Abstract
Background
An increasing percentage of potential organ donors are infected with hepatitis C virus (HCV). Establishment of HCV infection in uninfected recipients is near-universal with the requirement for post-transplant antiviral treatment. The aim of this study was to determine if antivirals combined with an HCV entry blocker given before and for 7 days after transplant would be safe and reduce the likelihood of HCV infection in recipients of organs from HCV-infected donors.
Methods
HCV-uninfected organ recipients without pre-existing liver disease were treated with ezetimibe 10 mg (an HCV entry inhibitor) and glecaprevir/pibrentasvir 300 mg/120 mg one dose before and daily for 7 days after transplantation from HCV-infected donors under age 70 without HIV or HBV co-infection. HCV RNA was assessed daily for 14 days and then weekly to 12 weeks post-transplant. The primary endpoint was prevention of chronic HCV infection by intention-to-treat as evidenced by undetectable serum HCV RNA 12 weeks after transplant (registration NCT04017338).
Findings
30 patients (23 male, median age 61) received transplants (13 lung, 10 kidney, 6 heart and 1 kidney-pancreas) from 18 HCV-infected donors. The median donor viral load was 5.11 log10IU/mL (range 1.18–7.13, IQR 4.55–5.63 log10IU/mL) and included different HCV genotypes (9 genotype 1, 2 genotype 2, 5 genotype 3 and 2 genotype unknown). All 30 of 30 (100%) patients met the primary endpoint with undetectable HCV RNA at 12 weeks post-transplant and remain HCV RNA negative at last follow-up (median 36 weeks, range 14–54, IQR 25–47 weeks post-transplant). Low-level viremia was transiently detectable in 20 (67%) of 30 recipients in the early post-transplant period but never beyond day 11. Treatment was well tolerated with no dose reductions or treatment discontinuations; there were 27 serious adverse events in 18 (60%) of 30 patients with one grade 3 ALT elevation possibly related to treatment. Transient ALT and CK elevations during treatment resolved with treatment completion. Two recipients died of unrelated causes and neither was ever viremic for HCV.
Interpretation
Ezetimibe combined with glecaprevir/pibrentasvir given one dose before and for 7 days after transplant prevented establishment of chronic HCV infection in recipients of different organs from HCV-infected donors. This study demonstrates that an ultra-short course of DAAs and ezetimibe can prevent establishment of chronic HCV infection in the recipient, alleviating many of the concerns of using HCV-infected organs for transplantation.
Keywords: organ transplantation, hepatitis C virus (HCV), prevention, ezetimibe, direct-acting antivirals
Introduction
Organ transplantation is a life-saving therapy, but access is greatly limited by a shortage of donor organs. The ongoing opioid epidemic has led to an increase in hepatitis C virus (HCV) transmission and the overdose crisis has resulted in an increase in organ offers from HCV-infected donors1. Major advances in HCV therapy have opened the possibility of transplanting organs from HCV-infected donors to HCV-uninfected recipients2,3. Recent studies have shown that recipients of organs from HCV-infected donors can be promptly treated with a high probability of cure with 4- to 12-week courses of HCV therapy4–6. While this approach appears safe and effective, challenges have been encountered5,7,8. In addition, despite a full course of therapy, cases of relapse with complicated resistance profiles and even fibrosing cholestatic hepatitis have been reported7,9,10. To avoid problems encountered with treating HCV after transplantation, strategies to prevent infection would be preferable.
Virus present in residual blood and fluid in the donor organ at the time of transplantation infects the recipient liver promptly. We have previously shown that light-based therapy during ex-vivo organ perfusion prior to transplantation can reduce infectivity and lower initial viral levels in the recipient but is inadequate to entirely prevent infection7,11. Pre-emptive therapy with direct-acting antiviral agents (DAAs) that potently inhibit stages in the HCV lifecycle could prevent replication and spread of HCV upon infection. However, it would be preferable to block or limit HCV entry as well. HCV entry into hepatocytes is a complex process requiring multiple entry factors including CD8112, SRB1 (scavenger-receptor-B1)13, claudin-114, occludin15 and the Niemann-Pick C1-like receptor (NPC1L)16. NPC1L silencing or receptor blocking with antibodies, potently inhibits HCV entry. Ezetimibe, an approved cholesterol-lowering therapy, is a ligand for the NPC1L receptor, and in cell culture and a humanized mouse model, pre-treatment with ezetimibe restricts HCV entry16.
Preventative therapy with DAAs requires the use of regimens that work against all HCV genotypes and can be used in patients with end-stage organ disease. Glecaprevir/pibrentasvir combines an HCV protease inhibitor with an inhibitor of the non-structural 5A (NS5A) protein and treats all 6 HCV genotypes, leading to cure rates of 95–99% with 8 weeks of therapy in chronic HCV infection3,17. Glecaprevir/pibrentasvir is safe in patients with chronic kidney disease, including dialysis, a relevant concern after transplantation18.
In this study, we evaluated the use of glecaprevir/pibrentasvir combined with ezetimibe given as a single dose prior to transplant and for one week after surgery to prevent infection after organ transplantation from HCV-infected donors to uninfected recipients.
Methods
Study population
Donors
Donor inclusion criteria were age under 70 years (age under 45 years for kidney transplants) and NAT (nucleic acid test) positivity for HCV. Other donor parameters were evaluated according to standard metrics for organ selection. Donors positive for hepatitis B virus (NAT or hepatitis B surface antigen), HIV (NAT or serology), or HTLV 1 or 2 (serology) were excluded, but hepatitis B core antibody-positive donors were allowed.
Recipients
All patients (of any age) on the lung, heart, kidney or kidney-pancreas transplant waitlist at the Toronto General Hospital were eligible for the trial provided they tested negative for HCV RNA, did not have pre-existing liver disease (fibrosis > stage 2; or transaminases > 3x upper limit of normal), were not listed for liver transplantation, did not have a known allergy to glecaprevir/pibrentasvir or ezetimibe and provided written informed consent. Patients were approached during clinic visits or when admitted to hospital. They received educational materials about HCV and were given the opportunity to meet with the hepatology team before transplantation. For all but kidney transplants, patients provided written informed consent while on the waitlist and reaffirmed consent at the time of organ offer. For kidney transplants, patients from the top of the waitlist were offered participation in the trial at the time a kidney from an HCV-infected donor became available to avoid concerns regarding waitlist allocation priority. The kidney recipients spoke to a physician uninvolved in the transplant process about the study and received written study materials for review. Patients were given a minimum of 3 hours and up to 12 hours to review the information and ask questions before signing informed consent.
Procedures
Organs from HCV NAT-positive donors were selected based on standard criteria for transplantation. Organs were retrieved and transported in static cold preservation. Lungs were evaluated on clinical grounds and if deemed necessary, were evaluated and treated with 4 hours of normothermic ex-vivo lung perfusion (EVLP) using an acellular perfusate solution19. Based on our previous work showing that ultraviolet-C (UVC) light delivered during EVLP lowers HCV RNA levels and infectivity, UVC light was delivered to the circulating perfusate for the duration of EVLP7,11. Ex-vivo organ perfusion was not performed for organs other than lungs.
Once the transplant was confirmed, consented recipients received the initial dose of glecaprevir (300 mg)/pibentrasvir (120 mg) and ezetimibe (10 mg), orally 6 to 12 hours prior to the anticipated implantation of the donor organ. Recipients continued daily oral glecaprevir/pibrentasvir and ezetimibe for 7 additional days after transplant (total 8 doses). If required, treatment was given by nasogastric tube. The ezetimibe dose was chosen because 10 mg daily is the approved dose for human use. HCV RNA was measured with the Cobas 4800 HCV RNA assay (Roche Diagnostics, Basel, Switzerland) with a lower limit of quantification of 15 IU/mL daily for the first 2 weeks, then weekly for 12 weeks and again 6 months after transplantation. Prevention of established infection was defined as undetectable HCV RNA 12 weeks after transplantation. Tacrolimus was used for all recipients because of a significant interaction with glecaprevir/pibrentasvir and cyclosporine20. Other post-operative transplant care and immunosuppression followed standard transplant practice. The study was approved by the Research Ethics Board of Toronto General Hospital, University Health Network and by Health Canada. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
Outcomes
The primary endpoint was prevention of HCV infection as evidenced by undetectable serum HCV RNA 12 weeks after transplantation. Secondary endpoints included graft and patient survival, development of anti-HCV antibodies, development of HCV-specific immune responses (to be reported elsewhere), HCV viremia with and without EVLP, adverse events, incidence of acute liver dysfunction (including fibrosing cholestatic hepatitis), incidence of biopsy-proven acute rejection requiring treatment, organ function at 3, 6 and 12 months post-transplant as measured by 6-minute walk and forced expiratory volume in 1 second (FEV1) (lung), estimated glomerular filtration rate (eGFR) (kidney), ejection fraction (heart) and freedom from exogenous insulin (pancreas). Patients will be followed with annual HCV RNA for 5 years.
Statistical analysis
All HCV RNA values were log-transformed and values that were detectable but below the limit of quantification (15 IU/mL) were arbitrarily assigned a value of 7.5 IU/mL. As a post-hoc secondary analysis, baseline factors associated with transient viremia were evaluated using exact logistic regression with both dichotomous (viremic vs non-viremic) and ordinal (undetectable, detectable <15 IU/mL and quantifiable viremia) models. Because some recipients received organs from the same donors, the regression analysis was also fit to a generalized estimating equations (GEE) model, to account for the lack of independence between all samples. For the association with donor viral load and HCV genotype, the bivariate analysis was performed with both exact logistic regression and the GEE model to ensure that results were concordant. The study was designed with a target sample size of 40 patients to assess safety and efficacy. Efficacy and safety were evaluated using intention-to-treat analysis. The study was designed with futility rules to extend therapy to 14 days if more than 1 patient in the first 5 became infected and to extend to 4 weeks if more than 1 in the first 5 of a 14-day treatment became infected. A data safety and monitoring board reviewed the results of the study after the first 5 patients completed their treatment course and then every 3 months or upon reporting of an unexpected adverse event. The full protocol is available in the supplementary material. Data are reported on the first 30 recipients. Analyses were performed with SAS version 9.4, with two-sided p-values less than 0.05 considered statistically significant. The study was registered on ClinicalTrials.gov (NCT04017338), however there was a delay between initial submission and final posting of the trial due to an administrative error.
Role of Funding Sources
This study, including medication costs, was funded by a project grant from the Canadian Institutes of Health Research and from a grant through the Multi-Organ Transplant Centre at University Health Network. The sponsors had no role in study design, data collection, analysis, or writing of the manuscript. The corresponding authors (JF, MC, AH) had full access to all of the data and final responsibility to submit for publication. All authors reviewed the final manuscript and approved the decision to submit for publication.
Results
Study population
A total of 30 transplants were performed from 18 HCV NAT-positive donors to HCV-uninfected recipients including 13 lungs (6 single, 7 double), 10 kidneys, 6 hearts and 1 kidney-pancreas transplant between February 4 and November 11, 2019. EVLP with UVC was used in 7 of the 13 lung cases. A maximum of 4 organs (heart, kidney, kidney/pancreas) were used from any single donor. Characteristics of donors and recipients are shown in Table 1. Of 18 donors, median age was 35.5 (range 17–57, IQR 31–38) years, 14 (77%) were male and median donor HCV RNA was 5.11 log10IU/mL (range 1.18–7.13, IQR 4.55–5.63 log10IU/mL).
Table 1.
Baseline characteristics of organ donors and recipients
| Baseline Characteristics | Summary Measures |
|---|---|
|
Recipient Factors (n=30) | |
| Age [years], (median, IQR) | 61 (48–66) |
| Male, n(%) | 23 (77%) |
| Ethnicity | |
| White | 22 (73%) |
| Asian | 4 (13%) |
| Black | 3 (10%) |
| Hispanic | 1 (3%) |
| Organ received, n(%) | |
| Lung | 13 (43%) |
| Kidney | 10 (33%) |
| Heart | 6 (20%) |
| Kidney-Pancreas | 1 (3%) |
| Time from Consent to transplant, [Days], (median, IQR) | 7.5 (1–34) |
| Follow-up time post-transplant, [weeks], (median, range, IQR) | 27 (14–54, 25–47) |
|
Donor Factors (n=18) | |
| Age [years], (median, IQR) | 35.5 (31–39) |
| Male, n(%) | 14 (78%) |
| HCV RNA level [log10IU/mL], (median, range, IQR) | 5.11 (1.18–7.13, 4.55–5.63) |
| HCV genotype, n(%) | |
| 1a | 7 (39%) |
| 1b | 1 (6%) |
| 1 unspecified | 1 (6%) |
| 2 | 2 (11%) |
| 3 | 5 (28%) |
| Unknown* | 2 (11%) |
Genotyping was unsuccessful in 2 donor samples
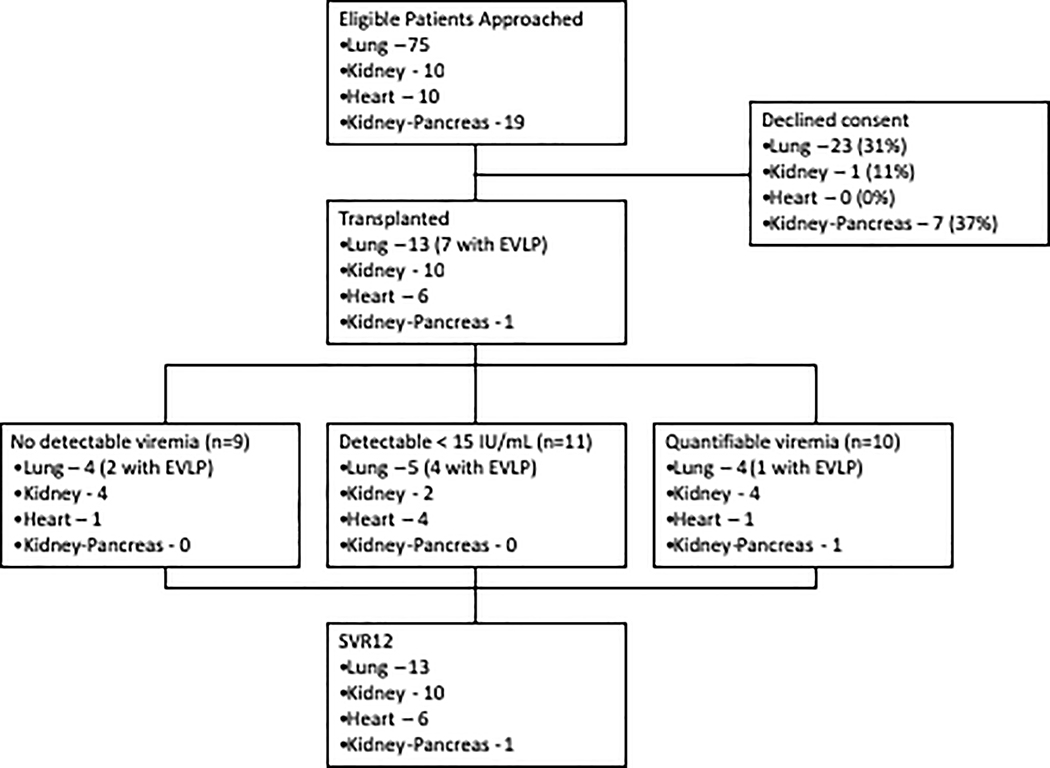
Of 113 eligible transplant candidates approached for the study, 82 (72.6%) consented to participate, of whom 30 (26.5%) ultimately received an organ from an HCV NAT-positive donor (Figure 1). The median age of recipients was 61 (range 26–76, IQR 48–66.8) years and 77% were male. Median time from informed consent to transplantation was 31 (IQR 10–49) days for lung recipients and 1 (IQR 1–18) day for heart recipients, compared to 50 (IQR 17–103) days and 66 (IQR 21–123) days from listing to lung and heart transplant from non-HCV-infected donors, respectively, during the study period (p<0.0001).
Figure 1. CONSORT Diagram –
The flow of patients from consent through transplantation is shown by organ-type.
HCV Infection
Of 30 transplant recipients, all 30 (100%) met the primary endpoint of undetectable HCV RNA at 12 weeks post-transplant and all were HCV RNA negative at last follow-up (median 36.1 weeks, range 14 to 54 weeks, IQR 25–47 weeks post-transplant). Establishment of chronic infection was prevented in all transplant recipients with the 8-day study regimen. All heart, lung and kidney/pancreas recipients and 5 kidney recipients completed treatment prior to discharge from hospital.
Early HCV Viremia
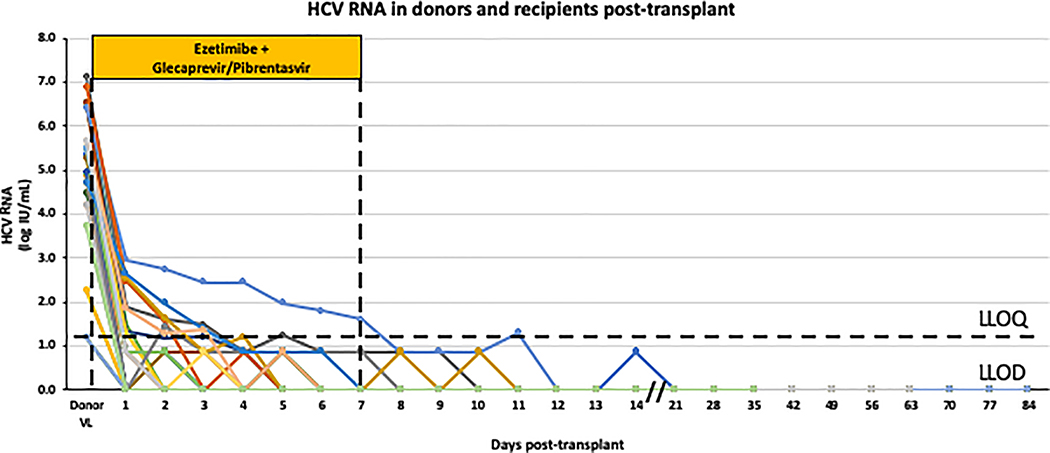
Among the 30 recipients, 9 (30%) never had detectable viremia, 11 (37%) had transiently detectable but unquantifiable HCV RNA and 10 (33%) had transiently quantifiable viremia with peak HCV RNA day 1 after transplantation (median 1.87 log10IU/mL, range 1.30 to 2.96, IQR 1.49–2.55 log10IU/mL). Individual HCV RNA levels are shown in Figure 2. Three patients had detectable HCV RNA beyond the 8 doses of antiviral therapy. Of these, 2 patients had a single detectable HCV RNA that was below the limit of quantification on Day 9 and 10 post-transplant, respectively, and HCV RNA has subsequently remained undetectable beyond 12 weeks of follow-up. The other individual had an HCV RNA of 2.96 log10IU/mL on post-operative day 1 that declined daily reaching unquantifiable but detectable levels on post-operative day 8. On post-operative day 11, 4 days after completing therapy, HCV RNA was 1.3 log10IU/mL but has been undetectable since (39 weeks post-transplant). All other patients had undetectable HCV RNA by the end of treatment and have remained HCV RNA negative.
Figure 2. HCV RNA levels in the donor and recipients post-transplant.
The HCV RNA levels are shown for the 18 organ donors and for each of the 30 organ recipients over time (each coloured line represents an individual recipient). HCV RNA levels that were detectable but below the limit of quantification (15 IU/mL) were arbitrarily set to 7.5 IU/mL. A dose of ezetimibe and glecaprevir/pibrentasvir was given before and for 7 days after transplantation; VL viral load; LLOQ lower limit of quantification; LLOD lower limit of detection
HCV antibody became detectable in 14 of 30 patients (47%). In two patients, HCV antibody was detectable at week 4 but was undetectable at week 12. There was no association with transient viremia and development of HCV antibodies.
Safety
Two lung transplant recipients died, 49- and 109-days post-transplant, from sepsis and subarachnoid hemorrhage, respectively (Supplementary Appendix page 9). The other 28 recipients are alive with functioning grafts. Including the deaths, 32 serious adverse events (SAE) occurred in 20 patients (Table 2). One SAE was deemed possibly related to the therapy; prolongation of hospital admission in a kidney recipient for transiently elevated liver enzymes (peak ALT 650 U/L at post-op day 11; INR and bilirubin normal). Work-up for causes of acute hepatitis was unrevealing, including persistently undetectable HCV RNA. Liver biopsy showed a non-specific acute hepatitis with moderate steatosis but no steatohepatitis and no fibrosis. ALT returned to normal day 27 after transplantation.
Table 2.
Safety Data
| Event | Recipients (n=30) |
|---|---|
| Serious Adverse Events* | |
| Total Events | 32 |
| Patients with SAE (n,%) | 23 (67%) |
| Treatment-related SAEs (n) | 1 |
| AEs requiring treatment discontinuation | 0 |
| Patients with episode of acute rejection requiring treatment* (n,%) | |
| Lung | 3 (23%) |
| Kidney | 0 |
| Heart | 4 (67%) |
| Kidney-Pancreas | 0 |
| Episodes of acute rejection requiring treatment* (n) | |
| Lung | 3 |
| Kidney | 0 |
| Heart | 7 |
| Kidney-Pancreas | 0 |
| Laboratory Adverse Event (n,%) | |
| ALT Elevation* | |
| Grade 1 | 9 (30%) |
| Grade 2 | 2 (7%) |
| Grade 3 | 4 (13%) |
| Grade 4 | 1 (3%) |
| Bilirubin Elevation | |
| Grade 1 | 1 (3%) |
| Grade 2 | 6 (20%) |
| Grade 3 | 1 (3%) |
| Grade 4 | 2 (7%) |
| Creatine Kinase Elevation* | |
| Grade 1 | 2 (7%) |
| Grade 2 | 14 (47%) |
| Grade 3 | 1 (3%) |
| Grade 4 | 0 |
see supplementary appendix for details of SAEs, rejection episodes, ALT and CK elevations
Because of the proximity to transplantation, documentation of non-serious AEs focused on laboratory abnormalities using Common Terminology Criteria for Adverse Events (Table 2, Supplementary Appendix page 9). No AEs led to dose reductions or study discontinuation. Transient transaminase elevations occurred in 16 recipients (53%) during dosing of study medications. ALT elevations did not correlate with HCV RNA detection. Total bilirubin elevations occurred in 10 (33%) patients, with grade 2 or higher elevations seen only in lung or heart recipients, which resolved by day 30 after transplantation. Creatine kinase (CK) levels were followed because of the association of rhabdomyolysis with ezetimibe use. CK levels were transiently elevated during follow-up in 17 (57%) patients on therapy (Supplementary Appendix page 9).
Organ function was evaluated at 12 weeks of follow-up, with a median left ventricular ejection fraction of 58% (range 55–68%, IQR 56–60%) in cardiac patients, median 6-minute walk of 480m (range 352–576m, IQR 403–538m) and FEV1 76% (range 42–108%, IQR 57–78%) of predicted for lung recipients and median eGFR of 74 (range 45–98, IQR 65–92) mL/minute for kidney recipients. The pancreas recipient does not require insulin injections. Graft function was similar to patients who received organs from HCV-uninfected donors during the study period (data not shown). At least one and up to 4 episodes of biopsy-proven acute rejection requiring treatment were documented in 4 heart and 3 lung recipients, all of which responded to pulse steroids and increased immunosuppression. Rejection was not observed in kidney or kidney-pancreas recipients.
Factors associated with viremia
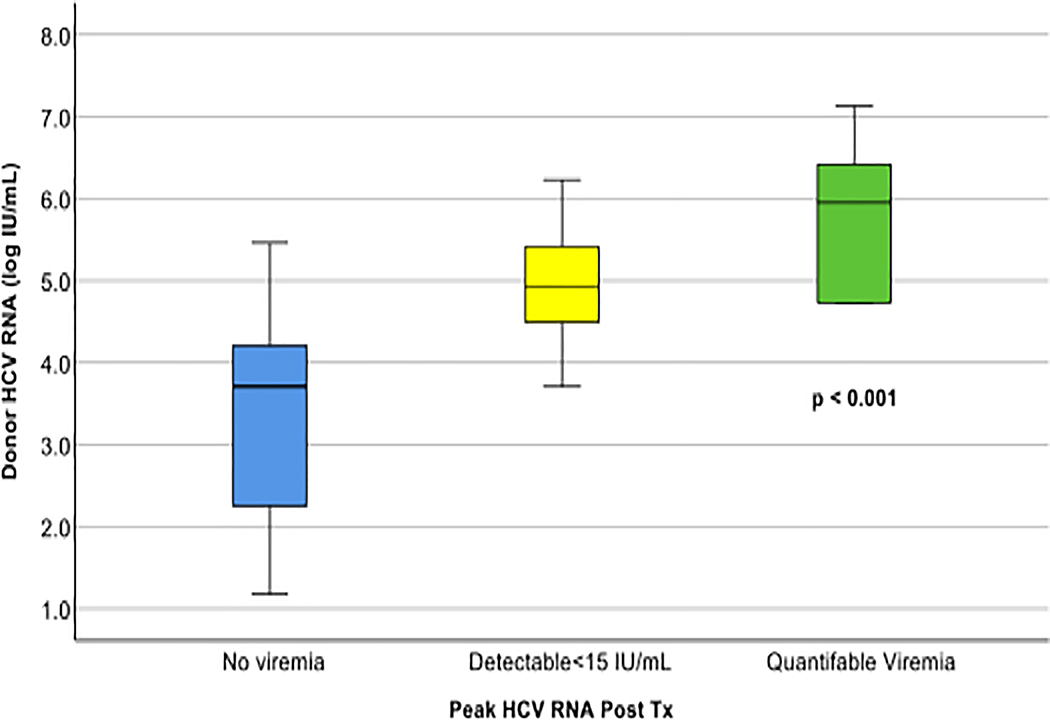
Post-hoc analyses showed that detection of viremia in the recipient was strongly associated with donor HCV RNA level (Figure 3). Recipients who never had detectable viremia had a median donor viral load of 3.71 (range 1.18–5.46, IQR 2.25–4.21) log10IU/mL, compared to 4.93 (range 3.71–6.52, IQR 4.49–5.42) log10IU/mL in recipients with detectable but unquantifiable HCV RNA and 6.04 (range 4.73–7.13, IQR 4.92–6.52) log10IU/mL in recipients with quantifiable levels of HCV RNA (p=0.0010).
Figure 3. Recipient day 1 post-transplant HCV RNA as a function of donor viral load.
Median and interquartile range of HCV RNA levels in the organ donors are shown for recipients with no documented viremia, detectable viremia below the limit of quantification (<15 IU/mL), and quantifiable viremia. HCV RNA levels were compared between groups using the Kruskal-Wallis test.
All 10 recipients of organs from genotype 3-infected donors had detectable viremia (p=0.049), which remained significant after controlling for donor viral load by exact or GEE regression (OR 7.09, 95% CI 1.05–48.0, p=0.045). Organ-type, recipient/donor age, sex and NG drug delivery were not associated with recipient viremia (Supplementary Appendix page 4–6). Recipients of organs from the same donor had similar outcomes, with all or none developing viremia. EVLP with UVC during perfusion was performed in 7 of 13 lung transplants based on clinical indications. Of these, one had quantifiable viremia whereas 3 of 6 with no EVLP/UVC had quantifiable viremia (p=0.27). The donor virus was not sequenced and thus it is unknown if any transmitted virus had pre-existing resistance-associated substitutions.
Discussion
The use of ezetimibe as an HCV entry blocker combined with the potent DAA combination of glecaprevir/pibrentasvir was able to prevent establishment of infection when given immediately prior to and for seven days after organ transplantation from HCV-infected donors to HCV-uninfected recipients. With this short course of therapy, most patients completed antiviral therapy prior to hospital discharge, and all remain free of HCV infection.
The use of HCV-infected organs for transplantation has become of increasing importance due to the devastating consequences of the ongoing overdose crisis1. The prevalence of HCV has been steadily rising among potential organ donors who die of overdose, many of whom are young without medical comorbidities4. Studies have documented the safety of using organs from HCV-infected donors with prompt initiation of antiviral therapy shortly after transplant. However, post-transplant treatment can be challenging due to drug interactions, post-operative complications and logistical challenges such as securing coverage for a full course of DAA therapy8,10,21. Although most studies have reported excellent outcomes, relapses after therapy have been documented with complex resistance profiles and even fibrosing cholestatic hepatitis, the most severe form of HCV infection7,9,10. In addition, other complications such as HCV-related glomerulonephritis and increased acute cellular rejection, have been reported in studies with long delays before starting treatment10,22. As such, prevention of transmission would be preferable.
Teasing apart whether transmission was truly prevented or if patients were infected but rapidly cured is difficult. Although 21 patients had transiently detectable viremia, over half were below the 15 IU/ml lower limit of quantification and viral levels steadily declined in all. This may reflect residual viral RNA from donor plasma present in the allograft at the time of implantation rather than active infection and replication. Consistent with this observation, the most significant corollate of viremia in the recipient was the donor viral load, as previously observed5. Detection of negative-strand HCV RNA would confirm active viral replication; however viral loads were too low for negative-strand virus detection23. Genotype 3 HCV was also associated with recipient viremia. Although genotype 3 infection has proven harder to cure with DAAs, glecaprevir/pibrentasvir is very active against this genotype and the small numbers limit strong conclusions as evidenced by the wide confidence intervals in the regression analysis 3,24. Clearance of low-level residual viremia after treatment completion may reflect immune control of infection or measurement of non-infectious virions, as has been observed with short-course DAA therapy for chronic HCV infection25. Appearance of HCV antibodies in half the recipients did not correlate with viremia. In at least some patients, the antibodies may reflect adoptive transfer from the donor, which has been observed in recipients of HCV antibody-positive/NAT-negative organs in the absence of transmission26.
The strategy used has some notable advantages over other approaches. The ultra-short course of antivirals allowed completion of therapy during the initial inpatient stay for heart and lung recipients, and at a significantly reduced cost compared to a standard course of treatment, making it feasible for the HCV treatment to become part of the cost of transplantation, similar to prophylactic therapy for other infections (cytomegalovirus, hepatitis B virus etc). Early studies used genotype-specific regimens, which can be problematic because the donor HCV genotype is rarely known4,27. The pangenotypic combination of sofosbuvir/velpatasvir has been used successfully, but sofosbuvir is contraindicated in patients who receive amiodarone, a relevant consideration for management of post-operative arrythmias, especially after heart or lung transplantation29 and although recently approved in the United States for use in renal impairment, sofosbuvir metabolites accumulate in patients with severe kidney impairment with unknown significance28. Glecaprevir/pibrentasvir is pangenotypic and can be safely used even for people on dialysis18. Although treatment was well tolerated, over half of the patients had ALT elevations during treatment. Notably, these did not correlate with HCV RNA and resolved after stopping therapy, raising the possibility that some ALT elevations were due to mild hepatotoxicity. Bilirubin elevations were associated with hepatic congestion in heart and lung transplant recipients and all resolved within 30 days of transplantation.
This study has some limitations. The relatively small sample size may limit generalizability; however, there were no virologic failures despite very high donor viral loads and multiple HCV genotypes across multiple organ types, increasing confidence that this approach is safe and effective. Although follow-up is limited, 25 patients have been followed for over 6 months, 8 for over one year and no late relapses in this study or other studies transplanting organs from HCV-positive donors into HCV-negative recipients have been reported giving us confidence that establishment of chronic infection has been prevented. This short-course strategy to prevent infection requires that the recipient liver is not already infected with HCV and thus this approach should not be used in HCV-discordant liver transplantation. The specific contribution of ezetimibe is unclear. However, in studies of short course sofosbuvir/velpatasvir, transmission was seen in 13 to 30% with the need for retreatment with one and sometimes a second full course of salvage DAA therapy30. Bethea and colleagues used a very similar protocol for heart transplants, starting with a pre-transplant dose of glecaprevir/pibrentasvir but without ezetimibe. They treated all patients for a standard 8-week course and all achieved SVR31. Notably, the mean peak HCV RNA levels in recipients was 1.76 log IU/mL after a dose of glecaprevir/pibrentasvir compared to 0.88 log IU/mL after a dose of glecaprevir/pibrentasvir with ezetimibe (p=0.029)31. With the very short treatment course used in this study, it was felt prudent to maximize the chance of preventing transmission and thus ezetimibe was given to all recipients. Fortunately, ezetimibe is inexpensive, has few drug interactions, and is well tolerated but it would be reasonable to consider a randomized trial to clarify its utility.
In conclusion, in this proof-of-concept study ezetimibe combined with glecaprevir/pibrentasvir, given one dose before and for 7 days after transplantation, prevented establishment of chronic HCV infection in recipients of different organs from HCV-infected donors suggesting that short-course therapy may be adequate to prevent HCV transmission.
Supplementary Material
Research in Context.
Evidence before this study
Therapy for hepatitis C virus (HCV) infection has improved dramatically in recent years with the development of potent, well-tolerated direct-acting antivirals (DAAs). The devastating effects of the opioid epidemic and overdose crisis in North America has seen a steady increase in the number of potential organ donors who test positive for HCV infection. Organs from these often otherwise healthy HCV-infected donors have traditionally not been used for transplantation because of poorer graft and patient survival. However, a number of recent studies have demonstrated that HCV can be effectively and safely treated after transplantation. The first pilot studies were performed in small numbers of kidney transplant recipients who were treated promptly with the DAA combination of grazoprevir/elbasvir. All patients responded to a full course of therapy, but the limited genotype coverage of this regimen has limited its broad use.
Subsequent studies evaluated the use of the pangenotypic regimen of sofosbuvir/velpatasvir in different organ recipients. Although results have been promising with high rates of HCV cure, challenges have arisen. Sofosbuvir is contraindicated in patients receiving amiodarone, which is frequently used to manage post-transplant arrythmias in heart and lung transplant recipients and although it was recently approved for use in advanced renal disease, drug metabolites accumulate in patients with severe renal impairment, with unknown significance. Furthermore, relapses after a full course of treatment have been reported with very complex resistance profiles and severe hepatitis, including fibrosing cholestatic hepatitis (FCH), the most severe form of HCV infection. Post-transplant treatment has also created logistical challenges, particularly securing coverage for a full course of DAA therapy, which leads to potential significant delays in treatment initiation. Prevention of HCV infection would be preferable.
We have previously shown that HCV transmission can be limited with the use of ultraviolet light (UV) during ex-vivo organ perfusion. UV exposure completely abrogated HCV infectivity in vitro and reduced initial viral loads in patients receiving organs from HCV-infected lungs. However, it was inadequate to entirely prevent infection in the majority of recipients leading us to search for new approaches to prevention.
Initial studies using pre-transplant DAA dosing reduced the probability of detecting viremia in the recipient, however very short courses of 1 or 4 days of sofosbuvir/velpatasvir had relatively high failure rates (13–30%). No studies with a DAA plus an entry blocker were identified on review of published literature (Pubmed – search terms: ‘Hepatitis C’ ‘entry inhibitor’ ‘entry blocker’ ‘ezetimibe’) and ongoing trials (ClinicalTrials.gov).
Added value of this study
Elegant studies had previously identified the Niemann-Pick-like C1-like receptor as an important entry factor for HCV and showed that ezetimibe, a ligand for this receptor, could limit HCV entry into hepatocytes in vitro and in humanized mice. We hypothesized that the use of an entry inhibitor combined with a potent DAA combination would prevent recipients from becoming infected upon receipt of an organ from an HCV-infected donor.
We treated recipients of different organs with a single dose of ezetimibe and the potent pangenotypic DAA combination of glecaprevir/pibrentasvir prior to transplant followed by 7 days of this combination post-transplant. The protocol was applied in 30 individuals who received organs (13 lung, 10 kidney, 6 heart, 1 kidney-pancreas) from 18 HCV-infected donors. All have reached at least 14 weeks of follow-up and none has developed chronic HCV infection. The treatment was well tolerated with only mild reversable ALT and creatine kinase elevations seen during treatment that resolved shortly after stopping. This study documents that when given with an entry blocker and before transplantation, much shorter than standard DAA durations can be used to prevent chronic infection in recipients of organs from HCV-infected donors.
Implications of all the available evidence
Collectively, studies show that organs from otherwise healthy HCV-infected donors can safely be used for transplantation into HCV-uninfected recipients. Although post-transplant treatment is relatively effective, prevention of chronic infection is clearly preferable. This study demonstrates that an ultra-short course of DAAs and ezetimibe can be completed before hospital discharge and prevent establishment of chronic HCV infection in the recipient. This strategy would alleviate most of the concerns with using HCV-infected organs for transplantation and thus would have a major impact on organ availability across North America and beyond.
Acknowledgments
Funding: The study was funded by grants from the Canadian Institutes of Health Research and the Organ Transplant Program, University Health Network.
NIH Funding:
J. Feld: U01DK082874
H. Dahari: R01AI078881, R01AI144112, R01AI146917, R01GM121600, and I01CX001398
J. Kim: U01 AI063594-11
H. Janssen: U01DK082874
Financial Support: This study was supported by a project grant from the Canadian Institutes for Health Research and from the Organ Transplant Program, University Health Network. No writing assistance was provided.
Footnotes
Data Sharing Agreement
Deidentified participant-level data will be available to others upon publication of the study. Requests for data should be sent to Jordan.feld@uhn.ca and upon review of the proposed protocol and signing of a data-sharing agreement, the data will be made available. The protocol and consent form will also be available upon email request. on episodes, ALT and CK elevations
| Research grants | Financial compensation for lecture activities | Consultancy | |
|---|---|---|---|
| JJF | AbbVie, Abbott, Gilead, Wako/Fujifilm- | AbbVie, Enanta, Gilead, Janssen, Roche | |
| MC | |||
| DK | |||
| HD | Eiger Biopharmaceuticals, Inc. | Replicor Inc. | CoCrystal Pharma Inc. |
| RVPR | |||
| NM | |||
| NK | |||
| IB | |||
| FO | |||
| MAZ | |||
| OC | |||
| KT | |||
| SJK | Alexion | Astellas | |
| JS | |||
| TR | |||
| MM | Novartis, Servier | ||
| CA | |||
| TW | Lung Bioengineering | ||
| GS | |||
| MS | |||
| HLAJ | |||
| SK | |||
| BH | Intercept, Cymabay, Mirum, Albireo | Intercept, Cymabay, Mirum, Albireo, Chemomab, Calliditas | |
| LS | Gilead | ||
| AH | Astellas, Roche, Qiagen | Astellas, Merck, Sanofi |
References
- 1.Durand CM, Bowring MG, Thomas AG, et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med. 2018;168(10):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015;373(27):2599–2607. [DOI] [PubMed] [Google Scholar]
- 3.Zeuzem S, Foster GR, Wang S, et al. Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N Engl J Med. 2018;378(4):354–369. [DOI] [PubMed] [Google Scholar]
- 4.Durand CM, Bowring MG, Brown DM, et al. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med. 2018;168(8):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolley AE, Singh SK, Goldberg HJ, et al. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med. 2019;380(17):1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg DS, Abt PL, Blumberg EA, et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;376(24):2394–2395. [DOI] [PubMed] [Google Scholar]
- 7.Cypel M, Feld JJ, Galasso M, et al. Prevention of viral transmission during lung transplantation with hepatitis C-viraemic donors: an open-label, single-centre, pilot trial. Lancet Respir Med. 2019. [DOI] [PubMed] [Google Scholar]
- 8.Levitsky J, Formica RN, Bloom RD, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 9.Kapila N, Al-Khalloufi K, Bejarano PA, Vanatta JM, Zervos XB. Fibrosing cholestatic hepatitis after kidney transplantation from HCV-viremic donors to HCV-negative recipients: A unique complication in the DAA era. Am J Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Kapila N, Menon KVN, Al-Khalloufi K, et al. HCV NAT positive solid organ allografts transplanted into HCV negative recipients: A real-world experience. Hepatology. 2019. [DOI] [PubMed] [Google Scholar]
- 11.Galasso M, Feld JJ, Watanabe Y, et al. Inactivating hepatitis C virus in donor lungs using light therapies during normothermic ex vivo lung perfusion. Nat Commun. 2019;10(1):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pileri P, Uematsu Y, Campagnoli S, et al. Binding of hepatitis C virus to CD81. Science. 1998;282(5390):938–941. [DOI] [PubMed] [Google Scholar]
- 13.Lavie M, Sarrazin S, Montserret R, et al. Identification of conserved residues in hepatitis C virus envelope glycoprotein E2 that modulate virus dependence on CD81 and SRB1 entry factors. J Virol. 2014;88(18):10584–10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans MJ, von Hahn T, Tscherne DM, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446(7137):801–805. [DOI] [PubMed] [Google Scholar]
- 15.Ploss A, Evans MJ, Gaysinskaya VA, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457(7231):882–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sainz B, Barretto N, Martin DN, et al. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18(2):281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017;17(10):1062–1068. [DOI] [PubMed] [Google Scholar]
- 18.Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and Pibrentasvir in Patients with HCV and Severe Renal Impairment. N Engl J Med. 2017;377(15):1448–1455. [DOI] [PubMed] [Google Scholar]
- 19.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364(15):1431–1440. [DOI] [PubMed] [Google Scholar]
- 20.Kosloski MP, Zhao W, Li H, et al. Drug-Drug Interactions of Tacrolimus or Cyclosporine With Glecaprevir and Pibrentasvir in Healthy Subjects. Clin Pharmacol Drug Dev. 2019;8(6):779–789. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg DS, Levitsky J. Transplanting livers from HCV-infected donors into HCV-negative recipients: Promise but mind the pitfalls. Am J Transplant. 2019;19(5):1264–1265. [DOI] [PubMed] [Google Scholar]
- 22.Molnar MZ, Nair S, Cseprekal O, et al. Transplantation of kidneys from hepatitis C-infected donors to hepatitis C-negative recipients: Single center experience. Am J Transplant. 2019;19(11):3046–3057. [DOI] [PubMed] [Google Scholar]
- 23.Komurian-Pradel F, Perret M, Deiman B, et al. Strand specific quantitative real-time PCR to study replication of hepatitis C virus genome. J Virol Methods. 2004;116(1):103–106. [DOI] [PubMed] [Google Scholar]
- 24.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N Engl J Med. 2015;373(27):2608–2617. [DOI] [PubMed] [Google Scholar]
- 25.Kohli A, Osinusi A, Sims Z, et al. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet. 2015;385(9973):1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vera ME, Volk ML, Ncube Z, et al. Transplantation of hepatitis C virus (HCV) antibody positive, nucleic acid test negative donor kidneys to HCV negative patients frequently results in seroconversion but not HCV viremia. Am J Transplant. 2018;18(10):2451–2456. [DOI] [PubMed] [Google Scholar]
- 27.Reese PP, Abt PL, Blumberg EA, et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann Intern Med. 2018;169(5):273–281. [DOI] [PubMed] [Google Scholar]
- 28.Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. [DOI] [PubMed] [Google Scholar]
- 29.Fontaine H, Lazarus A, Pol S, et al. Bradyarrhythmias Associated with Sofosbuvir Treatment. N Engl J Med. 2015;373(19):1886–1888. [DOI] [PubMed] [Google Scholar]
- 30.Gupta G, Yakubu I, Bhati C, et al. Ultra-short duration direct acting anti-viral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis c negative kidney transplant recipients. Am J Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Bethea ED, Gaj K, Gustafson JL, et al. Pre-emptive pangenotypic direct acting antiviral therapy in donor HCV-positive to recipient HCV-negative heart transplantation: an open-label study. Lancet Gastroenterol Hepatol. 2019;4(10):771–780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.