Abstract
Amidoallyl cations are appealing three-carbon synthons for the preparation of complex amine-containing carbocycles; however, methods to generate and utilize these reactive species are limited and underexplored compared to those for oxallyl cations. Here we disclose a bioinspired strain-driven ring opening of bicyclic methyleneaziridines to 2-amidopentadienyl cation intermediates that readily engage in Nazarov cyclizations. Advantages of this strategy include ease of generation and improved reactivity compared to 3-pentadienyl cations, control over the ultimate position of the alkene, the potential for high dr between vicinal stereocenters, and the ability to further elaborate the products to fully substituted aminocyclopentanes. Experimental and computational studies support a dual role for the Rh2Ln complex as both a nitrene transfer catalyst and a Lewis acid promoter, insight that provides a framework for the future development of asymmetric 2-imino-Nazarov cyclizations.
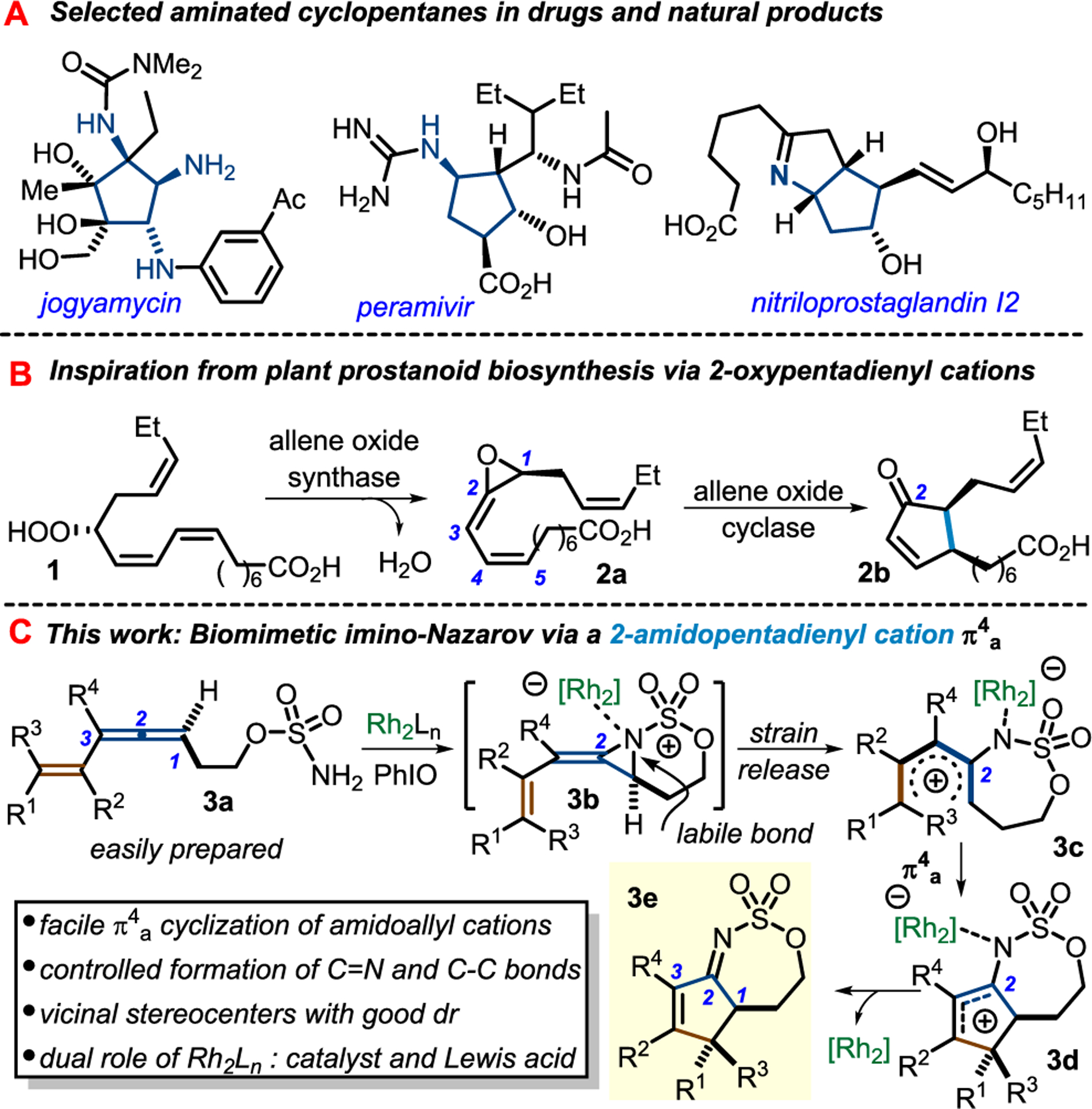
Flexible methods to construct functionalized, densely substituted carbocycles have long been of intense interest to the synthetic community, as these motifs occur frequently in useful bioactive molecules and natural products. For example, amine-bearing cyclopentanes are found in diverse natural products and pharmaceuticals, including the antiprotozoal compound jogyamycin, the anti-influenza drug peramivir, and nitriloprostaglandin I2 (PGI2), which is used in the treatment of arteriosclerosis, cardiac failure, and thrombosis (Scheme 1A).
Scheme 1.
Background and Proposed 2-Imino-Nazarov Reaction
The aza version of the classic Nazarov cyclization1 of divinyl ketones to prepare 2-cyclopenten-1-imines is challenging. This is due to better stabilization of the key pentadienyl cation in a “3-imino-Nazarov” reaction compared with the 3-oxyallyl cation intermediates implicated in a typical Nazarov reaction.2 Tius, Hsung, West, Huang, and others have reported creative solutions to overcome this issue;3 nonetheless, reaction development has been hampered by the dearth of methods for convenient generation of 3-amidoallyl cation intermediates.4 The aza-Piancatelli reaction, which moves the N to C1, has also been used to address this challenge;5 however, it benefits from a “push–pull” enol–iminium intermediate that ultimately furnishes the amine-substituted cyclopentenone product.
Our work was inspired by the biosynthesis of epi-jasmonic acid (Scheme 1B), which proceeds via a Nazarov-type electrocyclization of allene oxide 2a to furnish α,β-unsaturated cyclopentenone 2b. This enzyme-catalyzed process readily controls the site of unsaturation in the product and yields excellent dr and ee of the new vicinal stereocenters.6 We envisaged that a nitrogen version of this process could be readily mimicked by a tandem allene aziridination/electro- cyclization (Scheme 1C) from easily obtained eneallenes of the form 3a. Rh2-catalyzed nitrene transfer yields conjugated bicyclic methyleneaziridine 3b, analogous to 2a. The ring strain inherent in 3b (~35 kcal/mol) provides kinetically competent access to versatile 2-amidoallyl cation intermediate 3c without the need for stoichiometric Lewis or Brønsted acids7 or competing formation of iminocyclopropanes.7d,f Additionally, the modified 2-N positioning in 3c reduces stabilization of the pentadienyl cation relative to traditional 3-amidopentadienyl cations to facilitate productive cyclization. A 4π conrotatory electrocyclization of 3c to 3d and subsequent loss of the Rh complex furnishes 3e, ideally with high dr. The intermediacy of 3d enables the formation of 3e with predictable positioning of the alkene, as substrate-controlled elimination present in the typical Nazarov reaction is not operative here. In fact, no elimination or tautomerization is required from 3d in this oxidative manifold, which avoids loss of stereochemical information between the vicinal stereocenters installed in the conrotatory cyclization of 3c.8 This Communication describes our “2-imino-Nazarov” reaction, including factors influencing the mechanism and stereo-selectivity of the cyclization, the elaboration of the products to increase molecular complexity from simple precursors, and potential strategies to secure enantioenriched products.
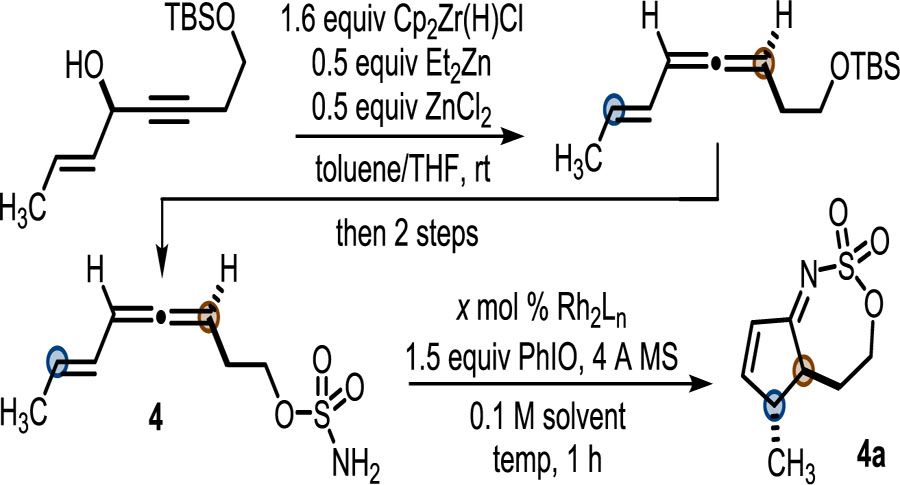
Hydrozirconation/Zr=O elimination of allyl propargyl alcohol with Schwartz’s reagent enables rapid access to eneallene 4 (Table 1).9 Preliminary studies found that sulfamates were the best nitrene precursors and that a two-carbon tether between the allene and the sulfamate was optimal. Nitrene transfer conditions previously reported by our group served as a starting point for investigating the feasibility of the 2-imino-Nazarov reaction of 4.7a Catalytic Rh2(TPA)4 (TPA = triphenylacetate) transformed 4 to 4a in 24% yield with >19:1 dr (entry 1). Varying the concentration (entries 2 and 3) had little impact; however, increasing the temperature to 50 °C (entry 1 vs 4) improved the yield of 4a to 41%. Adding the catalyst last, as opposed to the oxidant, was not beneficial (entry 5).
Table 1.
Selected Optimization Studies
 | |||||
|---|---|---|---|---|---|
| entry | solvent | catalyst (mol %) | temp (°C) | yield (%)a | notes |
| 1 | CH2CI2 | Rh2(TPA)4 (1) | rt | 24 | 15% rsm |
| 2 | CH2Cl2 (0.2 M) | Rh2(TPA)4 (1) | rt | 28 | – |
| 3 | CH2Cl2 (0.05 M) | Rh2(TPA)4 (1) | rt | 33 | – |
| 4 | CH2Q2 | Rh2(TPA)4 (1) | 50 | 41 | – |
| 5 | CH2Q2 | Rh2(TPA)4 (1) | 50 | 33 | catalyst added last |
| 6 | CH2Q2 | Rh2(TPA)4 (1) | 50 | 21 | PhI(OAc)2 |
| 7 | MeNO2 | Rh2(TPA)4 (1) | 45 | 23 | – |
| 8 | CH2CI2 | Rh2(TPA)4 (5) | 50 | 55 | LUMO = −2.90 eV |
| 9 | CH2CI2 | Rh2(TPA)4 (5) | 50 | 58 | 0.05 equiv of PhIO × 3, added every 20 min |
| 10 | CICH2CH2CI | Rh2(TPA)4 (5) | 80 | 49 | – |
| 11 | CH2CI2 | Rh2(OAc)4 (5) | 50 | 42 | LUMO = −2.68 eV |
| 12 | CH2CI2 | Rh2(esp)2 (5) | 50 | 41 | LUMO = −2.71 eV |
NMR yields using mesitylene as an internal standard.
Switching the oxidant to PhI(OAc)2 (entry 6) or the solvent to MeNO2 (entry 7) gave low yields of 21% and 23%, respectively. However, increasing the loading of Rh2(TPA)4 to 5 mol % (entries 8 and 9) in CH2Cl2 improved the yield of 4a to 58%; further increases in temperature in DCE (entry 10) were not beneficial. Interestingly, Rh2(OAc)4 (entry 11) and Rh2(esp)2 (entry 12) were inferior to Rh2(TPA)4. Computations of the LUMO energies for Rh2(TPA)4 (–2.90 eV), Rh2(OAc)4 (−2.68 eV), and Rh2(esp)2 (−2.71 eV) suggested that Lewis acidity of the catalyst was important to reaction success (see the Supporting Information (SI) for details).
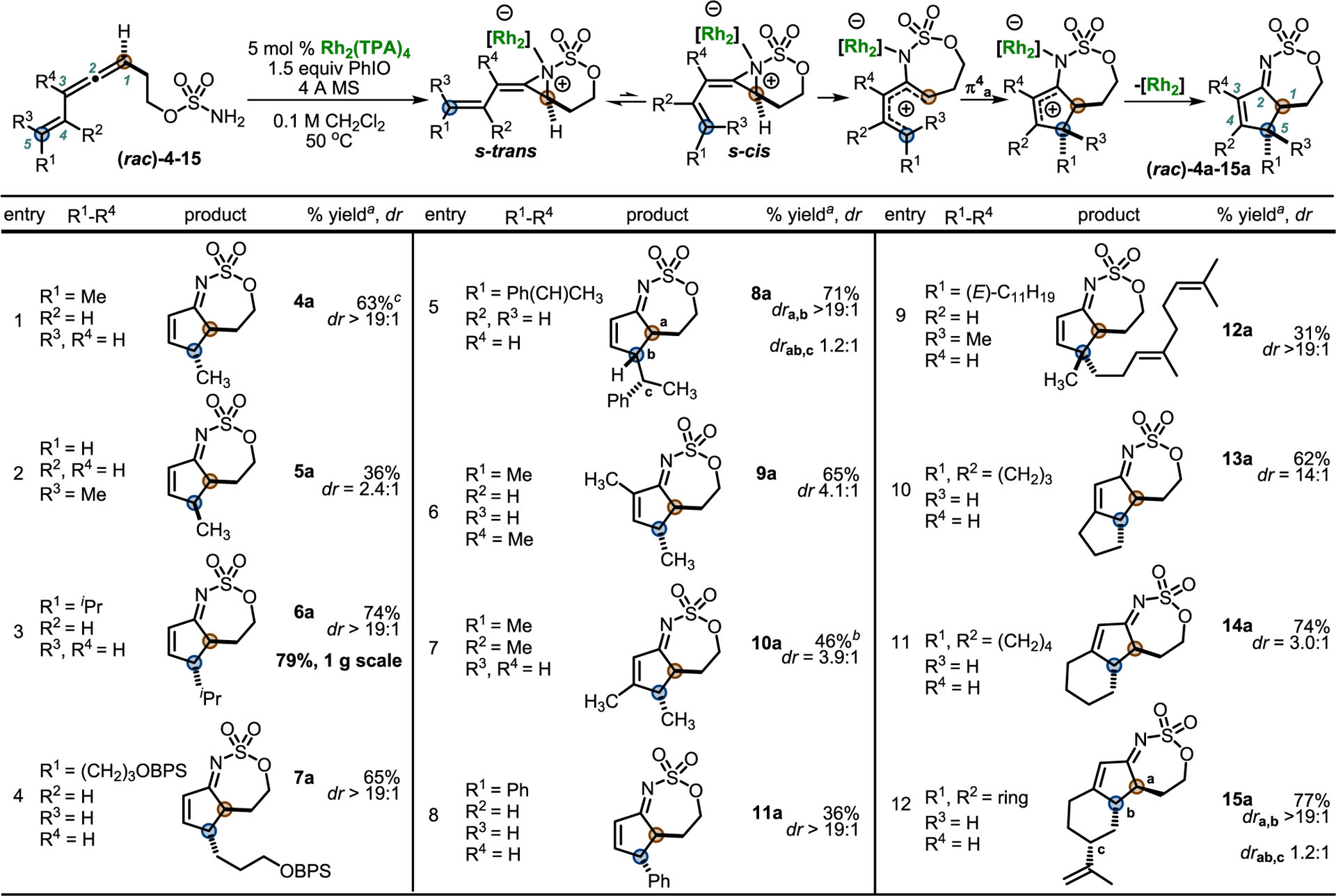
With the optimized conditions in hand, the scope of the tandem allene aziridination/2-imino-Nazarov reaction was explored (Table 2). All of the substrates were either racemic or a 1:1 racemic mixture of diastereomers. Freshly prepared 4 provided 4a in 63% yield with >19:1 dr, bearing an anti relationship between the hydrogens at the two newly formed stereocenters through a thermally allowed conrotatory 4π electrocyclization (Table 2, entry 1). Engaging cis-eneallene 5 gave 5a in moderate yield (entry 2), favoring the syn isomer (NOESY-NMR studies detailed in the SI); we considered that the lower dr compared to 4a could result from isomerization of the alkene geometry in 5, partial epimerization of 5a under the reaction conditions, or a competing “non-Nazarov” pathway. Control experiments confirmed that the alkene of 5 does not isomerize in the presence of Rh2TPA4 or PhIO, while epimerization of 5a is unlikely, as we would expect a similar dr for 4a if this pathway were operative. Isomerization of intermediate species or a competing non-Nazarov pathway cannot be ruled out.
Table 2.
Scope of the Tandem Allene Aziridination/2-Imino-Nazarov Reaction
|
Isolated yields
Using 1 mol % Rh2(TPA)4.
The yield decreased as allene aged.
Isopropyl substitution was tolerated in 6 to give 6a in 74% yield with excellent dr (entry 3). To our delight, the preparation of 6a could be run on a 1 g scale to give a 79% yield with >19:1 dr. Protected alcohols were suitable precursors, with 7 giving 7a in 65% yield with >19:1 dr (entry 4). The ability of the external stereocenter in 8 to control the dr in the all-carbon stereotriad in 8a was investigated (entry 5); while the dr between a and b was >19:1, the dr between a/b and c was only moderately improved to 1.2:1.
Substitution at C3 (R4) and C4 (R2) in 9 and 10 (entries 6 and 7) gave 9a and 10a in good yields; NOE correlations for 10a showed anti stereochemistry in the major diastereomer. Here, moderate dr may result from isomerization of either the precursor or intermediates, although a competing non-Nazarov pathway is also possible. Extending the conjugation of the alkene in styrenyl allene 11 (entry 8) resulted in an unoptimized 36% yield of 11a with >19:1 dr, though this substrate was prone to decomposition at high temperatures. Increased steric congestion in eneallenes 12 (entry 9), where both R1 and R3 are alkyl substituents, gave lower yields but excellent dr in forming a challenging all-carbon quaternary center in 12a. Eneallenes 13–15, where both R1 and R2 are substituted, gave good yields of the 2-cyclopenten-1-imines 13a–15a. As the alkene in these cases cannot undergo isomerization, the less-than-perfect dr is puzzling. Prolonged exposure of 14a as a mixture to the reaction conditions revealed no change in dr over 24 h, while resubjecting diastereomerically pure anti-14a (>19:1 dr) to the reaction conditions gave no change in the dr, providing support for lack of epimerization of the imine itself (see the SI for details). The isopropenyl substituent on cyclohexene 15a promotes high anti selectivity between the adjacent stereocenters a and b.
Density functional theory calculations (see the SI for computational details) were carried out to gain more insight into the mechanism of this unusual 2-imino-Nazarov reaction (Figure 1). The fate of methyleneaziridine 16, which is readily formed upon reaction of eneallene 4a with Rh2 catalyst in the presence of PhIO, was explored first. Exothermic coordination of the aziridine nitrogen to the Rh2 catalyst leads to INT1, which evolves to amidoallyl cation INT2 through TS1, a saddle point associated with aziridine ring opening (ΔE⧧ = 13.6 kcal/mol). The intermediacy of this achiral intermediate was supported by subjecting enantioenriched 4 (92% ee; see the SI for details) to the standard conditions and noting degradation of the axial chirality to only 12% ee in the product 4a. Ring closure converts INT2 into bicyclic INT3 via TS2, which is associated with the formation of a new C–C bond. The lower barrier for this step (ΔE⧧ = 5.0 kcal/mol) is consistent with its computed high exothermicity (ΔER = −39.8 kcal/mol); however, the barrier is similar to that for an aza-Piancatelli reaction with the N at C1, highlighting the favorable electronic benefits of positioning the N at C2 versus C3.5 Decoordination of the Rh2 fragment gives the observed product 4a and releases the catalyst. The reaction profile for the corresponding (Z)-eneallene isomer 16-Z was computed to be higher in energy than that computed for 16 along the entire reaction coordinate. In addition, the E/Z isomerization barriers computed for either INT1 or INT2 (>20 kcal/mol; see Figure S-2 in the computational portion of the SI) are much higher than the barriers associated with the ring opening and subsequent cyclization. This finding could help to explain the higher yields noted with 4 versus 5.
Figure 1.
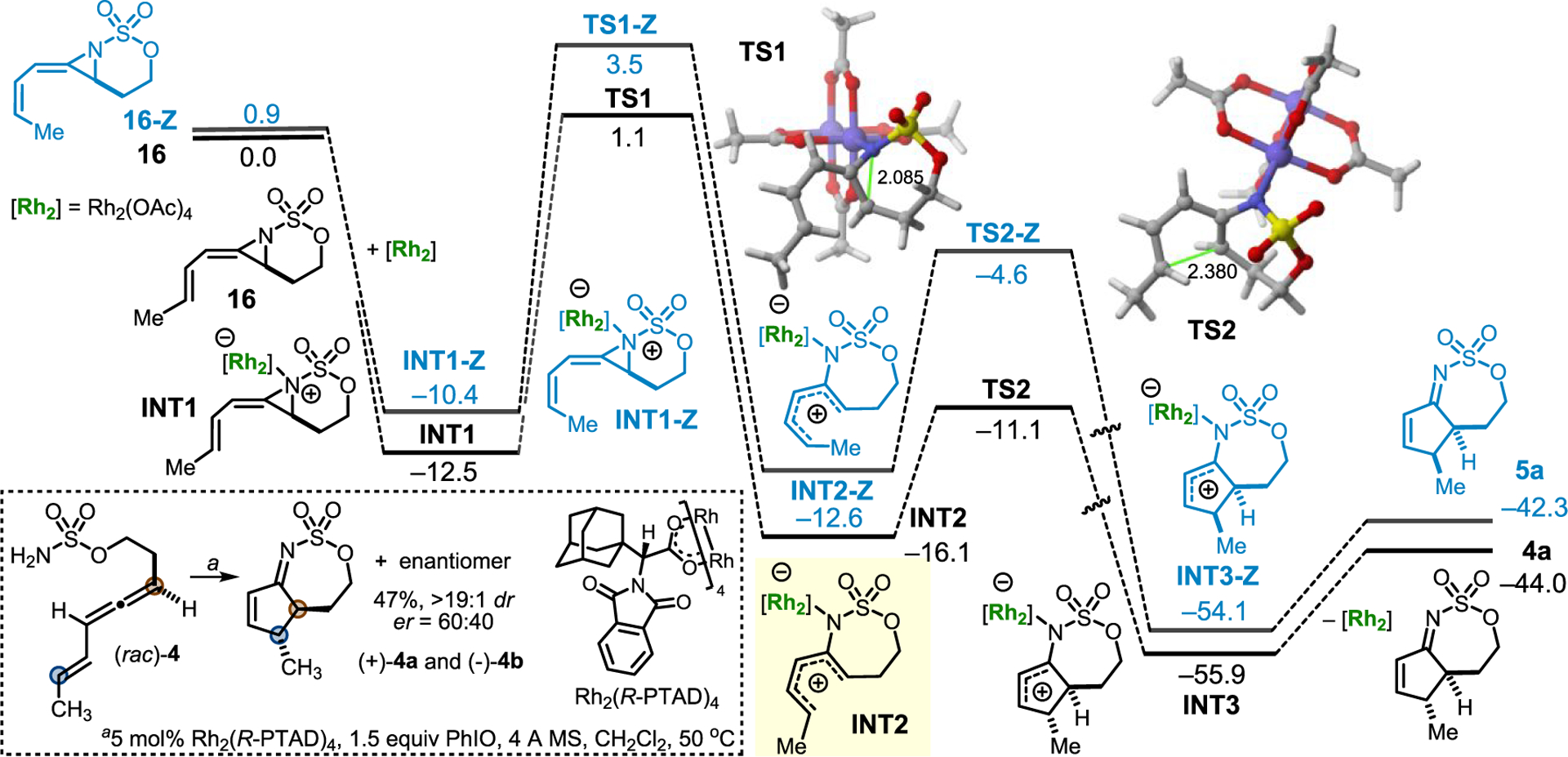
Computed reaction profile for the 2-imino-Nazarov reaction of aziridine 16. Relative energies and bond distances are given in kcal/mol and angstroms, respectively. All of the data were computed at the SMD(CH2Cl2)-B3LYP-D3/def2-TZVPP//SMD(CH2Cl2)-B3LYP-D3/def2-SVP level.
The dual role of the Rh2Ln nitrene transfer catalyst as a Lewis acid promoter was intriguing; while these modes of reactivity are precedented individually, there are few examples where both features of a Lewis acidic dinuclear Rh catalyst are utilized in a synergistic fashion.10 During optimization studies, higher catalyst loadings of the most Lewis acidic Rh2Ln complex gave more efficient cyclization. Dissociation of Rh2(OAc)4 prior to cyclization had a prohibitively large computed energy barrier, leading to the hypothesis that the use of a racemic allene with a chiral Rh2Ln catalyst might generate enantioenriched products. Indeed, subjecting (rac)-4 (Figure 1 inset) to the reaction conditions using Rh2(R-PTAD)4 produced (+)-4a and (−)-4b in a 60:40 er, a promising result considering the large distance between the catalyst and the site of the stereodetermining C–C bond formation event. Efforts to identify better chiral Rh2Ln catalysts and counteranions for accessing enantioenriched, densely functionalized amino-cyclopentenes are currently underway. This result also provides compelling evidence that Rh2 is involved in promoting cyclization as a Lewis acid, in addition to facilitating the nitrene transfer.
Finally, the 2-cyclopenten-1-imine products of tandem allene aziridination/2-imino-Nazarov reaction proved to be flexible intermediates for the preparation of complex aminated cyclopentanes. As shown in Scheme 2, a variety of transformations were successfully carried out on 6a (see the SI for conditions). Global reduction of 6a with NaBH3CN produced 17 in 82% yield with 4.8:1 dr. Boc protection of the amine, separation of the diastereomers, and double displacement with NaI/NaH generated fused pyrrolidine 18 in 64% yield over two steps (>19:1 dr), showcasing one of many potential uses of the tether.6a Higher-order cuprates, such as Bu2Cu(CN)Li2, gave diastereoselective 1,4-addition to 6a; subsequent protonation of the metalloenamine and imine reduction afforded 19 with >19:1 dr. Lastly, to demonstrate the application of 6a to the synthesis of fully substituted aminated cyclopentanes, nucleophilic epoxidation with H2O2 and catalytic NaOH provided 20 in 58% yield with >19:1 dr.
Scheme 2.
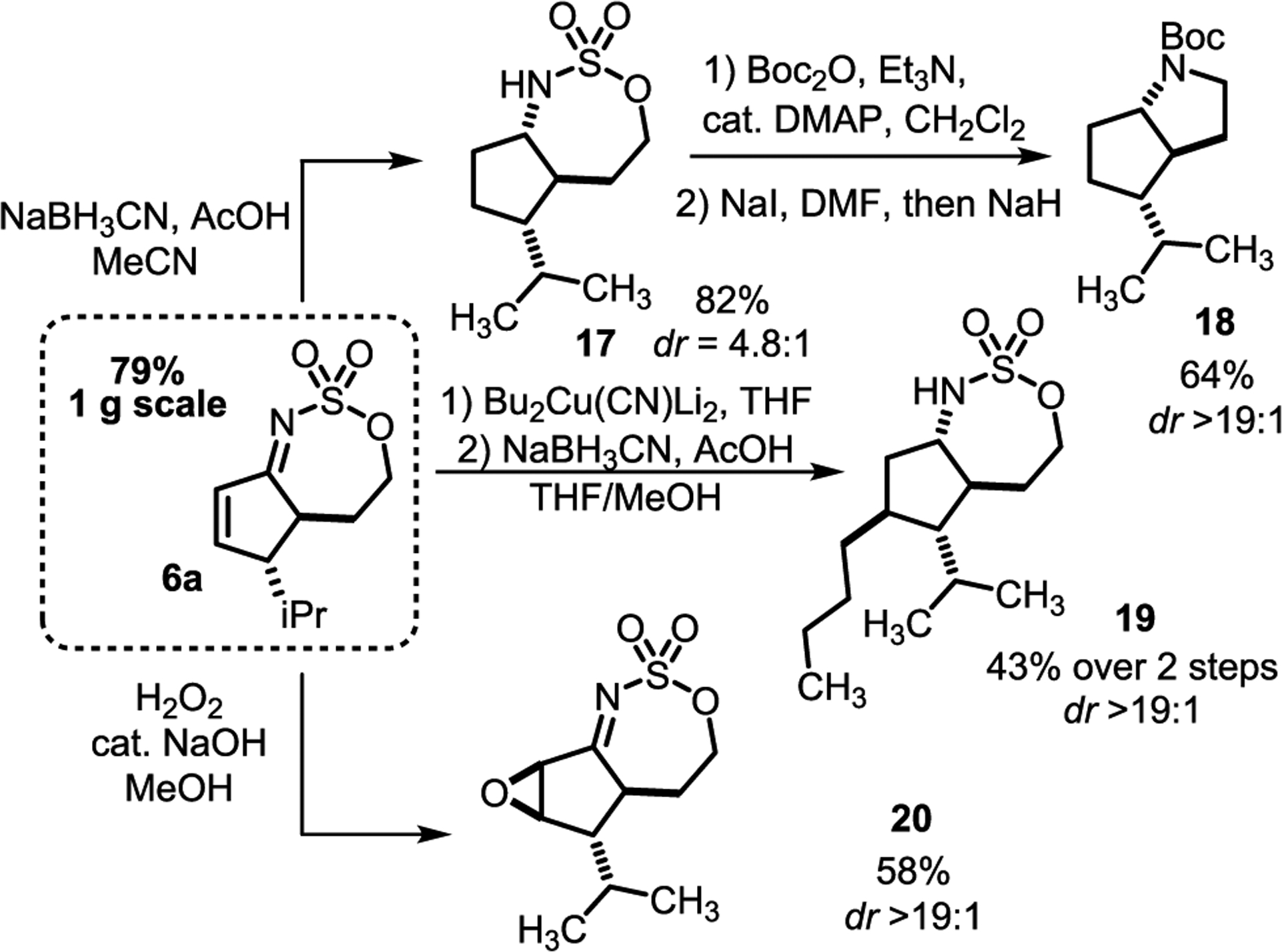
Flexible Transformations of 6a
In conclusion, we have developed an efficient 2-imino-Nazarov cyclization reaction from simple precursors. Stereo- controlled, site-selective Rh2-catalyzed eneallene aziridination initiates an electrocyclization that furnishes structurally diverse α,β-iminocyclopentene scaffolds in good yields and dr. Investigation of the reaction mechanism suggested the formation of discrete achiral intermediates, implying that strain-promoted methyleneaziridine ring opening to give a 2- amidopentadienyl cation is operative. Computations indicated that the nitrene transfer catalyst remains associated during cyclization as a mild Lewis acid, providing a new framework for developing asymmetric 2-imino-Nazarov electrocyclizations.
Supplementary Material
ACKNOWLEDGMENTS
J.M.S. thanks the National Institutes of Health (NIH) (1R01GM132300-01) for support of this work. The NMR facilities at UW-Madison are funded by the National Science Foundation (NSF) (CHE-9208463 and CHE-9629688) and the NIH (RR08389-01). The Q-Exactive mass spectrometer was acquired from an NIH-S10 award (NIH-1S10OD020022-1). I.F. acknowledges financial support from the Spanish MINECO-FEDER (Grants CTQ2016-78205-P and CTQ2016-81797-REDC). Dr. Charlie Fry of UW-Madison is thanked for assistance with NMR spectroscopy, and Dr. Martha Vestling of UW-Madison is thanked for assistance with collection of HRMS data. J.R.C. thanks Dr. Steven Schmid for helpful early discussions and Amirah Mat Lani for a generous gift of PhIO.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c02441.
Experimental procedures and spectroscopic data for all new compounds (PDF)
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.0c02441
Contributor Information
Israel Fernádez, Departmento de Quimíca Orgánica I and Centro de Innovacíon en Quimíca Avazanda (ORFEO–CINQA), Facultad de Ciencias Químicas, Universidad Complutense de Madrid, 28040 Madrid, Spain.
Jennifer M. Schomaker, Department of Chemistry, University of Wisconsin, Madison, Wisconsin 53706, United States.
REFERENCES
- (1).Selected reviews on Nazarov reactions:; (a) Vinogradov MG; Turova OV; Zlotin SG Nazarov reaction: current trends and recent advances in the synthesis of natural compounds and their analogs. Org. Biomol. Chem 2017, 15, 8245. [DOI] [PubMed] [Google Scholar]; (b) Di Grandi MJ Nazarov-like cyclization reactions. Org. Biomol. Chem 2014, 12, 5331. [DOI] [PubMed] [Google Scholar]; (c) Frontier AJ; Collison C The Nazarov cyclization in organic synthesis. Recent advances. Tetrahedron 2005, 61, 7577. [Google Scholar]; (d) Tius MA Allene ether Nazarov cyclization. Chem. Soc. Rev 2014, 43, 2979. [DOI] [PubMed] [Google Scholar]
- (2).Substituent effects and retro-Nazarov reactivity:; Harmata M; Lee DR The Retro-Nazarov Reaction. J. Am. Chem. Soc 2002, 124, 14328. [DOI] [PubMed] [Google Scholar]
- (3).Examples of state-of-the-art imino-Nazarov reactions:; (a) Fan T; Wang A; Li J-Q; Ye J-L; Zheng X; Huang P-Q Versatile one-pot synthesis of polysubstituted cyclopent-2-enimines from α,η-unsaturated amides: imino-Nazarov reaction. Angew. Chem., Int. Ed 2018, 57, 10352. [DOI] [PubMed] [Google Scholar]; (b) Ma Z-X; He S; Song W; Hsung RP –Aryl-substituted allenamides in an imino-Nazarov cyclization cascade catalyzed by Au(I). Org. Lett 2012, 14, 5736. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bonderoff SA; Grant TN; West FG; Tremblay M Nazarov reactions of vinyl cyclopropylamines: an approach to the imino-Nazarov problem. Org. Lett 2013, 15, 2888. [DOI] [PubMed] [Google Scholar]; (d) Suárez-Pantiga S; Rubio E; Alvarez-Rúa; González, J. M. Intermolecular reaction of internal alkynes and imines: propargyl tosylates as key partners in a gold-catalyzed [4 + 1] unusual cyclization leading to cyclopent-2-enimines. Org. Lett 2009, 11, 13. [DOI] [PubMed] [Google Scholar]; (e) Tius MA; Chu CC; Nieves-Colberg R An imino Nazarov cyclization. Tetrahedron Lett. 2001, 42, 2419. [Google Scholar]; (f) Bow WF; Basak AK; Jolit A; Vicic DA; Tius MA Enamine-iminium ion Nazarov cyclization of α-ketoenones. Org. Lett 2010, 12, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) William R; Wang S; Ding F; Arviana EN; Liu X-W Interrupted imino-Nazarov cyclization of 1-aminopentadienyl cation and related cascade process. Angew. Chem., Int. Ed 2014, 53, 10742. [DOI] [PubMed] [Google Scholar]
- (4).Other methods to generate amidoallyl cations:; (a) Schmid R; Schmid H Silberioneninduzierte Reaktion von 3-Chlor-2-pyrrolidi-no-cyclohexen mit 1,3-Dienen. Helv. Chim. Acta 1974, 57, 1883. [Google Scholar]; (b) Kim H; Ziani-Cherif C; Oh J; Cha JK New [4 + 3] cycloaddition approach to cis-2,8-disubstituted oxocanes. J. Org. Chem 1995, 60, 792. [Google Scholar]; (c) Kende AS; Huang H Asymmetric [4 + 3] cycloadditions from chiral d-chloro imines. Tetrahedron Lett. 1997, 38, 3353. [Google Scholar]; (d) De Kimpe N; Stevens C Silver ion-induced reactions of i-haloimines. Tetrahedron 1990, 46, 6753. [Google Scholar]; (e) De Kimpe N; Palamareva M; Verhe R; Debuyck L; Schamp N Silver-induced conversion of α-chloro ketimines into 2,2-dimethyl-3-(N-alkyl)imino-8-oxabicyclo[3.2.1]oct-6-enes. Presumptive evidence for the [3 + 4] cycloaddition of intermediate 2-aminoallylcarbenium ions with furan. J. Chem. Res 1986, 190. [Google Scholar]; (f) Saputra MA; Dange NS; Cleveland AH; Malone JA; Fronczek FR; Kartika R Regioselective functionalization of enamides at the l-Carbon via unsymmetrical 2-amidoallyl cations. Org. Lett 2017, 19, 2414. [DOI] [PubMed] [Google Scholar]; (g) Schlegel M; Schneider C Lewis acid-catalyzed nucleophilic addition of indoles to in situ-generated 2-amidoallyl cations. J. Org. Chem 2017, 82, 5986. [DOI] [PubMed] [Google Scholar]
- (5).Selected references on Piancatelli and aza-Piancatelli reactions:; (a) Veits GK; Wenz DR; Read de Alaniz J Versatile method for the synthesis of 4-aminocyclopentenones: Dysprosium(III) triflate catalyzed aza-Piancatelli rearrangement. Angew. Chem., Int. Ed 2010, 49, 9484. [DOI] [PubMed] [Google Scholar]; (b) Yu D; Thai VT; Palmer LI; Veits GK; Cook JE; Read de Alaniz J; Hein JE Importance of off-cycle species in the acid-catalyzed aza-Piancatelli rearrangement. J. Org. Chem 2013, 78, 12784. [DOI] [PubMed] [Google Scholar]; (c) Nieto Faza O; Silva López C;Álvarez R; de Lera ÁR Theoretical study of the electrocyclic ring closure of hydroxypenta-dienyl cations. Chem. - Eur. J 2004, 10, 4324. [DOI] [PubMed] [Google Scholar]; (d) Davis RL; Tantillo DJ Theoretical studies on pentadienyl cation electrocyclizations. Curr. Org. Chem 2010, 14, 1561. [Google Scholar]
- (6).Mechanistic studies of strain-promoted 2-oxypentadienyl cation cyclization of allene oxides in nature:; (a) Turner JG; Ellis C; Devoto A The jasmonate signal pathway. Plant Cell 2002, 14, S153. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) López CS; Faza ON; York DM; de Lera ÁR Theoretical study of the vinyl allene oxide to cyclopent-2-en-1-one rearrangement: Mechanism, torquoselectivity and solvent effects. J. Org. Chem 2004, 69, 3635. [DOI] [PubMed] [Google Scholar]; (c) González-Pérez AB; Grechkin A; de Lera ÁR A unifying mechanism for the rearrangement of vinyl allene oxide geometric isomers to cyclopentenones. Org. Biomol. Chem 2014, 12, 7694. [DOI] [PubMed] [Google Scholar]
- (7).Amidoallyl cations via allene amination and methyleneaziridine ring opening:; (a) Gerstner NC; Adams CS; Tretbar M; Schomaker JM Stereocontrolled syntheses of seven-membered carbocycles by tandem allene aziridination/[4 + 3] reaction. Angew. Chem., Int. Ed 2016, 55, 13240. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Prié G; Prévost N; Twin H; Fernandes SA; Hayes JF; Shipman A A Lewis acid catalyzed intramolecular [4 + 3] cycloaddition route to polycyclic systems that contain a seven-membered ring. Angew. Chem., Int. Ed 2004, 43, 6517. [DOI] [PubMed] [Google Scholar]; (c) Griffin K; Montagne C; Hoang CT; Clarkson GJ; Shipman M Lewis acid promoted intramolecular (3 + 2) ‘cycloadditions’ of methyleneaziridines with alkene and alkyne acceptors. Org. Biomol. Chem 2012, 10, 1032. [DOI] [PubMed] [Google Scholar]; (d) Feast GC; Page LW; Robertson J The intramolecular amination of allenes. Chem. Commun 2010, 46, 2835. [DOI] [PubMed] [Google Scholar]; (e) Robertson J; Feast GC; White LV; Steadman VA; Claridge TDW Structure and reactivity of bicyclic methylene aziridines prepared by intramolecular aziridination of allenes. Org. Biomol. Chem 2010, 8, 3060. [DOI] [PubMed] [Google Scholar]; (f) Stoll AH; Blakey SB Rhodium catalyzed allene amination: Diastereose- lective synthesis of aminocyclopropanes via a 2-amidoallylcation intermediate. J. Am. Chem. Soc 2010, 132, 2108. [DOI] [PubMed] [Google Scholar]; (g) Stoll AH; Blakey SB Rhodium catalyzed allene amidation: a facile entry into 2-amidoallylcations for unusual [3 + 3] annulation reactions. Chem. Sci 2011, 2, 112. [Google Scholar]; (h) Takahashi H; Yasui S; Tsunoi S; Shibata I Catalytic cycloaddition of 2-methyleneaziridines with 1,1-Dicyanoal-kenes. Org. Lett 2014, 16, 1192. [DOI] [PubMed] [Google Scholar]
- (8).Eneallene-oxidation-initiated Nazarov cyclization:; (a) Kim SJ; Cha JK An efficient cyclopentenone formation via an allene oxide. Tetrahedron Lett. 1988, 29, 5613. [Google Scholar]; (b) Doutheau A; Gore J; Malacria M Preparation et Epoxydation de Trienes-1,2,4ynes-6 (alleneenynes). Tetrahedron 1977, 33, 2393. [Google Scholar]; (c) Dulcere J-P; Grimaldi J; Santelli M Synthesis of silyl-substituted vinylallenes. Tetrahedron Lett. 1981, 22, 3179. [Google Scholar]; (d) Malona JA; Cariou K; Spencer WT; Frontier AJ Total synthesis of (±)-rocaglamide via oxidation-initiated Nazarov cyclization. J. Org. Chem 2012, 77, 1891. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Spencer WT; Levin MD; Frontier AJ Oxidation-initiated Nazarov cyclization of vinyl alkoxyallenes. Org. Lett 2011, 13, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Fradette RJ; Kang M; West FG Oxidation-initiated cyclizations of pentadienyl ethers: an alternative entry to the Nazarov reaction. Angew. Chem., Int. Ed 2017, 56, 6335. [DOI] [PubMed] [Google Scholar]
- (9).Methods to prepare eneallenes:; (a) Pu X; Ready JM Direct and stereospecific synthesis of allenes via reduction of propargylic alcohols with Cp2Zr(H)Cl. J. Am. Chem. Soc 2008, 130, 10874. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Myers AG; Zheng B New and stereospecific synthesis of allenes in a single step from propargylic alcohols. J. Am. Chem. Soc 1996, 118, 4492. [Google Scholar]
- (10).Rh2Ln catalysts as Lewis acids:; (a) Dequirez G; Ciesielski J; Retailleau P; Dauban P Catalytic intermolecular alkene oxy-amination with nitrenes. Chem. - Eur. J 2014, 20, 8929. [DOI] [PubMed] [Google Scholar]; (b) Ciesielski J; Dequirez G; Retailleau P; Gandon V; Dauban P Rhodium-catalyzed alkene difunctionalization with nitrenes. Chem. - Eur. J 2016, 22, 9338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


