Abstract
Background
At present, novel coronavirus disease 2019 (COVID-19) has become a serious global public health problem. The current meta-analysis aimed to find risk factors for the COVID-19-related death, helping to enhance the efficacy and reduce the mortality of COVID-19.
Methods
We searched PubMed, Embase, medRxiv, and Cochrane Library for articles published between January 1, 2020, and April 13, 2020. We statistically analyzed the risk factors of the COVID-19 deceased with meta-analysis.
Results
A total of 2401 patients in 15 articles were included in this study. Meta-analysis showed that 66.6% of COVID-19 deceased were male, with a median age of 69.9 years. Common symptoms of deceased included fever (70.6–100%), dyspnea (38.89–85.7%), cough (22.4–78%), and fatigue (22–61.9%). The incidence of hypertension, chronic cardiovascular disease, diabetes, and chronic cerebrovascular disease among the COVID-19 deceased were 38.56% (95% confidence interval (CI) 25.84 ~ 52.12%), 17.54% (95% CI 13.38 ~ 21.69%), 22.2% (95% CI 19.30 ~ 25.10%), and 15.58% (95% CI 10.05 ~ 21.12%), respectively. Compared with the surviving COVID-19 patients, the deceased had lower platelet levels (mean difference (MD) = − 39.35, 95% CI − 55.78 ~ − 22.93) and higher C-reactive protein (CRP) (MD = 80.85, 95% CI 62.53 ~ 99.18) and lactate dehydrogenase (LDH) (MD = 246.65, 95% CI 157.43 ~ 335.88) at admission. The most common complications of the deceased were acute respiratory distress syndrome (ARDS) (OR = 100.36, 95% CI 64.44 ~ 156.32) and shock (OR = 96.60, 95% CI 23.80 ~ 392.14).
Conclusion
Most of the COVID-19 deceased were elderly males. Fever, dyspnea, dry cough, fatigue, hypertension, chronic cardiovascular and cerebrovascular disease, diabetes, and laboratory examinations showed low levels of platelet content, increased CRP and LDH were associated with the risk of dying. ARDS and shock were risk factors for death in COVID-19 patients.
Keywords: Death, COVID-19, Severe acute respiratory syndrome coronavirus 2, Meta-analysis
Introduction
Since the outbreak of the new coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], formerly known as 2019-nCoV) in December 2019 in Wuhan, Hubei Province, China [1–3], confirmed cases have appeared in countries around the world. Genome sequencing and phylogenetic analysis show that SARS-CoV-2 is a new type of human-infected β-coronavirus. Bats are presumed to be the original host of this zoonotic virus, but there is no evidence that there is an intermediate host that promotes human infection with the virus [4]. According to the Coronavirus disease 2019 (COVID-19) Situation Report–84 [5] released by the World Health Organization (WHO) on April 13, there were 1,773,084 confirmed cases of COVID-19 and 111,652 deaths worldwide. Patients infected with SARS-CoV-2 were prone to death when they develop severe pneumonia, pulmonary edema, ARDS, or multiple organ failure (such as shock, acute heart injury, and acute kidney injury) [6]. The increasing number of deaths forces us to find out the clinical characteristics of patients at risk of dying, so that clinical workers can take corresponding rescue measures in the early stage to reduce the occurrence of death.
At present, more and more retrospective studies have reported the clinical information of COVID-19 dead patients. Here we will provide systematic assessment and details, not only to assess the disease coexistence of all dead patients, the results of laboratory examinations, but also to compare the potential disease risks between survival and death patients. These results may assist management in developing prevention policies to address COVID-19 and its key results.
Methods
Search strategy and selection criteria
Literature search in the PubMed, Embase, medRxiv (https://www.medrxiv.org/), and Cochrane Library from January 1, 2020 to April 13, 2020, using a combination of the following keywords: “SARS-CoV-2” or “2019-nCoV” or “COVID-19” or “new coronavirus” or “Wuhan Coronavirus” and “die” or “death” or “fatality” or “mortality” or “decease” or “survive” or “survivors”. Restrict publication language to English. In addition, to ensure the comprehensiveness and accuracy of the research, we also consulted the references of the included literature. This work was independently completed by two authors (Peishan Qiu and Yunjiao Zhou). Disagreements were resolved by the third investigator (Fan Wang). All the search results were evaluated according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.
Inclusion and exclusion criteria
The inclusion criteria of the meta-analysis were as follows: (1) the cases in each study were patients diagnosed with COVID-19, (2) involving the death group or non-survivor group and the survivor group, (3) at least one outcome reported among demographical characteristics, comorbidities, and clinical manifestations of COVID-19 deceased. Studies such as (a) repetitive publications, (b) reviews, editorials, case reports, letters, and family-based studies, and (c) studies with fewer than five cases were excluded.
Data extraction and paper quality evaluation
Data extraction and literature quality evaluation were completed by two investigators (Peishan Qiu and Yunjiao Zhou) independently. Disagreements were resolved with a third investigator (Fan Wang) or by consensus. All included papers were evaluated using the Newcastle–Ottawa scale [7]. The following variables were extracted: the authors, the baseline details, clinical manifestations, laboratory examinations, and clinical outcomes.
Statistical analysis
Single-arm meta-analysis was performed using R 3.6.3 software. Review Manager 5.3 (RevMan version 5.3) software was used to compare the clinical characteristics and laboratory results of COVID-19 deceased and surviving patients. The odds ratio (OR, 95% confidence intervals (CI)) was used to describe the ratio of the probability of the complications occurring in dead patients vs. survivor. Mean difference (MD, 95%CI) was used to describe the differences in the mean of laboratory examinations between dead and surviving COVID-19 patients. Heterogeneity among the studies was assessed by the Cochran Chi-square and I2 test. When I2 < 50%, a fixed-effect model was used, otherwise a random-effect model was selected. When there was statistical heterogeneity among the results, a further sensitivity analysis was performed to determine the source of heterogeneity. The publication bias was assessed using a funnel plot and quantified using Egger’s linear regression test. P < 0.05 was statistically significant (two sided).
Results
Characteristics of included studies
As shown in Fig. 1, the total number of records initially determined based on the search strategy was 2679. After deleting 462 duplicates, we deleted another 2134 articles by reading the title and abstract of the article. We eliminated 68 articles by reading the full-text articles of the remaining 83 studies, 35 of which had no clinical data of death, 30 of which did not provide clinical data related to our study, and 3 of which included less than 5 cases. Finally, there were 15 articles included in our meta-analysis [8–22].
Fig. 1.
Flow diagram of the number of studies screened and included in the meta-analysis
Clinical manifestation of dead patients with COVID-19
The clinical data study included 15 studies [8–22] with a total of 2401 cases, including 904 deceased. The epidemiology, baseline characteristics, and clinical symptoms of all deaths included are summarized in Table 1. The median age of COVID19 deaths was 69.9 years, with 66.6% males. The main clinical symptoms of COVID19 dead patients on admission were fever (70.6–100%), dyspnea (38.89–85.7%), cough (22.4–78%), fatigue (22–61.9%), expectoration (22.2–57.1%), myalgia (2.78–33.3%), diarrhea (4–38.1%), anorexia (23.4–56.5%). Other rare symptoms included chest urgency (2.4–49%), headache (4.7–23.8%), sore throat (2.4–4.7%), hemoptysis (4–5.56%), nausea and vomiting (4–4.7%), abdominal pain (3.5–5%), and dizziness (9%).
Table 1.
Demographics, baseline characteristics, and clinical symptoms of COVID-19 deceased included in the meta-analysis
| Study | Author | Cao JL [8] | Chen T [9] | Deng Y [10] | Du RH [11] | Du YZ [12] | Hang Y [13] | Korean [14] | Li Xu [15] | NERC [16] | Ruan QR [17] | Tang N [18] | Tu WJ [19] | Wang L [20] | Yang XB [21] | Zhou F [22] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dead | Number of patients | 17 | 113 | 109 | 21 | 85 | 36 | 54 | 25 | 66 | 68 | 134 | 25 | 65 | 32 | 54 | |
| Age | median (IQR), y | 72 (63–81) | 68(62–77) | 69 (62–74) | 70.2 (65–75.4) | 65.8 (56.3–75.3) | 69.22 (62.74–75.71) | 75.5 (66–80) | 71.83 (63.34–80.32) | 77 (35–93) | 67 (15–81) | 68.7 (61–76.4) | 70 (64–80) | 76 (70–83) | 64.6 (57–72.15) | 69 (63–76) | |
| Sex | Female | 4 (23.5) | 30 (27) | 36 (33) | 11 (52.4) | 23 (27.1) | 11 (30.56) | 21 (38.89) | 15 (60) | 29 (43.9) | 19 (28) | 44 (32.8) | 6 (24) | 26 (40) | 11 (34) | 16 (30) | |
| Male | 13 (76.5) | 83 (73) | 73 (67) | 10 (47.6) | 62 (72.9) | 25 (69.44) | 33 (61.1) | 10 (40) | 37 (56.1) | 49 (72) | 90 (67.2) | 19 (76) | 39(60) | 21 (66) | 38 (70) | ||
| Signs and symptoms | Fever | 12 (70.6) | 104 (92) | 95 (87.2) | 21 (100) | 78 (91.8) | 34 (94.44) | – | – | - | 59 (87) | – | – | 56 (87.5) | 31 (97) | 51 (94) | |
| Cough | 8 (47.1) | 79 (70) | 47 (43.1) | 14 (66.7) | 19 (22.4) | 28 (77.78) | – | – | - | 51 (75) | – | – | 30 (46.9) | 25 (78) | 39 (72) | ||
| Fatigue | 9 (52.9) | 64 (57) | 30 (27.5) | 13 (61.9) | 50 (58.8) | 17 (47.22) | – | – | - | 15 (22) | – | – | 26 (40.6) | – | 15 (28) | ||
| Anorexia | – | 31 (27) | – | – | 48 (56.5) | – | – | – | – | – | – | – | 15 (23.4) | – | – | ||
| Myalgia | 5 (29.4) | 21 (19) | 30 (27.5) | 7 (33.3) | 14 (16.5) | 1 (2.78) | – | – | – | 9 (13) | – | – | 1 (1.6) | 4 (12.5) | 8 (15) | ||
| Dyspnoea | – | 70 (62) | 77 (70.6) | 18 (85.7) | 60 (70.6) | 14 (38.89) | – | – | – | 59 (87) | – | – | 38 (59.4) | 21 (66) | – | ||
| Chest tightness | – | 55 (49) | – | – | 2 (2.4) | – | – | – | – | – | – | – | 15 (23.4) | – | – | ||
| Expectoration | – | 35 (31) | 35 (32.1) | 12 (57.1) | 32 (37.6) | 8 (22.22) | – | – | – | 29 (43) | – | – | 17 (26.6) | – | 14 (26) | ||
| Pharyngalgia | – | 4 (4) | – | – | 2 (2.4) | – | – | – | – | – | – | – | 3 (4.7) | – | – | ||
| Haemoptysis | – | 4 (4) | 5 (4.6) | – | – | 2 (5.56) | – | – | – | 3 (4) | – | – | – | – | – | ||
| Diarrhoea | 3 (17.6) | 27 (24) | 19 (17.4) | 8 (38.1) | 16 (18.8) | 3 (8.33) | – | – | – | – | – | – | 8 (12.5) | – | 2 (4) | ||
| Nausea | – | 8 (7) | – | – | – | – | – | – | – | – | – | – | 1 (1.6) | – | 3 (6) | ||
| Vomiting | – | 6 (5) | – | – | 4 (4.7) | – | – | – | – | – | – | – | – | 1 (3) | 3 (6) | ||
| Abdominal pain | – | 6 (5) | – | – | 3 (3.5) | – | – | – | – | – | – | – | – | – | - | ||
| Headache | – | 11 (10) | 6 (5.5) | 5 (23.8) | 4 (4.7) | – | – | – | – | – | – | – | – | 2 (6) | – | ||
| Dizziness | – | 10 (9) | – | – | – | – | – | – | – | – | – | – | 2 (3.1) | – | – | ||
| NOS | score | 8 | 9 | 6 | 8 | 9 | 6 | 6 | 7 | 6 | 9 | 7 | 9 | 7 | 9 | 9 | |
Data are median (IQR) and n (%)
COVID-19 coronavirus disease 2019
Prevalence of underlying diseases in COVID-19 deaths
Through the current meta-analysis, we found that the prevalence rate of underlying diseases among COVID-19 dead patients was 72.21% (95% CI 62.85 ~ 79.96%).
Hypertension
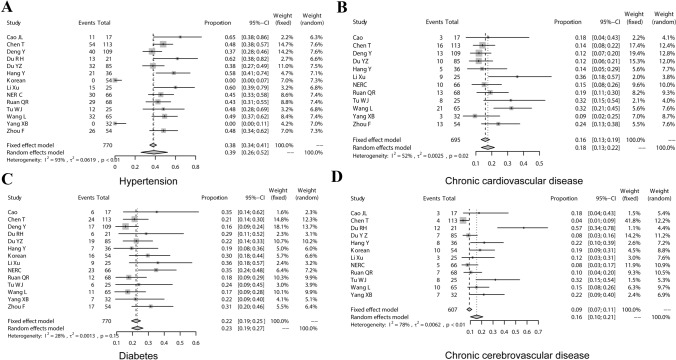
In a comprehensive analysis of 14 studies [8–17, 19–22], the pooled prevalence of hypertension in the COVID-19 deceased was estimated as 38.56% (95% CI 25.84 ~ 52.12%) (Fig. 2a). According to the results of the I2 index (93%) and the Chi-square test, there was high and significant heterogeneity between the studies (P < 0.01), also no publication bias was present according to Eggers’s test (t = 0.083162, P = 0.9351).
Fig. 2.
Meta-analysis of the prevalence of comorbidities in COVID-19 deaths. a Hypertension; b chronic cardiovascular disease; c diabetes; d chronic cerebrovascular disease
Chronic cardiovascular disease
To estimate the pooled prevalence of the cardiovascular disease in COVID-19 deceased, 14 studies were evaluated [8–17, 19–22]. The incidence was 23.48% (95% CI 16.75 ~ 30.20%), with high and significant heterogeneity (I2 = 83%). There was publication bias by Eggers's test (t = 3.2692, P = 0.006713). Through sensitivity analysis, it was found that the heterogeneity significantly decreased (I2 = 52%) after the exclusion of data from the “Korean” group [14] and the “Du RH” group [11]. The prevalence of meta-analysis after excluding studies affecting heterogeneity was 17.54% (95% CI 13.38 ~ 21.69%) (Fig. 2b), and there was no publication bias according to Eggers’s test (t = 3.1061, P = 0.01113).
Diabetes
The pooled prevalence of diabetes in COVID-19 death patients was estimated to be 22.2% (95% CI 19.30 ~ 25.10%). The heterogeneity of the study was low (I2 = 28.4%, P = 0.1519) (Fig. 2c). A fixed model was used to perform a meta-analysis of data from 14 studies [8–17, 19–22].
Chronic cerebrovascular disease
After the combination of 12 studies [8, 9, 11–17, 19–21], the random-effects model was used to calculate the prevalence of the cerebrovascular disease in COVID-19 deceased was 15.58% (95% CI 10.05 ~ 21.12%, I2 = 77.7%, P < 0.01) (Fig. 2d). The heterogeneity of the study was high, using a random-effects model. The sensitivity analysis was relatively stable.
Other comorbidities
The results of the meta-analysis showed that the incidence of chronic lung disease in COVID-19 deceased was 14.2% (95% CI 11.27 ~ 17.21%). The prevalence of tumors (4.28%, 95% CI 2.21 ~ 6.99%), chronic kidney disease (4.17%, 95% CI 2.6 ~ 5.74%), and chronic liver disease (2.86%, 95% CI 1.6 ~ 4.48%) were slightly increased in COVID-19 deaths. There was no publication bias by Egger’s test.
Characteristics of laboratory examinations of COVID-19 death patients
The purpose of this study was to explore the changes in laboratory indexes related to the poor prognosis of COVID-19 by comparing the laboratory examinations of the final dead patients with COVID-19 and the surviving patients (Table 2).
Table 2.
Comparison of laboratory results between COVID-19 deceased and surviving patients
| No. of studies | MD (95%CI) | P value | Heterogeneity | Model used | ||
|---|---|---|---|---|---|---|
| I2 | Ph | |||||
| White blood cell count, × 109/L | 7 | 3.77 (2.90, 4.64) | < 0.00001 | 60% | 0.02 | Random |
| Neutrophil count, × 109/L | 3 | 4.57 (2.92, 6.23) | < 0.00001 | 71% | 0.03 | Random |
| Lymphocyte count, × 109/L | 8 | − 0.37 (− 0.47, − 0.26) | < 0.00001 | 80% | < 0.0001 | Random |
| Haemoglobin, g/L | 5 | − 0.30 (− 2.84, 2.24) | 0.82 | 0 | 0.93 | Fixed |
| Platelets, × 109/L | 6 | − 39.35 (− 55.78, − 22.93) | < 0.00001 | 65% | 0.01 | Random |
| d-dimer, mg/L | 6 | 2.96 (1.93, 3.98) | < 0.00001 | 0% | 0.61 | Fixed |
| CRP, mg/L | 6 | 80.85 (62.53, 99.18) | < 0.00001 | 73% | 0.002 | Random |
| PCT, ng/mL | 4 | 0.15 (− 0.04, 0.34) | 0.12 | 86% | 0.0008 | Random |
| ALT, U/L | 6 | 5.07 (0.26, 9.88) | 0.04 | 74% | 0.002 | Random |
| AST, U/L | 5 | 14.26 (11.70, 16.81) | < 0.00001 | 48% | 0.11 | Fixed |
| BUN, mmol/L | 3 | 4.00 (3.33, 4.67) | < 0.00001 | 0% | 0.52 | Fixed |
| Scr, μmol/L | 6 | 21.20 (16.79, 25.61) | < 0.00001 | 13% | 0.33 | Fixed |
| LDH, U/L | 4 | 246.65 (157.43, 335.88) | < 0.00001 | 85% | 0.0002 | Random |
| CK, U/L | 4 | 48.98 (3.53, 94.43) | 0.03 | 78% | 0.004 | Random |
| Alb, g/L | 4 | − 4.00 (− 5.17, − 2.83) | < 0.00001 | 0% | 0.77 | Fixed |
| Tbil, mmol/L | 4 | 3.92 (1.94, 5.91) | 0.0001 | 59% | 0.06 | Random |
| IL-6, pg/mL | 5 | 27.57 (17.45, 37.69) | < 0.00001 | 96% | < 0.00001 | Random |
| PT, seconds | 6 | 1.13 (0.67, 1.60) | < 0.00001 | 60% | 0.03 | Random |
| APTT, seconds | 3 | 0.64 (− 0.17, 1.44) | 0.12 | 22% | 0.28 | Fixed |
COVID-19 Coronavirus disease 2019, MD mean difference, CI confidence interval, CRP C-reactive protein, PCT procalcitonin, ALT alanine transaminase, AST aspartate transaminase, BUN blood urea nitrogen, Scr serum creatinine, LDH lactate dehydrogenase, CK creatine kinase, Alb albumin, Tbil total bilirubin, IL-6 interleukin-6, PT prothrombin time, APTT activated partial thromboplastin time
P ≤ 0.05 was considered to be statistically significant
Through meta-analysis, we found that compared with patients who were also infected with SARS-CoV-2 but whose final outcome was alive, laboratory examinations showed a slightly higher leucocytes count (MD = 3.77, 95% CI 2.90 ~ 4.64), neutrophil count increased slightly (MD = 4.57, 95% CI 2.92 ~ 6.23), lymphocyte count decreased (MD = -0.37, 95% CI − 0.47 ~ − 0.26), platelets count decreased significantly (MD = − 39.35, 95% CI − 55.78 ~ − 22.93), d-dimer slightly increased (MD = 2.96, 95% CI 1.93 ~ 3.98), CRP increased significantly (MD = 80.85, 95% CI 62.53 ~ 99.18), alanine transaminase (ALT) slightly increased (MD = 5.07, 95% CI 0.26 ~ 9.88), aspartate transaminase (AST) significantly increased (MD = 14.26, 95% CI 11.70 ~ 16.81), blood urea nitrogen (BUN) slightly increased (MD = 4.00, 95% CI 3.33 ~ 4.67), serum creatinine (Scr) was significantly increased (MD = 21.20, 95% CI 16.79–25.61), and LDH was abnormally increased (MD = 246.65, 95% CI 157.43 ~ 335.88), creatine kinase (CK) increased (MD = 48.98, 95% CI 3.53 ~ 94.43), plasma albumin decreased (MD = − 4.00, 95% CI − 5.17 ~ − 2.83), total bilirubin (Tbil) slightly increased (MD = 3.92, 95% CI 1.94 ~ 5.91), interleukin-6 (IL-6) significantly increased (MD = 27.57, 95% CI 17.45 ~ 37.69), prothrombin time (PT) slightly increased (MD = 1.13, 95% CI 0.67 ~ 1.60). However, there was no significant difference in hemoglobin, procalcitonin (PCT), and activated partial thromboplastin time (APTT) between the two groups.
Occurrence of complications of COVID-19 death patients
We compared the incidence of complications between dead and surviving patients after the SARS-CoV-2 infection. A total of eight studies [8–10, 17, 19–22] were included in the meta-analysis.
Acute respiratory distress syndrome (ARDS)
A total of eight studies were included [8–10, 17, 19–22]. The results showed that compared with the patients who survived COVID-19, the most common complication of the patients who eventually died was ARDS (OR = 75.05, 95% CI 33.35 ~ 168.86, P < 0.00001). The heterogeneity of the study was high (I2 = 70%), and Egger's test showed publication bias (P = 0.001). The sensitivity analysis showed that the heterogeneity of the study was significantly reduced (I2 = 0%) after excluding the data of the “Yang XB” group [21]. Meta-analysis showed that ARDS was a risk factor for death in COVID-19 patients (OR = 100.36, 95% CI 64.44 ~ 156.32, P < 0.00001) (Fig. 3a). The Egger’s test showed that there was no publication bias (P = 0.61).
Fig. 3.
Meta-analysis of the comparison of complications between COVID-19 dead patients and surviving patients. a Acute respiratory distress syndrome (ARDS); b shock
Shock
A total of six studies were included [8–10, 19, 20, 22]. We found that dead patients were more likely to develop shock (OR = 51.55, 95% CI 8.32 ~ 319.28, P < 0.0001, I2 = 80%, P = 0.001) than COVID-19 survivors, but the heterogeneity of the study was high. The sensitivity analysis showed that the heterogeneity of this study decreased (I2 = 52%, P = 0.08) after excluding the “Wang L” group [20]. Therefore, the shock was a risk factor for death in patients COVID-19 (OR = 96.60, 95% CI 23.80 ~ 392.14, P < 0.00001) (Fig. 3b).
Other complications
Compared with the surviving patients, the incidence of infection (OR = 30.25, 95% CI 6.86 ~ 133.39, P < 0.00001, I2 = 82%, P < 0.0001), acute renal failure (AKI) (OR = 28.11, 95% CI 10.73 ~ 73.66, P < 0.00001, I2 = 69%, P = 0.002), acute heart injury (OR = 27.73, 95% CI 11.00 ~ 69.89, P < 0.00001, I2 = 78%, P = 0.0002) and acute liver injury (OR = 3.15, 95% CI 1.29 ~ 7.70, P = 0.01, I2 = 75%, P = 0.003) increased in patients with COVID-19 death.
Discussion
This meta-analysis included data from 15 laboratory-confirmed COVID-19 studies. Through our analysis, we found that 66.6% (602/904) of the dead patients were male, and the median age was 69.9 years old, in line with Huang et al. [3]. Common symptoms of COVID-19 deaths include fever, dyspnea, cough, and fatigue. Hypertension, chronic cardiovascular disease, diabetes, and chronic cerebrovascular disease are common comorbidities among COVID-19 deceased. COVID-19 patients with complications such as ARDS and shock are more likely to die. Laboratory examinations showed low levels of platelet content, increased CRP and LDH were associated with death in patients with COVID-19.
The elderly and men were the susceptible factors of COVID-19. For the high mortality rate of COVID-19 among the elderly, we believe that it may be related to the high prevalence of comorbidities among the elderly. Studies of the Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV also found more infections in men than women [23, 24]. On the one hand, it is generally believed that women have stronger innate and adaptive immune responses than men [25], which may be due to the protection of X chromosomes and sex hormones [26]. For this reason, women are less likely to affect a variety of bacteria and viruses than men. On the other hand, men tend to have more bad habits than women (such as smoking, drinking, more underlying diseases), which may also be the reason why men with COVID-19 are more likely to die.
Chronic diseases have become a global economic burden [27]. Our study revealed that the prevalence of chronic underlying diseases in dead patients with COVID-19 was 72.21% (95% CI 62.85–79.96%). Compared to subjects with no comorbidities, severe pandemic influenza cases were significantly increased in patients with obesity, cardiovascular diseases, and hypertension [28]. The study of the data extracted from the severe MERS-CoV cases suggested that diabetes and hypertension were equally prevalent in approximately 50% of the patients [23]. The state of hyperglycemia and insulin resistance in diabetic patients would weaken the synthesis of pro-inflammatory cytokines such as interferon-γ and interleukin and their downstream acute phase reactants [29], which makes diabetic patients more susceptible to SARS-CoV-2. Simultaneously, viral infection may cause sharp fluctuations in blood glucose levels in patients with diabetes, which will adversely affect the rehabilitation of patients. The angiotensin-converting enzyme 2 (ACE2) has been proved to be the receptor for SARS-CoV-2 to enter the host cells [6]. A study analyzed the lung transcriptome samples of more than 700 patients with comorbidities associated with severe COVID-19 and found that ACE2 was highly expressed in these patients compared to the control individuals [30]. Hence, patients with comorbidities may have higher chances of developing severe COVID-19. Lippi et al. believed that hypertension was a clinical predictor of COVID-19 severity in elderly patients [31]. Our research showed that the incidence of hypertension in patients with COVID-19 death was 38.56% (95% CI 25.84 ~ 52.12%). Tikellis et al. found that the expression of ACE2 in patients with hypertension might be decreased [32], increasing the level of angiotensin II, which would promote the development of ARDS [33].
In a study of the pathophysiology of ARDS, increased inflammation, and lung injury in rat models were associated with increased age, which was caused by an imbalance in the lung renin-angiotensin system (RAS) [34]. We observed that patients who died were more likely to have ARDS than those who survived with COVID-19 (OR = 75.05, 95% CI 33.35–168.86). And as mentioned earlier, the elderly were the majority of the dead. ACE2 can antagonize the activation of the classical RAS and mediate vasodilation, anti-inflammatory, and anti-proliferation [35]. Therefore, ACE2 has a protective effect on chronic underlying diseases and ARDS [33]. But when SARS-COV-2 binds to the ACE2 receptor and enters alveolar epithelial cells through membrane fusion, the expression of ACE2 in alveolar epithelial cells is down-regulated by mechanisms such as internalization, shedding, and viral replication [36, 37]. Moreover, the COVID-19 deceased were also more likely to have a shock. The electrolyte disorder and the decrease of systemic circulation blood volume during the shock would lead to the injury of various organs in the whole body and accelerate the death of the patients [38]. We found that patients with dyspnea were more likely to develop death. Dyspnea is a sign of decreased lung function and hypoxia. Therefore, when patients have symptoms of dyspnea, clinicians must be vigilant to prevent further deterioration of the condition. Interestingly, studies had shown that high fever (≥ 39 ℃) was positively related to the occurrence of ARDS, but negatively related to death [39, 40]. Although our study also showed that the most common symptom of COVID-19 deceased was fever. Unfortunately, we could not clarify the inevitable relationship between the death of COVID-19 patients and the degree of fever based on the available data.
Our analysis of the biochemical results showed that some indices were abnormally elevated in the blood of COVID-19 deceased, including LDH, AST, and Scr, which indicated the injury of the myocardium, liver, and kidney. This result is consistent with the extensive distribution of SARS-CoV-2 receptors ACE2, and can also partially explain why some patients died from multiple organ failure [6]. SARS-CoV-2 can cause tissues and organs injury by direct and/or indirect action [41]. The direct injury is to infect corresponding cells by identifying the ACE2 receptor. Another important cause for multiple organ failure and even death in critical patients is the release of cytokines from the inflammatory storm, causing systemic immune injury [42]. During the early stage of the inflammatory storm, the d-dimer increases significantly, which is the result of inflammation activating plasmin. The inflammation progresses and the presence of hypoxia will result in an overall hypercoagulable state or even disseminated intravascular coagulation. The inflammatory storm is also the main cause of eventual death in many patients with advanced SARS-CoV and MERS-CoV infection [43, 44]. In our study, we also observed a significant increase in IL-6 among COVID-19 deceased. IL-6 is a good predictor of disease severity and prognosis because it is expressed longer than other cytokines (such as TNF and IL-1) [45].
There are several limitations to our analysis and review. First, most of the included studies were cross-sectional studies with insufficient demonstration ability and lack of randomized controlled trials with optimized design. Second, our evidence was mostly from China. It would be better to include as many studies with a broad geographic scope and multiple races, to get a more comprehensive understanding of COVID-19 deceased. Third, the clinical data of patients provided by some studies were not comprehensive enough. Fourth, the actual cause of COVID-19 deceased might be obscured by unmeasured or unknown confounders. Despite our relatively large sample size, well-designed trials of high quality are needed to overcome inherent statistical bias and confirming the relationship between death and COVID-19.
Conclusion
According to the results of the current study, the majority of COVID-19 deaths have occurred in elderly men. Typical symptoms (fever, dyspnea, dry cough, and fatigue), chronic underlying diseases (hypertension, cardiovascular disease, diabetes, and cerebrovascular disease), associated laboratory abnormalities (low platelet count, increased CRP, and LDH), and complications (ARDS and shock) are risk factors for death in COVID-19 patients. Pay close attention to these risk factors and identify critical patients early. Personalized treatment regimens are needed to enhance the efficacy and reduce the risk of COVID-19 related death.
Author contributions
All the authors designed the study. Peishan Qiu, Yunjiao Zhou, and Fan Wang designed the literature search and searched the articles. Peishan Qiu, Yunjiao Zhou, Haizhou Wang, and Meng Zhang contributed to the data extraction process. All the authors analysed the data. Peishan Qiu wrote the first draft of article. All the authors revised the article and approved the final version.
Funding
This work was supported by the following projects:
National Natural Science Foundation of China (81,401,306) and Clinical Research Project of Guangzhou Medical University (No. 2017[160]) and Guangdong Province Science and Technology Program (No. 2016ZC0143).
National Natural Science Foundation of China (81472735); Wuhan University (2042019kf0206); National Basic Research Program of China (973program, 2015CB932600).
Availability for data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This is a review article and no human or animal participants were recruited to this study.
Human and animal rights
This is a review article. All data used were from published articles, which were following the ethical standards of the institutional review board/international ethics committee for each center and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
All data used in this article were from studies that had obtained the informed consent of all individual participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peishan Qiu, Yunjiao Zhou and Fan Wang contributed equally to this work.
Contributor Information
Xingfei Pan, Email: panxf0125@163.com.
Qiu Zhao, Email: qiuzhao@whu.edu.cn.
Jing Liu, Email: liujing_GI@whu.edu.cn.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui DS, IA E, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus disease 2019 (COVID-19) Situation Report–84 (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/). Accessed 13 Apr 2020
- 6.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 8.Cao J, Tu WJ, Cheng W et al (2020) Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clin Infect Dis [DOI] [PMC free article] [PubMed]
- 9.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;11:1261. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:200524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hang Y YR, Xu Y, Gong P (2020) Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv
- 14.Korean Society of Infectious D, Korea Centers for Disease C, Prevention Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35:e132. doi: 10.3346/jkms.2020.35.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan. China. Int J Infect Dis. 2020;94:128. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Covid-19 National Emergency Response Center E, Case Management Team KCfDC, Prevention Coronavirus disease-19: the first 7,755 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020;11:85–90. doi: 10.24171/j.phrp.2020.11.2.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Med. 2020;46:846. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu WJ, Cao J, Yu L et al (2020) Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med 46:1117–1120 [DOI] [PMC free article] [PubMed]
- 20.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Channappanavar R, Fett C, Mack M, et al. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 26.Gal-Oz ST, Maier B, Yoshida H, et al. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun. 2019;10:4295. doi: 10.1038/s41467-019-12348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Liu C, Zhang L, et al. How to control the economic burden of treating cardio-cerebrovascular diseases in China? Assessment based on system of health accounts 2011. J Glob Health. 2020;10:010802. doi: 10.7189/jogh.10.010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb Perspect Med. 2012;2:a007724. doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto BG, Oliveira AE, Singh Y et al (2020) ACE2 Expression is Increased in the Lungs of Patients with Comorbidities Associated with Severe COVID-19. J Infect Dis 222:556–563 [DOI] [PMC free article] [PubMed]
- 31.Lippi G, Wong J, Henry BM. Hypertension and its severity or mortality in Coronavirus Disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 32.Tikellis C, Bernardi S, Burns WC. Angiotensin-converting enzyme 2 is a key modulator of the renin-angiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens. 2011;20:62–68. doi: 10.1097/MNH.0b013e328341164a. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schouten LR, Helmerhorst HJ, Wagenaar GT, et al. Age-dependent changes in the pulmonary renin-angiotensin system are associated with severity of lung injury in a model of acute lung injury in rats. Crit Care Med. 2016;44:e1226–e1235. doi: 10.1097/CCM.0000000000002008. [DOI] [PubMed] [Google Scholar]
- 35.Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdecchia P, Cavallini C, Spanevello A, et al. COVID-19: ACE2centric infective disease? Hypertension. 2020;76:294. doi: 10.1161/HYPERTENSIONAHA.120.15353. [DOI] [PubMed] [Google Scholar]
- 37.Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuccari S, Damiani E, Domizi R, et al. Changes in cytokines, haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with CytoSorb. Blood Purif. 2020;49:107–113. doi: 10.1159/000502540. [DOI] [PubMed] [Google Scholar]
- 39.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Intern Med. 2020;180:934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schell-Chaple HM, Puntillo KA, Matthay MA, et al. Body temperature and mortality in patients with acute respiratory distress syndrome. Am J Crit Care. 2015;24:15–23. doi: 10.4037/ajcc2015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu B, Li M, Zhou Z, et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castrucci MR. Factors affecting immune responses to the influenza vaccine. Hum Vaccin Immunother. 2018;14:637–646. doi: 10.1080/21645515.2017.1338547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lew TW, Kwek TK, Tai D, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 44.Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.