Abstract
The nitration of azolo[1,5-a]pyrimidin-7-amines with several nitration agents (such as acetic nitric anhydride, nitronium tetrafluoroborate, and a mixture of concentrated nitric acid and sulfuric acid) has been investigated. It has been shown that, depending on the conditions, the nitration of pyrazolopyrimidin-7-amines bearing electron-withdrawing groups in the pyrazole ring leads to nitration products in the pyrimidine and/or pyrazole ring. The nitration of triazolo[1,5-a]pyrimidin-7-amines with “nitrating mixture” has been optimized, thus allowing us to obtain a series of 6-nitro[1,2,4]triazolo[1,5-a]pyrimidin-7-amines, followed by their reduction into the corresponding [1,2,4]triazolo[1,5-a]pyrimidin-6,7-diamines (yields 86–89%). The latter have been subjected to heterocyclization by a variety of electrophilic compounds (such as CS2, glyoxal, triethyl orthoformate) with the formation of five- or six-membered annulated cycles.
Introduction
Polycyclic systems containing the pyrimidine ring have long been attracting attention of chemists due to a remarkable role played by many fused pyrimidines (purines, pteridines, and other derivatives) in biology and drug design. During the last decade, the synthesis and properties of azolo[1,5-a]pyrimidines have the most actively been studied. Indeed, according to the SciFinder database, more than 1000 original papers and patents have been published over the last 5 years (2015–2019), thus indicating great interest in this class of compounds. These azolo[1,5-a]-pyrimidines have been found to exhibit antiviral, anti-inflammatory, antibacterial, antifungal, antiparasitic, antitumor, and other biological activities.1
At the same time, reports on the synthesis of fused polycyclic derivatives, bearing the core fragment of azolo[1,5-a]pyrimidines, remain scarcely presented in the literature, and a limited number of synthetic methods for obtaining of azolo[b]purines have so far been described.2
Another important class of fused pyrimidines of natural origin is presented by pteridines, which are known to act as immune system activators, pigments, and enzyme cofactors, while their synthetic derivatives proved to exhibit various types of biological activity.3
A few research studies dedicated to azolo-annulated pteridines have been carried out during recent years. They include the synthesis of azolo-annulated [1,5-c,f], [3,4-b], [4,3-a,c,e,f,g] pteridines, as well as the series of azolo[1,5-a]tetrahydropyrazines.4 There are experimental data on anti-inflammatory and antibacterial activities of pyrazolo[4.3-g]pteridines;4b 7-amino-azolo[4,3-f]- and [1,5-f]pteridinones have been established to be antitumour agents,4c while 4,5-dihydro[1,2,4]triazolo[4,3-f]pteridines are known to act as inhibitors of the BRD4 chromatin-binding protein,5 which is a biological target for many diseases, including cancer,6 inflammation,7 diabetes,8 and viral infections.9 These data demonstrate an extremely wide range of plausible therapeutic applications of these compounds.4d However, the data on the synthesis and biological activity of the azolo[1,5-a]pteridines remain to be scarcely available.
Therefore, the development of new methods for the synthesis of annulated derivatives of azolo[1,5-a]pyrimidines appears to be a relevant task for organic and medicinal chemistry. We have developed earlier a synthetic approach to azolo[1,5-a]pyrimidine-7-amines, which were used as starting materials in the present work.10
Results and Discussion
It is well known that the direct incorporation of the nitro group into the pyrimidine ring usually requires the presence of several electron-donating groups. Indeed, it has been shown by Makisumi that the nitration of [1,2,4]triazolo[1,5-a]pyrimidin-7-amine (1a) with fuming nitric acid in the presence of sulfuric and acetic acids at 10–15 °C does not lead to the desired product.11 However, Lynch et al. have reported the successful nitration of pyrazolo[1,5-a]pyrimidine with acetyl nitrate (a mixture of fuming nitric acid and acetic anhydride) at 5–10 °C, leading to 6-nitropyrazolo[1,5-a]pyrimidine,12 which indicates that the appropriate nitration conditions can also be selected for 1,2,4-triazolo[1,5-a]pyrimidine-7-amine (1a).
Since attempts to use acetyl nitrate and nitronium tetrafluoroborate as nitration agents have failed, we have selected a mixture of HNO3 and H2SO4 in the ratio 1:6, where an excess of sulfuric acid reduces the oxidizing properties of nitric acid while also contributing to its complete conversion to a nitronium cation (Scheme 1).
Scheme 1. Nitration of [1,2,4]Triazolo[1,5-a]pyrimidin-7-amine (1a).
We have found that an increase in the reaction time up to 24 h at room temperature does not afford the target nitration product (only trace quantities of the latter are formed). However, the optimization of the reaction temperature has allowed us to obtain the desired product 2a in high yield and reduce significantly the reaction time without provoking secondary processes at a wide temperature interval. Conditions 6 (Table 1) appear to be optimal and can be used for the nitration of triazolopyrimidines 1b–d (Scheme 2).
Table 1. Nitration Reaction Parameters.
| entry | nitration agent | T (°C) | time (h) | yield (%)a |
|---|---|---|---|---|
| 1 | HNO3/Ac2O (1:2) | rt | 3 | nrb |
| 2 | NO2BF4 in acetonitrile | rt | 24 | nrb |
| 3 | NO2BF4 in acetonitrile | 82 | 12 | nrb |
| 4 | 3 equiv HNO3/H2SO4 (1:6)d | rt | 24 | tracec |
| 5 | 3 equiv HNO3/H2SO4 (1:6)d | 60 | 15 | 89 |
| 6 | 3 equiv HNO3/H2SO4 (1:6)d | 80 | 4 | 92 |
| 7 | 3 equiv HNO3/H2SO4 (1:6)d | 100 | 3 | 87 |
Isolated yield.
nr = no reaction.
Detected by TLC.
Molar ratio.
Scheme 2. Nitration of [1,2,4]Triazolo[1,5-a]pyrimidin-7-amines (1a–d).
In all cases, the nitration process proceeds smoothly, affording the target products in good yields. No oxidation of the amino group has been observed, thus allowing to avoid the application of any protective groups. In the case of azolopyrimidine 1b, the methylthio group is completely oxidized into the corresponding sulfone 2b under the described conditions, while a smaller excess of the nitrating mixture causes the formation of a mixture of methyl sulfoxide and methyl sulfonyl derivatives (for details, see Supporting Information S50 and S51).
Under the same reaction conditions, the nitration of pyrazolopyrimidine-7-amines 3a,b (Scheme 3) bearing electron-withdrawing groups in the pyrazole ring (Table 1, entry 6) leads to nitration products on the pyrimidine and pyrazole rings (Scheme 3a), even at room temperature for 3 h.
Scheme 3. Nitration of Pyrazolo[1,5-a]pyrimidine-7-amines.
We also found that treatment of 3a with an excess of nitration mixture (2 equiv) using potassium nitrate leads exclusively to the nitration product according to the pyrazole ring 5 (compound 5 have been previously described13); the use of acetyl nitrate as a nitrating agent made it possible to selectively obtain the nitration product in the pyrimidine ring 6 (Scheme 3b).
In the literature, cases described of such pyrazole behavior contained an ethoxycarbonyl fragment in the third position of the ring.14 Presumably, the reaction mechanism consists in the hydrolysis of the corresponding carboxyl derivative, its decarboxylation, and subsequent nitration; this is indirectly confirmed by the discovery of trace amounts of amide and decarboxylated derivatives in the mass spectra of compounds 4 and 5 (for details, see Supporting Information S41–S48).
Subsequent reduction of the obtained nitro derivatives 2a–d in an H2 atmosphere at a pressure of 5 bar with a Pd/C (5%) catalyst leads to the formation of the corresponding heteroaromatic diamines 7a–d with high yields (86–89%) (Scheme 4).
Scheme 4. Reduction of 6-Nitro-[1,2,4]triazolo[1,5-a]pyrimidin-7-amines (2a–d) in the Pd/C–H2 System.
Thus, we have proposed methods for obtaining a number of diamines 7 not previously described in the literature, which can act as “building blocks” in the synthesis of condensed systems containing a triazolo[1,5-a]pyrimidine fragment.
Subsequently, we examined the synthetic potential of the obtained heteroaromatic 1,2-diamines.
It was found that the boiling of diamines 7 in formic acid (97%), which ceases at the stage of formylation of the amino group in position 6, does not lead to the formation of an annulated imidazole ring. However, according to the literature, this approach has been successfully implemented for related structures containing an alkylamino group at position 7 of a heterocyclic system.2c Another method presented in the literature is the interaction of pyrazolo[1,5-a]pyrimidin-6,7-diamines with orthoesters under microwave activation and in the absence of the solvent.2a As an alternative to this approach, we propose a synthesis method (Scheme 5) that does not require microwave activation, consisting of the cyclocondensation of [1,2,4]triazolo[1,5-a]pyrimidine-6,7-diamines (7) with triethyl orthoformate in acetic acid. Target products 8a–d were isolated with high yields (71–91%).
Scheme 5. Synthesis of Triazolo[5,1-b]purines.
In this case, the optimal conditions for obtaining the structures 8 consist in cyclization with triethyl orthoformate (300 mol %) in glacial acetic acid (in the case of 8b a mixture of acetic acid with DMF), with the use of a smaller amount of orthoester, leading to the formation of acylation products.
The possibility of one-pot cyclization of diamines 7 with carbon disulfide followed by the selective alkylation of the formed thiones to the corresponding derivatives 9 (Scheme 6) was also shown.
Scheme 6. One-Pot Synthesis of Compounds 9a–d.
Only one example of the synthesis of related structures can be found in the literature, in which the authors claim that alkylation proceeds exclusively at the nitrogen atom in the 3 position; however, this contradicts the data obtained by us.2b Using the 1H–13C HSQC and 1H–13C HMBC correlation methods with the example of compound 9c, it is shown that the alkylation proceeds at the sulfur atom (Figure 1; for details, see Supporting Information S33–S38).
Figure 1.
Key interactions in 1H–13C HSQC and 1H–13C HMBC spectra of compound 9c (δ, ppm).
In the spectrum of 1H–13C HSQC, the position of signals of the carbon atoms of the isobutyl substituent was uniquely determined: methyl (21.4 ppm), methine (28.0 ppm), and methylene (39.7 ppm), whose protons have characteristic signals in the 1H spectra. The signal of the C-4 atom (141.1 ppm), which interacts with the singlet of the pyrimidine proton H-4 (9.14 ppm), was also established.
The position of the signals of atoms C-5a (153.2 ppm), C-1a (144.6 ppm), and C-3a (122.1 ppm) was established using the spectrum of 1H–13C HMBC according to the presence of interaction with the H-4 proton signal, which can be identified due to the special features of the environment. Under the condition of alkylation on one of the nitrogen atoms of the imidazole ring in the HMBC spectrum, the interaction of the methylene proton signal of the isobutyl substituent with the signal of the C-1a (144.6 ppm) or C-3a (122.1 ppm) atom would be observed; however, only the interaction with the signal of the C-2 atom (156.9 ppm) is additionally detected for these protons. From the foregoing, we can form an unambiguous conclusion about the presence of a structure having an alkyl substituent at the sulfur atom.
Using the example of diamine 7b, the possibility of synthesizing 2H-[1,2,4]triazolo[1,5-a][1,2,3]triazolo[4,5-e]pyrimidinine is shown as a result of diazotization, followed by subsequent intramolecular azo coupling (Scheme 7). The alternative approach proposed by us for the synthesis of this heterocyclic system is especially relevant due to being previously presented in the literature in a single example.15
Scheme 7. Example of the Synthesis of Azolo Azapurine 10.
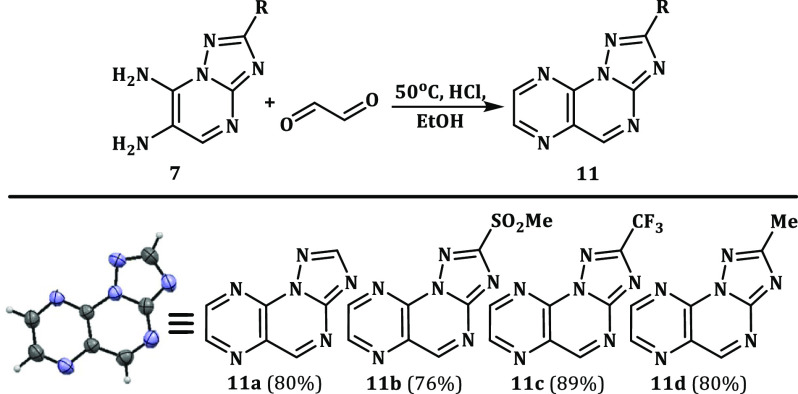
As noted earlier, no data on the synthesis and properties of azolo[1,5-a]pteridines are available in the literature. In this connection, we developed a method for the synthesis of these systems, involving cyclocondensation of diamines 7 with glyoxal (Scheme 8).
Scheme 8. Preparation of Triazolo[1,5-a]pteridines 11a–d.
In all cases, heterocyclization proceeds smoothly and with high yields (76–89%). In the absence of acid catalysis, the reaction is complicated by the formation of a poorly soluble precipitate, the main component of which is comprised of the dimeric derivative of the starting diamine (for details, see Supporting Information S52 and S53).
Conclusions
Thus, we have developed a method for the synthesis of 6,7-diamino-1,2,4-triazolo[1,5-a]pyrimidines, which are convenient matrixes for the construction of various kinds of polynuclear condensed systems of interest as materials for medicine and technology.
Experimental Section
One-dimensional 1H, 13C, and 19F NMR spectra as well as two-dimensional 1H–13C HSQC and 1H–13C HMBC experiments were acquired on a Bruker DRX-400 instrument (400, 101, and 376 MHz, respectively), a Bruker DRX-500 instrument (500, 126, and 470 MHz, respectively) or a Bruker Avance NEO 600 instrument (600 and 151 MHz, respectively), equipped with a Prodigy broadband gradient cryoprobe using DMSO-d6 as a solvent and TMS as an internal standard. High-resolution mass spectrometry (HRMS) was performed using a Bruker Daltonik MaXis Impact HD quadrupole time-of-flight mass spectrometer (positive (POS)/negative (NEG) electrospray ionization or atmospheric-pressure chemical ionization from the MeCN or MeCN/DMSO solution, at a flow rate of 180 μL·h–1 with parameters optimized for small molecule detection based on a preinstalled method for infusion analysis). Elemental analysis was performed on a PerkinElmer PE 2400 elemental analyzer. Melting points were determined in open capillaries using a Stuart SMP3 apparatus. All solvents and commercially available reactants/reagents were used as received. Noncommercial starting materials were prepared as described below or according to the literature procedures.
General Procedure for the Synthesis of Compounds 2a–d
The corresponding triazolopyrimidine-7-amine 1a–d (10 mmol) was added portionwise to a mixture of 1.25 mL (30 mmol) of fuming HNO3 and 9.60 mL (180 mmol) of concentrated H2SO4 with stirring and cooling (ice bath). The reaction mixture was gradually heated to 80 °C under reflux and kept for 4 h, then cooled to room temperature, poured into ice, and carefully neutralized with aqueous ammonia to pH ∼ 7. The precipitate was filtered off, thoroughly washed with H2O, and air-dried.
6-Nitro-[1,2,4]triazolo[1,5-a]pyrimidine-7-amine (2a)
Obtained from 1a (1.35 g, 10 mmol, 1 equiv) in a mixture of fuming HNO3 (1.25 mL, 30 mmol, 3 equiv) and H2SO4 (9.60 mL, 180 mmol, 18 equiv). Pale yellow solid; 1.65 g, 92% yield; mp 263–265 °C. 1H NMR (DMSO-d6, 400 MHz): δ 8.65 (s, 1H), 9.22 (s, 1H), δ 9.51 (br s, 2H). 13C{1H} NMR (DMSO-d6, 101 MHz): δ 118.4, 145.9, 152.1, 155.7, 156.2. Anal. calcd for C5H4N6O2: C, 33.34; H, 2.24; N, 46.66. Found: C, 33.24; H, 2.06; N, 46.53.
2-(Methylsulfonyl)-6-nitro-[1,2,4]triazolo[1,5-a]pyrimidine-7-amine (2b)
Obtained from 1b (1.81 g, 10 mmol, 1 equiv) in a mixture of fuming HNO3 (1.25 mL, 30 mmol, 3 equiv) and H2SO4 (9.60 mL, 180 mmol, 18 equiv). White solid; 2.16 g, 84% yield; mp 265–267 °C. 1H NMR (DMSO-d6, 400 MHz): δ 3.51 (s, 3H), 9.31 (s, 1H), 9.44 (br s, 2H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 41.8, 119.7, 146.6, 153.9, 156.2, 164.8. Anal. calcd for C6H6N6O4S: C, 27.91; H, 2.34; N, 32.55. Found: C, 28.12; H, 2.41; N, 32.58.
6-Nitro-2-(trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyrimidine-7-amine (2c) + H2O
Obtained from 1c (2.03 g, 10 mmol, 1 equiv) in a mixture of fuming HNO3 (1.25 mL, 30 mmol, 3 equiv) and H2SO4 (9.60 mL, 180 mmol, 18 equiv). White solid; 2.32 g, 87% yield; mp 233–235 °C. 1H NMR (DMSO-d6, 400 MHz): δ 9.34 (s, 1H), 9.99 (br s, 2H). 13C{1H} NMR (DMSO-d6, 101 MHz): δ 118.9 (q, J = 271.0 Hz), 119.6, 146.4, 153.7, 155.6 (q, J = 39.2 Hz), 156.4. 19F NMR (DMSO-d6, 470 MHz): δ 97.69 (s). Anal. calcd for C6H3F3N6O2 + H2O: C, 27.08; H, 1.89; N, 31.58. Found: C, 27.11; H, 2.07; N, 31.36. HRMS (ESI-POS) m/z: [M + H]+ calcd for C6H4F3N6O2+ 249.0342; found 249.0338.
2-Methyl-6-nitro-[1,2,4]triazolo[1,5-a]pyrimidine-7-amine (2d) + H2O
Obtained from 1d (1.49 g, 10 mmol, 1 equiv) in a mixture of fuming HNO3 (1.25 mL, 30 mmol, 3 equiv) and H2SO4 (9.60 mL, 180 mmol, 18 equiv). Yellow solid; 1.76 g, 83% yield; mp 254–256 °C. 1H NMR (DMSO-d6, 400 MHz): δ 2.49 (s, 3H), 9.15 (s, 1H), 9.52 (br s, 2H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 14.7, 118.3, 145.3, 151.8, 156.0, 165.7. Anal. calcd for C6H6N6O2 + H2O: C, 33.97; H, 3.80; N, 39.61. Found: C, 33.87; H, 3.99; N, 39.78. HRMS (ESI-POS) m/z: [M + H]+ calcd for C6H7N6O2+ 195.0625; found 195.0625.
7-Aminopyrazolo[1,5-a]pyrimidine-3-carbonitrile (3b)
A solution of 108 mg (1 mmol) of 3-amino-4-cyanopyrazole in dioxane 5 mL was stirred at 50 °C and treated by adding 3,3-diethoxypropionitrile (150 μL, 1 mmol), then 36% HCl solution (86 μL, 1 mmol). The reaction mixture was refluxed for 2 h, the suspension was cooled to room temperature, and the precipitate was filtered off and air-dried. The dry residue was dissolved in H2O, the solution was stirred and adjusted with aqueous ammonia to pH ∼ 8, and the precipitate was filtered off and dried in a vacuum desiccator over P2O5. Beige solid; 132 mg, 83% yield, mp >300 °C. 1H NMR (DMSO-d6, 500 MHz): δ 6.35 (d, J = 5.5 Hz, 1H), 8.24 (d, J = 5.5 Hz, 1H), 8.33 (br s, 2H), 8.64 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 78.4, 91.4, 114.4, 146.6, 149.1, 151.2, 152.2. Anal. calcd for C7H5N5: C, 52.83; H, 3.17; N, 44.01. Found: C, 52.78; H, 2.99; N, 44.25.
3,6-Dinitropyrazolo[1,5-a]pyrimidine-7-amine (4)
The corresponding triazolopyrimidine-7-amine 3a,b (1 mmol) was added portionwise to a mixture of 125 μL (3 mmol) of fuming HNO3 and 960 μL (18 mmol) of concentrated H2SO4 with stirring and cooling (ice bath). The reaction mixture was kept at room temperature for 3 h, then poured into crushed ice, and carefully neutralized with aqueous ammonia to pH ∼ 7; the precipitate was filtered off, washed with H2O and EtOH, and dried in a vacuum desiccator over P2O5.
Obtained from 3a (206 mg, 1 mmol, 1 equiv) in a mixture of fuming HNO3 (125 μL, 3 mmol, 3 equiv) and H2SO4 (960 μL, 18 mmol, 18 equiv). Bright yellow solid; 202 mg, 90% yield; mp 282–284 °C. Obtained from 3b (159 mg, 1 mmol, 1 equiv) in a mixture of fuming HNO3 (125 μL, 3 mmol, 3 equiv) and H2SO4 (960 μL, 18 mmol, 18 equiv). Bright yellow solid; 211 mg, 94% yield; mp 283–285 °C. 1H NMR (DMSO-d6, 600 MHz): δ 9.07 (s, 1H), 9.28 (s, 1H), 9.73 (br s, 1H), 10.07 (br s, 1H). 13C{1H} NMR (DMSO-d6, 151 MHz): δ 120.1, 124.2, 143.8, 144.3, 146.1, 152.4. Anal. calcd for C6H4N6O4: C, 32.15; H, 1.80; N, 37.50. Found: C, 32.38; H, 1.77; N, 37.29.
3-Nitropyrazolo[1,5-a]pyrimidine-7-amine (5)10
To a solution of 206 mg (1 mmol) of pyrazolopyrimidin-7-amine 3a in 640 μL (12 mmol) of concentrated H2SO4 with stirring and cooling (ice bath) was added 202 mg (2 mmol) of KNO3. The reaction mixture was kept at room temperature for 3 h, poured into crushed ice, and carefully neutralized with aqueous ammonia to pH ∼ 7; the precipitate was filtered off, washed thoroughly with H2O and EtOH, and dried under reduced pressure at 110 °C over P2O5. Bright yellow solid; 111 mg, 62% yield; mp >300 °C. 1H NMR (DMSO-d6, 500 MHz): δ 6.49 (d, J = 5.6 Hz, 1H), 8.36 (d, J = 5.6 Hz, 1H), 8.54 (br s, 2H), 8.92 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 93.9, 121.0, 142.2, 143.8, 149.1, 153.8. Anal. calcd for C6H5N5O2: C, 40.23; H, 2.81; N, 39.10. Found: C, 40.49; H, 2.85; N, 38.74.
Ethyl 7-Amino-6-nitropyrazolo[1,5-a]pyrimidine-3-carboxylate (6)
Fuming nitric acid (1 mL) was carefully added dropwise to acetic anhydride (2 mL) with cooling (ice bath) and kept at room temperature for 1 h, then cooled again. To the mixture 206 mg of pyrazolopyrimidin-7-amine 3a was added and allowed to stand for 3 h. The reaction mixture was poured into crushed ice and allowed to stand overnight; the precipitate was filtered, washed with H2O, and dried in a vacuum desiccator over P2O5. Pale yellow solid; 75 mg, 30% yield; mp >300 °C. 1H NMR (DMSO-d6, 400 MHz): δ 1.31 (t, J = 7.1 Hz, 3H), 4.30 (q, J = 7.1 Hz, 2H), 8.66 (s, 1H), 9.15 (s, 1H), 9.53 (br s, 1H), 9.81 (br s, 1H). 13C{1H} NMR (DMSO-d6, 101 MHz): δ 14.3, 59.8, 104.2, 118.0, 145.7, 148.0, 148.2, 149.4, 161.3. Anal. calcd for C9H9N5O4: C, 43.03; H, 3.61; N, 27.88. Found: C, 43.08; H, 3.71; N, 27.82.
General Procedure for the Synthesis of Compounds 7a–d
A mixture of corresponding 6-nitrotriazolopyrimidine-7-amine 2a–d (1 mmol) and 10% (by weight) Pd/C (5 mol %) in DMF (for 2a,b) or EtOH (for 2c,d) was hydrogenated in an autoclave at 50 °C and 5–7 bar pressure of hydrogen for 5–7 h. The resulting solution/suspension was heated until the precipitate was completely dissolved and filtered hot from Pd/C; the solvent was removed by distillation; and the precipitate was washed off with CHCl3, filtered, and air-dried.
[1,2,4]Triazolo[1,5-a]pyrimidine-6,7-diamine (7a)
Obtained from 2a (1.80 g, 10 mmol, 1 equiv), Pd/C (0.18 g, 10% by weight 2a) in DMF (50 mL). Beige solid; 1.32 g, 88% yield; mp >300 °C. 1H NMR (DMSO-d6, 400 MHz): δ 4.50 (br s, 2H), 7.52 (br s, 2H), 8.08 (s, 1H), 8.30 (s, 1H). 13C{1H} NMR (DMSO-d6, 101 MHz): δ 115.7, 138.9, 141.8, 151.2, 154.0. Anal. calcd for C5H6N6: C, 40.00; H, 4.03; N, 55.97. Found: C, 39.93; H, 3.81; N, 55.82.
2-(Methylsulfonyl)-[1,2,4]triazolo[1,5-a]pyrimidine-6,7-diamine (7b)
Obtained from 2b (2.58 g, 10 mmol, 1 equiv), Pd/C (0.26 g, 10% by weight 2b) in DMF (50 mL). Beige solid; 1.96 g, 86% yield; mp 265–267 °C. 1H NMR (DMSO-d6, 500 MHz): δ 3.42 (s, 3H), 4.81 (br s, 2H), 7.91 (br s, 2H), 8.17 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 41.9, 118.2, 139.1, 142.3, 150.2, 163.2. Anal. calcd for C6H8N6O2S: C, 31.58; H, 3.53; N, 36.82. Found: C, 31.77; H, 3.68; N, 36.67.
2-(Trifluoromethyl)-[1,2,4]triazolo[1,5-a]pyrimidine-6,7-diamine (7c)
Obtained from 2c (2.48 g, 10 mmol, 1 equiv), Pd/C (0.25 g, 10% by weight 2c) in DMF (50 mL). Beige solid; 1.92 g, 88% yield; mp 226–228 °C. 1H NMR (DMSO-d6, 500 MHz): δ 4.77 (br s, 2H), 7.84 (br s, 2H), 8.16 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 117.9, 119.7 (q, J = 270.6 Hz), 139.0, 142.2, 150.6, 153.8 (q, J = 37.9 Hz). 19F NMR (DMSO-d6, 376 MHz): δ 98.40 (s). Anal. calcd for C6H5F3N6: C, 33.04; H, 2.31; N, 38.53. Found: C, 33.12; H, 2.34; N, 38.32.
2-Methyl-[1,2,4]triazolo[1,5-a]pyrimidine-6,7-diamine (7d)
Obtained from 2d (1.94 g, 10 mmol, 1 equiv), Pd/C (0.19 g, 10% by weight 2d) in DMF (50 mL). Beige solid; 1.46 g, 89% yield; mp 261–263 °C. 1H NMR (DMSO-d6, 400 MHz): δ 2.39 (s, 3H), 4.42 (br s, 2H), 7.43 (br s, 2H), 7.96 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 14.8, 115.4, 138.6, 141.1, 151.8, 163.0. Anal. calcd for C6H8N6: C, 43.90; H, 4.91; N, 51.19. Found: C, 43.93; H, 4.84; N, 51.44.
General Procedure for the Synthesis of Compounds 8a–d
To a solution (in case of 8d suspension) of the corresponding triazolopyrimidin-6,7-diamine 7a–d (1 mmol) in 4 mL of glacial AcOH (for 8b in 6 mL of a mixture of AcOH/DMF 1:2), 0.50 mL (3 mmol) of triethyl orthoformate was added. The reaction mixture was kept at 80 °C (for 8b at 90 °C) for 2–3 h, the suspension/solution was cooled to room temperature, and the target product was isolated by the method indicated for each particular compound.
1H-[1,2,4]Triazolo[5,1-b]purine (8a)
The precipitate was filtered, washed with CHCl3, and dried under reduced pressure at 110 °C over P2O5. Obtained from 7a (150 mg, 1 mmol, 1 equiv) in a mixture of glacial AcOH (4 mL) and (EtO)3CH (0.50 mL, 3 mmol, 3 equiv). Beige solid; 130 mg, 81% yield; mp >300 °C. 1H NMR (DMSO-d6, 600 MHz): δ 8.63 (s, 1H), 8.64 (s, 1H), 9.20 (s, 1H), 13.92 (br s, 1H). 13C{1H} NMR (DMSO-d6, 151 MHz): δ 119.1, 142.4, 144.5, 144.8, 154.0, 154.6. Anal. calcd for C6H4N6: C, 45.00; H, 2.52; N, 52.48. Found: C, 45.24; H, 2.60; N, 52.33.
7-(Methylsulfonyl)-1H-[1,2,4]triazolo[5,1-b]purine (8b)
The suspension was diluted with 3 mL of H2O, and the precipitate was filtered and dried under reduced pressure at 110 °C over P2O5. Obtained from 7b (228 mg, 1 mmol, 1 equiv) in a mixture of DMF (4 mL), glacial AcOH (2 mL), and (EtO)3CH (0.50 mL, 3 mmol, 3 equiv). Beige solid; 169 mg, 71% yield; mp >300 °C. 1H NMR (DMSO-d6, 500 MHz): δ 3.53 (s, 3H), 8.76 (s, 1H), 9.39 (s, 1H), 14.18 (br s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 40.1, 120.3, 144.1, 144.5, 145.3, 153.2, 163.3. Anal. calcd for C7H6N6O2S: C, 35.29; H, 2.54; N, 35.28. Found: C, 35.53; H, 2.31; N, 35.29.
7-(Trifluoromethyl)-1H-[1,2,4]triazolo[5,1-b]purine (8c)
The solvent was removed under reduced pressure, and the precipitate was washed off with CHCl3, filtered, and dried under reduced pressure at 110 °C over P2O5. Obtained from 7c (218 mg, 1 mmol, 1 equiv) in a mixture of glacial AcOH (4 mL) and (EtO)3CH (0.50 mL, 3 mmol, 3 equiv). Pale yellow-beige solid; 189 mg, 83% yield; mp 297–299 °C. 1H NMR (DMSO-d6, 400 MHz): δ 8.75 (s, 1H), 9.38 (s, 1H), 14.17 (br s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 119.6 (q, J = 270.4 Hz), 120.1, 144.0, 144.2, 145.2, 153.5, 153.8 (q, J = 38.6 Hz). 19F NMR (DMSO-d6, 376 MHz): δ 98.54 (s). Anal. calcd for C7H3F3N6: C, 36.85; H, 1.33; N, 36.84. Found: C, 36.88; H, 1.44; N, 36.72.
7-Methyl-1H-[1,2,4]triazolo[5,1-b]purine (8d)
The precipitate was filtered, washed with CHCl3, and dried under reduced pressure at 110 °C over P2O5. Obtained from 7d (164 mg, 1 mmol, 1 equiv) in a mixture of glacial AcOH (4 mL) and (EtO)3CH (0.50 mL, 3 mmol, 3 equiv). Sandy solid; 159 mg, 91% yield; mp >300 °C. 1H NMR (DMSO-d6, 500 MHz): δ 2.55 (s, 3H), 8.61 (s, 1H), 9.12 (s, 1H), 13.84 (br s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 14.7, 118.4, 141.1, 143.7, 144.3, 154.0, 163.2. Anal. calcd for C7H6N6: C, 48.27; H, 3.47; N, 48.25. Found: C, 48.16; H, 3.38; N, 48.42.
General Procedure for the Synthesis of Compounds 9a–d
To a solution/suspension of the corresponding diamine 7a–c (1 mmol) in 2 mL of DMF, 73 μL (1.2 mmol) of CS2 was added and kept close for 5 h and then excess CS2 and dissolved H2S were distilled off using a rotary evaporator. To the reaction mixture, 109 μL (1 mmol) of isobutyl bromide and 139 μL (1 mmol) of triethylamine were added, the mixture was heated to 80 °C and kept for 2 h, and the solvent was removed under reduced pressure. The dry residue was washed with H2O, filtered, and air-dried. Then, the dry residue was boiled in benzene for 1 h, the suspension was cooled, and the precipitate was filtered and air-dried.
2-[(iso-Butyl)sulfanyl]-1H-[1,2,4]triazolo[5,1-b]purine (9a)
Obtained from 7a (150 mg, 1 mmol, 1 equiv), CS2 (73 μL, 1.2 mmol, 1.2 equiv), isobutyl bromide (109 μL, 1 mmol, 1 equiv), and triethylamine (139 μL, 1 mmol, 1 equiv) in DMF (2 mL). White solid; 182 mg, 73% yield; mp >300 °C. 1H NMR (DMSO-d6, 500 MHz): δ 1.05 (d, J = 6.7 Hz, 6H), 2.04 (m, 1H), 3.33 (d, J = 6.8 Hz, 2H) 8.59 (s, 1H), 8.99 (s, 1H), 14.02 (br s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 21.4, 28.1, 39.7, 121.0, 139.2, 144.5, 153.2, 154.3, 155.6. Anal. calcd for C10H12N6S: C, 48.37; H, 4.87; N, 33.85. Found: C, 48.46; H, 4.90; N, 33.69.
2-[(iso-Butyl)sulfanyl]-7-(methylsulfonyl)-1H-[1,2,4]triazolo[5,1-b]purine (9b)
Obtained from 7b (228 mg, 1 mmol, 1 equiv), CS2 (73 μL, 1.2 mmol, 1.2 equiv), isobutyl bromide (109 μL, 1 mmol, 1 equiv), and triethylamine (139 μL, 1 mmol, 1 equiv) in DMF (2 mL). Beige solid; 221 mg, 68% yield; mp 250–251 °C. 1H NMR (DMSO-d6, 500 MHz): δ 1.05 (d, J = 6.7 Hz, 6H), 2.06 (m, 1H), 3.36 (d, J = 6.8 Hz, 2H) 3.51 (s, 3H), 9.15 (s, 1H), 14.28 (br s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 21.4, 28.1, 39.8, 42.1, 122.3, 141.4, 144.7, 152.9, 157.0, 163.5. Anal. calcd for C11H14N6O2S2: C, 40.48; H, 4.32; N, 25.75. Found: C, 40.66; H, 4.38; N, 25.58.
2-[(iso-Butyl)sulfanyl]-7-(trifluoromethyl)-1H-[1,2,4]triazolo[5,1-b]purine (9c)
Obtained from 7c (218 mg, 1 mmol, 1 equiv), CS2 (73 μL, 1.2 mmol, 1.2 equiv), isobutyl bromide (109 μL, 1 mmol, 1 equiv), and triethylamine (139 μL, 1 mmol, 1 equiv) in DMF (2 mL). Beige solid; 281 mg, 89% yield; mp 285–287 °C. 1H NMR (DMSO-d6 with 0.05% v/v TMS, 400 MHz): δH δ = 1.06 (6H, d, J = 6.6 Hz, SCH2CH(CH3)2); δ = 2.06 (1H, m, SCH2CH(CH3)2); δ = 3.35 (2H, d, J = 6.8 Hz, SCH2CH(CH3)2); δ = 9.14 (1H, s, H-4); δ = 14.26 (1H, br s, NH). 13C NMR (DMSO-d6, 125 MHz): δC 21.4 (s, SCH2CH(CH3)2), 28.0 (s, SCH2CH(CH3)2), 39.7 (s, SCH2CH(CH3)2), 119.6 (q, J = 270.4 Hz, C7), 122.1 (s, C3a), 141.1 (s, C4), 144.6 (s, C1a), 153.2 (s, C5a), 154.0 (q, J = 38.5 Hz, CF3), 156.9 (s, C2). 19F NMR (DMSO-d6, 470 MHz): δ 98.44 (s). Anal. calcd for C11H11F3N6S: C, 41.77; H, 3.51; N, 26.57. Found: C, 41.90; H, 3.59; N, 26.36.
2-[(iso-Butyl)sulfanyl]-7-methyl-1H-[1,2,4]triazolo[5,1-b]purine (9d)
Obtained from 7d (164 mg, 1 mmol, 1 equiv), CS2 (73 μL, 1.2 mmol, 1.2 equiv), isobutyl bromide (109 μL, 1 mmol, 1 equiv), and triethylamine (139 μL, 1 mmol, 1 equiv) in DMF (2 mL). Beige solid; 220 mg, 84% yield; mp >300 °C. 1H NMR (DMSO-d6, 400 MHz): δ 1.04 (d, J = 6.7 Hz, 6H), 2.04 (m, 1H), 2.53 (s, 3 H), 3.32 (d, J = 6.8 Hz, 2H), 8.89 (s, 1H), 13.93 (br s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 14.7, 21.4, 28.1, 39.7, 120.5, 138.4, 144.1, 153.6, 155.5, 163.3. Anal. calcd for C11H14N6S: C, 50.36; H, 5.38; N, 32.04. Found: C, 50.40; H, 5.39; N, 31.99.
7-(Methylsulfonyl)-2H-[1,2,4]triazolo[1,5-a][1,2,3]triazolo[4,5-e]pyrimidine (10)
To a suspension of 0.228 mg (1 mmol) of diamine 7b in 4 mL of ice-cold AcOH, 104 mg of NaNO2 was added portionwise. The mixture was kept for 1 h, and then three drops of 2 M HCl were added. The precipitate was filtered and dried in a vacuum desiccator over KOH. Yellow sandy solid; 139 mg, 58%; mp 248–250 °C. 1H NMR (DMSO-d6, 500 MHz): δ 3.55 (s, 3H), 9.85 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 42.1, 130.2, 143.9, 151.3, 154.3, 163.6. Anal. calcd for C6H5N7O2S: C, 30.13; H, 2.11; N, 40.99. Found: C, 30.15; H, 2.29; N, 40.82
General Procedure for the Synthesis of Compounds 11a–d
A mixture of the corresponding diamine 7a–d (1 mmol), 86 μL (1 mmol) of 36% HCl and 115 μL (1 mmol) of 40% solution of glyoxal in water were kept at 50 °C. The reaction progress was monitored by TLC (in the CH3Cl/CH3OH (9:1) system) for the disappearance of a spot of the starting diamine (Rf ∼ 0.1) and formation of a spot of the product (Rf ∼ 0.85). The target product was isolated by the method indicated for each particular compound.
[1,2,4]Triazolo[1,5-a]pteridine (11a)
The suspension was cooled in an ice bath, the precipitate was filtered. Obtained from 7a (150 mg, 1 mmol, 1 equiv), 40 wt % solution of glyoxal in water (115 μL, 1 mmol, 1 equiv), 36% HCl (86 μL, 1 mmol, 1 equiv), in EtOH (5 mL). Light brown solid; 138 mg, 80% yield; mp >216–218 °C. 1H NMR (DMSO-d6, 500 MHz): δ 8.83 (s, 1H), 9.22 (d, J = 2.2 Hz, 1H), 9.24 (d, J = 2.2 Hz, 1H), 9.66 (s, 1H). 13C{1H} NMR (DMSO-d6, 101 MHz): δ 130.1, 142.2, 145.6, 149.6, 154.8, 155.3, 159.5. Anal. calcd for C7H4N6: C, 48.84; H, 2.34; N, 48.82. Found: C, 48.74; H, 2.51; N, 48.63.
2-(Methylsulfonyl)-[1,2,4]triazolo[1,5-a]pteridine (11b)
The suspension was cooled in an ice bath, and the precipitate was filtered. Obtained from 7b (228 mg, 1 mmol, 1 equiv), 40 wt % solution of glyoxal in water (115 μL, 1 mmol, 1 equiv) and 36% HCl (86 μL, 1 mmol, 1 equiv) in EtOH (5 mL). Light brown solid; 190 mg, 76% yield; mp >229–231 °C. 1H NMR (DMSO-d6, 500 MHz): δ 3.58 (s, 3H), 9.32 (s, 2H), 9.85 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 42.0, 131.8, 142.5, 146.8, 150.2, 155.0, 162.1, 164.2. Anal. calcd for C8H6N6O2S: C, 38.40; H, 2.42; N, 33.59. Found: C, 38.27; H, 2.27; N, 33.69.
2-(Trifluoromethyl)-[1,2,4]triazolo[1,5-a]pteridine (11c)
The reaction mixture was neutralized with a 5% alcohol solution of KOH to pH ∼ 7 and diluted with 5 mL of MeCN; the precipitate was filtered off and washed with 2 mL of MeCN. The resulting filtrate was evaporated completely and dried in a vacuum desiccator over P2O5. The dry residue was dissolved in MeCN and filtered through a thin layer of silica gel, the filtrate was evaporated, and the precipitate was washed with Et2O and dried in air. Obtained from 7c (28 mg, 1 mmol, 1 equiv), 40 wt % solution of glyoxal in water (115 μL, 1 mmol, 1 equiv), 36% HCl (86 μL, 1 mmol, 1 equiv), in EtOH (5 mL). Brown solid; 214 mg, 89% yield; mp >170–172 °C. 1H NMR (DMSO-d6, 500 MHz): 9.32 (s, 2H), 9.85 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 119.2 (q, J = 270.6 Hz), 131.6, 142.3, 146.7, 150.1, 154.7 (q, J = 39.2 Hz), 155.2, 161.9. 19F NMR (DMSO-d6, 470 MHz): 98.14 (s). Anal. calcd for C8H3F3N6: C, 40.01; H, 1.26; N, 35.00. Found: C, 39.92; H, 1.11; N, 34.88.
2-Methyl-[1,2,4]triazolo[1,5-a]pteridine (11d)
The suspension was cooled in an ice bath, and the precipitate was filtered. Obtained from 7d (164 mg, 1 mmol, 1 equiv), 40 wt % solution of glyoxal in water (115 μL, 1 mmol, 1 equiv), 36% HCl (86 μL, 1 mmol, 1 equiv), in EtOH (5 mL). Beige solid; 148 mg, 80% yield; mp >226–228 °C. 1H NMR (DMSO-d6, 500 MHz): δ 2.61 (s, 3H), 9.17 (d, J = 2.1 Hz, 1H), 9.20 (d, J = 2.1 Hz, 1H), 9.58 (s, 1H). 13C{1H} NMR (DMSO-d6, 126 MHz): δ 14.8, 130.6, 141.7, 145.2, 149.6, 155.0, 159.0, 164.6. Anal. calcd for C8H6N6: C, 51.61; H, 3.25; N, 45.14. Found: C, 51.63; H, 3.16; N, 45.15.
Acknowledgments
The research was financially supported by the Russian Science Foundation (project no. 19-13-00234). Analytical studies were carried out using equipment of the Center for Joint Use “Spectroscopy and Analysis of Organic Compounds” at the Postovsky Institute of Organic Synthesis of UB RAS.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01849.
The authors declare no competing financial interest.
Supplementary Material
References
- a Oukoloff K.; Lucero B.; Francisco K. R.; Brunden K. R.; Ballatore C. 1,2,4-Triazolo[1,5-a]pyrimidines in drug design. Eur. J. Med. Chem. 2019, 165, 332–346. 10.1016/j.ejmech.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rusinov V. L.; Charushin V. N.; Chupakhin O. N. Biologically active azolo-1,2,4-triazines and azolopyrimidines. Russ. Chem. Bull. 2018, 67, 573–599. 10.1007/s11172-018-2113-8. [DOI] [Google Scholar]; c Yang F.; Yu L.-Z.; Diao P.-C.; Jian X.-E.; Zhou M.-F.; Jiang C.-S.; You W.-W.; Ma W.-F.; Zhao P. L. Novel [1,2,4]triazolo[1,5-a]pyrimidine derivatives as potent antitubulin agents: design, multicomponent synthesis and antiproliferative activities. Bioorg. Chem. 2019, 92, 103260 10.1016/j.bioorg.2019.103260. [DOI] [PubMed] [Google Scholar]; d Wan Y.; Wu W.; Guo S.; He S.; Tang X.; Wu X.; Nandakumar K. S.; Zou M.; Li L.; Chen X.; Liu S.; Yao X. [1,2,4]Triazolo[1,5-a]pyrimidine derivative (Mol-5) is a new NS5-RdRp inhibitor of DENV2 proliferation and DENV2-induced inflammation. Acta Pharmacol. Sin. 2019, 1–13. 10.1038/s41401-019-0316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Wang S.; Shen D.; Zhao L.; Yuan X.; Cheng J.; Yu B.; Zheng Y.; Liu H. Discovery of [1,2,4]triazolo[1,5-a]pyrimidine derivatives as new bromodomain-containing protein 4 (BRD4) inhibitors. Chin. Chem. Lett. 2020, 31, 418–422. 10.1016/j.cclet.2019.08.029. [DOI] [Google Scholar]; f Yuan S.; Wang S.; Zhao M.; Zhang D.; Chen J.; Li J.-X.; Zhang J.; Song Y.; Wang J.; Yu B.; Liu H. Brønsted acid-promoted “on–water” C(sp3)-H functionalization for the synthesis of isoindolinone/[1,2,4]triazolo[1,5-a]pyrimidine derivatives targeting the SKP2-CKS1 interaction. Chin. Chem. Lett. 2020, 31, 349–352. 10.1016/j.cclet.2019.07.019. [DOI] [Google Scholar]; g Abd El-Aleam R. H.; George R. F.; Lee K. J.; Keeton A. B.; Piazza G. A.; Kamel A. A.; El-Daly M. E.; Hassan G. S.; Abdel-Rahman H. M. Design and synthesis of 1,2,4-triazolo[1,5-a]pyrimidine derivatives as PDE 4B inhibitors endowed with bronchodilator activity. Arch. Pharm. 2019, 352, 1900002 10.1002/ardp.201900002. [DOI] [PubMed] [Google Scholar]; h Combs D.; Langevine C. M.; Qiu Y.; Zusi F. C. U.S. Patent WO2005011609A2, 2004.
- a Castillo J.-C.; Estupiñan D.; Nogueras M.; Cobo J.; Portilla J. 6-(aryldiazenyl)pyrazolo[1,5-a]pyrimidines as strategic intermediates for the synthesis of pyrazolo[5,1-b]purines. J. Org. Chem. 2016, 81, 12364–12373. 10.1021/acs.joc.6b02431. [DOI] [PubMed] [Google Scholar]; b Sheikhi-Mohammareh S.; Shiri A. An Alternative Regioselective Approach for the Synthesis of Highly Functionalized derivatives of Pyrazolo[5,1-b]purine Scaffold. J. Heterocycl. Chem. 2018, 55, 2055–2060. 10.1002/jhet.3242. [DOI] [Google Scholar]; c Savateev K. V.; Ulomsky E. N.; Borisov S. S.; Voinkov E. K.; Fedotov V. V.; Rusinov V. L. 8-Alkyl[1,2,4]Triazolo[5,1-b]Purines. Chem. Heterocycl. Compd. 2014, 50, 880–887. 10.1007/s10593-014-1542-z. [DOI] [Google Scholar]
- Carmona-Martínez V.; Ruiz-Alcaraz A. J.; Vera M.; Guirado A.; Martínez-Esparza M.; García-Peñarrubia P. Therapeutic potential of pteridine derivatives: A comprehensive review. Med. Res. Rev. 2019, 39, 461–516. 10.1002/med.21529. [DOI] [PubMed] [Google Scholar]
- a Abdallah T.; Darwish M.; Hassaneen H. A Novel Synthesis of 1,2,4-Triazolopteridines. Molecules 2002, 7, 494–500. 10.3390/70600494. [DOI] [Google Scholar]; b Abdel-Mohsen S. A.; El-Emary T. I.; El-Kashef H. S. Synthesis, Anti-inflammatory and Antibacterial Activities of Novel Pyrazolo[4,3-g]pteridines. Chem. Pharm. Bull. 2016, 64, 476–482. 10.1248/cpb.c16-00044. [DOI] [PubMed] [Google Scholar]; c Hou Y.; Zhu L.; Li Z.; Shen Q.; Xu Q.; Li W.; Liu Y.; Gong P. Design, synthesis and biological evaluation of novel 7-amino-[1,2,4]triazolo[4,3-f]pteridinone, and 7-aminotetrazolo[1,5-f]pteridinone derivative as potent antitumor agents. Eur. J. Med. Chem. 2019, 163, 690–709. 10.1016/j.ejmech.2018.12.009. [DOI] [PubMed] [Google Scholar]; d Bi X.; Li J.; Li J.; Shi W.; Dai Y.; Li Q.; Zhang W.; Huang W.; Qian H.; Jiang C. Design, synthesis and biological evaluation of novel 4,5-dihydro-[1,2,4]triazolo[4,3-f]pteridine derivatives as potential BRD4 inhibitors. Bioorg. Med. Chem. 2019, 27, 2813–2821. 10.1016/j.bmc.2019.05.006. [DOI] [PubMed] [Google Scholar]; e Brown D. J.; Shinozuka K. Triazolopteridines. I. Simple s-Triazolo[4,3-c]pteridines and their derived [1,5-c] isomers. Aust. J. Chem. 1981, 34, 189–194. 10.1071/CH9810189. [DOI] [Google Scholar]; f Giudice M. R. D.; Borioni A.; Mustazza C.; Gatta F. New [g]-fused [1,2,4]triazolo[1,5-c]pyrimidines: Synthesis of pyrido[3,2-e] and [4,3-e][1,2,4]triazolo[1,5-c]pyrimidine, pyrimido[5,4-e][1,2,4]triazolo[1,5-c]pyrimidine and [1,2,4]triazolo[1,5-c]pteridine derivatives. J. Heterocycl. Chem. 1994, 31, 1503–1507. 10.1002/jhet.5570310637. [DOI] [Google Scholar]; g Ivachtchenko A. V.; Golovina E. S.; Kadieva M. G.; Kysil’ V. M.; Mitkin O. D. , Antagonists of serotonin 5-HT6 receptors. iv. synthesis and structure-activity interactions in amines containing the 3-(arylsulfonyl)-2-(methylthio)pyrazolo[1,5-a]pyrimidine fragment. Pharm. Chem. J. 2013, 46, 595–602. 10.1007/s11094-013-0853-1. [DOI] [Google Scholar]; h Mokaber-Esfahani M.; Eshghi H.; Shiri A.; Akbarzadeh M.; Mirzaei M. Synthesis of [1,2,4]triazolo[3,4-b]pteridines as a novel class of heterocyclic compounds. J. Chem. Res. 2015, 39, 216–219. 10.3184/174751915X14271341601550. [DOI] [Google Scholar]
- a Chung C. Small Molecule Bromodomain Inhibitors. Prog. Med. Chem. 2012, 51, 1–55. 10.1016/B978-0-12-396493-9.00001-7. [DOI] [PubMed] [Google Scholar]; b Filippakopoulos P.; Qi J.; Picaud S.; Shen Y.; Smith W. B.; Fedorov O.; Morse E. M.; Keates T.; Hickman T. T.; Felletar I.; Philpott1 M.; Munro S.; McKeown M. R.; Wang Y.; Christie A. L.; West N.; Cameron M. J.; Schwartz B.; Heightman T. D.; La Thangue N.; French C. A.; Wiest O.; Kung A. L.; Knapp S.; Bradner J. E. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J.; Shi J.; Wang E.; Rappaport A. R.; Herrmann H.; Sison E. A.; Magoon D.; Qi J.; Blatt K.; Wunderlich M.; Taylor M. J.; Johns C.; Chicas A.; Mulloy J. C.; Kogan S. C.; Brown P.; Valent P.; Bradner J. E.; Lowe S. W.; Vakoc C. R. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 2011, 478, 524–528. 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.; Li S.; Markova D. Z.; Liang A.; Kepler C. K.; Huang Y.; Zhou J.; Yan J.; Chen W.; Huang D.; Xu K.; Ye W. Bromodomain-containing protein 4 inhibition alleviates matrix degradation by enhancing autophagy and suppressing NLRP3 inflammasome activity in NP cells. J. Cell. Physiol. 2020, 235, 5736–5749. 10.1002/jcp.29508. [DOI] [PubMed] [Google Scholar]
- a Thompson P. J.; Shah A.; Apostolopolou H.; Bhushan A. BET Proteins Are Required for Transcriptional Activation of the Senescent Islet Cell Secretome in Type 1 Diabetes. Int. J. Mol. Sci. 2019, 20, 4776 10.3390/ijms20194776. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang Q.; Sun Y.; Li T.; Liu L.; Zhao Y.; Li L.; Zhang L.; Meng Y. Function of BRD4 in the pathogenesis of high glucose-induced cardiac hypertrophy. Mol. Med. Rep. 2018, 19, 499–507. 10.3892/mmr.2018.9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A. R. RSV Reprograms the CDK9•BRD4 Chromatin Remodeling Complex to Couple Innate Inflammation to Airway Remodeling. Viruses 2020, 12, 472 10.3390/v12040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazizov D. A.; Fedotov V. V.; Gorbunov E. B.; Ulomskiy E. N.; Yeltsov O. S.; Rusinov G. L.; Rusinov V. L. Effective method for the synthesis of azolo[1,5-a]pyrimidin-7-amines. Chem. Heterocycl. Compd. 2019, 55, 573–577. 10.1007/s10593-019-02498-2. [DOI] [Google Scholar]
- Makisumi Y. Synthesis of Potential Anticancer Agents. VII. 6-Nitro- and 6-Amino-s-triazolo[2,3-a]pyrimidines. Chem. Pharm. Bull. 1961, 9, 873–877. 10.1248/cpb.9.873. [DOI] [Google Scholar]
- Lynch B. M.; Khan M. A.; Sharma S. C.; Teo H. C. Pyrazolo[1,5-a]Pyrimidine: Synthesis and Regiospecific Electrophilic Substitution in the Pyrazole and/or Pyrimidine Rings. Can. J. Chem. 1975, 53, 119–124. 10.1139/v75-016. [DOI] [Google Scholar]
- L’Oreal. U.S. Patent US6099593, 2000.
- Huppatz J. Systemic Fungicides. The Synthesis of Pyrazolo[1,5-a]pyrimidine Analogues of Carboxin. Aust. J. Chem. 1985, 38, 221–230. 10.1071/CH9850221. [DOI] [Google Scholar]
- Gorbunov E. B.; Rusinov G. L.; Ulomskii E. N.; Isenov M. L.; Charushin V. N. Synthesis of 2H-azolo[1,5-a][1,2,3]triazolo[4,5-e]pyrimidines. Chem. Heterocycl. Compd. 2015, 51, 491–495. 10.1007/s10593-015-1725-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.