Abstract
Robust epidemiological and biological evidence supports a causal link between prenatal Zika Virus (ZIKV) infection and congenital brain abnormalities including microcephaly. However, it remains uncertain if ZIKV infection in pregnancy also increases the risk for other adverse fetal and birth outcomes. In a prospective cohort study we investigated the influence of ZIKV on the prevalence of prematurity, low birth weight, small-for-gestational-age, and fetal death as well as microcephaly (i.e., overall and disproportionate) in the offspring of women attending a high-risk pregnancy clinic during the recent ZIKV outbreak in Brazil. During the recruitment period (01 March 2016–23 August 2017), urine samples were tested for ZIKV by RT-PCR from all women attending the high-risk pregnancy clinic at Jundiaí University Hospital and from the neonates after delivery. Of the 574 women evaluated, 44 (7.7%) were ZIKV RT-PCR positive during pregnancy. Of the 409 neonates tested, 19 (4.6%) were ZIKV RT-PCR positive in the first 10 days of life. In this cohort, maternal ZIKV exposure was not associated with increased risks of prematurity, low birth weight, small-for-gestational-age, or fetal death. However, relative to ZIKV-negative neonates, ZIKV-positive infants had a five-fold increased risk of microcephaly overall (RR 5.1, 95% CI 1.2–22.5) and a ten-fold increased risk of disproportionate microcephaly (RR 10.3, 95% CI 2.0–52.6). Our findings provide new evidence that, in a high-risk pregnancy cohort, ZIKV RT-PCR positivity in the neonate at birth is strongly associated with microcephaly. However, ZIKV infection during pregnancy does not appear to influence the risks of prematurity, low birth weight, small-for-gestational-age or fetal death in women who already have gestational comorbidities. The results suggest disproportion between neonatal head circumference and weight may be a useful screening indicator for the detection of congenital microcephaly associated with ZIKV infection.
Subject terms: Epidemiology, Risk factors
Introduction
Zika Virus (ZIKV) has been detected sporadically in entomological and immunologic surveillance studies across Africa and Southeast Asia since the 1950s1,2. This mosquito-borne infection, which was previously considered to cause only mild disease3, spread to the Americas in 20144,5. By August 2016, Brazil had reported 174,000 suspected and 78,500 confirmed cases of this flavivirosis, 5,500 of which were in the state of São Paulo6–8. The rapid dissemination of this single-stranded RNA virus and member of the Flaviviridae family9 in the Americas has been primarily attributed to the expansion of its principal vector, the mosquito Aedes aegypti10. ZIKV has also been shown to be transmitted sexually11,12 and has the potential to be transmitted in other body fluids, including breast milk13,14.
The association between maternal flavivirus infections and adverse birth outcomes has recently become more evident. For symptomatic dengue virus infections in pregnancy, a 2016 systematic review and meta-analysis15 reported positive associations with preterm delivery and low birth weight, while a matched case–control study in Brazil (2006–2012) found an increased risk of stillbirth16.
In the case of ZIKV, robust epidemiological and biological evidence now supports a causal link between prenatal ZIKV infection and a range of congenital brain abnormalities including microcephaly17–21. Case control study evidence from Recife in the Northeast of Brazil has shown that intra-uterine ZIKV exposure is associated with microcephaly22,23, and prospective cohort data in symptomatic women with suspected ZIKV in Rio de Janeiro have shown an association between congenital ZIKV infection and abnormal neurological examination and/or brain imaging at birth24,25, Studies quantifying the risk of other adverse fetal outcomes (i.e., prematurity, low birth weight, small-for-gestational age, and fetal death) associated with ZIKV infection during pregnancy are lacking.
Microcephaly occurs as the result of an insult that disturbs early brain growth26. In the case of ZIKV, microcephaly is associated with fetal brain disruption sequence (FBDS)27,28, a condition arising from a disturbance in brain tissue formation during the second or third trimester of pregnancy with subsequent fetal skull collapse resulting from decreased intracranial hydrostatic pressure29. Differentiation between proportionate and disproportionate microcephaly (i.e., disproportion between neonatal head circumference and weight) has been made in some ZIKV studies25,30, however its importance in helping to characterise the Congenital Zika Syndrome has still not been established.
The scientific community and the World Health Organization (WHO)20 have called for the urgent analysis of data from all ZIKV pregnancy cohorts to further understand the full spectrum of adverse outcomes associated with this infection in pregnancy; as well as maximise the availability of data for use in future meta-analyses31. In response to this call, this prospective cohort study in Jundiaí, São Paulo, compared the prevalence of five well-defined adverse fetal outcomes (i.e., prematurity, low birthweight, small-for-gestational-age, fetal death and microcephaly) in a group of ZIKV-positive and ZIKV-negative women and infants.
Methods
Study design and participants
This prospective cohort study from the Jundiaí Zika Cohort was initiated at Jundiaí University Hospital in São Paulo State, Brazil. The municipality of Jundiaí, located 60 km to the northwest of São Paulo city, has 409,000 inhabitants32 and one of the highest Human Development Indices of all the municipalities in the state33. The maternity department at the University Hospital is the only public maternity facility in the municipality and performs approximately 300–400 deliveries per month, an estimated two-thirds of the total births in Jundiaí34. The incidence and prevalence of ZIKV infection in Jundiaí during the study period has not been determined. However, in 2016, in the State of Sao Paulo, 9,845 cases of ZIKV were reported to the Brazilian Notifiable Disease Registry (SINAN)35. This would give a crude incidence of 0.2 cases of ZIKV per 1,000 inhabitants in the state of Sao Paulo in the year 2016. However, this is likely to be a gross underestimation as this figure only represents cases that accessed health services and were reported by health professionals, and, therefore, is likely to be biased to those with more severe clinical presentations.
During the recruitment period (1 March 2016–23 August 2017), all women attending the high-risk pregnancy clinic (i.e., due to the presence of risk factors threatening the life or health of the pregnant woman or her fetus)36,37 at Jundiaí University Hospital at any stage of pregnancy were considered eligible and offered the opportunity to participate in the study. Specifically, a pregnant woman was defined as high risk if she had sociodemographic risk factors (e.g., low or high BMI, extremes of age, drug use); had a previous adverse reproductive history (e.g., previous stillbirth, recurrent miscarriages); had a current obstetric illness (e.g., pre-eclampsia) or had an intercurrent disease during pregnancy (e.g., epilepsy, diabetes mellitus)37. The reasons for choosing this study population are explored in detail in our Cohort Profile38 but are summarised as follows (1) to try to maximise follow-up adherence, (2) to provide an appropriate location for the collection of samples and (3) to optimise newborn data by ensuring a large proportion would be born in Jundiai University Hospital. Over the duration of follow-up, clinical teams cared for pregnant women in accordance with standard Brazilian Ministry of Health protocols. At the time of enrolment, women of any stage of pregnancy were eligible to participate. The only exclusion criteria were women with life-threatening conditions and those with severe learning difficulties who could not give informed consent.
At enrolment, detailed demographic, medical and antenatal information, as well as examination findings were compiled by research nurses who interviewed the women and reviewed their antenatal records. Data was initially collected using an in-house data collection tool and subsequently, in August 2016, when a standardised tool was created by the WHO39,40, this was updated accordingly and immediately put into use. For the additional variables that were added from the WHO protocol, pregnant women who had been enrolled and had their baseline questionnaire administered prior to August 2016 were asked to answer any new questions, where possible, during follow-up visits. Data was entered digitally into the Cohort’s database (created using Salesforce™ Brasil online platform) and stored on a secured server.
Women had urine collected by research nurses at the time of recruitment and 2–3 weeks later for ZIKV detection. Subsequently, sample collection was repeated on a 2–3 monthly basis during routine antenatal visits. Trained volunteers carried out pre-arranged weekly follow-up telephone calls and asked the women whether they had experienced any symptoms consistent with ZIKV infection during that time. If symptoms were reported, the women were advised to report to the hospital for clinical review and collection of urine. Antenatal ultrasound scanning was carried out in months 3, 5, 7 and 8 in women who remained ZIKV negative and monthly in women who had a positive ZIKV RT-PCR and/or developed ZIKV-like symptoms during pregnancy according to the WHO definition41. All antenatal scans were carried out at the São Paulo Radiology Centre by sonographers specialising in fetal medicine and using Voluson 730 Expert/Voluson E6, GE equipment. At the time of delivery in hospital, both women and neonates had urine collected. Neonatal urine was collected using sterile urine bags.
Laboratory procedures
All laboratory procedures were performed on de-identified samples. In the present study, due to severe financial constraints, ZIKV detection was limited to analysis by nucleic acid amplification testing of maternal and neonatal urine specimens collected during gestation and 10 days post-partum. Studies have shown that ZIKV is consistently detectable in urine for at least several weeks while its presence in serum is of shorter duration9,42,43.
For ZIKV detection, total RNA was extracted from urine by the commercial QIAamp Viral RNA Kit (Qiagen®), following the manufacturer’s instructions and stored at − 80 °C until used. ZIKV specific reverse transcription (RT) and quantitative polymerase chain reaction (qPCR) were performed with GoTaq® 1-Step RT-qPCR System (Promega®) on ABI Prism 7,500 SDS Real-Time cycler (Applied Biosystems). The ZIKV specific primers and probes designed by Lanciotti et al.9, are complementary to the non-structural 5 Protein (polymerase). The RT cycle consisted of a 10 min cycle at 50 °C and a 15 min cycle at 95 °C. The PCR consisted of forty cycles of 15 s at 95 °C and a 1 min cycle at 60 °C. Three positive controls (RNA extracted from positive ZIKV samples) and two negative controls (H2O) were included. We considered positive samples those that presented a threshold cycle (Ct) lower than 38.5 (as per Lanciotti et al.)44. In cases where the results were inconclusive, repetitions were performed with serially diluted samples.
Anthropometric measures
Anthropometric measurements at birth (i.e., neonatal weight, length and head circumference) were obtained for all live-born infants, and the equipment used was consistent for all. Weight was assessed using digital scales, length using a recumbent baby length scale and head circumference using a standardised non-elastic tape measure. Z-scores for weight, length and head circumference were determined using the online Intergrowth calculator, which takes into account gestational age and sex45–47. Gestational age was estimated using first trimester ultrasound (USS) when available and by last menstrual period (LMP) when USS was unavailable. If USS or LMP-estimated gestational ages were not available, infant gender was missing, or anthropometric measures were not recorded at birth (i.e., if the neonate was delivered outside of the clinic), the infant and mother were excluded from the analytical cohort. Preterm birth was defined as any baby born alive before 37 completed weeks of pregnancy48. Low birth weight was defined as a birth weight of < 2500 g. Small-for-gestational-age (SGA) was defined as infants with birth weight z-scores of < − 1.28 at birth (equivalent to 10th percentile) and extreme SGA as a birth weight of < − 1.88 z-scores (equivalent to the 3rd percentile)49. Microcephaly was defined as a head circumference z-score of < − 2 and severe microcephaly as a z-score < − 350,51. Disproportionate microcephaly was defined, as per the National Birth Defects Network definition51, as a head circumference z-score of < − 2 with a proportionally normal birth weight z-score of > − 2. Fetal loss was defined as a death prior to expulsion or complete extraction from the pregnant woman at any gestational age as per WHO/ICD-1052.
Case definitions
Infants were considered to have been exposed to ZIKV during pregnancy if their mothers had at least one positive ZIKV RT-PCR urine sample during pregnancy and to have vertical ZIKV transmission if they had a positive ZIKV PCR urine sample within 10 days of birth53,54. Women were considered to be symptomatic for ZIKV if they met the WHO case definition for suspected ZIKV41, defined as a person presenting with rash and/or fever and at least one of the following signs or symptoms: arthralgia or arthritis or conjunctivitis (non-purulent/hyperaemic).
Statistical analysis
The sample size was calculated using an estimated prevalence of cases of microcephaly among neonates of ZIKV RT-PCR positive pregnant women of 2%. A final analytical cohort size of n = 531 would give 80% power to detect a crude relative risk of two with a probability of type I error (α) of 5%. Although initially the Jundiai Zika Cohort aimed to enrol 500 pregnant women, recruitment continued for longer than initially anticipated to try to capture possible seasonal differences in the incidence of ZIKV disease. Criteria for the final selection of women to be included in the analytical cohort for this particular study were women with high-risk pregnancies who were tested for ZIKV using RT-PCR and whose babies had anthropometric measures at birth. Categorical variables were compared among women and infants with positive and negative ZIKV RT-PCR results using the Chi-square test except where there were less than 5 in any cell in which case Fisher’s exact test was used. Measures of association (Crude Risk Ratios) and 95% confidence intervals were calculated directly by comparing the prevalence of adverse fetal outcomes in the ZIKV exposed and unexposed groups. All statistical analyses were carried out using STATA™ version 15.1 software.
Ethical approval and informed consent
This study received ethical approval by the research ethics committee of Jundiaí Medical School, protocol number 1446577 and methods were carried out specifically in accordance with the guidelines and regulations set thereby. Participating women provided written, informed consent for themselves and for future follow-up of their child.
Results
Study participants
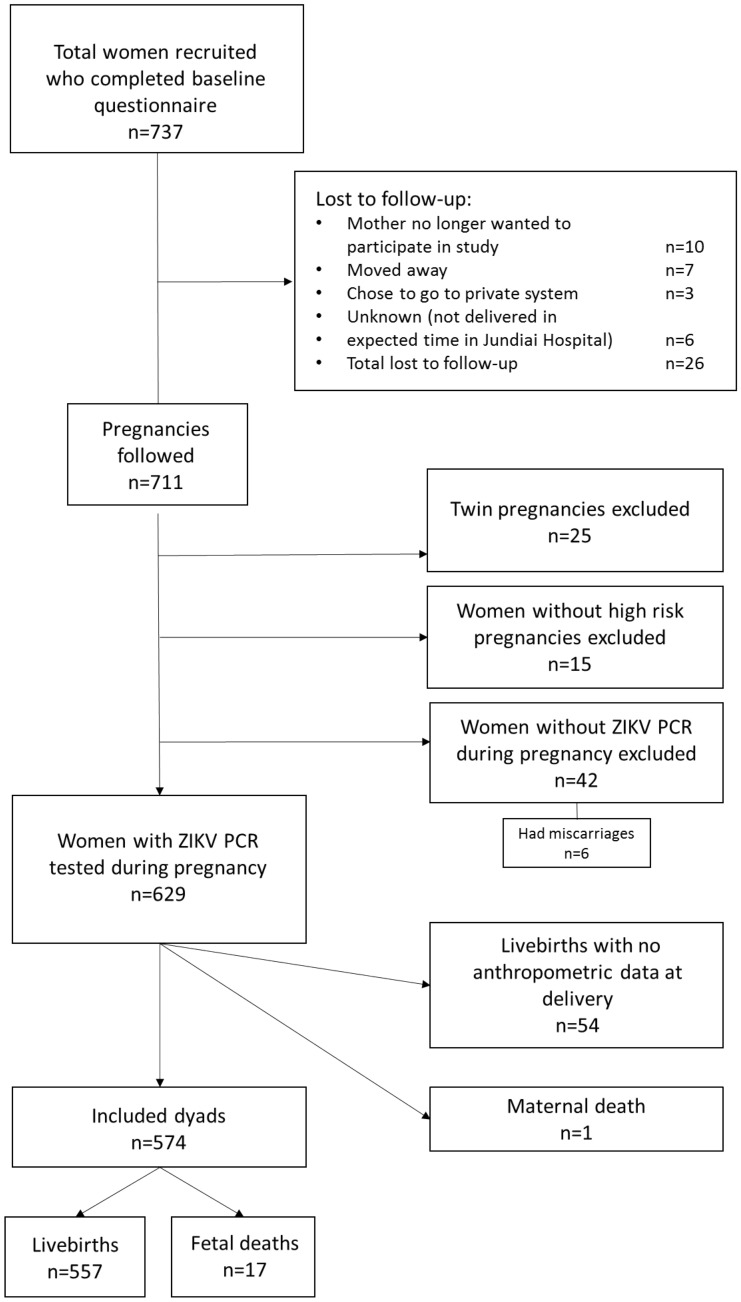
A total of 737 women were initially enrolled in the study between March 2016 and August 2017 and completed the baseline questionnaire. Of these, 26 were lost to follow-up (see Fig. 1). Women with missing data pertaining to risk factors and outcomes of interest were also excluded from this analysis. In addition, for the purposes of answering this particular study question, twin pregnancies and 15 women who were recruited because of suspected ZIKV infection but who did not have other risk factors were excluded from the analysis. This resulted in 695 women who remained eligible and were followed. At the end of the follow-up period 62 women, including 6 who had miscarried, had not had a urine ZIKV RT-PCR test during pregnancy. Of the 618 women whose urine was ZIKV tested during pregnancy, 42 of their infants did not have anthropometric information collected at birth and there was one maternal death. This resulted in a total of 574 dyads, comprising 557 livebirths and 17 fetal deaths, in the analytical cohort (Fig. 1). ZIKV testing following delivery was available from 409 neonates, 71.3% of the maternal cohort.
Figure 1.
Flow diagram showing participants of the Jundiai Zika Cohort at each stage of the study, recruitment period: 01 March 2016–23 August 2017, Jundiai, São Paulo, Brazil.
During the 126.4 person-years of pregnancy follow-up time (mean 11.5 weeks, maximum 34.4 weeks) 44 women (7.7%) had a positive ZIKV urine test during pregnancy. The majority (61.4%, n = 27) tested positive in the 3rd trimester (36.4%, n = 16 in the 2nd trimester, 2.3%, n = 1 in the 1st trimester).
The baseline characteristics were similar between the women with and without detectable ZIKV infections in pregnancy (Table 1). In both groups, the majority of women were aged 20–34 years, had completed high school but had not undertaken any higher education studies. The ethnic make-up of the cohort is consistent with the demographic profile of the state of São Paulo55; approximately half of the women self-identified as white, a third mixed race (i.e., “parda”), 10% black, and around 2% indigenous or Asian. The majority women were married or lived with their partner; 60% reported that their pregnancy was unplanned. The cohort Caesarean section rate approached 50%, comparable to the Caesarean section rates in high-risk pregnancies in the public healthcare system in Brazil56. The majority of women were recruited in the 3rd trimester of pregnancy. Almost one third of the women had a diagnosis of diabetes and around 20% had hypertension during pregnancy.
Table 1.
Maternal characteristics of participants of the Jundiaí Zika Cohort, March 2016 to August 2017, Jundiaí, São Paulo, Brazil.
| ZIKV RT-PCR positive women (n = 44) | ZIKV RT-PCR negative women (n = 530) | p-valuea | |
|---|---|---|---|
| Age | |||
| 13–19 years | 10 (22.7%) | 84 (15.9%) | 0.47 |
| 20–34 years | 26 (59.1%) | 331 (62.5%) | |
| 35–46 years | 8 (18.2%) | 115 (21.7%) | |
| Missing | 0 | 0 | |
| Education | |||
| ≤ 8 years | 9 (20.9%) | 90 (17.5%) | 0.76 |
| 9–11 years | 12 (27.3%) | 118 (22.9%) | |
| 12 years | 16 (36.4%) | 229 (44.5%) | |
| > 12 years | 6 (14.0%) | 78 (15.2%) | |
| Missing | 1 (2.3%) | 15 (2.8%) | |
| Ethnicity/race | |||
| White | 23 (52.3%) | 278 (53.8%) | 0.97* |
| Mixed race | 16 (36.4%) | 177 (34.2%) | |
| Black | 4 (9.1%) | 52 (10.1%) | |
| Other (Asian/indigenous) | 1 (2.3%) | 10 (1.9%) | |
| Missing | 0 | 13 (2.5%) | |
| Relationship with partner | |||
| Married/co-habiting | 35 (79.6%) | 395 (76.0%) | 0.60 |
| Single/divorced/widowed | 9 (20.5%) | 125 (24.0%) | |
| Missing | 0 | 10 (1.9%) | |
| Type of delivery | |||
| Vaginal/forceps | 23 (52.3%) | 257 (50.7%) | 0.86 |
| C-section | 21 (47.7%) | 250 (49.3%) | |
| Missing | 0 | 23 (4.3%) | |
| Trimester when recruited | |||
| 1st | 2 (4.7%) | 26 (5.0%) | 0.97* |
| 2nd | 15 (34.9%) | 186 (35.8%) | |
| 3rd | 26 (60.5%) | 308 (59.2%) | |
| Missing | 1 (2.3%) | 10 (1.9%) | |
| Diabetes | |||
| Yes | 14 (32.6%) | 165 (32.7%) | 0.99 |
| No | 29 (67.4%) | 340 (67.3%) | |
| Missing | 1 (2.3%) | 25 (4.7%) | |
| Hypertension | |||
| Yes | 8 (18.2%) | 102 (20.2%) | 0.75 |
| No | 36 (81.8%) | 403 (79.8%) | |
| Missing | 0 | 25 (4.7%) | |
Percentages for all categories were calculated with exclusion of those with missing data from the denominator.
aAll p-values calculated using Chi2 test except for those labelled with asterisk which were calculated using Fisher’s exact test. The ‘missing’ category was not included as a category when the p-value was estimated.
Fetal outcomes
In this high-risk pregnancy cohort, there was no significant difference in gestational age at birth between infants born to ZIKV positive or negative women (Table 2). Premature birth occurred in 9.1% of the ZIKV exposed and 13.3% of the unexposed women. Further, the proportion of early term (i.e., 37–38 weeks) and term and post-term (i.e., > 39 weeks) were similar in the exposed and unexposed groups.
Table 2.
Gestational age at birth in weeks of live-born infants in the Jundiaí Zika Cohort March 2016 to August 2017, Jundiaí, São Paulo, Brazil.
| Gestational age (completed weeks) | ZIKV exposed (n = 44) | ZIKV unexposed (n = 513) | Crude RR (95% CI) |
|---|---|---|---|
| < 37 (preterm) | 4 (9.1%) | 68 (13.3%) | 0.7 (0.3–1.8) |
| 37–38 (early term) | 21 (47.7%) | 222 (43.4%) | 1.1 (0.8–1.5) |
| ≥ 39 (term and post-term) | 19 (43.2%) | 222 (43.4%) | 1.0 (0.7–1.4) |
| Missing | 0 | 1 (0.2%) |
Low birth weight (< 2,500 g) was recorded for 9.1% of infants from ZIKV-positive women and 11.1% from ZIKV-negative women (Table 3). Defining SGA as birth weight z-score of < − 1.28, 9.1% and 9.7% of infants from ZIKV-positve and negative women, met this definition respectively.
Table 3.
Birth weight-related outcomes of infants born in the Jundiaí Zika Cohort from March 2016 to August 2017, Jundiaí, São Paulo, Brazil.
| Birth weight | ZIKV exposed (n = 44) | ZIKV unexposed (n = 513) | Crude RR (95% CI) | |
|---|---|---|---|---|
| Birthweight | VLBW (< 1,500 g) | 1 (2.3%) | 10 (1.9%) | 1.2 (0.2–8.9) |
| LBW (1,500–2,499 g) | 3 (6.8%) | 47 (9.2%) | 0.7 (0.24–2.3) | |
| Normal (2,500–4,000 g) | 39 (88.6%) | 435 (84.8%) | 1.0 (0.9–1.2) | |
| Large (> 4,000 g) | 1 (2.3%) | 21 (4.1%) | 0.6 (0.1–4.0) | |
| Total LBW | 4 (9.1%) | 57 (11.1%) | 0.8 (0.3–2.1) | |
| SGA | Extreme SGA (z-score < − 1.88) | 2 (4.5%) | 15 (2.9%) | 1.6 (0.4–6.6) |
| SGA (− 1.88 < z-score < − 1.28) | 2 (4.5%) | 36 (7.0%) | 0.6 (0.2–2.6) | |
| Not SGA | 40 (90.9%) | 463 (90.3%) | 1.0 (0.9–1.1) | |
| Total SGA | 4 (9.1%) | 50 (9.7%) | 0.9 (0.4–2.5) |
VLBW very low birth weight, LBW low birth weight, SGA small for gestational age (birth weight < − 1.28 z-scores).
All of the 17 women whose pregnancies ended in miscarriage or stillbirth, were ZIKV RT-PCR negative during gestation; it was not possible to obtain samples from these fetuses to determine their ZIKV status. Twelve infants (2.2%) were born with microcephaly, defined as a head circumference z-score of < − 2, and two (0.9%) of the infants had severe microcephaly (z-score < − 3). Microcephaly occurred in 4.5% infants born to ZIKV-positive women as compared to 1.9% of infants born to ZIKV-negative women (RR 2.3, 95% CI 0.5–10.3) (Table 4). Disproportionate microcephaly (defined as microcephaly with a birth weight z-score > − 2) occurred in two (4.5%) of the infants from ZIKV-positive women vs. versus five (1%) infants from ZIKV-negative women (RR 4.7 95% CI 0.9–23.3). Overall, adverse fetal outcomes occurred in 22.7% of pregnancies from ZIKV-positive women and 24.3% in ZIKV-negative women.
Table 5.
Prevalence and relative risk of adverse outcomes among infants with presumed congenital Zika Virus infection (infant ZIKV PCR positive at birth) in the Jundiai Zika Cohort from March 2016 to August 2017, Jundiaí, SP, Brazil.
| Infant ZIKV RT-PCR positive at birth (n = 19) | Infant ZIKV RT-PCR negative at birth (n = 390) | Crude RR (95% CI) | |
|---|---|---|---|
| All adverse outcomes | 4 (21.1%) | 86 (22.1%) | 1.0 (0.4–2.3) |
| SGA | 2 (10.5%) | 42 (10.8%) | |
| LBW | 2 (10.5%) | 38 (9.7%) | (0.3–3.7) |
| Microcephaly | 2 (10.5%) | 8 (2.1%) | |
| Disproportionate | 2 (10.5%) | 4 (1.0%) | 1.1 (0.3–4.1) |
| Proportionate | 0 | 4 | 5.1 (1.2–22.5) |
| Preterm | 1 (5.3%) | 48 (12.3%) | 10.3 (2.0–52.6) |
Categories are not mutually exclusive. Microcephaly was defined as infants with head circumference z-scores of < − 2 at birth. Severe microcephaly was defined as head circumference z-score of < − 3 at birth. Proportionate microcephaly was defined as infants with both head circumference and birth weight z-scores of < − 2 at birth and disproportionate microcephaly as head circumference z-score of < − 2 with birth weight z-score of > − 2.
SGA small for gestational age (birth weight < 10th percentile for sex and gestational age or < − 1.28 z-scores), LBW low birth weight (birth weight < 2,500 g). Babies who had a positive ZIKV PCR within 10 days of birth were considered to be positive for this analysis.
Table 4.
Prevalence and relative risk of adverse outcomes among infants exposed (maternal ZIKV PCR positive) and unexposed to Zika Virus during pregnancy in the Jundiai Zika Cohort from March 2016 to August 2017, Jundiaí, SP, Brazil.
| Variable | ZIKV RT-PCR positive women (n = 44) | ZIKV RT-PCR negative women (n = 513) | Crude RR (95% CI) |
|---|---|---|---|
| All negative outcomes | 10 (22.7%) | 129 (24.3%) | 0.9 (0.5–1.6) |
| SGA | 4 (9.1%) | 50 (9.7%) | 0.9 (0.4–2.5) |
| LBW | 4 (9.1%) | 57 (11.1%) | 0.8 (0.3–2.1) |
| Microcephaly | 2 (4.5%) | 10 (1.9%) | 2.3 (0.5–10.3) |
| Disproportionate | 2 (4.5%) | 5 (1.0%) | 4.7 (0.9–23.3) |
| Proportionate | 0 | 5 (0.8%) | – |
| Preterm | 4 (9.1%) | 68 (13.3%) | 0.7 (0.3–1.8) |
| Fetal death | 0 | 17 (3.3%) | – |
Categories are not mutually exclusive. Microcephaly was defined as infants with head circumference z-scores < − 2 at birth. Severe microcephaly was defined as head circumference z-score of < − 3 at birth. Proportionate microcephaly was defined as infants with both head circumference and birth weight z-scores of < − 2 at birth and disproportionate microcephaly as head circumference z-score of < − 2 with birth weight z-score of > − 2. SGA = small for gestational age (birth weight < 10th percentile for sex and gestational age or < − 1.28 z-scores).
LBW low birth weight (birthweight < 2,500 g).
For the 409 neonates with known ZIKV RT-PCR status, 19 (4.6%) had a positive ZIKV RT-PCR in the first 10 days of life. This analysis showed that the risk of microcephaly among neonates with detectable ZIKV RT-PCR at birth was five times the risk compared to the ZIKV-negative neonates (RR 5.1, 95% CI 1.2–22.5). Additionally, the risk of disproportionate microcephaly among neonates with positive ZIKV RT-PCR at birth was ten times the risk compared to neonates with negative ZIKV RT-PCR (RR 10.3, 95% CI 2.0–52.6). There were no statistical differences between the outcomes of preterm birth, low birth weight and SGA according to infant ZIKV RT-PCR status at birth. Overall, adverse outcomes occurred in 4 (21.1%) infants with positive ZIKV RT-PCR after birth compared to 86 (22.1%) infants with negative ZIKV RT-PCR after birth.
Discussion
Using data collected prospectively in the Jundiaí Zika Cohort during the 2015–2017 outbreak of ZIKV in Brazil, the results of this study demonstrate that neonates who are positive for ZIKV in their urine within 10 days of birth have a fivefold greater risk of having microcephaly and a tenfold increased risk of having disproportionate microcephaly as compared to ZIKV-negative neonates. Conversely, we observed no differences in the risks of preterm birth, low birth weight or SGA in infants with or without detectable ZIKV in urine. These results aligned with our findings in comparing fetal and birth outcomes in the offspring of pregnant women with and without detectable ZIKV in their urine during pregnancy. Overall, our findings provide preliminary evidence that aside from the central nervous system sequelae, ZIKV does not appear to have substantive deleterious effects on pregnancy progression or fetal growth in high risk pregnancies.
Microcephaly was detected in a minority of ZIKV-positive neonates and in even a smaller percentage of babies from ZIKV-positive women. This low rate of progression to microcephaly is consistent with other studies carried out in Rio de Janeiro25, the French territories in the Americas30 and the USA57–59. Also similar to the Rio de Janeiro study, no significant differences were noted in our cohort between ZIKV-positive and negative groups in rates of preterm birth or SGA. In our study, fetal demise was a rare outcome that was only observed in ZIKV-negative women. Further analysis of large study populations and meta-analyses are needed to confirm that ZIKV is not typically a cause of fetal demise.
The strengths of our study are its prospective design, large sample size, utilization of a well-defined study population (attending a single high volume referral centre), the fact that women were recruited independently of them having ZIKV symptoms (providing us with a large comparison group) and the fact that all women received equivalent care under the same team of physicians.
The limitations of the study in part relate to the pressing nature of the ZIKV and microcephaly epidemic and the urgency to start the investigation, like many other cohorts in Brazil, prior to formal funding being secured.
The recruitment of only high-risk pregnant women brought advantages, as discussed earlier, in both logistics and maximisation of follow-up rates, however, these advantages also introduced limitations in external validity when generalising findings to the general pregnant population of Brazil. Moreover, as recruitment was carried out in a specific population of pregnant women that were users of a particular health service, it is possible that there are systematic differences between those recruited and those not recruited, which we were not able to measure, and these may have introduced bias. However, when comparing the sociodemographic profile of the pregnant women in our cohort and the profile of pregnant women living and using public maternity facilities in the State of São Paulo at the time of the study, we can see that they are quite similar. Additional comparative investigations are needed to determine if maternal and/or congenital ZIKV infection increases the risk of adverse pregnancy outcomes in standard risk pregnancies.
Another study limitation relates to the narrow window in which ZIKV can be detected in body fluids. In this study, urine samples were prioritised as they have been shown to be superior to blood samples in their window for ZIKV RT-PCR detection9,42,43. However, it is possible that some women in our cohort (particularly those who were asymptomatic) were indeed infected with ZIKV during their gestation but their viral load may have become undetectable by RT-PCR by the time their urine was collected and analysed. This may have contributed to the observed lack of concordance in ZIKV detection between women and their babies (Table S3) and the identification of microcephaly in babies from women who were ZIKV-negative during pregnancy. Overall, these results reinforce the conclusion that has also been reached by other studies, that the absence of ZIKV detection in urine and other biological fluids is not definitive for the absence of infection60,61. The concomitant use of serology, not available in the present investigation, may have provided additional validation of maternal exposure to this virus, although serological tests for ZIKV antibody also have significant inherent problems, notably cross-reactivity with other flaviviruses62, and the fact that individuals previously exposed to DENV do not mount a ZIKV IgM response61.
Ultimately, it is possible that this led to an underestimation of the risk of adverse outcomes related to ZIKV infection during pregnancy as the extent of exposure to ZIKV among pregnant women in the study may have been underestimated. However, if this was the case, our effects measures would have in fact been larger and more significant.
An additional limitation to our analysis is the absence of toxoplasmosis, rubella, cytomegalovirus, Herpes virus, syphilis, and parvovirus B19 (TORCH) antibody screening in our population. Negative findings would have offered stronger evidence that microcephaly in our cohort was due exclusively to ZIKV infection. However, a similar study carried out over the same time period in Paraíba in the Northeast of Brazil found that a substantial attributable risk of microcephaly was due to ZIKV (35–87% of microcephaly occurring during the time of the investigation was attributable to ZIKV)63.
In summary, our results show that microcephaly, and more specifically disproportionate microcephaly, is associated with congenital ZIKV infection. Nevertheless, exposure to ZIKV does not appear to increase the risk of other adverse fetal outcomes above the baseline risks observed in ZIKV-negative women with high risk pregnancies. The results suggest disproportion between neonatal head circumference and weight may be a useful screening indicator for detection of congenital microcephaly associated with ZIKV infection.
Supplementary information
Acknowledgements
We are grateful to the contribution of the rest of the Jundiaí Zika Cohort Group including Alexandra Siqueira Melo, Ana Paula Antuns Pascalicchio Bertozzi, Anderson Pereira Soares, André Prado Grion, Andrea Cristina Botelho Silva, Antônio Fernandes Moron, Clóvis Antônio Lopes Pinto, Cristiane Martins, Danila Soares Tambalo, Danila Vedovello, Diego Lima, Dora Fix Ventura, Eduardo Massad, Eduardo Roberto Bagni, Fabiana Martins Soares de Souza, Fernanda Cangerana, Fernanda Guerra Velasco, Fernando N. Arita, Francisco del Moral Hernandez, Geovanne Ribeiro dos Santos, Hérbene José Figuinha Milani, Heydi Segundo Tabares, Juliana Paula Gomes de Almeida, Kallene Vidal, Luiz Baran, Magda Maria Sales Carneiro Sampaio, Mayana Zatz , Marcelo Costa, Márcia Borges Machado, Margareth Martha Arilha Silva, Maria de Fátima Rizzo, Maria Manoela Duarte Rodrigues, Marielton P. Cunha, Max Damico, Mirella Barboni, Mirella Nayane B. Leite, Patricia Carvalho Loiola, Paolo Marinho de Andrade Zanotto, Rafael Izbicki, Renata Chrystina Bianchi de Barros, Rita de Cássia Aguirre B. Dezena, Renato Pereira de Souza, Sandra Helena Alves Bonon, Shahab Zaki Pour, Sergio Rosemberg, Sergio Vranjec, Silvia Maria Ribeiro Oyama, Stéphanno Gomes Pereira Sarmento, Tânia Mendes Quintella , Tânia Ritti Ferraretto, Tathiana Ghisi de Souza, Thamirys Cosmo Gillo Fajardo, Valtenice França and Waldinei Merces Rodrigues.
Author contributions
N.S.C.: conceptualization, data curation, formal analysis, methodology, investigation, validation, visualization, writing-original draft and review and editing. E.B.B.: methodology, resources, supervision, validation, visualization, writing-review and editing. E.S.P.: formal analysis, methodology, supervision, validation, visualization, writing-review and editing. M.F.A.: formal analysis, funding acquisition, methodology, resources, supervision, validation, visualization, writing-review and editing. R.E.G.: conceptualization, investigation, project administration, resources, supervision. L.C.R.: methodology, resources, funding acquisition. S.D.P.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation.
Data availability
The data that support the findings of this study are available on request from the Principal Investigator [SP]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-69235-0.
References
- 1.Bell BP, Boyle CA, Petersen LR. Preventing Zika virus infections in pregnant women: An urgent public health priority. Am. J. Public Health. 2016;106:589–590. doi: 10.2105/ajph.2016.303124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faye O, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014;8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao-Lormeau VM, et al. Guillain–Barre syndrome outbreak associated with Zika virus infection in French Polynesia: A case–control study. Lancet (London, England) 2016;387:1531–1539. doi: 10.1016/s0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ECDC. Rapid Risk Assessment. Zika virus epidemic in the Americas: Potential association with microcephaly and Guillain–Barre syndrome. (Stockholm, 2015).
- 5.Albuquerque M, et al. The microcephaly epidemic and Zika virus: Building knowledge in epidemiology. Cad. Saude Publ. 2018;34:e00069018. doi: 10.1590/0102-311x00069018. [DOI] [PubMed] [Google Scholar]
- 6.Pan American Health Organisation . Zika Suspected and Confirmed Cases Reported by Countries and Territories in the Americas Cumulative Cases, 2015–2016. Washington: PAHO/WHO; 2016. [Google Scholar]
- 7.Pan American Health Organisation . Zika—Epidemiological Report Brazil. Washington: PAHO/WHO; 2017. [Google Scholar]
- 8.Secretaria de Vigilancia em Saude—Ministerio da Saude—Brasil. Boletim Epidemiologico. Brasilia: Ministry of Health, Brazil, 2016. https://portalarquivos2.saude.gov.br/images/pdf/2016/setembro/16/2016-028---Dengue-SE32.pdf
- 9.Lanciotti RS, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman EB, Kramer LD. Zika virus mosquito vectors: Competence, biology, and vector control. J. Infect. Dis. 2017;216:S976–s990. doi: 10.1093/infdis/jix405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Ortenzio E, et al. Evidence of sexual transmission of Zika virus. N. Engl. J. Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- 12.Moreira J, Peixoto TM, de Siqueira AM, Lamas CC. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. 2017 doi: 10.1016/j.cmi.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Cavalcanti MG, et al. Zika virus shedding in human milk during lactation: An unlikely source of infection? Int. J. Infect. Dis. 2017;57:70–72. doi: 10.1016/j.ijid.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Colt S, et al. Transmission of Zika virus through breast milk and other breastfeeding-related bodily-fluids: A systematic review. PLoS Negl. Trop. Dis. 2017;11:e0005528. doi: 10.1371/journal.pntd.0005528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paixao ES, Teixeira MG, Mda CC, Rodrigues LC. Dengue during pregnancy and adverse fetal outcomes: A systematic review and meta-analysis. Lancet Infect. Dis. 2016;16:857–865. doi: 10.1016/s1473-3099(16)00088-8. [DOI] [PubMed] [Google Scholar]
- 16.Paixao ES, et al. Symptomatic dengue infection and the risk of stillbirth in Brazil, 2006–2012: A matched case–control study. Lancet Infect. Dis. 2017;17:957–964. doi: 10.1016/S1473-3099(17)30366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects-reviewing the evidence for causality. N. Engl. J. Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 18.Paixao ES, Barreto F, Mda GT, Mda CC, Rodrigues LC. History, epidemiology, and clinical manifestations of Zika: A systematic review. Am. J. Public Health. 2016;106:606–612. doi: 10.2105/ajph.2016.303112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues LC. Microcephaly and Zika virus infection. Lancet (London, England) 2016;387:2070–2072. doi: 10.1016/s0140-6736(16)00742-x. [DOI] [PubMed] [Google Scholar]
- 20.Costello A, et al. Defining the syndrome associated with congenital Zika virus infection. Bull. World Health Organ. 2016;94:406–406a. doi: 10.2471/blt.16.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besnard M, et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro. Surveill. 2016 doi: 10.2807/1560-7917.es.2016.21.13.30181. [DOI] [PubMed] [Google Scholar]
- 22.de Araujo TV, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case–control study. Lancet Infect. Dis. 2016 doi: 10.1016/s1473-3099(16)30318-8. [DOI] [PubMed] [Google Scholar]
- 23.de Araujo TV, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: Final report of a case–control study. Lancet Infect. Dis. 2018;18:328–336. doi: 10.1016/S1473-3099(17)30727-2. [DOI] [PubMed] [Google Scholar]
- 24.Brasil P, et al. Zika virus infection in pregnant women in Rio de Janeiro—Preliminary report. N. Engl. J. Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brasil P, et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashwal S, Michelson D, Plawner L, Dobyns WB. Practice parameter: Evaluation of the child with microcephaly (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73:887–897. doi: 10.1212/WNL.0b013e3181b783f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corona-Rivera JR, et al. Report and review of the fetal brain disruption sequence. Eur. J. Pediatr. 2001;160:664–667. doi: 10.1007/s004310100813. [DOI] [PubMed] [Google Scholar]
- 28.da Silva AAM, et al. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg. Infect. Dis. 2016;22:1953–1956. doi: 10.3201/eid2211.160956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore CA, Weaver DD, Bull MJ. Fetal brain disruption sequence. J. Pediatr. 1990;116:383–386. doi: 10.1016/S0022-3476(05)82825-2. [DOI] [PubMed] [Google Scholar]
- 30.Hoen B, et al. Pregnancy outcomes after ZIKV infection in French Territories in the Americas. N. Engl. J. Med. 2018;378:985–994. doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- 31.Wilder-Smith A, et al. Understanding the relation between Zika virus infection during pregnancy and adverse fetal, infant and child outcomes: A protocol for a systematic review and individual participant data meta-analysis of longitudinal studies of pregnant women and their infants and children. BMJ Open. 2019;9:e026092. doi: 10.1136/bmjopen-2018-026092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brazilian Institute of Geography and Statistics (IBGE). Cidades. Jundiai. Panorama. 2017. https://cidades.ibge.gov.br/brasil/sp/jundiai/panorama. Accessed 20 Nov 2017.
- 33.Prefeitura Jundiai (Jundiai local government). Jundiai. A Cidade. Perfil. 2015. https://www.jundiai.sp.gov.br/a-cidade/perfil/. Accessed 22 Nov 2017
- 34.DATASUS—Departamento de Informatica do SUS. Informações de Saúde—Estatísticas vitais—nascidos vivos. Ministry of Health, Brasilia (2015).
- 35.Centro de Vigilância Epidemiológica “Prof. Alexandre Vranjac” Situação epidemiológica das arboviroses no estado de São Paulo: Dengue; Chikungunya; Zika vírus. BEPA. 2016;13(147):31–37. [Google Scholar]
- 36.NIH—Eunice Kennedy Shriver National Institute of Child Health and Human Development. High Risk Pregnancy. 2017. https://www.nichd.nih.gov/health/topics/high-risk. Accessed 27 Mar 2018.
- 37.Ministerio da Saúde. Gestante de Alto Risco. Brasilia: Ministerio da Saúde, 2001. https://bvsms.saude.gov.br/bvs/publicacoes/gestantes.pdf
- 38.Sanchez Clemente N, et al. Cohort profile: the Jundiaí Zika cohort (JZC), a pregnancy and birth cohort in São Paulo state, Brazil. BMJ Open. 2019;9:e027947. doi: 10.1136/bmjopen-2018-027947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organisation. Harmonization of ZIKV research protocols to address key public health concerns. Geneva; 2016. https://www.who.int/reproductivehealth/zika/ZIKV-Protocol-Summary-for-WHO-v4-2.pdf [DOI] [PubMed]
- 40.Van Kerkhove MD, et al. Harmonisation of Zika virus research protocols to address key public health concerns. Lancet Glob. Health. 2016;4:e911–e912. doi: 10.1016/s2214-109x(16)30255-8. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organisation. Zika virus disease—Interim case definition. Emergency Preparedness Response. (Geneva, 2016).
- 42.Bingham AM, et al. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease—Florida, 2016. MMWR Morb. Mortal Wkly. Rep. 2016;65:475–478. doi: 10.15585/mmwr.mm6518e2. [DOI] [PubMed] [Google Scholar]
- 43.Waehre T, Maagard A, Tappe D, Cadar D, Schmidt-Chanasit J. Zika virus infection after travel to Tahiti, December 2013. Emerg. Infect. Dis. 2014;20:1412–1414. doi: 10.3201/eid2008.140302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, Ldel CS. Phylogeny of Zika Virus in Western Hemisphere, 2015. Emerg. Infect. Dis. 2016;22:933–935. doi: 10.3201/eid2205.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Intergrowth-21st. INTERGROWTH-21st Newborn Size at Birth Chart. 2015. https://intergrowth21.tghn.org/articles/intergrowth-21st-newborn-size-birth-chart/. Accessed 07 Oct 2016.
- 46.Intergrowth-21st. Newborn biometry by Intergrowth-21st standards/references. Online calculator. 2018. https://intergrowth21.ndog.ox.ac.uk.
- 47.Villar J, Cheikh Ismail L, Victora CG, et al. International standard for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet (London, England) 2014;6(384):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organisation. Preterm Birth. 2017. https://www.who.int/mediacentre/factsheets/fs364/en/.
- 49.World Health Organisation. Intrauterine growth retardation in newborn children. 2018. https://who.int/ceh/indicators/iugrnewborn.pdf.
- 50.World Health Organisation. Screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero. Geneva; 2016. https://apps.who.int/iris/bitstream/10665/204475/1/WHO_ZIKV_MOC_16.3_eng.pdf?ua=1
- 51.National Birth Defects Prevention Network. Congenital Microcephaly. 2016. https://www.nbdpn.org/docs/NBDPN_Case_Definition_-_Surveillance_Microcephaly_2016Feb28_Final_DRAFT.pdf
- 52.World Health Organisation. International classification of diseases, and related health problems. 10th revision. In (ed. WHO) 1–1243. (Geneva, Switzerland, 1993).
- 53.National Center on Birth Defects and Developmental Disabilities Centers for Disease Control and Prevention . Zika and Pregnancy—Evaluation and Testing. Atlanta: CDC; 2018. [Google Scholar]
- 54.Krow-Lucal ER, Biggerstaff BJ, Staples JE. Estimated incubation period for Zika Virus disease. Emerg. Infect. Dis. 2017;23:841–845. doi: 10.3201/eid2305.161715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brazilian Institute of Geography and Statistics (IBGE). Características Étnico-raciais da População. Rio de Janeiro: Ministério do Planejamento, Desenvolvimento e Gestão, 2013. https://biblioteca.ibge.gov.br/visualizacao/livros/liv63405.pdf
- 56.Reis Z, et al. Association between risk pregnncy and route of delivery with maternal and neonatal outcomes. Rev. Bras. Ginecol. Obstet. 2014;36:65–71. doi: 10.1590/S0100-72032014000100004. [DOI] [PubMed] [Google Scholar]
- 57.Honein MA, et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317:59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- 58.Pomar L, et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: Prospective cohort study in French Guiana. BMJ (Clin. Res. Ed.) 2018;363:k4431. doi: 10.1136/bmj.k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shapiro-Mendoza CK, et al. Pregnancy outcomes after maternal zika virus infection during pregnancy—US Territories, January 1, 2016–April 25, 2017. MMWR Morbid. Mortal. Wkly. Rep. 2017;66:615–621. doi: 10.15585/mmwr.mm6623e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barzon L, et al. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin. Infect. Dis. 2018;66:1173–1180. doi: 10.1093/cid/cix967. [DOI] [PubMed] [Google Scholar]
- 61.Petridou C, et al. Zika virus infection in travellers returning to the United Kingdom during the period of the outbreak in the Americas (2016–2017): A retrospective analysis. Travel. Med. Infect. Dis. 2019;29:21–27. doi: 10.1016/j.tmaid.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Stettler K, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science (New York, N.Y.) 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 63.Krow-Lucal E, et al. Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraíba, Brazil, in 2015–2016: A case–control study. Lancet Child Adolesc. Health. 2018;2:205–213. doi: 10.1016/S2352-4642(18)30020-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the Principal Investigator [SP]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.