Abstract
Background
Surgical injury stimulates the systemic inflammatory response. The magnitude of the postoperative systemic inflammatory response has been shown to be significantly associated with short and long-term outcomes following surgery of varying severity. Different anesthetic techniques for surgery may have an impact on the postoperative systemic inflammatory response and on the rate of the postoperative infective complications.
The aim of the present systematic review was to examine the relationship between perioperative anesthesia, the postoperative systemic inflammatory response and postoperative infective complications in patients undergoing surgery.
Methods
This was carried out using PubMed and other established databases from 1987 up to March 2018. In particular, randomized controlled studies and systemic inflammation markers, interleukin 6 and C-reactive protein were examined.
Results
Overall, 60 controlled, randomized clinical trials were included in the review. The mean or median values of both interleukin 6 and C-reactive protein were taken for each study and the mean value was calculated for each anesthetic group at sampling points of 12–24 and 24–72 hours for interleukin 6 and C-reactive protein respectively. When taking the magnitude of surgery into account, TIVA using propofol was significantly associated with a reduction in particular C-reactive protein (P = .04). However, there were no other specific anesthetic methods including general, regional and combined anesthetics that were associated with a reduction in either interleukin 6 or C-reactive protein.
Conclusion
There is some evidence that anesthetic regimens may reduce the magnitude of the postoperative systemic inflammatory response. However, the studies were heterogeneous and generally of low quality.
Future, well conducted, adequately powered studies are required to clarify the effect of anesthesia on the postoperative systemic inflammatory response and infective complications.
INTRODUCTION
The surgical stress response is defined as the systemic reaction of the human body to a surgical procedure. It has long been recognized that a surgical injury results in stereotypical changes of the neuroendocrinological, metabolic, immunological and hematological systems in humans [1]. The neuroendocrine response to surgery involves the stimulation of the sympathetic nervous system and resultant tachycardia, hypertension and stimulation of the hypothalamic–pituitary adrenal axis. This induces the release of hormones such as adenocorticotropic hormone (ACTH), catecholamines (norepinephrine and epinephrine) and cortisol. Increasing circulating concentrations of such mediators are associated with the suppression of pro-inflammatory T cell responses [2]. For example, an increase in the white cell count is associated with a decrease in the number of lymphocytes including CD4 + and CD8 + and is proposed to have a detrimental effect on the postoperative immunity [3].
Furthermore, the production of pro-inflammatory cytokines including interleukin (IL) IL-1, IL-6, IL-8 and tumor necrosis factor (TNF) alpha by innate immune cells such as neutrophils and macrophages, interacting with damaged cells and platelets, leads to the production of acute phase proteins from the liver such as C-reactive protein (CRP), fibrinogen and complement proteins. The existence of other factors including the pre-existing co-morbid condition, adjuvant chemo or radio therapy, blood transfusion and type of surgical procedure may amplify the surgical stress response [4]. An exaggerated postoperative systemic inflammatory response (SIR) is associated with increased postoperative morbidity and mortality [5], [6].
In terms of routine clinical assessment of the magnitude of surgical injury, circulating concentrations of IL-6 and CRP are particularly useful in the 12–24 hour and 24–96 hour periods respectively following surgical injury [7]. Indeed, in colorectal surgery, postoperative threshold concentrations of CRP > 150 mg/L on day 3 and 4 are associated with increased postoperative infections precluding safe discharge [8], [9].
In addition to host factors, and surgical factors, different anesthetic techniques used in surgery may have a differential effect on the postoperative SIR and postoperative complications [10]. Some anesthetic techniques may affect the immune system by decreasing the levels of pro-inflammatory cytokines and modify the function of innate and adaptive immune cells. For example, the immunomodulatory effect of propofol has been reported in several studies and more favorable than inhalational agents and that combined regional anesthesia has a greater effect than single use of general anesthesia in reducing the surgery induced inflammatory response. Furthermore, the modification of perioperative anesthetic technique may play an important role in cancer patients to reduce the incidence of metastasis and improve the long-term survival [11], [12].
The aim of the present systematic review and meta-analysis was to examine the relationship between different anesthetic techniques and the magnitude of the postoperative SIR in particular that of IL-6 and CRP, and the postoperative infective complications in patients undergoing surgery of different degrees of severity. The results of this review may help to delineate which anesthetic techniques reduce the magnitude of the systemic inflammatory response.
METHODS
Outcomes of Interest
The primary outcome of interest was the impact of anesthesia on the postoperative SIR in particular IL-6 and CRP in patients following surgery. The secondary outcome of interest was the impact of anesthesia on postoperative complications, in particular infective complications, following surgery.
Literature Search
A systematic search of the scientific literature was conducted from 1987 until March 2018 using PubMed, the Excerpta Medica Database (EMBASE), Web of Science databases and the Cochrane Database of Systematic Reviews (CDSR).
Study Selection and Data Extraction
The following search terms were used in free text and medical subject heading (MeSH) together with the usual Boolean meaning of “OR” and “AND” including (“anaesthesia and analgesia”/ OR analgesia, epidural/ OR analgesia, patient controlled/ OR anesthesia/ OR anesthesia, general/ OR anesthesia, inhalation/ OR balanced anesthesia/ OR anesthesia, endotracheal/ OR anesthesia, intravenous [Mesh]) AND “systemic inflammation OR stress response OR systemic inflammatory response” [Mesh]) AND (“General Surgery”[Mesh] OR “Surgical Procedures, Operative”[Mesh] AND “IL-6” AND “CRP” AND “postoperative complication”.
A search of the bibliographies of selected papers was carried out to identify any relevant articles missed during the primary search. The duplicated studies were removed manually. Additional studies were hand-searched from the reference list of included studies. The literature search and data extraction were carried out by a single author (AA). Any uncertainty regarding the inclusion, or otherwise, of a paper was discussed with the senior author (DM). Data on study characteristics including authors, year of publication, country of origin, number of patients, type and severity of surgery, anesthetic agents used type of complications and inflammatory response markers were extracted to preconstructed tables for each individual study. Study quality was assessed using the Jadad scale.
Study Eligibility Criteria
The study question was performed according to the PICO classification including; Population: patients undergoing surgery. Intervention: anesthetic technique. Comparison: different general and regional anesthetic techniques (general anesthesia; general plus regional anesthesia; regional anesthesia; miscellaneous adjuvants). Outcome: IL-6, CRP and postoperative infective complications.
Only controlled, randomized clinical trials published in the English language, including, patients older than 18 years, undergoing surgery of any type were included in the review.
All titles and abstracts were reviewed to assess their relevance for inclusion. There were no restrictions in terms of ethnicity, and stage of cancer or surgical approach.
Meta-Analysis
In the present review, some studies were amenable to meta-analysis using random or fixed effects model to calculate the combined mean difference and its 95% confidence interval in postoperative IL-6 and CRP. Where data were expressed as a median and range or interquartile range, the calculation of mean and standard deviation was derived from the methods of Hozo et al. and Wan et al. [13], [14].
With regards to the effect of anesthesia on the postoperative complications, ORS and 95% confidence interval (CI) were obtained from each study and shown in a forest plot graph and combined using a random effects model.
In the present review, the majority of studies were heterogeneous and therefore the use of random effects model was considered more appropriate than fixed effects model as it was not assumed that they shared a common effect.
Meta-analysis was performed by using the Review Manager software version 5.3 (RevMan v5.3 Nordic Cochrane Collaboration). Statistical heterogeneity was determined by the I2 test.
Evaluation of Clinical Trial Studies
The methodological quality of each study was evaluated using the Jadad scale tool, also known as the Oxford quality scoring system. This is a 3-question, 5-point system with superior validity and reliability evidence compared with other scoring systems [15].
Points for randomization, double-blinding, and description of withdrawals and dropouts are included within the score with points omitted for inappropriate description of randomization or blinding. Studies scoring ≥ 3 points are considered to represent satisfactory methodological quality, with studies scoring, ≤ 2 points considered to be of low quality. Studies in which double-blinding is not possible may be assessed as high quality if the total score ≥ 2 points [16], [17].
RESULTS
Study Selection Process
The results of the literature review are shown in the PRISMA Flow Diagram (Fig 1 [18];).
Fig 1.
Flow diagram chart illustrated the process of article selection. Some studies measured both IL-6 and CRP and showing the postoperative complications and 8 studies showing only the postoperative complications.
In total, 395 studies were identified through the databases. Records were excluded including 165 review articles, 30 articles not in English, 20 animal studies and 2 studies which include non-infective complications. In addition, studies not meeting the inclusion criteria, such as those not reporting IL-6 or CRP or reporting these markers at time points out with the study specifications, were excluded.
Sixty studies examined the impact of different anesthetic techniques on the postoperative SIR and postoperative infective complications. The mean or median values of IL-6 and CRP were taken for each study and the mean value was calculated for each anesthetic group at sampling points of 12–24 and 24–72 hours for IL-6 and CRP respectively.
The Effect of General Anesthesia on the Postoperative SIR
In total, 12 studies compared different types of general anesthetic (GA) agents (intravenous or inhalational) on the postoperative SIR (Table 1). The mean peak IL-6 and CRP were 484 pg/mL (n = 425) and 107 mg/L (n = 195) respectively. Note: The mean peak IL-6 was 86 pg/mL if the study of Li et al., is excluded from the results.
Table 1.
The relationship between the general anesthesia and postoperative systemic inflammatory response in patients undergoing different types of surgery in the context of a randomized controlled trial
| Author (s) | Country | Type of surgery | Severity of surgery | Patients (n) |
Anaesthetics used | Inflammatory response marker | Post-operative sampling point | Findings | Comments | Quality of study |
|---|---|---|---|---|---|---|---|---|---|---|
| [19] | Egypt | Minor elective surgery. | Minor | 40 | Halothane group. Isoflurane group. |
IL-6⁎ | 24 h | Halothane group, IL-6 = 30 pg/mL Isoflurane group, IL-6 = 31 pg/mL |
No significant difference between groups. | Low range of quality score. |
| [20] | Germany | Minimal invasive partial diskectomy. | Moderate | 48 | TIVA§ with propofol and sufentanil compared with BAL§ with sevoflurane. | IL-6 | 24 h | TIVA, IL-6 = 15 pg/mL, P < .05 BAL, IL-6 = 35 pg/mL, P < .05 |
Significant reduction in IL-6 in TIVA group versus BAL group. | Low range of quality score. |
| [12] | China | Open cholecystectomy. | Moderate | 40 | TIVA with propofol and remifentanil compared with BAL|| with isoflurane. |
IL-6 | 12 h | TIVA group, IL-6 = 13.7 ± 4.5 pg/mL, P < .001 IA group, IL-6 = 15.5 ± 5.2 pg/mL, P < .001 |
Significant reduction in IL-6 in TIVA versus IA group. | Low range of quality score. |
| [21] | Sweden | Colorectal cancer. | Moderate | 50 | TIVA with propofol and remifentanil compared with inhalational anaesthesia with sevoflurane and fentanyl. | IL-6 | 24 h | TIVA, 24 h, IL-6 = 505 (129.4-1370) pg/mL. Inhalational, 24 h, IL-6 = 370 (198-810) pg/mL. |
No significant difference between groups. | Low range of quality score. |
| [22] | Brazil | Otorhinological surgery. | Minor | 34 | TIVA with propofol compared with inhaled anaesthesia with isoflurane. | IL-6 | 24 h | Propofol, 24 h, IL-6 = 22 pg/mL Isoflurane, 24 h, IL-6 = 20 pg/mL |
No significant difference between groups. | Low range of quality score. |
| [25] | Korea | Cardiopulmonary bypass surgery. | Major | 112 | Group P = propofol with sufentanil. Group S = sevoflurane with sufentanil. |
CRP† | 24 h | Group P, 24 h, CRP = 80 (13.108, (1.483-24.733) mg/L, P = .05 Group S, 24 h, CRP = 120 (13.108, (1.483-24.733) mg/L, P = .05 |
Significant reduction in CRP in group P versus group S. | Low range of quality score. |
| [23] | Romania | Colorectal cancer. | Moderate | 60 | TIVA with propofol compared with inhalational anaesthesia with isoflurane. | IL-6 | 24 h | TIVA + propofol, 24 h, IL-6 = 88 (5.8-349) pg/mL, P = .6 Inhalational, 24 h, IL-6 = 101(23-428) pg/mL, P = .6 |
No significant difference between groups. | Low range of quality score. |
| [24] | UK | Cardiopulmonary bypass surgery. | Major | 40 | Group P = Propofol and fentanyl group. Group I = Isoflurane and fentanyl group. |
IL-6 | 24 h | Group P, 24 h, IL-6 = 25.8 (4.4) pg/mL, P < .001 Group I, 24 h, IL-6 = 34.5 (6.1) pg/mL, P < .001 |
Significant reduction in IL-6 in group P versus group I. | High range of quality score. |
| CRP | 24 h | Group P, 24 h, CRP = 15.7 (4) mg/L, P < .001 Group I, 24 h, CRP = 25.8 (3.2) mg/L, P < .001 |
Significant reduction in CRP in group P versus group I. | |||||||
| [26] | Japan | Thoracoabdominal esophagectomy. | Major | 20 | Group P = propofol anaesthesia followed by propofol sedation. Group S = sevoflurane anesthesia followed by midazolam sedation. |
CRP | 48 h | Group P, 48 h, CRP = 143 ± 3.9 mg/L, P < .05 Group S, 48 h, CRP = 204 ± 4 mg/L, P < .05 |
Significant reduction in CRP in group P versus group S. | Low range of quality score. |
| [28] | China | Open esophagectomy | Major | 30 | TIVA with propofol compared with dexmedetomedine. | IL-6 | 24 h | TIVA + propofol, 24 h, IL-6 = 310 pg/mL, P < .05 Dexmedetomedine, 24 h, IL-6 = 180 pg/mL |
Significant reduction in IL-6 in dexmedetomedine group versus TIVA + Propofol group. | Low range of quality score. |
| [29] | Brasil | Mini-cardiopulmonary bypass surgery. | Major | 23 | TIVA + DEX ‡ group = Propofol, sufentanil and DEX. TIVA group = propofol and sufentanil. |
IL-6 | 24 h | TIVA + DEX group, 24 h, IL-6 = 130 pg/mL, P < .0001 TIVA group, 24 h, IL-6 = 160 pg/mL, P < .0001 |
Significant reduction in IL-6 in TIVA + DEX group versus TIVA group. | High range of quality score. |
| CRP | 24 h | TIVA + DEX group, 24 h, CRP = 150 mg/L TIVA group, 24 h, CRP = 120 mg/L |
No significant difference between groups. | |||||||
| [27] | China | Tibial fracture surgery. | Moderate | 60 | Control group = patients received propofol with remifentanil. Etomidate group = patients received etomidate with remifentanil. |
IL-6 | 24 h | Control, 24 h, IL-6 = 9000 ± 0.48 pg/mL, P = .001 Etomidate, 24 h, IL-6 = 3240 ± 1.24pg/mL, P = .001 |
Significant reduction in IL-6 in etomidate group versus control group. | Low range of quality score. |
⁎IL-6, Interleukin 6; †CRP, C-reactive protein; ‡DEX, dexmedetomedine; §TIVA, total intravenous anesthesia; ||BAL, balanced inhalational anesthesia.
Studies Comparing Inhalational Anesthetic Drugs
One study with minor severity of surgery (n = 40) reported no significant effect on the mean peak IL-6 when halothane plus nitrous oxide was compared with isoflurane plus nitrous oxide for maintenance of anesthesia after induction with propofol and fentanyl (30 pg/mL versus 31 pg/mL, P-value not given) [19].
Studies Comparing Total Intravenous Anesthesia (TIVA) to Inhalational Anesthesia
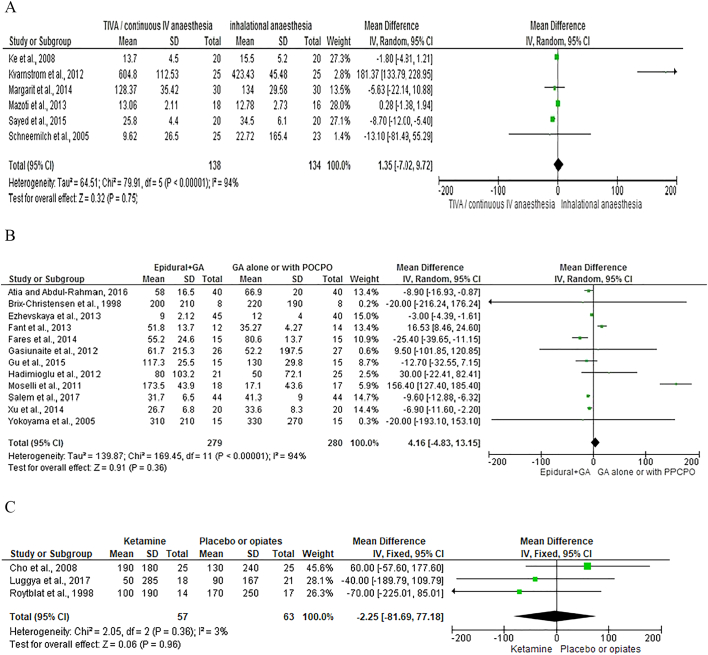
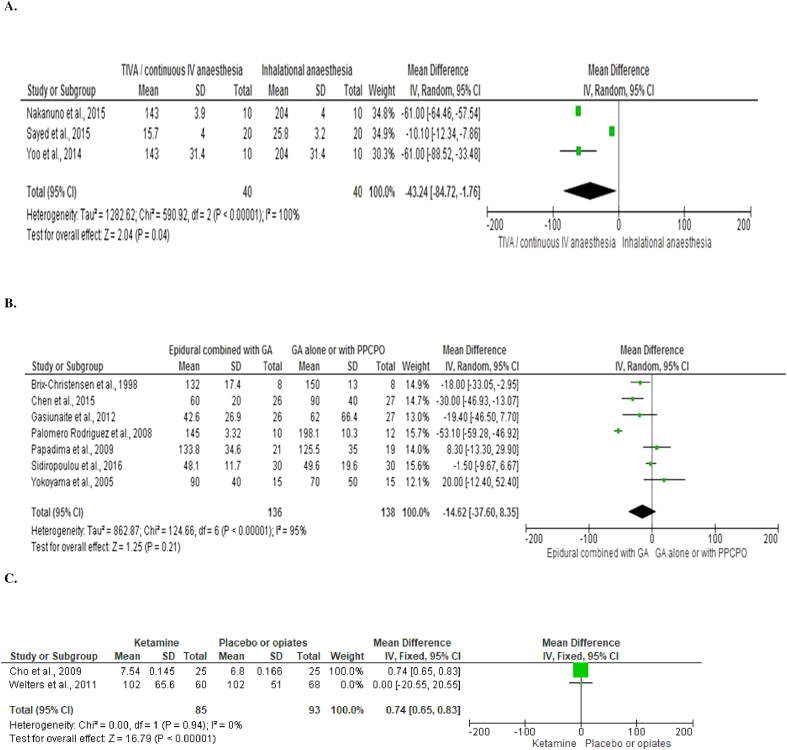
Six studies [12], [20], [21], [22], [23], [24] with 272 patients compared the use of TIVA to inhalational anesthesia and measured IL-6 at 12 to 24 hours after surgery (Fig 2, A). On meta-analysis using a random effects model, TIVA was associated with a non-significant difference in IL-6 concentration (mean difference = − 1.35, 95% CI–7.02, 9.72, P = .75). There was a wide variation in heterogeneity between studies (I2 = 94%, P < .00001).
Fig 2.
Forest graph of studies that compared the use of different anesthetics on the plasma level of IL-6 following surgery of varying severity.
A. Total intravenous anesthesia compared with inhalational anesthesia.
B. Epidural anesthesia in combination with general anesthesia to either general anesthesia alone or with postoperative patient controlled parenteral opiates.
C. Ketamine compared with placebo or opiates.
Three studies [24], [25], [26] with 172 patients compared the use of TIVA to inhalational anesthesia and measured CRP at 24 to 48 hours after surgery (Fig 3, A). On meta-analysis using a random effects model, TIVA was associated with a significant difference in CRP concentration (mean difference = − 43.24, 95% CI–84.72, − 1.76, P = .04). There was a wide variation in heterogeneity between studies (I2 = 100%, P < .00001).
Fig 3.
Forest graph of studies that compared the use of different anesthetics on the plasma level of CRP following surgery of varying severity.
A. Total intravenous anesthesia compared with inhalational anesthesia.
B. Epidural anesthesia in combination with general anesthesia to either general anesthesia alone or with postoperative patient controlled parenteral opiates.
C. Ketamine compared with placebo or opiates.
Of note, patients in the study by Nakanuno et al received postoperative sedation with either propofol or midazolam resulting in longer duration of drug administration than in other studies where anesthetic agents were only administered during surgery. If this study is removed from the meta-analysis, TIVA is associated with a non-significant difference in CRP concentration.
Studies Comparing Drugs Used in TIVA
One study in emergency orthopedic surgery (n = 60), reported a significant reduction of the mean peak IL-6 in patients given TIVA using etomidate versus TIVA with propofol (3240 pg/mL versus 9000 pg/mL, P = .001) [27]. It should be noted that etomidate inhibits the conversion of 11-deoxycortisol to cortisol resulting in transient HPA axis suppression. Another study in patients undergoing esophagectomy (n = 30), reported a significant reduction of the mean peak IL-6 in patients given TIVA using dexmedetomedine compared to TIVA with propofol (180 pg/mL versus 310 pg/mL, P < .05) [28]. In a further study (n = 23), a significant reduction of the mean peak IL-6 was observed when dexmedetomedine was added to propofol TIVA compared with propofol TIVA alone in mini-cardiopulmonary bypass surgery (130 vs 160 pg/mL, P < .0001) although the mean peak CRP was not different in both groups (150 vs 120 mg/L, P > .05) [29].
The Effect of Regional Anesthesia/Analgesia on the Postoperative SIR
A total of 24 studies including 1034 patients compared the effects of different regional or neuraxial anesthetic or analgesic techniques on the postoperative SIR (Table 2.A, Table 2.B).
Table 2.A.
The relationship between combined general and regional or neuraxial anesthesia/analgesia and general anesthesia alone (including postoperative intravenous opiate analgesia) on the postoperative systemic inflammatory response in patients undergoing different types of surgery in the context of a randomized controlled trial
| Author (s) | Country | Type of surgery | Severity of surgery | Patients (n) |
Anesthetics used | Inflammatory response marker | Post-operative sampling point | Findings | Comments | Quality of study |
|---|---|---|---|---|---|---|---|---|---|---|
| [35] | Denmark | Coronary artery bypass grafting surgery. | Major | 16 | Group I = TEA‡ combined with inhalational anesthesia. Group II = high dose fentanyl group. |
IL-6⁎ | 24 h | Group I, IL-6 = 200 pg/mL. Group II = IL-6 = 230 pg/mL. |
No significant difference between groups. | Low range of quality score. |
| CRP† | 48 h | Group I, CRP = 132 mg/L (± 17.4) Group II = CRP = 150 mg/L (± 13) |
No significant difference between groups. | |||||||
| [36] | Japan | Esophageal cancer. | Major | 30 | Group E = GA§ with continuous epidural infusion for postoperative analgesia compared with group G = intraoperative GA and postoperative IV morphine infusion. | IL-6 | 24 h | Group E, 24 h, IL-6 = 310 pg/mL Group G, 24 h, IL-6 = 330 pg/mL |
No significant difference between groups. | Low range of quality score. |
| CRP | 24 and 72 h | Group E, 24 h, CRP = 90 mg/L 72 h, CRP = 100 mg/L Group G, 24 h, CRP = 70 mg/L 72 h, CRP = 100 mg/L |
No significant difference between groups. | |||||||
| [30] | Taiwan | Colon cancer. | Moderate | 60 | Thoracic epidural analgesia with lidocaine compared with IV infusion with lidocaine and control group. | IL-6 | 12 h | Control,12 h, IL-6 = 29 pg/mL, P < .0001 TEA, 12 h, IL-6 = 14 pg/mL, P < .0001 IV group, 12 h, IL-6 = 20 pg/mL, P < .0001 |
Significant reduction in IL-6 in TEA group versus other groups and IV group was better than the control group. | Low range of quality score. |
| [33] | Netherlands | Coronary artery bypass surgery. | Major | 60 | AG = alfentanil group HDRG = high-dose remifentanil group. LDRG = low-dose remifentanil group. TEA = thoracic epidural analgesia in combination with propofol-TCI technique. |
IL-6 | 18 h | AG, IL-6 = 0.18 pg/mL, P = .006 HDRG, IL-6 = 0.14 pg/mL, P = .006 LDRG, IL-6 = 0.15 pg/mL, P = .006 TEA, IL-6 = 0.46 pg/mL, P = .006 |
Significant increase in IL-6 in TEA group versus other groups. | Low range of quality score. |
| CRP | 24,48 and 72 h | AG, 24 h, CRP = 80 mg/L 48 h, CRP = 170 mg/L 72 h, CRP = 120 mg/L HDRG, 24 h, CRP = 70 mg/L 48 h, CRP = 180 mg/L 72 h, CRP = 120 mg/L LDRG, 24 h, CRP = 80 mg/L 48 h, CRP = 220 mg/L 72 h, CRP = 145 mg/L TEA, 24 h, CRP = 50 mg/L 48 h, CRP = 200 mg/L 72 h, CRP = 135 mg/L |
No significant difference between groups. | |||||||
| [47] | Spain | Coronary artery bypass graft surgery with cardiopulmonary bypass. | Major | 22 | GA = GA with postop IV morphine infusion TEA with bupivacaine combined with GA. |
CRP | 24 and 36 h | GA, 24 h, CRP = 200 mg/L, P = .047 36 h, CRP = 250 mg/L TEA, 24 h, CRP = 160 mg/L, P = .047 36 h, CRP = 200 mg/L |
Significant reduction in CRP in TEA group versus GA group. | High range of quality score. |
| [48] | Greece | Abdominal colectomy. | Major | 40 | Group G = GA with postop PCA# Group C = GA combined with epidural analgesia. |
CRP | 24 h | Group G, 24 h, CRP = 120.40 mg/L (125.53 ± 35.03) Group C, 24 h, CRP = 139 mg/L (133.87 ± 34.65), P = .045 |
Significant increase in CRP in group C versus group G. | Low range of quality score. |
| [37] | Italy | Colon cancer. | Moderate | 35 | IEA = GA with intraoperative epidural analgesia compared with IA = GA with IV analgesia. | IL-6 | 24 h | IEA, 24 h, IL-6 = 173.5 pg/mL. IA, 24 h, IL-6 = 171.2 pg/mL. |
No significant difference between groups. | Low range of quality score. |
| [38] | Turkey | Renal transplantation surgery. | Major | 46 | Group I = GA alone. Group II = EA || combined with GA. |
IL-6 | 24 h | Group I, 24 h, IL-6 = 80 pg/mL, P < .05 Group II, 24 h, IL-6 = 50 pg/mL, P < .05 |
Significant reduction in IL-6 in group II versus group I. | Low range of quality score. |
| [39] | Lithuania | Laparoscopic colorectal surgery. | Moderate | 53 | GA compared with combined GA with EA. | IL-6 | 24 h | GA, 24 h, IL-6 = 52.2 (197.56) pg/mL. EA, 24 h, IL-6 = 61.78 (215.31) pg/mL. |
No significant difference between groups. | Low range of quality score. |
| CRP | 24 and 48 h | GA, 24 h, CRP = 128.6 (0) mg/L 48 h, 62.07 (66.43) mg/L EA, 24 h, CRP = 64 (38.47) mg/L 48 h, 42.62 (26.98) mg/L |
No significant difference between groups. | |||||||
| [40] | Philadelphia | Major spinal surgery. | Major | 85 | Group E = EA and endotracheal anesthesia with sevoflurane during surgery and continuous epidural analgesia with ropivacaine, fentanyl and epinephrine after surgery. Group G = GA with sevoflurane and fentanyl and systemically administered opioids after surgery. |
IL-6 | 24 h | Group E, 24 h, IL-6 = 9 pg/mL Group G, 24 h, IL-6 = 12 pg/mL |
No significant difference between groups. | Low range of quality score. |
| [41] | Sweden | Radical retro- pubic prostatectomy. | Moderate | 26 | Group E = PCEA⁎⁎ received epidural analgesia using LA¶ during operation and a combination of LA and opioids after operation. Group P = PCIA# has IV opioid-based analgesia. |
IL-6 | 24 h | Group E, 24 h, IL-6 = 35.7 pg/mL, P = .953 Group P, 24 h, IL-6 = 29.1 pg/mL, P = .953 |
No significant difference between groups. | Low range of quality score. |
| CRP | 24 and 72 h | Group E, 24 h, CRP = 69 (36) mg/L, P = .907 72 h, CRP = 98 (68) mg/L, P = .515 Group P, 24 h, CRP = 67 (25) mg/L, P = .907 72 h, CRP = 112 (32) mg/L, P = .515 |
No significant difference between groups. | |||||||
| [42] | Egypt | Ivor Lewis esophagectomy | Major | 30 | Group I = GA and postoperative PCA# morphine Group II = Thoracic epidural analgesia combined with GA. |
IL-6 | 20 h | Group I, 20 h, IL-6 = 80.6 ± 13.7, P = .033 Group II, 20 h, IL-6 = 55.2 ± 24.6, P = .033 |
Significant reduction in IL-6 in group II versus group I. | Low range of quality score. |
| [43] | China | Colon cancer. | Moderate | 40 | PEA = Thoracic propofol epidural anesthesia GA with PCA IV sufentanil |
IL-6 | 24 h | TPEA, 24 h, IL-6 = 26.75 (6.84) pg/mL, P = .007 GA, 24 h, IL-6 = 33.60 (8.32) pg/mL, P = .007 |
Significant reduction in IL-6 in TPEA versus GA group. | Low range of quality score. |
| [31] | UK | Laparoscopic colorectal surgery. |
Moderate | 120 | PCA compared with spinal analgesia. | IL-6 | 24 h | PCA, 24 h, IL-6 = 58 pg/mL, Spinal, 24 h, IL-6 = 42 pg/mL |
No significant difference between groups. | Low range of quality score. |
| [49] | China | Colon cancer. | Moderate | 53 | G = GA with postoperative PCIV opiate E = GA combined with EA. |
CRP | 48 h | GA,48 h, CRP = 90 mg/L, P < .01 Epidural, 48 h, CRP = 65 mg/L, P < .01 |
Significant reduction in CRP in EA group versus GA group. | Low range of quality score. |
| [44] | China | Esophageal carcinoma undergoing thoracic surgery. | Major | 57 | Group I = GA + PCIA Group II = GA + PCEA Group III = GA + TEA + PCIA Group IV = GA + TEA + PCEA |
IL-6 | 24 h | Group I, 24 h, IL-6 = 140 ± 56.3 pg/mL, P = .46 Group II, 24 h, IL-6 = 128.7 ± 29.7 pg/mL, Group III, 24 h, IL − 6 = 130 ± 29.8 pg/mL, P = .46 Group IV, 24 h, IL-6 = 117.3 ± 25.5 pg/mL, P = .46 |
No significant difference between groups. | Low range of quality score. |
| [50] | Greece | Laparoscopic cholecystectomy. |
Minor | 60 | GA compared with lumbar epidural anesthesia and GA. | CRP | 24 h | GA, 24 h, CRP = 49.68 ± 19.69 mg/L EGA, 24 h, CRP = 48.15 ± 11.73 mg/L |
No significant difference between groups. | High range of quality score. |
| [45] | Egypt | Major abdominal surgery. | Major | 80 | Group I = combined TIVA with TEA. Group II = GA with TIVA†† |
IL-6 | 24 h | Group I, IL-6, 24 h = 58 ± 16.59 pg/mL, P = .033 Group II, IL-6, 24 h = 66.93 ± 20.06 pg/mL, P = .033 |
Significant reduction in IL-6 in group I versus group II. | Low range of quality score. |
| [34] | Turkey | Laparoscopic cholecystectomy. |
Minor | 60 | TEA = combination of GA and thoracic epidural analgesia divided into four groups: Group S = saline, Group F = fentanyl, Group B = bupivacaine and group L = levobupivacaine were infused with saline, saline and fentanyl, bupivacaine and fentanyl, and levobupivacaine and fentanyl, respectively via epidural catheter before surgical incision. | IL-6 | 24 h | Group S, 24 h, IL-6 = 17 pg/mL Group F, 24 h, IL-6 = 17 pg/mL Group B, 24 h, IL-6 = 15 pg/mL Group L, 24 h, IL-6 = 14 pg/mL. |
No significant difference between groups. | Low range of quality score. |
| [32] | China | Minimally invasive mitral valve surgery. | Major | 30 | Group A = patients received intercostal nerve block combined with GA. Group B = patients received GA alone. |
IL-6 | 24 h | Group A, 24 h, IL-6 = 1300 pg/mL, P < .001 Group B, 24 h, IL-6 = 2200 pg/mL, P < .001 |
Significant reduction in IL-6 in group A versus group B. | Low range of quality score. |
| [46] | Egypt | Coronary artery bypass graft surgery. | Major | 88 | GA = GA alone. TEA + GA = thoracic epidural analgesia combined with GA. |
IL-6 | 24 h | GA, 24 h, IL-6 = 41.38 pg/mL TEA + GA, 24 h, IL-6 = 31.7 pg/mL |
Significant reduction in IL-6 in TEA combined with GA group versus GA group. | High range of quality score. |
IL-6, Interleukin 6; † CRP, C-reactive protein; ‡ TEA, thoracic epidural anesthesia; § GA, general anesthesia; || EA, epidural anesthesia; ¶ LA, local anesthesia; # PCIA/PCA, patient-controlled intravenous analgesia; ⁎⁎ PCEA, patient-controlled epidural analgesia; †† TIVA, total intravenous anesthesia.
Table 2.B.
The relationship between regional anesthesia and postoperative systemic inflammatory response in patients undergoing different types of surgery in the context of a randomized controlled trial
| Author (s) | Country | Type of surgery | Severity of surgery | Patients (n) |
Anesthetics used | Inflammatory response marker | Post-operative sampling point | Findings | Comments | Quality of study |
|---|---|---|---|---|---|---|---|---|---|---|
| [51] | Turkey | Anorectal Surgery. | Minor | 58 | ITGA = intratracheal GA‡ compared with regional (saddle block) anesthesia. | CRP † | 24 h | ITGA, CRP = 15.08 ± 14.36 mg/L, P = .531 Regional, CRP = 18.06 ± 21.01 mg/L, P = .531 |
No significant difference between groups. | Low range of quality score. |
| [53] | Greece | Total knee arthoplasty. | Moderate | 56 | Group A = Spinal anesthesia followed by IV morphine analgesia. Group B = EA§ followed by epidural analgesia. |
IL-6 ⁎ | 24 h | Group A, 24 h, IL-6 = 0.67 pg/mL Group B, 24 h, IL-6 = 0.73 pg/mL |
No significant difference between groups. | Low range of quality score. |
| CRP | 24 and 48 h | Group A, 24 h, CRP = 5.5 mg/L 48 h, CRP = 93.5 mg/L Group B, 24 h, CRP = 6.2 mg/L 48 h, CRP = 85.8 mg/L |
No significant difference between groups. | |||||||
| [52] | Turkey | Major lower extremity surgery. | Major | 60 | Group E = EA group. Group G = standard GA group. |
CRP | 24 h | Group E, 24 h, CRP = 62.1 ± 31.2 mg/L, P = .917 Group G, 24 h, CRP = 64.1 ± 38.4 mg/L, P = .917 |
No significant difference between groups. | Low range of quality score. |
⁎IL-6, Interleukin 6; †CRP, C-reactive protein; ‡GA, general anesthesia; §EA, epidural anesthesia.
Studies comparing regional anesthetic techniques in patients also receiving general anesthesia
One study in colonic resection compared thoracic epidural to intravenous lidocaine and a placebo control group in patients undergoing GA with desflurane maintenance for colonic surgery, finding a significant difference in IL-6 concentration 12 hours after surgery (P < .0001) with the lowest in the epidural group (14 pg/mL), followed by the IV lidocaine group (20 pg/mL) and the highest in the placebo control group (29 pg/mL) [30].
Only one study, in patients undergoing laparoscopic colorectal resection, (n = 120) compared the combination of GA plus spinal anesthesia (bupivacaine and diamorphine) to GA plus postoperative patient controlled analgesia (PCA) with morphine and did not show any significant effect on the mean peak CRP (42 mg/L versus 58 mg/L, P-value not given) [31]. In addition, a single study in cardiac surgery (n = 30), compared GA with or without intercostal nerve block, reporting significantly lower peak IL-6 in the combined intercostal / GA group (2200 pg/mL versus 1300 pg/mL, P < .001) [32].
One randomized study (n = 60) compared the effect of four different anesthetic techniques on the inflammatory response to cardiac surgery with CPB. All patients received TIVA with Propofol plus either; alfentanil infusion; high dose remifentanil infusion; low dose remifentanil infusion; or low dose remifentanil infusion plus thoracic epidural. An increase in the mean peak IL-6 was seen in the group receiving low dose remifentanil infusion plus thoracic epidural (P = .006), although the mean peak difference of CRP was not statistically significant between the groups [33]. A further study in patients undergoing laparoscopic cholecystectomy under GA (n = 60), reported no significant difference in mean peak IL-6 when four different thoracic epidural analgesia regimens were compared; saline; fentanyl; fentanyl plus bupivacaine; or fentanyl plus levobupivacaine (P value not given) [34].
Twelve studies [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46] with 529 patients compared the use of epidural anesthesia in combination with GA to GA alone or with postoperative patient controlled parenteral opiates and measured IL-6 20 to 24 hours after surgery (Fig 2, B). On meta-analysis using a random effects model, epidural was associated with a non-significant difference in IL-6 concentration (mean difference = 4.16, 95% CI -4.83-13.15, P = .36). There was a wide variation in heterogeneity between studies (I2 = 94%, P < .00001).
Seven studies (281 patients) [35], [36], [39], [47], [48], [49], [50] compared the use of epidural anesthesia in combination with GA to GA alone or with postoperative patient controlled parenteral opiates and measured CRP 24 to 72 hours after surgery (Fig 3, B). On meta-analysis using a random effects model, epidural was associated with a non-significant difference in CRP concentration (mean difference = − 14.62, 95% CI -37.60-8.35, P = .21). There was a wide variation in heterogeneity between studies (I2 = 95%, P < .00001).
Studies Comparing General Anesthesia with Central Neuraxial Anesthesia
One study in patients undergoing hemorrhoidectomy (n = 58), showed no significant difference of the mean peak CRP when “saddle block” spinal anesthesia without GA was compared to GA (18 mg/L versus 15 mg/L, P = .531) [51]. In another study of patients undergoing major lower limb surgery (n = 60), there were no significant difference of the mean peak CRP in patients given epidural anesthesia without GA versus GA (62.1 mg/L versus 64.1 mg/L, P = 917) [52].
Studies Comparing Central Neuraxial Anesthetic Techniques Without GA
A single study in patients undergoing total knee arthroplasty (n = 56) reported no significant difference of the mean peak IL-6 (0.67 pg/mL versus 0.73 pg/mL, P = .626) and CRP at 24 hours (5.5 mg/L versus 6.2 mg/L, P = .443) when spinal anesthesia was compared to epidural anesthesia [53].
The Effect of Miscellaneous And Adjuvant Drugs With General and Regional Anesthesia on the Postoperative SIR
The addition of some adjuvant drugs with general and regional anesthesia may play a role in mitigating the inflammatory mediators. Sixteen studies were included with the results shown in Table 3.
Table 3.
The relationship between the effects of adjuvant drugs with general anesthetics on the postoperative systemic inflammatory response in patients undergoing different types of surgery in the context of a randomized controlled trial
| Author (s) | Country | Type of surgery | Severity of surgery | Patients (n) |
Anesthetics used | Inflammatory response marker | Post-operative sampling point | Findings | Comments | Quality of study |
|---|---|---|---|---|---|---|---|---|---|---|
| [58] | Israel | Coronary artery bypass grafting surgery. | Major | 31 | Control group = large dose of fentanyl. Ketamine group = small dose of ketamine added to GA‡. |
IL-6 ⁎ | 24 h | Control, IL-6 = 170 pg/mL. P < .05 Ketamine, IL-6 = 100 pg/mL, P < .05 |
Significant reduction in IL-6 in ketamine group versus control group. | High range of quality score. |
| [68] | China | Colorectal cancer. | Moderate | 40 | Control group received only PCEA§ with morphine and ropivacaine. Clonidine group received preoperative epidural clonidine and postoperative PCEA with clonidine + morphine + ropivacaine. |
IL-6 | 12–24 h | Control, 12 h, IL-6 = 25 pg/mL, P < .0001 24 h, IL6 = 9 pg/mL, P < .0001 Clonidine, 12 h, IL-6 = 16 pg/mL, P < .0001 24 h, IL6 = 7 pg/mL, P < .0001 |
Significant reduction in IL-6 in clonidine group versus control. | Low range of quality score. |
| [64] | Japan | Esophageal cancer surgery. | Major | 14 | Control group = did not receive PGE1|| PGE1 group = received IV PGE1 during anesthesia. |
IL-6 | 24 h | Control, IL-6 = 66.7 (35.5–159.3) pg/mL, P < .05 PGE1, IL-6 = 32.8 (17.9–86.9) pg/mL, P < .05 |
Significant reduction in IL-6 in PGE1 group versus control. | High range of quality score. |
| [69] | Japan | Lower open abdominal surgery. | Major | 40 | Different doses of pre-incisional epidural neostigmine with mepivacaine before the induction of GA | IL-6 | 24 h | Control, IL-6 = 8000% (0.27 ± 0.10) N-0.05 mg, IL6 = 9000% (0.12 ± 0.04) N-0.1 mg, IL-6 = 13,000% (0.40 ± 0.19) N-0.15 mg, IL-6 = 13,000% (0.66 ± 0.37) |
No significant difference between groups. | Low range of quality score. |
| [62] | China | Colorectal cancer. | Moderate | 40 | Pre-incisional IV pentoxifylline compared to control group. | IL-6 | 12–24 h | Control, 12 h, IL-6 = 50 pg/mL, P < .0001 24 h, IL6 = 21 pg/mL, P < .0001 PTX, 12 h, IL-6 = 23 pg/mL, P < .0001 24 h, IL-6 = 17 pg/mL, P < .0001 |
Significant reduction in IL-6 in pentoxifylline group versus control group. | Low range of quality score. |
| [55] | China | Total knee joint replacement surgery. | Moderate | 37 | Control group = placebo was given 1 hour before surgery. All patients received epidural combined with isoflurane anesthesia during operation and PCEA postoperatively. Study group = oral rofecoxib 1 hour before surgery. |
IL-6 | 12 h | Control,12 h, IL-6 = 63 pg/mL, P < .05 Rofecoxib, 12 h, IL-6 = 38 pg/mL, P < .05 |
Significant reduction in IL-6 in rofecoxib group versus control group. | Low range of quality score. |
| [59] | Korea | Off-pump coronary artery bypass graft surgery. | Major | 50 | Control group = saline during induction of anesthesia with sevoflurane. Ketamine group = 0.5 mg kg-1 of ketamine during induction of anesthesia. |
IL-6 | 24 h | Control, IL-6 = 130 pg/mL. Ketamine, IL-6 = 190 pg/mL. |
No significant difference between groups. | High range of quality score. |
| CRP† | 24–48 h | Control, 24 h, CRP = 70 mg/L 48 h, CRP = 150 md/L Ketamine, 24 h, CRP = 73 mg/L 48 h, CRP = 160 mg/L |
No significant difference between groups. | |||||||
| [65] | Turkey | Cardiopulmonary bypass surgery. | Major | 24 | Intra-operative amiodarone group compared with control. | IL-6 | 24 h | Control, 24 h, IL-6 = 45.72 ± 17.35) pg/mL. Amiodarone, 24 h, IL-6 = 52.09 ± 4.40) pg/mL |
No significant difference between groups. | High range of quality score. |
| CRP | 24 h | Control, 24 h, CRP = 105.13 (105.13 ± 0.57) mg/L Amiodarone, 24 h, CRP = 99.25 (99.25 ± 19.27) mg/L |
No significant difference between groups. | |||||||
| [61] | UK | Coronary artery bypass surgery with cardiopulmonary bypass. | Major | 128 | Ketamine based anesthetics compared with standard anesthesia with propofol and sufentanil. | CRP | 24 h | Ketamine, 24 h, CRP = 102 (65.6) mg/L, P = .299 Propofol, 24 h, CRP = 102 (51) mg/L, P = .299 |
No significant difference between groups. | Low range of quality score. |
| [67] | Japan | Cardiopulmonary bypass surgery. | Major | 37 | Group D = Dexmedetomedine group. Group S = Saline group. |
IL-6 | 24 h | Group D, 24 h, IL-6 = 20 pg/mL, P = .0026 Group S, 24 h, IL-6 = 56 pg/mL, P = .0026 |
Significant reduction in IL-6 in group D versus group S. | High range of quality score. |
| CRP | 24,48 and 72 h | Group D, 24 h, CRP = 52.5 mg/L 48 h, CRP = 72.5 mg/L 72 h, CRP = 53.9 mg/L Group S, 24 h, CRP = 58.9 mg/L 48 h, CRP = 64.7 mg/L 72 h, CRP = 39.8 mg/L |
No significant difference between groups. | |||||||
| [63] | Korea | Laparoscopic gastrectomy. | Moderate | 39 | Saline group, were infused with an equal volume of normal saline. Clinical dose group were infused with a loading dose of 0.5 mg/kg esmolol followed by infusion at a constant rate of 30 μg/kg/min, subclinical dose group were infused with a loading dose of 0.25 mg/kg esmolol and followed by constant infusion of 15 μg/kg/min. |
CRP | 24 h | Saline, 24 h, CRP = 59 mg/L, P = .043 Clinical, 24 h, CRP = 24 mg/L, P = .043 Subclinical, 24 h, CRP = 44 mg/L |
Significant reduction in CRP in clinical dose group versus saline group. | High range of quality score. |
| [66] | Iran | Coronary artery bypass graft surgery with cardiopulmonary bypass surgery. | Major | 81 | Selenium group = IV bolus of 600 μg Se before induction of anesthesia. Placebo group = normal saline. |
IL-6 | 24 h | Selenium, 24 h, IL-6 = 100 pg/mL, P = .17 Placebo, 24 h, IL-6 = 106 pg/mL, P = .17 |
No significant difference between groups. | High range of quality score. |
| CRP | 24 and 48 h | Selenium, 24 h, CRP = 100 mg/L, P = .075 48 h, CRP = 123 mg/L, P = .11 Placebo, 24 h, CRP = 106 mg/L, P = .075 48 h, CRP = 130 mg/L, P = .11 |
No significant difference between groups. | |||||||
| [54] | China | Percutaneous nephrolithotomy. | Minor | 120 | Parecoxib group and control group. | IL-6 | 24 h | Control, 24 h, IL-6 = 26 pg/mL, P < .05 Parecoxib, 24 h, IL-6 = 17 pg/mL, P < .05 |
Significant reduction in IL-6 in parecoxib versus control group. | High range of quality score. |
| CRP | 24, 48 and 72 h | Control, 24 h, CRP = 24 mg/L, P < .05 48 h, CRP = 28 mg/L, P < .05 72 h, CRP = 34 mg/L, P < .05 Parecoxib, 24 h, CRP = 17 mg/L, P < .05 48 h, CRP = 19 mg/L, P < .05 72 h, CRP = 23 mg/L, P < .05 |
Significant reduction in CRP in parecoxib versus control group. | |||||||
| [57] | China | Thoracoscopic lobectomy. | Major | 92 | Control group = patients received saline. Nalbuphine HCL group = patients received IV nalbuphine HCL prior to induction of anesthesia. |
IL-6 | 24 h | Control group, 24 h, IL-6 = 153.36 ± 6.77 pg/mL, P < .001 Nalbuphine HCL group, 24 h, IL-6 = 126.49 ± 6.68 pg/mL, P < .001 |
Significant reduction in IL-6 in nalbuphine group versus control group. | Low range of quality score. |
| [56] | China | Laparoscopic cholecystectomy. | Minor | 113 | Control group = patients received sufentanil. Observation group = patients received oxycodone HCL. |
IL-6 | 24 h | Control, 24 h, IL-6 = 55.16 ± 8.05 pg/mL, P < .05 Observation, 24 h, IL-6 = 43.17 ± 6.66 pg/mL, P < .05 |
Significant reduction in IL-6 in observation group versus control group. | Low range of quality score. |
| [60] | USA | Abdominal or perineal surgery. | Major | 39 | Ketamine group and placebo group. | IL-6 | 24 h | Ketamine, 24 h, IL-6 = 50 ± 285 pg/m, P = .402 Placebo, 24 h, IL-6 = 90 ± 167 pg/mL, P = .402 |
No significant difference between groups. | High range of quality score. |
* IL-6, Interleukin 6; †CRP, C-reactive protein; ‡GA, general anesthesia; §PCEA, patient-controlled epidural analgesia; ||PGE1, prostaglandin E1.
Studies of Cyclo-Oxygenase Inhibitors Administered Perioperatively
Two studies reported the impact of cyclo-oxygenase (COX) 2 inhibitors. In the first study (n = 120), a single dose of IV Parecoxib 40 mg was administered in patients who had undergone percutaneous nephrolithotomy on the day of surgery followed by 40 mg every 12 hours for 48 hours demonstrating significant reduction of the mean peak IL-6 (17 pg/mL versus 26 pg/mL, P < .05) and CRP (19.7 mg/L versus 28.6 mg/L, P < .05) [54]. In the second study, 37 patients undergoing total knee replacement were randomized to receive pre-operative oral Rofecoxib or placebo one hour before surgery. Both groups received GA plus epidural during surgery with patient controlled epidural analgesia postoperatively. Mean peak IL-6 was reduced significantly in the Rofecoxib group (38 pg/mL versus 63 pg/mL, P < .05) [55].
Studies Comparing Opioid Regimens as Part of GA
Two studies reported the impact of different opioids during and after anesthesia on the postoperative systemic inflammatory response. In the first study (n = 113), there was a significant reduction in the mean peak IL-6 in those treated with oxycodone versus sufentanil in patients undergoing resection of rectal carcinoma under TIVA (43 pg/mL versus 55 pg/mL, P < .05) [56]. In the second study (n = 92), IV nalbuphine was associated with a significantly lower mean peak IL-6 when administered prior to induction of anesthesia in patients underwent to thoracoscopic lobectomy (126.49 pg/mL versus 153.36 pg/mL, P < .001) [57].
Studies of Ketamine Administered as an Analgesic Adjunct
Three studies [58], [59], [60] with 120 patients compared the use of ketamine to either placebo or opiates during GA and measured IL-6 at 24 hours after surgery (Fig 2, C). On meta-analysis using a fixed effects model, ketamine was associated with a non-significant difference in IL-6 concentration (mean difference = − 2.25, 95% CI -81.69-77.18, P = .96). There was minimal heterogeneity between studies (I2 = 3%, P = .36).
Two studies [59], [61] with 178 patients compared the use of ketamine to either placebo or opiates during GA and measured CRP at 24 hours after surgery (Fig 3, C). On meta-analysis using a fixed effects model, ketamine was associated with a significant difference in CRP concentration (mean difference = 0.74, 95% CI 0.65–0.83, P < .001). There was minimal heterogeneity between studies (I2 = 0%, P = .94).
Other/Miscellaneous
Six studies investigated the impact of other / miscellaneous adjuvant drugs on the postoperative SIR during GA. In a study of 40 patients randomized to receive IV pentoxyphylline infusion or placebo before GA for colorectal surgery, mean peak IL-6 levels were reduced when compared with control (20 pg/mL versus 35.5 pg/mL, P < .0001) [62]. In patients undergoing laparoscopic gastrectomy (n = 39), those who received a clinical dose of the beta blocker esmolol had a lower mean peak CRP versus placebo (24 mg/L versus 59 mg/L, P = .043) [63].
In a study comparing prostaglandin E1 (PGE1) to placebo (n = 14), there was a significant reduction of the mean peak IL-6 when a small dose of PGE1 was added during anesthesia (33 pg/mL versus 67 pg/mL, P < .05) [64]. In a study of cardiac surgery on cardiopulmonary bypass (n = 24), there was no significant difference in the mean peak IL-6 (52 pg/mL versus 45.72 pg/mL, P < .01) and CRP (99.3 mg/L versus 105.1 mg/L, P < .01) between patients who received amiodarone versus control [65]. In another cardiac surgery study (n = 81), there was no significant difference in the mean peak IL-6 (100 pg/mL versus 106 pg/mL, P = .17) and CRP (111.5 mg/L versus 118 mg/L, P = .11) between patients who received IV selenium before induction of anesthesia and placebo [66]. Finally, a further study of patients requiring cardiopulmonary bypass (n = 37) which compared a short infusion of dexmedetomedine to placebo for 10 minutes after aortic cross clamp during CBP in addition to TIVA with propofol reported a significant association with lower peak IL-6 concentrations in the treatment arm (20 pg/mL versus 56 pg/mL, P = .0026). Of note, both groups received 1 g methylprednisolone during surgery [67].
Epidural Adjuncts
Two studies reported the impact of adjuvant drugs used in epidural infusions on the postoperative SIR. The first study compared epidural using ropivacaine and morphine with the addition of clonidine during GA to epidural ropivacaine and morphine without clonidine in patients undergoing colorectal surgery, reporting a significantly reduced mean peak IL-6 in the treatment group (n = 40) (11.5 pg/mL versus 17 pg/mL) [68]. In a further study in patients undergoing open gynecological surgery (n = 40), there was no significant difference in mean peak IL-6 when different doses of epidural neostigmine were administered before induction of GA [69].
The effect of regional and general anesthetic techniques on postoperative complications
Fourteen studies including 1755 patients reported the impact of general and regional anesthetic techniques on postoperative complications across a variety of surgical specialities and severities (Table 4).
Table 4.
Comparison between different types of anesthesia on the postoperative infective complications following different types of surgery in the context of a randomized controlled trial
| Author (s) | Country | Type of surgery | Severity of surgery | Patients (n) |
Type of complications | Anesthetics used | Findings | Comments | Quality of study |
|---|---|---|---|---|---|---|---|---|---|
| [70] | California | Intra-thoracic, intra-abdominal or major (non-cerebral) vascular surgery. | Major | 53 |
⁎ Pneumonia ⁎ Sepsis |
Group I = EA† and postoperative analgesia. Group II = GA⁎and parenteral narcotic administration for postoperative pain relief. |
Group I, 1 case of pneumonia and one case of sepsis. Group II, 9 cases of pneumonia and 4 cases of sepsis. |
Significant reduction in postoperative complications in group I compared with group II. | Low range of quality score. |
| [71] | France | Major abdominal surgery. | Major | 153 | ⁎ Pulmonary complication. | Group I = GA with IV fentanyl and postoperative analgesia with subcutaneous morphine. Group II = GA combined with epidural bupivacaine and epidural bupivacaine with morphine for postoperative pain relief. |
Group I, 23 cases with pulmonary complication. Group II, 21 cases with pulmonary complication. |
No significant difference between the groups. | Low range of quality score. |
| [72] | UK | Coronary artery bypass graft surgery. | Major | 408 | ⁎ Lower respiratory tract infection. | Group TEA‡ = GA with perioperative TEA. Group GA = GA with postoperative opioid analgesia. |
Group TEA, 31 cases of lower respiratory tract infection. Group GA, 59 cases of lower respiratory tract infection. |
Significant reduction in lower respiratory tract infection in TEA group compared with GA group. | High range of quality score. |
| [37] | Italy | Colon cancer | Moderate | 35 |
⁎ Anastomosis leakage (AL). ⁎ Pneumonia ⁎ Ileus |
IEA = GA with intraoperative epidural analgesia compared with IA = GA with IV analgesia. | IEA group, one case of AL, 5 cases of pneumonia and 2 cases of ileus. IA, no cases of AL or ileus and 4 cases of pneumonia. |
No significant difference between the groups. | Low range of quality score. |
| [73] | The Netherlands | Cardiac surgery. | Major | 654 | ⁎ Pneumonia | Group I = GA alone. Group II = combined GA and TEA. |
Group I, 19 cases of Pneumonia. Group II, 30 cases of Pneumonia. |
No significant difference between the groups. | High range of quality score. |
| [75] | South Korea | Ivor Lewis operation for esophageal cancer. | Major | 48 |
⁎ Anastomosis leakage (AL). ⁎ Sepsis |
Group S = sevoflurane. Group P = TIVA§ with propofol and remifentanil. |
Group S, 1 case of AL and 2 cases of sepsis. Group P, 2 cases of AL with no cases of sepsis. |
No significant difference between the groups. | Low range of quality score. |
| [39] | Lithuania | Laparoscopic colorectal surgery. | Moderate | 53 | ⁎ Anastomotic permeability. | GA compared with combined GA with EA. | GA group, anastomotic permeability is 14.8% GA + EA group, anastomotic permeability is 11.5% |
No significant difference between the groups. | Low range of quality score. |
| [42] | Egypt | Ivor Lewis esophagectomy | Major | 30 |
⁎ Anastomosis leakage (AL). ⁎ Pneumonia ⁎ Septic shock |
Group I = GA Group II = Thoracic epidural analgesia combined with GA. |
GA group, 4 cases of AL, 6 cases of pneumonia and 2 cases of septic shock. GA + TEA, 1 case of AL, 2 cases of pneumonia and one case of septic shock. |
No significant difference between the groups. | Low range of quality score. |
| [49] | China | Colon cancer. | Moderate | 53 |
⁎ Anastomosis leakage (AL). ⁎ Wound infection. ⁎ Urinary tract infection (UTI). |
GA alone compared with GA combined with epidural anesthesia. | GA group, 1 case of AL, 1 case of wound infection and with no case of UTI. GA + EA, no case of AL, 1 case of wound infection and with no case of UTI. |
No significant difference between the groups. | Low range of quality score. |
| [31] | UK | Laparoscopic colorectal surgery. | Moderate | 120 | ⁎ Ileus | PCA || compared with spinal analgesia. | PCA group, 11 cases of ileus. Spinal analgesia group, 2 cases of ileus. |
Significant reduction in ileus in spinal analgesia compared with PCA. | Low range of quality score. |
| [26] | Japan | Thoraco-abdominal esophagectomy. | Major | 20 | ⁎ Anastomosis leakage (AL). | Group P = propofol anesthesia followed by propofol sedation. Group S = sevoflurane anesthesia followed by midazolam sedation. |
Group P, no cases with AL. Group S, 2 cases with AL. |
No significant difference between the groups. | Low range of quality score. |
| [76] | China | Laparoscopic radical hysterectomy for cervical cancer. | Moderate | 58 |
⁎Wound infection. ⁎Urinary tract infection (UTI). |
Group S = sevoflurane. Group P = TIVA with propofol. |
Group S, no cases have shown with wound infection but 3 cases with UTI. Group P,, no cases have shown with wound infection but 1 case with UTI. |
No significant difference between the groups. | Low range of quality score. |
| [77] | Slovenia | Craniotomy | Major | 40 | ⁎Wound infection. | Group P = propofol. Group S = Sevoflurane. |
Group P, one case of wound infection. Group S, one case of wound infection. |
No significant difference between the groups. | High range of quality score. |
| [74] | India | Abdominal laparotomy. | Major | 60 | ⁎Anastomosis leakage (AL). | TEB group = patients received GA along with thoracic epidural block. GA group = patients received GA alone. |
Group TEB, 2 of AL Group GA, 1 of AL. |
No significant difference between the groups. | High range of quality score. |
GA, general anesthesia; †EA, epidural anesthesia; ‡TEA, thoracic epidural anesthesia; §TIVA, total intravenous anesthesia; ||PCA, patient-controlled analgesia.
Infective Complications
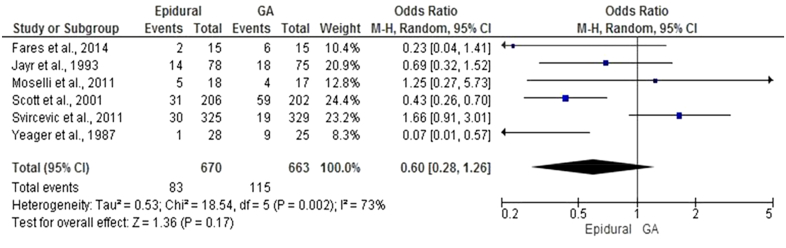
Eight studies [37], [42], [49], [70], [71], [72], [73], [74] with 1446 patients compared the use of epidural anesthesia in combination with GA to GA alone and reported rates of infective complications after surgery (Fig 4, A). On meta-analysis using a random effects model, epidural was associated with a non-significant difference in infective complications (OR = 0.98, 95% CI 0.49–1.95, P = .94). There was a wide variation in heterogeneity between studies (I2 = 69%, P = .002).
Fig 4.
Comparison of anesthetic techniques reporting infective complications after surgery.
A. Forest graph of studies that compared the use of epidural to general anesthesia and reported rates of infective complications after surgery.
B. Forest graph of studies that compared the use of total intravenous anesthesia to inhalational anesthesia and reported rates of infective complications after surgery.
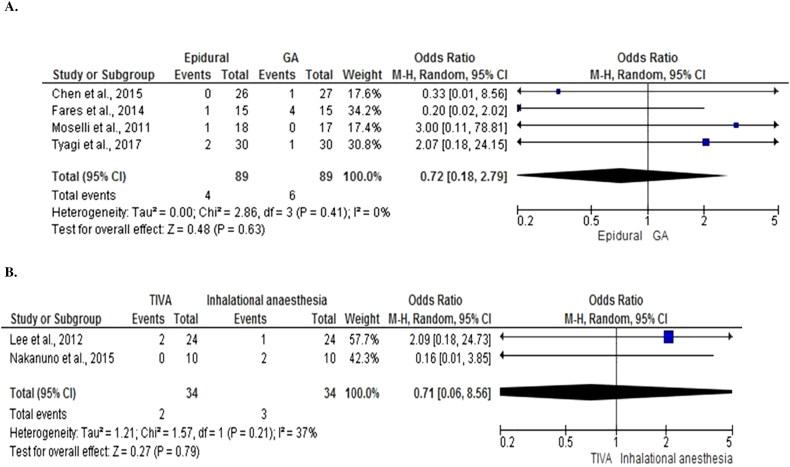
Four studies [26], [75], [76], [77] with 166 patients compared the use of anesthetic maintenance with TIVA to inhalational agents and reported rates of infective complications after surgery (Fig 4, B). On meta-analysis using a random effects model, TIVA was associated with a non-significant difference in infective complications (OR = 0.47, 95% CI 0.14–1.56, P = .21). There was minimal heterogeneity between studies (I2 = 0%, P = 82).
Lower Respiratory Tract Infection
Six studies [37], [42], [70], [71], [72], [73] with 166 patients compared the use of epidural anesthesia in combination with GA to GA and reported rates of lower respiratory tract infection after surgery (Fig 5). On meta-analysis using a random effects model, epidural was associated with a non-significant difference in lower respiratory tract infections (OR = 0.60, 95% CI 0.28–1.26, P = .17). There was a wide variation in heterogeneity between studies heterogeneity (I2 = 73%, P = .002).
Fig 5.
Forest graph of studies that compared the use of epidural anesthesia in combination with general anesthesia to general anesthesia and reported rates of lower respiratory tract infection after surgery.
Anastomotic Leak
Four studies [37], [42], [49], [74], 1 in esophagectomy and 3 in colorectal surgery, with 178 patients compared the use of epidural anesthesia in combination with GA to GA and reported rates of anastomotic leak (Fig 6, A). On meta-analysis using a random effects model, epidural was associated with a non-significant difference in anastomotic leak (OR = 0.72, 95% CI 0.18–2.79, P = .63). There was minimal heterogeneity between studies (I2 = 0%, P = .41).
Fig 6.
Comparison of anesthetic techniques reporting anastomotic leak after surgery.
A, Forest graph of studies that compared the use of epidural anesthesia in combination with general anesthesia to general anesthesia.
B, Forest graph of studies that compared the use of total intravenous anesthesia to inhalational anesthesia.
Two studies [26], [75] both in esophagectomy, with 68 patients compared anesthetic maintenance with TIVA to inhalational agents and reported rates of anastomotic leak (Fig 6, B). On meta-analysis using a random effects model, TIVA was associated with a non-significant difference in anastomotic leak (OR = 0.71, 95% CI 0.06–8.56, P = .79). There was minimal heterogeneity between studies (I2 = 37%, P = .21).
A single study (n = 53) in laparoscopic colorectal surgery compared epidural anesthesia in combination with GA to GA alone and reported no significant difference in anastomotic permeability (11.5% versus 14.8%, P > .05) [39].
Wound Infection
One study (n = 58) comparing TIVA with propofol to inhalational anesthesia in laparoscopic hysterectomy for cervical cancer reported no significant difference in wound infection rates, with no wound infection in either group [76]. A further study (n = 40) comparing TIVA with propofol to inhalational anesthesia in craniotomy also reported no significant difference in wound infection rates with 1 wound infection in each group [77].
Ileus
A single study (n = 35) in colonic cancer resection compared epidural anesthesia in combination with GA to GA including remifentanil and reported no significant difference in rates of postoperative ileus (2 versus 0, P > .05) [37]. A further study (n = 120) in laparoscopic colorectal surgery compared GA plus spinal anesthesia (bupivacaine and diamorphine) to GA plus postoperative analgesia with PCA morphine, reporting a significant reduction in rates of ileus in the group of patients given spinal opioid (2 versus 11, P < .05) [31].
DISCUSSION
In the present systematic review and meta-analysis, there were 60 randomized controlled, clinical studies that examined the relationship between anesthesia and the objective markers of the postoperative SIR following surgical operations of varying severity. The majority of the studies involved in this review had a small study population (< 50 patients per trial arm). The majority of studies measured IL-6 in the postoperative period; however there was considerable variability in the values reported. In contrast, fewer studies reported CRP values with less variability. Irrespective, the majority of studies did not report a significant difference in the magnitude of the postoperative systemic inflammatory response when different general and regional anesthetic techniques were compared. Only 14 randomized studies reported the influence of anesthesia on postoperative infective complications and the results from the present meta-analysis did not find any difference in postoperative complications between different anesthetic groups.
There is good evidence that both IL-6 and CRP reflect the magnitude of surgical injury [7]. For example, laparoscopic surgery, compared with open surgery, is associated with a smaller surgical injury and lower peak IL-6 and CRP. Furthermore, it has been established that there are certain threshold values of CRP that when measured are associated with the development of postoperative infective complications, particularly in colorectal surgery, but increasingly in other surgical specialities [4], [9]. However, although not routinely measured in clinical laboratories, the majority of studies in the present review examined IL-6 in the postoperative period. It is likely that the peak IL-6 measurement, rather than CRP, was made as it could be sampled earlier in the postoperative period. Therefore, given the relationship between peak CRP and infective complications, it would be important that in future studies peak CRP is measured when anesthetic regimens are being tested, especially in the context of postoperative complications.
Experimental and clinical studies have long suggested that the choice of anesthetic agents may influence the immune system, in particular, that some anesthetic regimens may be associated with less immunosuppression. This is likely to be very important in cancer surgery [78]. With the enhanced recovery protocols now being used in cancer surgery there is an opportunity to move towards standardized anesthetic and perioperative care protocols that are known to reduce the magnitude of the postoperative systemic inflammatory response and therefore reduce the relative postoperative immunosuppression, with the aim of reducing postoperative morbidity, and disease recurrence in the context of cancer surgery.
From the results of the present systematic review and meta-analysis it would appear that total intravenous anesthesia, in particular the use of propofol, was associated with a consistent moderation of the postoperative systemic inflammatory response (CRP not IL-6) in moderate to major severity of surgery. Therefore, it may be that intravenous anesthetic regimens in moderate to major severity of surgery have the potential to reduce the postoperative systemic inflammatory response. Indeed, it is of interest that there is some experimental evidence that propofol, a GABA receptor agonist, is less immunosuppressive compared with inhalational anesthesia. For example, it has been reported that propofol preserves NK function, inhibits COX-2 and the production of PGE-2 and pro-inflammatory cytokines such as IL-1, TNF-α and IL-6 [10], [77], [79], [80], [81]. In contrast, inhalational anesthetics such as sevoflurane and isoflurane may increase the pro inflammatory cytokines especially IL-6, inhibit neutrophil function and reduce lymphocyte proliferation [12], [82].
The administration of dexmedetomedine, an alpha 2 receptor agonist, an adjunct to general anesthesia leads to a significant decrease in plasma concentration of IL-6 but without any significant effect on CRP level [83]. In addition to the anesthetic effect of dexmedetomedine, it also exhibits some clinical benefits among them the anti-inflammatory, sedative, analgesic and anxiolytic effects [84].
Ketamine, an NMDA receptor antagonist, is thought to have both anti-inflammatory and sedative effects with a suppressive effect of NK cell function and pain transmission [80], [81]. It produces an analgesic effect in low or small sub-anesthetic dose. However, the results of meta-analysis reported that the use of ketamine at analgesic doses did not show any significant reduction in IL-6 concentration but it shows a significant reduction in CRP concentrations.
The efficacy of combining epidural with general anesthesia as compared with general anesthetic alone has been reported by multiple clinical studies to maintain postoperative immune function and provide better pain control during perioperative period [85]. Epidural anesthesia can be associated with negative effects such as hypotension resulting in excessive fluid administration and local complications such as insertion site / epidural infection. However, the results of the present meta-analysis suggest that the use of epidural with general versus general anesthesia alone has no significant impact on either postoperative IL-6 or CRP.
Some other drugs, used before or after induction of anesthesia as adjuvant therapy, appear to have a significant effect in reducing the mean peak of IL-6 and CRP. Among these are anti-inflammatory drugs including corticosteroids, NSAIDs, and selective COX-2 inhibitors, and other agents not typically known for their anti-inflammatory effects including nalbuphine, oxycodone, epidural clonidine, pentoxifylline and esmolol. Further work is required to define the role, if any, of these agents in the perioperative period.
The main limitation of this review is the small number of sample size in each arm. In addition, the majority of the studies reported low quality of evidence along with high level of heterogeneity and this may affect the overall summary estimate of the meta-analysis. The severity of surgical injury was variable from mild to moderate to severe, and a variety of different surgical procedures and specialities were included, and this may have had an effect on the efficacy of the anesthetic agent examined. Patients at higher risk of postoperative complications and patients undergoing higher severity of surgery may be more likely to receive additional anesthetic techniques such as epidurals resulting in the potential for unmeasured confounding by indication.
In general, when carrying out such systematic review and meta-analysis the sample size is important since it determines the precision of the estimates and the power of the study to determine whether or not there is a real effect. Therefore, where there were small numbers of studies with few observations then the conclusions that can be made from such a systematic review and meta-analysis is limited.
Further studies are required controlling for the anesthetic agent(s) administered, the severity of surgery and the postoperative biomarker used.
In conclusion, this systematic review and meta-analysis reported the current randomized controlled trials evidence of the association between general anesthesia, regional anesthesia or both combined to moderate the magnitude of the postoperative SIR as well as infective complications. There was a suggestion that TIVA using propofol or ketamine at analgesic doses is associated with a reduction in the magnitude of the postoperative systemic inflammatory response as measured by CRP although not IL6. However, there were no other observed differences in anesthetic techniques which favored a reduction in the magnitude of the postoperative SIR and infective complications.
Further, adequately powered studies in patients undergoing moderate / major severity of surgery using postoperative CRP measurements are required to clarify the effect of perioperative anesthesia on the postoperative SIR and infective complications. Such work is of clinical importance due to the associations between postoperative systemic inflammation and postoperative morbidity.
AUTHOR CONTRIBUTION
The authors A.A., C.R., and D.M. contributed for study concepts and design. The authors A.A., S.M., and D.M. contributed for data acquisition, analysis and interpretation. All authors contributed for the manuscript preparation, editing and review.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
FUNDING SOURCES
Funding of this work was received from Ministry of Health, Riyadh, Kingdom of Saudi Arabia.
References
- 1.Desborough J.P. The stress response to trauma and surgery. BJA: British Journal of Anaesthesia. 2000;85(1):109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Finnerty, C.C., Mabvuure, N.T., Ali, A., Kozar, R.A., Herndon, D.N. The surgically induced stress response. JPEN Journal of parenteral and enteral nutrition. 2013; 37 (5). [DOI] [PMC free article] [PubMed]
- 3.Dabrowska A., Slotwinski R. The immune response to surgery and infection. Central-European Journal of Immunology : bimonthly of the Polish Society for Immunology coedited by eleven other central european immunological societies. 2014;39:532–537. doi: 10.5114/ceji.2014.47741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watt D.G., Ramanathan M.L., McSorley S.T., Walley K., Park J.H., Horgan P.G. Clinicopathological determinants of an elevated systemic inflammatory response following elective potentially curative resection for colorectal Cancer. Ann Surg Oncol Annals of Surgical Oncology. 2017;24(9):2588–2594. doi: 10.1245/s10434-017-5987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannoudis P.V., Dinopoulos H., Chalidis B., Hall G.M. Surgical stress response. JINJ Injury. 2006;37:S3–S9. doi: 10.1016/S0020-1383(07)70005-0. [DOI] [PubMed] [Google Scholar]
- 6.McSorley S.T., Horgan P.G., McMillan D.C. The impact of preoperative corticosteroids on the systemic inflammatory response and postoperative complications following surgery for gastrointestinal cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;101:139–150. doi: 10.1016/j.critrevonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Watt D.G., Horgan P.G., McMillan D.C. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery Surgery. 2015;157(2):362–380. doi: 10.1016/j.surg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 8.McSorley S.T., Ramanathan M.L., Horgan P.G., McMillan D.C. Postoperative C-reactive protein measurement predicts the severity of complications following surgery for colorectal cancer. Int J Colorectal Dis International Journal of Colorectal Disease. 2015;30(7):913–917. doi: 10.1007/s00384-015-2229-3. [DOI] [PubMed] [Google Scholar]
- 9.McDermott F.D., Heeney A., Kelly M.E., Steele R.J., Carlson G.L., Winter D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102(5):462–479. doi: 10.1002/bjs.9697. [DOI] [PubMed] [Google Scholar]
- 10.Cassinello F., Prieto I., del Olmo M., Rivas S., Strichartz G.R. Cancer surgery: how may anesthesia influence outcome? J Clin Anesth. 2015;27(3):262–272. doi: 10.1016/j.jclinane.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Perry N.J.S., Buggy D., Ma D. Can anesthesia influence Cancer outcomes after surgery?Can anesthesia influence Cancer outcomes after surgery?Can anesthesia influence Cancer outcomes after surgery? JAMA Surg. 2019;154(4):279–280. doi: 10.1001/jamasurg.2018.4619. [DOI] [PubMed] [Google Scholar]
- 12.Ke J.J., Zhan J., Feng X.B., Wu Y., Rao Y., Wang Y.L. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care. 2008;36(1):74–78. doi: 10.1177/0310057X0803600113. [DOI] [PubMed] [Google Scholar]
- 13.Hozo, S.P., Djulbegovic, B., & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology. 2005; 5 (13). [DOI] [PMC free article] [PubMed]
- 14.Wan, X., Wang, W., Liu, J., & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014; 14 (135). [DOI] [PMC free article] [PubMed]
- 15.Olivo S.A., Macedo L.G., Gadotti I.C., Fuentes J., Stanton T., Magee D.J. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 16.Chung J.H., Kang D.H., Jo J.K., Lee S.W. Assessing the quality of randomized controlled trials published in the journal of Korean medical science from 1986 to 2011. J Korean Med Sci. 2012;27(9):973–980. doi: 10.3346/jkms.2012.27.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. IJSU International Journal of Surgery. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Helmy S.A.K., Al-Attiyah R.J. The effect of halothane and isoflurane on plasma cytokine levels. Anaesthesia Anaesthesia. 2000;55(9):904–910. doi: 10.1046/j.1365-2044.2000.01472-2.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneemilch C.E., Ittenson A., Ansorge S., Hachenberg T., Bank, U Effect of 2 anesthetic techniques on the postoperative proinflammatory and anti-inflammatory cytokine response and cellular immune function to minor surgery. J Clin Anesth. 2005;17(7):517–527. doi: 10.1016/j.jclinane.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Kvarnstrom A.L., Sarbinowski R.T., Bengtson J.P., Jacobsson L.M., Bengtsson A.L. Complement activation and interleukin response in major abdominal surgery. Scand J Immunol. 2012;75(5):510–516. doi: 10.1111/j.1365-3083.2012.02672.x. [DOI] [PubMed] [Google Scholar]
- 22.Mazoti M.A., Braz M.G., de Assis Golim M., Braz L.G., Dias N.H., Salvadori D.M. Comparison of inflammatory cytokine profiles in plasma of patients undergoing otorhinological surgery with propofol or isoflurane anesthesia. Inflamm Res. 2013;62(10):879–885. doi: 10.1007/s00011-013-0643-y. [DOI] [PubMed] [Google Scholar]
- 23.Margarit S.C., Vasian H.N., Balla E., Vesa S., Ionescu D.C. The influence of total intravenous anaesthesia and isoflurane anaesthesia on plasma interleukin-6 and interleukin-10 concentrations after colorectal surgery for cancer a randomised controlled trial. Eur J Anaesthesiol. 2014;31(12):678–684. doi: 10.1097/EJA.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 24.Sayed S., Idriss N.K., Sayyed H.G., Ashry A.A., Rafatt D.M., Mohamed A.O. Effects of propofol and isoflurane on haemodynamics and the inflammatory response in cardiopulmonary bypass surgery. Br J Biomed Sci. 2015;72(3):93–101. doi: 10.1080/09674845.2015.11666803. [DOI] [PubMed] [Google Scholar]
- 25.Yoo Y.C., Shim J.K., Song Y., Yang S.Y., Kwak Y.L. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014;86(2):414–422. doi: 10.1038/ki.2013.532. [DOI] [PubMed] [Google Scholar]
- 26.Nakanuno R., Yasuda T., Hamada H., Yoshikawa H., Nakamura R., Saeki N. Propofol for anesthesia and postoperative sedation resulted in fewer inflammatory responses than sevoflurane anesthesia and midazolam sedation after Thoracoabdominal Esophagectomy. Hiroshima J Med Sci. 2015;64(3):31–37. [PubMed] [Google Scholar]
- 27.Li R., Fan L., Ma F., Cao Y., Gao J., Liu H. Effect of etomidate on the oxidative stress response and levels of inflammatory factors from ischemia-reperfusion injury after tibial fracture surgery. Exp Ther Med. 2017;13(3):971–975. doi: 10.3892/etm.2017.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang W., Han C., Jiang W., Ding W., Gu D., Tan Y. A comparion of the effects of dexmedetomedine and propofol on stress response in patients undergoing open esophagectomy under total intravenous anesthesia: a randomized controlled trail. Int J Clin Exp Med International Journal of Clinical and Experimental Medicine. 2016;9(3):6545–6550. [Google Scholar]
- 29.Bulow N.M., Colpo E., Pereira R.P., Correa E.F., Waczuk E.P., Duarte M.F. Dexmedetomidine decreases the inflammatory response to myocardial surgery under mini-cardiopulmonary bypass. Braz J Med Biol Res. 2016;49(4) doi: 10.1590/1414-431X20154646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo C.P., Jao S.W., Chen K.M., Wong C.S., Yeh C.C., Sheen M.J. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth. 2006;97(5):640–646. doi: 10.1093/bja/ael217. [DOI] [PubMed] [Google Scholar]
- 31.Day A.R., Smith R.V.P., Scott M.J.P., Fawcett W.J., Rockall T.A. Randomized clinical trial investigating the stress response from two different methods of analgesia after laparoscopic colorectal surgery. Br J Surg. 2015;102(12):1473–1479. doi: 10.1002/bjs.9936. [DOI] [PubMed] [Google Scholar]
- 32.Zhan Y., Chen G., Huang J., Hou B., Liu W., Chen S. Effect of intercostal nerve block combined with general anesthesia on the stress response in patients undergoing minimally invasive mitral valve surgery. Exp Ther Med. 2017;14(4):3259–3264. doi: 10.3892/etm.2017.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heijmans J., Fransen E., Buurman W., Maessen J., Roekaerts P. Comparison of the modulatory effects of four different fast-track anesthetic techniques on the inflammatory response to cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2007;21(4):512–518. doi: 10.1053/j.jvca.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Ozcan S., Ozer A.B., Yasar M.A., Erhan O.L. Effects of combined general anesthesia and thoracic epidural analgesia on cytokine response in patients undergoing laparoscopic cholecystectomy. Niger J Clin Pract. 2016;19(4):436–442. doi: 10.4103/1119-3077.183308. [DOI] [PubMed] [Google Scholar]
- 35.Brix-Christensen V., Tonnesen E., Sorensen I.J., Bilfinger T.V., Sanchez R.G., Stefano G.B. Effects of anaesthesia based on high versus low doses of opioids on the cytokine and acute-phase protein responses in patients undergoing cardiac surgery. Acta Anaesthesiol Scand. 1998;42(1):63–70. doi: 10.1111/j.1399-6576.1998.tb05082.x. [DOI] [PubMed] [Google Scholar]
- 36.Yokoyama M., Itano Y., Katayama H., Morimatsu H., Takeda Y., Takahashi T. The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy. Anesth Analg. 2005;101(5):1521–1527. doi: 10.1213/01.ANE.0000184287.15086.1E. [DOI] [PubMed] [Google Scholar]
- 37.Moselli N.M., Baricocchi E., Ribero D., Sottile A., Suita L., Debernardi F. Intraoperative epidural analgesia prevents the early proinflammatory response to surgical trauma. Results from a prospective randomized clinical trial of intraoperative epidural versus general analgesia. Ann Surg Oncol. 2011;18(10):2722–2731. doi: 10.1245/s10434-011-1700-9. [DOI] [PubMed] [Google Scholar]
- 38.Hadimioglu N., Ulugol H., Akbas H., Coskunfirat N., Ertug Z., Dinckan A. Combination of epidural anesthesia and general anesthesia attenuates stress response to renal transplantation surgery. Transplant Proc. 2012;44(10):2949–2954. doi: 10.1016/j.transproceed.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Gasiunaite, D., Sipylaite, J., Kontrimaviciute, E., Poskus, E. Impact of anesthesia method on cortisol and interleukin-6 concentration changes during and after laparoscopic colorectal surgery. Acta medica Lituanica. 2012; 19 (3).
- 40.Ezhevskaya A.A., Mlyavykh S.G., Anderson D.G. Effects of continuous epidural anesthesia and postoperative epidural analgesia on pain management and stress response in patients undergoing major spinal surgery. Spine (Phila Pa 1976) 2013;38(15):1324–1330. doi: 10.1097/BRS.0b013e318290ff26. [DOI] [PubMed] [Google Scholar]
- 41.Fant F., Tina E., Sandblom D., Andersson S.O., Magnuson A., Hultgren-Hornkvist E. Thoracic epidural analgesia inhibits the neuro-hormonal but not the acute inflammatory stress response after radical retropubic prostatectomy. Br J Anaesth. 2013;110(5):747–757. doi: 10.1093/bja/aes491. [DOI] [PubMed] [Google Scholar]
- 42.Fares, K.M., Mohamed, S.A., Hamza, H.M., Sayed, D.M., Hetta, D.F. Effect of thoracic epidural analgesia on pro-inflammatory cytokines in patients subjected to protective lung ventilation during Ivor Lewis esophagectomy. Pain physician. 2014; 17 (4). [PubMed]
- 43.Xu Y.J., Chen W.K., Zhu Y., Wang S.L., Miao C.H. Effect of thoracic epidural anaesthesia on serum vascular endothelial growth factor C and cytokines in patients undergoing anaesthesia and surgery for colon cancer. Br J Anaesth. 2014;113(Suppl. 1):i49–i55. doi: 10.1093/bja/aeu148. [DOI] [PubMed] [Google Scholar]
- 44.Gu C.Y., Zhang J., Qian Y.N., Tang Q.F. Effects of epidural anesthesia and postoperative epidural analgesia on immune function in esophageal carcinoma patients undergoing thoracic surgery. Mol Clin Oncol. 2015;3(1):190–196. doi: 10.3892/mco.2014.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atia, A., Abdel-Rahman, K. Combined Thoracic Epidural with General Anesthesia vs. General Anesthesia Alone for Major Abdominal Surgery: Anesthetic Requirements and Stress Response. J Anesth Clin Res. 2016; 7 (616): 2.
- 46.Salem, A.E., Hagras, M.M., Mohamed, A., Hassaan, M. Thoracic Epidural Analgesia Lessens Inflammatory Response to Coronary Artery Bypass Grafting Surgery. Journal of Anesthesia & Intensive Care Medicine. 2017; 2 (4).
- 47.Palomero Rodriguez M.A., Suarez Gonzalo L., Villar Alvarez F., Varela Crespo C. Moreno Gomez Limon, I., Criado Jimenez, A. thoracic epidural anesthesia decreases C-reactive protein levels in patients undergoing elective coronary artery bypass graft surgery with cardiopulmonary bypass. Minerva Anestesiol. 2008;74(11):619–626. [PubMed] [Google Scholar]
- 48.Papadima A., Boutsikou M., Lagoudianakis E.E., Kataki A., Konstadoulakis M., Georgiou L. Lymphocyte apoptosis after major abdominal surgery is not influenced by anesthetic technique: a comparative study of general anesthesia versus combined general and epidural analgesia. J Clin Anesth. 2009;21(6):414–421. doi: 10.1016/j.jclinane.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Chen W.K., Ren L., Wei Y., Zhu D.X., Miao C.H., Xu J.M. General anesthesia combined with epidural anesthesia ameliorates the effect of fast-track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. Int J Colorectal Dis. 2015;30(4):475–481. doi: 10.1007/s00384-014-2098-1. [DOI] [PubMed] [Google Scholar]
- 50.Sidiropoulou I., Tsaousi G.G., Pourzitaki C., Logotheti H., Tsantilas D., Vasilakos D.G. Impact of anesthetic technique on the stress response elicited by laparoscopic cholecystectomy: a randomized trial. J Anesth. 2016;30(3):522–525. doi: 10.1007/s00540-016-2148-7. [DOI] [PubMed] [Google Scholar]
- 51.Buyukkocak U., Daphan C., Caglayan O., Aydinuraz K., Kaya T., Saygun O. Effects of different anesthetic techniques on serum leptin, C-reactive protein, and cortisol concentrations in anorectal surgery. Croat Med J. 2006;47(6):862–868. [PMC free article] [PubMed] [Google Scholar]
- 52.Kahveci K., Ornek D., Doger C., Aydin G.B., Aksoy M., Emre C. The effect of anesthesia type on stress hormone response: comparison of general versus epidural anesthesia. Niger J Clin Pract. 2014;17(4):523–527. doi: 10.4103/1119-3077.134058. [DOI] [PubMed] [Google Scholar]
- 53.Chloropoulou P., Iatrou C., Vogiatzaki T., Kotsianidis I., Trypsianis G., Tsigalou C. Epidural anesthesia followed by epidural analgesia produces less inflammatory response than spinal anesthesia followed by intravenous morphine analgesia in patients with total knee arthroplasty. Med Sci Monit. 2013;19:73–80. doi: 10.12659/MSM.883749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Z., Jiang H., Zhao H., Liu Z., Dong Z., Zhu B. Efficacy of parecoxib on the level of Il-6, CRP, and postoperative pain relief after percutaneous nephrolithotomy. Int J Clin Exp Med International Journal of Clinical and Experimental Medicine. 2016;9(10):19454–19460. [Google Scholar]
- 55.Feng Y., Ju H., Yang B., An H. Effects of a selective cyclooxygenase-2 inhibitor on postoperative inflammatory reaction and pain after total knee replacement. J Pain. 2008;9(1):45–52. doi: 10.1016/j.jpain.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Liu T., Tang Y.-B., Jia X.-D., Wu W.-H., Yu D.-W., Zhou M.-T. Effect of oxycodone hydrochloride injection preemptive analgesia on serum inflammatory factors, neurotransmitter index and immune function in patients with laparoscopic cholecystectomy. Journal of Hainan Medical University. 2017;23(17):58–61. [Google Scholar]
- 57.Zhang Y., Jiang Q., Li T. Nalbuphine analgesic and anti-inflammatory effects on patients undergoing thoracoscopic lobectomy during the perioperative period. Exp Ther Med. 2017;14(4):3117–3121. doi: 10.3892/etm.2017.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roytblat L., Talmor D., Rachinsky M., Greemberg L., Pekar A., Appelbaum A. Ketamine attenuates the Interleukin-6 response after cardiopulmonary bypass. Anesthesia & Analgesia Anesthesia & Analgesia. 1998;87(2):266–271. doi: 10.1097/00000539-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Cho J.E., Shim J.K., Choi Y.S., Kim D.H., Hong S.W., Kwak Y.L. Effect of low-dose ketamine on inflammatory response in off-pump coronary artery bypass graft surgery. Br J Anaesth. 2009;102(1):23–28. doi: 10.1093/bja/aen325. [DOI] [PubMed] [Google Scholar]
- 60.Luggya T.S., Roche T., Ssemogerere L., Kintu A., Kasumba J.M., Kwizera A. Effect of low-dose ketamine on post-operative serum IL-6 production among elective surgical patients: a randomized clinical trial. Afr Health Sci. 2017;17(2):500–507. doi: 10.4314/ahs.v17i2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welters I.D., Feurer M.K., Preiss V., Muller M., Scholz S., Kwapisz M. Continuous S-(+)-ketamine administration during elective coronary artery bypass graft surgery attenuates pro-inflammatory cytokine response during and after cardiopulmonary bypass. Br J Anaesth. 2011;106(2):172–179. doi: 10.1093/bja/aeq341. [DOI] [PubMed] [Google Scholar]
- 62.Lu, C.H., Chao, P.C., Borel, C.O., Yang, C.P., Yeh, C.C., Wong, C.S., et al. Preincisional intravenous pentoxifylline attenuating perioperative cytokine response, reducing morphine consumption, and improving recovery of bowel function in patients undergoing colorectal cancer surgery. Anesthesia and analgesia. 2004; 99 (5): 1465–71; table of contents. [DOI] [PubMed]
- 63.Kim Y., Hwang W., Cho M.L., Her Y.M., Ahn S., Lee J. The effects of intraoperative esmolol administration on perioperative inflammatory responses in patients undergoing laparoscopic gastrectomy: a dose-response study. Surg Innov. 2015;22(2):177–182. doi: 10.1177/1553350614532534. [DOI] [PubMed] [Google Scholar]
- 64.Nakazawa K., Narumi Y., Ishikawa S., Yokoyama K., Nishikage T., Nagai K. Effect of prostaglandin E1 on inflammatory responses and gas exchange in patients undergoing surgery for oesophageal cancer. Br J Anaesth. 2004;93(2):199–203. doi: 10.1093/bja/aeh184. [DOI] [PubMed] [Google Scholar]
- 65.Rahman A., Akbulut H., Bayar M.K., Ozden M., Burma O., Uysal A. The effects of intra-operative amiodarone loading on the systemic inflammatory response syndrome induced by cardiopulmonary bypass. Anadolu Kardiyol Derg. 2009;9(4):318–324. [PubMed] [Google Scholar]
- 66.Sedighinejad A., Imantalab V., Mirmansouri A., Mohammadzadeh Jouryabi A., Kanani G., Nassiri Sheikhani N. Effects of low-dose selenium on the inflammatory response in coronary artery bypass graft surgery: a clinical trial. Iran Red Crescent Med J. 2016;18(8) doi: 10.5812/ircmj.37918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ueki M., Kawasaki T., Habe K., Hamada K., Kawasaki C., Sata T. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69(7):693–700. doi: 10.1111/anae.12636. [DOI] [PubMed] [Google Scholar]
- 68.Wu, C.T., Jao, S.W., Borel, C.O., Yeh, C.C., Li, C.Y., Lu, C.H., et al. The effect of epidural clonidine on perioperative cytokine response, postoperative pain, and bowel function in patients undergoing colorectal surgery. Anesthesia and analgesia. 2004; 99 (2): 502–9, table of contents. [DOI] [PubMed]
- 69.Masaki E., Saito H., Shoji K., Matsushima M. Postoperative analgesic effect of epidural neostigmine and plasma cortisol and IL-6 responses. J Clin Anesth. 2004;16(7):488–492. doi: 10.1016/j.jclinane.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Yeager M.P., Glass D.D., Neff R.K., Brinck-Johnsen T. Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology. 1987;66(6):729–736. doi: 10.1097/00000542-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Jayr, C., Thomas, H., Rey, A., Farhat, F., Lasser, P., Bourgain, J.L. Postoperative pulmonary complications. Epidural analgesia using bupivacaine and opioids versus parenteral opioids. Anesthesiology. 1993; 78 (4): 666–76; discussion 22A. [DOI] [PubMed]
- 72.Scott N.B., Turfrey D.J., Ray D.A.A., Nzewi O., Sutcliffe N.P., Lal A.B. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesthesia & Analgesia Anesthesia & Analgesia. 2001;93(3):528–535. doi: 10.1097/00000539-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Svircevic V., Nierich A.P., Moons K.G., Diephuis J.C., Ennema J.J., Brandon Bravo Bruinsma G.J. Thoracic epidural anesthesia for cardiac surgery: a randomized trial. Anesthesiology. 2011;114(2):262–270. doi: 10.1097/ALN.0b013e318201d2de. [DOI] [PubMed] [Google Scholar]
- 74.Tyagi A., Bansal A., Das S., Sethi A.K., Kakkar A. Effect of thoracic epidural block on infection-induced inflammatory response: a randomized controlled trial. J Crit Care. 2017;38:6–12. doi: 10.1016/j.jcrc.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Lee J.J., Kim G.H., Kim J.A., Yang M., Ahn H.J., Sim W.S. Comparison of pulmonary morbidity using sevoflurane or propofol-remifentanil anesthesia in an Ivor Lewis operation. J Cardiothorac Vasc Anesth. 2012;26(5):857–862. doi: 10.1053/j.jvca.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 76.Liu S., Gu X., Zhu L., Wu G., Zhou H., Song Y. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Medicine. 2016;95(49) doi: 10.1097/MD.0000000000005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Markovic-Bozic J., Karpe B., Potocnik I., Jerin A., Vranic A. Novak-Jankovic. V Effect of propofol and sevoflurane on the inflammatory response of patients undergoing craniotomyBMC Anesthesiol. 2016;16:18. doi: 10.1186/s12871-016-0182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dang Y., Shi X., Xu W., Zuo M. The effect of anesthesia on the immune system in colorectal Cancer patients. Can J Gastroenterol Hepatol. 2018;2018 doi: 10.1155/2018/7940603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.González-Correa J.A., Cruz-Andreotti E., Arrebola M.M., López-Villodres J.A., Jódar M., De La Cruz J.P. Effects of propofol on the leukocyte nitric oxide pathway: in vitro and ex vivo studies in surgical patients. Naunyn Schmiedebergs Arch Pharmacol. 2008;376(5):331–339. doi: 10.1007/s00210-007-0220-4. [DOI] [PubMed] [Google Scholar]
- 80.Piegeler T., Beck-Schimmer B. Anesthesia and colorectal cancer–the perioperative period as a window of opportunity? Eur J Surg Oncol. 2016;42(9):1286–1295. doi: 10.1016/j.ejso.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 81.Cruz, F.F., Rocco, P.R., Pelosi, P. Anti-inflammatory properties of anesthetic agents. Critical care (London, England). 2017; 21 (1): 67. [DOI] [PMC free article] [PubMed]
- 82.Lee Y.M., Song B.C., Yeum K.J. Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int. 2015;2015 doi: 10.1155/2015/242709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li B., Li Y., Tian S., Wang H., Wu H., Zhang A. Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: a meta-analysis. Sci Rep. 2015;5:12342. doi: 10.1038/srep12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li, J., Yan, H.-T., Che, J.-X., Bai, S.-R., Qiu, Q.-M., Ren, L., et al. Effects of Neurolytic Celiac Plexus Block on Liver Regeneration in Rats with Partial Hepatectomy. PloS one. 2013; 8 (9). [DOI] [PMC free article] [PubMed]
- 85.Song P., Dong T., Zhang J., Li J., Lu W. Effects of different methods of anesthesia and analgesia on immune function and serum tumor marker levels in critically ill patients. Exp Ther Med. 2017;14(3):2206–2210. doi: 10.3892/etm.2017.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]