Abstract
Background:
Attention-deficit/hyperactivity disorder (ADHD) symptoms have been linked with eating behaviors and obesity adolescence and young adulthood. Yet, little is known about whether these associations occur during early childhood and few studies have examined these associations prospectively.
Objectives:
To assess magnitude and direction of associations between childhood ADHD symptoms and eating behaviors.
Methods:
Participants were from the Newborn Epigenetics Study (N = 470, M age = 4 years). Multivariable linear regression models were used to examine cross-sectional associations between ADHD symptoms and eating behaviors. Latent Change Score (LCS) modeling was performed to examine prospective association among a subset of children with available follow-up data. (N = 100, M age = 7 years).
Results:
The cross-sectional results showed that attention problem (AP) and hyperactivity (HY) were positively associated with food responsiveness, emotional overeating, desire to drink, and slowness in eating. AP, but not HY, was inversely associated with enjoyment of food. Results of the LCS models revealed AP and HY were both positively associated with prospective changes in emotional overeating and satiety responsiveness. AP was further positively associated with prospective changes in food responsiveness. The reverse relationship predicting changes in ADHD symptoms from earlier assessments of eating behaviors was not significant.
Conclusion:
Results suggest a link between ADHD symptoms, and obesity-related eating behaviors in early childhood, highlighting the need to address self-regulation and healthy eating behaviors in the prevention of childhood obesity.
Keywords: ADHD, eating behaviors, self-regulation, childhood, latent change score
INTRODUCTION
The prevalence rate of overweight and obesity among preschoolers (aged 2-5 years) is of high public health concern. Overweight and obesity during early childhood has been associated with numerous morbidities and if continued through adulthood increases the risk of chronic disease and some cancers.1-9 Thus, understanding the factors contributing to early childhood obesity is critical to advance prevention efforts.
Although childhood obesity is a condition driven by numerous factors at multiple levels of influence, a growing body of research has begun to examine the link that Attention Deficit Hyperactivity Disorder (ADHD) and related symptoms have with obesity. The association between ADHD and obesity was implied in early reports of an elevated prevalence of ADHD among adult bariatric patients,10 as well as in subsequent studies showing children and adolescents diagnosed with ADHD have a higher risk for obesity than their counterparts without ADHD.11,12 At the same time, population-based studies examining ADHD symptoms (not the disorder per-se) have also found a monotonic relationship between increasing ADHD symptoms and increasing Body Mass Index (BMI).13
Related also are studies examining domains of executive cognitive functions (poor inhibitory control, attention, or short-term memory), which are often diminished among children with elevated ADHD symptoms. Specifically, these studies have shown significant associations between poorer performance on tasks of executive functions and higher BMI or greater weight gain during childhood and early adulthood.13-17 Meta-analyses of these studies have shown that across studies, ADHD and diminished executive functioning capacities are related to a higher BMI and greater risk for obesity in childhood; however the effect sizes observed have been small.14,18,19 On the other hand, the relationship between ADHD and eating disorders showed stronger effects.20,21 Thus, it may be that ADHD and related decrements in cognitive functions are more closely aligned with the development of problematic eating behaviors, which then increases the risk of obesity through development.
Childhood ADHD has been associated prospectively with eating disorders that occur in adolescence, 22-24 and childhood ADHD symptoms have been associated with later loss of control over eating in adulthood.25 In a recent cross-sectional study of a sample of children in China (mean age of 10.6 years), ADHD symptoms were found to be related to emotional eating (over and under eating), but not with BMI.26 Similarly, in a cross-sectional study of children (mean age = 4 years) from the Rhea Cohort (Crete, Greece), investigators found that food approach eating behaviors (food responsiveness and emotional overeating) were positively associated with ADHD symptoms, including impulsivity, inattention and hyperactivity.27 Fewer studies have investigated the association between ADHD symptoms and eating behaviors prospectively. Exceptions are studies of clinical samples of children with ADHD that find elevated comorbidities with eating disorders during adolescence among cases compared to controls22-24 and at least two longitudinal cohort studies.28,29 With regard to the latter, Sonneville et al. (2015) followed children from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort from mid-childhood (mean age = 7.5 years) through mid-adolescence (age range = 14-16 years) and found that ADHD symptoms in childhood predicted binge eating behavior during adolescence.28 Likewise, Bjorklund et al., (2018) assessed predictors of food responsiveness in a large community sample of Norwegian 6-year old children, followed at age 8 and 10 years of age, and found that greater symptoms of ADHD at 6 years of age significantly predicted greater food responsiveness at 8 and 10 years of age accounting for a number of other covariates.29
These prospective studies, though limited, are extremely important since they imply a possible direction of association between ADHD and eating behaviors. That is, ADHD symptoms predicting subsequent problematic eating problems and obesity and not the reverse. However, these studies do not explicitly test the possibility that problematic eating behaviors and obesity may precede ADHD and related symptoms or if there are potential bi-directional relationships. In adults, adiposity during midlife is a risk factor for later cognitive problems, like dementia.30 It is possible that children with obesity and related eating behavior problems may develop subsequent problems with executive functions or behavioral symptoms related to ADHD; however, many of the studies in children have examined ADHD symptoms and executive functions as risk factors for subsequent eating problems and obesity.31 Nevertheless, additional studies that evaluate directional and possible bi-directional relationships are needed to help bring additional clarity to this association between ADHD symptoms and eating behaviors.
Given there are few studies linking ADHD symptoms and obesogenic eating behaviors, additional research is needed to clarify the direction and magnitude of this potential relationship. Further, no studies that we are aware of have investigated this relationship between ADHD symptoms and eating behaviors prospectively in young pre-school age children. The current study had two primary aims. First, we examined the degree to which previous cross-sectional findings replicated in our sample of preschool aged children recruited from the Newborn Epigenetic Study Cohort (NEST). Based on the extant literature, we hypothesized that ADHD symptoms in early childhood would be associated with eating behaviors, particularly food responsiveness, which relates to eating in response to external cues and was associated with ADHD symptoms in the study by Bjorklund discussed above.29 Second, in a subsample of children with available longitudinal data, we investigated relationships between ADHD and eating behaviors prospectively. Given that extant literature in children suggests that ADHD symptoms would predict changes in eating behaviors over time we hypothesized this directional relationship; however, we tested reverse and bi-directional associations as well.
METHODS
Study Sample
Participants were part of the Newborn Epigenetic Study (NEST), a pre-birth cohort initiated in 2005 to examine the effects of early exposures on epigenetic profiles and development outcomes, which has been further described in previous papers.32,33 The Institutional Review Board (IRB) at Duke University approved this study (IRB numbers Pro00043781, Pro00014548, and Pro00064859). Of the 3,545 approached, 2,546 were successfully enrolled (72%). Participation was not associated with educational level, maternal age, maternal BMI, and although Asian pregnant mothers were less likely to participate, participation was similar with respect to mothers of other racial/ethnic backgrounds. Women were recruited from prenatal clinics serving Duke University Hospital and Durham Regional Hospital Obstetrics facilities from April 2005 to June 2011. Attempts were made either by mail or recruitment in prenatal clinics to invite all women to participate in NEST who were eligible. Eligibility criteria included: English or Spanish speaking, age ≥ 18 years, pregnant, and had intentions to use one of the two obstetrics facilities enabling access to labor and birth outcome data.
Between January 2011 and June 2013, mothers who had children between the ages of 2 and 6 years of age (N = 1272) were sent a survey that included measures assessing ADHD symptoms and eating behaviors, and 531 (42%) returned their survey. In 2016 - 2018, a subset of women (n = 218) from the initial enrollment cohort were re-contacted for a follow-up sub-study where mothers repeated some of the assessments that were completed in the 2011-2013 survey. Overall, 470 mothers who had completed at least one survey between 2011 and 2013 or between 2016 and 2018 were included in the present cross-sectional analyses. The children’s mean age for these 470 mother-child pairs was 4 years of age (SD = 1.93). Comparing the full baseline sample (n = 2,175) to the cross-sectional sample (n = 470), there was a significant difference in terms of maternal ethnicity, as there were significantly fewer Hispanic/Latino mothers represented in the follow-up cross-sectional sample (χ2 (2) = 82.33, p < .001). This was likely due to the fact that a Spanish language surveys were not available at the follow-up assessments. In addition, mothers represented in the cross-sectional follow-up sample had higher educational attainment (χ2 (3) = 67.90, p < .001) and were approximately 8.5 months older at delivery than those in the baseline sample (M = 28.89 vs 28.16, t (807.1) = 2.52, p = .01). No differences were found in terms of child’s sex (χ2 (1) = 0.814, p = .37) or with respect to pre-pregnancy BMI (t (658.97) = 1.13, p = .26).
Of these 470 mother-child pairs, 100 mother-child pairs were participants in both the earlier 2011 to 2013 survey and follow-up 2016 to 2018 survey. The mean age of children in this prospective set of 100 children was 7 years of age (SD = 1.52). Like the cross-sectional sample, this sample of 100 mother-child pairs differed from the full baseline sample in terms of maternal ethnicity (fewer Hispanics; χ2 (2) = 16.78, p < .001) and education of mothers (higher educational attainment; χ2 (3) = 10.30, p = .02), but did not differ with respect to child’s sex, χ2 (1) = 1.31, p = .252, maternal age at delivery, t (108.87) = 1.02, p = .31, or pre-pregnancy BMI, t (104.9) = 1.68, p = .10.
The cross-sectional sample of 470 children did not statistically differ from the prospective sample of 100 children in terms of maternal race (χ2 = 1.40, p = .50), education level (χ2 = 3.31, p = .35), child’s sex (χ2 = 1.06, p = .30), or age at delivery, t (154.35) = 0.03, p = .95. Mothers in the cross-sectional sample had significantly higher pre-pregnancy BMI (M = 28.94) than mothers in the prospective sample (M = 26.79), t (173.79) = 2.33, p = .02.
Measures
Child ADHD symptoms
Parent Rating Scales from The Behavior Assessment System for Children 2nd edition (BASC-2), a valid and reliable comprehensive measure of a child’s problem behaviors and appropriate for use in children age 2 to 11 years, was used to assess ADHD related symptoms from the clinical problems subscales: hyperactivity (HY) and attention problem (AP).34 These clinical subscales have been shown to discriminate between children with ADHD and healthy controls.35 For the purposes of this study, raw scores were examined for HY and AP subscales. Raw scores are used in the analyses since they are more precise and uniform, relative to age and sex adjusted T-scores, and are generally recommended for correlative and longitudinal analyses.36,37 T-scores are presented for descriptive purposes, with scores between 60 and 70 considered “at risk” and scores ≥70 considered “clinically significant (values correspond to the 85th and 98th age-adjusted percentile rank)38. Higher AP and HY scores suggest more problematic behaviors. The BASC-2 includes a set of age appropriate questions regarding preschool behavior and child behavior with fewer questions for HY and AP behaviors for the preschool version. Thus, the average score was used to equate the two versions. Internal reliability in the sample of 470 was .80 for the HY subscale and .77 for the AP subscale. Internal reliability in the sample of 100 was .86 for the HY subscale and .84 for the AP subscale at the follow-up time point when children were ~7 years of age.
Child Eating Behaviors
The Children’s Eating Behavior Questionnaire (CEBQ) was used to assess child eating behaviors.39 Items for the CEBQ were developed from focus groups and interviews with parents of children aged 1 to 12 years (mean age: 3.8 (± 1.7) years). The scale has been used in studies of children with an age range of 2 to 11 years.40-42 Items have Likert scale response options ranging from 1 (never) to 5 (always). Although eight eating behaviors are assessed in the CEBQ, we restricted our analyses to include four food approaching behaviors: food responsiveness (FR), enjoyment of food (EF), emotional overeating (EOE), desire to drink (DD); and two food avoidant behaviors: satiety responsiveness (SR) and slowness in eating (SE), which were assessed in both evaluation time-points. Food approaching behaviors reflect a general avidity toward eating and food, e.g., “if allowed, my child would eat too much” (FR); “my child loves food” (EF); “my child eats more when worried” (EOE); and “if given the chance, my child would always be having a drink (DD)”; whereas food avoidant behaviors reflect how easily a child reaches satiety/fullness, such as “my child gets full before his/her meal is finished” (SR) or the speed of eating, such as “my child takes more than 30 minutes to finish a meal” (SE). The CEBQ has been shown to have high internal consistency, good test-retest reliability, and stability over time. 39,43 For the study sample, Cronbach’s alpha for the sample of 470 for each of the subscales range from .65 to .90. For the study sample of 100 Cronbach’s alpha for each of the subscales range from .70 to .88 at the follow-up time point when children were ~7 years of age. Many of these particular subscales have been also correlated with BMI.44
Covariates and other variables
Child heights and weights were measured and used to calculate BMI-for-age z-scores (BMI) using reference data from the Centers for Disease Control and Prevention45. Child BMI was measured either during a study visit or a well-child clinic visit, and measurements used in analyses were selected to occur during the same year as the CEBQ data. Maternal sociodemographic information and child’s characteristics were either extracted from medical records (child’s sex) or were reported during enrollment or follow-up surveys completed by the mother (race, education level). Mothers and children’s ages were calculated based on survey date, mother’s birth date and delivery date obtained from medical records.
Statistical analyses
First, we examined the cross-sectional associations between ADHD symptoms and concurrent eating behaviors using multivariable linear regression. Each CEBQ construct was regressed on the two BASC subscales, AP and HY, separately. The distribution of the emotional overeating subscale was skewed and thus was log transformed. Potential confounders were added to the regression models, which included maternal race, education, child’s age, sex and BMI z-scores. SAS 9.4 was used for data management, while STATA 14.1 was used for regression analyses.
Next, we examined prospective relationships between ADHD symptoms and eating behaviors using latent change score (LCS) modeling. LCS modeling is a structural equation modeling method that is widely used in longitudinal studies and cognitive research.46-51 In addition to being able to identify relationships between baseline levels and changes in individual trajectories for one trait of interest, LCS modeling is also useful for disentangling the directional relationship between two traits of interest.47 Mplus 7 was used for LCS modeling.
As shown in Figure 1, the conceptual framework for LCS within this study captures the relationship among and between variables at time 1 (during the preschool years) and time 2 (during the childhood years) for six different relationships of interest: 1) covariance between ADHD symptoms and eating behaviors at time 1 (path A); 2) the effect of ADHD symptoms at time 1 on the change of ADHD symptoms from time 1 to time 2 (path B); 3) the effect of ADHD symptoms at time 1 on the change in eating behavior from time 1 to time 2 (path C); 4) the effect of eating behaviors at time 1 on the change of ADHD symptoms from time 1 to time 2 (path D); 5) the effect of eating behaviors at time 1 on the change of eating behaviors from time 1 to time 2 (path E); 6) and the covariance between changes in ADHD and eating behaviors in school age (path F). For this particular study we were most interested in paths C and D, which test whether baseline ADHD symptoms lead to changes in eating behavior or vice versa. The same set of covariates used the cross-sectional models were used in the prospective model.
Figure 1.
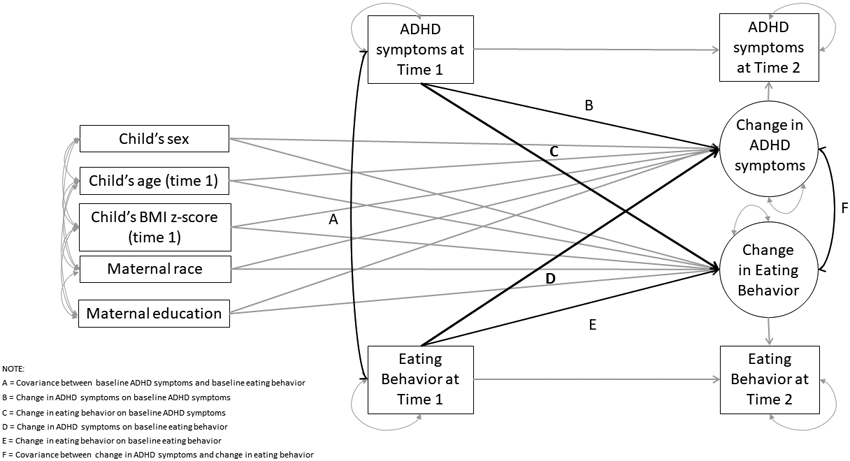
Conceptual framework for Latent Change Score (LCS) modeling of ADHD symptoms and eating behaviors. Path A: covariance between ADHD symptoms and eating behaviors at baseline; Path B: effect of ADHD symptoms at time 1 on the change of ADHD symptoms from time 1 to time 2; Path C: effect of ADHD symptoms at time 1 on the change in eating behavior from time 1 to time 2; Path D: effect of eating behaviors at time 1 on the change of ADHD symptoms from time 1 to time 2; Path E: effect of eating behaviors at time 1 on the change of eating behaviors from time 1 to time 2; Path F: covariance between changes in ADHD and eating behaviors in school age
Additionally, we conducted supplementary analyses to disentangle relationships between ADHD symptoms, eating behaviors, and child BMI. Multivariable regressions and LCS modeling were performed for cross-sectional and prospective analyses separately.
RESULTS
Characteristics of the overall analytic sample are described in Table 1. Within the cross-sectional sample, the mean age was 33 years (SD = 5.8) for mothers and 4 years (SD = 1.9) for children. Most women reported their race/ethnicity was “Black” (51%) or “White” (41%). Nearly half of the mothers reported their highest level of education as “college graduate” (47%). Based on BMI cut-offs, fifty-two children (11%) included in analyses were overweight, and 87 (19%) were obese. Children’s mean T-score was 50.12 (SD = 9.2) for BASC AP subscale and 47.9 (SD = 10.4) for BASC HY subscale, with n = 79 (17%) and n = 51 (11%) children having T-scores of 60 or above (“at risk” or “clinically significant” range) for AP and HY, respectively. With regard to eating behaviors, children’s mean scores of CEBQ ranged from 1.4 (SD = 0.6) for emotional overeating scale to 3.6 (SD = 0.8) for the enjoyment of food scale.
Table 1.
Descriptive statistics of the sample characteristics (N = 470)
| Cross-sectional Sample | ||
|---|---|---|
| N | % or Mean ± SD | |
| Maternal characteristics | ||
| Age at delivery, (years) | 470 | 33.1 ± 5.8 |
| Race, n (%) | ||
| White | 197 | 41.9 |
| Black | 239 | 50.9 |
| Other | 34 | 7.2 |
| Maternal education level, n (%) | ||
| High school graduate or lower | 249 | 53.0 |
| College graduate or higher | 221 | 47.0 |
| Child characteristics | ||
| Age, (years) | 470 | 3.7 ± 1.9 |
| Sex, n (%) | ||
| Boy | 237 | 50.4 |
| Girl | 233 | 49.6 |
| Weight status, n (%) | ||
| Underweight (Less than the 5th percentile) | 45 | 9.6 |
| Normal or Healthy Weight (5th - 85th percentile) | 286 | 60.9 |
| Overweight (85th - 95th percentile) | 52 | 11.1 |
| Obese (95th percentile or greater) | 87 | 18.5 |
| ADHD symptoms (BASC-2) | ||
| Attention problem (AP) | ||
| Raw score | 470 | 1.1 ± 0.5 |
| T score | 470 | 50.1 ± 9.2 |
| Hyperactivity (HY) | ||
| Raw score | 470 | 0.9 ± 0.5 |
| T score | 470 | 47.9 ± 10.4 |
| Eating behaviors (CEBQ) | ||
| Food Responsiveness (FR) | 470 | 2.3 ± 0.9 |
| Enjoyment of food (EF) | 470 | 3.6 ± 0.8 |
| Emotional overeating (EOE) | 470 | 1.4 ± 0.6 |
| Desire to drink (DD) | 468 | 3.3 ± 1.2 |
| Satiety responsiveness (SR) | 470 | 2.9 ± 0.7 |
| Slowness in eating (SE) | 470 | 2.8 ± 0.7 |
SD - standard deviation; BMI - body mass index.
Associations between ADHD symptoms and concurrent eating behaviors are presented in Table 2. Controlling for maternal race, education, child’s age, sex, and child BMI z-scores, attention problem (AP) scores were significantly positively associated with food responsiveness (B = 0.19, 95% CI 0.03 to 0.34), emotional overeating (B = 0.08, 95% CI 0.02 to 0.14), desire to drink (B = 0.28, 95% CI 0.08 to 0.48), slowness in eating (B = 0.17, 95% CI 0.04 to 0.29) and significantly negatively associated with enjoyment of food (B =−0.20, 95% CI −0.35 to −0.05). Hyperactivity (HY) was significantly positively associated with food responsiveness (B = 0.42, 95% CI 0.24 to 0.60), emotional overeating (B = 0.09, 95% CI 0.02 to 0.15), desire to drink (B = 0.51, 95% CI 0.29 to 0.74), slowness in eating (B = 0.18, 95% CI 0.04 to 0.33), and satiety responsiveness (B = 0.16, 95% CI 0.002 to 0.31).
Table 2.
Cross-sectional associations between ADHD symptoms and eating behaviors (N=470)
| Attention Problem (AP) |
Hyperactivity (HY) |
|||||
|---|---|---|---|---|---|---|
| Eating behaviors (CEBQ) | B | 95% CI | p | B | 95% CI | p |
| Food Responsiveness (FR) | 0.19 | [ 0.03, 0.34 ] | .02 | 0.42 | [0.24, 0.60] | <.001 |
| Enjoyment of Food (EF) | −0.20 | [−0.35, −0.05] | .01 | −0.02 | [−0.19, 0.15 ] | .83 |
| Emotional Overeating (EOE)a | 0.08 | [0.02, 0.14 ] | .01 | 0.09 | [0.02, 0.15] | .01 |
| Desire to Drink (DD) | 0.28 | [0.08, 0.48] | .01 | 0.51 | [ 0.29, 0.74 ] | <.001 |
| Satiety Responsiveness (SR) | 0.11 | [ −0.02, 0.24 ] | .11 | 0.16 | [0.002, 0.31 ] | .049 |
| Slowness in Eating (SE) | 0.17 | [ 0.04, 0.29 ] | .01 | 0.18 | [ 0.03, 0.32 ] | .02 |
B - Unstandardized coefficient; CI - confidence interval; p - p value.
Models adjusted for maternal race, education, child’s age, sex and BMI-z score.
Bolds indicate statistically significant at p <0.05.
Emotional overeating scores were not normally distributed, so to improve normality log transformed scores were used in multivariable regression models.
Table 3 displays paths illustrated in Figure 1. Path C shows that greater AP symptoms at time 1 was significantly associated with greater changes from time 1 to time 2 in food responsiveness (BΔ = 0.42, p = .01), emotional overeating (BΔ = 0.41, p <.001), and satiety responsiveness (BΔ = 0.43, p < .001). Path C for HY symptoms shows that greater HY symptoms at time 1 was also associated with greater change from time 1 to time 2 in emotional overeating and satiety responsiveness (BΔ = 0.35, p = .01; BΔ = 0.42, p = .01, respectively). We did not observe any significant association with respect to path D: paths predicting changes from time 1 to time 2 in ADHD symptoms in relation to time 1 eating behaviors. Taken together, the results suggest that early ADHD symptoms have a significant association with changes in eating behaviors from early childhood (~age 4) to later childhood (~age 7), but not vice versa.
Table 3.
Prospective associations between ADHD symptoms and eating behaviors using latent change score (LCS) modeling (N=100)
|
LCS path |
ADHD Symptoms | Model 1 Food Responsiveness |
Model 2 Enjoyment of Food |
Model 3 Emotional Overeating |
Model 4 Desire to Drink |
Model 5 Satiety Responsiveness |
Model 6 Slowness in Eating |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | B | SE | p | B | SE | p | B | SE | p | B | SE | p | ||
| Path A | Attention Problem (AP) | 0.10 | 0.05 | .04 | −0.06 | 0.04 | .17 | 0.05 | 0.03 | .10 | 0.15 | 0.07 | .03 | 0.03 | 0.04 | .43 | 0.05 | 0.04 | .15 |
| Hyperactivity (HY) | 0.04 | 0.04 | .37 | −0.05 | 0.04 | .15 | 0.05 | 0.02 | .06 | 0.12 | 0.06 | .04 | 0.04 | 0.04 | .19 | 0.06 | 0.03 | .05 | |
| Path B | Attention Problem (AP) | −0.43 | 0.10 | <.001 | −0.41 | 0.10 | <.001 | −0.44 | 0.10 | <.001 | −0.42 | 0.10 | <.001 | −0.42 | 0.10 | <.001 | −0.43 | 0.10 | <.001 |
| Hyperactivity (HY) | −0.51 | 0.08 | <.001 | −0.53 | 0.08 | <.001 | −0.50 | 0.08 | <.001 | −0.51 | 0.08 | <.001 | −0.51 | 0.08 | <.001 | −0.54 | 0.08 | <.001 | |
| Path C | Attention Problem (AP) | 0.42 | 0.16 | .01 | −0.06 | 0.14 | .67 | 0.41 | 0.12 | <.001 | 0.13 | 0.17 | .43 | 0.43 | 0.12 | <.001 | 0.11 | 0.13 | .38 |
| Hyperactivity (HY) | 0.20 | 0.18 | .28 | −0.25 | 0.17 | .13 | 0.35 | 0.14 | .01 | −0.11 | 0.20 | .60 | 0.42 | 0.14 | .01 | 0.26 | 0.16 | .09 | |
| Path D | Attention Problem (AP) | 0.01 | 0.06 | .92 | 0.07 | 0.06 | .26 | 0.06 | 0.10 | .51 | −0.02 | 0.04 | .62 | −0.06 | 0.07 | .38 | −0.02 | 0.08 | .85 |
| Hyperactivity (HY) | −0.02 | 0.04 | .56 | −0.04 | 0.04 | .41 | −0.07 | 0.07 | .32 | −0.03 | 0.03 | .40 | −0.02 | 0.05 | .69 | 0.07 | 0.06 | .21 | |
| Path E | Attention Problem (AP) | −0.77 | 0.09 | <.001 | −0.54 | 0.09 | <.001 | −0.76 | 0.11 | <.001 | −0.06 | 0.08 | <.001 | −0.75 | 0.09 | <.001 | −0.75 | 0.10 | <.001 |
| Hyperactivity (HY) | −0.72 | 0.09 | <.001 | −0.56 | 0.09 | <.001 | −0.75 | 0.12 | <.001 | −0.58 | 0.08 | <.001 | −0.76 | 0.09 | <.001 | −0.78 | 0.10 | <.001 | |
| Path F | Attention Problem (AP) | 0.02 | 0.04 | .64 | −0.05 | 0.03 | .16 | −0.07 | 0.03 | .02 | 0.10 | 0.04 | .02 | 0.01 | 0.03 | .95 | 0.01 | 0.03 | .90 |
| Hyperactivity (HY) | 0.08 | 0.03 | .01 | 0.02 | 0.03 | .51 | 0.07 | 0.02 | .01 | 0.05 | 0.03 | .12 | 0.01 | 0.02 | .61 | −0.04 | 0.02 | .11 | |
B - Unstandardized coefficient; SE - standard error; p - p value.
Models adjusted for maternal race, education; child’s age, sex and BMI-z score at baseline.
Bolds indicate statistically significant at p <0.05.
Path A: Covariance between baseline ADHD symptoms and baseline eating behavior
Path B: Change in ADHD symptoms on baseline ADHD symptoms
Path C: Change in eating behavior on baseline ADHD symptoms
Path D: Change in ADHD symptoms on baseline eating behavior
Path E: Change in eating behavior on baseline eating behavior
Path F: Covariance between change in ADHD symptoms and change in eating behavior
In supplementary analyses we also examined the degree to which ADHD symptoms and eating behaviors were related to children’s BMI. Controlling for maternal race, education, child’s age and sex, cross-sectional results (Suppl. Table S1) show children’s BMI to be positively associated with food responsiveness (B = 0.57, 95% CI 0.28 to 0.85), enjoyment of food (B = 0.33, 95% CI 0.03 to 0.63), and emotional overeating (B = 1.51, 95% CI 0.66 to 2.37) and negatively associated with satiety responsiveness (B = −0.56, 95% CI −0.92 to −0.19) and slowness in eating (B = −0.55, 95% CI −0.87 to −0.22). While ADHD symptoms and BMI were not significantly associated in the cross-sectional analyses, the results from LCS showed that greater AP and HY symptoms at time 1 (preschool age) were associated with greater changes in BMI from the preschool period to later childhood (time 2; Suppl. Figure S1, Table S2, AP: BΔBMI=0.98, p = .048; HY: BΔBMI=1.43, p = .01). On the other hand, higher BMI at time 1 was not statistically associated with changes in ADHD symptoms from time 1 to time 2.
DISCUSSION
A growing body of literature suggests links between ADHD symptoms and obesity-related eating behaviors. Notwithstanding, there are only a few studies that examined these association in an early age, and even fewer that analyzed this link prospectively. A main finding of the current study was that we were able to generalize findings from Leventakou et al. within the Rhea Cohort, who in their cross-sectional study found that food responsiveness and emotional overeating were positively associated with ADHD symptoms. Additionally, we were able to determine the direction of effect in the relationship between ADHD symptoms and eating behaviors in a subsample of children with available longitudinal data, as results from our prospective analyses indicated that ADHD symptoms precede eating behaviors, but not the reverse.
The present study expanded previous findings in several ways. First, using the CEBQ, our results support current literature suggesting positive associations between core symptoms of ADHD (e.g. inattention and hyperactivity) and food responsiveness and emotional overeating.26,27,29 A number of studies have presented evidence linking ADHD and loss of control (LOC) eating in adolescence and young adults, demonstrating shared mechanisms (e.g. impulsivity) underlying the relationship between ADHD and binge eating behaviors.25,52 In our young sample, we demonstrate eating patterns that may be vulnerabilities for LOC eating, such as food responsiveness and emotional overeating, are associated with attention problems and hyperactive-impulsive symptoms. Given that binge eating broadly, and LOC eating, specifically, was not assessed in our sample, it is possible that some of these young children were already engaging in binge eating behavior. Alternatively, children with elevated ADHD symptoms and elevated food responsiveness or emotional overeating may be a particularly vulnerable group for interventions to prevent the onset of binge eating.
While studies have generally documented a relationship between greater ADHD symptoms and food approach behaviors, like food responsiveness, some studies have also found greater ADHD symptoms to be related to food avoidant behavior, such as food fussiness in children27 and restrictive eating in adults.53 Some of our findings also line up with these previous results in that we too found greater ADHD symptoms during preschool age were prospectively related to greater satiety responsiveness (a food avoidant behavior) from the preschool to early childhood period. It may be that symptoms of ADHD are related to a diverse set of eating behavior phenotypes that contribute to risk for obesity over development. In this later case, children with ADHD symptoms may be less interested in or forget about eating when distracted by other activities appearing to their parents as reaching satiety earlier than other children (e.g., “full before his/her meal is finished”).
Results from the LCS models indicated that ADHD symptoms in early childhood predicted greater changes in eating behaviors in preschool aged children, but not vice versa, suggesting a direction of effect and a potential causal relationship between ADHD symptoms and obesogenic eating behaviors. The findings from these main models controlled for BMI, but supplementary models exploring the prospective relationship between early ADHD symptoms and BMI showed that higher preschool ADHD symptoms predicted greater changes in BMI, but earlier BMI did not predict greater changes in ADHD symptoms. These findings align with previous studies that have also found that ADHD symptoms, across the range of development, correlate with a higher BMI.54,55 Taken together, findings from current study suggest that this relationship between ADHD and obesogenic eating behaviors and greater changes in BMI begin in early in childhood, maintain over development and support a potential causal relationship such that ADHD symptoms increase the risk for elevated BMI and obesity during early development.
The mechanisms linking ADHD and problematic eating behaviors and BMI or obesity need further attention and investigation.56 Twin and siblings studies suggest that there may be a shared genetic association between obesity and ADHD symptoms or executive functions57-59 and genome-wide association studies also show overlap in genetic loci between these two traits.60,61 It may be that a similar overlap exists between ADHD and eating behaviors too since there has been an observed genetic component to eating behaviors, such as satiety and food responsiveness.62,63 In addition to the potential genetic mechanism underlying these associations, there may also be neurobiological ones as well, such as those associated with dopamine regulation which is related to impulse regulation, eating behavior problems and obesity. ADHD symptoms may also influence eating behaviors by disturbing sleep patterns. Sleep is disrupted among children with ADHD64 and problematic eating may ensue to compensate for low energy levels resulting from a lack of sleep. These and other mechanisms deserve further investigation to better understand this link between ADHD, obesogenic eating behavior, and childhood obesity.
To our knowledge, this is the first study to examine the association between ADHD symptoms and eating behaviors in early childhood using both cross-sectional and prospective designs. Using valid measures of ADHD and eating behaviors, our results support a link between early ADHD symptoms and eating patterns among young children. More importantly, the present study provides prospective evidence of this association using a novel analytic tool (LCS), which enabled us to test bi-directional associations and disentangle the directions of effects between these traits. Extending these models to changes in BMI confirm the suspected relationship between ADHD symptoms as a risk factor for greater childhood weight gain.
Along with these strengths, however, the findings should also be interpreted within the context of some limitations. First, the study was conducted in a community sample based in the southeastern US, which may make it difficult to generalize findings to other populations, especially to clinical samples (i.e., children diagnosed with ADHD). Second, although we were able to examine prospective associations and causal links can be inferred with greater confidence than with cross-sectional methods, further experimental studies would be needed to determine causation. Additionally, LCS tested relations from two discrete time points, so we are unable to currently predict whether these associations continue across the life span and to what degree they impact future obesity risk. Finally, we primarily used parent-reported measures in the study to assess children’s ADHD symptoms and eating behaviors. This could lead to biased reporting, for example, if mothers foresee a link between ADHD symptoms in their child an eating behaviors or seek to explain their child’s eating behaviors on other measures. Future prospective studies that employ other methods independent of self-report, such as the use of the NIH Toolbox or other tests to assess executive functions underlying ADHD symptoms, could further help bring clarity to this important link related to early childhood eating behaviors and risk for obesity.
Despite its limitations, this study provides insights into the association between ADHD symptoms and eating behaviors, suggesting that ADHD symptoms precede problematic eating behavior. These findings have significant clinical relevance, particularly for pediatricians and primary care providers, who are often the first professionals to speak with parents about emerging behavioral difficulties and healthy eating habits. If these associations are replicated, providers will likely want to speak with parents about the impact that early ADHD symptoms can have on eating behaviors, and the risk for dysregulated eating and subsequent obesity. Moving forward, a better understanding of the underlying factors and mechanisms contributing to the association between ADHD and eating patterns in early childhood is needed, especially for the development of tailored intervention and prevention strategies for childhood obesity. In addition, prospective studies are necessary to further advancement our understanding of the role that ADHD-related phenotypes have on both eating behaviors and risk for obesity during childhood.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Aging 1R21AG041048 [BFF], National Institute of Environmental Health Sciences R01ES016772 [CH], R21ES014947 [CH], P30ES011961 pilot project [SKM], P01ES022831 [SKM, SHK and BFF], the US Environmental Protection Agency (RD-83543701 [SKM, SHK and BFF]), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD084487 [BFF and SHK]), the National Institute of Drug Abuse (K24DA023464 [SHK]), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK085173 [CH and SKM]), and the Duke Cancer Institute [SKM and CH]. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Conflict of interest: the authors report no conflicts of interest.
REFERENCES
- 1.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018;319(16):1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999-2016. Pediatrics. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuire S Institute of Medicine. 2012. Accelerating progress in obesity prevention: solving the weight of the nation Washington, DC: the National Academies Press; Adv Nutr. 2012;3(5):708–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin RM. The Surgeon General’s vision for a healthy and fit nation. Public Health Rep. 2010;125(4):514–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groner JA, Joshi M, Bauer JA. Pediatric precursors of adult cardiovascular disease: noninvasive assessment of early vascular changes in children and adolescents. Pediatrics. 2006;118(4):1683–1691. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–17 e12. [DOI] [PubMed] [Google Scholar]
- 7.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999-2008. Pediatrics. 2012;129(6):1035–1041. [DOI] [PubMed] [Google Scholar]
- 8.van Geel M, Vedder P, Tanilon J. Are overweight and obese youths more often bullied by their peers? A meta-analysis on the correlation between weight status and bullying. Int J Obes. 2014;38(10):1263–1267. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5(4):282–304. [DOI] [PubMed] [Google Scholar]
- 10.Altfas JR. Prevalence of attention deficit/hyperactivity disorder among adults in obesity treatment. BMC Psychiatry. 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122(1):e1–6. [DOI] [PubMed] [Google Scholar]
- 12.Byrd HC, Curtin C, Anderson SE. Attention-deficit/hyperactivity disorder and obesity in US males and females, age 8-15 years: National Health and Nutrition Examination Survey 2001-2004. Pediatr Obes. 2013;8(6):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuemmeler BF, Ostbye T, Yang C, McClernon FJ, Kollins SH. Association between attention-deficit/hyperactivity disorder symptoms and obesity and hypertension in early adulthood: a population-based study. Int J Obes. 2011;35(6):852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Penalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: A systematic review and meta-analysis. Am J Psychiatry. 2016;173(1):34–43. [DOI] [PubMed] [Google Scholar]
- 15.Cortese S, Tessari L. Attention-Deficit/Hyperactivity Disorder (ADHD) and obesity: Update 2016. Curr Psych Rep. 2017;19(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortese S, Faraone SV, Bernardi S, Wang S, Blanco C. Adult attention-deficit hyperactivity disorder and obesity: epidemiological study. Br J Psychiatry. 2013;203(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis C, Levitan RD, Smith M, Tweed S, Curtis C. Associations among overeating, overweight, and attention deficit/hyperactivity disorder: a structural equation modelling approach. Eat Behav. 2006;7(3):266–274. [DOI] [PubMed] [Google Scholar]
- 18.Pearce AL, Leonhardt CA, Vaidya CJ. Executive and reward-related function in pediatric obesity: A meta-analysis. Child Obes. 2018;14(5):265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nigg JT, Johnstone JM, Musser ED, Long HG, Willoughby MT, Shannon J. Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: New data and meta-analysis. Clin Psychol Rev. 2016;43:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunault P, Frammery J, Montaudon P, et al. Adulthood and childhood ADHD in patients consulting for obesity is associated with food addiction and binge eating, but not sleep apnea syndrome. Appetite. 2019;136:25–32. [DOI] [PubMed] [Google Scholar]
- 21.Cortese S, Bernardina BD, Mouren MC. Attention-deficit/hyperactivity disorder (ADHD) and binge eating. Nutr Rev. 2007;65(9):404–411. [DOI] [PubMed] [Google Scholar]
- 22.Biederman J, Petty CR, Monuteaux MC, et al. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. Am J Psychiatry. 2010;167(4):409–417. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimasu K, Barbaresi WJ, Colligan RC, et al. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population-based birth cohort study. J Child Psychol Psychiatry. 2012;53(10):1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikami AY, Hinshaw SP, Arnold LE, et al. Bulimia nervosa symptoms in the multimodal treatment study of children with ADHD. Int J Eat Disord. 2010;43(3):248–259. [DOI] [PubMed] [Google Scholar]
- 25.Egbert AH, Wilfley DE, Eddy KT, et al. Attention-Deficit/Hyperactivity Disorder symptoms are associated with overeating with and without loss of control in youth with overweight/obesity. Child Obes. 2018;14(1):50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong L, Shi H, Li X. Associations among ADHD, abnormal eating and overweight in a non-clinical sample of Asian children. Sci Rep. 2017;7(1):2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leventakou V, Micali N, Georgiou V, et al. Is there an association between eating behaviour and attention-deficit/hyperactivity disorder symptoms in preschool children? J Child Psychol Psychiatry. 2016;57(6):676–684. [DOI] [PubMed] [Google Scholar]
- 28.Sonneville KR, Calzo JP, Horton NJ, et al. Childhood hyperactivity/inattention and eating disturbances predict binge eating in adolescence. Psychol Med. 2015;45(12):2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjorklund O, Belsky J, Wichstrom L, Steinsbekk S. Predictors of eating behavior in middle childhood: A hybrid fixed effects model. Dev Psychol. 2018;54(6):1099–1110. [DOI] [PubMed] [Google Scholar]
- 30.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–1064. [DOI] [PubMed] [Google Scholar]
- 31.Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12(9):740–755. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Murphy SK, Murtha AP, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012;7(7):735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyo C, Murtha AP, Schildkraut JM, et al. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health. 2011;11(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allison Bender H, Auciello D, Morrison CE, MacAllister WS, Zaroff CM. Comparing the convergent validity and clinical utility of the Behavior Assessment System for Children-Parent Rating Scales and Child Behavior Checklist in children with epilepsy. Epilepsy Behav. 2008;13(1):237–242. [DOI] [PubMed] [Google Scholar]
- 35.Harrison JR, Vannest KJ, Reynolds CR. Behaviors that discriminate ADHD in children and adolescents: primary symptoms, symptoms of comorbid conditions, or indicators of functional impairment? J Atten Disord. 2011;15(2):147–160. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach TM. Manual for the child behavior checklist/4-18 and 1991 profile. Burlington, VT: : Dept. of Psychiatry, University of Vermont; 1991. [Google Scholar]
- 37.Moeller J A word on standardization in longitudinal studies: Don’t. Front Psychol. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds CR, Kamphaus RW. Behavior assessment for children (BASC-2). Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 39.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–970. [DOI] [PubMed] [Google Scholar]
- 40.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008;88(1):22–29. [DOI] [PubMed] [Google Scholar]
- 41.Jaddoe VW, van Duijn CM, van der Heijden AJ, et al. The Generation R Study: design and cohort update until the age of 4 years. Eur J Epidemiol. 2008;23(12):801–811. [DOI] [PubMed] [Google Scholar]
- 42.Jaddoe VW, van Duijn CM, van der Heijden AJ, et al. The Generation R Study: design and cohort update 2010. Eur J Epidemiol. 2010;25(11):823–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashcroft J, Semmler C, Carnell S, van Jaarsveld CH, Wardle J. Continuity and stability of eating behaviour traits in children. Eur J Clin Nutr 2008;62(8):985–990. [DOI] [PubMed] [Google Scholar]
- 44.Webber L, Hill C, Saxton J, Van Jaarsveld CH, Wardle J. Eating behaviour and weight in children. Int J Obes. 2009;33(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuczmarski RJ. CDC growth charts; United States. 2000. [PubMed]
- 46.Werheid K, Kohncke Y, Ziegler M, Kurz A. Latent change score modeling as a method for analyzing the antidepressant effect of a psychosocial intervention in Alzheimer’s disease. Psychother and Psychosom. 2015;84(3):159–166. [DOI] [PubMed] [Google Scholar]
- 47.Kievit RA, Brandmaier AM, Ziegler G, et al. Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Dev Cogn Neurosci. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol. 2009;60:577–605. [DOI] [PubMed] [Google Scholar]
- 49.McArdle J, Nesselroade J. Using multivariate data to structure developmental change In: Life-span developmental psychology: Methodological contributions. U Virginia, Dept of Psychology, Charlottesville, VA, US: Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc; 1994:223–267. [Google Scholar]
- 50.McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data In: New methods for the analysis of change. U Virginia, Charlottesville, VA, US: Washington, DC, US: American Psychological Association; 2001:139–175. [Google Scholar]
- 51.Quinn JM, Wagner RK, Petscher Y, Lopez D. Developmental relations between vocabulary knowledge and reading comprehension: a latent change score modeling study. Child Dev. 2015;86(1):159–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinblatt SP. Are eating disorders related to Attention Deficit/Hyperactivity Disorder? Curr Treat Options Psychiatry. 2015;2(4):402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaisari P, Dourish CT, Rotshtein P, Higgs S. Associations Between Core Symptoms of Attention Deficit Hyperactivity Disorder and Both Binge and Restrictive Eating. Front Psychiatry. 2018;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raziel A, Sakran N, Goitein D. [The relationship between attention deficit hyperactivity disorders (ADHD) and obesity]. Harefuah. 2014;153(9):541–545, 557. [PubMed] [Google Scholar]
- 55.Martinez de Velasco R, Barbudo E, Perez-Templado J, Silveira B, Quintero J. Review of the association between obesity and ADHD. Actas Esp Psiquiatr. 2015;43(1):16–23. [PubMed] [Google Scholar]
- 56.Cortese S The Association between ADHD and Obesity: Intriguing, Progressively More Investigated, but Still Puzzling. Brain Sci. 2019;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Q, Hartman CA, Kuja-Halkola R, Faraone SV, Almqvist C, Larsson H. Attention-deficit/hyperactivity disorder and clinically diagnosed obesity in adolescence and young adulthood: a register-based study in Sweden. Psychol Med. 2019;49(11):1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Do EK, Haberstick BC, Williams RB, et al. The role of genetic and environmental influences on the association between childhood ADHD symptoms and BMI. Int J Obes. 2019;43(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood AC, Vainik U, Engelhardt LE, et al. Genetic overlap between executive functions and BMI in childhood. Am J Clin Nutr. 2019;110(4):814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albayrak O, Putter C, Volckmar AL, et al. Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(4):295–305. [DOI] [PubMed] [Google Scholar]
- 62.Llewellyn CH, van Jaarsveld CH, Plomin R, Fisher A, Wardle J. Inherited behavioral susceptibility to adiposity in infancy: a multivariate genetic analysis of appetite and weight in the Gemini birth cohort. Am J Clin Nutr. 2012;95(3):633–639. [DOI] [PubMed] [Google Scholar]
- 63.Wardle J, Carnell S. Appetite is a heritable phenotype associated with adiposity. Ann Behav Med. 2009;38 Suppl 1:S25–30. [DOI] [PubMed] [Google Scholar]
- 64.Lunsford-Avery JR, Krystal AD, Kollins SH. Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clin Psychol Rev. 2016;50:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


