Abstract
Pancreatic cancer is a lethal disease in a large part due to the systemic nature at the time of diagnosis. In those patients who undergo a potentially curative resection of pancreatic cancer, the overwhelming majority will have systemic relapse. Circulating tumor cells are an important mediator of the development of metastases. Circulating tumor cells have been identified in patients with clinically localized resectable pancreatic cancer and exist as several phenotypes. Mesenchymal and stem cell–like phenotypes of circulating tumor cells predict early recurrence and worse survival. This review focuses on the current understanding of circulating tumor cells in pancreatic cancer and how this information can be used in developing more effective therapy in the future.
Introduction
Pancreatic cancer, known as pancreatic ductal adenocarcinoma (PDAC), is a highly lethal disease. Unlike most other solid tumor types, the incidence of and mortality from pancreatic cancer are on the rise, and by 2030, it is expected to be the second deadliest cancer in the United States [1]. The 5-year survival for a patient with this diagnosis is only 8% [2], and the median survival with optimal chemotherapy regimens is reported to be in the 8–11-month range in 2 randomized clinical trials [3,4]. The reasons for the lethality of pancreatic cancer are multifactorial but primarily relate to the systemic nature of the disease. At the time of diagnosis, 60%–80% of patients are found to have metastatic disease (stage IV) and are thus unlikely to achieve long-term survival.
The most common sites of distant spread include liver, lung, and extraregional nodal metastases and peritoneal carcinomatosis. The only chance for a cure for pancreatic cancer is through an oncologic resection, after which additional survival benefit is achieved with multimodality systemic therapy [5,6]. Unfortunately, only 15% of patients present with localized resectable disease at the time of diagnosis. Even among this relatively favorable group who undergoes optimal therapy of surgical resection and chemotherapy, 76% will have a systemic relapse (Fig 1) [7]. Taken together, these observations demonstrate the highly systemic nature of pancreatic cancer.
Fig 1.
Patterns of progression following resection: This figure depicts the common sites of progression following a potentially curative resection of localized disease. The most common pattern of treatment failure is systemic, accounting for 76% of relapses in this large cohort of 531 patients. Specifically, liver alone or liver and combined systemic locations account for the majority of metastatic relapse.
In this context, it is clear that a profound advancement of survival for PDAC will only come from the development of 2 clinical areas: early detection, such that cancers can be identified and resected in the preinvasive state, and improved systemic control of micrometastatic disease in those patients diagnosed with localized cancer. Recently, with respect to early detection, a multianalyte blood test called CancerSEEK, which detects circulating tumor DNA and protein markers, has shown promising results for the detection of PDAC and 7 other commonly occurring cancers [8]. The potential integration of this approach into standard clinical practice will be determined with the completion of currently ongoing prospective validation studies in a large cohort of individuals.
In this review, we will discuss the tumor biology of pancreatic cancer as it relates to systemic spread, summarize select literature that implicates a role for circulating tumor cells (CTCs) and disseminated tumor cells (DTCs) in establishing metastases, and outline a concept of how this information could be used to improve survival in those patients undergoing a potentially curative resection.
The Biology of Pancreatic Cancer
Pancreatic cancer develops from 2 types of precursor lesions: microscopic pancreatic intraepithelial neoplasms (PanINs) and macroscopic mucin-producing cystic neoplasms [[9], [10], [11]], the latter of which include intraductal papillary mucinous neoplasms (IPMNs) and the less common mucinous cystic neoplasms. It is postulated that carcinogenesis occurs in a stepwise manner through a series of progressive dysplastic changes that culminate in high-grade dysplasia (grade 3 PanIN or IPMN with high-grade dysplasia, formerly called carcinoma in situ) and ultimately invasive cancer [10,[12], [13], [14]]. The predominant pattern to progression is through PanIN, for which no available imaging test can accurately detect and discriminate high-grade lesions [15]. The sequence of progression is thought to be analogous to the widely accepted adenoma to carcinoma progression in colorectal cancer. However, the process of PDAC tumorigenesis remains poorly understood; in fact, some evidence suggests that this may not be as linear a process as has been proposed [16,17]. It should be noted that most low-grade lesions will never progress to high-grade neoplasms or invasive cancer.
Pancreatic cancer is a relatively acellular tumor in which the cancer cells typically account for less than 50% of all cells within the tumor. The remaining cells consist of a dense stroma that creates a desmoplastic matrix of tissue surrounding the cancer cells with collagen, activated fibroblasts, pancreatic stellate cells, and immune cell infiltration [15]. This matrix has been shown to support tumor aggressiveness and creates a barrier to chemotherapy and effective immune cell function [18]. This barrier is both physical and biological. For example, the matrix creates a hypoxic environment favorable to cancer cell metabolism and promotes an epithelial-to-mesenchymal transition (EMT) of cancer cells [19]. This is significant, as EMT is thought to be a necessary step in the invasive capacity of cells [[20], [21], [22], [23], [24]]. The dense stromal reaction can be seen early in PDAC tumorigenesis and may play a role in early tumor dissemination [11]. In a screening study of patients who were at high risk of developing pancreatic cancer and underwent resection, evidence of stromal changes could be seen surrounding PanINs [25].
Direct evidence of early tumor dissemination can be seen in the pathological assessment of surgical specimens obtained from the resection of patients with localized disease. In the Johns Hopkins series of patients with resected PDAC, 78% exhibit regional lymph node metastases, 85% have perineural sheath invasion, and 64% have microvascular invasion (unpublished results). The ability of these cells to invade the microvasculature is nicely demonstrated in this clear mount photomicrograph published by Hruban (Fig 2) [26,27].
Fig 2.
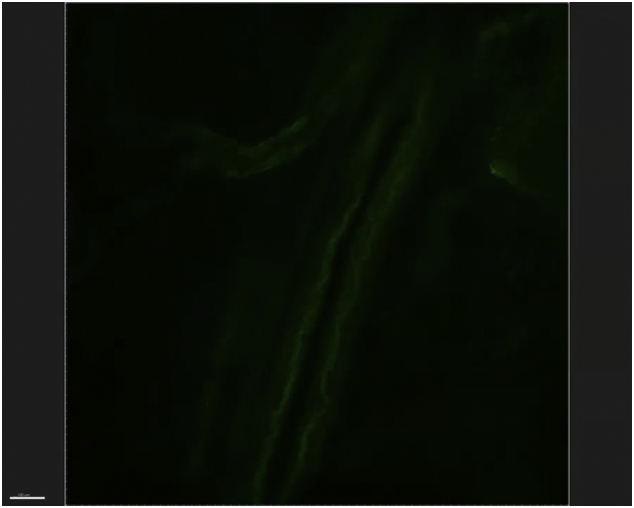
Invasion of microvasculature and pancreatic cancer: This figure depicts a “clear mount,” in which the green-labeled cancer cells can be seen invading a small vessel. Created by Dr Ralph Hruban at Johns Hopkins, this figure illustrates the propensity of pancreatic cancer toward systemic spread. Printed with permission.
(Fig 2—please link to video).
Interestingly, it has been shown that a high proportion of patients with preinvasive IPMN exhibit circulating epithelial cells (CECs). In this study, circulating epithelial cells were identified in the blood of IPMN patients in the absence of malignancy, and a statistically significant correlation between Pdx-1–positive CECs and high-grade dysplasia at the time of surgery [28] was found. Moreover, in a separate study, PanIN-like lesions were demonstrated in the liver of a mouse model of pancreatic cancer [23].
General Mechanism of Metastases
Research over the past decade has provided insight into the process of cancer metastasis. One prevailing theory that is supported with ever-increasing evidence suggests that cancer dissemination occurs through shedding of cancer cells into the circulation. These cells, called CTCs, are presumed to be the source of metastatic disease [22]. The presence of CTCs has been demonstrated in a wide range of epithelial malignancies, including prostate, ovarian, breast, colorectal, gastric, bladder, renal, lung, and pancreatic cancers [29]. Notably, small early-stage tumors may shed the same number of cells as the later-stage tumors, but the latter may provide humoral factors such as adhesion molecules, chemokines, and proteinases that promote the further growth of CTCs [30,31].
The process of blood-borne metastases is complex and involves phenotypic changes in cancer cells and the support of noncancer cells. For a cell to transition from the primary tumor to seed a metastatic deposit, it must gain the ability to invade into the circulation, avoid anoikis, survive the physically and biologically harsh environment of the circulation, gain access to host tissue, and proliferate within that tissue (Fig 3) [32]. The current model of blood-borne metastasis accounts for the relatively inefficient process of establishing metastases via CTC. In this model, numerous cells extravasate into circulation from the tumor, and most will be dead within minutes. An important step in this process is the EMT [[20], [21], [22], [23], [24]],a reversible phenotypic change whereby a polarized epithelial-like cancer cell loses its polarity and ability to adhere to adjacent cells, taking on the properties of a mesenchymal cell. This transition is marked by the loss of surface expression of E-cadherin, present on epithelial cells, and the expression of N-cadherin and vimentin, characteristic of mesenchymal cells [21].
Fig 3.
Steps of metastasis: This figure demonstrates the basic steps proposed to occur in cancer metastasis. A small percentage of cancer cells within the primary tumor undergo a phenotypic change to become more mesenchymal-like. This change is called an EMT and is associated with cells developing the ability to grow in the absence of contact with the basement membrane, migrate into the circulatory system, and survive the harsh environment of circulation. These cells, now called CTCs, leave circulation through a poorly understood process and take up residence in the stroma of distant organs. Once there, these cells are called DTCs, which evade the immune system and remain quiescent. DTCs can become activated and form distant tumors clinically, called metastatic disease. Image from Gkountala et al. Biol Direct. 2016. Creative Commons License: http://creativecommons.org/licenses/by/4.0/.
Cancer cells that have undergone EMT are typically a small fraction of the total cells within a tumor and have the ability to migrate and invade the basement membrane, withstand hypoxic conditions, and resist many cytotoxic agents. The mechanism through which they survive circulation and extravasate into the extracellular matrix is poorly understood. Cancers liberate thousands of these cells daily [[33], [34], [35]], but only a few manage to implant in the distant organs and proliferate, giving rise to metastasis [36]. At least a small subset of these cancer cells that partially or completely undergo EMT possesses stem cell–like properties, such as the ability of prolonged quiescence, self-renewal, and generation of a tumor with characteristics similar to the parent tumor [[20], [21], [22]].
That few cells are required for development of metastasis was demonstrated in an animal model in which cancer cells from melanomas were labeled with 125iodine-iodo-deoxyuridine and injected in mice. The vast majority of cells died, and only 0.1% survived for more than 24 hours; thus, the metastatic foci that eventually formed arose from only 0.01% of the original cell population [37]. Similarly, Baccelli et al isolated CTCs from the blood samples of more than a hundred patients with metastatic breast cancer and transplanted the cells in the medullary cavity of immunocompromised mice. Metastases developed only in mice in which more than 1,100 CTCs were transplanted, leading to the conclusion that cells with the potential to create metastasis (metastasis-initiating cells) may be limited in the broad CTC population. Importantly, the metastatic cells isolated in mice were ER +, PR +, and HER2 −, mirroring the cells in patient primary tumors. By examining the surface markers, the authors identified the metastasis-initiating cell population as EPCAM +, CD44 +, CD47 +, and MET +/− [33]. Other studies that demonstrate the ability of CTCs to establish metastases, albeit at low efficiency, include the finding that CTCs from patients with small cell lung cancer are capable of establishing tumors when transferred into immunocompromised mice [38].
Once in the extracellular matrix, CTCs are called DTCs and can reside there for years, evading the immune system. The establishment of a metastatic lesion occurs with a reverse phenotypic change—mesenchymal to epithelial transition—back to an epithelial-type cell, followed by proliferation into a metastatic tumor deposit. In the case of PDAC, the metastatic lesions have been shown to share the same genetic features as the primary tumor [39]. The signal cascade that regulates dormancy overgrowth and the triggers for growth after prolonged dormancy are as yet unknown. It is known, however, that the growth of DTCs into a metastatic lesion requires signals from the stroma of the metastatic deposit or even passenger stromal cells from the primary tumor. In addition, signaling from immune cells, particularly neutrophils, is important to metastatic growth. Other elements may also come into play, such as factors secreted by the primary tumors, contributing to the creation of the appropriate microenvironment in specific organs [30,31].
The Role of Circulating Tumor Cells as a Biomarker in PDAC
The development of methods for global genetic analysis, expression profiling, and protein expression has led to the identification of novel biomarkers that have the potential to direct therapy. This innovation has provided necessary tools for a precision approach to the treatment of pancreatic cancer. The application of targeted therapies has changed the potential role and mechanism of a biopsy. Currently, the main use of a biopsy in patients with resectable pancreatic cancer is for the establishment of a tissue diagnosis required for those patients to be considered for neoadjuvant therapy. A traditional biopsy is a “snapshot” in time over the entire natural history and treatment course of the disease. Because traditional biopsy is invasive, it is neither feasible nor practical to perform serial biopsies to guide treatment in real time.
Tumors continuously evolve, with variable clones potentially driving progression in the natural course of the disease and under the pressure of treatment. This poses a significant challenge in the choice and monitoring of therapy; an effective treatment might be introduced early and produce a significant initial response but may then fail to maintain results as resistant clones survive and multiply. Moreover, PDAC consists of multiple cellular clones, and each has the potential for a unique biological behavior [40]. This tumor heterogeneity also creates the potential for sampling error with the use of a traditional biopsy. The inability to detect the aggressive clones among all clones that drive outcome has clinical implications in terms of guiding management in a precision approach.
Liquid biopsy has the potential to overcome limitations of traditional biopsy because it provides the opportunity to gain access to biomarkers through a minimally invasive method, such as a blood draw, with little discomfort and virtually no risks. This enables real-time analysis with multiple samples over time to monitor tumor progression and response to therapy. Finally, it may ultimately be able to represent the biomarkers of all clones of the primary tumor, metastatic deposits, and subclinical disease.
Circulating tumor DNA (ctDNA) is commonly used for the liquid biopsy of tumors. The ability of ctDNA-containing plasma to be stored for long periods and relative ease of analysis make this a popular form of liquid biopsy over CTC analysis. Indeed, the measurement of ctDNA has found many potential uses in the management of pancreatic cancer. However, ctDNA is limited in its ability to directly evaluate and potentially intervene in the metastatic process because it mainly represents the genome of dying cells, not those that are currently dividing and driving disease progression. On the other hand, CTCs are the probable source of metastatic lesions. Hence, they provide the potential for direct assessment of tumor biology [14]. In addition, each CTC likely represents a unique clone present in the tumor and, as such, is not an “average” of all mutations, as is the case with ctDNA.
Despite these advantages, CTC analysis faces multiple challenges. Compared to ctDNA, CTCs are less prevalent in plasma, making any assay based on CTCs less sensitive for identification of subclinical disease. Also, in most cases, blood samples of CTCs must be isolated immediately and cannot be stored as an unprocessed sample.
Conflicting results have been found related to the correlation between CTC counts and prognosis in pancreatic cancer. CTC positivity has been correlated with worse prognosis in patients with pancreatic cancer in many studies [41,42]. In a study by de Albuquerque et al, a longer progression-free survival (PFS) was seen in patients without CTCs than in those with CTCs [43]. Similarly, Zhang et al followed their patients for 1½ years and found a correlation between CTC positivity and both the development of metastases and worse survival. Interestingly, they observed that CTC counts increased 10 days after the surgical procedure, and they postulated that dormant tumor cells are stimulated and reactivated by surgery [44]. Conversely, Z'graggen et al found a correlation between the prevalence of CTCs in blood and tumor stage, but this finding did not translate into worse survival [45]. Khoja et al and Dotan et al, in 2 different studies, did not find a statistically significant association between CTCs and PFS or overall survival; however, both acknowledged that these results could be due to their small sample sizes [46,47]. More recently, a study including 179 patients with pancreatic lesions concluded that CECs do not help determine the presence of malignancy, stage, or prognosis in the case of the manifest presence of PDAC or cystic lesions. However, only a single blood sample, prior to treatment, was analyzed, and the same authors reported that the other studies that did find a correlation counted circulating cells from multiple blood samples taken before, after, and during therapy [48].
The results of 2 large meta-analyses, each with more than 600 patients, which assessed the correlation of CTCs with outcome in pancreatic cancer, have been published and demonstrate a correlation between CTC positivity and poor outcomes. One of these concluded that CTCs strongly predict disease course in pancreatic cancer patients, with a worse overall survival (HR = 1.64, 95% CI 1.39–1.94, P < 0.00001) and recurrence-free survival (HR = 2.36, 95% CI 1.41–3.96, P < 0.00001). CTCs were associated with unfavorable prognostic outcomes in all the analyzed groups (before, during, and after treatment), and post-treatment CTC positivity was the most predictive of poor outcome (PFS/recurrence-free survival HR = 8.36, 95% CI 3.22–21.67, P < .0001). The other meta-analysis, with 623 patients, also concluded that CTC-positive patients had worse progression-free survival (HR = 1.89, 95% CI 1.25–4.00, P < 0.001) and overall survival (HR = 1.23, 95% CI 0.88–2.08, P < 0.001) [49,50].
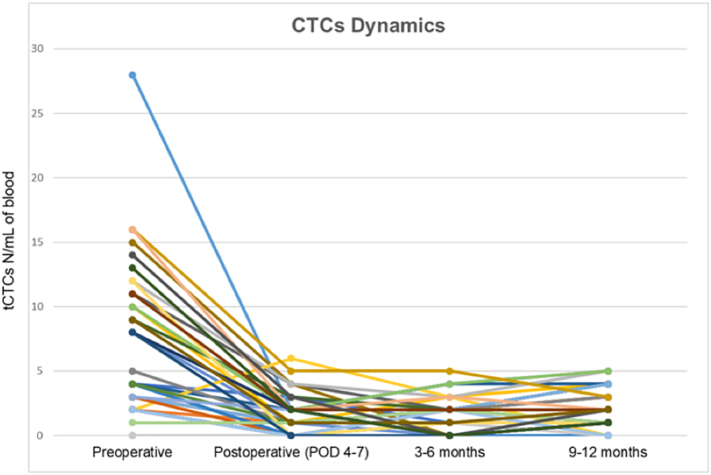
Gemenetzis et al recently published a prospective longitudinal study (CLUSTER Trial NCT2974764) in which 136 patients with pancreatic cancer were followed with liquid biopsies. Measurement of CTC concentration in peripheral blood was performed at fixed intervals, starting prior to surgical resection, at 4 and 6 postoperative days, and every 2 to 3 months thereafter. CTCs were isolated based on size (> 8 μm) and then stratified into groups of epithelial CTCs if only expressing cytokeratin or mixed epithelial/mesenchymal CTCs if also expressing vimentin [51]. Tumor cells were identified in the blood of 131 (96%) patients. Chemotherapy-naive patients at the time of surgery (58%) had significantly higher CTC numbers before resection when compared to patients post neoadjuvant therapy (42%). Both groups had a significant decrease in the number of CTCs after surgery (Fig 4). However, patients that developed early disease recurrence within 1 year from surgery had significantly higher pre- and postoperative CTC counts, with a higher proportion of mixed epithelial/mesenchymal phenotype CTCs, suggesting that these cells have a more aggressive biology. In line with these findings, patients who underwent exploration with aborted resection due to occult abdominal metastatic disease had a significantly higher number of CTCs than patients in whom resection was completed [52].
Fig 4.
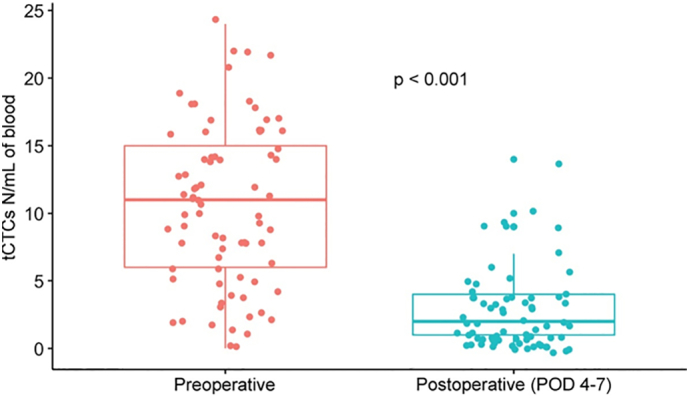
Change in CTC in response to therapy: This figure depicts 1 example of change in CTC in response to standard therapy. This graph is taken from results of the CLUSTER trial and demonstrates a significant drop in CTCs following oncologic resection. Printed with permission.
The Role of CTC and DTC in Relapse
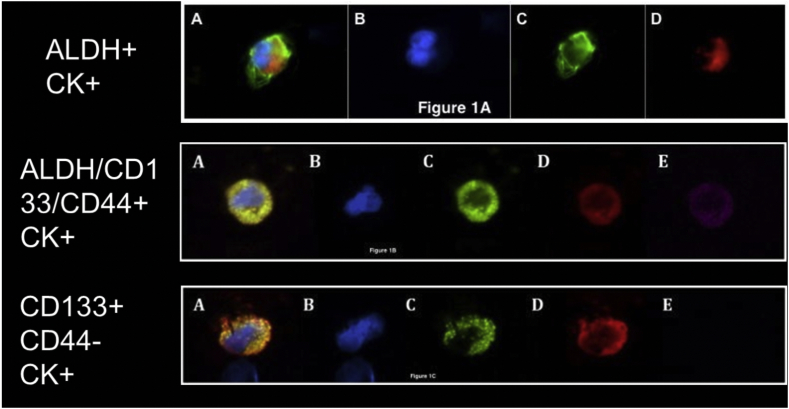
Recent work in the isolation and characterization of CTC and DTC in patients with pancreatic cancer has provided insight into the mechanisms of disease relapse. Cancer stem cells, also called tumor-initiating cells, have been found to constitute a small percentage of all cells within cancer (<0.01) but are necessary for driving growth [53]. They are defined by their ability to remain dormant for years, self-renew, and differentiate into all cell types. In animal studies, cancer stem cells are able to establish tumors with much greater efficiency than samples from bulk tumor [54]. Work in PDAC has revealed the presence of cancer stem cells in this disease expressing CD133, CD44, and aldehyde dehydrogenase (ALDH) [55]. Our group reported the presence of a CK +/ALDH + phenotype in 77% of all patients with CTCs at the time of resection (Fig 6) [56]. This phenotype predicted worse overall and disease-free survival (HR 3.4, 95% CI 1.2–9.8, P = 0.03). In further stratification of this cohort, patients with CTCs that were “triple-positive” for CK/CD133/CD44 (6.45, HR 6.45; 95% CI 2.1–19.7) had a much higher risk of recurrence compared to those with CTCs positive for CK/CD133 and negative for CD44. These results support the notion that at least a subset of CTCs has tumor-initiating cell properties. However, direct evidence for this has not yet been reported. Direct proof of the ability of CTCs from pancreatic cancer to establish metastatic lesions will, in part, require a demonstration of their ability to grow in culture. This feat has yet to be accomplished with CTCs from pancreatic cancer, but CTC culture has been reported in some other tumor types, such as colorectal and breast cancers [48,57,58].
Fig 6.
Stem cell–like/tumor-initiating cell–like features of CTC in pancreatic cancer:
A, Circulating tumor cells from pancreatic cancer patients demonstrating (A) pan-cytokeratin–positive, ALDH-positive CTC (merge); (B) DAPI (blue); (C) aldehyde dehydrogenase (green); and (D) pan-cytokeratin (red).
B, Circulating tumor cells from pancreatic cancer patients demonstrating (A) pan-cytokeratin–positive, CD133-positive, CD44-positive CTC (merge); (B) DAPI (blue); (C) CD133 (green); (D) pan-cytokeratin (red); and (E) CD44 (pink).
C, Circulating tumor cells from pancreatic cancer patients demonstrating (A) pan-cytokeratin–positive, CD133-positive, CD44-negative CTC (merge); (B) DAPI (blue); (C) CD133 (green); (D) pan-cytokeratin (red); and (E) CD44 negativity (absence of pink).
Printed with permission.
Clinical experience shows that relapse of pancreatic cancer can occur years after resection and systemic therapy. Two recent publications lend insight into a mechanism for this observation. A subset of patients in the CLUSTER trial mentioned above was found to have no evidence of disease recurrence at 1 year but maintained a steady baseline level of mainly epithelial CTCs (Fig 5) [52]. As the half-life of CTCs has been measured to be on the order of minutes in animal models [35,59], these findings suggest the existence of an unknown reservoir of these persistent CTCs, which likely includes extravascular niches of long-lived, dormant, or slow-growing DTCs within distant organs, lymph nodes, bone marrow, and circulation. Direct evidence of such cells and their mechanism of immune evasion was recently published by Pommier et al [60]. In this elegant study, investigators demonstrated isolated DTCs that lacked major histocompatibility complex I (MHC I) expression within the liver parenchyma of patients with pancreatic cancer. This observation raised the possibility that these DTCs evaded CD8 + T cell–mediated clearance. Indeed, using a mouse model of pancreatic cancer, the investigators demonstrated that in animals preimmunized against the tumor, very few micrometastatic lesions were seen at 5 days compared to the heavy tumor burden seen in nonimmunized mice. Moreover, as time progressed, nonimmunized mice developed visible metastatic lesions, whereas the immunized mice only harbored single DTCs. These DTCs differed from the cancer cells in the macroscopic metastatic lesions in that they did not express the epithelial makers CK19 and E-cadherin, nor did they express MHC I. The DTCs were found to be quiescent, with no expression of Ki67. Additionally, analysis of the DTCs by single-cell expression profiling revealed a strong upregulation of the pathway related to a response to ER stress. Furthermore, it was shown that downregulation of MHC I is mediated through the unresolved ER stress [61].
Fig 5.
Persistent CTC in the absence of clinical disease: This figure demonstrates 1 of the most interesting observations of the CLUSTER Trial. CTCs were present in a subset of patients who were at least 1 year out from an oncologic resection but had no clinical evidence of recurrence.
These reports demonstrate the ability to identify subclinical disease and the mechanism by which this disease can persist for years in patients with resected pancreatic cancer.
Implications of CTC and DTC in the Treatment of Localized Pancreatic Cancer
Clinical experience, reinforced by direct scientific evidence, supports the concept that pancreatic cancer is almost always a systemic disease at the time of diagnosis. To cure a patient with clinically localized pancreatic cancer, 2 battles will need to be won—a local battle, fought by surgical resection and, in some cases, a combination of surgery and radiation, and a systemic battle, fought by systemic therapy. Thus, it is helpful in the context of surgical decision making to think of a patient as having both a solid and liquid phase of a tumor.
With respect to the local battle, a successful R0 (microscopically margin-negative) oncologic resection renders a patient free of the solid phase. However, the liquid phase remains, as evidenced by the presence of CTCs and DTCs after surgery. Regarding the systemic battle, CTCs have been observed in patients with localized, resectable disease [62], raising the possibility that detection and characterization of these cells could eventually inform treatment decisions. For example, a patient with a high CTC burden prior to any therapy might benefit more from neoadjuvant therapy than a patient with a low CTC burden, who might have a favorable outcome with surgery alone. However, further studies are required before such an approach could be considered.
Both adjuvant and neoadjuvant therapies are likely effective at eradicating the bulk of liquid disease, yet an essential subset of cells in the form of DTCs remains. These cells evade the immune system as a result of their lack of MHC I and likely other features yet to be determined. Their ability to lie dormant as nonproliferating quiescent cells renders them resistant to standard cytotoxic chemotherapy. The majority of our present understanding of pancreatic cancer biology is based on the primary tumor, not DTCs. We propose that what is important in disease relapse is not what we take out during surgery but what we leave behind in a liquid form. CTCs and DTCs have a unique biology and a unique tumor microenvironment, and certainly must possess some vulnerabilities that can be leveraged in developing novel therapies. To make a significant, positive impact on survival, future translational research will need to focus on the biology of DTCs and CTCs and potential therapeutic targets they express.
Conclusion
Pancreatic cancer is a highly deadly disease as a result of its systemic nature at the time of diagnosis in current clinical practice. Perhaps the single most important advancement in improving survival will be through the early detection of precancerous lesions and earlier surgical resection. In this regard, methods of early detection through liquid biopsy, such as CancerSEEK, have been developed, showing promising results. The true impact of this assay will be determined after ongoing prospective validation studies are completed. For those patients already diagnosed with localized pancreatic cancer, on the other hand, improved survival will only come with major advances in systemic control.
A paradigm for the management of these patients envisions that they have 2 phases of disease: a solid and a liquid phase. In many cases, the solid phase of the disease is successfully treated through surgical resection. However, the liquid, or systemic, phase, represented by DTCs and CTCs, is then left behind. Members of a subset of these cells are long-lived, evade the immune system, are resistant to current cytotoxic therapies, and are capable of establishing relapse. The focus of future surgical translational research should be the disease that is left behind: micrometastatic disease that consists of DTCs and CTCs. Through a better understanding of these cells, there can be hope for the development of innovative therapies that target their vulnerabilities. Even in the event that these cells are found to lack targetable features, a large survival advantage could be gained if they can be kept in their quiescent state through alternative, novel therapies, converting pancreatic cancer into a chronic disease where the majority of patients can achieve long-term survival.
Author Contributions
All authors contributed equally to the concept and design, writing of the manuscript, literature evaluation, and final editing.
Conflict of Interest
Authors have no conflicts of interest to disclose.
Funding Sources
There is no funding for this review to declare.
References
- 1.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Noone A.M., Cronin K.A., Altekruse S.F. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results program, 1992-2013. Cancer Epidemiol Biomarkers Prev. 2017;26(4):632–641. doi: 10.1158/1055-9965.EPI-16-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T., Desseigne F., Ychou M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011 doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff D.D., Ramanathan R.K., Borad M.J. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011 doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans D.B., Varadhachary G.R., Crane C.H. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 6.Varadhachary G.R., Wolff R.A., Crane C.H. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 7.Groot V.P., Rezaee N., Wu W. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018 doi: 10.1097/SLA.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J.D., Li L., Wang Y. Science (80- ) 2018. Detection and localization of surgically resectable cancers with a multi-analyte blood test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruban R.H., Takaori K., Klimstra D.S. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. In: American. Journal of Surgical Pathology. 2004 doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 10.Pea A., Yu J., Rezaee N. Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2017 doi: 10.1097/SLA.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J., Walter K., Omura N. Unlike pancreatic cancer cells pancreatic cancer associated fibroblasts display minimal gene induction after 5-aza-2′-deoxycytidine. PLoS One. 2012 doi: 10.1371/journal.pone.0043456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Chiaro M., Beckman R., Ateeb Z. Ann Surg. 2019. Main duct dilatation is the best predictor of high-grade dysplasia or invasion in intraductal papillary mucinous neoplasms of the pancreas. [DOI] [PubMed] [Google Scholar]
- 13.Del Chiaro M., Schulick R.D. Main-duct intraductal papillary mucinous neoplasm. High cancer risk in duct diameter of 5 to 9 mm. Ann Surg. 2017;266(6):e86. doi: 10.1097/SLA.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 14.Yu J., Sadakari Y., Shindo K. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2017;66(9):1677–1687. doi: 10.1136/gutjnl-2015-311166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J., Li A., Hong S.M., Hruban R.H., Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aune D., Greenwood D.C., Chan D.S. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(4):843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 17.Kandel S., Kloeters C., Meyer H., Hein P., Hilbig A., Rogalla P. Whole-organ perfusion of the pancreas using dynamic volume CT in patients with primary pancreas carcinoma: acquisition technique, post-processing and initial results. Eur Radiol. 2009;19(11):2641–2646. doi: 10.1007/s00330-009-1453-z. [DOI] [PubMed] [Google Scholar]
- 18.Junttila M.R., de Sauvage F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 19.Cirri P., Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1(4):482–497. https://www.ncbi.nlm.nih.gov/pubmed/21984967 [PMC free article] [PubMed] [Google Scholar]
- 20.Brabletz T., Jung A., Spaderna S., Hlubek F., Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 21.Mani S.A., Guo W., Liao M.J. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morel A.P., Lievre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhim A.D., Mirek E.T., Aiello N.M. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Erkan M., Reiser-Erkan C., Michalski C.W. The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr Mol Med. 2012;12(3):288–303. doi: 10.2174/156652412799218921. https://www.ncbi.nlm.nih.gov/pubmed/22272725 [DOI] [PubMed] [Google Scholar]
- 26.Hong S.M., Noe M., Hruban C.A., Thompson E.D., Wood L.D., Hruban R.H. A “clearer” view of pancreatic pathology: a review of tissue clearing and advanced microscopy techniques. Adv Anat Pathol. 2019;26(1):31–39. doi: 10.1097/PAP.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 27.Noe M., Rezaee N., Asrani K. Immunolabeling of cleared human pancreata provides insights into three-dimensional pancreatic anatomy and pathology. Am J Pathol. 2018;188(7):1530–1535. doi: 10.1016/j.ajpath.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poruk K.E., Valero V., 3rd, He J. Circulating epithelial cells in intraductal papillary mucinous neoplasms and cystic pancreatic lesions. Pancreas. 2017;46(7):943–947. doi: 10.1097/MPA.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 29.Allard W.J., Matera J., Miller M.C. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 30.Husemann Y., Geigl J.B., Schubert F. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan R.N., Riba R.D., Zacharoulis S. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gkountela S., Aceto N. Stem-like features of cancer cells on their way to metastasis. Biol Direct. 2016:1–14. doi: 10.1186/s13062-016-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baccelli I., Schneeweiss A., Riethdorf S. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013 doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 34.Nagrath S., Sequist L.V., Maheswaran S. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stott S.L., Lee R.J., Nagrath S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2(25):25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pantel K., Speicher M.R. The biology of circulating tumor cells. Oncogene. 2016;35(10):1216–1224. doi: 10.1038/onc.2015.192. [DOI] [PubMed] [Google Scholar]
- 37.Fidler I.J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45(4):773–782. https://www.ncbi.nlm.nih.gov/pubmed/5513503 [PubMed] [Google Scholar]
- 38.Hodgkinson C.L., Morrow C.J., Li Y. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20(8):897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 39.Makohon-Moore A.P., Zhang M., Reiter J.G. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet. 2017;49(3):358–366. doi: 10.1038/ng.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makohon-Moore A., Iacobuzio-Donahue C.A. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer. 2016;16(9):553–565. doi: 10.1038/nrc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bidard F.C., Huguet F., Louvet C. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24(8):2057–2061. doi: 10.1093/annonc/mdt176. [DOI] [PubMed] [Google Scholar]
- 42.Kurihara T., Itoi T., Sofuni A. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15(2):189–195. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 43.de Albuquerque A., Kubisch I., Breier G. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology. 2012;82(1):3–10. doi: 10.1159/000335479. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Wang F., Ning N. Patterns of circulating tumor cells identified by CEP8, CK and CD45 in pancreatic cancer. Int J Cancer. 2015;136(5):1228–1233. doi: 10.1002/ijc.29070. [DOI] [PubMed] [Google Scholar]
- 45.Z'Graggen K., Centeno B.A., Fernandez-del Castillo C., Jimenez R.E., Werner J., Warshaw A.L. Biological implications of tumor cells in blood and bone marrow of pancreatic cancer patients. Surgery. 2001;129(5):537–546. doi: 10.1067/msy.2001.113819. [DOI] [PubMed] [Google Scholar]
- 46.Dotan E., Alpaugh R.K., Ruth K. Prognostic significance of MUC-1 in circulating tumor cells in patients with metastatic pancreatic adenocarcinoma. Pancreas. 2016;45(8):1131–1135. doi: 10.1097/MPA.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoja L., Backen A., Sloane R. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106(3):508–516. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cauley C.E., Pitman M.B., Zhou J. Circulating epithelial cells in patients with pancreatic lesions: clinical and pathologic findings. J Am Coll Surg. 2015;221(3):699–707. doi: 10.1016/j.jamcollsurg.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma X.L., Li Y.Y., Zhang J. Prognostic role of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(15):6015–6020. doi: 10.7314/apjcp.2014.15.15.6015. https://www.ncbi.nlm.nih.gov/pubmed/25124566 [DOI] [PubMed] [Google Scholar]
- 50.Han L., Chen W., Zhao Q. Prognostic value of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35(3):2473–2480. doi: 10.1007/s13277-013-1327-5. [DOI] [PubMed] [Google Scholar]
- 51.Poruk K.E., Valero V., 3rd, Saunders T. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann Surg. 2016;264(6):1073–1081. doi: 10.1097/SLA.0000000000001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gemenetzis G., Groot V.P., Yu J. Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: results of the prospective CLUSTER study. Ann Surg. 2018;268(3):408–420. doi: 10.1097/SLA.0000000000002925. [DOI] [PubMed] [Google Scholar]
- 53.Kreso A., Dick J.E. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 54.Quintana E., Shackleton M., Sabel M.S., Fullen D.R., Johnson T.M., Morrison S.J. Efficient tumour formation by single human melanoma cells. Nature. 2008 doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee C.J., Dosch J., Simeone D.M. Pancreatic cancer stem cells. J Clin Oncol. 2008;26(17):2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 56.Poruk K.E., Blackford A.L., Weiss M.J. Circulating tumor cells expressing markers of tumor-initiating cells predict poor survival and cancer recurrence in patients with pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23(11):2681–2690. doi: 10.1158/1078-0432.CCR-16-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franken B., de Groot M.R., Mastboom W.J. Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res. 2012;14(5):R133. doi: 10.1186/bcr3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pantel K., Deneve E., Nocca D. Circulating epithelial cells in patients with benign colon diseases. Clin Chem. 2012;58(5):936–940. doi: 10.1373/clinchem.2011.175570. [DOI] [PubMed] [Google Scholar]
- 59.Meng S., Tripathy D., Frenkel E.P. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10(24):8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 60.Pommier A., Anaparthy N., Memos N. Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science (80- ) 2018;360(6394) doi: 10.1126/science.aao4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sosa M.S., Bragado P., Aguirre-Ghiso J.A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kulemann B., Pitman M.B., Liss A.S. Circulating tumor cells found in patients with localized and advanced pancreatic cancer. Pancreas. 2015 doi: 10.1097/MPA.0000000000000324. [DOI] [PubMed] [Google Scholar]