Abstract
Background:
In almost all countries, incidence rates of liver cancer are 100–200% higher in males than in females. However, this difference is predominantly driven by hepatocellular carcinoma (HCC), which accounts for 75% of liver cancer cases. Intrahepatic cholangiocarcinoma (ICC) accounts for 12% of cases and has rates only 30% higher in males. Hormones are hypothesized to underlie observed sex differences. We investigated whether prediagnostic circulating hormone and sex hormone binding globulin (SHBG) levels were associated with liver cancer risk, overall and by histology, by leveraging resources from five prospective cohorts.
Methods:
Seven sex steroid hormones and SHBG were quantitated using gas chromatography-tandem mass spectrometry (GC-MS/MS) and competitive electrochemiluminescence immunoassay, respectively, from baseline serum/plasma samples of 191 post-menopausal female liver cancer cases (HCC n=83, ICC n=56) and 426 controls, matched on sex, cohort, age, race/ethnicity, and blood collection date. Odds ratios (ORs) and 95% confidence intervals (CIs) for associations between a one-unit increase in log2 hormone value (approximate doubling of circulating concentration) and liver cancer were calculated using multivariable-adjusted conditional logistic regression.
Results:
A doubling in the concentration of 4-androstenedione was associated with a 50% decreased liver cancer risk (OR=0.50,95%CI=0.30–0.82), while SHBG was associated with a 31% increased risk (OR=1.31,95%CI=1.05–1.63). Examining histology, a doubling of estradiol was associated with a 40% increased risk of ICC (OR=1.40,95%CI=1.05–1.89), but not HCC (OR=1.12,95%CI=0.81–1.54).
Conclusions:
This study provides the first evidence that higher levels of 4-androstenedione may be associated with lower, and SHBG with higher, liver cancer risk in women. However, this study does not support the hypothesis that higher estrogen levels decrease liver cancer risk. Indeed, estradiol may be associated with an increased ICC risk.
Keywords: cohort study, mass spectrometry, sex hormones, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, human
Introduction
Liver cancer incidence rates have notable sex differences worldwide, with rates among males being 100–200% higher than those among females. However, these differences are largely determined by the dominant histologic type of liver cancer, hepatocellular carcinoma (HCC), which accounts for approximately 75% of cases. The second most common histologic type of liver cancer, intrahepatic cholangiocarcinoma (ICC), accounts for approximately 12% of cases and has incidence rates that are only 30% higher in men (1, 2). Established risk factors that vary in prevalence by sex, including hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, alcohol consumption, smoking, and obesity, cannot fully explain the male predominance of liver cancer (3).
Sex steroid hormones have been proposed as an explanation for the sex differences, with a hypothesized beneficial role for estrogens and a detrimental role for androgens in liver cancer pathogenesis (4, 5). This hypothesis is supported by epidemiologic studies, which have reported an association between oophorectomy and increased liver cancer risk (6, 7). Similarly, animal models have reported that dosing female rodents with testosterone or removing their ovaries increases liver cancer development, while dosing male rodents with estrogen or castrating them reduces liver cancer development (8–10). Several small studies have investigated circulating hormones, primarily testosterone or estradiol, in relation to HCC risk in males (11–15). These studies have reported that testosterone levels are higher in HCC cases. Only one prospective study, has examined this hypothesis in a population that included females, concluding that sex hormone binding globulin (SHBG), but not testosterone, was associated with increased HCC risk (16). However, the study was not powered to examine the associations separately for females.
To date, no prospective study has examined circulating sex steroid hormones in relation to liver cancer risk among female HCC or ICC cases. Additionally, all prior epidemiologic studies of sex steroid hormones and liver cancer have been conducted outside the US (11–16), and endogenous hormone levels have been shown to vary by geography (17). Thus, we investigated whether prediagnostic circulating sex steroid hormone concentrations were associated with liver cancer risk in US females.
Methods
Study Population.
The current study was nested in the Liver Cancer Pooling Project (7) and leveraged the resources from five prospective parent cohort studies: Multiphasic Health Checkup Study (MHC) (18); New York University Women’s Health Study (NYUWHS) (19); Nurses’ Health Study (NHS) (20); Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) (21); and Women’s Health Initiative (WHI) (22) (Supplemental Table S1). All studies received Institutional Review Board and data sharing approvals from their host institutions and the NCI.
The current study was restricted to post-menopausal women at blood draw, as there are known differences in circulating sex steroid hormones between pre- and post-menopausal women (23). Menopausal status was determined through a combination of self-report, age at blood draw, and levels of estrone and estradiol. Liver cancer, defined as incident primary liver cancer (International Classification of Diseases, 10th edition [ICD-10] diagnostic code C22), was ascertained by linkage to state cancer registries or medical/pathology record review. Cases were further classified using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) morphology codes 8170–8175 for HCC and 8032–8033, 8041, 8050, 8070–8071, 8140–8141, 8160, 8260, 8480, 8481, 8490, and 8560 for ICC.
Controls were individually matched to cases using incidence-density sampling based on parent cohort, age, race/ethnicity, and date of baseline blood collection (±3-months). A total of 191 post-menopausal cases and 426 controls were included in the analysis: 101 participants from MHC, 44 from NYUWHS, 40 from NHS, 80 from the PLCO, and 352 from WHI.
Laboratory Methods.
Steroid hormone assays were performed at the Pharmacogenomics Laboratory of Laval University (Quebec, Canada) (24). All samples were quantitated for estradiol and testosterone, using a gas chromatography selected reaction monitoring–tandem mass spectrometry assay (GC-MS/MS; Figure 1). In studies with sufficient volume available, samples were also quantitated for estrone, dehydroepiandrosterone (DHEA), 4-androstenedione, and Δ−5-androstenediol using GC-MS/MS (Supplemental Table S2). For hormone values below the lower limit of quantification (LLOQ), a value of ½ LLOQ was assigned. Blinded quality control (QC) replicate samples were included in each batch (n=81 total QC samples). Within and between batch coefficients of variation (CVs) were <17%, and the intraclass correlation coefficient (ICC) was >0.80 for all hormones.
Figure 1.
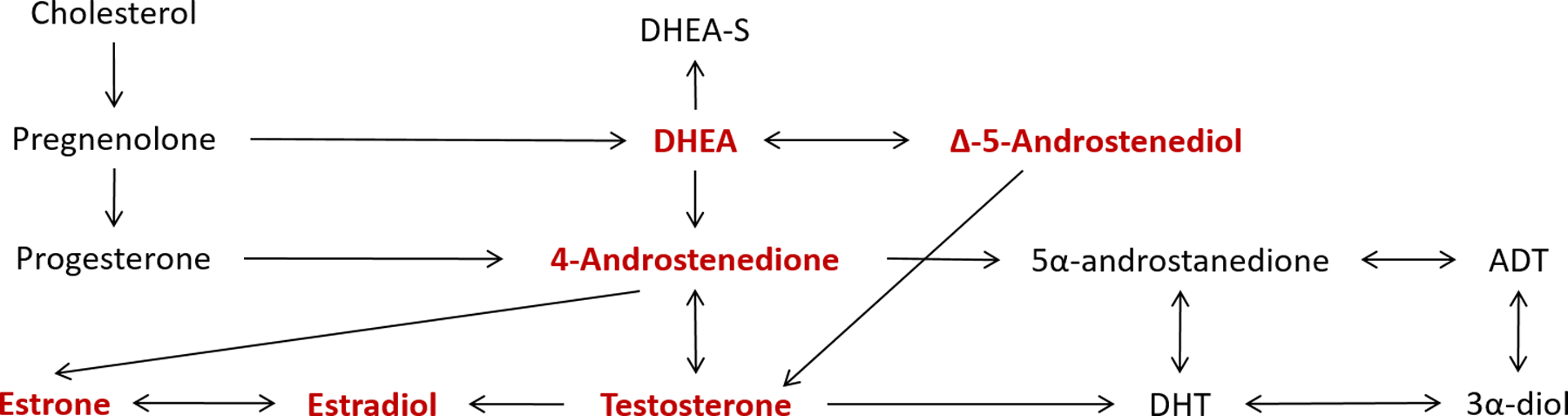
Schematic of sex steroid hormone metabolism. Quantitated sex steroid hormones are highlighted in red. Sex hormone binding globulin (SHBG) is not shown, as it is not part of the sex steroid metabolism pathway.
SHBG was quantitated in all samples at the Clinical and Epidemiologic Research Laboratory of Boston Children’s Hospital (Boston, MA) using a competitive electrochemiluminescence immunoassay on the Roche E Modular system (Roche Diagnostics, Indianapolis, IN). For SHBG, the within and between batch CVs were <3%, and the ICC was 0.99.
In addition to the individual hormones, testosterone:estradiol ratio, androstenedione:estrone ratio, free estradiol (25), and free testosterone (26) were calculated. Circulating hormone concentrations were categorized in quartiles, based on the control distribution. Tests of linear trend were performed using quartile-specific hormone concentration medians. As continuous hormone values were right skewed, values were also log2 transformed, which corresponds to a doubling of circulating hormone concentration per unit change.
To determine HBV status, hepatitis B surface antigen (HBsAg) was assayed using the Bio-Rad GS HBsAg 3.0 enzyme immunoassay (Bio-Rad Laboratories, Redmond, WA, USA). For determination of HCV status, antibody to HCV (anti-HCV) was assessed using the Ortho HCV Version 3.0 ELISA test system (Ortho-Clinical Diagnostics, Inc.).
Data and Sample Collection.
MHC collected non-fasting blood samples from Kaiser Permanente Northern California members who underwent a multiphasic health checkup in Oakland or San Francisco. Serum samples were initially stored at −23°C, and then since 1980, stored at −40°C (18). NYUWHS collected non-fasting blood samples from during the baseline visit; serum samples were stored at −80°C (19). NHS collected blood samples via mail, and participants reported fasting status. Plasma samples were stored at −130°C (20). PLCO collected blood samples from participants in the screening intervention study arm during baseline visit. Fasting status was not ascertained; serum samples were stored at −70°C (21). WHI collected overnight fasting blood samples during the baseline visit. Serum samples were initially frozen at −70°C; after arriving at the biorepository, samples were stored at −80°C (22). Prior studies have shown long-term stability of sex steroid hormones at ≤−20°C (27–29).
Statistical Analysis.
Geometric mean hormone concentration levels were adjusted for parent study and age; ANOVA was used to compare cases and controls. Differences in potential covariates between cases and controls were assessed by either the chi-square or Fisher’s exact test for categorical variables and the Wilcoxon-Mann-Whitney test for continuous variables. Odds ratios (ORs) and 95% confidences intervals (CIs) were estimated using conditional logistic regression to examine the association between circulating hormone concentrations and liver cancer risk. Liver cancer cases were also stratified by histology (HCC vs. ICC), where sample size permitted (n>5 cases in each quartile).
Values for missing covariates were derived using a single imputation: race (5.2% of cases, 6.1% of controls), BMI (4.2%, 3.5%), smoking status (5.8%, 6.8%), alcohol consumption (6.3%, 7.0%), HBV status (0.5%, 0.2%), HCV infection (0.5%, 0.2%), diabetes (4.2%, 3.8%), and menopausal hormone therapy (MHT) use (7.3%, 6.8%). We also examined results utilizing a complete case analysis (i.e., whereby only observations with complete covariates were maintained) or creating a ‘missing’ covariate category, and results did not differ (data not shown). Confounding was assessed by first determining whether covariates were associated with exposure among the controls and with the outcome among the unexposed (i.e., the lowest quartile of each hormone concentration) (30). If a potential confounder was associated with the exposure and outcome, then each covariate was removed from the full model one at a time to evaluate if the log odds ratio changed by ≥10%. If the change occurred for any hormone, the variable was considered a confounder and remained in the model. All potential covariates met these criteria and were included in the final models: age (continuous), BMI (<25, 25-<30, ≥30 kg/m2), smoking (never, former, current), current alcohol use, HBsAg, anti-HCV, self-reported diabetes, and current MHT use. Using likelihood ratio tests, effect measure modification of the relationships between log2 transformed hormones and liver cancer were assessed by the covariates included in the final models. There was no evidence of effect measure modification by any of the covariates (all Ps≥0.05). All tests were 2-sided. Analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
Sensitivity Analysis.
In addition to calculating quartiles based on the overall control distribution, we calculated study-specific quartiles, and tests of linear trend were performed based on the quartile score. To evaluate the possibility of reverse causation, we conducted three lag analyses excluding case-control pairs whose case was diagnosed within 2, 5, and 10 years of the date of blood donation. As WHI accounted for roughly half of the participants, we examined associations between estradiol, testosterone, and SHBG excluding these participants. To examine if there was residual confounding by BMI, we examined BMI as a continuous variable. Finally, we excluded individuals with hormone concentrations below the LLOQ.
Results
As shown in Table 1, controls were more likely than cases to have a BMI <25 kg/m2 (43.0% vs. 34.0%) and to drink alcohol at study baseline (60.1% vs. 48.7%). Controls were less likely to be anti-HCV(+) (2.1% vs. 18.8%) and have diabetes (5.2% vs. 13.1%). Cases had higher mean concentrations of estrogens than controls (e.g., estradiol 7.46 vs. 6.70 pg/mL), while controls had higher mean concentrations of most androgens (e.g., testosterone 0.17 vs. 0.18 ng/mL).
Table 1.
Characteristics of study participants by case-control status.
| Controls (n=426) | Cases (n=191) | |||||
|---|---|---|---|---|---|---|
| Characteristic | N (%) | N (%) | P-value | |||
| Mean age at baseline (SD), years | 62.5 | (6.9) | 62.8 | (7.2) | 0.5 | |
| Race | ||||||
| White | 324 | (76.1) | 141 | (73.8) | ||
| Black | 47 | (11.0) | 22 | (11.5) | ||
| Asian/Pacific Islander | 27 | (6.3) | 14 | (7.3) | ||
| American Indian/Alaska Native | 2 | (0.5) | 1 | (0.5) | ||
| Other | 26 | (6.1) | 13 | (6.8) | 1.0 | |
| Body mass index (kg/m2) | ||||||
| <25.0 | 183 | (43.0) | 65 | (34.0) | ||
| 25.0 – <30.0 | 151 | (35.5) | 72 | (37.7) | ||
| ≥ 30.0 | 92 | (21.6) | 54 | (28.3) | 0.07 | |
| Smoking status | ||||||
| Never | 237 | (55.6) | 98 | (51.3) | ||
| Former | 146 | (34.3) | 71 | (37.2) | ||
| Current | 43 | (10.1) | 22 | (11.5) | 0.6 | |
| Current alcohol drinker | ||||||
| No | 170 | (39.9) | 98 | (51.3) | ||
| Yes | 256 | (60.1) | 93 | (48.7) | 0.008 | |
| Chronic hepatitis B virus infection (HBsAg) | ||||||
| No | 420 | (98.6) | 186 | (97.4) | ||
| Yes | 6 | (1.4) | 5 | (2.6) | 0.3 | |
| Chronic hepatitis C virus infection (anti-HCV) | ||||||
| No | 417 | (97.9) | 155 | (81.2) | ||
| Yes | 9 | (2.1) | 36 | (18.8) | <0.001 | |
| Diabetes | ||||||
| No | 404 | (94.8) | 166 | (86.9) | ||
| Yes | 22 | (5.2) | 25 | (13.1) | <0.001 | |
| Current menopausal hormone therapy use | ||||||
| No | 283 | (66.4) | 128 | (67.0) | ||
| Yes | 143 | (33.6) | 63 | (33.0) | 0.9 | |
| Parent study | ||||||
| Multiphasic Health Checkup Study (MHC) | 71 | (16.7) | 30 | (15.7) | ||
| New York University Women’s Health Study (NYUWHS) | 33 | (7.8) | 11 | (5.8) | ||
| Nurses’ Health Study (NHS) | 28 | (6.6) | 12 | (6.3) | ||
| Prostate, Lung, Colorectal, and Ovarian Cancer Trial (PLCO) | 60 | (14.1) | 20 | (10.5) | ||
| Women’s Health Initiative (WHI) | 234 | (54.9) | 118 | (61.8) | 0.5 | |
| Hormone Concentrations, age- and study-adjusted mean (95% CI)1 | ||||||
| Estradiol (pg/mL) | 6.70 | (6.52, 6.88) | 7.46 | (6.90, 8.07) | 0.2 | |
| Estrone (pg/mL) | 31.29 | (30.34, 32.26) | 39.04 | (36.13, 42.18) | 0.4 | |
| Testosterone (ng/mL) | 0.18 | (0.17, 0.18) | 0.17 | (0.16, 0.17) | 0.4 | |
| Dehydroepiandrosterone (ng/mL) | 1.71 | (1.68, 1.74) | 1.40 | (1.37, 1.43) | 0.05 | |
| 4-Androstenedione (ng/mL) | 0.46 | (0.46, 0.47) | 0.41 | (0.41, 0.42) | 0.04 | |
| Δ−5-androstenediol (pg/mL) | 272.76 | (264.97, 280.82) | 276.80 | (268.43, 285.43) | 0.8 | |
| Sex hormone binding globulin (nmol/L) | 78.47 | (77.53, 79.41) | 96.66 | (95.26, 98.07) | <0.001 | |
| Free estradiol (pg/mL) | 0.12 | (0.12, 0.12) | 0.12 | (0.11, 0.13) | 1.0 | |
| Free testosterone (pg/mL) | 1.67 | (1.62, 1.72) | 1.33 | (1.29, 1.36) | <0.001 | |
| Testosterone:Estradiol (pg/mL) | 26.67 | (25.51, 27.88) | 21.78 | (20.01, 23.71) | 0.04 | |
| Free testosterone:Free estradiol (pg/mL) | 13.86 | (13.23, 14.53) | 10.67 | (9.81, 11.61) | 0.01 | |
| 4-Androstenedione:Estrone | 0.01 | (0.01, 0.01) | 0.02 | (0.01, 0.02) | 0.03 | |
Standardized to 62.5 years, mean age among controls.
Estradiol was not associated with liver cancer or HCC risk. However, a doubling in the concentration of estradiol was associated with a 40% increased ICC risk (OR per one unit increase in log2 estradiol=1.40,95%CI=1.05–1.89;P=0.02;Table 2). Results were consistent when we examined quartiles of estradiol concentration (OR quartile 4 vs. 1=5.72,95%CI=1.18–27.76;Ptrend=0.04), although the referent quartile only included five cases. The association between free estradiol and ICC was similar to the estradiol-ICC association. No associations were seen with estrone.
Table 2.
Adjusted1 odds ratios (OR) and 95% confidence intervals (CI) for associations between circulating sex steroid hormone concentrations and liver cancer risk.
| Liver Cancer | Hepatocellular Carcinoma2 | Intrahepatic Cholangiocarcinoma2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | OR | 95% CI | Controls | Cases | OR | 95% CI | Controls | Cases | OR | 95% CI | ||||
| Estradiol (pg/mL) | |||||||||||||||
| Q1 | 0 to 3.22 | 108 | 38 | 1.00 | 34 | 12 | 1.00 | 26 | 5 | 1.00 | |||||
| Q2 | >3.22 to 6.25 | 105 | 48 | 1.18 | (0.63, 2.22) | 44 | 23 | 2.28 | (0.59, 8.86) | 31 | 15 | 2.87 | (0.72, 11.53) | ||
| Q3 | >6.25 to 14.58 | 107 | 54 | 1.39 | (0.71, 2.72) | 53 | 24 | 1.13 | (0.28, 4.62) | 33 | 17 | 4.61 | (0.97, 21.94) | ||
| Q4 | >14.58 | 106 | 51 | 1.50 | (0.73, 3.06) | 49 | 24 | 2.52 | (0.57, 11.22) | 29 | 17 | 5.72 | (1.18, 27.76) | ||
| p-value for trend3 | 0.2 | 0.5 | 0.04 | ||||||||||||
| Continuous (log2) | 426 | 191 | 1.08 | (0.93, 1.25) | 180 | 83 | 1.12 | (0.81, 1.54) | 119 | 54 | 1.40 | (1.05, 1.89) | |||
| Estrone (pg/mL) | |||||||||||||||
| Q1 | 0 to 13.64 | 48 | 17 | 1.00 | 7 | 2 | — | 6 | 1 | — | |||||
| Q2 | >13.64 to 23.86 | 48 | 20 | 1.18 | (0.50, 2.77) | 11 | 6 | — | 7 | 3 | — | ||||
| Q3 | >23.86 to 45.13 | 48 | 17 | 0.87 | (0.32, 2.34) | 18 | 4 | — | 11 | 4 | — | ||||
| Q4 | >45.13 | 48 | 19 | 0.92 | (0.32, 2.63) | 14 | 6 | — | 14 | 5 | — | ||||
| p-value for trend3 | 0.8 | 0.7 | 0.9 | ||||||||||||
| Continuous (log2) | 192 | 73 | 1.11 | (0.83, 1.49) | 50 | 18 | 1.32 | (0.29, 5.90) | 38 | 13 | 1.26 | (0.58, 2.71) | |||
| Testosterone (ng/mL) | |||||||||||||||
| Q1 | 0 to 0.12 | 107 | 50 | 1.00 | 50 | 20 | 1.00 | 32 | 21 | 1.00 | |||||
| Q2 | >0.12 to 0.17 | 107 | 52 | 1.23 | (0.71, 2.13) | 47 | 21 | 1.95 | (0.74, 5.13) | 37 | 11 | 0.31 | (0.10, 0.95) | ||
| Q3 | >0.17 to 0.25 | 106 | 40 | 0.79 | (0.44, 1.42) | 50 | 19 | 1.28 | (0.42, 3.87) | 24 | 11 | 0.56 | (0.19, 1.64) | ||
| Q4 | >0.25 | 106 | 49 | 0.99 | (0.57, 1.74) | 33 | 23 | 2.44 | (0.77, 7.68) | 32 | 13 | 0.51 | (0.19, 1.38) | ||
| p-value for trend3 | 0.6 | 0.3 | 0.4 | ||||||||||||
| Continuous (log2) | 426 | 191 | 0.90 | (0.75, 1.08) | 180 | 83 | 1.34 | (0.93, 1.94) | 125 | 56 | 0.74 | (0.49, 1.12) | |||
| Dehydroepiandrosterone (ng/mL) | |||||||||||||||
| Q1 | 0 to 1.16 | 48 | 20 | 1.00 | 11 | 6 | — | 12 | 3 | — | |||||
| Q2 | >1.16 to 1.82 | 48 | 21 | 1.57 | (0.66, 3.76) | 13 | 7 | — | 12 | 6 | — | ||||
| Q3 | >1.81 to 2.82 | 48 | 19 | 1.10 | (0.45, 2.67) | 15 | 4 | — | 6 | 2 | — | ||||
| Q4 | >2.82 | 48 | 13 | 0.64 | (0.24, 1.70) | 11 | 1 | — | 11 | 3 | — | ||||
| p-value for trend3 | 0.3 | 0.2 | 0.2 | ||||||||||||
| Continuous (log2) | 192 | 73 | 0.75 | (0.52, 1.08) | 50 | 18 | 0.14 | (0.01, 2.77) | 41 | 14 | 2.98 | (0.48, 18.68) | |||
| 4-Androstenedione (ng/mL) | |||||||||||||||
| Q1 | 0 to 0.36 | 48 | 25 | 1.00 | 12 | 5 | — | 11 | 4 | — | |||||
| Q2 | >0.36 to 0.51 | 48 | 23 | 1.04 | (0.46, 2.39) | 15 | 7 | — | 11 | 5 | — | ||||
| Q3 | >0.51 to 0.69 | 48 | 13 | 0.54 | (0.22, 1.32) | 15 | 4 | — | 11 | 3 | — | ||||
| Q4 | >0.69 | 47 | 12 | 0.27 | (0.09, 0.80) | 8 | 2 | — | 9 | 2 | — | ||||
| p-value for trend3 | 0.01 | 0.7 | 0.4 | ||||||||||||
| Continuous (log2) | 191 | 73 | 0.50 | (0.30, 0.82) | 50 | 18 | 0.69 | (0.07, 6.68) | 42 | 14 | 0.48 | (0.09, 2.69) | |||
| Δ−5-androstenediol (pg/mL) | |||||||||||||||
| Q1 | 0 to 194.30 | 48 | 20 | 1.00 | 16 | 7 | — | 14 | 3 | — | |||||
| Q2 | >194.30 to 355.56 | 48 | 17 | 1.32 | (0.50, 3.47) | 15 | 4 | — | 11 | 5 | — | ||||
| Q3 | >355.56 to 497.24 | 48 | 18 | 1.20 | (0.44, 3.24) | 12 | 7 | — | 10 | 2 | — | ||||
| Q4 | >497.24 | 47 | 18 | 0.83 | (0.28, 2.46) | 7 | 0 | — | 6 | 4 | — | ||||
| p-value for trend3 | 0.8 | 0.4 | 0.5 | ||||||||||||
| Continuous (log2) | 191 | 73 | 0.95 | (0.64, 1.40) | 50 | 18 | 15.33 | (0.22, 1078.12) | 41 | 14 | 1.64 | (0.54, 4.94) | |||
| Sex hormone binding globulin (nmol/L) | |||||||||||||||
| Q1 | 0 to 53.76 | 107 | 43 | 1.00 | 38 | 12 | 1.00 | 34 | 21 | 1.00 | |||||
| Q2 | >53.76 to 81.61 | 106 | 30 | 0.78 | (0.43, 1.42) | 41 | 10 | 1.11 | (0.31, 4.01) | 32 | 8 | 0.33 | (0.11, 0.96) | ||
| Q3 | >81.61 to 130.10 | 107 | 46 | 1.24 | (0.70, 2.21) | 48 | 19 | 2.54 | (0.76, 8.57) | 33 | 12 | 0.76 | (0.26, 2.22) | ||
| Q4 | >130.10 | 106 | 72 | 1.81 | (0.94, 3.47) | 53 | 42 | 4.84 | (1.31, 17.82) | 26 | 15 | 1.27 | (0.39, 4.18) | ||
| p-value for trend3 | 0.06 | 0.01 | 0.9 | ||||||||||||
| Continuous (log2) | 426 | 191 | 1.31 | (1.05, 1.63) | 180 | 83 | 1.60 | (1.14, 2.22) | 125 | 56 | 1.13 | (0.72, 1.78) | |||
| Free estradiol (pg/mL) | |||||||||||||||
| Q1 | 0 to 0.06 | 106 | 37 | 1.00 | 30 | 16 | 1.00 | 28 | 2 | — | |||||
| Q2 | >0.06 to 0.13 | 107 | 51 | 1.52 | (0.78, 2.96) | 51 | 23 | 1.32 | (0.36, 4.80) | 32 | 17 | — | |||
| Q3 | >0.13 to 0.23 | 106 | 47 | 1.36 | (0.69, 2.69) | 57 | 20 | 0.70 | (0.20, 2.47) | 29 | 14 | — | |||
| Q4 | >0.23 | 107 | 56 | 1.88 | (0.91, 3.90) | 42 | 24 | 1.28 | (0.31, 5.36) | 36 | 23 | — | |||
| p-value for trend3 | 0.2 | 1.0 | 0.04 | ||||||||||||
| Continuous (log2) | 426 | 191 | 1.01 | (0.87, 1.17) | 180 | 83 | 0.89 | (0.62, 1.26) | 125 | 56 | 1.43 | (1.04, 1.95) | |||
| Free testosterone (pg/mL) | |||||||||||||||
| Q1 | 0 to 0.94 | 107 | 64 | 1.00 | 47 | 35 | 1.00 | 34 | 16 | 1.00 | |||||
| Q2 | >0.94 to 1.56 | 106 | 49 | 0.86 | (0.50, 1.49) | 56 | 16 | 0.45 | (0.17, 1.17) | 29 | 17 | 1.39 | (0.50, 3.88) | ||
| Q3 | >1.56 to 2.60 | 106 | 33 | 0.57 | (0.32, 1.03) | 42 | 14 | 0.50 | (0.17, 1.47) | 29 | 9 | 0.66 | (0.20, 2.15) | ||
| Q4 | >2.60 | 107 | 45 | 0.69 | (0.38, 1.23) | 35 | 18 | 0.59 | (0.19, 1.87) | 33 | 14 | 0.88 | (0.32, 2.44) | ||
| p-value for trend3 | 0.1 | 0.3 | 0.5 | ||||||||||||
| Continuous (log2) | 426 | 191 | 0.81 | (0.68, 0.96) | 180 | 83 | 0.90 | (0.66, 1.23) | 125 | 56 | 0.76 | (0.53, 1.08) | |||
| Testosterone:Estradiol | |||||||||||||||
| Q1 | 0 to 12.67 | 105 | 55 | 1.00 | 53 | 24 | 1.00 | 33 | 26 | 1.00 | |||||
| Q2 | >12.67 to 27.26 | 105 | 54 | 0.93 | (0.52, 1.65) | 53 | 22 | 1.40 | (0.52, 3.78) | 34 | 18 | 0.47 | (0.17, 1.27) | ||
| Q3 | >27.26 to 56.71 | 105 | 38 | 0.60 | (0.32, 1.12) | 41 | 20 | 1.59 | (0.51, 5.02) | 34 | 7 | 0.25 | (0.09, 0.76) | ||
| Q4 | >56.71 | 104 | 41 | 0.78 | (0.39, 1.56) | 27 | 15 | 2.02 | (0.56, 7.21) | 24 | 5 | 0.29 | (0.07, 1.09) | ||
| p-value for trend3 | 0.2 | 0.3 | 0.01 | ||||||||||||
| Continuous (log2) | 419 | 188 | 0.89 | (0.78, 1.02) | 174 | 81 | 1.09 | (0.82, 1.44) | 125 | 56 | 0.70 | (0.55, 0.90) | |||
| Free testosterone:Free estradiol | |||||||||||||||
| Q1 | 0 to 6.52 | 104 | 58 | 1.00 | 54 | 25 | 1.00 | 33 | 25 | 1.00 | |||||
| Q2 | >6.52 to 15.49 | 105 | 52 | 0.82 | (0.46, 1.47) | 49 | 25 | 1.27 | (0.46, 3.48) | 37 | 17 | 0.54 | (0.20, 1.45) | ||
| Q3 | >15.49 to 31.02 | 106 | 46 | 0.60 | (0.32, 1.11) | 45 | 21 | 0.94 | (0.31, 2.83) | 32 | 9 | 0.24 | (0.08, 0.75) | ||
| Q4 | >31.02 | 104 | 33 | 0.56 | (0.27, 1.15) | 26 | 10 | 1.09 | (0.29, 4.13) | 23 | 5 | 0.36 | (0.10, 1.28) | ||
| p-value for trend3 | 0.07 | 0.9 | 0.02 | ||||||||||||
| Continuous (log2) | 419 | 189 | 0.87 | (0.76, 0.99) | 174 | 81 | 0.99 | (0.76, 1.29) | 125 | 56 | 0.72 | (0.56, 0.91) | |||
| Androstenedione:Estrone | |||||||||||||||
| Q1 | 0 to 0.01 | 47 | 25 | 1.00 | 17 | 9 | — | 16 | 7 | — | |||||
| Q2 | >0.01 to 0.02 | 48 | 18 | 0.63 | (0.26, 1.57) | 17 | 6 | — | 10 | 5 | — | ||||
| Q3 | >0.02 to 0.03 | 47 | 18 | 0.61 | (0.22, 1.64) | 8 | 2 | — | 9 | 1 | — | ||||
| Q4 | >0.03 | 48 | 12 | 0.27 | (0.08, 0.91) | 8 | 2 | — | 6 | 1 | — | ||||
| p-value for trend3 | 0.05 | 0.2 | 1.0 | ||||||||||||
| Continuous (log2) | 190 | 73 | 0.75 | (0.55, 1.02) | 50 | 19 | 0.03 | (0.00, 200.30) | 41 | 14 | 0.78 | (0.38, 1.62) | |||
Models are adjusted for age (continuous), matching factors, BMI, smoke status, alcohol status, HBV, anti-HCV, diabetes, and current menopausal hormone therapy use.
Models with categories of <5 cases are suppressed.
P-values for linear trend were calculated using the Wald test.
A doubling in the concentration of 4-androstenedione was associated with a 50% reduction in liver cancer risk (OR=0.50,95%CI=0.30–0.82;P=0.006;Table 2). The highest quartile of 4-androstenedione was associated with a 73% reduced liver cancer risk (OR=0.27,95%CI=0.09–0.80;Ptrend=0.01). Similar inverse trends were observed for HCC and ICC, although neither association attained statistical significance. A doubling in the concentration of free testosterone was modestly associated with a decreased liver cancer risk (OR=0.81,95%CI=0.68–0.96;P=0.01). Similar, although not statistically significant, associations with free testosterone were observed for both HCC and ICC. No other associations were observed between androgens and liver cancer risk.
For SHBG, a doubling in the concentration was associated with a 31% increased liver cancer risk (OR=1.31,95%CI=1.05–1.63;P=0.02;Table 2). A similar association was also observed for SHBG and HCC risk (OR=1.60,95%CI=1.14–2.22;P=0.006). The highest quartile of SHBG was associated with a 4.8-times increased HCC risk (OR=4.84,95%CI=1.31–17.82;Ptrend=0.01). There was little to no association with ICC risk (OR per one unit increase in log2 SHBG=1.13,95%CI=0.72–1.78;P=0.6).
Androgen:estrogen ratio metrics were associated with a decreased ICC risk (Table 2). A doubling of the testosterone:estradiol ratio was associated with 30% decreased ICC risk (OR=0.70,95%CI=0.55–0.90;P=0.009). Results were similar for the ratio of free testosterone:free estradiol.
Demographics by parent study are shown in Supplemental Table S3. To examine potential differences by parent study, we conducted a sensitivity analysis using study-specific quartiles of hormone concentrations; results were similar (Supplemental Table S4). Adjustment for covariates had little effect on the observed estimates, as evidenced in the minimally adjusted models (Supplemental Table S5). Follow-up in the parent cohorts varied from 16–45 years (Supplemental Table S1); the mean duration between blood draw and liver cancer diagnosis was 7.7 years.
Having an undiagnosed liver cancer at baseline could, in theory, affect hormone concentrations. Thus, we conducted lag analyses excluding case-control pairs whose case was diagnosed within 2, 5, and 10 years from parent study baseline; results were similar (Supplemental Table S6). When we excluded WHI, adjusted for BMI as continuous, or excluded hormone values below the LLOQ, results were similar (data not shown).
Conclusion
In an analysis combining data from five large prospective cohort studies, higher prediagnostic concentrations of circulating 4-androstenedione were associated with 50% reduction in liver cancer risk in post-menopausal females, while higher levels of SHBG were associated with a 31% increased risk. Additionally, higher levels of estradiol and free estradiol were associated with a 40–43% increased risk of ICC, but not HCC.
This is the first study to examine the association between prediagnostic sex steroid hormone levels and liver cancer risk in post-menopausal women. Prior studies conducted in Asian populations reported that testosterone levels were higher in male HCC cases compared to controls (11–14). However, a primary risk factor for liver cancer in Asia is HBV, and it has been reported that HBV X protein enhances androgen receptor-responsive gene expression in a manner that is dose-dependent on circulating androgen levels, possibly accounting for a portion of the male-predominance of HBV-related HCC (31). In a study from Shanghai, China, the highest level of serum testosterone was associated with a 13-fold increased liver cancer risk among HBsAg-positive male participants, but there was no association among HBsAg-negative participants (11). Two European studies have investigated hormones and SHBG, one in a population of males with cirrhosis and one in a general population study that included females (15, 16). Similar to the current report, these studies found that SHBG, but not testosterone, was associated with increased HCC risk.
4-androstenedione is the only circulating androgen that has higher concentrations in pre-menopausal women than in men (23). In pre-menopausal women with oophorectomy or in post-menopausal women, circulating 4-androstenedione is decreased by 50% (32). 4-androstenedione serves as a prohormone and can be converted to testosterone by 17β-hydroxysteroid dehydrogenase or to estrone by aromatase. Additionally, 4-androstenedione itself has weak androgenic activity, which is approximately 10% that of testosterone (23). Reasons for an inverse association between circulating 4-androstenedione concentration and liver cancer risk are not completely understood. In experimental studies of both male and female rodents, hepatocarcinogenesis has been promoted by 4-androstenedione, which is thought to cause positive regulation of male gene expression through STAT5b (33–35). However, the animal studies administered exogenous androstenedione compounds via oral gavage. Thus, the relevance to humans is questionable. Alternatively, androgens are known to induce immunosuppression, which is thought to be related to the female preponderance of autoimmune diseases (36). In a study examining modulation of thymocyte (i.e., precursor of T lymphocytes) proliferation, testosterone and DHT inhibited, while androstenedione increased, thymocyte proliferation (37). Taken together, these studies suggest that testosterone and DHT promote immunosuppression, which is typically seen in males, while androstenedione enhances immunostimulation, suggesting a female-type immune response that may be beneficial in suppressing liver cancer development.
SHBG levels are typically twice as high in women as in men, due to estrogen promotion and androgen inhibition of SHBG production (23). Two prior studies have found similar associations to those reported herein between circulating SHBG and liver cancer risk (15, 16), but the mechanisms underlying this association are unclear. Studies of HCV-infected individuals reported that SHBG levels were positively associated with severity of liver disease in men (38), but this association was not observed in women (39). However, it has been hypothesized that SHBG may be a marker of liver damage. The mechanism underlying this correlation between SHBG and liver damage has been suggested to be through IGF-1, which downregulates SHBG production (40). Concentrations of IGF-1 have been shown to decrease preceding HCC diagnosis (41, 42). In our lag analysis, the association between SHBG and liver cancer risk was robust, even after excluding participants that developed liver cancer within 10 years of blood draw. However, this could suggest that IGF-1 and SHBG are dysregulated early in hepatocarcinogenesis.
In contrast to the hypothesis that higher levels of estrogens decrease liver cancer risk, we report that higher levels of estradiol and free estradiol were associated with an increased ICC risk and had no association with HCC. A prior study from Thailand similarly reported higher estradiol in male cholangiocarcinoma patients (n=54) compared to controls (n=68) (43) but did not examine women and nor stratify by cholangiocarcinoma topography (i.e., intrahepatic versus extrahepatic). Additional experimental studies have implicated factors that affect estrogenic regulation in ICC development. In a normal liver, cholangiocytes have undetectable levels of estrogen receptors, but ICC tumors express both estrogen receptor-α and -β (44–46). Experimental evidence suggests that estrogen, potentially mediated through interleukin-6 or vascular endothelial growth factor, promotes cholangiocarcinogenesis, while selective estrogen receptor modulators can inhibit growth (47–49). Taken together, these observations raise the provocative hypothesis that estrogen could partially explain the sex differences reported in incidence rates of ICC and HCC.
Strengths of this study include use of state-of-the-art quantitation of sex steroid hormones, serum samples collected prior to incident diagnosis of cancer, and large sample size. Prior studies utilized radioimmunoassay for hormone quantitation (11–16, 43). While correlations between radioimmunoassay and mass spectrometry technologies are high, mass spectrometry is considered to be the gold standard for hormone quantification, as it has greater sensitivity and accuracy of detection (50). The study design, which entailed pooling data across five prospective cohort studies, allowed for the first study of the association between hormones and liver cancer risk in a female population. The sample size also allowed for stratification by liver cancer type – HCC and ICC. However, the samples sizes for HCC and ICC were still somewhat limited, which made it difficult to determine differences in associations by liver cancer histology.
Limitations of the current study include lack of information on preexisting liver disease and generalizability. We utilized information and serum samples collected prior to diagnosis among women enrolled in prospective cohort studies. However, none of these studies were specifically designed to assess liver cancer outcomes and provided no information on possible preexisting liver diseases. As underlying disease processes might affect hormone concentrations, we conducted a lag analysis. Results were similar, suggesting that preexisting liver disease did not substantially affect our findings. However, we cannot definitively say that potential preexisting liver disease was excluded in these lag analyses. Further, this study is only among post-menopausal females, thus may not be generalizable to men and pre-menopausal women. The great majority of liver cancer among women in the US, however, occurs among post-menopausal women. Studying risk factors for liver cancer among women is of vital as the forecast rates of liver cancer in women are increasing faster than among men (51). Thus, women will share a larger burden of liver cancer in the future.
In summary, this study does not support the hypothesis that higher levels of estrogens decrease, or androgens increase, liver cancer risk among postmenopausal women. We report that among women, higher 4-androstenedione levels were associated with a decreased liver cancer risk, while higher SHBG levels were associated with an increased risk. Further, higher levels of circulating estradiol and free estradiol were associated with a significantly increased risk of ICC, but not HCC. These results provide evidence of etiological heterogeneity by liver cancer histologic type with regard to the roles of estrogens and androgens. While intriguing, these findings need to be replicated in additional populations.
Supplementary Material
Acknowledgements:
For the Nurses’ Health Study, we would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
For the Women’s Health Initiative, the full list of investigators that have contributed can be found on the following website: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Funding: NIH Intramural Research Program, National Cancer Institute (JL Petrick, AA Florio, LE Beane-Freeman, JN Hofmann, CM Kitahara, ND Freedman, BI Graubard, J Koshiol, LM Liao, MS Linet, MP Purdue, C Schairer, R Sinha, KA McGlynn). National Institutes of Health grants CA047988 (I Lee, JE Buring), CA182913 (I Lee, JE Buring), HL043851 (I Lee, JE Buring), HL080467 (I Lee, JE Buring), HL099355 (I Lee, JE Buring), K07 CA188126 (X Zhang), DK098311 (AT Chan), CA186107 (AT Chan), CA87969 (AT Chan), CA167552 (AT Chan), UM1 CA182934 (A Zeleniuch-Jacquotte), P30 CA016087 (A Zeleniuch-Jacquotte), P30 ES000260 (A Zeleniuch-Jacquotte), U01 CA164974 (L Rosenberg, JR Palmer), and R01 CA058420 (L Rosenberg, JR Palmer). American Cancer Society Research Scholar Grant RSG NEC-130476 (X Zhang). The WHI program (J Wactawski-Wende, TE Rohan) is funded by the National Institutes of Health contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The HPFS and NHS programs (X Zhang) were support by the National Cancer Institute, National Institutes of Health grant numbers UM1CA186107, P50CA127003, P01CA87969, and UM1CA167552.
Abbreviations:
- MHC
Multiphasic Health Checkup Study
- NYU
New York University
- NHS
Nurses’ Health Study
- PLCO
Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
- WHI
Women’s Health Initiative
- NCI
National Cancer Institute
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- OR
odds ratio
- CI
confidence interval
- DHEA
dehydroepiandrosterone
- 4-dione
4-androstenedione
- 5-diol
Δ−5-androstenediol
- T
testosterone
- DHT
dihydrotestosterone
- ADT
androsterone
- 3β-diol
5-α -androstane-3β,17β-diol
- E1
estrone
- E2
estradiol
- SHBG
sex hormone binding globulin
- p
progesterone
- LLOQ
lower limit of quantification
- HBsAg
hepatitis B surface antigen
- anti-HCV
antibody to HCV
- MHT
menopausal hormone therapy
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Altekruse SF, Devesa SS, Dickie LA, McGlynn KA, Kleiner DE. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag 2011;38:201–205. [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dyke AL, Shiels MS, Jones GS, Pfeiffer RM, Petrick JL, Beebe-Dimmer JL, Koshiol J. Biliary tract cancer incidence and trends in the United States by demographic group, 1999–2013. Cancer 2019;125:1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGlynn KA, Petrick JL, London WT: Chaper 33. Liver Cancer In: Thun MJ, Linet MS, Cerhan JR, Haiman C, Schottenfeld D, eds. Schottenfeld and Fraumeni cancer epidemiology and prevention. Fourth edition. ed. New York, NY: Oxford University Press, 2018; xix, 1308 pages. [Google Scholar]
- 4.De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol 2002;193:59–63. [DOI] [PubMed] [Google Scholar]
- 5.Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology 2010;78 Suppl 1:172–179. [DOI] [PubMed] [Google Scholar]
- 6.Yu MW, Chang HC, Chang SC, Liaw YF, Lin SM, Liu CJ, Lee SD, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology 2003;38:1393–1400. [DOI] [PubMed] [Google Scholar]
- 7.McGlynn KA, Sahasrabuddhe VV, Campbell PT, Graubard BI, Chen J, Schwartz LM, Petrick JL, et al. Reproductive factors, exogenous hormone use and risk of liver cancer among U.S. women: Results from the Liver Cancer Pooling Project. Br J Cancer 2015;(In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vesselinovitch SD, Itze L, Mihailovich N, Rao KV. Modifying role of partial hepatectomy and gonadectomy in ethylnitrosourea-induced hepatocarcinogenesis. Cancer Res 1980;40:1538–1542. [PubMed] [Google Scholar]
- 9.Nakatani T, Roy G, Fujimoto N, Asahara T, Ito A. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn J Cancer Res 2001;92:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamada Y, Kiso S, Yoshida Y, Chatani N, Kizu T, Hamano M, Tsubakio M, et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol 2011;301:G1031–1043. [DOI] [PubMed] [Google Scholar]
- 11.Yuan JM, Ross RK, Stanczyk FZ, Govindarajan S, Gao YT, Henderson BE, Yu MC. A cohort study of serum testosterone and hepatocellular carcinoma in Shanghai, China. Int J Cancer 1995;63:491–493. [DOI] [PubMed] [Google Scholar]
- 12.Yu MW, Cheng SW, Lin MW, Yang SY, Liaw YF, Chang HC, Hsiao TJ, et al. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst 2000;92:2023–2028. [DOI] [PubMed] [Google Scholar]
- 13.Yu MW, Chen CJ. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res 1993;53:790–794. [PubMed] [Google Scholar]
- 14.Tanaka K, Sakai H, Hashizume M, Hirohata T. Serum testosterone:estradiol ratio and the development of hepatocellular carcinoma among male cirrhotic patients. Cancer Res 2000;60:5106–5110. [PubMed] [Google Scholar]
- 15.Ganne-Carrie N, Chastang C, Uzzan B, Pateron D, Trinchet JC, Perret G, Beaugrand M. Predictive value of serum sex hormone binding globulin for the occurrence of hepatocellular carcinoma in male patients with cirrhosis. J Hepatol 1997;26:96–102. [DOI] [PubMed] [Google Scholar]
- 16.Lukanova A, Becker S, Husing A, Schock H, Fedirko V, Trepo E, Trichopoulou A, et al. Prediagnostic plasma testosterone, sex hormone-binding globulin, IGF-I and hepatocellular carcinoma: etiological factors or risk markers? Int J Cancer 2014;134:164–173. [DOI] [PubMed] [Google Scholar]
- 17.Falk RT, Fears TR, Hoover RN, Pike MC, Wu AH, Nomura AM, Kolonel LN, et al. Does place of birth influence endogenous hormone levels in Asian-American women? Br J Cancer 2002;87:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler JL, Ramcharan S, Feldman R, Siegelaub AB, Campbell B, Friedman GD, Dales LG, et al. Multiphasic checkup evaluation study. 1. Methods and population. Prev Med 1973;2:197–206. [DOI] [PubMed] [Google Scholar]
- 19.Toniolo PG, Pasternack BS, Shore RE, Sonnenschein E, Koenig KL, Rosenberg C, Strax P, et al. Endogenous hormones and breast cancer: a prospective cohort study. Breast Cancer Res Treat 1991;18 Suppl 1:S23–26. [DOI] [PubMed] [Google Scholar]
- 20.Belanger CF, Hennekens CH, Rosner B, Speizer FE. The nurses’ health study. Am J Nurs 1978;78:1039–1040. [PubMed] [Google Scholar]
- 21.Kramer BS, Gohagan J, Prorok PC, Smart C. A National Cancer Institute sponsored screening trial for prostatic, lung, colorectal, and ovarian cancers. Cancer 1993;71:589–593. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 23.Yen SSC, Strauss JF, Barbieri RL. Yen & Jaffe’s reproductive endocrinology : physiology, pathophysiology, and clinical management. Seventh edition ed. Philadelphia, PA: Elsevier/Saunders, 2014: xvi, 942 pages. [Google Scholar]
- 24.Caron P, Turcotte V, Guillemette C. A chromatography/tandem mass spectrometry method for the simultaneous profiling of ten endogenous steroids, including progesterone, adrenal precursors, androgens and estrogens, using low serum volume. Steroids 2015;104:16–24. [DOI] [PubMed] [Google Scholar]
- 25.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- 26.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- 27.Holl K, Lundin E, Kaasila M, Grankvist K, Afanasyeva Y, Hallmans G, Lehtinen M, et al. Effect of long-term storage on hormone measurements in samples from pregnant women: the experience of the Finnish Maternity Cohort. Acta Oncol 2008;47:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas HV, Key TJ, Allen DS, Moore JW, Dowsett M, Fentiman IS, Wang DY. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer 1997;75:1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroud LR, Solomon C, Shenassa E, Papandonatos G, Niaura R, Lipsitt LP, Lewinn K, et al. Long-term stability of maternal prenatal steroid hormones from the National Collaborative Perinatal Project: still valid after all these years. Psychoneuroendocrinology 2007;32:140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008: x, 758 p. [Google Scholar]
- 31.Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, Chen PJ, Yang WJ, et al. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A 2007;104:2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder PJ. The role of androgens in women. J Clin Endocrinol Metab 2001;86:1006–1007. [DOI] [PubMed] [Google Scholar]
- 33.Blystone CR, Elmore SA, Witt KL, Malarkey DE, Foster PM. Toxicity and carcinogenicity of androstenedione in F344/N rats and B6C3F1 mice. Food Chem Toxicol 2011;49:2116–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toxicology and carcinogenesis studies of androstenedione (CAS No. 63-05-8) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser 2010:1, 7–31,33–171 passim. [PubMed] [Google Scholar]
- 35.Rooney JP, Ryan N, Chorley BN, Hester SD, Kenyon EM, Schmid JE, George BJ, et al. From the Cover: Genomic Effects of Androstenedione and Sex-Specific Liver Cancer Susceptibility in Mice. Toxicol Sci 2017;160:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock 2000;14:81–90. [DOI] [PubMed] [Google Scholar]
- 37.Yao G, Shang XJ. A comparison of modulation of proliferation of thymocyte by testosterone, dehydroisoandrosterone and androstenedione in vitro. Arch Androl 2005;51:257–265. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen HV, Mollison LC, Taylor TW, Chubb SA, Yeap BB. Chronic hepatitis C infection and sex hormone levels: effect of disease severity and recombinant interferon-alpha therapy. Intern Med J 2006;36:362–366. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar M, Lai JC, Sawinski D, Zeigler TE, Cedars M, Forde KA. Sex hormone levels by presence and severity of cirrhosis in women with chronic hepatitis C virus infection. J Viral Hepat 2019;26:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bezemer ID, Rinaldi S, Dossus L, Gils CH, Peeters PH, Noord PA, Bueno-de-Mesquita HB, et al. C-peptide, IGF-I, sex-steroid hormones and adiposity: a cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 2005;16:561–572. [DOI] [PubMed] [Google Scholar]
- 41.Mazziotti G, Sorvillo F, Morisco F, Carbone A, Rotondi M, Stornaiuolo G, Precone DF, et al. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective study. Cancer 2002;95:2539–2545. [DOI] [PubMed] [Google Scholar]
- 42.Major JM, Stolzenberg-Solomon RZ, Pollak MN, Snyder K, Virtamo J, Albanes D. Insulin-like growth factors and liver cancer risk in male smokers. Br J Cancer 2010;103:1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunsawong T, Singsuksawat E, In-chon N, Chawengrattanachot W, Thuwajit C, Sripa B, Paupairoj A, et al. Estrogen is increased in male cholangiocarcinoma patients’ serum and stimulates invasion in cholangiocarcinoma cell lines in vitro. J Cancer Res Clin Oncol 2012;138:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvaro D, Alpini G, Onori P, Franchitto A, Glaser SS, Le Sage G, Folli F, et al. Alfa and beta estrogen receptors and the biliary tree. Mol Cell Endocrinol 2002;193:105–108. [DOI] [PubMed] [Google Scholar]
- 45.Alvaro D, Alpini G, Onori P, Franchitto A, Mancino MG, Glaser S, Drudi-Metalli V, et al. Estrogen Regulation of Cholangiocyte Proliferation In: Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000-2013. [Google Scholar]
- 46.Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol 2006;169:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isse K, Specht SM, Lunz JG 3rd, Kang LI, Mizuguchi Y, Demetris AJ. Estrogen stimulates female biliary epithelial cell interleukin-6 expression in mice and humans. Hepatology 2010;51:869–880. [DOI] [PubMed] [Google Scholar]
- 48.Mancino A, Mancino MG, Glaser SS, Alpini G, Bolognese A, Izzo L, Francis H, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis 2009;41:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampson LK, Vickers SM, Ying W, Phillips JO. Tamoxifen-mediated growth inhibition of human cholangiocarcinoma. Cancer Res 1997;57:1743–1749. [PubMed] [Google Scholar]
- 50.Hsing AW, Stanczyk FZ, Belanger A, Schroeder P, Chang L, Falk RT, Fears TR. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev 2007;16:1004–1008. [DOI] [PubMed] [Google Scholar]
- 51.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. Journal of Clinical Oncology 2016;34:1787–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


