistant recurrent OC patients who received no more than two prior chemotherapy regimens.
Another phase III trial (NCT01081262), studying different chemotherapy regimens with or without bevacizumab as the first-line therapy in treating patients with mucinous epithelial OC, was closed early due to slow accrual.44 An ongoing phase III trial (NCT03635489) is evaluating the efficacy and safety of bevacizumab plus chemotherapy in Chinese participants with newly diagnosed advanced OC.
For CC, phase II trials (e.g., NCT00548418) demonstrated that the combination of chemotherapy and bevacizumab in patients with recurrent or persistent CC had an objective response rate (ORR) of 59–88%.45–47 Furthermore, a phase III trial, GOG-0240 (NCT00803062), revealed an improvement in the median PFS (2.2 months longer, P = 0·0002) and OS (3.5 months longer, P = 0.007) among patients receiving chemotherapy plus bevacizumab compared with those receiving chemotherapy alone.48 Based on this trail, the FDA approved bevacizumab in combination with standard chemotherapy for metastatic, persistent, or recurrent CC. For locally advanced CC, a phase II trial (NCT00369122) showed concurrent cisplatin-based chemoradiotherapy and bevacizumab had an ORR of 68.7%.49 Another phase II/III trial (JCOG1311) has been initiated to compare different chemotherapy regimens with or without bevacizumab in stage IVb, recurrent or persistent CC.50
Currently, there are limited results of phase III studies assessing the efficacy of bevacizumab for patients with EC. In a phase II trial (NCT00301964) for persistent or recurrent EC, the single-agent bevacizumab therapy was shown to have an ORR of 13.5%, with the median PFS and OS being 4.2 and 10.5 months, respectively.51 Another phase II trial (NCT00879359) for advanced or recurrent EC showed that bevacizumab in combination with chemotherapy had an ORR of 73%, presenting a median PFS of 18 months and a median OS of 58 months.52 For patients with high-risk EC, postoperative bevacizumab added to chemotherapy and pelvic radiotherapy resulted in a high OS rate (at 2 years) of 96.7% and a disease-free survival rate of 79.1%, which was reported in a phase II trial (NCT01005329).53 However, bevacizumab plus chemotherapy failed to demonstrate a significant increase in PFS of patients with advanced or recurrent EC, reported by the MITO END-2 trial (NCT01770171) in 2019.54
Grade 3 or worse adverse events (AEs) occurring at a higher incidence (incidence ≥ 2%) in patients receiving chemotherapy plus bevacizumab compared with chemotherapy alone (from data of those phase III trials) included fatigue, hypertension, neutropenia, thrombocytopenia, proteinuria, nausea, headache, dyspnea, epistaxis, abdominal pain, hyponatremia, pain in extremity, and palmar-plantar erythrodysaesthesia syndrome.55
Pazopanib
Pazopanib is an oral TKI of VEGFR-1/-2/-3, PDGF receptor (PDGFR) -α/-β, and c-Kit.56–58 Pazopanib showed promising activity in phase I/II trials for patients with platinum-sensitive recurrent OC with increased ORR and PFS.59–61 A phase III trial, AGO-OVAR16 (NCT00866697), investigated the efficacy and safety of pazopanib (800 mg daily) as maintenance therapy after first-line chemotherapy in patients with newly diagnosed stage II–IV OC. The study showed that the pazopanib maintenance significantly improved the median PFS (5.6 months longer, P = 0.0021).62 In subgroup analyses, the PFS benefit with maintenance pazopanib was observed in most subgroups except East Asian patients. To gain further insight, a concurrent study (NCT01227928) similar in design to AGO-OVAR16 was undertaken in the East Asian population, showing that pazopanib maintenance therapy was not associated with a benefit in PFS or OS. There was no satisfactory explanation for this result yet. However, the final analysis of the OVAR16 study was reported in 2019. No difference was observed in the median OS between pazopanib arm and placebo arm.63 Grade 3 or worse AEs occurring at a higher incidence in the combined treatment arm compared with placebo included hypertension, neutropenia, diarrhea, thrombocytopenia, increased alanine aminotransferase, and palmar-plantar erythrodysesthesia. A phase I/II trial (NCT02055690) recently reported that combination of pazopanib and fosbretabulin (a prodrug with vascular-disrupting activity) might potentially improve survival outcomes compared with pazopanib alone.64 However, this trial was prematurely stopped due to serious cardiac toxicity.
Currently, there are limited data of clinical trials investigating pazopanib for patients with CC or EC. A phase II trial evaluated pazopanib in the treatment of recurrent or persistent carcinosarcoma of the uterus with a result of no response.65
Nintedanib
Nintedanib is another oral TKI of VEGFR-1/-2/-3, FGF receptor (FGFR)-1/-2/–3, and PDGFR-α/β. A phase II trial in platinum-sensitive recurrent OC patients showed an improvement in PFS rate in nintedanib maintenance arm than placebo arm (16.3% vs. 5.0%, P = 0.06).66 Subsequently, a phase III trial, AGO-OVAR12 (NCT01015118), investigated the combination of nintedanib (200 mg daily) with first-line chemotherapy in patients with newly diagnosed stage IIb–IV OC. The median PFS was 0.6 month longer in the nintedanib arm than that in the placebo arm (P = 0.024).67 Increased incidences of AEs, including hypertension, gastrointestinal perforation, and bleeding, were reported in the nintedanib arm. The final result of OS is pending. However, for bevacizumab-resistant OC population, single-agent nintedanib was shown to have minimal activity with an ORR of 7.4% in a phase II trial (NCT01669798).68
We found limited clinical data of phase II/III trials investigating the activity of nintedanib in EC and CC. One phase II trial, GOG-0229K (NCT01225887), evaluated nintedanib in the treatment of advanced, recurrent, or metastatic EC. It showed modest activity with an ORR of 9.4%.69
Cediranib
Cediranib is a TKI of VEGFR-1/-2/-3 and c-Kit.70,71 Given the activity of cediranib in OC showed by early-phase trials,72 a phase III trial, ICON6 (NCT00532194), investigated the combination of cediranib (20 mg orally daily) with chemotherapy and as maintenance treatment in patients with platinum-sensitive recurrent OC. The median PFS was 2.3 months longer in the cediranib maintenance arm than that in the placebo arm (P < 0·0001).73 The data of OS have not been updated. Currently, there are no differences in immature results of median OS across the arms. Increased incidences of diarrhea, neutropenia, hypertension, and voice changes were noted in arms with cediranib.
A phase II study, GOG 229J (NCT01132820), showed cediranib as a monotherapy treatment for recurrent or persistent EC was well-tolerated, with a median PFS of 3.65 months and a median OS of 12.5 months.74 Cediranib showed sufficient activity to warrant further investigation for recurrent EC. However, we found limited clinical data for patients with CC.
Trebananib
Trebananib is a peptide-Fc fusion protein that binds angiopoietin-1/-2, preventing the interaction of angiopoietin with the Tie2 receptor.75 Trebananib has shown single-agent activity and prolonged PFS in recurrent OC in early-phase trials.76,77 There are three completed phase III trials assessing trebananib in recurrent or newly diagnosed advanced OC. TRINOVA-1 trial (NCT01204749) investigated the addition of trebananib (15 mg/kg intravenously weekly) to single-agent weekly paclitaxel in recurrent OC with platinum-free interval ≤12 months. As a result, the median PFS was 1.8 months longer in the trebananib arm than that in the placebo arm (P < 0.0001).78 Subsequently, TRINOVA-2 (NCT0128125) evaluated the addition of trebananib to PLD in patients with recurrent OC, and it showed that trebananib did not significantly prolong PFS. However, the addition of trebananib to PLD improved ORR compared with placebo arm (46% vs. 21%, P < 0.001).79 TRINOVA-3 trial (NCT01493505) showed that the addition of trebananib to fist-line chemotherapy did not improve PFS or produce new safety signals for patients with newly diagnosed advanced OC.80 The result of OS was not mature. The major toxic effect associated with trebananib treatment was edema.78,81
For recurrent or persistent EC, a phase II trial (NCT01210222) showed an ORR of 3.1%, with insufficient single-agent activity to warrant further investigation of trebananib.82
Other antiangiogenic agents
Apatinib is a small-molecule TKI by binding to the VEGFR-2 ATP-binding site, which is taken orally.83,84 Given the promising results of a phase III study in Chinese gastric cancer patients,85 apatinib had been actively investigated as a salvage treatment for other advanced solid tumor, including OC.84 A phase II study of apatinib in patients with recurrent OC indicated that apatinib (500 mg daily) was a feasible treatment with an ORR of 41.4%.86 Grade 3 AEs were hand–foot syndrome, hypertension, and neutropenia. Another phase II trial (NCT02867956) demonstrated that apatinib plus etoposide showed promising efficacy and manageable toxicities in patients with platinum-resistant or -refractory OC with an ORR of 54%.87 An ongoing phase III trial in China (NCT04000295) is further evaluating the efficacy and safety of apatinib in patients with platinum-resistant recurrent OC compared with chemotherapy.
Sunitinib and brivanib are oral TKIs of VEGFR and PDGFR. Sunitinib was an FDA-approved drug for renal cell cancer and gastrointestinal stromal tumors. The safety and efficacy of sunitinib in OC were evaluated in several phase II trials with reported ORR ranging from 3.3% to 16.7%.88–91 In metastatic or recurrent EC, sunitinib showed promising activity in a phase II trial (NCT00478426) with an ORR of 18.1%.92 However, sunitinib had insufficient activity as a single agent in advanced or metastatic CC to warrant further investigation.93 Two phase II trials demonstrated that brivanib was well-tolerated and worthy of further investigation in persistent or recurrent EC/CC with an ORR of 7% and 8%, respectively.94,95
For the development of antiangiogenic agents and other targeted therapies, the addition of bevacizumab to conventional chemotherapy in OC is a very important step. However, most of the analysis reported so far showed that antiangiogenic agents led to no significant improvement in OS for patients with gynecological cancers. Thus, identification of predictive biomarkers for antiangiogenic agents and development of other targeted drugs are anticipated.
Poly (ADP-ribose) polymerase (PARP) inhibitors
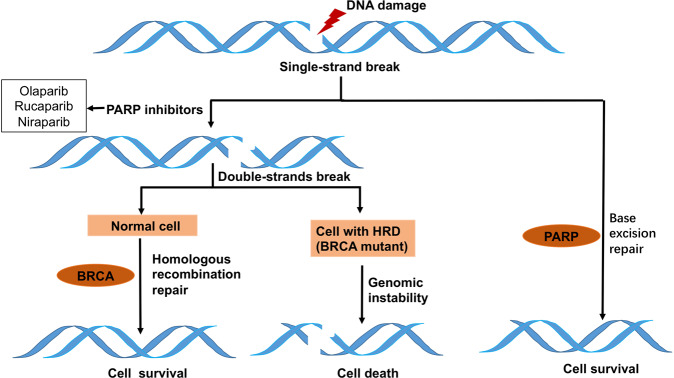
PARP is a sort of nuclear enzyme with 17 identified members.96 PARP-1 and 2 are involved in DNA repair.97 PARP-1, with a structure of the N-terminal zinc-finger DNA-binding domain, the central automodification domain and the C-terminal catalytic domain, was originally found involved in the base-excision repair (BER) pathway, which is important in the repair of single-stranded DNA breaks (SSBs).98 Therefore, inhibition of PARP-1 leads to the accumulation of DNA SSBs and ultimately results in DNA double-strand breaks (DSBs) during DNA replication.99 DSBs are the most lethal DNA insults. Nonhomologous end joining (NHEJ) and homologous-recombination repair (HRR) are the two main DSB repair pathways in humans.100 The preferred pathway is HRR, since it is more accurate. Thus, in cells with functional HRR, PARP inhibition will not result in cell death since DSBs will be precisely and effectively repaired. However, in cells with homologous-recombination deficiency (HRD), such as those with BRCA1/2 mutations, DSBs are left unrepaired or repaired by the error-prone NHEJ pathway, which result in genomic instability and ultimately cell death.101 This mechanism of synthetic lethality in HRD cells (Fig. 2) makes PARP inhibitors a novel targeted and personalized cancer treatment.102,103
Fig. 2.
Base-excision repair/single-strand break pathway and the mechanism of synthetic lethal interactions. Inhibition of PARP-1 causes the accumulation of DNA SSBs and ultimately results in DSBs during DNA replication. In cells with HRD, DSBs are left unrepaired or repaired by the error-prone NHEJ pathway, which result in genomic instability and ultimately cell death
In gynecological cancers, germline and somatic BRCA1/2 mutations (gBRCAm and sBRCAm) occur in ~10–15% of OC patients, and even more frequently in patients with high-grade serous OC (HGSOC), which is the most common type of OC.22,104,105 In addition, genomic alterations in other homologous-recombination (HR) genes including ATM, BRIP1, PALB2, and RAD51C are being studied.106 The comprehensive genomic analysis has identified that ~50% of high-grade serous tumors (including OC and EC) exhibit HRD.107,108 Moreover, the presence of HRD predicts a favorable response to platinum therapies and to PARP inhibitors. PARP inhibitors are also known to sensitize DNA-damaging agents, including carboplatin.109 Based on the above facts, PARP inhibitors are supposed to be groundbreaking therapeutic strategies for patients with gynecological cancers, especially for OC.110
Several PARP inhibitors, including olaparib, rucaparib, niraparib, veliparib, and talazoparib are actively investigated in clinical trials. The development of PARP inhibitors is productive. Olaparib is the first PARP inhibitor applied in clinic and approved by FDA for cancer treatment, followed by rucaparib and niraparib. The results from phase II/III clinical trials, assessing PARP inhibitors in gynecological cancers, are summarized in Tables 4 and 5. The ongoing clinical trials without results are listed in Table 6.
Table 4.
Phase III trials (with results) of PARP inhibitors in gynecological cancers
| ID | Cancer/condition | No. | Intervention | mPFS (Mos.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|
| NCT01844986 SOLO-1 | OC/BRCAm | 319 | (1) Placebo | 13.8 | 12.3 | 121 |
| (2) Olaparib | Not reached, P < 0.0001 | 20.8 | ||||
| NCT01874353 SOLO-2 | OC/recurrent, BRCAm | 295 | (1) Placebo | 5.5 | 8.08 | 120 |
| (2) Olaparib | 19.1, P < 0.0001 | 17.95 | ||||
| NCT02477644 PAOLA-1 | OC/stage III–IV | 806 | (1) Bevacizumab+ placebo | 16.6 | 31 | 122 |
| (2) Bevacizumab+ olaparib | 22.1, P < 0·0001 | 31 | ||||
| NCT01847274 NOVA | OC/platinum-sensitive recurrent | 553 | (1) Placebo | HRD:10.4; All:8.2 | 15.08 | 138 |
| (2) Niraparib | HRD: 21.9; All:13.8, *P < 0.0001 | 29.97 | ||||
| NCT02655016 PRIMA | OC/stage III–IV | 733 | (1) Placebo | 8.2 | 18.9 | 140 |
| (2) PC + Niraparib | 13.8, P < 0·0001 | 70.5 | ||||
| NCT01968213 ARIEL3 | OC/platinum-sensitive recurrent | 564 | (1) Placebo | BRCAm: 5.4; HRD: 5.4 | 10.58 | 136 |
| (2) Rucaparib | BRCAm: 16.6; HRD: 13.6, **P < 0.0001 | 21 | ||||
| NCT02470585 GOG-3005 | OC/stage III–IV, HGSOC | 1140 | (1) Placebo | BRCAm: 22.0; HRD: 20.5 | 32 | 150 |
| (2) Veliparib combination only | - | 27 | ||||
| (3) Veliparib throughout | BRCAm: 34.7; HRD: 31.9, ***P < 0.0001 | 45 |
HRD homologous-recombination deficiency, HGSOC high-grade serous ovarian cancer. *P-value of both HRD cohort and all population are <0.0001. ** and *** P-value of both BRCAm and HRD cohorts are <0.0001
Table 5.
Phase II trials (with results) of PARP inhibitors in gynecological cancers
| ID | Cancer/condition | No. | Intervention | ORR (%) | mPFS (mon.) | mOS (mon.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|---|---|
| NCT00494442 STUDY9 | OC/advanced, BRCAm | 58 | Olaparib | 33.3 | – | – | 36.4 | 411 |
| NCT00753545 STUDY19 | OC/serous, recurrent | 265 | (1) Placebo: BRCAm/BRCAwt | 4.2 | 4.3/5.5, P < 0.0001 | 34.9/30.2, P = 0.025 | 8.6 | 115,412 |
| (2) Olaparib: BRCAm/BRCAwt | 12.3 | 11.2/7.4, P = 0.0075 | 26.6/24.5, P = 0.37 | 22.8 | ||||
| NCT00679783 STUDY 20 | OC/recurrent, HGSOC | 91 | Olaparib: BRCAm/BRCAwt | 41/24 | 7.4/6.4 | – | 16 | 111 |
| NCT00628251 STUDY12 | OC/advanced, BRCAm | 98 | (1) Olaparib (200 mg twice daily) | 25 | 5 | 9 | 15.6 | 413 |
| (2) Olaparib (400 mg twice daily) | 31.3 | 5 | 11 | 18.8 | ||||
| (3) PLD | 18.2 | 4.8 | 13, All P > 0.5 | 15.6 | ||||
| NCT01078662 STUDY42 | OC/BRCAm | 193 | Olaparib | 31.1 | 7.03 | 16.62 | 30.2 | 118 |
| NCT01081951 | OC/advanced or platinum-sensitive recurrent | 173 | (1) PC | – | 9.6 | – | 20.99 | 414 |
| (2) Olaparib + PC | 12.2, P = 0.0012 | 25.33 | ||||||
| NCT01116648 | OC/platinum-sensitive recurrent | 90 | (1) Olaparib | 48.7 | 8.2 | 33.3 | – | 124,128 |
| (2) Cediranib + olaparib | 79.6 | 16.5, P = 0.007 | 44.2, P = 0.11 | 70 | ||||
| NCT02354586 QUADRA | OC/HGSOC, recurrent, HRD | 47 | Niraparib | 28 | 5.5 | 19 | 56 | 141 |
| NCT02657889 KEYNOTE-162 | OC/platinum-resistant recurrent | 62 | Niraparib + pembrolizumab | 18 | 3.4 | Not mature | – | 143 |
| NCT02354131 ENGOT-ov24 | OC/platinum-sensitive recurrent | 97 | (1) Niraparib | 30 | 5.5 | Not mature | – | 142 |
| (2) Niraparib + bevacizumab | 62 | 11.9, P < 0.0001 | 65 | |||||
| NCT01891344 ARIEL2 | OC/platinum-sensitive recurrent, HRD | 204 | Rucaparib: BRCAm | 80 | 12.8 | – | 24.5 | 133 |
| BRCAwt, LOH-high | 29.3 | 5.7 | ||||||
| BRCAwt, LOH-low | 10 | 5.2 | ||||||
| NCT01482715 STUDY10 | OC/BRCAm | 42 | Rucaparib | 59.5 | 76.2 | 134 | ||
| NCT01306032 | OC/HGSOC, BRCAm | 75 | (1) Cyclophosphamide | 19.4 | 3 | – | 0 | * |
| (2) Cyclophosphamide+ veliparib | 11.8 | 3, P = 0.68 | 8.11 | |||||
| NCT01540565 | OC/BRCAm | 52 | Veliparib | 26 | 8.18 | – | 20 | 146 |
| NCT01266447 | CC/persistent or recurrent | 27 | Veliparib + topotecan + filgrastim | 7 | 2 | 8 | 59.3 | 151 |
BRCAwt BRCA wild-type. LOH genomic loss of heterozygosity. *Unpolished data found in ClinicalTrials.gov
Table 6.
Ongoing phase II–III trials of PARP inhibitors in gynecological cancers (not including novel combination therapy)
| ID | Cancer/condition | Setting | No. | Start date | Intervention | Phase/assignment | Status |
|---|---|---|---|---|---|---|---|
| NCT02282020 SOLO-3 | OC/platinum-sensitive recurrent, BRCAm | Maintenance | 266 | 2015.2 | Olaparib vs. single-agent chemotherapy | III/randomized, parallel | Active, not recruiting |
| NCT03402841 OPINION | OC/platinum-sensitive recurrent, without BRCAm | Maintenance | 279 | 2018.1 | Olaparib | III/single group | Active, not recruiting |
| NCT03534453 L-MOCA | OC/platinum-sensitive recurrent | Maintenance | 300 | 2018.5 | Olaparib | III/single group | Active, not recruiting |
| NCT02855944 ARIEL4 | OC/recurrent | Monotherapy | 345 | 2016.9 | Rucaparib vs. chemotherapy | III/randomized, crossover | Recruiting |
| NCT04227522 MAMOC | OC/advanced | Maintenance | 190 | 2020.1 | Rucaparib vs. placebo | III/randomized, parallel | Not yet recruiting |
| NCT03519230 | OC/platinum-sensitive recurrent | Maintenance | 216 | 2018.5 | Pamiparib vs. placebo | III/randomized, parallel | Recruiting |
| NCT03709316 | OC/advanced | Maintenance | 381 | 2018.6 | Nirapairb vs. placebo | III/randomized, parallel | Recruiting |
| NCT03863860 | OC/platinum-sensitive recurrent | Maintenance | 216 | 2019.1 | Fluzoparib vs. placebo | III/randomized, parallel | Not yet recruiting |
| NCT04169997 | OC/advanced | Maintenance | 393 | 2020.2 | IMP4297 vs. placebo | III/randomized, parallel | Recruiting |
| NCT02489006 | OC/recurrent | Neoadjuvant | 24 | 2016.7 | Olaparib vs. platinum-based chemotherapy | II/ randomized, parallel | Recruiting |
| NCT03470805 | OC/recurrent, after PLD | Maintenance | 9 | 2018.6 | Olaparib | II/ single group | Active, not recruiting |
| NCT04377087 | OC/recurrent | Delayed maintenance | 75 | 2020.5 | Olaparib | II/ single group | Not recruiting |
| NCT03016338 | EC/recurrent | – | 44 | 2017.11 | Niraparib | II/ single group | Recruiting |
| NCT03644342 | CC/metastatic invasive | Concurrently | 20 | 2019.7 | Niraparib + radiotherapy | II/ single group | Recruiting |
| NCT03891576 | OC/platinum-sensitive recurrent | Maintenance | 105 | 2019.10 | Niraparib | II/ single group | Not yet recruiting |
| NCT04217798 | OC/platinum-resistant or -refractory | Maintenance | 32 | 2020.1 | Niraparib + etoposide | II/ single group | Not yet recruiting |
| NCT03617679 | EC/metastatic and recurrent | Maintenance | 138 | 2019.3 | Rucaparib vs. placebo | II/randomized, parallel | Recruiting |
| NCT03795272 | CC/locally advanced | Maintenance | 162 | 2019.11 | Rucaparib vs. placebo | II/randomized, parallel | Withdrawn |
| NCT04171700 LODESTAR | Solid tumor/HRD | – | 220 | 2019.11 | Rucaparib | II/ single group | Recruiting |
| NCT03509636 | OC/recurrent, BRCAm | – | 113 | 2018.4 | Fluzoparib | II/ single group | Active, not recruiting |
Olaparib
Olaparib is the best studied PARP inhibitor and approved by FDA for the maintenance treatment of selected advanced or recurrent OC patients. Early-phase clinical trials of olaparib demonstrated activity signals in patients with OC, with favorable tolerance and response rates.58,111–113 Following these promising results,114 a notable randomized placebo-controlled phase II trial, Study 19 (NCT00753545), evaluated olaparib as maintenance monotherapy for patients with platinum-sensitive recurrent OC. The median PFS was significantly longer in the olaparib arm compared with placebo (3.6 months longer, P < 0.001).115 A retrospective preplanned analysis suggested that patients with BRCAm gained the greatest PFS benefits from olaparib treatment (6.9 months longer, P < 0.0001). An exploratory post hoc analysis of Study 19 also suggested a numerical improvement in the OS.116 Although the PFS benefit was less in patients without BRCAm (1.9 months longer, P = 0.0075), this significant benefit suggested that a proportion of patients without BRCAm might also benefit from olaparib treatment.117 Another single-arm phase II trial, Study 42 (NCT01078662), evaluated olaparib as treatment for cancer patients with gBRCAm, including ovarian, breast, prostate, and pancreatic cancer. The ORR was 31.1% in platinum-resistant recurrent OC cohort. Stable disease (SD) was seen in 40% of patients, confirming significant activity.118,119 Based on these findings, the FDA approved single-agent olaparib as recurrence therapy for patients with advanced OC with gBRCAm who have received three or more lines of chemotherapy in 2014.
Several large randomized phase III trials of olaparib in gynecological cancers (mainly in OC) are currently in progress. The following three of the phase III trials reported promising results in OC. SOLO-2 trial (NCT01874353) evaluated the efficacy of olaparib as maintenance therapy in platinum-sensitive recurrent OC patients with BRCAm who had received at least two lines of previous chemotherapy. The results demonstrated a statistically significant improvement in investigator-assessed median PFS in the olaparib arm compared with placebo (13.6 months longer, P < 0·0001). At the time of the analysis of PFS, OS data were not mature with 24% of events.120 Based on this trial, the FDA approved olaparib as maintenance therapy for women with recurrent OC who are in complete or partial response to platinum-based chemotherapy in 2017. Another phase III trial, SOLO-1 (NCT01844986), evaluated the efficacy of olaparib as maintenance therapy in newly diagnosed advanced OC patients with BRCAm.121 After a median follow-up of 41 months, the risk of disease progression or death was 70% lower with olaparib than with placebo (P < 0.001). The estimated median PFS was not reached in the olaparib arm versus 13.8 months in the placebo arm (P < 0.0001). At the time of the analysis, OS data were not mature. Following this study, the FDA approved olaparib as maintenance therapy of advanced OC patients with BRCAm, who are in complete or partial response to first-line platinum-based chemotherapy in 2018. At the ESMO Congress 2019, new findings of a phase III trial, PAOLA-1/ENGOT-ov25 (NCT02477644), were presented. This is the first phase III trial to evaluate efficacy and safety of a PARP inhibitor plus bevacizumab as first-line maintenance therapy in advanced OC not restricted by surgical outcome or BRCA status. According to the results, patients with newly diagnosed OC had significantly improved the median PFS with addition of olaparib to bevacizumab maintenance treatment, as compared to placebo plus bevacizumab following first-line chemotherapy (5.5 months longer, P < 0.0001).122 Moreover, the PFS benefit in subgroups of patients with BRCAm and patients with other HRD was even more obvious (19.5 months longer and 11.5 months longer, respectively). In PAOLA-1 trial, the rate of AEs leading to treatment discontinuation is the highest figure reported across PARP inhibitor trials. However, there was no impact in QOL.
The FDA-recommended olaparib dose is 300 mg (two 150 mg tablets) taken orally twice daily. The most common serious AEs reported in SOLO-1 and SOLO-2 were anemia and neutropenia.
There are other three ongoing phase III trials of olaparib (as monotherapy) registered in the ClinicalTrials.gov database without available results, including SOLO-3 (NCT02282020), OPINION (NCT03402841), and L-MOCA (NCT03534453) (Table 6).
A phase II trial (NCT01116648) evaluated the efficacy and toxicity of the combination of cediranib and olaparib compared to olaparib alone in platinum-sensitive recurrent OC, based on the data from early clinical trial.123–126 This novel combination of angiogenesis inhibitor and PARP inhibitor improved the median PFS by 8.3 months compared with PARP inhibitor alone (P = 0.007).124,127 In the updated analysis in 2019, subset analyses within stratum defined by BRCA status demonstrated that this combination therapy significantly improved both median PFS (23.7 vs. 5.7 months, P = 0.002) and median OS (37.8 vs. 23.0 months, P = 0.047) in gBRCAwt/unknown patients.128 It encouraged the novel combination therapy of different targeted agents explored as a potential treatment strategy. Currently, we found only clinical case reports about efficacy of olaparib in other gynecological cancers (e.g., EC).129
Rucaparib
Rucaparib is a potent, oral, small-molecule PARP inhibitor.130,131 Rucaparib was FDA-approved in 2016 as monotherapy for the treatment of recurrent OC patients with BRCAm who have been treated with two or more chemotherapies. This approval was grounded on the proportion of patients with a favorable ORR observed in a pooled population of patients with BRCAm high-grade OC from the Study 10 and ARIEL2 trials.132–135 ARIEL2 (NCT01891344) is a phase II trial, assessing rucaparib as recurrence therapy for patients with platinum-sensitive OC. The median PFS after rucaparib treatment was 7.6 months longer in the BRCAm subgroup (P < 0.0001).
In a phase III trial, ARIEL3 (NCT01968213), assessed the efficacy and safety of rucaparib as maintenance therapy in patients with platinum-sensitive recurrent OC. The median PFS in patients with BRCAm was 11.2 months longer in the rucaparib arm than that in the placebo arm (P < 0·0001). In patients with HRD, it was 8.2 months longer (P < 0·0001). In the intention-to-treat (ITT) population, the median PFS was 5·4 months longer in patients in the rucaparib arm than that in the placebo arm (P < 0·0001).136 Based on this study, the FDA approved rucaparib for the maintenance treatment of recurrent OC patients who are in a complete or partial response to platinum-based chemotherapy. The ongoing ARIEL4 trial (NCT02855944) is another phase III study of rucaparib compared with chemotherapy in recurrent OC patients with BRCAm after two or more prior lines of therapy. The combination of rucaparib with other novel therapies (e.g., immune checkpoint inhibitor) is investigated for OC and EC in Phase I/II trials (NCT03101280, NCT03572478). A new phase III trial, MAMOC (NCT04227522), is going to investigate rucaparib maintenance therapy after bevacizumab maintenance following first-line chemotherapy in advanced OC.
The FDA-recommended rucaparib dose is 600 mg (two 300 mg tablets) taken orally twice daily. The most common serious AEs reported in ARIEL3 were anemia, pyrexia, vomiting, and small intestinal obstruction.
Niraparib
Niraparib is another FDA-approved PARP inhibitor.137 A phase III trial, ENGOT-OV16/NOVA (NCT01847274), evaluated the efficacy of niraparib as maintenance treatment for patients with platinum-sensitive recurrent OC. The results showed that niraparib increased PFS regardless of BRCA status when compared with placebo. Patients in the niraparib arm had significantly longer median PFS than those in the placebo arm, including 21.0 vs. 5.5 months in the gBRCAm cohort, 12.9 months vs. 3.8 months in the non-gBRCAm cohort for patients who had tumors with HRD, and 9.3 months vs. 3.9 months in the overall non-gBRCAm cohort (P < 0.001 for all three comparisons).138 Based on this study, niraparib was approved by FDA in 2017 as maintenance therapy for adult patients with recurrent OC who are in complete or partial response to platinum-based chemotherapy.138 Furthermore, A retrospective subanalysis demonstrated the safety and efficacy of niraparib in the subgroup of patients aged ≥70 years in this trial, suggesting that the use of niraparib should be considered in this population.139 Findings from another phase III trial, PRIMA (NCT02655016), were presented at the ESMO Congress 2019, and recently reported. This study evaluated the efficacy of niraparib following first-line chemotherapy in patients with newly diagnosed advanced OC and had similar findings with NOVA trial. Patients in the niraparib arm had substantial improvement in the median PFS compared to those in placebo arm (5.6 months longer, P < 0.0001). In the HRD cohort, the improvement of the median PFS was even greater in treatment group (21.9 vs. 10.4 months, P < 0.0001).140 Another phase III trial (NCT03709316) of niraparib in advanced OC is under way (Table 6). Several other phase II trials are studying the potential role of niraparib in different clinical settings. QUADRA trial (NCT02354586) assessed the activity of single-agent niraparib as the fourth or later line treatment for patients with platinum-sensitive recurrent HGSOC.141 This study met the primary endpoint, with an ORR of 28% in HRD-positive population. The median PFS in this population was 5.5 months. The median OS was 26 months in the BRCAm population, 19.0 months in the HRD-positive population, and 15.5 months in the HRD-negative population. NSGO-AVANOVA2/ENGOT-OV24 trial (NCT02354131) showed that niraparib (300 mg orally daily) plus bevacizumab (15 mg/kg intravenously every 3 weeks) significantly improved the median PFS compared with niraparib alone in patients with platinum-sensitive recurrent OC (5.4 months longer, P < 0.00001).142 TOPACIO/KEYNOTE-162 trial (NCT02657889) evaluated niraparib (200 mg orally daily) combined with pembrolizumab (an immune checkpoint inhibitor, 200mg intravenously on day 1 of each 21-day cycle) in patients with recurrent OC. The ORR was 18%, with a disease control rate of 65%. This novel combination therapy was tolerable, and responses in patients without HRD were higher than expected with either agent as monotherapy.143
The FDA-recommended niraparib dose is 300 mg taken orally once daily. The most common serious AEs reported in NOVA and PRIMA were thrombocytopenia, anemia, and neutropenia. Disutility analyses showed no significant QOL impairment associated with these toxic effects.144
Veliparib
Veliparib is a potent small-molecule inhibitor of PARP-1/2.145 Early-phase trials demonstrated activity of veliparib among OC patients with BRCAm to provide rationale for further clinical development.109,146–149 New results from a phase III trial, VELIA/GOG-3005 (NCT02470585), were reported at the ESMO Congress 2019. It assessed the efficacy of veliparib (150 mg orally twice daily) added to first-line chemotherapy and continued as maintenance monotherapy in patients with previously untreated advanced HGSOC. In the BRCAm cohort, the median PFS was 12.7 months longer in the veliparib-throughout arm than in the control arm (P < 0.001). In the HRD cohort, it was 11.4 months longer (P < 0.001). And in the ITT population, the median PFS was 5.2 months longer (P < 0.001). AEs reported with veliparib were predominantly gastrointestinal and hematologic. The most common AE leading to the discontinuation of veliparib was nausea.150
For the treatment of CC, there was a phase I/II trial (NCT01266447) that assessed veliparib in combination with topotecan for patients with recurrent or persistent CC, showing minimal clinical activity with an ORR of 7%.151 Another phase I trial (NCT01281852) investigated veliparib in combination with cisplatin and paclitaxel in patients with recurrent or metastatic CC.152 The results demonstrated an ORR of 34%, illustrating the potential of PARP inhibitors as a combination therapy in CC.
Other PARP inhibitors
Talazoparib is a potent PARP inhibitor showing antitumor cytotoxicity at much lower concentrations than other agents, with an ORR of 42% in early-phase clinical trials for advanced OC with BRCAm.153,154
Pamiparib is a highly selective oral PARP-1/2 inhibitor capable of penetrating the brain.155 In a phase I trial of pamiparib combined with tislelizumab (an immune checkpoint inhibitor) in advanced solid tumors, 9 (26%) of the 34 patients with OC achieved clinical responses.156 A phase II trial (NCT03933761) is assessing the clinical benefit rate of pamiparib in fusion-positive, reversion-negative HGSOC with BRCAm.
Fluzoparib is a novel PARP inhibitor undergoing clinical trials with potent anticancer activities.157,158 Two ongoing phase III trials (NCT03519230 and NCT03863860) are investigating the efficacy of pamiparib and fluzoparib as maintenance therapy in recurrent OC, respectively.
In summary, PARP inhibitors are acting as an exciting new option for patients with OC by significantly increasing both PFS and OS, especially for those with HRDs. However, cost effectiveness and drug resistance remain to be improved.159,160 In the future, it is necessary to identify more indications and predictive biomarkers.161,162 Moreover, numerous ongoing clinical trials of novel combination therapies are guiding the future direction of targeted therapy strategies (Tables 13 and 14).163,164
Table 13.
Ongoing phase III trials of novel combination targeted therapy in gynecological cancers
| ID | Cancer/condition | No. | Start date | Target | Intervention | Status |
|---|---|---|---|---|---|---|
| NCT02502266 COCOS | OC/platinum-resistant or -refractory recurrent, BRCAm | 680 | 2016.2 | VEGF, PARP | Cediranib + olaparib vs. cediranib vs. chemotherapy | Recruiting |
| NCT02446600 | OC/platinum-sensitive recurrent | 549 | 2016.2 | VEGF, PARP | Cediranib + olaparib vs. olaparib vs. chemotherapy | Active, not recruiting |
| NCT03522246 ATHENA | OC/stage III–IV | 1012 | 2018.5 | PARP, PD-1 | Rucaparib + nivolumab vs. rucaparib + placebo vs. nivolumab + placebo vs. placebo | Recruiting |
| NCT03602859 ENGOT-0V44 /FIRST | OC/stage III–IV | 912 | 2018.10 | PARP, PD-1 | Dostarlimab + niraparib vs. niraparib + placebo vs. placebo | Recruiting |
| NCT03884101 ENGOT-en9 | EC/recurrent or stage III–IV | 720 | 2019.4 | VEGF, PD-1 | Lenvatinib + pembrolizumab vs. chemotherapy | Recruiting |
| NCT03740165 KEYLYNK-001/ENGOT-ov43 | OC/fist-line treatment | 1086 | 2018.12 | VEGF, PARP, PD-1 | Pembrolizumab + olaparib vs. pembrolizumab + placebo vs. placebo, plus PC + bevacizumab | Recruiting |
| NCT03737643 DUO-O | OC/stage III–IV | 1056 | 2019.1 | VEGF, PARP, PD-1 | Durvalumab + olaparib vs. durvalumab + placebo vs. placebo, plus PC + bevacizumab | Recruiting |
| NCT03806049 NSGO/AVANOVA-Triplet | OC/platinum-sensitive recurrent | 337 | 2019.6 | VEGF, PARP, PD-1 | Niraparib + bevacizumab + dostarlimab vs. niraparib + bevacizumab vs. chemotherapy | Not yet recruiting |
Table 14.
Ongoing phase II trials of novel combination therapy in gynecological cancers
| ID | Cancer/condition | No. | Started date | Targets | Drugs | Design | Status |
|---|---|---|---|---|---|---|---|
| NCT02345265 | OC/recurrent | 70 | 2015.12 | VEGF, PARP | Cediranib + olaparib | Single group | Active, not recruiting |
| NCT02502266 | OC/ platinum-resistant recurrent | 680 | 2016.2 | VEGF, PARP | Cediranib + olaparib vs. cediranib vs. olaparib | Randomized parallel | Recruiting |
| NCT02889900 CONCERTO | OC/platinum-resistant recurrent | 62 | 2017.1 | VEGF, PARP | Cediranib + olaparib | Single group | Active, not recruiting |
| NCT03117933 OCTOVA | OC/platinum-resistant recurrent | 138 | 2017.3 | VEGF, PARP | Paclitaxel vs. cediranib + paclitaxel vs. cediranib + olaparib | Randomized parallel | Active, not recruiting |
| NCT0331574 BARCCO | OC/recurrent | 100 | 2017.6 | VEGF, PARP | Paclitaxel vs. cediranib + olaparib | Randomized parallel | Recruiting |
| NCT03326193 | OC/advanced | 105 | 2018.1 | VEGF, PARP | Niraparib + bevacizumab | Single group | Active, not recruiting |
| NCT03462212 MITO25 | OC/advanced, high grade | 234 | 2018.2 | VEGF, PARP | Rucaparib + bevacizumab + chemotherapy vs. rucaparib + chemotherapy vs. bevacizumab + chemotherapy | Randomized parallel | Recruiting |
| NCT03570437 COPELIA | EC/advanced | 129 | 2018.5 | VEGF, PARP | Paclitaxel vs. cediranib + paclitaxel vs. cediranib + olaparib | Randomized parallel | Recruiting |
| NCT03476798 | CC or EC/recurrent | 70 | 2018.6 | VEGF, PARP | Rucaparib + bevacizumab | Single group | Recruiting |
| NCT03660826 | EC/recurrent, refractory, or metastatic | 120 | 2018.9 | VEGF, PARP | Cediranib vs. olaparib vs. cediranib + olaparib | Randomized parallel | Active, not recruiting |
| NCT03895788 | OC/recurrent | 24 | 2019.1 | VEGF, PARP | Niraparib + brivanib | Single group | Recruiting |
| NCT02476798 Clovis-001 | CC or EC/recurrent | 70 | 2019.6 | VEGF, PARP | Rucaparib + bevacizumab | Single group | Active, not recruiting |
| NCT04376073 ANNIE | OC/platinum-sensitive recurrent | 40 | 2020.5 | VEGF, PARP | Niraparib + anlotinib | Single group | Recruiting |
| NCT02921269 | CC/recurrent | 22 | 2017.3 | VEGF, PD-1 | Atezolizumab + bevacizumab | Single group | Not yet recruiting |
| NCT03572478 | EC/metastatic or recurrent | 60 | 2018.8 | VEGF, PD-1 | Rucaparib vs. nivolumab vs. rucaparib + nivolumab | Randomized parallel | Recruiting |
| NCT03526432 | EC/advanced, recurrent or persistent | 55 | 2018.8 | VEGF, PD-1 | Atezolizumab + bevacizumab | Single group | Recruiting |
| NCT03367871 | CC/recurrent, persistent, or metastatic | 39 | 2018.12 | VEGF, PD-1 | Pembrolizumab + bevacizumab | Single group | Recruiting |
| NCT03816553 | CC/recurrent, persistent, or metastatic | 49 | 2019.1 | VEGF, PD-1 | Camrelizumab + apatinib | Single group | Recruiting |
| NCT04068974 | OC/platinum-resistant recurrent | 28 | 2019.8 | VEGF, PD-1 | Camrelizumab + apatinib | Single group | Not yet recruiting |
| NCT04197219 | EC/recurrent | 26 | 2020.1 | VEGF, PD-1 | Pembrolizumab + axitinib | Single group | Not yet recruiting |
| NCT03797326 | Advanced solid tumors | 180 | 2019.2 | VEGF, PD-1 | Pembrolizumab + lenvatinib | Single group | Recruiting |
| NCT04236362 | OC | 30 | 2020.1 | EGFR, PD-1 | TQB2450 + anlotinib | Single group | Not yet recruiting |
| NCT02571725 | OC/recurrent, BRCAm | 50 | 2016.2 | PARP, PD-1 | Olaparib + tremelimumab | Single group | Recruiting |
| NCT02912572 | EC/recurrent | 70 | 2016.12 | PARP, PD-1 | Talazoparib + avelumab | Non-randomized parallel | Recruiting |
| NCT03330405 Javelin Parp Medley | OC/platinum-sensitive recurrent | 242 | 2017.10 | PARP, PD-1 | Talazoparib + avelumab | Non-randomized parallel | Recruiting |
| NCT03572478 | EC/ metastatic or recurrent | 60 | 2018.8 | PARP, PD-1 | Rucaparib + nivolumab vs. nivolumab vs. rucaparib | Randomized parallel | Recruiting |
| NCT03651206 ROCSAN | OC/recurrent | 196 | 2019.1 | PARP, PD-1 | Niraparib/dostarlimab + niraparib vs. chemotherapy | Randomized parallel | Active, not recruiting |
| NCT03824704 | OC/HGSOC or endometroid | 139 | 2019.5 | PARP, PD-1 | Rucaparib + nivolumab | Single group | Recruiting |
| NCT04068753 STAR | CC/platinum-resistant recurrent | 150 | 2019.6 | PARP, PD-1 | Dostarlimab + niraparib | Single group | Active, not recruiting |
| NCT03951415 DOMEC | EC/recurrent | 55 | 2019.7 | PARP, PD-1 | Durvalumab + olaparib | Single group | Recruiting |
| NCT03955471 MOONSTONE | OC/progressive or recurrent | 68 | 2019.9 | PARP, PD-1 | Dostarlimab + niraparib | Single group | Recruiting |
| NCT04034927 | OC/recurrent | 170 | 2019.10 | PARP, PD-1 | Olaparib vs. olaparib + tremelimumab | Randomized parallel | Recruiting |
| NCT02953457 | OC/recurrent or refractory, BRCAm | 39 | 2017.6 | PARP, PD-1, CTLA-4 | Olaparib + durvalumab + tremelimumab | Single group | Recruiting |
| NCT02484404 | Advanced solid tumors | 384 | 2015.6 | VEGF, PARP, PD-1 | Olaparib + cediranib + durvalumab | Non-randomized parallel | Recruiting |
| NCT02873962 | OC/recurrent | 76 | 2016.11 | VEGF, PARP, PD-1 | Nivolumab + bevacizumab vs. nivolumab + bevacizumab+ rucaparib | Non-randomized sequential | Recruiting |
| NCT03574779 OPAL | OC/recurrent | 41 | 2019.1 | VEGF, PARP, PD-1, | Dostarlimab + niraparib + bevacizumab | Single group | Active, not recruiting |
| NCT04015739 BOLD | OC/recurrent | 63 | 2019.2 | VEGF, PARP, PD-1 | MEDI4736 + olaparib+ bevacizumab | Single group | Recruiting |
| NCT03694262 EndoBARR | EC/persistent or progressive | 30 | 2019.7 | VEGF, PARP, PD-1 | Atezolizumab + rucaparib + bevacizumab | Single group | Recruiting |
| NCT04361370 OPEB-01 | OC/platinum-resistant recurrent | 44 | 2020.4 | VEGF, PARP, PD-1 | Olaparib + pembrolizumab + bevacizumab | Single group | Active, not recruiting |
| NCT03699449 AMBITON | OC/platinum-resistant recurrent | 68 | 2018.11 | VEGF, PARP, PD-1, CTLA-4 | Olaparib + cediranib vs. durvalumab + olaparib vs. durvalumab + chemotherapy vs. durvalumab + tremelimumab + chemotherapy | Randomized parallel | Recruiting |
| NCT02208375 | EC or OC/recurrent | 150 | 2014.11 | PARP, mTOR, AKT | Olaparib + vistusertib vs. olaparib + capivasertib | Randomized parallel | Active, not recruiting |
| NCT03462342 CAPRI | OC/recurrent | 86 | 2018.3 | PARP, ATR | Olaparib + AZD6738 | Single group | Recruiting |
| NCT04065269 ATARI | Gynecological cancers, AR1A loss | 40 | 2019.11 | PARP, ATR | AZD6738 vs. AZD6738 + olaparib | Randomized parallel | Recruiting |
| NCT04239014 DUETTE | OC/platinum-sensitive recurrent | 192 | 2020.3 | PARP, ATR | AZD6738 vs. AZD6738 + olaparib vs. placebo + olaparib | Randomized parallel | Not yet Recruiting |
| NCT03579316 | OC/recurrent | 70 | 2018.12 | PARP, Wee | Adavosertib vs. adavosertib + olaparib | Randomized parallel | Recruiting |
| NCT03924245 | OC/platinum-resistant recurrent | 73 | 2019.12 | PARP, HDAC | Olaparib + entinostat | Single group | Active, not recruiting |
| NCT02764333 | OC/platinum-resistant recurrent | 29 | 2016.5 | PD-1, cancer vaccine | Durvalumab + TPIV200 | Single group | Active, not recruiting |
| NCT03946358 | CC/HPV + | 47 | 2019.9 | PD-1, cancer vaccine | Atezolizumab + UCPVax (vaccine) | Single group | Not yet Recruiting |
| NCT03015129 | EC | 80 | 2017.1 | PD-1, CTLA-4 | Durvalumab + tremelimumab vs. durvalumab | Randomized parallel | Recruiting |
| NCT03026062 | OC/platinum-resistant recurrent | 100 | 2017.5 | PD-1, CTLA-4 | Durvalumab vs. durvalumab + tremelimumab | Randomized parallel | Recruiting |
| NCT03277482 | Gynecological cancer | 32 | 2018.2 | PD-1, CTLA-4 | Durvalumab + tremelimumab + radiotherapy | Single group | Recruiting |
| NCT03355976 | OC/advanced, recurrent, or metastatic | 62 | 2018.4 | PD-1, CTLA-4 | Nivolumab + ipilimumab vs. nivolumab | Randomized parallel | Recruiting |
| NCT03894215 | CC/recurrent | 200 | 2019.3 | PD-1, CTLA-4 | Balstilimab + AGEN 1884 | Randomized parallel | Recruiting |
| NCT02734004 TRU-D | OC/stage III–IV | 24 | 2019.7 | PD-1, CTLA-4 | Durvalumab + tremelimumab | Single group | Recruiting |
| NCT04380805 | CC/recurrent or metastatic | 40 | 2020.5 | PD-1, CTLA-4 | AK104 | Single group | Active, not recruiting |
| NCT03439085 | CC/recurrent or metastatic | 77 | 2018.11 | PD-1, HPV vaccine | Durvalumab + MEDI0457 | Single group | Recruiting |
| NCT04096911 | CC | 20 | 2019.7 | PD-1, HPV vaccine | Sintilimab + quadrivalent HPV vaccine | Single group | Recruiting |
| NCT03835819 | EC/advanced or recurrent | 35 | 2019.9 | PD-1, ADC | Pembrolizumab + mirvetuximab soravtansine | Single group | Recruiting |
| NCT03113487 | OC/recurrent | 28 | 2018.2 | PD-1, p53 | Pembrolizumab + p53MVA | Single group | Recruiting |
HDAC histone deacetylase
PI3K/AKT/mTOR pathway blockade
The phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling is one of the critical intracellular pathways that regulates important cell activities, such as cell growth, survival, proliferation, differentiation, metabolism, apoptosis, and angiogenesis.165 PI3K is plasma membrane-associated lipid kinases, composed of regulatory subunit (PIK3R) and catalytic subunit (PIK3CA) that mediate receptor binding, activation, and localization of the enzyme.166 In normal conditions, PI3K can be activated by a variety of stimuli, including growth factors, cytokines, and hormones.167 Activation of AKT regulates a number of downstream targets. mTOR is a serine/threonine protein kinase and the best-described downstream target of AKT, composed of mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2).168 mTORC1 is sensitive to inhibition by rapamycin, and its analogs and mTORC2 exerts a positive feedback activation on AKT.169 There are also endogenous negative regulators of the PI3K pathway, such as the tumor suppressor—phosphatase and tensin homologue (PTEN).170 The PI3K/Akt/mTOR pathway is also involved in cross talk with other signaling pathways, including the Ras/Raf/MEK and estrogen receptor (ER) pathways.171 The overview of the PI3K/AKT/mTOR signaling pathway is included in Fig. 1. In cancer, this pathway can be aberrantly activated via a number of mechanisms, including loss of tumor-suppressor function, exposure to carcinogens, mutations/amplifications of PI3K, and mutations/amplifications of AKT. The deregulation of the PI3K/ AKT/mTOR pathway occurs in many cancers.172–174 As for gynecological cancers, this pathway is overactivated in OC (~70%),175–177 as well as EC and CC.178–180 In EC, the mutation rates of PI3K and PTEN were high, especially in the POLE subgroup.20 In vitro model of CC, mTOR inhibitors markedly reduced the expression level of HPV E7 protein, inducing apoptosis.181 Based on the preclinical evidence, the PI3K/AKT/mTOR pathway emerges as a potential therapeutic target in cancer, as well as gynecological malignancy.176,182,183 There are many drugs being tested in each part of this pathway: PI3K inhibitors, mTOR inhibitors, AKT inhibitors, and dual inhibitors on PI3K/mTOR or PI3K/AKT. mTOR inhibitors (everolimus and temsirolimus) and PI3K inhibitors (idelalisib, alpelisib and copanlisib) have been FDA-approved to be effective in the advanced cancer treatment, such as breast cancer, renal cell carcinoma, and lymphoma.164 Despite there are a number of preclinical/clinical data on PI3K/AKT/mTOR pathway inhibitors, currently there is no FDA-approved indication in gynecological cancers.
mTOR inhibitors
The most tested drugs in the PI3K/AKT/mTOR pathway are those blocking mTOR activity. Temsirolimus, everolimus, and ridaforolimus are the most-studied mTOR inhibitors in gynecological cancers. The results of completed clinical trials (phase II) investigating the safety and efficacy of them in gynecological cancers are summarized in Table 7.
Table 7.
Completed phase II trials of PI3K/AKT/mTOR pathway inhibitors in gynecological cancers
| ID | Cancer/condition | No. | Intervention | ORR (%) | CBR (%) | mPFS (mon.) | mOS (mon.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| NCT001460979 | EC/advanced | 22 | Temsirolimus | 10 | 35 | 3.0 | 21.3 | – | 415 |
| AGO-GYN8 | OC/advanced | 22 | 4.8 | 38.1 | 3.4 | 21.9 | |||
| NCT00429793 | OC/recurrent | 54 | Temsirolimus | 9.3 | – | 3.1 | 11.6 | 9.26 | 416 |
| NCIC IND 160 | EC/recurrent or metastatic | 23 | Temsirolimus | 26 | 89 | – | – | – | 188 |
| NCT00723255 | EC/recurrent | 53 | Temsirolimus + bevacizumab | 24.5 | 40 | 5.6 | 16.9 | 63.27 | 417 |
| NCT00729686 | EC/advanced or recurrent | 71 | (1) Temsirolimus | 22 | 52.4 | 4.9 | 10.8 | 36 | 187 |
| (2) Temsirolimus + hormone therapy | 14.3 | – | 61.9 | ||||||
| NCT00072176 NCIC CTG | EC/locally advanced, recurrent, or metastatic | 60 | (1) Temsirolimus + hormone therapy | 14 | 89 | 7.33 | – | 33.33 | 418 |
| 2) Temsirolimus + chemotherapy | 4 | 50 | 3.25 | 33.33 | |||||
| NCT00977574 GOG-86P | EC/stage III–IV or recurrent | 349 | (1) Bevacizumab + PC | 59.5 | – | – | 34 | 42.8 | 197 |
| (2) Temsirolimus + PC | 55.3 | 25 | 50.4 | ||||||
| (3) Bevacizumab + IC | 52.9 | 25.2 | 46.5 | ||||||
| NCT01026792 NCIC IND199 | CC/advanced or metastatic | 38 | Temsirolimus | 3 | 60.6 | 3.52 | – | 40.5 | 419 |
| NCT00087685 | EC/progressive or recurrent | 35 | Everolimus | 21 | 45.1 | – | – | – | 192 |
| NCT01068249 | EC/recurrent | 38 | Everolimus + letrozole | 32 | 40 | 3 | 14 | 31.6 | 194 |
| NCT01797523 | EC/recurrent | 58 | Everolimus + letrozole + metformin | 29 | 66.7 | – | – | – | 193 |
| NCT02283658 | OC/ER + , recurrent | 20 | Everolimus + letrozole | 16 | 37 | 3.9 | 13 | 63 | 420 |
| NCT00739830 | EC/stage III–IV | 130 | (1) Hormone or chemotherapy | 4 | 17 | 1.9 | – | 34 | 421 |
| (2) Ridaforolimus | 0 | 35 | 3.6 | 57 | |||||
| NCT00122343 | EC/recurrent | 45 | Ridaforolimus | 11 | 19 | – | – | 33 | 422 |
| NCT00770185 | EC/recurrent | 35 | Ridaforolimus | 8.8 | 62 | – | – | 37.1 | 423 |
| – | EC/progressive | 45 | Ridaforolimus | 7.4 | 33 | – | – | 35.6 | 424 |
| NCT01935973 | EC/recurrent or persistent | 26 | GSK2141795 + trametinib | 8.3 | – | – | – | 61 | 203 |
| NCT02538627 | CC/persistent or recurrent | 35 | GSK2141795 + trametinib | 7.1 | 44 | 3.6 | 14.8 | 57 | 202 |
| NCT01307631 | EC/recurrent | 37 | MK2206 | 5.5 | 33 | – | 8 | 37.84 | 205 |
| NCT01397877 ENDOPIK | EC/advanced or recurrent | 40 | BKM120 | 0 | 60 | 4.5 | 21 | 209 | |
| NCT02193633 | OC/HGSOC | 27 | Vistusertib + chemotherapy | 52 | 78 | 5.8 | – | – | 198 |
| NCT01587040 | EC/advanced or recurrent | 67 | Pilaralisib | 6 | 13.4 | – | – | 52.9 | 210 |
| NCT01420081 | EC/recurrent | 40 | Gedatolisib | 16 | 5 | 3.6 | – | 212 |
CBR clinical benefit rate = complete response + partial response + stable disease, ER+ estrogen receptor positive
Consistent with preclinical findings,171,184–186 initial clinical trials demonstrated promising activities of mTOR inhibitors in EC. Temsirolimus, an intravenous mTORC1 inhibitor (25 mg weekly), showed efficacy as monotherapy for advanced and recurrent EC with ORRs of 22–25%.187–189 Ridaforolimus is another intravenous mTORC1 inhibitor, administrated at a dose of 12.5 mg daily for 5 consecutive days every 2 weeks, showing a modest therapeutic efficacy as a single agent.190 A phase II trial studied the efficacy and tolerability of ridaforolimus in recurrent and advanced EC with an ORR of 8.8% and a SD of 52.9%.191 Everolimus, an oral mTORC1 inhibitor (10 mg daily), was evaluated in a phase II study (NCT00087685) for the treatment of patients with recurrent or persistent EC, showing an ORR of 0% and a SD of 43%.192 However, everolimus was reported to have the best effects in recurrent EC when combined with hormonal therapy (e.g., letrozole, an aromatase inhibitor), showing ORRs of 29–32%.193,194 Given that mTOR inhibitors are cytostatic cell cycle agents with a benefit mainly in terms of disease stabilization rather than disease response (tumor shrinkage), we found only modest effects of mTOR inhibitors as monotherapy in OC and CC based on current clinical evidence.195 Reasons to these disappointing results might be: (1) one pathway blockade is insufficient; combined therapies are needed; (2) analogs of rapamycin selectively inhibit mTORC1; the other mTOR complex, mTORC2, is a positive regulator of AKT; (3) predictive biomarkers are required to identify population who can get most benefit from this pathway blockade. Considering the evidence from preclinical studies showing promising activity of mTOR inhibitors in combination with chemotherapy, a number of clinical trials assessed the efficacy of the addition of mTOR to cytotoxic drugs, as well as novel combination of different targeted therapies. A Phase II trial (NCT01031381), evaluating everolimus plus bevacizumab in recurrent OC, reported that 28% patients were progression-free at 6 months. Patients with both platinum-sensitive and -resistant disease showed response. Overall, the regimen was well-tolerated.196 A randomized Phase II trial (NCT00977574) compared the efficacy of temsirolimus in combination with chemotherapy (carboplatin and paclitaxel) to bevacizumab plus chemotherapy in advanced or recurrent EC. Patients treated by temsirolimus plus chemotherapy had an ORR of 55.3%, and a median OS of 25 months. However, the results reported no improvement in comparison to bevacizumab plus chemotherapy.197 A phase I trial (NCT02193633) investigated the efficacy of vistusertib (a dual mTORC1/mTORC2 inhibitor) in combination with paclitaxel in OC, showing an ORR of 52% and a median PFS of 5.8 months.198 Currently, no specific predictive biomarker has been recognized. Tumors with PI3K or PTEN mutations did not necessarily respond to mTOR inhibitors.199 Common treatment-related AEs of mTOR inhibitors include stomatitis, mucositis, pneumonitis, rash, fatigue, anemia, diarrhea, nausea, vomiting, hyperglycemia, and immunosuppression.
AKT inhibitors
GSK2141795 and MK2206 are inhibitors targeting AKT, acting upstream of mTOR.200,201 A phase II trial tested dual inhibition of PI3K and Ras signaling by combining the AKT inhibitor (GSK2141795, 50 mg orally daily) and the MEK inhibitor (trametinib, 1.5 mg orally daily) in recurrent CC, with AEs including gastrointestinal events, fatigue, and rash. One patient had an unconfirmed partial response, with an ORR of 7.1%. Eight patients (57.1%) had stable disease.202 However, the combination of trametinib and GSK2141795 was shown to have high levels of toxicity in EC at this dose. And the preliminary efficacy is disappointing in another phase II trial (NCT01935973).203,204 Moreover, a two-arm, PIK3CA mutation stratified phase II trial (NCT01307631) in recurrent EC demonstrated limited single-agent activity of MK2206 (200 mg orally weekly) in both PIK3CA mutant and wild-type populations.205 Afuresertib, another AKT inhibitor, combined with chemotherapy showed an acceptable safety profile in patients with platinum-resistance OC in a phase I study.206 A phase II trial of afuresertib plus weekly paclitaxel in platinum-resistance OC (NCT04374630, PROFECTA-II) is under way.
PI3K inhibitors
BKM120 (buparlisib) is an oral pure PI3K inhibitor. It was shown to have antitumor activity in preclinical and early trials.207,208 However, a phase II trial (NCT01397877) demonstrated that the BKM120 (100 mg orally daily) was associated with a minimal antitumor activity as monotherapy in advanced or recurrent EC.209 Another oral PI3K inhibitor, pilaralisib (600-mg capsules or 400-mg tablets daily), also had minimal success in a phase II trial in advanced or recurrent EC.210 PF-04691502 and gedatolisib (PF-05212384) are potent, dual PI3K/mTOR inhibitors.211 A randomized phase II non-comparative trial (NCT01420081) was conducted in patients with recurrent EC following platinum-containing chemotherapy. Clinical benefit response criteria were only met in the gedatolisib/stathmin-low arm.212 Common treatment-related AEs include nausea, mucositis, decreased appetite, diarrhea, fatigue, vomiting, rash, and stomatitis.
In summary, the role of the PI3K/AKT/mTOR pathway inhibitors in gynecological cancers is not yet clear. The reasons for the unsatisfactory results may be related to the feedback loops and compensatory activation of Ras pathway. Even though the presented clinical results are controversial,213 there are amount of preclinical studies and clinical trials in progress, mainly combining PI3K signaling blockade with other therapies or different targeted agents.214 For example, a randomized phase II trial (NCT02397083) is designed to study how everolimus works with the levonorgestrel-releasing intrauterine system for early-stage EC. Another phase II trial (NCT03008408) is to learn if the combination of everolimus, letrozole, and ribociclib (a CDK4/6 inhibitor) can help to control recurrent or progressive EC. Dual mTORC inhibition continues to be assessed in advanced or recurrent OC (NCT03648489). Furthermore, for the future of this pathway targeted therapy, studies of predictive biomarkers might be very helpful and important.
Human epidermal growth factor receptor-targeted inhibitors
Human epidermal growth factor receptors (HERs), also known as erythroblastic leukemia viral oncogene (erbB) family, include HER1(Erb1, EGFR), HER2 (Erb2), HER3 (Erb3), and HER4 (Erb4).215 Structural features of HER proteins include extracellular ligand-binding domain, transmembrane domain, and intracellular protein tyrosine kinase domain.216 When ligands bind to the extracellular domain, HERs form homodimers or heterodimers with other members of the family.217 As an exception, HER2 does not bind any ligand, but it has the most favorable kinase activity. HER3 lacks tyrosine kinase activity.218 Dimerization of ligand-activated HERs initiates a cascade of downstream signaling, such as PI3K/AKT/mTOR, Ras/Raf/MAPK (the mitogen-activated protein kinase pathway), and JAK/ STAT (the signal transducer and activation of the transcription pathway), which regulate from cell division to death, motility to adhesion (Fig. 3).216 Overexpression of EGFR and HER2 protein and amplification of HER2 oncogene play an important role in carcinogenesis, associated with breast, lung, gastric, ovarian, endometrial, and bladder cancer.219–222 HER2 is also related to increased recurrence and poor prognosis in some cancers.223 Thus, EGFR and HER2 are promising targets for treatment of cancer.224–227
Fig. 3.
The HER signal transduction pathway and therapeutic interventions
HER-targeted drugs include monoclonal antibodies and small-molecule inhibitors. Monoclonal antibodies against the extracellular domain of the HER receptor include cetuximab, nimotuzumab, trastuzumab, pertuzumab, and ado-trastuzumab emtansine (T-DM1).227–229 Cetuximab and nimotuzumab bind to the extracellular domain of the EGFR. Trastuzumab obstructs HER2 homodimerization. HER2 overexpression is required for trastuzumab to be effective.230 Pertuzumab inhibits HER2 heterodimerization and does not require HER2 overexpression to be effective.231 T-DM1 is trastuzumab conjugated to emtansine (a microtubule inhibitor), which inhibits microtubule assembly in the cytoplasm and thus leads to cell death.232 Small-molecule inhibitors are TKIs including gefitinib, erlotinib, lapatinib, and afatinib against intracellular kinase domain to prevent signaling.233 Among them, gefitinib and erlotinib are inhibitors selective for EGFR.234,235 Lapatinib and afatinib inhibit both EGFR and HER2.236 Most of them have been approved by FDA as targeted therapies for certain advanced or recurrent cancers with selected biomarkers, such as breast cancer, colorectal cancer and non-small cell lung cancer (NSCLC).
As for gynecological cancers, HER2 is an important oncogene in high grade and stage EC, especially in uterine serous carcinoma.237,238 In OC, the rate of HER2 overexpression is highly variable (ranging from 2% to 66%), and the rate of EGFR overexpression is 30–70%.239,240 In CC, the rate of EGFR overexpression ranges from 6% to 90%.241,242 However, unlike in NSCLC,243 the clinical significance of EGFR/HER2 gene amplification or protein overexpression and the efficacy of HER-targeted therapy are still controversial in gynecological cancers (Table 8).
Table 8.
Completed phase II–III trials of HER-targeted therapy in gynecological cancers
| ID | Cancer/condition | Phase | No. | Intervention | ORR (%) | mPFS (mon.) | mOS (mon.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| NCT02095119 | CC/recurrent or metastatic | I/II | 17 | Nimotuzumab | 0 | 5.43 | 9.9 | – | 425 |
| NCT00997009 MITO CERV-2 | CC/recurrent | II | 108 | (1) PC | 84.6 | 5.2 | 17.7 | – | 244 |
| (2) PC + cetuximab | 76.4 | 7.6, P = 0.20 | 17, P = 0.27 | ||||||
| NCT10101192 | CC/advanced, persistent, or recurrent | II | 27 | Cetuximab + cisplatin | 29.6 | 3.91 | 8.77 | – | 245 |
| NCT00499031 | CC/persistent or recurrent | II | 38 | Cetuximab | 0 | 1.97 | 6.7 | 42.86 | 426 |
| NCT00086892 | OC/platinum-sensitive recurrent | II | 29 | Cetuximab | 32.1 | 9.4 | – | – | 248 |
| NCT01684878 | OC/platinum-resistant, with low tumor | III | 156 | (1) Placebo + chemotherapy | 8.7 | 2.6 | 8.4 | 37.66 | 253,254 |
| PENELOPE | HER3 mRNA expression | (2) Pertuzumab + chemotherapy | 13.1 | 4.3, P = 0.14 | 10.2, P = 0.60 | 43.42 | |||
| NCT02004093 | OC/recurrent | II | 149 | (1) Chemotherapy | - | 9.3 | Not reached | 16.2 | * |
| (2) Pertuzumab + chemotherapy | 8.0, P = 0.3967 | 28.2 | 26.7 | ||||||
| NCT00096993 | OC/platinum-resistant recurrent | II | 103 | (1) Placebo + chemotherapy | 4.6 | 2.6 | 13.1 | 61.54 | 252 |
| (2) Pertuzumab + chemotherapy | 13.8 | 2.9, P = 0.07 | 13.0, P = 0.65 | 35.38 | |||||
| NCT02004093 | OC/platinum-sensitive recurrent | II | 149 | (1) PC | – | 9.3 | Not yet estimable | 16.22 | 255 |
| (2) PC + pertuzumab | – | 8.5 | 28.2 | 26.67 | |||||
| NCT00189579 | OC/recurrent or refractory, HER2 + | II | 41 | Trastuzumab | 7.3 | 2.0 | – | – | 239 |
| NCT00006089 | EC/recurrent or stage III–IV, HER2 + | II | 34 | Trastuzumab | 0 | 1.8 | 6.8 | – | 249 |
| NCT01367002 | EC/advanced or recurrent, serous | II | 61 | (1) Chemotherapy | 75 | 8.0 | – | 51 | 250 |
| (2) Trastuzumab + chemotherapy | 44 | 12.6, P = 0.005 | |||||||
| NCT00023699 | OC/persistent or recurrent | II | 30 | Gefitinib | 0 | 1.23 | 3.7 | – | 427 |
| NCT00189358 | OC/platinum-resistant recurrent | II | 56 | Gefitinib + tamoxifen | 0 | 1.9 | 8.4 | 3.6 | 428 |
| - | CC/advanced or metastatic | II | 28 | Gefitinib | 0 | 1.2 | 3.6 | – | 266 |
| NCT00113373 | OC/recurrent | II | 28 | Lapatinib | 0 | 8.0 | – | 40 | 258 |
| NCT00436644 | OC/platinum-resistant recurrent | II | 18 | Lapatinib + topotecan | 5.6 | 3.5 | 15.5 | 22.2 | 260 |
| NCT00888810 | OC/recurrent | II | 39 | Lapatinib + topotecan | 14 | – | – | – | 259 |
| NCT00096447 | EC/persistent or recurrent | II | 30 | Lapatinib | 3.3 | 1.82 | 7.33 | 33.3 | 256 |
| NCT00430781 | CC/metastatic | II | 230 | (1) Lapatinib | 5 | 4.0 | 9.1 | 28.95 | 257 |
| (2) Pazopanib | 9 | 4.2, P < 0.013 | 11.8, P = 0.045 | 37.84 | |||||
| NCT00263822 | OC/no progression after first-line PC | III | 835 | (1) Erlotinib | – | 12.7 | 50.8 | 67 | 262 |
| (2) Observation | 12.4, P = 0.525 | 59.1, P = 0.903 | |||||||
| NCT00030446 | OC/recurrent | II | 50 | Erlotinib + carboplatin | 57 | – | – | 38 | 429 |
| NCT00126542 | OC/recurrent | II | 13 | Erlotinib + bevacizumab | 15 | 4.1 | 11 | – | 261 |
| NCT00130520 | OC/advanced | II | 40 | Erlotinib + bevacizumab | 23.1 | 4 | – | 30 | 398 |
| NCT00059787 | OC/advanced | II | 56 | Erlotinib + chemotherapy | 29 | 34.3 | – | 35.71 | 430 |
| NCT00217529 | OC/advanced | I/II | 159 | Erlotinib + chemotherapy | Terminated because of gastrointestinal toxicity. | 431 | |||
| NCT00031993 | CC/recurrent or persistent | II | 28 | Erlotinib | 0 | Only 1 patient PFS > 6 mths | 432 | ||
*Unpublished data found in ClinicalTrials.gov
Cetuximab
Cetuximab was demonstrated to have no additional benefit beyond chemotherapy in several phase II trials for CC.244,245 Moreover, in a phase II trial, the combination of cetuximab and topotecan induced a high rate of serious adverse reactions in the treatment of advanced CC.246 Another randomized phase II trial, MITO CERV-2 (NCT00997009), studied the efficacy of cetuximab plus carboplatin and paclitaxel in advanced or recurrent CC, showing no significant improvement in either the median PFS or the median OS.247 For OC, a phase II trial (NCT00086892) demonstrated modest activity of cetuximab in combination with carboplatin in patients with platinum-sensitive recurrent OC with an ORR of 32.1% and an increased incidence of hypersensitivity reactions.248 There is limited information about the clinical efficacy of cetuximab in EC.
Trastuzumab
Trastuzumab treatment revealed no responses in a phase II trial with HER2-positive EC (NCT00006089).249 However, another randomized phase II trial (NCT01367002) of paclitaxel and carboplatin with or without trastuzumab in primary stage III or IV or recurrent HER2-positive uterine serous carcinomas showed an improvement in the median PFS in the trastuzumab combination arm (4.6 months longer, P = 0.005). In the population with primary advanced-stage disease, the median PFS was 17.9 months in the trastuzumab combination arm versus 9.3 months in the chemotherapy alone arm. In the population with recurrent disease, the median PFS was 9.2 versus 6 months, respectively.250 For patients with HER2 overexpression OC, trastuzumab showed modest activity with an ORR of 7.3% in a phase II trial.239 A clinical study in china demonstrated that the combination of abraxane and trastuzumab might have promising efficacy and adverse reaction in the treatment of recurrent OC, showing a control rate of 86.4%.251 However, there is limited information about the clinical efficacy of trastuzumab for CC.
Pertuzumab
A randomized phase II trial (NCT00096993) of chemotherapy (gemcitabine) with or without pertuzumab in patients with platinum-resistant OC demonstrated an increased ORR in the pertuzumab combination arm.252 Furthermore, a phase III trial, PENELOPE (NCT01684878), evaluated the addition of pertuzumab to chemotherapy in patients with platinum-resistant OC with low tumor HER3 mRNA expression. However, the differences in the median PFS and OS were not statistically significant.253,254 In unselected patients with platinum-sensitive recurrent OC, a phase II trial (NCT02004093) showed that the addition of pertuzumab to carboplatin-based chemotherapy did not substantially prolong PFS.255 Also, there is limited information about the clinical efficacy of pertuzumab for EC and CC.
TKIs
In clinical trials of small-molecule inhibitors, a phase II trial (NCT00096447) tested the efficacy of lapatinib and explored biological characteristics in persistent or recurrent EC.256 The analysis demonstrated that lapatinib had limited activity in unselected cases in EC, as well as in OC and CC.257–260 A phase II trial assessed the activity and tolerability of the combination of bevacizumab and erlotinib in recurrent OC with an ORR of 15%.261 Furthermore, a phase III trial (NCT00263822), evaluating the efficacy of maintenance erlotinib in OC patients after first-line chemotherapy, showed no improvement in PFS or OS.262 Moreover, this study failed to show a consistent correlation between EGFR mutational status/protein expression and clinical outcomes. For CC, a phase II trial evaluated the efficacy of erlotinib combined with chemoradiation in treating patients with locally advanced CC, showing a promising activity with a complete response of 94.4%.263 Other HER-targeted TKIs (e.g., gefitinib, canertinib, and vandetanib) showed minimal clinical activities in gynecological cancers in current clinical trials.242,264–267
Even though the present clinical evidences are not very satisfying, HER-targeted therapies continue to be investigated in gynecological cancers for their potent value for biomarker-selected patients (e.g., NCT01388621, NCT01367002, NCT02039791, NCT00292955, NCT03469531, NCT00317772, NCT01953926). Furthermore, preclinical data suggested the potential of novel combination strategies involving HER-targeted therapy, which are also investigated in ongoing clinical trials.227,268–270
Other molecular targeted therapies
Ras/Raf/MEK
In the Ras/Raf/MEK signaling pathway, Ras activation is the first process in activation of the mitogen-activated protein kinases (MAPKs) cascade.271 Upon Ras activation, Raf is recruited to the cell membrane where subsequent changes in Raf phosphorylation status result in activating MEK kinases (MEK1 and MEK2).272 MEK1 and MEK2 furtherly trigger Erk1 and Erk2. Finally, Erks regulate the activity of several transcription factors that induce the expression of multiple genes required for important cell activities (Fig. 4).273 The dysregulation of this pathway exists in many human tumors, making it an attractive antitumor target. Intensive preclinical researches have led to identifying Raf kinase inhibitors, as well as inhibitors of its downstream effector MEK kinase.274–276 The results of the completed phase II trials, which evaluated the efficacy of the Ras/Raf/MEK pathway inhibitors in treating gynecological cancers, are summarized in Table 9.
Fig. 4.
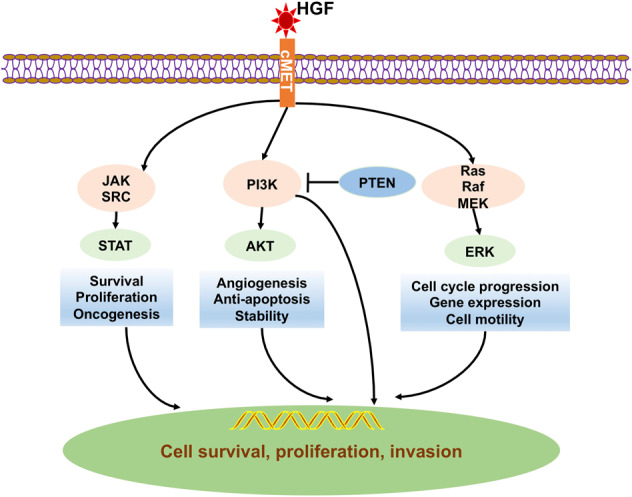
The HGF/c-MET signal transduction pathway and therapeutic interventions
Table 9.
Phase II trials (with results) of molecular targets in gynecological cancers
| ID | Cancer/condition | No. | Target | Intervention | ORR (%) | mPFS (mon.) | mOS (mon.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| NCT01936363 | OC | 63 | MEK | (1) Pimasertib + XL765 | 12.5 | 9.99 | – | 50 | * |
| (2) Pimasertib + placebo | 12.1 | 12.71 | 56.25 | ||||||
| NCT00551070 | OC/recurrent, low-grade serous | 52 | Selumetinib | 15 | – | – | 63.46 | 298 | |
| NCT01011933 | EC/recurrent or persistent | 54 | Selumetinib | 6 | 2.3 | 8.5 | 64 | 297 | |
| NCT02538627 | CC/recurrent or persistent | 35 | Trametinib + Uprosertib | 7.1 | 3.6 | 14.8 | 57 | 202 | |
| NCT01935973 | EC/ recurrent or persistent | 26 | Trametinib + Uprosertib | 8.3 | PFS at 6 months = 14% | 61 | 203 | ||
| NCT01047891 TRIAS | OC/platinum-resistant recurrent | 185 | Raf | (1) Sorafenib + topotecan | 31 | 6.7 | 17.1 | 59 | 290 |
| (2) Placebo + topotecan | 12 | 4.4, P = 0.0018 | 10.1, P = 0.017 | 51 | |||||
| NCT00390611 | OC/first-line treatment | 85 | (1) Sorafenib + PC | 69 | 15.4 | 36.5 | 27.91 | 288 | |
| (2) PC | 74 | 16.3, P = 0.38 | Not reached | 23.81 | |||||
| NCT00096200 | OC/platinum-sensitive recurrent | 36 | (1) Sorafenib + PC | 61 | 16.8 | 25.9 | 21.43 | 291 | |
| (2) Sorafenib | 15 | 5.6, P = 0.012 | 25.6, P = 0.974 | 16.67 | |||||
| NCT00791778 | OC/maintenance | 246 | (1) Sorafenib | – | 12.7 | – | 21.14 | 289 | |
| (2) Placebo | 15.7 | 20.33 | |||||||
| NCT00093626 | OC/third-line therapy | 11 | Sorafenib | – | 2.00 | 11.78 | low | 286 | |
| NCT00436215 | OC/recurrent | 55 | Sorafenib + bevacizumab | 19 | 6.1 | 45.45 | * | ||
| NCT00281515 | OC/stage IIb–IV | 105 | Ras | (1) Lonafarnib + PC | – | 11.5 | 20.6 | – | 278 |
| (2) PC | 16.4, P = 0.0141 | 43.4, P = 0.012 | |||||||
| NCT01164995 M10MKO | OC/p53 mutated refractory | 21 | Wee1 | Adavosertibc (AZD1775) | 43 | 5.3 | 12.6 | – | 341 |
| NCT01039207 | OC/recurrent or persistent | 31 | c-MET | Rilotumumab | 3.2 | PFS at 6 months = 6.5% | 45.16 | 322 | |
| NCT01716715 | OC/recurrent | 111 | (1) Cabozantinib | 7 | 5.3 | 19.4 | – | 324 | |
| (2) Paclitaxel | 24.1 | 5.5 | Not reached | ||||||
| NCT02315430 NRG-GY001 | OC/recurrent | 13 | Cabozantinib | 0 | 3.6 | 8.1 | – | 323 | |
| NCT00940225 | OC | 70 | Cabozantinib | 15 | 4.9 | – | 74.5 | 433 | |
| NCT02059265 | OC/ recurrent or persistent | 35 | Src | Dasatinib | 3.6 | 2.1 | 17.7 | 57.14 | 331 |
| NCT01196741 | OC/platinum-resistant recurrent | 107 | (1) Saracatinib + placitaxel | 29 | 4.7 | – | 57.97 | 434 | |
| (2) Placebo + placitaxel | 43 | 5.3, P = 0.99 | 51.43 | ||||||
| NCT01175343 | OC/platinum-resistant recurrent | 45 | Notch | RO4929097 | 0 | 1.3 | – | 22.73 | 339 |
*Unpublished data found in ClinicalTrials.gov
Lonafarnib is an orally protein farnesyltransferase inhibitor for H-ras, K-ras-4B, and N-ras.277 The addition of lonafarnib into first-line chemotherapy was investigated in a phase II trial (NCT00281515), and no effect was observed on prolonging PFS or OS in advanced OC.278 Sorafenib is a non-selective oral multikinase inhibitor with effects on angiogenesis through inhibition of the VEGF receptor.279,280 In addition, the antitumor effect of sorafenib is thought to be mediated through its inhibition of the Ras/Raf/MEK pathway, which is also frequently activated in advanced OC.281,282 It has been evaluated in more than 100 clinical trials in different cancer types, especially in large phase III studies in renal and liver cancers.283–285 It has been approved by the FDA for the treatment of renal, thyroid, and hepatocellular carcinoma. In OC, sorafenib showed antitumor activity in xenograft models and clinical studies.286,287 However, the results from a phase II trial (NCT00390611) in first-line treatment and maintenance therapy of OC showed no effect on prolonging PFS in sorafenib combination arm versus chemotherapy alone.288 The similar results were reported in patients with OC in complete remission (NCT00791778).289 On the other hand, a phase II trial (NCT01047891) demonstrated that sorafenib combined with topotecan as maintenance therapy significantly improved in the median PFS (2.3 months longer, P = 0.0018) and OS (7.1 months longer, P = 0.017) in patients with platinum-resistant recurrent OC.290 In patients with platinum-sensitive recurrent OC, sorafenib in combination with carboplatin and paclitaxel was reported to show promising activity with an ORR of 61%.291 Sorafenib was tested in early-phase clinical trial for CC patients receiving concurrent chemoradiation.292 However, it ended with early closure.
Selumetinib is an oral selective inhibitor of MEK1 and MEK2. It has shown activity against several advanced cancers.293,294 Since mutational alterations were found in the MAPK pathway in OC and EC, selumetinib-related clinical trials were conducted in gynecological cancers.281,295,296 For EC, selumetinib is well-tolerated in patients with recurrent or persistent disease, but with limited single-agent activity with an ORR of 6% (NCT01011933).297 A phase II trial (NCT00551070) demonstrated the potential activity of selumetinib in the treatment of recurrent low-grade OC with an ORR of 15%. It was suggested that inhibitors of the MAPK pathway should be further investigated in OC patients.298 Subsequently, an ongoing randomized phase II/III trial (NCT02101788) continues to study how well trametinib (another MEK inhibitor) works and compares it to the standard treatment in treating patients with low-grade OC. Trametinib, combined with GSK2141795 (an AKT inhibitor), has previously been tested in phase I and II studies.299 However, phase II clinical trials assessing this combination in EC (NCT01935973) or OC (NCT02538627) showed no clinical benefit.202,203 A phase II trial tested the combination of trametinib and GSK2141795 in recurrent CC with no confirmed response and a SD of 57%.202
In summary, while a powerful preclinical rationale suggests that inhibition of Ras/Raf/MEK signaling has promising potent as an antitumor targeted therapy, the clinical efficacy of this strategy in gynecological cancers is currently limited.
JAK/STAT
The janus kinase/signal transducer and activator of the tran-ions (JAK/STAT) pathway has been proved to mediate the action of cytokines, interferons and growth factors, and their control of gene expression.300 Activation of the JAK/STAT pathway and overexpression of STAT have been seen in many malignancies such as colorectal and breast cancers.301,302 Therefore, the JAK/STAT pathway is being focused as a potential target in cancer therapies. Ruxolitinib is an FDA-approved drug of JAK for treatment of patients with polycythemia vera.303 Preclinical studies demonstrated that ruxolitinib reduced OC cell viability.304,305 It enhanced the sensitivity of OC cells to other anticancer agents, and suppressed ovarian tumor growth in mice.306,307 These results supported the clinical investigation of ruxolitinib in OC patients. A phase I/II trial (NCT02713386) is trying to explore the effect of ruxolitinib phosphate when given together with paclitaxel and carboplatin in treating patients with stage III–IV OC.
HGF/c-MET
Tyrosine kinase receptor c-MET (cellular–mesenchymal to epithelial transition factor) is activated by hepatocyte growth factor (HGF) and it can trigger important cellular processes.308 Upon binding by HGF, MET is dimerized and activates cellular processes through the Ras/Raf/MEK and PI3K/AKT/mTOR pathways (Fig. 4).309,310 In a limited number of tumors, MET genetic lesions or mutations lead to the constitutive activation of MET.311,312 However, in a majority of malignancies, aberrant MET signaling derives from the upregulation of HGF transcription, leading to receptor and ligand overexpression.313–315
Since the publications of pioneer studies, the HGF/c-MET system has gained growing attention with its role in the pathogenesis of gynecological cancers.316,317 In a study analyzing 1115 advanced cancer patients, MET amplification was detected in 2.6% patients with solid tumors.318 But in OC, MET overexpression was detected in more than 20% (range from 22% to 41%) ovarian clear cell adenocarcinomas.319,320 And increased expression of HGF and c-Met signaling is associated with a poor prognosis of EC patients.321 Therefore, targeting the interaction of c-MET and HGF would be beneficial in treating gynecological cancers. Despite there are massive preclinical data on the HGF/c-MET axis, currently there is no FDA-approved indication of this targeted therapy in cancers.
The most tested drugs in HGF/c-MET axis are those blocking c-MET activity. Rilotumumab and cabozantinib are the most-studied c-MET inhibitors in gynecological cancers. The results of the completed clinical trials (phase II) investigating the safety and efficacy of them in gynecological cancers are summarized in Table 9. A phase II trial (NCT01039207) evaluated the rilotumumab in the treatment of persistent or recurrent OC. Only 1/31 achieved objective response, and only two patients got 6-month PFS.322 A phase II trial (NCT02315430) evaluated cabozantinib in treating patient with recurrent clear cell OC with no response.323 Another phase II trial (NCT01716715) compared cabozantinib versus weekly paclitaxel in treatment of persistent OC, with even worse OS and ORR in cabozantinib arm.324 These results do not warrant further evaluation of rilotumumab or cabozantinib as a single agent in targeted therapy of OC. There is currently limited information of the clinical efficacy of these agents in EC and CC.
Src
Sarcoma proto-oncogene tyrosine kinase (Src) is a downstream component of many growth factor receptors, such as VEGFR, EGFR, and c-MET.325 Src is thought to increase chemotherapy resistance through activating Ras and AKT.326 Preclinical studies showed that inhibiting Src resulted in enhancing apoptosis caused by cytotoxic drugs, such as paclitaxel, carboplatin, and gemcitabine.327,328 Src has been found to be overexpressed in gynecological cancers and promote resistance against chemotherapy.328,329 Dasatinib and saracatinib are the most-studied highly selective Src inhibitors in gynecological cancers.330 In a phase II trial (NCT01196741), it was reported that saracatinib did not improve activity of weekly paclitaxel in platinum-resistant OC.331 Another phase II trial (NCT02059265) showed that dasatinib had minimal activity as a single agent in patients with recurrent OC.332 Even though no obvious activity has been seen as a single agent, Src inhibitors used in combination with other antitumor agents are promising.
Notch
Notch signaling is a primordial, evolutionarily conserved cell-fate determination pathway that has great relevance to multiple aspects of cancer biology, from cancer stem cell to tumor immunity.333,334 Previous studies have shown that the Notch pathway is associated with the epithelial–mesenchymal transition (EMT) processes in OC and CC.335–338 Currently, several classes of Notch inhibitors have been developed, mainly composed of gamma-secretase inhibitors (GSIs), siRNA, and monoclonal antibodies against Notch pathways.336 RO4929097 is a GSI, which had insufficient activity as a single agent in platinum-resistant OC in a phase II clinical trial (NCT01175343).339
Cell cycle checkpoints
Wee1 is a kinase controlling G/M and S phase checkpoints via phosphorylation of the cyclin-dependent kinases. Ataxia-telangiectasia-mutated-and-Rad3-related kinase (ATR) plays an important role in the DNA damage response to replication stress, preventing the entry of cells with damaged DNA into mitosis (e.g., when the cancer cells are challenged by chemotherapy).340 These functions of Wee1 and ATR make them potential therapeutic targets. The activities of ATR inhibitors (e.g., AZD6738) and Wee1 inhibitors (e.g., AZD1775) have been investigated in early-phase trials in gynecological cancers341 (Tables 9 and 14).
Antibody–drug conjugates
Antibody–drug conjugates (ADCs) are complex engineered molecules composed of a monoclonal antibody conjugated to payload (e.g., cytotoxic drugs) via stable linkers.342,343 By binding to the antigens on the tumor cell surface, the ADCs release the drug components intracellularly and lead to the death of tumor cell. This site-selective drug delivery can reduce toxicities for patients by limiting the exposure of normal tissues to the cytotoxic drugs.344
Mirvetuximab soravtansine is an ADC for treatment of folate receptor α (FRα)-expressing tumors, comprising a humanized FRα-binding monoclonal antibody, a cytotoxic maytansinoid effector molecule DM4, and a cleavable disulfide linker.345–347 The FRα mediates the endocytotic uptake of folate, which has a role in amino acid, DNA and RNA metabolism as well as in methylation reactions.348 FRα is overexpressed in several cancers, including ovarian, lung, renal, endometrial, colorectal and breast cancers.349 Thus, it is a promising target for ADC design. The FRα expression in tumor is a response-predictive biomarker for patient selection. Preclinical studies showed it to have potent antitumor activities in OC xenografts.350 Phase I trials of mirvetuximab soravtansine in OC were conducted.347,351 In a population of patients with FRα-positive and platinum-resistant OC, mirvetuximab soravtansine showed an ORR of 26% and a median PFS of 4.8 months.352 However, the phase III FORWARD I trial (NCT02631876), comparing the safety and efficacy of mirvetuximab soravtansine to chemotherapy in platinum-resistant OC, was terminated because it did not meet prespecified primary endpoints. Another newly registered phase III trial (NCT04209855) is going to compare the efficacy chemotherapy in platinum-resistant OC with a high-level of FRα expression.
Tisotumab vedotin is a monomethyl auristatin E (MMAE) bearing ADC conjugated to an anti-tissue factor (TF) monoclonal antibody via a protease cleavable linker. TF is involved with tumor cell signaling and angiogenesis. Ongoing phase I/II trials GEN701/GEN702 (NCT02001623, NCT02552121), investigated tisotumab vedotin in solid tumor, including cervical, ovarian, endometrial, and other solid cancers. In the preliminary data released, 11/34 (32.4%) patients with CC achieved a response.353,354
Other ADCs continue to be investigated in a number of ongoing clinical trials (e.g., NCT03748186, NCT03835819, NCT01631552, NCT03657043, NCT03319628, NCT02988817, NCT02751918, NCT02606305, NCT02208375, NCT02996825).
Programmed death protein-1 pathway blockade
Another class of novel alternative therapy in cancer treatment is the immunotherapeutic drug, particularly the agent that inhibits the immune checkpoint. Programmed death protein-1 (PD-1) is an immune checkpoint molecule which is more commonly studied in immunotherapy researches of gynecological cancers. It plays an important role in T-cell coinhibition and exhaustion, and subsequently helps tumor cells evade immune surveillance.355 Thus, monoclonal antibodies were developed as a promising cancer therapy targeting at blockading the PD-1 pathway in tumor progression. Although immune checkpoint inhibitors do not target to kill tumor cells directly, they play an antitumor role by enhancing T-cell functions (Fig. 5). The expression of immunosuppressive PD-1 ligands (PD-L1 or PD-L2) on the surface of tumor cells is an important predictive biomarker of response to PD-1 blockade.356,357 It is also indicated that mismatch repair-deficient (dMMR) tumors, including dMMR EC, are sensitive to PD-1 blockade.358 Anti-PD-1 agents (pembrolizumab and nivolumab) and anti-PD-L1 agents (atezolizumab, avelumab, and durvalumab) were FDA-approved drugs for several kinds of advanced-stage cancers, such as melanoma, NSLC and renal cell carcinoma.359–361 In 2017, pembrolizumab was approved by FDA for the treatment of patients with unresectable or metastatic solid tumors with a biomarker referred to as microsatellite instability-high (MSI-H) or dMMR.362 These biomarkers are most commonly found in colorectal, gastrointestinal, and endometrial cancers.362–364 Successively, pembrolizumab was approved in certain condition of CC and EC (Table 1), basing on findings from two phase II trials (KEYNOTE 158 and KEYNOTE 146).365,366 The results of the completed phase I/II trials of anti-PD-1/PD-L1 agents for ovarian, cervical, and endometrial cancers are summarized in Table 10. And other ongoing phase II/III trials investigating anti-PD-1/PD-L1 therapy (not in addition to other targeted agents) in gynecological cancers are listed in Tables 11 and 12.
Fig. 5.
The immune checkpoint blockades. Antigen presenting cells (APC) take up antigen (Ag) released from tumor cells and present it to T cells. PD-1 receptors inhibit immune responses by engagement of PD-L1 and PD-L2. Therefore, monoclonal antibody blockading the PD-1 pathway results in enhancing antitumor immunity
Table 10.
Completed phase I/II trials of anti-PD-1/PD-L1 in gynecological cancers
| ID | Cancer/condition | Phase | No. | Intervention | ORR (%) | mPFS (mon.) | mOS (mon.) | SAEs (%) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| – | OC/platinum-resistant recurrent | II | 20 | Nivolumab | 15 | 3.5 | 20 | 40 | 378 |
| NCT02873962 | OC/recurrent | II | 38 | Nivolumab + bevacizumab | 21 | 9.4 | – | – | 377 |
| NCT00729664 | OC/advanced | I | 17 | Nivolumab | 5.9 | – | – | 5 | 435 |
| NCT02488759 CheckMate 358 trial | CC/recurrent or metastatic | I/II | 19 | Nivolumab | 26 | – | 21.9 | – | 375 |
| NCT02257528 | CC/persistent or recurrent | II | 26 | Nivolumab | 4 | – | – | 24 | 376 |
| NCT02674062 KEYNOTE100 | OC/advanced or recurrent | II | 376 | Pembrolizumab | 7.4–9.9 | 2.1 | 17.6 | 19.7 | 373 |
| NCT02657889 KEYNOTE-162 | OC/recurrent | I/II | 62 | Pembrolizumab+ niraparib | 18 | Not reached | – | – | 143 |
| NCT02537444 | OC/recurrent | II | 78 | (1) ACP-196 | 2.9 | – | – | 21 | * |
| KEYNOTE191 | (2) ACP-196+ pembrolizumab | 9.1 | 41 | ||||||
| NCT02628067 KEYNOTE 158 | CC/advanced | II | 98 | Pembrolizumab | 12.2 | 2.1 | 9 | 12.2 | 365 |
| - | EC/dMMR recurrent or persistent | II | 9 | pembrolizumab | 56 | – | Not reached | 0 | 370 |
| NCT02501096 KEYNOTE 146 | EC/advanced | II | 54 | Pembrolizumab+ lenvatinib | 39.6 | 7.4 | – | 30 | 366 |
| NCT02054806 KEYNOTE 028 | EC/advanced, PD-L1( + ) | Ib | 24 | Pembrolizumab | 13 | – | – | 16.7 | 367 |
| NCT02054806 KEYNOTE028 | OC/advanced, PD-L1( + ) | Ib | 26 | Pembrolizumab | 11.5 | 1.9 | 13.8 | 3.8 | 368 |
| CC/advanced, PD-L1( + ) | 24 | 17 | – | – | 21 | 369 | |||
| NCT02431559 | OC/platinum-resistant recurrent | I/II | 40 | Durvalumab+ PLD | 15 | 5.5 | – | 57.5 | * |
| NCT01772004 JAVELIN Solid Tumor | OC/recurrent or refractory | Ib | 124 | Avelumab | 9.7 | 2.7 | 10.8 | 6.5 | 381 |
| NCT02912572 | EC/MSS | II | 33 | Avelumab | 27.6 | – | – | 19 | 380 |
| EC/POLE or MSI | 6.25 | ||||||||
| NCT01375842 | EC/advanced or recurrent | Ia | 15 | Atezolizumab | 13.3 | 1.7 | 9.6 | 13.3 | 379 |
| NCT01375842 | OC/recurrent | I | 12 | Atezolizumab | 22.2 | 2.9 | 11.3 | 25.0 | 436 |
| EC/recurrent | 15 | 13.3 | 1.4 | 9.6 | 43.3 |
dMMR mismatch repair-deficient, MSS microsatellite stable, MSI microsatellite instable, POLE polymerase-ε. *Unpublished date found in clinicaltrials.gov
Table 11.
Ongoing phase II trials of anti-PD-1/PD-L1 in gynecological cancers (not including novel combination therapy)
| ID | Cancer/condition | No. | Start date | Intervention | Design | Status |
|---|---|---|---|---|---|---|
| NCT02725489 | Women’s cancers | 13 | 2016.6 | Durvalumab | Non-randomized parallel | Not yet recruiting |
| NCT02811497 METADUR | OC/platinum-resistant recurrent | 60 | 2016.9 | Durvalumab + azacitidine | Single group | Recruiting |
| NCT03899610 | OC/advanced | 24 | 2019.7 | Durvalumab + tremelimumab + chemotherapy | Single group | Recruiting |
| NCT03357757 LATENT | Virus associated cancer | 39 | 2018.2 | Avelumab + valproic acid | Single group | Recruiting |
| NCT03503786 MITO END-3 | EC/advanced or recurrent | 120 | 2018.4 | Avelumab + PC vs. avelumab | Randomized parallel | Not yet recruiting |
| NCT02440425 | OC/platinum-resistant recurrent | 43 | 2015.8 | Pembrolizumab + paclitaxel | Single group | Active, not recruiting |
| NCT02635360 | CC/advanced | 88 | 2016.1 | Pembrolizumab maintenance/throughout, plus chemoradiation | Randomized parallel | Recruiting |
| NCT02608684 PemCiGem | OC/platinum-resistant recurrent | 21 | 2016.2 | Pembrolizumab + standard treatment | Single group | Active, not recruiting |
| NCT02530154 | OC/stage III–IV | 30 | 2016.7 | Pembrolizumab + PC | Single group | Recruiting |
| NCT02899793 | EC/recurrent or metastatic | 25 | 2016.9 | Pembrolizumab | Single group | Recruiting |
| NCT02865811 | OC/platinum-resistant recurrent | 26 | 2016.9 | Pembrolizumab + doxorubicin | Single group | Active, not recruiting |
| NCT02901899 | OC/recurrent | 38 | 2016.11 | Pembrolizumab + gemcitabine | Single group | Recruiting |
| NCT02900560 | OC/platinum-resistant recurrent | 34 | 2016.12 | Pembrolizumab + azacytidine vs. pembrolizumab | Non-randomized parallel | Active, not recruiting |
| NCT02834975 | OC/advanced | 40 | 2016.12 | Pembrolizumab + PC | Single group | Recruiting |
| NCT03192059 PRIMMO | CC or EC | 43 | 2017.7 | Pembrolizumab | Single group | Recruiting |
| NCT02549209 | EC/recurrent | 46 | 2017.8 | Pembrolizumab + PC | Single group | Recruiting |
| NCT03126812 | OC/stage IV | 15 | 2017.11 | Pembrolizumab as neoadjuvant | Single group | Recruiting |
| NCT03275506 NEOPEMBROV | OC/stage IV | 45 | 2018.2 | Pembrolizumab + chemotherapy vs. chemotherapy | Non-randomized parallel | Recruiting |
| NCT03029403 | OC/advanced | 42 | 2018.2 | Pembrolizumab + DPX-Survivac (vaccine) + cyclophosphamide | Non-randomized parallel | Recruiting |
| NCT03410784 MITO28 | OC/advanced | 72 | 2018.4 | Pembrolizumab + PC | Single group | Not yet recruiting |
| NCT03276013 TOPIC | EC/advanced, recurrent or metastatic | 51 | 2018.5 | Pembrolizumab + doxorubicin | Single group | Recruiting |
| NCT03539328 MITO27 | OC/platinum-resistant recurrent | 138 | 2018.6 | Pembrolizumab + chemotherapy vs. chemotherapy | Randomized parallel | Not yet recruiting |
| NCT03732950 | OC/recurrent | 30 | 2019.3 | Pembrolizumab | Single group | Recruiting |
| NCT03430700 PROMPT | OC/platinum-resistant recurrent | 28 | 2019.5 | Pembrolizumab + paclitaxel | Single group | Recruiting |
| NCT04375956 | OC/platinum-resistant recurrent | 100 | 2020.5 | Pembrolizumab | Single group | Not yet recruiting |
| NCT04238988 | CC/locally advanced | 45 | 2020.3 | Pembrolizuma + PC | Single group | Not yet recruiting |
| NCT03340376 | CC/recurrent | 48 | 2017.8 | Atezolizumab vs. atezolizumab + doxorubicin vs. doxorubicin | Randomized parallel | Recruiting |
| NCT03612791 | CC/advanced | 190 | 2018.6 | Atezolizumab + radiotherapy vs. radiotherapy | Randomized parallel | Recruiting |
| NCT03614949 | CC/recurrent, persistent, or metastatic | 26 | 2019.1 | Atezolizumab | Single group | Recruiting |
| NCT02498600 | OC/recurrent | 96 | 2015.6 | Nivolumab vs. nivolumab + ipilimumab | Randomized parallel | Active, not recruiting |
| NCT03241745 | EC/metastatic or recurrent | 40 | 2017.8 | Nivolumab | Single group | Recruiting |
| NCT03808857 | CC/recurrent or metastatic | 80 | 2019.2 | GB226 | Single group | Recruiting |
| NCT03972722 | CC/recurrent or metastatic | 89 | 2019.5 | GLS-010 | Single group | Recruiting |
| NCT04188860 | CC/recurrent | 34 | 2019.12 | Camrelizumab + paclitaxel | Single group | Recruiting |
| NCT04368273 | CC/advanced | 30 | 2020.5 | Toripalimab | Single group | Not yet recruiting |
| NCT03104699 | CC/advanced | 211 | 2017.4 | Balstilimab | Single group | Active, not recruiting |
Table 12.
Ongoing phase III trials of anti-PD-1/PD-L1 in gynecological cancers (not including novel combination therapy)
| ID | Cancer/condition | No. | Start date | Intervention | Status |
|---|---|---|---|---|---|
| NCT02580058 JAVELIN Ovarian 200 | OC/platinum-resistant, or- refractory recurrent | 566 | 2015.12 | Avelumab + PLD vs. avelumab vs. PLD | Active, not recruiting |
| NCT02891824 ATALANTE | OC/platinum-sensitive recurrent | 405 | 2016.9 | Atezolizumab vs. placebo, plus PC + bevacizumab | Recruiting |
| NCT03038100 IMagyn050 | OC/stage III–IV | 1300 | 2017.3 | Atezolizumab vs. placebo, plus PC + bevacizumab | Active, not recruiting |
| NCT03353831 | OC/platinum-resistant recurrent | 664 | 2018.9 | Atezolizumab vs. placebo, plus paclitaxel or PLD | Recruiting |
| NCT03556839 | CC/stage IVb | 404 | 2018.9 | Atezolizumab vs. placebo, plus PC + bevacizumab | Recruiting |
| NCT03603184 AtTEnd | EC/advanced | 550 | 2018.10 | Atezolizumab vs. placebo, plus PC | Recruiting |
| NCT03635567 KEYNOTE-826 | CC/persistent, recurrent, or metastatic | 600 | 2018.10 | Pembrolizumab vs. placebo, plus PC + bevacizumab | Recruiting |
| NCT03914612 | EC/advanced or recurrent | 810 | 2019.7 | Pembrolizumab vs. placebo, plus PC | Recruiting |
| NCT04221945 | CC/locally advanced | 980 | 2020.4 | Pembrolizumab vs. placebo, plus chemoradiation | Recruiting |
| NCT03830866 CALLA | CC/locally advanced | 714 | 2019.2 | Durvalumab vs. placebo, plus chemoradiation | Recruiting |
| NCT03981796 RUBY | EC/recurrent or stage III–IV | 470 | 2019.7 | Dostarlimab vs. placebo, plus PC | Recruiting |
| NCT03912415 FERMATA | CC/advanced | 316 | 2019.9 | Prolgolimab vs. placebo, plus PC + bevacizumab | Not yet recruiting |
Anti-PD-1 agents
A phase Ib KEYNOTE-028 trial (NCT02054806) of pembrolizumab (10 mg/kg intravenously every 2 weeks) as a treatment of PD-L1–positive solid tumors showed that pembrolizumab was associated with a 17% ORR in CC cohort, a 13% ORR in EC cohort, and a 11.5% ORR in OC cohort, respectively.367–369 In KEYNOTE 158 trial (NCT02628067), pembrolizumab was investigated in a single cohort of recurrent or metastatic CC, resulting in an ORR of 12.2%. In the population of patients with PD-L1–positive tumors, the ORR was 14.6%. No response was observed in patients with PD-L1–negative tumors. The median OS was 9.4 months in the total population and 11 months in the PD-L1–positive tumor population.365 On the ground of this trial, the FDA-approved pembrolizumab for patients with recurrent or metastatic CC with disease progression on or after chemotherapy whose tumors expressed PD-L1, in 2018. As for EC, a phase II study evaluated the clinical efficacy of pembrolizumab in nine patients with recurrent or persistent EC with dMMR, and the results indicated that the ORR was 56%, the 12-month OS rate was 89%, and the median OS had not been reached.370 For EC patients without MSI or PD-L1 expression status, another phase II KEYNOTE 146 trial (NCT02501096) assessed the activity and safety of lenvatinib plus pembrolizumab in patients with biomarker-unselected advanced EC.371 Lenvatinib is an oral multikinase inhibitor targeting VEGFR, FGFR, PDGFR, RET, and KIT.372 An interim report of KEYNOTE 146 showed this combination of PD-1 blockade and inhibition of angiogenesis (as well as VEGF-mediated immune suppression) was associated with antitumor activity with an ORR of 35.6%.366 In September 2019, the FDA granted accelerated approval to the combination of pembrolizumab and lenvatinib for the treatment of patients with advanced EC without MSI-H or dMMR and who have disease progression following prior systemic therapy, but were not candidates for curative surgery or radiation. For patient with recurrent OC, single-agent pembrolizumab showed modest activity in a phase II trial (NCT02674061) with an ORR of 7.4–9.9%.373 A phase I/II trial (NCT02657889) demonstrated that niraparib combined with pembrolizumab was tolerable and had promising antitumor activity for platinum-resistant current OC with an ORR of 18% and a disease control rate of 65%.143 Furthermore, a recent study identified two determinants of response to the combination of pembrolizumab and niraparib: the presence of mutational signature 3 as a surrogate of HRD and a positive immune score as a surrogate of interferon-primed, CD8-exhausted effector T cells in the tumor microenvironment. Presence of one or both tumor features was associated with significantly prolonged PFS while absence of both was associated with no response.374
Nivolumab is another well-known anti-PD-1 drug. As indicated by a phase I/II trial (NCT02488759), nivolumab had a promising activity in metastatic CC with an ORR of 26%.375 However, another phase II trial (NCT02257528) demonstrated that single-agent nivolumab exhibited low antitumor activity in recurrent CC with an ORR of 4% and a SD of 36%.376 In patients with platinum-resistant recurrent OC, early-phase trials showed that monotherapy of anti-PD-1 agents had promising activity.377,378
Dostarlimab (TSR-042) is an investigational humanized anti-PD-1 monoclonal antibody. It demonstrated robust clinical activity in patients with previously treated recurrent or advanced EC in both MSI-H and MSS subgroups. It is being evaluated in combination of bevacizumab and niraparib in patients with platinum-resistant OC (NCT03574779).
Anti-PD-L1 agents
In a phase Ia trial (NCT01375842) assessing atezolizumab (10 mg/kg intravenously every 3 weeks) in advanced/recurrent EC, the ORR was 13.3% (2/15) in all populations. Both these two patients were in population with PD-L1 status >5% of tumor-infiltrating immune cells (2/5). Moreover, a trend for higher PFS and OS was noticed with higher PD-L1 expression.379 A phase II trial (NCT02912572) of avelumab (10 mg/kg intravenously every 2 weeks) in patients with microsatellite stable (MSS), microsatellite instable (MSI), and POLE-mutated recurrent/persistent EC demonstrated an ORR of 6.25% in the MSS cohort and an ORR of 27.6% in the MSI/POLE cohort.380 As demonstrated in these clinical outcomes, PD-L1 status, dMMR, MSI, and POLE mutation were predictive biomarkers to identify the EC population who could benefit from PD-1 blockade. However, in patients with recurrent OC, a single-agent trial of anti-PD-L1 agents demonstrated only modest efficacy.381
Several clinical trials are further conducted to combine chemotherapy or other targeted therapies with anti-PD-1/PD-L1 agents in treatment of gynecological cancers. A phase II trials showed that the combination of durvalumab (10 mg/kg intravenously every 2 weeks) and doxorubicin was associated with an ORR of 15% in platinum-resistant recurrent OC. A great number of clinical trials have been designed and registered to investigate the efficacy and safety of anti-PD-1/PD-L1 agents combined with other targeted therapy in cancer treatment.
Currently, we find limited results from phase III trials investigating the safety and efficacy of anti-PD-1/PD-L1 agents in gynecological cancers. The reported interim results in OC are somehow disappointing. JAVELIN Ovarian 100 (NCT02718417), a phase III study of avelumab in combination with chemotherapy treating previously untreated OC patients, was terminated in 2018. The decision of termination was based on the results of a planned interim analysis that showed futility of efficacy. It was further reported that another ongoing phase III study of avelumab for platinum-resistant/refractory recurrent OC, JAVELIN Ovarian 200 (NCT02580058), did not meet prespecified primary endpoints of OS or PFS in patients. As of January 2020, there are dozens of ongoing phase III trials involving anti-PD-1/PD-L1 drugs in gynecological cancers, registered on ClinicalTrials.gov. The ongoing phase III trials are listed in Table 12.
Even though the preliminary results of phase III JAVELIN Ovarian trials are unsatisfying, anti-PD-1/PD-L1 drugs (either used as monotherapy or used in combination with chemotherapy, other immune checkpoint inhibitors, cancer vaccines or other targeted therapies) are still expected to be promising approaches, especially in the treatment of CC and EC.382–384
Selective estrogen receptor downregulators
In EC, type I (endometrioid histologies), the most common type, is associated with an excess estrogen exposure in the absence of counteractive effects of progesterone, mostly with expressing estrogen and/or progesterone receptors (ER/PR).385–387 Hormonal therapy is an alternative treatment to control metastatic or recurrent disease.388,389 In addition to the conventional progestin therapy, inhibition of estrogen-induced proliferation by anti-estrogenic agents has been evaluated in EC, including selective estrogen receptor modulators (SERMs) or downregulators (SERDs) and aromatase inhibitors.390,391
Fulvestrant, the main SERD, has an anti-proliferative effect through down regulation of ER and plays an antitumor role as both hormonal therapy and targeted therapy. Fulvestrant was approved by FDA for the treatment of postmenopausal metastatic ER/PR-positive breast cancer, not yet for gynecological cancers.392 A phase II trial (NCT00334295) evaluated the activity and toxicity of fulvestrant, in patients with advanced or recurrent ER/PR-positive EC.393 It demonstrated an ORR of 11.4% in the ITT group, with a median PFS of 2.3 months and a median OS of 13.2 months. However, another phase II trial showed minimal activity of fulvestrant in advanced, recurrent, or persistent EC.394 No patient demonstrated a complete or partial response in the 22 ER-negative patients, with a stable disease rate of 18% as the best response. The median PFS and OS were 2 and 3 months, respectively. In the 31 ER-positive patients, the ORR and stable disease rate were 16% and 29%, with a median PFS of 10 months and a median OS of 26 months, respectively. As for OC, fulvestrant was associated with a low ORR of 8% and a stable disease rate of 35% in ER-positive, multiply recurrent OC.395 The effect of anti-estrogenic agents in advanced or recurrent EC needs further investigations. Furthermore, combining hormonal therapy with targeted therapies is a novel strategy in treating certain gynecological cancers, which is being assessed in several ongoing clinical trials (e.g., NCT03643510, NCT03294694, NCT02730923, NCT02476955, and NCT02188550).
Conclusion
From the large amount of clinical trials on targeted agents and molecular drugs, we can see the great enthusiasm in targeted therapies. Consequently, it has led to significant breakthrough in personalized medicine of antitumor treatment strategy, including gynecological cancers. According to current clinical evidence, PARP inhibitors have made a remarkable progress in treatment of OC depending on the identification of disease with HRD (e.g., BRCAm). As for EC, given the identification of hormone-dependent histological type and POLE/MSI molecular subtypes, the activity of PI3K/AKT/mTOR, PD-1, and hormone receptor-targeted therapies might be promising in treatment of patients with EC. Since CC is mostly associated with persistent infection of virus, immune-targeted therapies (e.g., anti-PD-1/PD-L1 agents) are expected to be prospective treatment strategy. For the future research, as we discussed previously, specific biomarkers are the keys to the tumor response of targeted drugs. Moreover, novel combination therapies, coinhibition of different targets, are worth conducting to overcome the drug resistance in cancer cells. A number of phase II/III clinical trials of novel combination strategies have been in progress (Tables 13 and 14).
Acknowledgements
This study was supported by National Major Scientific and Technological Project for “Significant New Drugs Development” in China (2018ZX09733001).
Author contributions
X.Z. substantially contributed to the conception and design of the work. Q.W. and H.L.P. did the literature research and data retrieval. Q.W. drafted the article and revised it. X.R.Q. and M.W. reviewed the draft. All authors approved the submitted version.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Qiao Wang, Hongling Peng
References
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2019. CA: Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Matulonis UA, et al. Ovarian cancer. Nat. Rev. Dis. Prim. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA: Cancer J. Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowtell DD, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat. Rev. Cancer. 2015;15:668. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow RE, et al. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol. Oncol. 1999;72:278–287. doi: 10.1006/gyno.1998.5145. [DOI] [PubMed] [Google Scholar]
- 6.Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol. Med. 2017;14:9–32. doi: 10.20892/j.issn.2095-3941.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo N, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann.Oncol. 2010;Suppl 5:v23–v30. doi: 10.1093/annonc/mdq244. [DOI] [PubMed] [Google Scholar]
- 8.Christie, E. L. & Bowtell, D. D. L. Acquired chemotherapy resistance in ovarian cancer. Ann. Oncol.28, viii13 (2017). [DOI] [PubMed]
- 9.Pignata S, et al. Treatment of recurrent epithelial ovarian cancer. Cancer. 2019;125(Suppl 24):4609–4615. doi: 10.1002/cncr.32500. [DOI] [PubMed] [Google Scholar]
- 10.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer J. clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 11.Munoz N, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 12.Vande Pol SB, Klingelhutz AJ. Papillomavirus E6 oncoproteins. Virology. 2013;445:115–137. doi: 10.1016/j.virol.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman A, Munger K. The papillomavirus E7 proteins. Virology. 2013;445:138–168. doi: 10.1016/j.virol.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. England J. Med. 356, 1915–1927 (2007). [DOI] [PubMed]
- 15.Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 2016;76:S49–S55. doi: 10.1016/j.jcv.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morice P, et al. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 18.Colombo N, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stelloo E, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. 2015;28:836–844. doi: 10.1038/modpathol.2015.43. [DOI] [PubMed] [Google Scholar]
- 20.Kandoth C, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr. Opin. Obstet. Gynecol. 2017;29:47–58. doi: 10.1097/GCO.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 22.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemann DW, et al. Differentiation and definition of vascular-targeted therapies. Clin. Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]
- 24.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 25.Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 26.Bridges EM, Harris AL. The angiogenic process as a therapeutic target in cancer. Biochem. Pharmacol. 2011;81:1183–1191. doi: 10.1016/j.bcp.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Miyasaka A, et al. PI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the HIF1-α/VEGF pathway in endometrial cancer. Gynecol. Oncol. 2015;138:174–180. doi: 10.1016/j.ygyno.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Graybill W, Sood AK, Monk BJ, Coleman RL. State of the science: emerging therapeutic strategies for targeting angiogenesis in ovarian cancer. Gynecologic Oncol. 2015;138:223–226. doi: 10.1016/j.ygyno.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518–529. doi: 10.1016/S0140-6736(15)01088-0. [DOI] [PubMed] [Google Scholar]
- 30.Kerbel RS. Tumor. Angiogenesis. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camerin GR, et al. VEGF gene polymorphisms and outcome of epithelial ovarian cancer patients. Future Oncol. 2017;13:409–414. doi: 10.2217/fon-2016-0299. [DOI] [PubMed] [Google Scholar]
- 32.Vergote I, et al. A phase 1b study of trebananib in combination with pegylated liposomal doxorubicin or topotecan in women with recurrent platinum-resistant or partially platinum-sensitive ovarian cancer. Gynecol. Oncol. 2014;135:25–33. doi: 10.1016/j.ygyno.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Haqshenas G, Doerig C. Targeting of host cell receptor tyrosine kinases by intracellular pathogens. Sci. Signal. 2019;12:eaau9894. doi: 10.1126/scisignal.aau9894. [DOI] [PubMed] [Google Scholar]
- 34.Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 35.Tewari KS, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian. Cancer. 2019;37:2317–2328. doi: 10.1200/JCO.19.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perren TJ, et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 37.Oza AM, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedlander M, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19:1126–1134. doi: 10.1016/S1470-2045(18)30343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aghajanian C, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aghajanian C, et al. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 2015;139:10–16. doi: 10.1016/j.ygyno.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman RL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–791. doi: 10.1016/S1470-2045(17)30279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bamias A, et al. Bevacizumab with or after chemotherapy for platinum-resistant recurrent ovarian cancer: exploratory analyses of the AURELIA trial. Ann. Oncol. 2017;28:1842–1848. doi: 10.1093/annonc/mdx228. [DOI] [PubMed] [Google Scholar]
- 43.Pujade-Lauraine E, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 44.Gore M, et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol. Oncol. 2019;153:541–548. doi: 10.1016/j.ygyno.2019.03.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monk BJ, et al. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J. Clin. Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zighelboim I, et al. Multicenter phase II trial of topotecan, cisplatin and bevacizumab for recurrent or persistent cervical cancer. Gynecol. Oncol. 2013;130:64–68. doi: 10.1016/j.ygyno.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki K, et al. Phase II trial of paclitaxel, carboplatin, and bevacizumab for advanced or recurrent cervical cancer. Gynecol. Oncol. 2019;154:554–557. doi: 10.1016/j.ygyno.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Tewari KS, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schefter TE, et al. A phase II study of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: preliminary results of RTOG 0417. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:1179–1184. doi: 10.1016/j.ijrobp.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa M, et al. A randomized phase II/III trial of conventional paclitaxel and carboplatin with or without bevacizumab vs dose-dense paclitaxel and carboplatin with or without bevacizumab, in stage IVB, recurrent or persistent cervical carcinoma: Japan Clinical Oncology Group Study (JCOG1311) Jpn J. Clin. Oncol. 2018;48:1096–1100. doi: 10.1093/jjco/hyy137. [DOI] [PubMed] [Google Scholar]
- 51.Aghajanian C, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2011;29:2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpkins F, et al. A phase II trial of paclitaxel, carboplatin, and bevacizumab in advanced and recurrent endometrial carcinoma (EMCA) Gynecol. Oncol. 2015;136:240–245. doi: 10.1016/j.ygyno.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Viswanathan AN, et al. NRG Oncology/RTOG 0921: a phase 2 study of postoperative intensity-modulated radiotherapy with concurrent cisplatin and bevacizumab followed by carboplatin and paclitaxel for patients with endometrial cancer. Cancer. 2015;121:2156–2163. doi: 10.1002/cncr.29337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorusso D, et al. Carboplatin-paclitaxel compared to carboplatin-paclitaxel-bevacizumab in advanced or recurrent endometrial cancer: MITO END-2—a randomized phase II trial. Gynecol. Oncol. 2019;155:406–412. doi: 10.1016/j.ygyno.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Oza AM, et al. Efficacy and safety of bevacizumab-containing therapy in newly diagnosed ovarian cancer: ROSiA single-arm phase 3B study. Int. J. Gynecol. Cancer. 2017;27:50–58. doi: 10.1097/IGC.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verheijen RB, et al. Clinical pharmacokinetics and pharmacodynamics of pazopanib: towards optimized dosing. Clin. Pharmacokinet. 2017;56:987–997. doi: 10.1007/s40262-017-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerklaan BM, et al. Phase I and pharmacological study of pazopanib in combination with oral topotecan in patients with advanced solid tumours. Br. J. Cancer. 2015;113:706–715. doi: 10.1038/bjc.2015.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hainsworth JD, Firdaus ID, Earwood CB, Chua CC. Pazopanib and liposomal doxorubicin in the treatment of patients with relapsed/refractory epithelial ovarian cancer: a phase Ib study of the Sarah Cannon Research Institute. Cancer Investig. 2015;33:47–52. doi: 10.3109/07357907.2014.998833. [DOI] [PubMed] [Google Scholar]
- 59.Friedlander M, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol. Oncol. 2010;119:32–37. doi: 10.1016/j.ygyno.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 60.Pignata S, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16:561–568. doi: 10.1016/S1470-2045(15)70115-4. [DOI] [PubMed] [Google Scholar]
- 61.Dinkic C, et al. Pazopanib (GW786034) and cyclophosphamide in patients with platinum-resistant, recurrent, pre-treated ovarian cancer—results of the PACOVAR-trial. Gynecol. Oncol. 2017;146:279–284. doi: 10.1016/j.ygyno.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 62.du Bois A, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol. 2014;32:3374–3382. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 63.Vergote I, et al. Overall survival results of AGO-OVAR16: a phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer. Gynecol. Oncol. 2019;155:186–191. doi: 10.1016/j.ygyno.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 64.Morgan RD, et al. Pazopanib and fosbretabulin in recurrent ovarian cancer (PAZOFOS): a multi-centre, phase 1b and open-label, randomised phase 2 trial. Gynecol. Oncol. 2020;156:545–551. doi: 10.1016/j.ygyno.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Campos SM, et al. A phase II evaluation of pazopanib in the treatment of recurrent or persistent carcinosarcoma of the uterus: a gynecologic oncology group study. Gynecol. Oncol. 2014;133:537–541. doi: 10.1016/j.ygyno.2014.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ledermann JA, et al. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J. Clin. Oncol. 2011;29:3798–3804. doi: 10.1200/JCO.2010.33.5208. [DOI] [PubMed] [Google Scholar]
- 67.du Bois A, et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17:78–89. doi: 10.1016/S1470-2045(15)00366-6. [DOI] [PubMed] [Google Scholar]
- 68.Secord AA, et al. Phase II trial of nintedanib in patients with bevacizumab-resistant recurrent epithelial ovarian, tubal, and peritoneal cancer. Gynecol. Oncol. 2019;153:555–561. doi: 10.1016/j.ygyno.2019.03.246. [DOI] [PubMed] [Google Scholar]
- 69.Dizon DS, et al. A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2014;135:441–445. doi: 10.1016/j.ygyno.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang W, McCormick A, Li J, Masson E. Clinical pharmacokinetics and pharmacodynamics of cediranib. Clin. Pharmacokinet. 2017;56:689–702. doi: 10.1007/s40262-016-0488-y. [DOI] [PubMed] [Google Scholar]
- 71.Kaplan AR, et al. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci. Transl. Med. 2019;11:eaav4508. doi: 10.1126/scitranslmed.aav4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirte H, et al. A phase 2 study of cediranib in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: a trial of the Princess Margaret, Chicago and California Phase II Consortia. Gynecol. Oncol. 2015;138:55–61. doi: 10.1016/j.ygyno.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Ledermann JA, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387:1066–1074. doi: 10.1016/S0140-6736(15)01167-8. [DOI] [PubMed] [Google Scholar]
- 74.Bender D, et al. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015;138:507–512. doi: 10.1016/j.ygyno.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 2013;73:1649–1657. doi: 10.1158/0008-5472.CAN-12-4697. [DOI] [PubMed] [Google Scholar]
- 76.Karlan BY, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. Lancet Oncol. 2012;30:362–371. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 77.Coxon A, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol. Cancer Ther. 2010;9:2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monk BJ, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:799–808. doi: 10.1016/S1470-2045(14)70244-X. [DOI] [PubMed] [Google Scholar]
- 79.Stark DP, et al. Quality of life with cediranib in relapsed ovarian cancer: The ICON6 phase 3 randomized clinical trial. Cancer. 2017;123:2752–2761. doi: 10.1002/cncr.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vergote I, et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:862–876. doi: 10.1016/S1470-2045(19)30178-0. [DOI] [PubMed] [Google Scholar]
- 81.Marth C, et al. ENGOT-ov-6/TRINOVA-2: Randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. Eur. J. Cancer. 2017;70:111–121. doi: 10.1016/j.ejca.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Moore KN, et al. A phase II trial of trebananib (AMG 386; IND#111071), a selective angiopoietin 1/2 neutralizing peptibody, in patients with persistent/recurrent carcinoma of the endometrium: an NRG/Gynecologic Oncology Group trial. Gynecol. Oncol. 2015;138:513–518. doi: 10.1016/j.ygyno.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao D, Hou H, Zhang X. Progress in the treatment of solid tumors with apatinib: a systematic review. OncoTargets Ther. 2018;11:4137–4147. doi: 10.2147/OTT.S172305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin M, et al. Successful maintenance therapy with apatinib inplatinum-resistant advanced ovarian cancer and literature review. Cancer Biol. Ther. 2018;19:1088–1092. doi: 10.1080/15384047.2018.1491500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 86.Miao M, et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol. Oncol. 2018;148:286–290. doi: 10.1016/j.ygyno.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 87.Lan CY, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol. 2018;19:1239–1246. doi: 10.1016/S1470-2045(18)30349-8. [DOI] [PubMed] [Google Scholar]
- 88.Campos SM, et al. A phase II trial of Sunitinib malate in recurrent and refractory ovarian, fallopian tube and peritoneal carcinoma. Gynecol. Oncol. 2013;128:215–220. doi: 10.1016/j.ygyno.2012.07.126. [DOI] [PubMed] [Google Scholar]
- 89.Baumann KH, et al. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann. Oncol. 2012;23:2265–2271. doi: 10.1093/annonc/mds003. [DOI] [PubMed] [Google Scholar]
- 90.Biagi JJ, et al. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: an NCIC Clinical Trials Group Study. Ann. Oncol. 2011;22:335–340. doi: 10.1093/annonc/mdq357. [DOI] [PubMed] [Google Scholar]
- 91.Chan JK, et al. A phase II evaluation of sunitinib in the treatment of persistent or recurrent clear cell ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group Study (GOG-254) Gynecol. Oncol. 2018;150:247–252. doi: 10.1016/j.ygyno.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castonguay V, et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: a study of the Princess Margaret, Chicago and California Consortia. Gynecol. Oncol. 2014;134:274–280. doi: 10.1016/j.ygyno.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 93.Mackay HJ, et al. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG Trial IND.184. Gynecol. Oncol. 2010;116:163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 94.Chan JK, et al. A phase II evaluation of brivanib in the treatment of persistent or recurrent carcinoma of the cervix: an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017;146:554–559. doi: 10.1016/j.ygyno.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powell MA, et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2014;135:38–43. doi: 10.1016/j.ygyno.2014.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Curtin NJ, Szabo C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol. Asp. Med. 2013;34:1217–1256. doi: 10.1016/j.mam.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Michèle R, et al. PARP inhibition: PARP1 beyond. Nat. Rev. Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair. 2007;6:923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J. Clin. Oncol. 2015;33:1397–1406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takata M, et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25:5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- 102.Drew Y. The development of PARP inhibitors in ovarian cancer: from bench to bedside. Br. J. Cancer. 2015;113:S3–S9. doi: 10.1038/bjc.2015.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bellio C, et al. PARP inhibition induces enrichment of DNA repair–proficient CD133 and CD117 positive ovarian cancer. Stem Cells. 2019;17:431–445. doi: 10.1158/1541-7786.MCR-18-0594. [DOI] [PubMed] [Google Scholar]
- 104.Kathryn A, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012;30:2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Network Atlas TCG. Integrated Genomic analyses of ovarian carcinoma. Nature. 2011;474:292–292. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cancer Genome Atlas Research Network. integrated genomic analyses of ovarian carcinoma. Nature474, 609–615 (2011). [DOI] [PMC free article] [PubMed]
- 107.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–1154. doi: 10.1158/2159-8290.CD-15-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ang YL, Tan DS. Development of PARP inhibitors in gynecological malignancies. Curr. Prob. Cancer. 2017;41:273–286. doi: 10.1016/j.currproblcancer.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 109.Donawho CK, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin. Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 110.Mirza MR, Pignata S, Ledermann JA. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann. Oncol. 2018;29:1366–1376. doi: 10.1093/annonc/mdy174. [DOI] [PubMed] [Google Scholar]
- 111.Gelmon KA, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 112.Fong PC, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 113.Lee CK, et al. Phase 1 trial of olaparib and oral cyclophosphamide in BRCA breast cancer, recurrent BRCA ovarian cancer, non-BRCA triple-negative breast cancer, and non-BRCA ovarian cancer. Br. J. Cancer. 2019;120:279–285. doi: 10.1038/s41416-018-0349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ang JE, et al. Efficacy of chemotherapy in BRCA1/2 mutation carrier ovarian cancer in the setting of PARP inhibitor resistance: a multi-institutional study. Clin. Cancer Res. 2013;19:5485–5493. doi: 10.1158/1078-0432.CCR-13-1262. [DOI] [PubMed] [Google Scholar]
- 115.Jonathan L, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 116.Matulonis UA, et al. Olaparib maintenance therapy in patients with platinum-sensitive, relapsed serous ovarian cancer and a BRCA mutation: overall survival adjusted for postprogression poly(adenosine diphosphate ribose) polymerase inhibitor therapy. Cancer. 2016;122:1844–1852. doi: 10.1002/cncr.29995. [DOI] [PubMed] [Google Scholar]
- 117.Ledermann J, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 118.Kaufman B, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Domchek SM, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol. Oncol. 2016;140:199–203. doi: 10.1016/j.ygyno.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pujade-Lauraine E, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 121.Moore K, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 122.Ray-Coquard I, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N. Engl. J. Med. 2019;381:2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 123.Liu JF, et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur. J. Cancer. 2013;49:2972–2978. doi: 10.1016/j.ejca.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu JF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karakashev S, et al. BET bromodomain inhibition synergizes with PARP inhibitor in epithelial ovarian cancer. Cell Rep. 2017;21:3398–3405. doi: 10.1016/j.celrep.2017.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang D, et al. Combined inhibition of PI3K and PARP is effective in the treatment of ovarian cancer cells with wild-type PIK3CA genes. Gynecol. Oncol. 2016;142:548–556. doi: 10.1016/j.ygyno.2016.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, et al. A phase 1 study optimizing the dosing of olaparib tablet formulation combined with cediranib in recurrent ovarian cancer. J. Clin. Oncol. 2015;33:5559–5559. [Google Scholar]
- 128.Liu JF, et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann. Oncol. 2019;30:551–557. doi: 10.1093/annonc/mdz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gockley AA, et al. Durable response in a woman with recurrent low-grade endometrioid endometrial cancer and a germline BRCA2 mutation treated with a PARP inhibitor. Gynecol. Oncol. 2018;150:219–226. doi: 10.1016/j.ygyno.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 130.Wilson RH, et al. A phase I study of intravenous and oral rucaparib in combination with chemotherapy in patients with advanced solid tumours. Br. J. Cancer. 2017;116:884–892. doi: 10.1038/bjc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kondrashova O, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7:984–998. doi: 10.1158/2159-8290.CD-17-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Drew Y, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br. J. Cancer. 2016;114:723–730. doi: 10.1038/bjc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Swisher EM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 134.Kristeleit R, et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin. Cancer Res. 2017;23:4095–4106. doi: 10.1158/1078-0432.CCR-16-2796. [DOI] [PubMed] [Google Scholar]
- 135.Oza AM, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 2017;147:267–275. doi: 10.1016/j.ygyno.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 136.Coleman RL, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–1961. doi: 10.1016/S0140-6736(17)32440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sandhu SK, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–892. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 138.Mirza MR, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 139.Fabbro M, et al. Efficacy and safety of niraparib as maintenance treatment in older patients (≥ 70 years) with recurrent ovarian cancer: Results from the ENGOT-OV16/NOVA trial. Gynecol. Oncol. 2019;152:560–567. doi: 10.1016/j.ygyno.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 140.González-Martín A, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 141.Moore KN, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–648. doi: 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed] [Google Scholar]
- 142.Mirza MR, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409–1419. doi: 10.1016/S1470-2045(19)30515-7. [DOI] [PubMed] [Google Scholar]
- 143.Konstantinopoulos PA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5:1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oza AM, et al. Quality of life in patients with recurrent ovarian cancer treated with niraparib versus placebo (ENGOT-OV16/NOVA): results from a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2018;19:1117–1125. doi: 10.1016/S1470-2045(18)30333-4. [DOI] [PubMed] [Google Scholar]
- 145.Burki TK. Veliparib for advanced ovarian cancer. Lancet Oncol. 2019;20:e616. doi: 10.1016/S1470-2045(19)30630-8. [DOI] [PubMed] [Google Scholar]
- 146.Coleman RL, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation— an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015;137:386–391. doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nishio S, et al. Phase 1 study of veliparib with carboplatin and weekly paclitaxel in Japanese patients with newly diagnosed ovarian cancer. Cancer Sci. 2017;108:2213–2220. doi: 10.1111/cas.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nishikawa T, et al. Phase 1 dose-escalation study of single-agent veliparib in Japanese patients with advanced solid tumors. Cancer Sci. 2017;108:1834–1842. doi: 10.1111/cas.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reiss KA, et al. A Phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin. Cancer Res. 2015;21:68–76. doi: 10.1158/1078-0432.CCR-14-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coleman RL, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 2019;381:2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kunos C, et al. A phase I-II evaluation of veliparib (NSC #737664), topotecan, and filgrastim or pegfilgrastim in the treatment of persistent or recurrent carcinoma of the uterine cervix: an NRG Oncology/Gynecologic Oncology Group study. Int. J. Gynecol. Cancer. 2015;25:484–492. doi: 10.1097/IGC.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thaker PH, et al. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: an NRG Oncology Study (NCT#01281852) Ann. Oncol. 2017;28:505–511. doi: 10.1093/annonc/mdw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dhawan MS, et al. Differential toxicity in patients with and without DNA repair mutations: phase i study of carboplatin and talazoparib in advanced solid tumors. Clin. Cancer Res. 2017;23:6400–6410. doi: 10.1158/1078-0432.CCR-17-0703. [DOI] [PubMed] [Google Scholar]
- 154.de Bono J, et al. Phase I, dose-escalation, two-part trial of the PARP Inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017;7:620–629. doi: 10.1158/2159-8290.CD-16-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lickliter, J. D. et al. A phase I dose-escalation study of BGB-290, a novel PARP1/2 selective inhibitor in patients with advanced solid tumors. Asco Meeting. (2016).
- 156.Friedlander M, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019;20:1306–1315. doi: 10.1016/S1470-2045(19)30396-1. [DOI] [PubMed] [Google Scholar]
- 157.Han Y, et al. Synergism of PARP inhibitor fluzoparib (HS10160) and MET inhibitor HS10241 in breast and ovarian cancer cells. Am. J. Cancer Res. 2019;9:608–618. [PMC free article] [PubMed] [Google Scholar]
- 158.Wang L, et al. Pharmacologic characterization of fluzoparib, a novel poly(ADP-ribose) polymerase inhibitor undergoing clinical trials. Cancer Sci. 2019;110:1064–1075. doi: 10.1111/cas.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang L, Wang Q, Xu Y, Han L. Advances in the treatment of ovarian cancer using PARP inhibitors and the underlying mechanism of resistance. Curr. Drug Targets. 2019;21:167–178. doi: 10.2174/1389450120666190925123507. [DOI] [PubMed] [Google Scholar]
- 160.Kubalanza K, Konecny GE. Mechanisms of PARP inhibitor resistance in ovarian cancer. Curr. Opin. Obstet. Gynecol. 2020;32:36–41. doi: 10.1097/GCO.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 161.Labidi-Galy SI, et al. Clinical factors associated with prolonged response and survival under olaparib as maintenance therapy in BRCA mutated ovarian cancers. Gynecol. Oncol. 2019;155:262–269. doi: 10.1016/j.ygyno.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 162.McCormick A, et al. Ovarian cancers harbor defects in nonhomologous end joining resulting in resistance to rucaparib. Clin. Cancer Res. 2017;23:2050–2060. doi: 10.1158/1078-0432.CCR-16-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Higuchi T, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol. Res. 2015;3:1257–1268. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Philip CA, et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer. 2017;17:638. doi: 10.1186/s12885-017-3639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Katso R, et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 166.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wullschleger S, Loewith R, Hall MNJC. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 168.Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin. Cell. Dev. Bio. 2014;36:79–90. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cheaib B, Auguste A, Leary A. The PI3K/Akt/mTOR pathway in ovarian cancer:therapeutic opportunities and challenges. Chinese J. Cancer. 2015;34:4–16. doi: 10.5732/cjc.014.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Papa A, et al. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell. 2014;157:595–610. doi: 10.1016/j.cell.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012;18:5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 172.Guo H, et al. The PI3K/AKT pathway and renal cell carcinoma. J. Genet. Genom. 2015;42:343–353. doi: 10.1016/j.jgg.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aziz SA, et al. Phosphatidylinositol-3-kinase as a therapeutic target in melanoma. Clin. Cancer Res. 2009;15:3029–3036. doi: 10.1158/1078-0432.CCR-08-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pivot X, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393:2591–2598. doi: 10.1016/S0140-6736(19)30653-1. [DOI] [PubMed] [Google Scholar]
- 175.Seiji M, Hiromasa K, Ryoko T, Tomoyuki SJGO. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015;137:173–179. doi: 10.1016/j.ygyno.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 176.Bugide S, et al. HPIP promotes epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer cells through PI3K/AKT pathway activation. Cell. Oncol. 2017;40:133–144. doi: 10.1007/s13402-016-0308-2. [DOI] [PubMed] [Google Scholar]
- 177.Ke Z, Caiping S, Qing Z, Xiaojing W. Sonic hedgehog-Gli1 signals promote epithelial-mesenchymal transition in ovarian cancer by mediating PI3K/AKT pathway. Med. Oncol. 2015;32:368. doi: 10.1007/s12032-014-0368-y. [DOI] [PubMed] [Google Scholar]
- 178.Mazloumi Gavgani F, et al. Class I phosphoinositide 3-kinase PIK3CA/p110alpha and PIK3CB/p110beta isoforms in endometrial cancer. Int. J. Mol. Sci. 2018;19:3931. doi: 10.3390/ijms19123931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bertelsen BI, et al. Molecular analysis of the PI3K-AKT pathway in uterine cervical neoplasia: frequent PIK3CA amplification and AKT phosphorylation. Int. J. Cancer. 2006;118:1877–1883. doi: 10.1002/ijc.21461. [DOI] [PubMed] [Google Scholar]
- 180.Kent CN, Guttilla Reed IK. Regulation of epithelial-mesenchymal transition in endometrial cancer: connecting PI3K, estrogen signaling, and microRNAs. Clin. Transl. Oncol. 2016;18:1056–1061. doi: 10.1007/s12094-016-1492-2. [DOI] [PubMed] [Google Scholar]
- 181.Oh KJ, Kalinina A, Park NH, Bagchi S. Deregulation of eIF4E: 4E-BP1 in differentiated human papillomavirus-containing cells leads to high levels of expression of the E7 oncoprotein. J. Virol. 2006;80:7079–7088. doi: 10.1128/JVI.02380-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang D, et al. Long non-coding RNA ANRIL indicates a poor prognosis of cervical cancer and promotes carcinogenesis via PI3K/Akt pathways. Biomed. Pharmacother. 2017;85:511–516. doi: 10.1016/j.biopha.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 183.Prasad SB, et al. PI3K/AKT pathway-mediated regulation of p27(Kip1) is associated with cell cycle arrest and apoptosis in cervical cancer. Cell. Oncol. 2015;38:215–225. doi: 10.1007/s13402-015-0224-x. [DOI] [PubMed] [Google Scholar]
- 184.Weigelt B, et al. PI3K pathway dependencies in endometrioid endometrial cancer cell lines. Clin. Cancer Res. 2013;19:3533–3544. doi: 10.1158/1078-0432.CCR-12-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.El-Kott AF, Shati AA, Al-Kahtani MA, Alqahtani S. Acylated ghrelin renders chemosensitive ovarian cancer cells resistant to cisplatin chemotherapy via activation of the PI3K/Akt/mTOR survival pathway. Anal. Cell. Pathol. 2019;2019:9627810. doi: 10.1155/2019/9627810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schrauwen S, et al. Dual blockade of PI3K/AKT/mTOR (NVP-BEZ235) and Ras/Raf/MEK (AZD6244) pathways synergistically inhibit growth of primary endometrioid endometrial carcinoma cultures, whereas NVP-BEZ235 reduces tumor growth in the corresponding xenograft models. Gynecol. Oncol. 2015;138:165–173. doi: 10.1016/j.ygyno.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 187.Fleming GF, et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a gynecologic oncology group study. Gynecol. Oncol. 2014;132:585–592. doi: 10.1016/j.ygyno.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oza AM, et al. Molecular correlates associated with a phase II study of temsirolimus (CCI-779) in patients with metastatic or recurrent endometrial cancer-NCIC IND 160. J. Clin. Oncol. 2006;24:3003–3003. [Google Scholar]
- 189.Santacana, M. et al. Biological effects of temsirolimus on the mTOR pathway in endometrial carcinoma: a Pharmacodynamic Phase II Study. Int. J. Gynecol. Cancer (2016). 10.1097/IGC.0000000000000715. Online ahead of print. [DOI] [PubMed]
- 190.Chon HS, et al. Phase I study of oral ridaforolimus in combination with paclitaxel and carboplatin in patients with solid tumor cancers. BMC Cancer. 2017;17:407. doi: 10.1186/s12885-017-3394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tsoref D, et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol. Oncol. 2014;135:184–189. doi: 10.1016/j.ygyno.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 192.Slomovitz BM, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Soliman PT, et al. Phase II study of everolimus, letrozole, and metformin in women with advanced/recurrent endometrial cancer. J. Clin. Oncol. 2016;34:5506–5506. [Google Scholar]
- 194.Slomovitz BM, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 2015;33:930–936. doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiu JW, et al. A phase I trial of ANG1/2-Tie2 inhibitor trebaninib (AMG386) and temsirolimus in advanced solid tumors (PJC008/NCImusical sharp9041) Investig. N. Drugs. 2016;34:104–111. doi: 10.1007/s10637-015-0313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Taylor SE, et al. Phase II study of everolimus (EV) and bevacizumab (BEV) in recurrent ovarian, peritoneal, and fallopian tube cancer. J. Clin. Oncol. 2016;34:5552–5552. doi: 10.1016/j.ygyno.2019.10.029. [DOI] [PubMed] [Google Scholar]
- 197.Aghajanian C, et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol. Oncol. 2018;150:274–281. [Google Scholar]
- 198.Basu B, et al. Vistusertib (dual m-TORC1/2 inhibitor) in combination with paclitaxel in patients with high-grade serous ovarian and squamous non-small-cell lung cancer. Ann. Oncol. 2018;29:1918–1925. doi: 10.1093/annonc/mdy245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mackay HJ, et al. Molecular determinants of outcome with mammalian target of rapamycin inhibition in endometrial cancer. Cancer. 2014;120:603–610. doi: 10.1002/cncr.28414. [DOI] [PubMed] [Google Scholar]
- 200.Aghajanian C, et al. A phase I, open-label, two-stage study to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral AKT inhibitor GSK2141795 in patients with solid tumors. Investig. N. Drugs. 2018;36:1016–1025. doi: 10.1007/s10637-018-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Winder A, et al. The allosteric AKT inhibitor, MK2206, decreases tumor growth and invasion in patient derived xenografts of endometrial cancer. Cancer Biol. Ther. 2017;18:958–964. doi: 10.1080/15384047.2017.1281496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu JF, et al. Results from a single arm, single stage phase II trial of trametinib and GSK2141795 in persistent or recurrent cervical cancer. Gynecol. Oncol. 2019;154:95–101. doi: 10.1016/j.ygyno.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 203.Westin SN, et al. Limited access safety lead-in of the MEK inhibitor trametinib in combination with GSK2141795, an AKT inhibitor, in patients with recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecol. Oncol. 2016;141:4–5. doi: 10.1016/j.ygyno.2019.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Westin SN, et al. Safety lead-in of the MEK inhibitor trametinib in combination with GSK2141795, an AKT inhibitor, in patients with recurrent endometrial cancer: an NRG Oncology/GOG study. Gynecol. Oncol. 2019;155:420–428. doi: 10.1016/j.ygyno.2019.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Myers AP, et al. Phase II, two-stage, two-arm, PIK3CA mutation stratified trial of MK-2206 in recurrent endometrial cancer (EC) J. Clin. Oncol. 2013;31:5524–5524. doi: 10.1002/ijc.32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Blagden SP, et al. Phase IB dose escalation and expansion study of AKT inhibitor afuresertib with carboplatin and paclitaxel in recurrent platinum-resistant ovarian cancer. Clin. Cancer Res. 2019;25:1472–1478. doi: 10.1158/1078-0432.CCR-18-2277. [DOI] [PubMed] [Google Scholar]
- 207.Bendell JC, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 208.Cao P, et al. PI3K p110alpha inhibition sensitizes cervical cancer cells with aberrant PI3K signaling activation to PARP inhibitor BMN673. Oncol. Rep. 2019;42:2097–2107. doi: 10.3892/or.2019.7313. [DOI] [PubMed] [Google Scholar]
- 209.Heudel PE, et al. Phase II study of the PI3K inhibitor BKM120 in patients with advanced or recurrent endometrial carcinoma: a stratified type I-type II study from the GINECO group. Br. J. Cancer. 2017;116:303–309. doi: 10.1038/bjc.2016.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Matulonis U, et al. Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with advanced or recurrent endometrial carcinoma. Gynecol. Oncol. 2015;136:246–253. doi: 10.1016/j.ygyno.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 211.Wainberg ZA, et al. A multi-arm phase i study of the PI3K/mTOR inhibitors PF-04691502 and gedatolisib (PF-05212384) plus irinotecan or the MEK inhibitor PD-0325901 in advanced cancer. Target Oncol. 2017;12:775–785. doi: 10.1007/s11523-017-0530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Del Campo JM, et al. A randomized phase II non-comparative study of PF-04691502 and gedatolisib (PF-05212384) in patients with recurrent endometrial cancer. Gynecol. Oncol. 2016;142:62–69. doi: 10.1016/j.ygyno.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 213.Roncolato F, et al. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2019;10:Cd012160. doi: 10.1002/14651858.CD012160.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kyriakopoulos CE, et al. A phase I study of tivantinib in combination with temsirolimus in patients with advanced solid tumors. Investig. N. Drugs. 2017;35:290–297. doi: 10.1007/s10637-016-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Normanno N, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 216.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 217.Zhang X, et al. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 218.Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp. C ell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 219.Christgen M, et al. Activating human epidermal growth factor receptor 2 (HER2) gene mutation in bone metastases from breast cancer. Virchows Arch. : Int. J. Pathol. 2018;473:577–582. doi: 10.1007/s00428-018-2414-1. [DOI] [PubMed] [Google Scholar]
- 220.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lau TS, et al. A loop of cancer-stroma-cancer interaction promotes peritoneal metastasis of ovarian cancer via TNFalpha-TGFalpha-EGFR. Oncogene. 2017;36:3576–3587. doi: 10.1038/onc.2016.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lin TC, et al. GALNT6 expression enhances aggressive phenotypes of ovarian cancer cells by regulating EGFR activity. Oncotarget. 2017;8:42588–42601. doi: 10.18632/oncotarget.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Slomovitz BM, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 2004;22:3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 224.Albagoush, S. A. & Limaiem, F. in StatPearls (StatPearls Publishing StatPearls Publishing LLC., 2019).
- 225.von Minckwitz G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Menderes G, et al. Efficacy of neratinib in the treatment of HER2/neu-amplified epithelial ovarian carcinoma in vitro and in vivo. Med. Oncol. 2017;34:91. doi: 10.1007/s12032-017-0956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Menderes G, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows promising antitumor activity in epithelial ovarian carcinoma with HER2/Neu expression. Gynecol. Oncol. 2017;146:179–186. doi: 10.1016/j.ygyno.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gianni L, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 229.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 230.Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 231.Agus DB, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 232.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xia W, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 234.Rawluk J, Waller CF. Gefitinib. Recent Results Cancer Res. 2018;211:235–246. doi: 10.1007/978-3-319-91442-8_16. [DOI] [PubMed] [Google Scholar]
- 235.Roskoski R., Jr Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol. Res. 2019;139:395–411. doi: 10.1016/j.phrs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 236.Voigtlaender M, Schneider-Merck T, Trepel M. Lapatinib. Recent Results Cancer Res. 2018;211:19–44. doi: 10.1007/978-3-319-91442-8_2. [DOI] [PubMed] [Google Scholar]
- 237.Konecny GE, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br. J. Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Buza N, English DP, Santin AD, Hui P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod. Pathol. 2013;26:1605–1612. doi: 10.1038/modpathol.2013.113. [DOI] [PubMed] [Google Scholar]
- 239.Bookman MA, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003;21:283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 240.Melissa H, Tasha SG, Tewari KS. A review of HER2-targeted therapy in breast and ovarian cancer: lessons from antiquity-CLEOPATRA and PENELOPE. Future Oncol. 2015;11:3113–3131. doi: 10.2217/fon.15.266. [DOI] [PubMed] [Google Scholar]
- 241.Cousin S, et al. Targeting ERBB2 mutations in solid tumors: biological and clinical implications. J. Hematol. Oncol. 2018;11:86. doi: 10.1186/s13045-018-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hyman DM, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554:189–194. doi: 10.1038/nature25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Petrelli F, Borgonovo K, Cabiddu M, Barni S. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small-cell lung cancer: a meta-analysis of 13 randomized trials. Clin. Lung cancer. 2012;13:107–114. doi: 10.1016/j.cllc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 244.Piccirillo MC, et al. D01*MITO (Multicentre Italian Trials in Ovarian cancer)—CERV 2 trial: a randomized phase II study of carboplatin and paclitaxel +/- cetuximab, in advanced and/or recurrent cervical cancer. Ann. Oncol. 2015;26:vi33–vi33. [Google Scholar]
- 245.Farley J, et al. Phase II study of cisplatin plus cetuximab in advanced, recurrent, and previously treated cancers of the cervix and evaluation of epidermal growth factor receptor immunohistochemical expression: a Gynecologic Oncology Group study. Gynecol. Oncol. 2011;121:303–308. doi: 10.1016/j.ygyno.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kurtz JE, et al. Cetuximab, topotecan and cisplatin for the treatment of advanced cervical cancer: A phase II GINECO trial. Gynecol. Oncol. 2009;113:16–20. doi: 10.1016/j.ygyno.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 247.Pignata S, et al. The MITO CERV-2 trial: A randomized phase II study of cetuximab plus carboplatin and paclitaxel, in advanced or recurrent cervical cancer. Gynecol. Oncol. 2019;153:535–540. doi: 10.1016/j.ygyno.2019.03.260. [DOI] [PubMed] [Google Scholar]
- 248.Secord AA, et al. Phase II trial of cetuximab and carboplatin in relapsed platinum-sensitive ovarian cancer and evaluation of epidermal growth factor receptor expression: a Gynecologic Oncology Group study. Gynecol. Oncol. 2008;108:493–499. doi: 10.1016/j.ygyno.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Fleming GF, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fader AN, et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor. Receptor 2/neu. 2018;36:2044–2051. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- 251.Yang Y, Lu Z-S, Zeng Z. Clinical efficacy and safety of combination of abraxane and trastuzumab in treatment of recurrent ovarian cancer. Pak. J. Pharm. Sci. 2018;31:2831–2834. [PubMed] [Google Scholar]
- 252.Makhija S, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J. Clin. Oncol. 2010;28:1215–1223. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 253.Lorusso D, et al. Patient-reported outcomes and final overall survival results from the randomized phase 3 PENELOPE trial evaluating pertuzumab in low tumor human epidermal growth factor receptor 3 (HER3) mRNA-expressing platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer. 2019;29:1141–1147. doi: 10.1136/ijgc-2019-000370. [DOI] [PubMed] [Google Scholar]
- 254.Kurzeder C, et al. Double-blind, placebo-controlled, randomized phase III trial evaluating pertuzumab combined with chemotherapy for low tumor human epidermal growth factor receptor 3 mRNA-expressing platinum-resistant ovarian cancer (PENELOPE) J. Clin. Oncol. 2016;34:2516–2525. doi: 10.1200/JCO.2015.66.0787. [DOI] [PubMed] [Google Scholar]
- 255.Kaye SB, et al. A randomized phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. Ann. Oncol. 2013;24:145–152. doi: 10.1093/annonc/mds282. [DOI] [PubMed] [Google Scholar]
- 256.Leslie KK, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol. Oncol. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Monk BJ, et al. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J. Clin. Oncol. 2010;28:3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 258.Garcia AA, et al. A phase II evaluation of lapatinib in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. Gynecol. Oncol. 2012;124:569–574. doi: 10.1016/j.ygyno.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lheureux S, et al. Expected benefits of topotecan combined with lapatinib in recurrent ovarian cancer according to biological profile: a phase 2 trial. Int. J. Gynecol. Cancer. 2012;22:1483–1488. doi: 10.1097/IGC.0b013e31826d1438. [DOI] [PubMed] [Google Scholar]
- 260.Weroha SJ, et al. Phase II trial of lapatinib and topotecan (LapTop) in patients with platinum-refractory/resistant ovarian and primary peritoneal carcinoma. Gynecol. Oncol. 2011;122:116–120. doi: 10.1016/j.ygyno.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nimeiri HS, et al. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol. Oncol. 2008;110:49–55. doi: 10.1016/j.ygyno.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Vergote IB, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J. Clin. Oncol. 2014;32:320–326. doi: 10.1200/JCO.2013.50.5669. [DOI] [PubMed] [Google Scholar]
- 263.Nogueira-Rodrigues A, et al. Phase 2 trial of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced cervical cancer. Cancer. 2014;120:1187–1193. doi: 10.1002/cncr.28471. [DOI] [PubMed] [Google Scholar]
- 264.Campos S, et al. Multicenter, randomized phase II trial of oral CI-1033 for previously treated advanced ovarian cancer. J. Clin. Oncol. 2005;23:5597–5604. doi: 10.1200/JCO.2005.08.091. [DOI] [PubMed] [Google Scholar]
- 265.Annunziata CM, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin. Cancer Res. 2010;16:664–672. doi: 10.1158/1078-0432.CCR-09-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Goncalves A, et al. A phase II trial to evaluate gefitinib as second- or third-line treatment in patients with recurring locoregionally advanced or metastatic cervical cancer. Gynecol. Oncol. 2008;108:42–46. doi: 10.1016/j.ygyno.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 267.Harter P, et al. Addition of vandetanib to pegylated liposomal doxorubicin (PLD) in patients with recurrent ovarian cancer. A randomized phase I/II study of the AGO Study Group (AGO-OVAR 2.13) Investig. N. Drugs. 2013;31:1499–1504. doi: 10.1007/s10637-013-0011-3. [DOI] [PubMed] [Google Scholar]
- 268.Sims AH, et al. Defining the molecular response to trastuzumab, pertuzumab and combination therapy in ovarian cancer. Br. J. Cancer. 2012;106:1779–1789. doi: 10.1038/bjc.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hassan W, Chitcholtan K, Sykes P, Garrill A. A combination of two receptor tyrosine kinase inhibitors, canertinib and PHA665752 compromises ovarian cancer cell growth in 3D cell models. Oncol. Ther. 2016;4:257–274. doi: 10.1007/s40487-016-0031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Su F, et al. SP1 promotes tumor angiogenesis and invasion by activating VEGF expression in an acquired trastuzumabresistant ovarian cancer model. Oncol. Rep. 2017;38:2677–2684. doi: 10.3892/or.2017.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Maekawa M, Nishida E, Tanoue T. Identification of the anti-proliferative protein Tob as a MAPK substrate. J. Biol. Chem. 2002;277:37783–37787. doi: 10.1074/jbc.M204506200. [DOI] [PubMed] [Google Scholar]
- 272.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat. Rev. Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 273.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 274.Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin. Cancer Res. 2008;14:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- 275.Zhong F, et al. Comprehensive genomic profiling of high-grade serous ovarian carcinoma from Chinese patients identifies co-occurring mutations in the Ras/Raf pathway withTP53. Cancer Med. 2019;8:3928–3935. doi: 10.1002/cam4.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Xu D, et al. Raf-ERK1/2 signalling pathways mediate steroid hormone synthesis in bovine ovarian granulosa cells. Reprod. Domest. Anim. = Zuchthyg. 2019;54:741–749. doi: 10.1111/rda.13419. [DOI] [PubMed] [Google Scholar]
- 277.Moorthy NS, Sousa SF, Ramos MJ, Fernandes PA. Farnesyltransferase inhibitors: a comprehensive review based on quantitative structural analysis. Curr. Medicinal Chem. 2013;20:4888–4923. doi: 10.2174/09298673113206660262. [DOI] [PubMed] [Google Scholar]
- 278.Meier W, et al. Randomized phase II trial of carboplatin and paclitaxel with or without lonafarnib in first-line treatment of epithelial ovarian cancer stage IIB-IV. Gynecol. Oncol. 2012;126:236–240. doi: 10.1016/j.ygyno.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 279.Wilhelm S, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 280.Ganesan P, et al. Phase I clinical trial of lenalidomide in combination with sorafenib in patients with advanced cancer. Investig. N. Drugs. 2014;32:279–286. doi: 10.1007/s10637-013-9966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Smolle E, Taucher V, Petru E, Haybaeck J. Targeted treatment of ovarian cancer-the multiple-kinase-inhibitor sorafenib as a potential option. Anticancer Res. 2014;34:1519–1530. [PubMed] [Google Scholar]
- 282.Siu LL, et al. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin. Cancer Res. 2006;12:144–151. doi: 10.1158/1078-0432.CCR-05-1571. [DOI] [PubMed] [Google Scholar]
- 283.Trump DLJUOS, Investigations O. 1. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;25:443–445. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 284.Haas NB, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kudo M, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018;3:424–432. doi: 10.1016/S2468-1253(18)30078-5. [DOI] [PubMed] [Google Scholar]
- 286.Bodnar L, Gornas M, Szczylik C. Sorafenib as a third line therapy in patients with epithelial ovarian cancer or primary peritoneal cancer: a phase II study. Gynecol. Oncol. 2011;123:33–36. doi: 10.1016/j.ygyno.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 287.Park GB, Ko HS, Kim D. Sorafenib controls the epithelialmesenchymal transition of ovarian cancer cells via EGF and the CD44HA signaling pathway in a cell typedependent manner. Mol. Med. Rep. 2017;16:1826–1836. doi: 10.3892/mmr.2017.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hainsworth JD, et al. Paclitaxel/carboplatin with or without sorafenib in the first-line treatment of patients with stage III/IV epithelial ovarian cancer: a randomized phase II study of the Sarah Cannon Research Institute. Cancer Med. 2015;4:673–681. doi: 10.1002/cam4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Herzog TJ, et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol. Oncol. 2013;130:25–30. doi: 10.1016/j.ygyno.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 290.Chekerov R, et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19:1247–1258. doi: 10.1016/S1470-2045(18)30372-3. [DOI] [PubMed] [Google Scholar]
- 291.Schwandt A, et al. Randomized phase II trial of sorafenib alone or in combination with carboplatin/paclitaxel in women with recurrent platinum sensitive epithelial ovarian, peritoneal, or fallopian tube cancer. Investig. N. Drugs. 2014;32:729–738. doi: 10.1007/s10637-014-0078-5. [DOI] [PubMed] [Google Scholar]
- 292.Milosevic MF, et al. Sorafenib increases tumor hypoxia in cervical cancer patients treated with radiation therapy: results of a phase 1 clinical study. Int J. Radiat. Oncol. Biol. Phys. 2016;94:111–117. doi: 10.1016/j.ijrobp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 293.Ho AL, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Janne PA, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 295.Chiu HC, et al. Epithelial to mesenchymal transition and cell biology of molecular regulation in endometrial carcinogenesis. J. Clin. Med. 2019;8:439. doi: 10.3390/jcm8040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ring KL, et al. Endometrial cancers with activating kras mutations have activated estrogen signaling and paradoxical response to MEK inhibition. Int. J. Gynecol. Cancer. 2017;27:854–862. doi: 10.1097/IGC.0000000000000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Coleman RL, et al. A phase II evaluation of selumetinib (AZD6244, ARRY-142886), a selective MEK-1/2 inhibitor in the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2015;138:30–35. doi: 10.1016/j.ygyno.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Farley J, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14:134–140. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bedard PL, et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin. Cancer Res. 2015;21:730–738. doi: 10.1158/1078-0432.CCR-14-1814. [DOI] [PubMed] [Google Scholar]
- 300.Groner B, von Manstein V. Jak Stat signaling and cancer: opportunities, benefits and side effects of targeted inhibition. Mol. Cell. Endocrinol. 2017;451:1–14. doi: 10.1016/j.mce.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 301.Borges S, et al. Involvement of a JAK/STAT pathway inhibitor: cytokine inducible SH2 containing protein in breast cancer. Adv. Exp. Med. Biol. 2008;617:321–329. doi: 10.1007/978-0-387-69080-3_30. [DOI] [PubMed] [Google Scholar]
- 302.Slattery ML, et al. JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Mol. Carcinog. 2013;52:155–166. doi: 10.1002/mc.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Passamonti F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18:88–99. doi: 10.1016/S1470-2045(16)30558-7. [DOI] [PubMed] [Google Scholar]
- 304.Shang AQ, et al. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer Biol. Ther. 2017;18:314–322. doi: 10.1080/15384047.2017.1310343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ruan Z, Yang X, Cheng W. OCT4 accelerates tumorigenesis through activating JAK/STAT signaling in ovarian cancer side population cells. Cancer Manag. Res. 2019;11:389–399. doi: 10.2147/CMAR.S180418. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 306.Han ES, et al. Therapeutic potential of ruxolitinib in human ovarian cancer. Gynecol. Oncol. 2016;141:45–46. [Google Scholar]
- 307.Wen W, et al. Increasing antitumor activity of JAK inhibitor by simultaneous blocking multiple survival signaling pathways in human ovarian cancer. Transl. Oncol. 2019;12:1015–1025. doi: 10.1016/j.tranon.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Basilico C, et al. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J. Biol. Chem. 2008;283:21267–21277. doi: 10.1074/jbc.M800727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 310.Gallo S, Sala V, Gatti S, Crepaldi T. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin. Sci. 2015;129:1173–1193. doi: 10.1042/CS20150502. [DOI] [PubMed] [Google Scholar]
- 311.Schmidt L, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 312.Lutterbach B, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res. 2007;67:2081–2088. doi: 10.1158/0008-5472.CAN-06-3495. [DOI] [PubMed] [Google Scholar]
- 313.Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 314.Kwon Y, et al. Effective inhibition of c-MET-mediated signaling, growth and migration of ovarian cancer cells is influenced by the ovarian tissue microenvironment. Oncogene. 2015;34:144–153. doi: 10.1038/onc.2013.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Han Z, et al. Alpha-ketobenzothiazole serine protease inhibitors of aberrant HGF/c-MET and MSP/RON kinase pathway signaling in cancer. ChemMedChem. 2016;11:585–599. doi: 10.1002/cmdc.201500600. [DOI] [PubMed] [Google Scholar]
- 316.Yamamoto S, et al. Gene amplification and protein overexpression of MET are common events in ovarian clear-cell adenocarcinoma: their roles in tumor progression and prognostication of the patient. Mod. Pathol. 2011;24:1146–1155. doi: 10.1038/modpathol.2011.70. [DOI] [PubMed] [Google Scholar]
- 317.Baykal C, et al. Overexpression of the c-Met/HGF receptor and its prognostic significance in uterine cervix carcinomas. Gynecol. Oncol. 2003;88:123–129. doi: 10.1016/s0090-8258(02)00073-2. [DOI] [PubMed] [Google Scholar]
- 318.Jardim DL, et al. Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson Phase I Clinic. Clin. Cancer Res. 2014;20:6336–6345. doi: 10.1158/1078-0432.CCR-14-1293. [DOI] [PubMed] [Google Scholar]
- 319.Yamashita Y, et al. Met is the most frequently amplified gene in endometriosis-associated ovarian clear cell adenocarcinoma and correlates with worsened prognosis. PLoS ONE. 2013;8:e57724. doi: 10.1371/journal.pone.0057724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wang H, et al. Expression and significance of CD44, CD47 and c-met in ovarian clear cell carcinoma. Int. J. Mol. Sci. 2015;16:3391–3404. doi: 10.3390/ijms16023391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li M, et al. HGF and c-Met in pathogenesis of endometrial carcinoma. Front. Biosci. 2015;20:635–643. doi: 10.2741/4328. [DOI] [PubMed] [Google Scholar]
- 322.Martin LP, et al. A phase II evaluation of AMG 102 (rilotumumab) in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2014;132:526–530. doi: 10.1016/j.ygyno.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Konstantinopoulos PA, et al. Phase II study of single-agent cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer (NRG-GY001) Gynecol. Oncol. 2018;150:9–13. doi: 10.1016/j.ygyno.2018.04.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Matulonis UA, et al. A randomized phase II study of cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2019;152:548–553. doi: 10.1016/j.ygyno.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yeatman TJ. A renaissance for SRC. Nat. Rev. Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 326.Pengetnze Y, et al. Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem. Biophys. Res. Commun. 2003;309:377–383. doi: 10.1016/j.bbrc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 327.Duxbury MS, et al. siRNA directed against c-Src enhances pancreatic adenocarcinoma cell gemcitabine chemosensitivity. J. Am. Coll. Surg. 2004;198:953–959. doi: 10.1016/j.jamcollsurg.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 328.Le XF, Bast RC., Jr Src family kinases and paclitaxel sensitivity. Cancer Biol. Ther. 2011;12:260–269. doi: 10.4161/cbt.12.4.16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol. Cancer Ther. 2005;4:217–224. [PubMed] [Google Scholar]
- 330.Simpkins F, et al. Dual Src and MEK inhibition decreases ovarian cancer growth and targets tumor initiating stem-like cells. Clin. Cancer Res. 2018;24:4874–4886. doi: 10.1158/1078-0432.CCR-17-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schilder RJ, et al. Phase II evaluation of dasatinib in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2012;127:70–74. doi: 10.1016/j.ygyno.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.McNeish IA, et al. A randomised, placebo-controlled trial of weekly paclitaxel and saracatinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancerdagger. Ann. Oncol. 2014;25:1988–1995. doi: 10.1093/annonc/mdu363. [DOI] [PubMed] [Google Scholar]
- 333.Takebe N, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Gomez-del Arco P, et al. Alternative promoter usage at the Notch1 locus supports ligand-independent signaling in T cell development and leukemogenesis. Immunity. 2010;33:685–698. doi: 10.1016/j.immuni.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zhou J, et al. Notch and TGFbeta form a positive regulatory loop and regulate EMT in epithelial ovarian cancer cells. Cell. Signal. 2016;28:838–849. doi: 10.1016/j.cellsig.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 336.Mamaeva V, et al. Inhibiting notch activity in breast cancer stem cells by glucose functionalized nanoparticles carrying gamma-secretase inhibitors. Mol. Ther. : J. Am. Soc. Gene Ther. 2016;24:926–936. doi: 10.1038/mt.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Shang C, Lang B, Meng LR. Blocking NOTCH pathway can enhance the effect of EGFR inhibitor through targeting CD133+ endometrial cancer cells. Cancer Biol. Ther. 2018;19:113–119. doi: 10.1080/15384047.2016.1250985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Chen C, et al. Prognostic roles of Notch receptor mRNA expression in human ovarian cancer. Oncotarget. 2017;8:32731–32740. doi: 10.18632/oncotarget.16387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Diaz-Padilla I, et al. A phase II study of single-agent RO4929097, a gamma-secretase inhibitor of Notch signaling, in patients with recurrent platinum-resistant epithelial ovarian cancer: a study of the Princess Margaret, Chicago and California phase II consortia. Gynecol. Oncol. 2015;137:216–222. doi: 10.1016/j.ygyno.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 340.Qiu Z, Oleinick NL, Zhang J. ATR/CHK1 inhibitors and cancer therapy. Radiother. Oncol. : J. Eur. Soc. Ther. Radiol. Oncol. 2018;126:450–464. doi: 10.1016/j.radonc.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Leijen S, et al. Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J. Clin. Oncol. 2016;34:4354–4361. doi: 10.1200/JCO.2016.67.5942. [DOI] [PubMed] [Google Scholar]
- 342.Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17:e254–e262. doi: 10.1016/S1470-2045(16)30030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Richardson DL, Seward SM, Moore KN. Antibody drug conjugates in the treatment of epithelial ovarian cancer. Hematol./Oncol. Clin. North Am. 2018;32:1057–1071. doi: 10.1016/j.hoc.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 344.Lee EK, Liu JF. Antibody-drug conjugates in gynecologic malignancies. Gynecol. Oncol. 2019;153:694–702. doi: 10.1016/j.ygyno.2019.03.245. [DOI] [PubMed] [Google Scholar]
- 345.Moore KN, et al. A review of mirvetuximab soravtansine in the treatment of platinum-resistant ovarian cancer. Future Oncol. 2018;14:123–136. doi: 10.2217/fon-2017-0379. [DOI] [PubMed] [Google Scholar]
- 346.Moore KN, et al. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRalpha)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 2018;151:46–52. doi: 10.1016/j.ygyno.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 347.Moore KN, et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in patients with solid tumors. Cancer. 2017;123:3080–3087. doi: 10.1002/cncr.30736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Ab O, et al. IMGN853, a folate receptor-alpha (FRalpha)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRalpha-expressing tumors. Mol. Cancer Ther. 2015;14:1605–1613. doi: 10.1158/1535-7163.MCT-14-1095. [DOI] [PubMed] [Google Scholar]
- 349.Cheung A, et al. Targeting folate receptor alpha for cancer treatment. Oncotarget. 2016;7:52553–52574. doi: 10.18632/oncotarget.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ponte JF, et al. Mirvetuximab soravtansine (IMGN853), a folate receptor alpha–targeting antibody-drug conjugate, potentiates the activity of standard of care therapeutics in ovarian cancer models. Neoplasia. 2016;18:775–784. doi: 10.1016/j.neo.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Moore KN, et al. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Phase I Expansion Study. J. Clin. Oncol. 2017;35:1112–1118. doi: 10.1200/JCO.2016.69.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Moore KN, et al. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Phase I Expansion Study. J. Clin. Oncol. 2017;35:1112–1118. doi: 10.1200/JCO.2016.69.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wolford JE, Tewari KS. Rational design for cervical cancer therapeutics: cellular and non-cellular based strategies on the horizon for recurrent, metastatic or refractory cervical cancer. Expert Opin. Drug Discov. 2018;13:445–457. doi: 10.1080/17460441.2018.1443074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.de Bono JS, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:383–393. doi: 10.1016/S1470-2045(18)30859-3. [DOI] [PubMed] [Google Scholar]
- 355.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 356.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature515, 563, (2014). [DOI] [PMC free article] [PubMed]
- 358.Le DT, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mok TSK, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 360.Rini BI, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 361.Hodi FS, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 362.Buderath P, et al. Prognostic significance of PD-1 and PD-L1 positive tumor-infiltrating immune cells in ovarian carcinoma. Int. J. Gynecol. Cancer. 2019;29:1389–1395. doi: 10.1136/ijgc-2019-000609. [DOI] [PubMed] [Google Scholar]
- 363.Pawlowska A, et al. Immunotherapies based on PD-1/PD-L1 pathway inhibitors in ovarian cancer treatment. Clin. Exp. Immunol. 2019;195:334–344. doi: 10.1111/cei.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Tan D, Sheng L, Yi QH. Correlation of PD-1/PD-L1 polymorphisms and expressions with clinicopathologic features and prognosis of ovarian cancer. Cancer Biomark. : Sect. A Dis. Markers. 2018;21:287–297. doi: 10.3233/CBM-170357. [DOI] [PubMed] [Google Scholar]
- 365.Chung HC, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 366.Makker V, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ott PA, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J. Clin. Oncol. 2017;35:2535–2541. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 368.Varga A, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol. Oncol. 2019;152:243–250. doi: 10.1016/j.ygyno.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 369.Frenel JS, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J. Clin. Oncol. 2017;35:4035–4041. doi: 10.1200/JCO.2017.74.5471. [DOI] [PubMed] [Google Scholar]
- 370.Fader AN, et al. Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer. Gynecol. Oncol. 2016;141:206–207. [Google Scholar]
- 371.Taylor MH, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J. Clin. Oncol. 2020;38:1154–1163. doi: 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Hao, Z. & Wang, P. Lenvatinib in management of solid tumors. Oncologist25, e302 (2019). [DOI] [PMC free article] [PubMed]
- 373.Matulonis UA, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019;30:1080–1087. doi: 10.1093/annonc/mdz135. [DOI] [PubMed] [Google Scholar]
- 374.Färkkilä A, et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat. Commun. 2020;11:1459. doi: 10.1038/s41467-020-15315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Naumann RW, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. J. Clin. Oncol. 2019;37:2825–2834. doi: 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Santin AD, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer ( NCT02257528/NRG-GY002) Gynecol. Oncol. 2020;157:161–166. doi: 10.1016/j.ygyno.2019.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Liu, J. F. et al. 937PDA phase II trial of combination nivolumab and bevacizumab in recurrent ovarian cancer. Ann. Oncol. 29, mdy285-146 (2018).
- 378.Hamanishi J, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 379.Fleming GF, et al. Clinical activity, safety and biomarker results from a phase Ia study of atezolizumab (atezo) in advanced/recurrent endometrial cancer (rEC) J. Clin. Oncol. 2017;35:5585–5585. [Google Scholar]
- 380.Konstantinopoulos PA, et al. Phase 2, two-group, two-stage study of avelumab in patients (pts) with microsatellite stable (MSS), microsatelite instable (MSI), and polymerase epsilon (POLE) mutated recurrent/persistent endometrial cancer (EC) J. Clin. Oncol. 2019;37:5502–5502. [Google Scholar]
- 381.Disis ML, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. J. Clin. Oncol. 2016;34:5533–5533. [Google Scholar]
- 382.Jiao S, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin. Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Lassen U. Combining PARP inhibition with PD-1 inhibitors. Lancet Oncol. 2019;20:1196–1198. doi: 10.1016/S1470-2045(19)30509-1. [DOI] [PubMed] [Google Scholar]
- 384.Westin SN, et al. Phase I trial of olaparib (PARP inhibitor) and vistusertib (mTORC1/2 inhibitor) in recurrent endometrial, ovarian and triple negative breast cancer. J. Clin. Oncol. 2018;36:5504–5504. [Google Scholar]
- 385.Lokich E, et al. Molecular markers in uterine serous cancer: correlation between endometrial biopsy and hysterectomy specimens. Gynecol. Oncol. Rep. 2019;29:98–101. doi: 10.1016/j.gore.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.van Kruchten M, et al. Hormone receptors as a marker of poor survival in epithelial ovarian cancer. Gynecol. Oncol. 2015;138:634–639. doi: 10.1016/j.ygyno.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 387.Zhang W, et al. Urolithin A suppresses the proliferation of endometrial cancer cells by mediating estrogen receptor-alpha-dependent gene expression. Mol. Nutr. Food Res. 2016;60:2387–2395. doi: 10.1002/mnfr.201600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.van Weelden WJ, Massuger L, Pijnenborg JMA, Romano A. Anti-estrogen treatment in endometrial cancer: a systematic review. Front. Oncol. 2019;9:359. doi: 10.3389/fonc.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Jerzak KJ, Duska L, MacKay HJ. Endocrine therapy in endometrial cancer: an old dog with new tricks. Gynecol. Oncol. 2019;153:175–183. doi: 10.1016/j.ygyno.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 390.Bogliolo S, et al. The role of fulvestrant in endometrial cancer. Expert Opin. Drug Metab. Toxicol. 2017;13:537–544. doi: 10.1080/17425255.2016.1244264. [DOI] [PubMed] [Google Scholar]
- 391.Blanco LZ,Jr, et al. Steroid hormone synthesis by the ovarian stroma surrounding epithelial ovarian tumors: a potential mechanism in ovarian tumorigenesis. Mod. Pathol. 2017;30:563–576. doi: 10.1038/modpathol.2016.219. [DOI] [PubMed] [Google Scholar]
- 392.Di Leo A, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 393.Emons G, et al. Phase II study of fulvestrant 250 mg/month in patients with recurrent or metastatic endometrial cancer: a study of the Arbeitsgemeinschaft Gynakologische Onkologie. Gynecol. Oncol. 2013;129:495–499. doi: 10.1016/j.ygyno.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 394.Covens AL, et al. Phase II study of fulvestrant in recurrent/metastatic endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2011;120:185–188. doi: 10.1016/j.ygyno.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 395.Argenta PA, et al. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol. Oncol. 2009;113:205–209. doi: 10.1016/j.ygyno.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 396.Gonzalez-Martin A, et al. Efficacy and safety results from OCTAVIA, a single-arm phase II study evaluating front-line bevacizumab, carboplatin and weeklypaclitaxel for ovarian cancer. Eur. J. Cancer. 2013;49:3831–3838. doi: 10.1016/j.ejca.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 397.Monk BJ, et al. Randomized phase II evaluation of bevacizumab versus bevacizumab plus fosbretabulin in recurrent ovarian, tubal, or peritoneal carcinoma: an NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016;34:2279–2286. doi: 10.1200/JCO.2015.65.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Chambers SK, et al. Overexpression of tumor vascular endothelial growth factor A may portend an increased likelihood of progression in a phase II trial of bevacizumab and erlotinib in resistant ovarian cancer. Clin. Cancer Res. 2010;16:5320–5328. doi: 10.1158/1078-0432.CCR-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Verschraegen CF, et al. Phase II study of bevacizumab with liposomal doxorubicin for patients with platinum- and taxane-resistant ovarian cancer. Ann. Oncol. 2012;23:3104–3110. doi: 10.1093/annonc/mds172. [DOI] [PubMed] [Google Scholar]
- 400.Musa F, et al. Phase II study of irinotecan in combination with bevacizumab in recurrent ovarian cancer. Gynecol. Oncol. 2017;144:279–284. doi: 10.1016/j.ygyno.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 401.Tew WP, et al. Randomized phase II trial of bevacizumab plus everolimus versus bevacizumab alone for recurrent or persistent ovarian, fallopian tube or peritoneal carcinoma: an NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2018;151:257–263. doi: 10.1016/j.ygyno.2018.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Tillmanns TD, et al. Phase II clinical trial of bevacizumab with albumin-bound paclitaxel in patients with recurrent, platinum-resistant primary epithelial ovarian or primary peritoneal carcinoma. Gynecol. Oncol. 2013;128:221–228. doi: 10.1016/j.ygyno.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 403.Eisenhauer EL, et al. A phase II study of gemcitabine, carboplatin and bevacizumab for the treatment of platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 2014;134:262–266. doi: 10.1016/j.ygyno.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 404.Aghajanian C, et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol. Oncol. 2018;150:274–281. [Google Scholar]
- 405.Alvarez EA, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2013;129:22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 406.Hagemann AR, et al. Phase II study of bevacizumab and pemetrexed for recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer. Gynecol. Oncol. 2013;131:535–540. doi: 10.1016/j.ygyno.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Richardson DL, et al. Paclitaxel with and without pazopanib for persistent or recurrent ovarian cancer: a randomized clinical trial. JAMA Oncol. 2018;4:196–202. doi: 10.1001/jamaoncol.2017.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Pignata S, Larusso D, Scambia G. MITO-11: A randomized multicenter phase II trial testing the addition of pazopanib to weekly paclitaxel in platinum resistant or -refractory advanced ovarian cancer (AOC) J. Clin. Oncol. 2014;32:5503–5503. [Google Scholar]
- 409.Karlan BY, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J. Clin. Oncol. 2012;30:362–371. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 410.Vergote I, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J. Clin. Oncol. 2013;31:5520–5520. [Google Scholar]
- 411.Audeh MW, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 412.Ledermann JA, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17:1579–1589. doi: 10.1016/S1470-2045(16)30376-X. [DOI] [PubMed] [Google Scholar]
- 413.Matulonis UA, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Ann. Oncol. 2016;27:1013–1019. doi: 10.1093/annonc/mdw133. [DOI] [PubMed] [Google Scholar]
- 414.Oza AM, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87–97. doi: 10.1016/S1470-2045(14)71135-0. [DOI] [PubMed] [Google Scholar]
- 415.Emons G, et al. Temsirolimus in women with platinum-refractory/resistant ovarian cancer or advanced/recurrent endometrial carcinoma. A phase II study of the AGO-study group (AGO-GYN8) Gynecol. Oncol. 2016;140:450–456. doi: 10.1016/j.ygyno.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 416.Behbakht K, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol. Oncol. 2011;123:19–26. doi: 10.1016/j.ygyno.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Einstein MH, et al. Phase II trial of temsirolimus and bevacizumab for initial recurrence of endometrial cancer. J. Clin. Oncol. 2012;30:5025–5025. [Google Scholar]
- 418.Oza AM, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J. Clin. Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Tinker AV, et al. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199) Gynecol. Oncol. 2013;130:269–274. doi: 10.1016/j.ygyno.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 420.Colon-Otero G, et al. Phase 2 trial of everolimus and letrozole in relapsed estrogen receptor-positive high-grade ovarian cancers. Gynecol. Oncol. 2017;146:64–68. doi: 10.1016/j.ygyno.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 421.Oza AM, et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J. Clin. Oncol. 2015;33:3576–3582. doi: 10.1200/JCO.2014.58.8871. [DOI] [PubMed] [Google Scholar]
- 422.Colombo N, et al. Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. Br J. Cancer. 2013;108:1021–1026. doi: 10.1038/bjc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Tsoref D, et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol. Oncol. 2014;135:184–189. doi: 10.1016/j.ygyno.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 424.Colombo N, et al. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer. J. Clin. Oncol. 2007;25:5516–5516. [Google Scholar]
- 425.Lucely C, et al. A pilot study of nimotuzumab plus single agent chemotherapy as second- or third-line treatment or more in patients with recurrent, persistent or metastatic cervical cancer. Cancer Biol. Ther. 2015;16:684–689. doi: 10.1080/15384047.2015.1026483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Santin AD, et al. Phase II trial of cetuximab in the treatment of persistent or recurrent squamous or non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol. Oncol. 2011;122:495–500. doi: 10.1016/j.ygyno.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Schilder RJ, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin. Cancer Res. 2005;11:5539–5548. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 428.Wagner U, et al. Gefitinib in combination with tamoxifen in patients with ovarian cancer refractory or resistant to platinum-taxane based therapy-a phase II trial of the AGO Ovarian Cancer Study Group (AGO-OVAR 2.6) Gynecol. Oncol. 2007;105:132–137. doi: 10.1016/j.ygyno.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 429.Hirte H, et al. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND.149) Gynecol. Oncol. 2010;118:308–312. doi: 10.1016/j.ygyno.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 430.Blank SV, et al. Erlotinib added to carboplatin and paclitaxel as first-line treatment of ovarian cancer: a phase II study based on surgical reassessment. Gynecol. Oncol. 2010;119:451–456. doi: 10.1016/j.ygyno.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Holmberg LA, Goff B, Veljovich D. Unexpected gastrointestinal toxicity from Docetaxel/Carboplatin/Erlotinib followed by maintenance Erlotinib treatment for newly diagnosed stage III/IV ovarian cancer, primary peritoneal, or fallopian tube cancer. Gynecol. Oncol. 2011;121:426. doi: 10.1016/j.ygyno.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 432.Schilder RJ, Sill MW, Yi-Chun L, Robert M. A phase II trial of erlotinib in recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Int. J. Gynecol. Cancer. 2009;19:929–933. doi: 10.1111/IGC.0b013e3181a83467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Vergote IB, et al. A phase 2 randomised discontinuation trial of cabozantinib in patients with ovarian carcinoma. Eur. J. Cancer. 2017;83:229–236. doi: 10.1016/j.ejca.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 434.McNeish IA, et al. A randomised, placebo-controlled trial of weekly paclitaxel and saracatinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer. Ann. Oncol. 2014;25:1988–1995. doi: 10.1093/annonc/mdu363. [DOI] [PubMed] [Google Scholar]
- 435.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Liu JF, et al. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol. Oncol. 2019;154:314–322. doi: 10.1016/j.ygyno.2019.05.021. [DOI] [PubMed] [Google Scholar]