Abstract
Adolescent animals are vulnerable to the effects of stress on brain development. We hypothesized that long-term effects of adolescent chronic stress are mediated by glucocorticoid receptor (GR) signaling. We used a specific GR modulator (CORT108297) to pharmacologically disrupt GR signaling in adolescent rats during exposure to chronic variable stress (CVS). Male and female rats received 30mg/kg of drug during a 2-week CVS protocol starting at PND46. Emotional reactivity (open field) and coping behaviors (forced swim test (FST)) were then tested in adulthood, 5 weeks after the end of the CVS protocol. Blood samples were collected two days before FST and serial samples after the onset of the swim test to determine baseline and stress response levels of HPA hormones respectively.
Our results support differential behavioral, physiological and stress circuit reactivity to adolescent chronic stress exposure in males and females, with variable involvement of GR signaling. In response to adolescent stress, males had heightened reactivity to novelty and exhibited marked reduction in neuronal excitation following swim stress in adulthood, whereas females developed a passive coping strategy in the FST and enhanced HPA axis stress reactivity. Only the latter effect was attenuated by treatment with the GR modulator C108297. In summary, our data suggest that adolescent stress differentially affects emotional behavior and circuit development in males and females, and that GR manipulation during stress can reverse at least some of these effects.
1. Introduction
Traumatic experiences during development are associated with numerous psychopathologies (e.g., depression and anxiety disorders). Early trauma can have a lasting impact on stress processing, evident as hypothalamic-pituitary adrenal (HPA) axis dysregulation (Oitzl et al., 2010). The adolescent brain is considered particularly susceptible to changes in environmental conditions (Brenhouse and Andersen, 2011). Areas involved in cognitive and affective processes, such as hippocampus, prefrontal cortex (PFC) and amygdala, are actively developing during this period, through synaptic pruning, myelination, and maturation of important cell signaling pathways (Andersen and Teicher, 2008, 2004; Brenhouse and Andersen, 2011). Several authors propose that adolescence is a critical period for the development of vulnerability to psychiatric disorders later in life (Eiland and Romeo, 2013; Leussis and Andersen, 2008). Moreover, traumatic or chronic stress during this phase is frequently associated with the onset of psychiatric disorders (Goodyer, 2000; Patel et al., 2007).
The actions of glucocorticoids in the brain and other organs are modulated by signaling through corticosteroid receptors, GR (glucocorticoid receptor) and MR (mineralocorticoid receptor) (Reul and de Kloet, 1985). GR and MR act cooperatively to modulate physiological and behavioral responses to stressors (de Kloet et al., 2008), with GR being of particular importance in mediating glucocorticoid feedback inhibition of the HPA axis (Myers et al., 2012). In rodents, early life stress as well as chronic exposure to stress in adulthood decrease the expression of GR in brain regions regulating feedback (Cattaneo and Riva, 2016; de Kloet et al., 2005; Herman et al., 2005), consistent with impaired HPA axis control. Of note, chronic exposure to glucocorticoids, acting through GR signaling, is linked to dendritic retraction, reduced cell growth and reduced neurogenesis (in hippocampus) (reviewed in Joëls et al., 2007), and it is thought that signaling through GRs plays a role triggering lasting effects of early life experience (reviewed in Champagne et al., 2009; Herbert et al., 2006). Notably, GR is expressed abundantly in the hippocampus, amygdala, and prefrontal cortex during adolescent development (as well as adulthood) (Eiland and Romeo, 2013; Herman et al., 2005; Herman and Cullinan, 1997), indicating that these structures may be susceptible to fluctuations in glucocorticoids during adolescent stress exposure.
Prior data from our group indicates that chronic variable stress (CVS) during adolescence can evoke changes in anxiety like-behavior and coping style in both males and females. Adult HPA axis responses to acute stress are blunted following adolescent stress in females (Wulsin et al., 2016), (whereas males are not affected (Cotella et al., 2019)). To further understand the mechanisms behind enduring effects of adolescent stress in males and females, we tested the hypothesis that adolescent chronic stress causes life-long changes in behavior, HPA axis reactivity and neurocircuit activation through GR activation during stress exposure. To test this hypothesis, we used a recently-developed GR specific modulator, CORT108297 (Hunt et al., 2015) to block GR activation during CVS. This compound specifically binds GR, having no affinity for the progesterone receptor or other steroid receptors (and thereby lacking potential complications associated with the currently-marketed GR antagonist, mifepristone (Sindelar et al., 2014). In the present study, we used this compound as a tool to specifically target GR signaling during the exposure to adolescent stress, in order to test the involvement of GR in defining the long-term effects previously observed in our model.
2. Materials and Methods
Male and female Sprague Dawley rats were bred in-house, weaned at postnatal day 21 (PND21) and pair-housed in standard clear cages ( 20 cm height x22cm width x 43cm length)) under a 12 h light/ 12 h dark cycle (lights on at 7:00 am) at constant room temperature (23+2 °C) with ad libitum access to food and water. No more than 2 littermates were included in each experimental group. All tests were performed during the light cycle, between 09:00 AM and 2:00 PM. All procedures and care performed in the animals were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
2.1. Adolescent chronic variable stress
Rats in the CVS group were subjected to 14 days of our standard CVS paradigm during late adolescence (PND 46 + 2) following previous work from our group (Jankord et al., 2011; Wulsin et al., 2016) with some modifications. The CVS protocol included a set of unpredictable variable stressors applied twice daily (AM and PM): 1) 1h shaker stress (100rpm), 2) 1h cold room (4°C), 3) 30 minute restraint, 4) 30 min hypoxia exposure (8% O2 and 92% N2) 5) 1h wet bedding. Swimming was not used as a CVS stressor to ensure that FST was a novel stressor. Following CVS, animals were allowed to recover for 5 weeks to be evaluated during adulthood. We did not include overnight stressors to avoid extending the time of exposure to stressors too far from the injection time. The timeline of the experiment is shown in Figure 1.A.
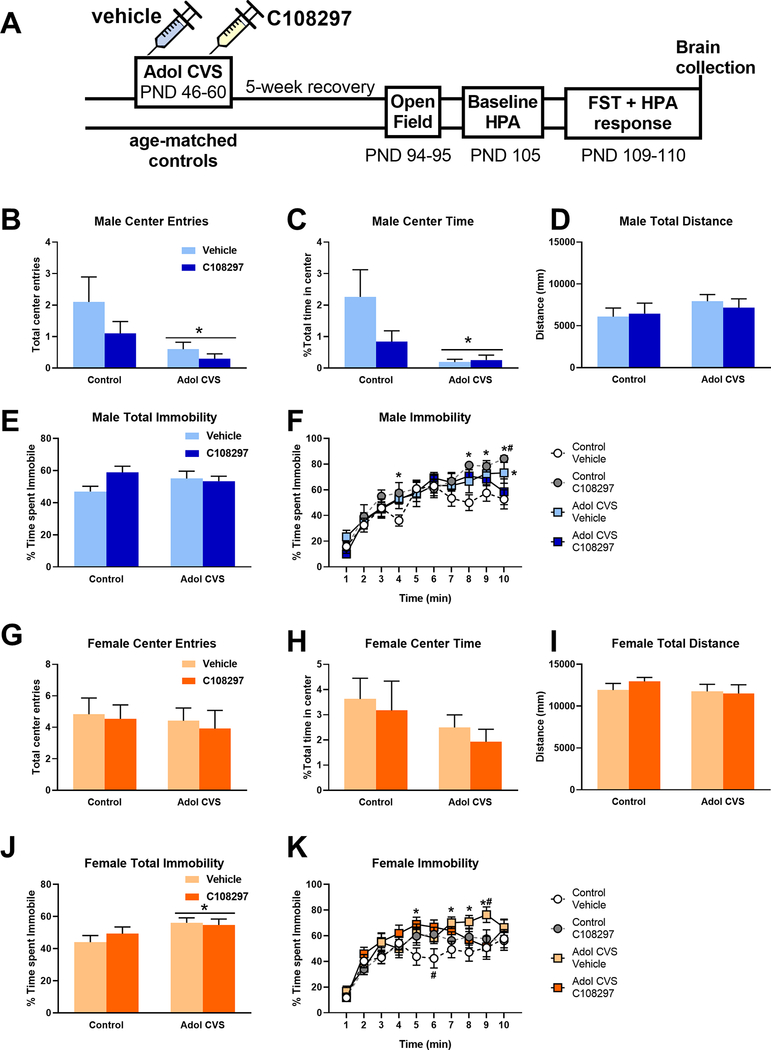
Figure 1:
A: Experimental timeline. Animals were subjected to adolescent CVS (adol) and concomitantly administered CORT108297 (30mg/Kg) or vehicle for 2 weeks starting at PND 46, and evaluation started after 5 weeks of recovery. Activity in the open field test arena after 5 min exploration: Total center entries (B: males; G: females), Total time in the center (C: males; H: females), total distance covered (D: males; I: females). *: significant result p < 0.05 Total percent of immobility in the forced swim test (FST) after 10 min exposure (E: males; J: females) and percent of immobility over time (F: males; K: females).. E, J: *: significant result p < 0.05. F, K: *: significant result p < 0.05 versus control vehicle group. #: significant result p < 0.05 versus CVS C108297 group (F: planned comparissons). Data are presented as mean ± s.e.m. (n=10 males per group, n=12 females per group, except for open field test, Control-C108297=11).
2.2. GR specific modulation
CORT108297 (30mg/kg) (Corcept therapeutics) or vehicle (polyethylene glycol) was subcutaneously injected daily at 9:00 AM throughout the duration of the CVS protocol. Biochemical assays document potent antagonistic actions of CORT108297 on GR (Sindelar et al., 2014). However actions appear to be more complicated in vivo, being depending on interactions with GR coactivator proteins. (Zalachoras et al., 2013). Consequently we refer to this drug as a GR modulator, given its capacity to also have actions as a partial agonist in some tissues or cellular contexts. AM stressors started between 30min to 1 h after the injection and PM stressors happened between 5 – 5.5 h after the injection (According to reports from Corcepts, the half-life of the compound is compatible with once-a-day oral dosing (https://www.sec.gov/Archives/edgar/data/1088856/000119312514100313/d667165d10k.htm). The resulting groups for each sex were: Control-Vehicle; Control-CORT108297; CVS-Vehicle and CVS-CORT108297.
2.3. Somatic markers
Body weight was recorded daily during the duration of the CVS protocol to administer the proper dose of C108297 every day. After the conclusion of CVS, rats were weighed once a week to keep track of the general health status of the animals as well as for possible extended effect of the experimental conditions. Data are presented as total body weight, percent of body weight gain from the first day of experiment during CVS and percent of body weight gain from the last day of CVS, measured once a week, to assess the recovery after CVS. At euthanasia, internal organs that are known to reflect chronic stress effects (adrenals, thymus and heart) were collected and weighed.
2.4. Open field evaluation
On PND94–95 rats were evaluated during a 5 minute exposure to an open field. Briefly, animals were placed on the corner of a 1m2 arena. Videos were acquired using Clever Capture Star software (CleverSys, Reston, VA) and then they were automatically scored using Clever Top Scan software (CleverSys, Reston, VA). The variables measured were total distance covered in the arena, total number of center entries and time spent in the center.
2.5. Forced swim test
At days 109–110 rats were evaluated in the forced swim test during 10 min (25+2 °C). Videos were acquired with Clever Capture Star software (CleverSys, Reston, VA and manually scored by a blinded observer. The method used was sampling the behavior each 5s (Slattery and Cryan, 2012). Behaviors considered: climbing, swimming, immobility and diving
2.6. Blood sampling and hormone assessment
At PND 105 rats were quickly tail bled (<3min) to get samples to determine baseline levels of stress hormones. The day of the FST (PND109) rats were bled from their tails 15, 30, 60 and 120 minutes after the onset of the test to assess the HPA stress response to a novel stressor. Samples were obtained by quickly clipping the distal tip of the tail of a freely moving rat with a razor blade and collecting ~250 ul of blood into EDTA (10ul, 100mM) containing tubes. Subsequent samples were obtained by gently removing the clot from the distal tail to recommence bleeding. All samples were collected within 3 min. In order to minimize hormonal variability due to circadian fluctuations, all procedures were performed during circadian nadir of the diurnal CORT rhythm and blood samples were collected before 1:00 PM. After collection, blood was centrifuged at 3500 × g, for 15 min at 4 °C and then plasma samples were extracted and kept at −20 °C until hormones level determination.
Corticosterone (CORT) and adrenocorticotropic hormone (ACTH) plasma levels were measured by radioimmunoassay as previously described (Smith et al., 2018; Wulsin et al., 2016). CORT concentration was determined using 125I RIA kits (MP Biomedicals Inc., Orangeburg NY). ACTH was determined by radioimmunoassay, using 125I ACTH as trace (Amersham Biosciences) (ACTH antiserum donated by Dr. William Engeland,University of Minnesota) at a 1:120,000 dilution (Ulrich-Lai et al., 2006). The ratio of plasma corticosterone to the log of plasma ACTH was calculated as an index of adrenal sensitivity to ACTH (Engeleand et al., 1981; Ulrich-Lai and Engeland, 2002).
2.7. Immunohistochemistry
Immediately after the last serial blood sample was taken, rats were euthanized with an overdose of sodium pentobarbital and subsequently transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PBS), pH 7.4. Brains were post-fixed in 4% paraformaldehyde at 4°C for 24h, then transferred to 30% sucrose in 0.01MPBS at 4°C. Brains were sliced into serial 35μm coronal sections using a freezing microtome (−20°C). Sections (1/12) were collected into wells containing cryoprotectant solution (30% Sucrose, 1% Polyvinyl-pyrolidone (PVP-40), and 30% Ethylene glycol, in 0.1 M PBS). For immunolabeling, sections were washed 6×5min in 0.01M PBS at room temperature (RT). After being rinsed, sections were incubated with 1% Sodium Borohydride in 0.1MPBS for 30 min at RT. After rinsing 6×5 min 0.1M PBS, they were incubated in 3% hydrogen peroxide diluted in 0.1M PBS for 20 min. Subsequently, brain slices were rinsed 6×5 min and 4×15 min in 0.1 M PBS and then incubated in blocking solution (4% normal goat serum (NGS), 0.4% TritonX-100, 0.2% bovine serum albumin (BSA) in 0.1M PBS, 2h at RT. Sections were then incubated with c-Fos rabbit polyclonal antibody (1:1000, Santa Cruz, sc-52) in blocking solution, overnight at RT. The next day, sections were rinsed (3 × 5 min) in 0.1M PBS at RT, followed by incubation with secondary antibody (biotinylated goat anti-rabbit, (1:400; Vector Laboratories, BA1000) in blocking solution at RT for 1h. Sections were again rinsed (3×5 min) in 0.1M PBS and then reacted with avidin-biotin horseradish peroxidase complex (1:800 in 0.1M PBS; Vector Laboratories) for 1 h at RT. Sections were then rinsed (3×5 min) in 0.1M PBS and then developed with a 8 min incubation in DAB-Nickel solution: 10mg 3,3′-diaminobenzidine (DAB) tablet (Sigma, DF905), 0.5 ml of a 2% aqueous nickel sulfate solution, 20ul of 30% hydrogen peroxide in 50ml of 0.1M PBS. Sections were finally washed in PBS, mounted on superfrost slides (Fisherbrand, Fisher), allowed to dry, dehydrated with xylene, and then coverslipped in DPX mounting medium (Sigma). Sections from 7 to 8 brains per experimental group were processed. For analysis, we counted 3 bilateral sections from equivalent coordinates covering the anterior, medial and posterior portions of the prefrontal cortex (PFC), nuclei in the amygdala: central amygdaloid nucleus (CeAm), medial amygdaloid nucleus (MeAm) and basolateral amygdala (BLA) and the subdivisions of the paraventricular nucleus of the thalamus (PVT): anterior (PVA), intermediate (PV) and posterior (PVP). In the case of the paraventricular nucleus of the hypothalamus (PVN) and the lateral ventral septum (LSV), we analyzed 4 anteroposterior bilateral sections from equivalent coordinates. Each brain region limit and coordinates were defined following a brain atlas (Paxinos and Watson, 2007). The number of Fos positive nuclei was counted with a semiautomatized method using ImageJ software (National Institutes of Health, Bethesda, MD). Counts of Fos immunoreactive cells were obtained from each area of interest using the Analyze Particle tool, using a defined common level of background intensity, nuclei circularity and size (previously validated manually). Once the number of Fos positive nuclei was determined in each section, the relative density of the population of immunopositive cells was calculated by dividing this number by the area measured in each case.
2.8. Comments on experimental Design and Statistical analysis
The main goal of our experiment was to assess the long-term effects of CVS during adolescence and how modulation of GR can influence the outcomes of stress. The need to carefully control stress conditions contraindicated strict attention to the estrus cycle, as doing so would introduce stressful handling/swabbing procedures in females only, and require alterations in timing of tests. In addition, we elected to analyze males and females independently, since 1) assessments were made in different testing sessions and 2) large inherent male/female differences exist in many of the tested endpoints (e.g., body and organ weight, HPA axis activation) that may occlude that ability to examine individual endpoints within sex. Therefore, the results were all analyzed within each sex and do not assess sex difference per se.
Data were analyzed using STATISTICA 7.0 (Statsoft, Inc.,Tulsa, USA), Prism 8 (GraphPad Software, La Jolla California USA) and Infostat (Di Rienzo et al., n.d.). For the analysis of the results from OFT, FST, baseline levels of hormones, a 2-way ANOVA (2×2 design: STRESS x TREATMENT) analysis was performed with a level of significance p < 0.05. HPA axis stress response and behavior on the FST over time were analyzed by repeated measurements ANOVA, STRESS x TREATMENT x TIME, with a level of significance of p < 0.05. In the cases where significant differences were found, a Tukey post-hoc test was performed. In some cases, we performed planned comparison to evaluate individual differences in cases where there was not a significant interaction between factors. When necessary, data were processed by logarithmic or square root transformations to allow parametric analysis. In the case of open field, the results were analyzed using general linearized models (GLM). Only statistics for significant results are discussed in the results sections. Extreme outliers were removed from analysis. The experiment was designed to have n=10 per group for males and 12 per group for females. These numbers varied later in the analysis for the different variables for different reasons. Open field: 1 video was damaged during recording so the group Control-C108297 had 11 animals. Cort and ACTH: samples with not enough blood to process or that presented technical issues during assays were lost (see figures for n numbers). Based on prior studies, 8 brains/group were processed for immunohistochemistry but technical limitations create variations in the final number analyzed in each group (see figures for n numbers).
3. Results
3.1. Behavior
3.1.1. Open Field
The behavioral response to 5-minute exposure to an open field arena was analyzed as a measure of emotional reactivity. In males, prior adolescent (adol) CVS reduced total time spent in the center F(1, 35)= 6.10, p<0.05, with no effect of drug nor interaction between factors. Both CVS groups were significantly different from the control-vehicle group (p<0.05, planned comparison). The control-C108297 group was not different from the corresponding vehicle group, nor from the corresponding CVS group. Adol CVS also reduced the number of total entries to the center of the arena, F(1, 35) = 6.44, p<0.05. There was no drug effect nor interaction between factors and no individual differences when analyzing planned comparisons. There was no effect of stress or drug on the time spent in the center zone in females, nor the total number of entries in it. Importantly, none of the factors had an effect on locomotor activity in either sex. Effects are shown in Figs. 1.B–D and 1.G–I.
3.1.2. Forced swim test
Behavioral adaptation during the forced swim test is summarized in Figures 1.E–F and 1.J–K. In males, there was no effect of stress or drug treatment on total immobility during the test, and no stress by drug interaction. When analyzed over time, we observed a significant stress by drug interaction, F(1, 36)= 4.338, p<0.05. Although the interaction over time was not significant, F(9, 324)= 1.772, p=0.073, we used planned comparisons to determine possible differential effects between groups. Increased immobility was observed in the CVS-vehicle group relative to the control-vehicle group only in the last time point (p<0.05). Meanwhile, the CVS-C108297 group was not different from the control-vehicle levels, suggesting a mild effect of the drug in limiting the impact of adolescent CVS. The control-C108297 group increased immobility relative to the control-vehicle group over several time points (p<0.05) and was significant with respect to the CVS-C108297 group at the last time point (p<0.05).
In females, there was an effect of adol CVS on total immobility F(1, 44)= 5.331, p<0.05 in the FST. A stress by drug by time interaction F(9, 396)= 2.357, p<0.05 was observed upon temporal analysis of FST behavior. In this analysis the CVS-vehicle group significantly differed from the control-vehicle group across several time points (p<0.05) near the end of the test, indicating a shift in the coping strategy of these animals compared to the corresponding controls. Furthermore, the CVS-vehicle group differed from the CVS-C108297 group only at one later time point (min=9, p<0.05), again suggesting a mild effect of GR modulation during adolescence on the effects of stress in adulthood in the females. The control animals treated with the drug during adolescence, similar to what was observed in the males, showed a significant increase of the immobility during minute 6 compared to the control vehicle group (p<0.05) but then they returned to similar levels to those of the corresponding control group.
3.2. HPA axis
3.2.1. Baseline HPA activity
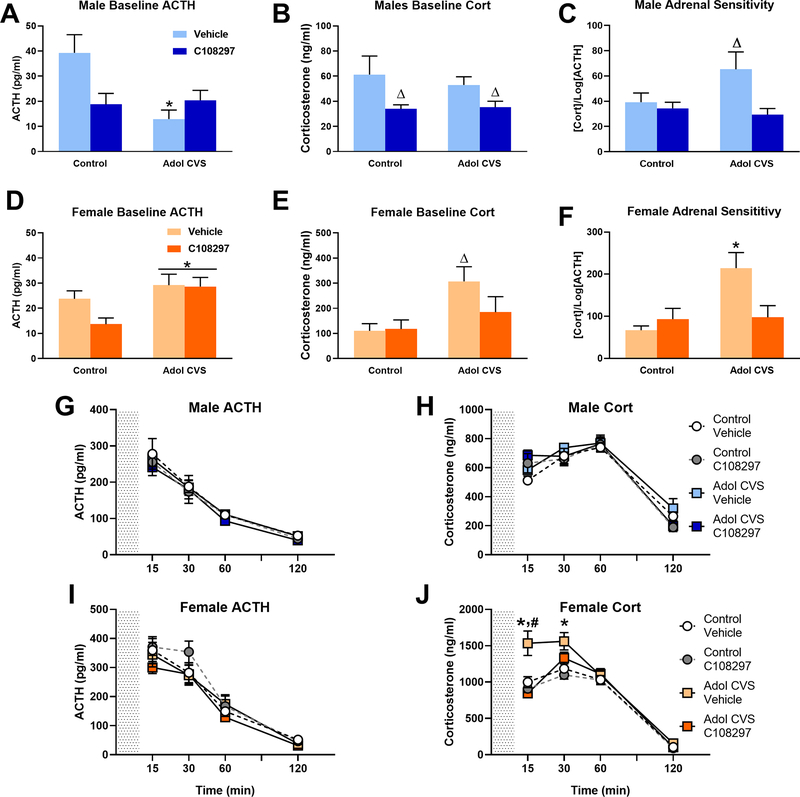
2 days before performing the forced swim test, a blood sample was obtained in order to determine the baseline levels of ACTH and corticosterone in the adult animals subjected to adolescent CVS and C108297 and their corresponding controls. When analyzing the effect on males, there was a significant stress by drug interaction F(1, 32)= 8.76, p<0.01 on baseline ACTH levels. The post hoc analysis revealed only a significant reduction of ACTH in the CVS-vehicle group compared to the corresponding unstressed group (p<0.05) (Fig 2.A). In the case of baseline corticosterone, C108297 had a main effect reducing the plasma levels of corticosterone F(1, 36)=6.88, p<0.05, with no effect of the stress. Planned comparisons confirmed that both groups treated with C108297 had lower corticosterone levels than the corresponding vehicle groups (Fig 2.B).
Figure 2: HPA axis regulation:
Animals were subjected to adolescent CVS (adol) and concomitantly administered CORT108297 (30mg/Kg) or vehicle for 2 weeks starting at PND 46, and evaluated after 6 weeks. (A-F) Baseline hormones concentration and index of adrenal sensitivity in males and females. Index of adrenal sensitivity was calculated as [Cort]/ log[ACTH] using baseline values. *: significant result p < 0.05 versus corresponding unstressed. Δ: significant result p < 0.05 versus corresponding control or vehicle group (planned comparisons). (G-J) Hormones concentration after 10 min exposure to FST (grey area). *: significant result p < 0.05 versus corresponding control group. #: significant result p < 0.05 vs CVS C108297. Data are presented as mean ± s.e.m. (n=10 per group for males and 12 for females except baseline ACTH and adrenal sensitivity of males: Ctrl-C108297=9, CVS-Vehic=9, CVS-C108297=8 and 7 respectively) and adrenal sensitivity of females (Ctrl-Vehic=11, CVS-Vehic=11 and CVS-C108297=11).
In females, adol CVS increased circulating ACTH F(1, 44)= 7.23, p<0.05 as well as corticosterone F(1, 44)=6.83, p<0.05. Planned comparisons revealed that the CVS-vehicle group had significantly higher levels of corticosterone than its corresponding control-vehicle group (p<0.05), an effect that was not observed in the CVS-C108297 group, which did not differ from control-vehicle or control-C108297 groups (Figs. 2.D or 2.E).
3.2.2. Index of adrenal sensitivity
The index of adrenal sensitivity was calculated as the concentration of baseline corticosterone over the logarithm of the baseline levels of ACTH. This gives an idea of how responsive the adrenal cortex is to the effects of ACTH (Engeland et al., 1981). In males, there was a main effect of drug on baseline adrenal sensitivity F(1,31)= 6.88, p<0.05, suggesting that adolescent treatment with C108297 reduced adrenal responsiveness. Planned comparisons indicated significantly higher adrenal sensitivity in the CVS-vehicle group than the rest of the groups (p<0.05).
There were main effects of stress and drug in females (F(1,38)=8.09, p< 0.01 and F(1,38)=7.55, p<0.01, respectively), as well as stress by drug interaction. F(1,38)= 5.88, p<0.05. There was a significant increase of adrenal responsivity index in the CVS-vehicle group compared to the control groups (p<0.05) (Tukey’s test), and this was no observed following treatment with C108297 during adolescence CVS (p<0.05). Results are shown in figures 2.C and 2.F.
3.2.3. HPA response to a novel acute stressor
In order to assess the HPA stress response in the adult animals subjected to adolescent stress and C108297 treatment and their corresponding controls, after being evaluated in the forced swim test, serial blood samples were taken at 15, 30, 60 and 120 minutes after the onset of the test. In males, there were no effects of stress or drug on ACTH and corticosterone in any of the experimental groups (Figs 2.G and 2.H).
In females, there were main effects of stress F(1, 44)=14.57, p<0.001 and drug F (1, 44)=11.63, p<0.01 on corticosterone response, as well as multiple interactions: stress by time F(3, 132)=3.839, p<0.015; drug by time F(3, 132)=7.212, p<0.001; stress by drug F(1, 44)=5.063, p<0.05; stress by drug by time F(3, 132)= 4.534, p<0.01. In contrast, there was no effect of stress or drug on ACTH release. Post hoc analysis indicated that the adol CVS-vehicle group had an exacerbated secretion of corticosterone at the 15 and 30 minute time points compared to the corresponding control group (p<0.05). Furthermore, treatment with C108297 significantly reduced this response at the 15 minute time point, indicating that treatment with C108297 during adolescent CVS prevented dysregulation of the HPA axis in adulthood in females (Figs 2.I and 2.J).
3.3. Somatic markers of stress
3.3.1. Body weight
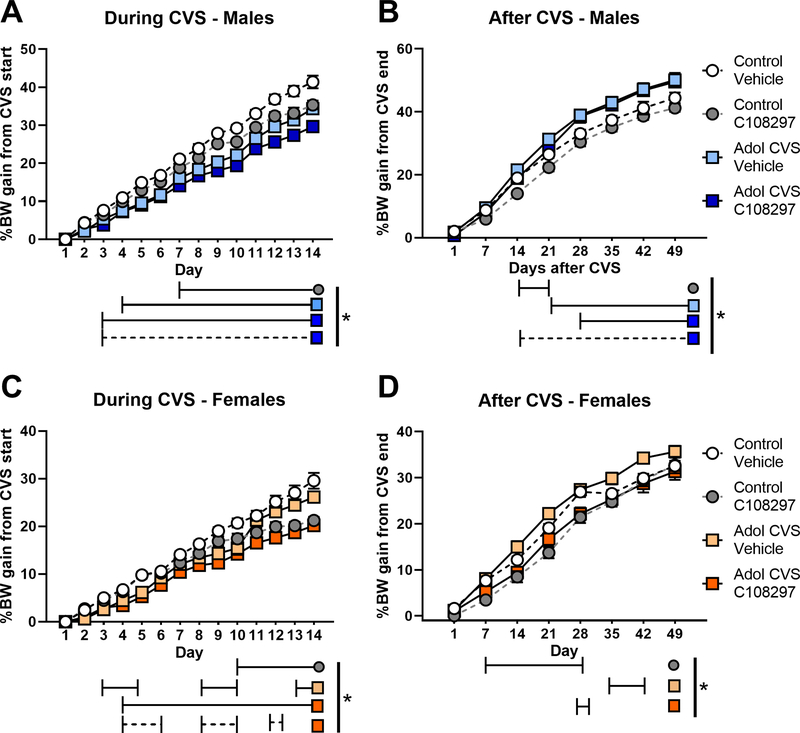
Body weight changes during CVS, post- CVS and across the whole experiment are summarized in Fig. 3. In males, there were significant main effects of stress F(1,36)=66.004, p<0.001, drug F(1.36)=14.791, p<0.001 and time F(12,432)=1534.22, p<0.001 during CVS. There were also significant stress by time F(12,432)=10.87, p<0.001 and drug by time F(12,432)=10.04, p<0.001 interactions. Planned comparisons indicated that treatment with C108297 decreased body weight gain in both adol CVS and control rats from day 7 to the end of CVS, suggesting an important metabolic effect of the drug itself. The CVS-vehicle group also showed reduced weight gain, consistent with a lasting chronic stress effect, from day 4 to the end of CVS. To accurately account for effects on normal body weight gain, the CVS-C108297 group was compared to the control vehicle group, given that C108297 alone affects body weight. The CVS-C108297 group had reduced weight gain relative to controls everyday from day 3 to the end of CVS. Finally, the CVS-C108297 group showed reduced weight gain compared to the corresponding control-C108297 group from day 3 to the end of CVS, suggesting an additive effect of drug and stress on metabolism (p<0.05) (Fig 3.A).
Figure 3: Body weight gain during (A, C) and after (B, D) CVS.
Animals were subjected to adolescent CVS (adol) and concomitantly administered CORT108297 (30mg/Kg) or vehicle for 2 weeks starting at PND 46. Body weight was taken weekly since before the beginning of CVS and daily during CVS (% weight gain calculated over CVS first day). After CVS, weight was taken weekly and % of weight gain was calculated from the last day of CVS. *: significant result p< 0.05 for planned comparisons or Tukey’s test (D). Solid lines represent comparisons versus Control-vehicle group. Dashed lines represent comparisons versus Control-C108297 group. Data are presented as mean ± s.e.m. (n=10 per group for males and 12 for females).
There was a significant main effect of stress on weight gain during recovery from CVS F(1,36)=20.01, p<0.001, indicating that both groups subjected to CVS increased rate of body weight gain after cessation of CVS. There was also a significant time effect F(7, 252)=1581.106, p<0.001 and a significant stress by time interaction F(7,252)=12.26, p<0.001 in males. The control group treated with C108297 had reduced weight gain rate only between 14 and 21 days after CVS compared to the control-vehicle group (p<0.05). On the contrary, the CVS-vehicle group and the CVS-C108297 group had continuous increased weight gain from day 21 and 14 respectively (p<0.05) comparing to the control-vehicle group. This is suggesting a metabolic compensatory effort in the animals subjected to CVS to recover growth rate (Fig. 3.B).
Despite the increase in the weight gain rate observed in the males after CVS, this was not reflected in absolute changes in body weight in the stressed group treated with C108297 since the groups treated with the compound had reduced absolute weight throughout the experiment (Appendix A, Figure A1.A).
In females (Fig. 3.C), there were main effects of stress F(1,44)=18.005, p< 0.001, drug F(1,44)=16.638, p<0.001 and time F(12,528)=901.39, p<0.001 on body weight gain during CVS, due to a general reduction in weight gain seem both in the CVS and C108297 treated females (p<0.05). There were significant stress by time F(12,528)=4.248, p=0.001 and drug by time F(12,528) =24.208, p<0.001 interactions. Weight gain was reduced in both C108297 groups. The control-C108297 group had reduced gain weight from day 10 until the end of the CVS (p<0.05). The CVS-vehicle group showed reduced weight gain between days 3 to 5, 8 to 10 and 13 to 14 (p<0.05) (suggesting possible cyclic effect related to interaction of stress with hormonal fluctuations in the females). Decreased weight gain was most pronounced in the CVS-C108297 group (reduced weight gain from day 4 on (p<0.05)), again suggesting an additive effect of stress and drug on metabolism. Weight gain in the CVS-C108297 group differed from the corresponding unstressed group on days 4 to 6, 8 to 10 and day 12 (p<0.05).
There were main effects of drug F(1,44) =18.229, p< 0.001 and time F(7,308)=813.12, p<0.001and weight gain after CVS, as well as several interactions: drug by time F(7,308)=3.818, p<0.001, stress by drug by time F(7,308)=2.316, p<0.05. Post hoc analysis indicated that controls treated with C108297 had reduced weight gain from days 7 until 21 after the end of CVS (p<0.05). Animals subjected to adol CVS and vehicle increased growth rate, but only for a limited time between days 35 and 42 (p<0.05). The CVS-C108297 group had reduced weight gain at day 28 after CVS (p<0.05), and did not differ from the control-C108297 group. Thus, the effect of the drug seems to have prevented the rebound on weight gain evoked by CVS (Fig. 3.D).
In the case of absolute body weight, C108297 evoked a reduction throughout the experiment (Appendix A Figure A1.B).
3.3.2. Other somatic effects
Wet weights of the adrenal glands, thymus and heart were measured to account for somatic effects of chronic stress. Since there was a difference in the body weight of the animals across the whole experiment due to the drug, the effect of adolescent CVS was analyzed by covaring the organs’ weight and the body weight measurement at the end of experiment.
The table shows the effects of stress and treatment on the somatic measurements. We did not observe effect of stress or C108297 on adrenal gland or thymus weight in males, nor covariation with the body weight. In the case of the heart, there was a main effect of stress F(1,34)=6.15, p<0.05 and positive covariation to body weight F(1,34)=18.82, p<0.001, correlation coefficient=1.99. Planned comparisons indicated that CVS evoked a long-term increase in the mass of the heart, regardless of the treatment. Results are shown in appendix A table A2.
In females, adrenal weights were not affected by stress or drug and were not affected by body weight differences. Regarding the thymus, there were no effects due to the experimental variables but there was a positive correlation with body weight F(1,40)=6.13, p<0.05, coefficient=1.29. Finally, regarding the heart, there were no experimental effects in the females, only positive correlation with body weight F(1,42)=26.76, p<0.001, coefficient=2.51. (Table A.2).
3.4. Fos activation
All animals were sacrificed immediately after the last blood sample collection (120 min after commencement of FST) to allow assessment of expression of Fos protein in HPA axis- and stress-regulatory circuitry. Fos is the product of the immediate early gene cfos, and is reliably induced from largely undetectable levels by acute stressor exposure. We assessed the ability of the FST procedure to recruit neurons in the prefrontal cortex, (PFC) (prelimbic (PL) and infralimbic (IL) subdivisions), the lateral septum, ventral part (LSV), the paraventricular nucleus of the hypothalamus (PVN), and the subdivisions of the amygdala, central (CeAm), basolateral (BLA), and medial (MeAM).
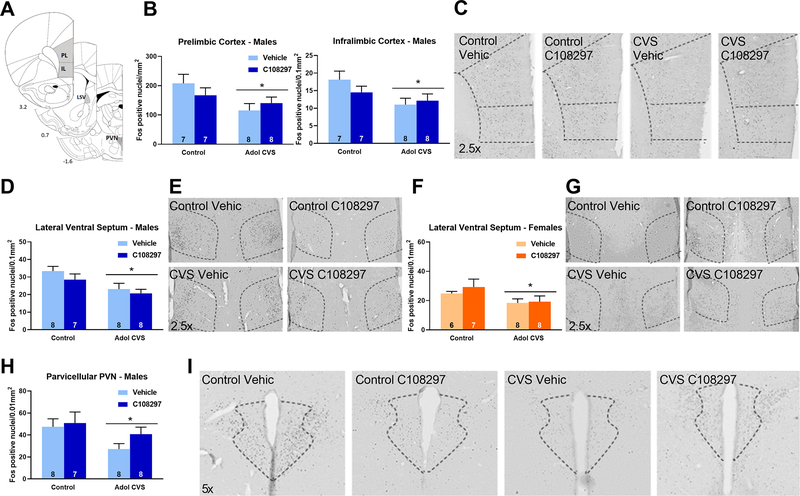
We observed that adolescent CVS reduced Fos-immunoreactive (ir) nuclei in males in the prelimbic cortex, stress F(1, 26)= 5.661, p<0.05, and in the infralimbic cortex, stress F(1, 26)=5.64, p<0.05. There was no effect of drug treatment and no stress by drug interaction (Figs. 4.B–C). Females did not show effects of adol CVS or C108297 in any PFC area (Table 1).
Figure 4: Fos immunoreactivity (Fos-ir) in the prelimbic and infralimbic divisions of the prefrontal cortex (PFC), lateral ventral septum (LSV) and parvicelullar division of the paraventricular nucleus of the hypothalamus (PVN).
Animals were subjected to adolescent CVS (adol) and concomitantly administered CORT108297 (30mg/Kg) or vehicle for 2 weeks starting at PND 46. Brains were collected 2h after the onset of FST, after 6 weeks of recovery from adol CVS. (A) Representative images from brain atlas showing the analyzed areas (Paxinos and Watson, 1998). (B-C) Fos-ir in the PFC of males and representative images. (D-E) Fos-ir in the LSV of males and representative images. (F-G) Fos-ir in the LSV of females and representative images. (H-I) Fos-ir in the PVN of males and representative images. *: significant result p < 0.05. Data are presented as mean ± s.e.m. n numbers are displayed in each bar.
Table 1: Fos immunoreactivity in the females.
The table only shows the regions with negative results. Animals were subjected to adolescent CVS (adol) and concomitantly administered CORT108297 (30mg/Kg) or vehicle for 2 weeks starting at PND 46. Brains were collected 2h after the onset of FST, after 6 weeks of recovery from adol CVS. Data are presented as mean ± s.e.m (n number).
| Female Fos Immunoreactivity | ||||
|---|---|---|---|---|
| Brain region (Fos-ir positive nuclei/area) | Control-Vehicle | Control-C108297 | CVS-Vehicle | CVS-C108297 |
| PL (Fos-ir/mm2) | 133.32 ± 15.12 (8) | 166.62 ± 30.25 (8) | 122.78 ± 12.87 (8) | 140.88 ± 26.61 (8) |
| IL (Fos-ir/0.1 mm2) | 13.77 ± 1.8 (8) | 14.41 ± 2.11 (8) | 12.473 ± 1.54 (8) | 14.17 ± 2.4 (8) |
| PVN (Fos-ir/0.01 mm2) | 45.96 ± 8.80 (6) | 43.20 ± 11.72 (6) | 28.14 + 7.73 (7) | 33.16 ± 8.62 (7) |
| MeAm (Fos-ir/0.1 mm2) | 17.01 ± 2.11 (7) | 16.92 ± 2.98 (7) | 13.8 ± 1.50 (8) | 17.90 ± 2.16 (8) |
| CeAm (Fos-ir/0.1 mm2) | 5.072 ± 1.09 (7) | 5.98 ± 1.42 (7) | 4.466 ± 0.81 (8) | 4.49 ± 0.83 (7) |
| BLA (Fos-ir/0.1 mm2) | 4.38 ± 0.82 (7) | 6.59 ± 1.30 (7) | 4.939 ± 0.58 (8) | 5.50 ± 0.66 (8) |
| PVA (Fos-ir/0.01 mm2) | 28.77 ± 5.99 (7) | 26.29 ± 6.21 (7) | 27.89 ± 3.72 (5) | 38.85 ± 11.37 (6) |
| PV (Fos-ir/0.01 mm2) | 34.56 ± 3.08 (5) | 27.67 ± 5.69 (7) | 24.37 ± 3.69 (5) | 36.15 ± 6.58 (6) |
| PVP (Fos-ir/0.01 mm2) | 30.41 ± 3.72 (6) | 23.05 ± 5.15 (7) | 32.18 ± 5.30 (5) | 27.89 ± 6.43 (7) |
In the lateral ventral septum, adol CVS caused a reduction of Fos immunoreactivity in response to the acute stressor in both sexes: males, stress F(1, 27)=9.726, p<0.01; females, stress F(1, 25)=4.710, p<0.05, with no effect of C108297 or stress by drug interaction (Figs. 4.D–E and 4.F–G).
Fos-ir was reduced in the parvicellular region of the paraventricular nucleus of the hypothalamus in males (main effect of stress F(1, 27)=4.421, p<0.05) (Figs 4.H–I) but not in females (Table 1). There was no stress by drug interaction.
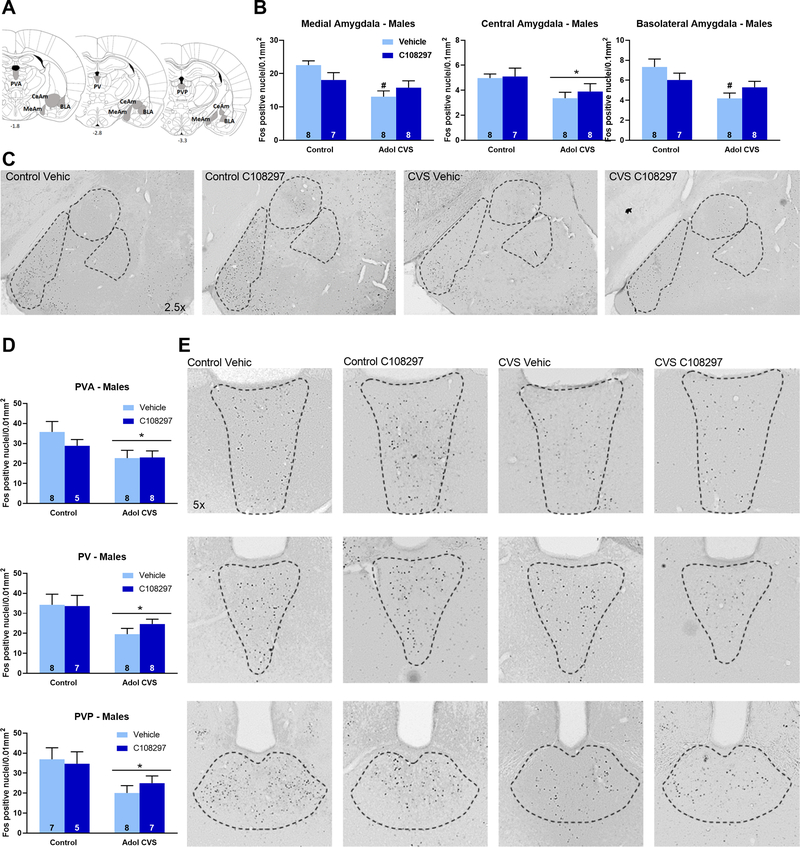
In the amygdala, there were main effects of stress on Fos-ir nuclei in the medial amygdaloid nucleus (MeAm), F (1, 27)=10.36, p<0.01, the central amygdaloid nucleus (CeAm), F(1, 27)=6.846, p<0.05 and the anterior part of the basolateral amygdaloid nucleus (BLA), F(1, 27)=8.572, p<0.01. In the case of MeAM and BLA, planned comparisons revealed that the CVS-vehicle group had significantly less Fos immunoreactive nuclei than the control-vehicle group in these areas (p<0.05 respectively), while the CVS-C108297 group did not differ from the respective unstressed group. This suggests a role of GR signaling during adolescence in defining the long-term effect of CVS on amygdala excitability in males, particularly in the medial and basolateral subdivisions. Effects are shown in (Figs. 5. B–C). There was no effect of stress or drug in females (Table 1).
Figure 5: Fos immunoreactivity (Fos-ir) in the amygdala (medial (MeAm), central (CeAm) and basolateral divisions) and the paraventricular thalamic nucleus (anterior (PVA), intermediate (PV) posterior (PVP) divisions).
Animals were subjected to adolescent CVS (adol) and concomitantly administered CORT108297 (30mg/Kg) or vehicle for 2 weeks starting at PND 46. Brains were collected 2h after the onset of FST, after 6 weeks of recovery from adol CVS. (A) Representative images from brain atlas showing the analyzed areas (Paxinos and Watson, 1998). (B-C): Fos-ir in the amygdala of males and representative images. (D-E) Fos-ir in the paraventricular thalamic nucleus of males and representative images. *: significant result p < 0.05. #: planned comparisons effect vs control vehicle group. Data are presented as mean ± s.e.m. n numbers are displayed in each bar.
There was a reduction in the number of Fos-ir nuclei in all the subdivisions of the paraventricular nucleus of the thalamus: PVA, stress F(1, 25)=4.871, p<0.05; PV, stress F(1, 27)=8.162, p<0.01; PVP, stress F(1, 23 =7.812, p<0.05. (Figs. 5.D–E). No effects were observed in the females (Table 1), and there were no interaction effects in either sex.
It is important to highlight the fact that adolescent CORT108297 did not affect Fos expression in either sex.
4. Discussion
The results of this study provide evidence supporting differential behavioral, physiological and stress circuit reactivity to adolescent chronic stress exposure in males and females, evident in tests of emotional reactivity, coping behavior, HPA axis function and neuronal activation by stressors. In response to adolescent stress, males had heightened reactivity to novelty and exhibited marked reduction in neuronal excitation following swim stress in adulthood. Females developed a passive coping strategy in the FST as a consequence of adolescent stress, and enhanced HPA axis stress reactivity to the FST. The latter effect was attenuated by treatment with the GR modulator C108297, suggesting that enhanced HPA axis reactivity was programmed by glucocorticoid signaling during adolescence.
Glucocorticoids are known to have powerful effects on metabolism, and the administration of C108297 in adolescence had a marked impact on body weight gain during the drug exposure period as well as persistent effects after administration had ceased. Moreover, C108297 and adolescent stress produced an additive effective on body weight gain during the stress period. Since exposure to C108297 was systemic, it is possible that modulation of GR signaling during adolescence is able to program peripheral and/or central mechanisms regulating metabolism.
4.1. Emotional reactivity in the open field test and coping in the forced swim test
Exposure to CVS in adolescence differentially affected behavioral responses in adult males and in females. The avoidance of the center area and reduced exploration in an open field is typically interpreted as an indicator of heightened emotional reactivity to the novel environment (Faraji et al., 2014). These data are consistent with prior findings from our group and others (Cotella et al., 2019; Green et al., 2013; Ilin and Richter-Levin, 2009; Jacobson-Pick and Richter-Levin, 2010; Luo et al., 2014; McCormick et al., 2008; Zhang and Rosenkranz, 2012), and suggest that prior stress reshapes behavioral responses to potential danger in males.
In the present study, performance in the open field test was not affected in females. These data contrast somewhat with a previous study from our group (Smith et al., 2018). This discrepancy may relate to the CVS protocol used: in the present work, animals did not receive periodic overnight isolation or crowding, which may constitute a more profound stress exposure for females (Note that this was done to avoid stressing the animals when the bioavailability of CORT108297 would be low). In addition, the daily injection of the GR modulator or vehicle over the course of 2 weeks is another factor to take into account, given this constitutes itself a minor chronic stress situation that has been argued to evoke responses in the brain (Moghaddam, 2002).
We replicated previous results showing increased FST immobility in adult females previously subjected to adolescent CVS (Wulsin et al., 2016). In males, we only observed a modest effect of adolescent CVS at the end of the test, which was also consistent with previous results (Cotella et al., 2019) but in a less marked way. Bourke and Neigh (2011) indicated that females but not males are vulnerable to the effects of adolescent stress on the FST as adults and another study failed to report effects in male rats (Toth et al., 2008), suggesting that females may be more sensitive to the impact of adolescent stress on subsequent behavioral coping.
Administration of C108297 modestly blocked effects of CVS on FST, but more interestingly, it evoked noticeable behavioral changes independent of stress. As was the case with body weight, it is possible that GR signaling during this developmental period has a role in defining coping strategies to aversive situations later in life.
4.2. HPA axis regulation
It is important to note that males and females exhibit marked differences in HPA axis function, with females secreting substantially higher levels of corticosterone (reviewed in Herman et al., 2016). This sex difference is largely fueled by elevated levels of corticosteroid binding globulin in females, which largely normalizes levels of ‘free’ corticosterone (Tinnikov, 1999), (compare figures 2B and E, 2H and J for example). Previous work from our group and others indicate substantial differences in HPA axis secretion across the estrus cycle (Carey et al., 1995; Figueiredo et al., 2002; Viau and Meaney, 1991). Our design was optimized to study stress as the primary independent variable and then the effect of the modulation of GR during stress and therefore could not adequately address the cycle, which would require daily stressful procedures (vaginal swabs) and variation of time points to correspond with components of the cycle (proestrus is time-limited). Thus, it is important to acknowledge that our study was (intentionally) not adequately powered for assessment of sex differences, and that effects observed in females transcend any variance caused by time of estrus.
Adolescent CVS reduced baseline AM ACTH in the males, but had no effect on corticosterone secretion. Though significant, the decrease in ACTH occurs in the low range of circulating ACTH, and may not be sufficient to differentially affect drive of the adrenal. In contrast, adolescent CVS increased baseline AM ACTH and corticosterone in females, the latter of which was blocked by treatment with C108297. Remarkably, C108297 reduced baseline levels of corticosterone in both sexes and prevented the increase in adrenal sensitivity caused by CVS.
The C108297 effect on corticosterone levels, in the absence of changes to ACTH levels, in both sexes, points to the possibility that C108297 exerts actions at the level of the adrenal gland to regulate adrenal gland reactivity. This absence of effects on ACTH secretion is consistent with prior findings indicating that C108297 does not regulate the expression of CRH in the PVN in intact rats (Zalachoras et al., 2013). Since no adrenal hypertrophy was noted, it is possible that prior CVS increased splanchnic innervation or reactivity, which can then regulate the sensitivity of the adrenal cortex to ACTH (Engeland and Arnhold, 2005). Thus, modulation of GR signaling during adolescence causes lasting changes in HPA axis function, perhaps due to modification of adrenal development and peripheral innervation occurring in adolescent life. As discussed in Romeo, 2010a, 2010b, it is still unknown how adrenal function is developing during adolescence and how this is affected by stress. Nevertheless, based on human literature, we know that there is a general thickening of the adrenal cortex during this life epoch, which has been mostly studied in association to pubertal changes in sex hormones (Hui et al., 2009; Nakamura et al., 2015). Relevance for stress response remains to be determined.
Exposure to adolescent CVS does not affect the magnitude of the male HPA axis response to an acute novel stressor in adulthood (5 weeks later) (Cotella et al., 2019; Smith et al., 2018). This contrasts with long-lasting sensitization seen in adults receiving CVS and tested 5 weeks later (Cotella et al., 2019), suggesting that if anything, in males, adolescence confers resilience to the long-term effect of CVS on HPA axis response to an acute stressor. On the contrary, adolescent stress sensitized HPA axis reactivity in adult females, suggesting a lasting impact on stress reactivity. The data suggest adolescent females may be susceptible rather than resilient to the effects of chronic stress. Of note, our previous study using a different CVS regimen (including overnight social stressors) caused females to be hyporesponsive to acute stress, indicating differential reorganization of stress susceptibility (Wulsin et al., 2016). These data agree with those of Pohl et al. (2007), exploring two different adolescent chronic stress protocols of different intensity in female rats. These studies indicate that enhanced corticosterone secretion was observed in the group exposed to a subjectively “milder” stress protocol (no cold water stress). Collectively the data suggest that adolescent females exhibit long-term stress susceptibility across a broad range of stressor intensities, with the characteristics of stress regimen dictating the magnitude and direction of behavioral outcomes.
4.3. C108297: Mechanism of Action
Administration of C108297 prevented HPA axis sensitization following CVS in females, indicating involvement of GR signaling. C108297 is known to have both agonistic and antagonistic properties in vivo (Viho et al., 2019; Zalachoras et al., 2013) and the exact mechanism of action is difficult to specify given its tissue specificity. Prior studies indicate that administration of C108297 for 5 days reduced corticosterone secretion following a forced swim challenge in previously unstressed rats (Solomon et al., 2014), suggesting a GR agonistic action in the context of HPA feedback. Moreover, Zalachoras et al. (2013) note that C108297 treatment enhances fear memory in the passive avoidance test in a manner similar to that of corticosterone, consistent with GR agonistic actions in the CNS. However, like mifepristone, C108297 antagonizes actions of exogenous corticosterone administration For example, CORT108297 in hippocampus reverses the repression of GR-regulated genes and blocks suppression of neurogenesis by corticosterone. In the PVN, like mifepristone, CORT 108297 enhances Fos expression after an acute stressor (Zalachoras et al., 2013). Therefore, actions of CORT 108297 likely depend on cellular context. Central GR antagonism via CORT108297 during adolescence may be sufficient to block effects of intermittent corticosterone responses on HPA axis responsiveness. Alternatively, it is possible that agonistic action on the HPA axis is preventing increases in corticosterone during repeated stress exposures across the CVS regimen, which may alter subsequent excitability of stress responsive neuronal populations. Dissection of agonist vs. antagonist properties of CORT 108297 in this context would require assessment of HPA axis drive throughout the CVS regimen. Furthermore, it is also possible that the observed outcomes are the result of combinatory actions of CORT 108297 of multiple tissues and brain regions.
C108297 reduces body weight gain and absolute body weight in both sexes and this effect remained weeks after cessation of the treatment. Prior studies have reported body weight reduction following administration of C108297 and other similar GR targeting drugs ( Asagami et al., 2011; Belanoff et al., 2010; Sindelar et al., 2014; Van Den Heuvel et al., 2016), suggesting their potential efficacy in the treatment of obesity. Initial studies attributed body weight effects to peripheral GR antagonist actions on metabolism, although more recent data also points to changes in food intake that differed from the mostly peripheral and milder effects that mifepristone exerts on metabolism (Van Den Heuvel et al., 2016). In our study, C108297 produces resistance to body weight gain during treatment as well as several weeks following discontinuation, consistent with an organizational action of adolescent glucocorticoids on metabolic trajectory, which could be related both long-term actions on fat tissue metabolism as well as to programming of central control of food intake.
4.4. Mapping of neuronal recruitment in response to forced swim test
Quantifying Fos immunoreactive cells after exposure to FST provides insight into how adolescent stress can program the recruitment of brain regions integrating the response to the FST challenge, particularly in the males. In general, adolescent stress blocked recruitment of several brain regions known to be involved in stress integration, including the prefrontal cortex, the lateral ventral septum, the paraventricular nuclei of the thalamus and hypothalamus, and the central, medial and basolateral divisions of the amygdala. Consistent with our data, previous studies also noted decreased prefrontal cortical activation using different modalities of adolescent stress and acute adult stimulus (Ishikawa et al., 2015). In adult males, fos RNA expression induced by exposure to a novel environmental stressor is known to decrease in many of these brain regions immediately following a week of CVS, and this reduction is still observed in the prelimbic cortex and the lateral ventral septum after a month (Ostrander et al., 2010). Our results suggest that neurophysiological adaptations to prior stress experience may render stress-integrative circuitry less responsive to novel challenges. This stands in contrast to females, where (except for the lateral septum) adolescent stress is ineffective in modulating Fos reactivity following the forced swim.
The combination of behavioral resilience and generalized reduction in post-FST Fos expression in males suggests that adolescent stress may act to limit neural responses to subsequent stress later in life and thereby buffer its overall impact. Prior studies indicate that adolescent male HPA axis responses to both acute restraint (Russell D. Romeo, 2010a; Viau et al., 2005) and CVS (Jankord et al., 2011) are more pronounced than adult responses and accompanied by higher Fos immunoreactivity in the PVN compared to adults at least in the case of acute exposure to stress (Viau et al., 2005). It has also been reported that prepubertal males have reduced Fos expression in the medial amygdala after an acute stressor compared to adults (Kellogg et al., 1998), indicating the relevance of developmental differences regarding the sensitivity of different brain regions to stress.
It is important to also consider the limited resilience evident in females. In our previous studies, the immediate impact of adolescent stress on the HPA axis response to acute stress in males (Jankord et al., 2011) is not observed in females (Wulsin et al., 2016). This is also consistent with previously reported reduced stress reactivity during adolescence in females (Russell D. Romeo, 2010a). Taken in cobination with the generalized absence of adolescent stress reduction in Fos expression observed in this sex in the current study, the data suggest that reduced stress reactivity during adolescence may result in enhanced neurocircuit activation in adulthood. A possible explanation could be that the failure to robustly engage central stress pathways during adolescent stress is a factor in limiting later stress resilience in females, resulting in more pronounced behavioral and physiological reactivity later in life.
5. Conclusion
The results presented here support the idea that experiencing chronic stress during adolescence evokes different effects within each sex. Furthermore, we also demonstrate that some of these enduring adolescent stress effects could be prevented by modulating the signaling through the glucocorticoid receptor during the exposure to stress, suggesting the involvement of these receptors in the development of the adult phenotype observed. Based on the disparities in the effects observed both in males and females, it is evident that either the organizational and/or the activational effects of sex hormones play a relevant part modulating the effects of stress during development and this may in part be mediated via glucocorticoid signaling, indicating an interesting avenue for future research. Remarkably, we observed that modulating this receptor system in unstressed animals also affected some of the adult outcomes, supporting the participation of glucocorticoid dynamics during this developmental phase as a programming factor for adult responses. Finally, differences in the effects due to variations in the chronic stress protocols and the dissection of the antagonistic versus the agonistic actions on GR are another important direction for further investigation.
Supplementary Material
Acknowledgements
We would like to thank Corcept Therapeutics for providing the CORT108297 compound, and in particular, to Drs. Joseph Belanoff, Hazel Hunt for their thoughtful comments on the manuscript. Thanks to Dr. William Engeland (University of Minnesota) for kindly donating the ACTH antiserum. We also would like to acknowledge other members of Dr Herman’s laboratory and other researchers in the Reading Campus of the University of Cincinnati, especially Dr. Matia Solomon, for their support and helpful comments on the results.
Funding: This project was funded by the National Institutes of Health (R01MH101729 and VA I01BX003858 to JPH, T32 DK059803 to EMC and SEM, F30-NS-095578 to ACW), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET- ARGENTINA), Postdoctoral Fellowship to EMC and a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation to RDM.
References
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 31, 183–91. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH, 2004. Delayed Effects of Early Stress on Hippocampal Development. Neuropsychopharmacology 29. [DOI] [PubMed] [Google Scholar]
- Asagami T, Belanoff JK, Azuma J, Blasey CM, Clark RD, Tsao PS, 2011. Selective Glucocorticoid Receptor (GR-II) Antagonist Reduces Body Weight Gain in Mice. J. Nutr. Metab 2011, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt HJ, Belanoff JK, Golding E, Gourdet B, Phillips T, Swift D, Thomas J, Unitt JF, Walters I, 2015. 1H-Pyrazolo[3,4-g]hexahydro-isoquinolines as potent GR antagonists with reduced hERG inhibition and an improved pharmacokinetic profile. Bioorganic Med. Chem. Lett 25, 5720–5725. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Blasey CM, Clark RD, Roe RL, 2010. Selective glucocorticoid receptor (type II) antagonist prevents and reverses olanzapine-induced weight gain. Diabetes. Obes. Metab 12, 545–7. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN, 2011. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav 60, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL, 2011. Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev 35, 1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER, 1995. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J. Endocrinol 144, 311–21. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Riva MA, 2016. Stress-induced mechanisms in mental illness: A role for glucocorticoid signaling. J. Steroid Biochem. Mol. Biol 160, 169–174. [DOI] [PubMed] [Google Scholar]
- Champagne DL, de Kloet ER, Joëls M, 2009. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Semin. Fetal Neonatal Med 14, 136–42. [DOI] [PubMed] [Google Scholar]
- Cotella EM, Scarponi Gómez A, Lemen P, Chen C, Fernández G, Hansen C, Herman JP, Paglini MG, 2019. Long-term impact of chronic variable stress in adolescence versus adulthood. Prog. Neuro-Psychopharmacology Biol. Psychiatry 88, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F, 2005. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci 6, 463–475. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Karst H, Joëls M, 2008. Corticosteroid hormones in the central stress response: quick-and-slow. Front. Neuroendocrinol 29, 268–72. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD, 2013. Stress and the developing adolescent brain. Neuroscience 249, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland W, Arnhold M, 2005. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine 28, 325–331 LA–English. [DOI] [PubMed] [Google Scholar]
- Engeland W, Byrnes GJ, Presnell K, Gann DS, 1981. Adrenocortical Sensitivity to Adrenocorticotropin (ACTH) in Awake Dogs Changes as a Function of the Time of Observation and after Hemorrhage Independently of Changes in ACTH. Endocrinology 108, 2149–2153. [DOI] [PubMed] [Google Scholar]
- Engeland WC, Dempsher DP, Byrnes GJ, Presnell K, Gann DS, 1981. The Adrenal Medullary Response to Graded Hemorrhage in Awake Dogs. Endocrinology 109, 1539–1544. [DOI] [PubMed] [Google Scholar]
- Faraji J, Soltanpour N, Jafari SY, Moeeini R, Pakdel S, Moharreri A, Metz GAS, 2014. Stress inhibits psychomotor performance differently in simple and complex open field environments. Horm. Behav 65, 66–75. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Dolgas CM, Herman JP, 2002. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology 143, 2534–40. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, 2000. First-episode major depression in adolescents: Affective, cognitive and endocrine characteristics of risk status and predictors of onset. Br. J. Psychiatry 176, 142–149. [DOI] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM, 2013. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male long-evans rats. Dev. Psychobiol 55, 849–859. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Grossman a B., Hastings MH, de Kloet ER, Lightman SL, Lupien SJ, Roozendaal B, Seckl JR, 2006. Do Corticosteroids Damage the Brain? J. Neuroendocrinol. 18, 393–411. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, 1997. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 20, 78–84. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B, 2016. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol 6, 603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H, 2005. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1201–13. [DOI] [PubMed] [Google Scholar]
- Hui X-G, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H, 2009. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J. Endocrinol 203, 241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilin Y, Richter-Levin G, 2009. Enriched Environment Experience Overcomes Learning Deficits and Depressive-Like Behavior Induced by Juvenile Stress. PLoS One 4, 4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa J, Nishimura R, Ishikawa A, 2015. Early-life stress induces anxiety-like behaviors and activity imbalances in the medial prefrontal cortex and amygdala in adult rats. [DOI] [PubMed]
- Jacobson-Pick S, Richter-Levin G, 2010. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav. Brain Res 214, 268–276. [DOI] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP, 2011. Stress vulnerability during adolescent development in rats. Endocrinology 152, 629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Karst H, Krugers HJ, Lucassen PJ, 2007. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front. Neuroendocrinol 28, 72–96. [DOI] [PubMed] [Google Scholar]
- Kellogg C., Awatramani G., Piekut D., 1998. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neuroscience 83, 681–689. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL, 2008. Is Adolescence a Sensitive Period for Depression ? Behavioral and Neuroanatomical Findings From a Social Stress Model 30, 22–30. [DOI] [PubMed] [Google Scholar]
- Luo X-M, Yuan S-N, Guan X-T, Xie X, Shao F, Wang W-W, 2014. Juvenile stress affects anxiety-like behavior and limbic monoamines in adult rats. Physiol. Behav 135, 7–16. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ, 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav. Brain Res 187, 228–238. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, 2002. Stress Activation of Glutamate Neurotransmission in the Prefrontal Cortex: Implications for Dopamine- Associated Psychiatric Disorders 1–13. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen J, Herman JP, 2012. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell. Mol. Neurobiol 32, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Fujishima F, Hui X, Felizola SJA, Shibahara Y, Akahira J, McNamara KM, Rainey WE, Sasano H, 2015. 3βHSD and CYB5A double positive adrenocortical cells during adrenal development/aging. Endocr. Res 40, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER, 2010. Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev 34, 853–866. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Flak JN, Richtand NM, Herman JP, 2009. Chronic stress produces enduring decreases in novel stress-evoked c-fos mRNA expression in discrete brain regions of the rat. Stress 12, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Flisher AJ, Hetrick S, McGorry P, 2007. Mental health of young people: a global public-health challenge. Lancet 369, 1302–13. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The rat brain in stereotaxic coordinates. Elsevier. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL, 2007. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav. Neurosci 121, 462–474. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, de Kloet ER, 1985. Two Receptor Systems for Corticosterone in Rat Brain: Microdistribution and Differential Occupation. Endocrinology 117, 2505–2511. [DOI] [PubMed] [Google Scholar]
- Romeo RD, 2010a. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front. Neuroendocrinol 31, 232–40. [DOI] [PubMed] [Google Scholar]
- Romeo RD, 2010b. Adolescence: A central event in shaping stress reactivity. Dev. Psychobiol 52, n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Sindelar DK, Carson MW, Morin M, Shaw J, Barr RJ, Need A, Alexander-Chacko J, Coghlan M, Gehlert DR, 2014. LLY-2707, a novel nonsteroidal glucocorticoid antagonist that reduces atypical antipsychotic-associated weight gain in rats. J. Pharmacol. Exp. Ther 348, 192–201. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF, 2012. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc 7, 1009–1014. [DOI] [PubMed] [Google Scholar]
- Smith BL, Morano RL, Ulrich-Lai YM, Myers B, Solomon MB, Herman JP, 2018. Adolescent environmental enrichment prevents behavioral and physiological sequelae of adolescent chronic stress in female (but not male) rats. Stress 21, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, Flak JN, Ulrich-Lai Y, Herman JP, 2014. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm. Behav 65, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnikov AA, 1999. Responses of Serum Corticosterone and Corticosteroid-Binding Globulin to Acute and Prolonged Stress in the Rat. Endocrine 11, 145–150. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A, 2008. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem 107, 522–532. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y, Engeland W, 2002. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76, 79–92. [DOI] [PubMed] [Google Scholar]
- Van Den Heuvel JK, Boon MR, Van Hengel I, Peschier-Van Der Put E, Van Beek L, Van Harmelen V, Van Dijk KW, Pereira AM, Hunt H, Belanoff JK, Rensen PCN, Meijer OC, 2016. Identification of a selective glucocorticoid receptor modulator that prevents both diet-induced obesity and inflammation. Br. J. Pharmacol 173, 1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M, 2005. Gender and Puberty Interact on the Stress-Induced Activation of Parvocellular Neurosecretory Neurons and Corticotropin-Releasing Hormone Messenger Ribonucleic Acid Expression in the Rat. Endocrinology 146, 137–146. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ, 1991. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 129, 2503–2511. [DOI] [PubMed] [Google Scholar]
- Viho E, Buurstede JC, Mahfouz A, Koorneef LL, van Weert LTCM, Houtman R, Hunt H, Kroon J, Meijer O, 2019. Corticosteroid action in the brain: the potential of selective receptor modulation. Neuroendocrinology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Wick-Carlson D, Packard BA, Morano R, Herman JP, 2016. Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology 65, 109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalachoras I, Houtman R, Atucha E, Devos R, Tijssen AMI, Hu P, Lockey PM, Datson N. a, Belanoff JK, Lucassen PJ, Joëls M, de Kloet ER, Roozendaal B, Hunt H, Meijer OC, 2013. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc. Natl. Acad. Sci 110, 7910–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA, 2012. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience 226, 459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.