Abstract
Astrocytes protect neurons during cerebral injury through several postulated mechanisms. Recent therapeutic attention has focused on enhancing or augmenting the neuroprotective actions of astrocytes but in some instances astrocytes can assume a neurotoxic phenotype. The signaling mechanisms that drive astrocytes toward a protective versus toxic phenotype are not fully known but cell–cell signaling via proteases acting on cell-specific receptors underlies critical mechanistic steps in neurodevelopment and disease. The protease activated receptor (PAR), resides in multiple brain cell types, and most PARs are found on astrocytes. We asked whether neuron-generated thrombin constituted an important astrocyte activation signal because our previous studies have shown that neurons contain prothrombin gene and transcribed protein. We used neuron and astrocyte mono-cell cultures exposed to oxygen-glucose deprivation and a model of middle cerebral artery occlusion. We found that ischemic neurons secrete thrombin into culture media, which leads to astrocyte activation; such astrocyte activation can be reproduced with low doses of thrombin. Media from prothrombin-deficient neurons failed to activate astrocytes and adding thrombin to such media restored activation. Astrocytes lacking PAR1 did not respond to neuron-generated thrombin. Induced astrocyte activation was antagonized dose-dependently with thrombin inhibitors or PAR1 antagonists. Ischemia-induced astrocyte activation in vivo was inhibited after neuronal prothrombin knockout, resulting in larger strokes. Restoring prothrombin to neurons with a lentiviral gene vector restored astrocyte activation and reduced stroke damage. We conclude that neuron-generated thrombin, released during ischemia, acts via PAR1 and may cause astrocyte activation and paracrine neuroprotection.
Keywords: astrocyte activation, ischemia, neuroprotection, thrombin
1 |. INTRODUCTION
Astrocytes protect neurons during cerebral injury through several postulated mechanisms, including but not limited to glutamate reuptake, secretion of growth factors and cytokines, secretion of toxin-resistance factors, and absorption of toxic free radicals (Faulkner et al., 2004; Hirayama & Koizumi, 2017; Pitt et al., 2017; Zamanian et al., 2012). Recent therapeutic attention has focused on enhancing or augmenting the neuroprotective actions of astrocytes (Jha et al., 2013; Lin et al., 2006). In contrast, new evidence suggests that in some instances astrocytes can assume a neurotoxic phenotype (Liddelow et al., 2017). The signaling mechanisms that drive astrocytes toward a protective versus a toxic phenotype are not fully known (Bao, Hua, Keep, & Xi, 2018; H. Wang, Ubl, Stricker, & Reiser, 2002; Y. Wang, Luo, & Reiser, 2007). Cell–cell signaling via proteases acting on cell-specific receptors underlies critical mechanistic steps in neurodevelopment and disease (Ben Shimon et al., 2015; Burda, Radulovic, Yoon, & Scarisbrick, 2013; De Luca, Virtuoso, Maggio, & Papa, 2017; Vaughan & Cunningham, 1993; Wagner et al., 1989; Yoon, Radulovic, Drucker, Wu, & Scarisbrick, 2015). The protease activated receptor (PAR), resides in multiple brain cell types, and the majority of PARs are found on astrocytes (Junge et al., 2004; H. Wang, Ubl, & Reiser, 2002) and endothelial cells, with lesser densities on neurons, pericytes, and oligodendroglia. Circulating serum proteases, notably thrombin, fibrinogen, plasmin, kallikreins, and activated protein C (APC), enter the central nervous system (CNS) via injured blood brain barrier (De Luca et al., 2017; Petersen, Ryu, & Akassoglou, 2018; Radulovic et al., 2015). The action of thrombin on PAR1 is limited by endogenous antagonists, including protease nexin-1(PN-1) (Choi, Suzuki, Kim, Wagner, & Cunningham, 1990; Mirante et al., 2013).
During translational studies of cerebral ischemia, we noted that neurons contained the gene for prothrombin (also called Factor II or FII) and that neurons actively transcribe the protein (Chen et al., 2012), a finding documented previously (Dihanich, Kaser, Reinhard, Cunningham, & Monard, 1991; Weinstein, Gold, Cunningham, & Gall, 1995). Prothrombin (FII) is converted to thrombin (activated FII, aFII) by factor Xa, which is also expressed in rat brain (Shikamoto & Morita, 1999; Thevenet, Angelillo-Scherrer, Price, & Hirt, 2009). We wondered whether protease actions, via PARs could represent a more generalized and important signaling mechanism within the CNS. That is, we asked whether injured neurons use thrombin when signaling glial cells in the neurovascular unit. Brain-derived thrombin has been proposed as a signaling molecule in neurodegeneration previously (Rohatgi, Sedehizade, Reymann, & Reiser, 2004), including studies of ischemic hippocampal slices (Stein et al., 2015). Using genomic and pharmacological methods in animal stroke models and in cell culture, we sought to determine whether neuron-generated thrombin acts on astrocytes to activate a protective astrocyte phenotype.
2 |. METHODS
2.1 |. Animals
All experiments were performed following protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Cedars-Sinai Medical Center as per the NIH Guide for the Care and Use of Laboratory Animals. Our methods also included randomization, blinding, and statistical procedures as per the recommended Stroke Therapy Academic Industry Roundtable (STAIR) and RIGOR guidelines (Fisher et al., 2009; Lapchak, Zhang, & Noble-Haeusslein, 2013). Prothrombin conditional knockout Mice (FIIf/f) mice were a generous gift from Dr. Eric Mullins at Children’s Hospital Research Foundation, Children’s Hospital Medical Center, Cincinnati, OH. The FIIf/f mice were crossed with C57BL/6-Tg (Nes-cre/ERT2)KEisc/J Mice (Stock no. 016261 Jackson Labs) to create NestinERT2FIIf/f line. Upon exposure to tamoxifen, Cre recombinase causes recombination between LoxP sites resulting in prothrombin knockout (Exons 3–9) in Nestin positive cells (see Figure S1). Wild-type C57/bl6-J mice were purchased from Jackson Labs and PAR1 knockout animals were kindly provided by Dr. Shaun Coughlin (University of California, San Francisco). All animals were aged 8–12 weeks at the beginning of the study. Genotyping of all the animals were confirmed by PCR (Primers list in Table S1).
2.2 |. Mouse middle cerebral occlusion model
Wild type (wt) and NestinCreERT2FIIf/f mice were injected intraperitoneally daily with (random assignment) vehicle or tamoxifen (Sigma H7904) (75 mg/kg dissolved in 10% EtOH and 90% corn oil) for 5 days. Both vehicle and tamoxifen injected animals were allowed 7 days for tamoxifen clearance (t1/2 14–16 hr). Another 7 days after end of the injections (total 14 days after injections began) animals were divided into two groups randomly. One group of animals underwent 2 hr middle cerebral artery occlusion (MCAo). The second group of animals were injected stereotaxically in the medial striatum (coordinates from bregma: Anterior–posterior +0.2 mm; medial-lateral left 2 mm; dorsal-ventral 3.5 mm) with lentivirus (source) containing mouse prothrombin gene or an empty vector and after 7 more days (total 21 days after injections) subjected to 2 hr MCAo. The viral vector was validated in HEK cells (Figure S5) and with gene sequencing. Similarly, age-matched PAR1−/− and wt animals were also subjected to 2 hr MCAo. The MCAo was induced as described previously (Rajput et al., 2014; Van Winkle et al., 2013). Briefly, animals were anesthetized with 4% isoflurane mixed in oxygen and nitrous oxide (30:70) and received 2 MDa dextran conjugated with fluorescein isothiocyanate (FITC-Dextran) (Sigma–Aldrich, FD2000S) in phosphate-buffered saline (PBS). Cerebral blood flow was monitored using laser Doppler flowmetry (LDF) by fixing a probe on the skull, 4-mm lateral to bregma (Moor Instruments, Devon, United Kingdom). Nylon 6–0 silicon-coated suture was inserted and advanced 10 mm from the carotid bifurcation into the internal carotid artery and MCA occlusion verified using LDF. After 2 hr the suture was removed, and reperfusion confirmed with LDF. Animals survived for 24 hr after stroke and neurological functions were examined during reperfusion and 24 hr after onset of ischemia using a rating scale we have validated previously (Van Winkle et al., 2013). Animals were killed with an overdose of ketamine and xylazine, and then intracardially perfused with saline and 4% paraformaldehyde. The brains were fixed and cryopreserved in 30% sucrose solution for sectioning. The surgeon, behavioral raters, and the histology image analysts were all blinded to the genomic and treatment status of the mice. Animals with >80% decrease in LDF after occlusion and complete recovery after suture withdrawal were included in this study. Samples sizes are based on our prior use of this model, from which we estimated a Cohen’s d between 1.2 and 1.3 for most measures: this yields a sample size between 6 and 8 given a power of 0.6, alpha 0.05 (R version 3.4.2, library pwr).
2.3 |. Primary neuronal culture
Primary neuronal cultures were prepared from the striatum of E16-E18 Sprague–Dawley rat (source) embryos and E16-E18 NestinCreERT2FIIf/f or C57BL/6J mouse embryos according to our previously published protocol (Rajput et al., 2014). Briefly, the striatum was carefully separated and isolated, cleaned of meninges, and finely minced. The tissue was digested in 0.25% trypsin for 5 min in a 37°C water bath with occasional gentle shaking. DNAse I was added to the cells at 37°C for another 5 min. The cells were suspended in 50 ml warmed Neurobasal medium and centrifuged for 5 min at 1000g. The cell pellet was resuspended in prewarmed complete media (phenol red free Neurobasal Media containing 10% horse serum, 1% Glutamax, 1% pyruvate, 1% penicillin/streptomycin, and 1% B27 supplement, source) and then filtered through a 70 μM diameter membrane, removed, suspended, and counted for total cell concentration before plating. Neurons were plated on 96-well or 6-well plates coated with poly-D-lysine. After 3 days the cells were treated with 5-fluoro-2′-deoxyuridine for 24 hr to suppress glia. Experiments were performed on confluent cultures after 10–14 days. For conditional knockdown of prothrombin in neuronal cultures from NestinCreERT2FII f/f mice, 7 days after isolation, the neurons were treated with 5 μM of 4-OH tamoxifen for 24 hr followed by two media changes in 3–4 days to clear any remaining tamoxifen from the medium.
2.4 |. Primary astrocyte cell culture
Primary astrocytes were isolated from cerebral cortices of P1/P2 Sprague–Dawley rats or P0/P1 NestinCreERT2FIIf/f or C57BL/6J or PAR1−/− mice. Astrocytes were isolated from the striatum, which was separated and isolated, cleaned of meninges, and finely minced. The tissue was digested in 0.25% trypsin for 5 min in a 37°C water bath with occasional gentle shaking. DNAse I was added to the cells at 37°C for another 5 min. The cells were suspended in 50 ml warmed astrocyte medium and centrifuged for 5 min at 1000g. The cell pellet was resuspended in prewarmed astrocyte media and then filtered through a 70 μM diameter membrane, removed, suspended, and counted for total cell concentration before plating. The cells were initially grown in T75 flasks in DMEM (phenol red free) containing 10% fetal bovine serum, 1% Glutamax, 1% pyruvate, and 1% penicillin/streptomycin and 1% N2 supplement. After 2 days the cells were shaken at 200 rpm for 10 min to remove the floating microglial cells. Cell were washed with PBS, dissociated with trypsin, and plated on 24-well plates at a concentration of 2.5–3 × 105 cells/well. This procedure leads to a cell culture in which at least 95% cells are astrocytes (see Figures S4 and S6).
2.5 |. Oxygen-glucose deprivation
Oxygen-glucose deprivation (OGD) experiments were performed using glucose-free and serum-free Neurobasal medium for neurons and glucose free and serum free DMEM media for astrocytes (source). For use, medium was bubbled with a combination of 95% N2/5% CO2 for 30 min at 37°C to create “OGD medium.” To initiate substrate deprivation, designated wells were washed with PBS and covered with OGD medium and immediately transferred to an incubator with 95% N2 and 5% CO2 at 37°C. At the end of the prespecified duration of OGD, the plates were removed, and medium was immediately changed back to normal media for respective cell types, neurons or glia. After OGD, the plates were returned to normal conditions (37°C incubator, room air) for 24 hr.
2.6 |. Neuronal and astrocyte culture experiments
We prepared conditioned neuronal OGD media using neurons from wt or from NestinCreERT2FIIf/f embryonic cultures lacking the prothrombin gene (FII KO) that were subjected to 2.5 hr OGD, after pilot data showed 2.5 hr of OGD kills ~80% of neurons. Conditioned neuronal OGD medium (nOM) was centrifuged at 2,000g for 10 min to remove cell debris; supernatant was removed and stored at −80°C. Wild type astrocytes cultured on 24-well plates were treated with nOM for one of several prespecified time points ranging from 0 to 120 min. After nOM treatment, the astrocytes were fixed with 4% PFA and stained for glial fibrillary acidic protein (GFAP) and Serpin-A3N to document activation. Then, nOM from FII KO neurons was supplemented with 30 U/ml of high purity bovine thrombin (BioPharm Laboratories LLC. Catalogue no. 91–035-100, thrombin specific activity->2,200 US units/mg powder) and added onto wt astrocytes for 0–120 min, after which the astrocytes were fixed for staining. For controls, we collected media from neurons not subjected to OGD (nM).
To assess thrombin-mediated astrocyte activation and the role of PAR1 receptors, primary cultures of rat astrocytes were treated with escalating doses of thrombin (0–100 U/ml) added into the culture media, after which the cultures were stained for astrocyte activation. We prepared nOM from primary rat neuronal cultures exactly as for mouse cells, but the duration of OGD was 2 hr. To inhibit or stimulate PAR1 in primary rat astrocyte cultures, we added nOM and various doses of thrombin inhibitor argatroban (1–100 μM) or PAR1 antagonist SCH-79797 (1–100 μM) or PAR1 peptide agonist TFLLR-NH2 (1–30 μM) (Tocris). For controls, we used nM in place of nOM. After 120 min, the media was switched to normal astrocyte growth media. We chose 120 min exposure time because preliminary data showed astrocytes were maximally activated if treated for 120 min with nOM (Figure S7). We cultured primary astrocytes from PAR1−/− mice on 24-well plates and treated them with nOM and drugs as above. To demonstrate that PAR1−/− astrocytes were able to activate at all, we treated additional cultures with H2O2 (100 μM) or glutamate (100 μM) for 120 min after which they were fixed with 4% PFA for staining (Figure 4). These treatment groups were needed to establish that the cells retained the capacity to mount a normal response to injury (free radical or excitotoxicity) in PAR−/− cells.
FIGURE 4.
Effect of PAR1 KO on in vivo astrocyte activation during focal cerebral ischemia. To assess the role of PAR1 in mediating astrocyte activation, we conducted 120 min MCAo in wt and PAR1 constitutive KO (PARKO) mice. We assessed the resulting damage and astrocyte activation 24 hr later. (a) We validated that the PARKO mice expressed an abnormal PAR1 receptor using PCR. The normal gene product is 175 kb while the mutated gene containing an insertion is 225 kb. The inserted segment blocks transcription of a functional PAR1 receptor (Connolly, Ishihara, Kahn, Farese, & Coughlin, 1996). (b) The area of vascular damage (leakage)—assessed as in Figure 1—was significantly reduced in PARKO compared to wt mice. Representative photomicrographs of the ischemic mouse hemisphere are shown above and mean ± SEM quantification below, n = 4, *two-tailed t test, p < .05. (c) Neuronal damage (assessed with Fluoro-Jade C as in Figure 1) was significantly increased in PARKO compared to wt animals. Representative photomicrographs of the ischemic mouse hemisphere are shown above and mean ± SEM quantification below, n = 4, ***two-tailed t test, p < .001. (d) Astrocyte activation markers GFAP and Serpin-A3N were significantly reduced in PARKO compared to wt mice after MCAo. ***Two-tailed t test, p < .001. Representative photomicrographs are shown in (e). (f) We added 120 min nOM or escalating doses of thrombin (1, 10, 30, and 50 U/ml) to stable astrocytes cultured from PARKO mice. We measured astrocyte activation after 120 min dwell time. There was no effect of nOM from wt or FII KO neurons, nor did any dose of thrombin activate astrocytes raised from PARKO mice. For a positive non-thrombin control, we used 100 μm glutamate and 100 μm H2O2, both of which significantly increased astrocyte activation, two-way ANOVA p < .0001, followed by Tukey’s post-hoc test for multiple comparisons, *p < .05 and ***p < .001
2.7 |. Mass spectrometry and proteomic analysis
To assess for prothrombin in nOM and nM, media was collected from FII KO and wt neurons and supplemented with 1X Protease inhibitor (Pierce™ 88,666). Protein was denatured by adding 2% SDS and then sonicating for 10 min with 10 s on/off cycles at an amplitude of 70%, followed by acetone precipitation. Precipitated protein was resuspended in 50 mM ammonium bicarbonate containing 0.1% RapiGest (Waters) and protein amount was determined using the bicinchoninic acid assay (BCA assay, Pierce). Then 200 μg protein per sample were reduced using 1 mM TCEP, alkylated with 5 mM iodoacetamide and digested with ~1:40 Trypsin-LyC combination (Promega), desalted and completely dried in a SpeedVac. Liquid chromatography (LC) MS/MS was carried out on a Dionex Ultimate 3000 NanoLC connected to an Orbitrap Elite (Thermo Fisher) equipped with an EasySpray ion source as previously reported (Kooij et al., 2014). The mobile phase A was comprised of 0.1% aqueous formic acid and mobile phase B was 0.1% formic acid in acetonitrile. Peptides were loaded onto the analytical column (PepMap RSLC C18 2 μm, 100 Å, 50 μm i.d. × 15 cm) at a flow rate of 300 nL/min using a linear AB gradient composed of 2–25% A for 185 min, 25–90% B for 5 min, then at isocratic hold at 90% for 5 min with re-equilibrating at 2% A for 10 min. Temperature was set to 40°C for both columns. Nanosource capillary temperature was set to 275°C and spray voltage set to 2 kV. MS1 scans were acquired in the Orbitrap Elite at a resolution of 60,000 FWHM with an AGC target of 1 × 106 ions over a maximum of 500 ms. Protein quantification was carried out by averaging the MS2 spectral count among biological replicates. MS2 spectra were acquired for the top 15 ions from each MS1 scan in normal scan mode in the ion trap with a target setting of 1 × 104 ions, an accumulation time of 100 ms, and an isolation width of 2 Da. Normalized collision energy was set to 35% and one microscan was acquired for each spectra (Luo et al., 2010). Preparative data analysis and peptide identification search were performed using the raw MS/MS files after they were converted to mzXML using MSConvert. The mzXML files were searched against the Swiss-Prot reviewed mouse FASTA database (ver. 2017–03; 32,741 proteins and decoys) (Apweiler et al., 2004) using the Sorcerer 2™-SEQUEST® algorithm (Sage-N Research). Target-decoy modeling of peptide spectral matches was performed using the following criteria: Fragment tolerance: 1.00 Da; Parent Tolerance: 0.040–0.160 Da; Fixed modification: +57 on C (carbamidomethyl); Variable modification: +16 on M (oxidation); Enzyme: Trypsin with three max missed cleavages. Post-search analysis was performed using Scaffold 3 version 1.4.1 (Proteome Software, Inc., Portland, OR) with protein and peptide probability thresholds set to 95%. Thrombin was identified using 14 unique peptide sequences. Quantification was based on total spectral counts.
2.8 |. Immunohistochemistry in sections
Using a sliding microtome, 30-μm-thick brain sections were cut and free-floating sections were collected in PBS. Brain sections were incubated in 0.2% Triton X-100 in PBS for 15 min and followed by three subsequent washes with PBS. Sections were then incubated in 5% normal goat serum for 1 hr at room temperature and followed by overnight incubation with rabbit anti-GFAP (1:2000) and goat antiSerpin-A3N (1:300) (Table S2). Following three subsequent washes in PBS, brain sections were incubated with Alexa 488 (Green) and Cy3 (Red) conjugated secondary antibodies for 1 hr at room temperature. The sections were washed, mounted on slides, and viewed under an Olympus (FV10i) microscope. To determine the GFAP and SerpinA3N intensity, images were taken at 1× magnification with laser intensities and gain constant for both the channels in all study groups. Three sections 50 μM apart were selected from each brain by an examiner blinded to the genotype and treatment group. Sections were analyzed using Image J (version 1.51). Images were converted to 8-bit grey scale and a region of interest (ROI) was drawn to include the ischemic region. Background intensity from sections stained with secondary antibody alone was subtracted. Threshold correction was performed for all treatment groups and kept consistent between experimental groups and replicates. Integrated fluorescence intensity from the ROI is reported as astrocyte activation (fluorescence intensity arbitrary units [a.u.]).
2.9 |. Immunocytochemistry in culture
Astrocytes cultures were fixed with 4% paraformaldehyde and stained for GFAP and Serpin-A3N. Briefly, coverslips were incubated in 0.02% Triton X-100 in PBS for 15 min and followed by three subsequent washes with PBS followed by incubation in 5% normal goat serum for 1 hr at room temperature and followed by overnight incubation with rabbit anti-GFAP (1:2000) and goat-Serpin-A3N (1:300). Coverslips were incubated with Alexa 488 and Cy3 conjugated secondary antibodies for 1 hr at room temperature. Cover slips were washed and mounted on slides and viewed as above. Each experiment was conducted in triplicate and the observer was blinded to the treatment conditions. Each coverslip was divided into four quadrant and 12–14 images at 10× resolution were captured from each quadrant. GFAP and Serpin-A3N florescence intensity was quantified using NIH Image J software as described previously (Lyden et al., 2018) (version 1.51).
2.10 |. Vascular leakage and neuronal injury
Vascular disruption was imaged as leakage of 2 MDa-dextran FITC and cellular injury was imaged using Fluoro-Jade C staining in 30 μm brain sections, imaged at low power using epifluorescence microscopy with a highly sensitive CCD camera (Apogee, Alta U32). The FITC-dextran signal was quantified using Image-Pro Plus (Media Cybernetics) as described previously (Chen et al., 2009). In brief, an operator blind to the subject’s group examined each section and adjusted the brightness and contrast levels to optimize the appearance of the fluorescence. The operator outlined the striatum using standard anatomic boundaries (Franklin & Paxinos, 1997; Paxinos & Watson, 1986), and applied semiautomatic thresholding and segmentation to measure the total area of fluorescence. For quantification of Fluoro-Jade C positive neurons, an operator blind to the treatment groups converted the images to 8-bit grey scale and used the pseudo flat field plugin render the fluorescence uniform in the all sections. Using the nucleus counter particle analysis plugin the number of neurons from each brain section were automatically counted and then summed over all sections.
2.11 |. Cell death assay
Cell death was measured using lactate dehydrogenase (LDH) activity in the media (Cytotoxicity Detection KitPLUS (LDH) Roche Applied Science, Germany catalog number 04744934001) as described (Rajput et al., 2014). Media was centrifuged at 15,000 rpm for 10 min to remove debris, then 100 μL media was transferred into a 96-well plate and 100 μL LDH reaction mixture was added. The plates were covered with aluminum foil and incubated with LDH substrate for 30 min at room temperature. Absorbance was measured using a spectrophotometer at 490 nm. After the remaining intact cells were lysed and assayed, the LDH activity in supernatant was normalized to the total LDH in the well and expressed as a percent.
2.12 |. Cell viability assay
Cell survival was quantified by measuring the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to produce a dark blue formazan. The colorimetric assay correlates with cellular metabolic activity due to function and integrity of mitochondria. Metabolism declines with decreasing numbers of cells but increases in the setting of cell proliferation. At the end of each experiment, the cell culture medium was removed and MTT was added to each culture well at final concentration of 0.5% MTT solution (wt/vol). After incubation for 3 hr at 37°C, the medium was replaced with solution containing 0.4 N HCI in 99% isopropanol for 1 hr. The formation of formazan was measured by recording the absorbance at a wavelength of 570 nm and a reference at 630 nm.
2.13 |. RNA-Seq analysis
Primary rat astrocytes were lysed using lysis media and total RNA extraction was performed using RNeasy mini kit (Qiagen, catalog no. 74104) according to the manufacturer’s instructions. Library construction was performed using the Illumina TruSeq Stranded mRNA library preparation kit. Briefly, total RNA samples were assessed for concentration using the Nanodrop 8000 (Thermo Scientific) and quality using the 2100 Bioanalyzer (Agilent). One microgram of total RNA per sample was used for poly-A mRNA selection using streptavidin-coated magnetic beads. cDNA was synthesized from enriched and fragmented RNA using reverse transcriptase (Super-Script II, Invitrogen) and random primers. The cDNA was further converted into double-stranded DNA, and the resulting dsDNA was enriched with PCR for library preparation. The PCR-amplified library was purified using Agencourt AMPure XP beads (Beckman Coulter). The concentration of the amplified library was measured with a NanoDrop spectrophotometer and an aliquot of the library resolved on an Agilent 2100 Bioanalyzer. Sample libraries were multiplexed and sequenced on a NextSeq 500 platform (Illumina) using 75 bp single end sequencing. On average, about 20 million reads were generated from each sample. Raw reads obtained from RNA-Seq was aligned to the transcriptome using STAR (version 2.5.0) (Dobin et al., 2013)/RSEM (version 1.2.25) (Li & Dewey, 2011) with default parameters, using Rnor 6.0 transcriptome reference downloaded from http://uswest.ensembl.org/. Expression counts for each gene in all samples were normalized by a modified trimmed mean of the M-values normalization method and unsupervised principal component analysis (PCA) was performed with DESeq2 Bioconductor package version 1.16.1 in R version 3.4.2. Each gene was fitted into a negative binomial generalized linear model, and the Wald test was applied to assess the differential expressions between two sample groups by DESeq2. Benjamini and Hochberg (B–H) procedure was applied to adjust for multiple hypothesis testing, and differential expression gene candidates were selected with a false-discovery rate (FDR) less than 5%. Venn plot was drawn by using VennDiagram package version 1.6.20. Likelihood ratio test integrated in DESeq2 was used to examine two models for the counts, a full model including all treatments (control, Thrombin 1 U, Thrombin 50 U, nM, nOM (60 min.DT) and nOM (120 min.DT)) and a reduced model without treatments to find the genes differentially expressed in any or all treatments. Differential expression gene with FDR < 1% after B–H correction were mean centered, and dendrograms were calculated by the hclust hierarchical clustering method and were plotted with heatmap.2 software in the g-plots package (version 3.0.1) in R version 3.4.2.
2.14 |. Statistical analysis
All results are summarized as mean ± SD without assumptions regarding normality. Where applicable, one-way or two-way ANOVA was used to test hypotheses, and for post-hoc correction for multiple comparisons we used Tukey’s procedure or other procedure as specified. Sample sizes for each experiment were based on prior results in our laboratory assuming power 60%, alpha 0.05, and an estimated a Cohen’s d between 0.5 and 1.3 for most measures (R version 3.4.2, library pwr) as well as Graph Pad Prism 7.0 was used.
3 |. RESULTS
3.1 |. Astrocytes respond to neuron-generated thrombin during ischemia to induce protection
Astrocytes transform from a resting state to an activated state following an injurious mechanism (e.g., ischemia or trauma) directly if the insult is sublethal (Sweeney et al., 2017), or via an indirect mechanism if adjacent cells, especially neurons, signal astrocytes to initiate protective functions (Y. Wang et al., 2007; Xing et al., 2014). To demonstrate a role for neuronal prothrombin in brain, we created an inducible knockout mouse to remove neuronally derived thrombin using Cre recombinase under the control of an estrogen response element (Figure S1). Adult knockout using an inducible method avoids confounding compensatory effects present in transgenic animals that constitutively lack a gene throughout the lifespan. After tamoxifen treatment and induced knockout, we performed standard MCAo as sketched in Figure 1a using our published method (Van Winkle et al., 2013). In animals treated with vehicle alone (intact neuronal prothrombin), we found robust activation in the ischemic zone, assessed with two different markers, GFAP and Serpin-A3N (Figure 1b,c). The toxic phenotype associated marker LCN2 did not increase (Figure 1b). After tamoxifen-induced knockout of neuronal prothrombin (FII KO), however, astrocyte activation failed to appear after MCAo (Figure 1b, c). This finding suggested that neuronal prothrombin was sufficient for astrocyte activation after ischemia. To confirm that prothrombin expression was sufficient to activate astrocytes, we restored prothrombin gene expression using a lentiviral vector (Figure S2) targeting neurons in FII KO mice. Restoration of prothrombin gene transduction partially restored astrocyte activation after ischemia in FII KO animals (Figure 1b,c). Neuronal prothrombin knockout was associated with significantly greater ischemic neuronal injury (Figure 1d,e) with no effect on vascular disruption (Figure 1f), suggesting that the loss of neuronal prothrombin resulted in greater ischemia-induced damage, perhaps related to reduced astrocyte mediated neuroprotection. To eliminate the possibility that lentiviral infection alone could induce nonspecific astrocyte activation, we evaluated viral infection in control experiments (Figure S3).
FIGURE 1.
Neuron-generated thrombin is critical for astrocyte mediated neuroprotection. (a) in NestinCreERT2FII f/f mice (Figure S1) we induced prothrombin knockout with tamoxifen (or vehicle, randomized) for 5 days. At random, 9 days later the animals received stereotactic injection of lentivirus containing the FII gene, or an empty vector, or no viral injections. All animals underwent MCAo 21 days after tamoxifen or vehicle and sacrifice 24 hr after MCAo. (b) Mid-parietal sections stained for GFAP (red) and lipocalin-2 (LCN2) (green) show robust GFAP expression in the ischemic territory of vehicle-treated NestinCreERT2FIIf/f mice, but less so after tamoxifen-induced FII KO. In the ischemic zone, there were few GFAP positive cells in FII KO mice (inset). Lentiviral FII gene replacement (Figure S2) restored GFAP; empty virus did not. (c) Loss of neuronal prothrombin resulted in significant loss of the GFAP and Serpin-A3N activation markers (see Figure S3d for LCN2 measures). Lentiviral prothrombin gene restoration showed astrocyte activation similar to tamoxifen-induced KO mice. *** Two-way ANOVA p < .001, Bonferroni comparison p < .05, n = 5). We validated the lentiviral vectors in several control animals (Figure S3b,c). (d, e) Increase in Fluoro-Jade C positive neurons was observed in FII KO compared to vehicle-treated NestinCreERT2FII f/f mice and wild type. The FII KO animals treated with tamoxifen and lentivirus showed significantly increased neuronal cell death compared to animals treated with control virus injection, and to true wt or vehicle-treated NestinCreERT2FII f/f mice. ***Two-way ANOVA p < .001, Sidak’s multiple comparison p < .05, n = 5 per group. Scale bar panel inset: 50 μm. (f) Leakage of FITC conjugated 2 MDa dextran demarcates regions of profound vascular damage. Neuronal FII KO had no effect on vascular disruption. Similarly, FII KO animals injected with FII lentivirus or empty virus showed no discernable changes in FITC-dextran leakage. Scale bar panel inset: 50 μm
3.2 |. Thrombin is generated by injured neurons to signal and activate astrocytes
We established that cultured mouse astrocytes do not express prothrombin (Figure S4). Then, to establish that nOM activated the astrocytes in a thrombin-dependent manner, we cultured cortical neurons with or without the prothrombin gene knocked-out, prepared nOM using 150 min OGD, and applied the conditioned nOM to astrocytes (Figure 2a). The nOM from wt and thrombin-deficient neurons appeared to activate astrocytes equivalently after short (15–60 min) dwell times (Figure 2b,c). After 120 min dwell time, however, thrombin-deficient nOM failed to activate astrocytes, compared to the wt media. Longer dwell times failed to show astrocyte activation (Figure S7).To confirm the specificity of this effect, we added 30 U/ml thrombin to the thrombin-deficient nOM and observed in a dwell-time-dependent manner that thrombin restored the astrocyte-activating property of nOM (Figure 2d). We confirmed the presence of thrombin in nOM using mass spectrometry, and confirmed it was below the limit of detection in the FII KO media (Figure 2e, Table S3 shows the spectral count from mass spectroscopy data and Table S4 shows the peptide sequences used to identify the protein). These data demonstrate that injured neurons secrete factors that activate astrocytes, one of which is thrombin. Thrombin begins to induce astrocyte activation by 2 hr of contact time. At shorter contact times, other factors appear to activate astrocytes, although to a significantly lesser degree (for both GFAP and serpin-A3N: ANOVA followed by Tukey’s multiple comparisons, p < .01 for 120 min vs. all other dwell times).
FIGURE 2.
Conditioned neuronal media containing thrombin induces astrocyte activation. (a) Cultured neurons from NestinCreERT2FII f/f mice were treated with tamoxifen (to induce FII KO) or vehicle for 24 hr followed by 3–4 days and at least two media changes to clear the tamoxifen. Conditioned neuronal OGD media (nOM) was collected after 150 min OGD and applied onto stable wt astrocyte cultures for varying dwell times (DT). (b) Astrocytes treated with nOM contained reactive astrocyte markers using GFAP (red), Serpin-A3N (green) and DAPI (blue); scale bar 50 μm. (b-i) astrocytes treated with non-OGD neuronal media (b-ii) astrocytes treated with nOM from vehicle-treated NestinCreERT2FII f/f showing increased expression and stellate transformation of astrocytes (b-iii) astrocytes treated with nOM from FII KO showing less expression of GFAP and Serpin-A3N. (b-iv) Astrocyte activation restored to cells treated as in (b-iii) by adding 30 U of thrombin to nOM derived from the FII KO cells. (c) We placed nOM derived from FII KO (labeled FII−/−) or the same cells without tamoxifen induction (labeled as “wt”) on astrocytes for varying dwell times before fixation and staining. Regardless of dwell time (15, 30, 60, and 120 min) we found significantly increased activation (GFAP or Serpin-A3N). After 120 min dwell time, FII KO significantly abrogated activation; at other dwell times there was a variable effect of FII KO on activation. Two-way ANOVA, p < .001, Tukey’s post-hoc comparisons p < .01. (d) We found a direct and linear relationship between dwell time and activation measured with GFAP and Serpin-A3N after 30 U/ml thrombin to nOM generated from FII KO neurons and measured activation after 15, 30, 60, or 120 min dwell time. Astrocyte activation was statistically significantly elevated after all dwell times, compared to control, and each dwell time was significantly greater than all lesser dwell times using two-way ANOVA (p < .001) followed by Tukey’s post-hoc test for multiple comparisons, p < .05. Data summarized as mean ± SEM. n = 1,200–1,500 astrocytes examined from three different cultures for each dwell time. (e) Mass spectrometry confirmed the nOM from wt contained prothrombin while nOM from FII KO neurons did not. Media was obtained from neuronal cell cultures of FII KO mice (as in Figure 1), after 150 min OGD (labeled nOM for OGD neuronal media) or normoxic conditions (labeled NM for neuronal media). Control was not different from any condition other than nOM obtained without tamoxifen-induced gene deletion. One-way ANOVA, p < .001, Bonferroni’s correction for multiple comparison (p < .01). OGD, oxygen-glucose deprivation
3.3 |. Astrocytes respond to neuron-generated thrombin via the PAR1 receptor
We sought to confirm thrombin’s astrocyte-activating effect by adding thrombin onto stable, nonischemic, cultured rat astrocytes. In pilot experiments, we demonstrated the effect of various OGD times and several thrombin doses on cultured rat neurons and astrocytes (- Figure S5): 80% of neurons and astrocytes were killed by 2 or 8 hr of OGD, respectively (Lyden et al., 2018). Cultured rat astrocytes did not express prothrombin (Figure S6). In response to applied thrombin, astrocyte activation markers were increased significantly in a dose-dependent manner (Figure 3a). The direct thrombin inhibitor argatroban blocked this effect when added to the culture coincidently with thrombin, consistent with our prior findings in stroke models (Lyden et al., 2014; Rajput et al., 2014). We prepared nOM from rat neuronal cultures after 120 min OGD (approach shown in Figure 2a) and applied the 120 min nOM to stable, nonischemic rat astrocyte cultures (Figure 3b). We selected the 120 min nOM and used a dwell time of 120 min on activated astrocytes after dose-ranging pilot studies (- Figure S7). Again, the activating effect could be inhibited by argatroban dose-dependently (Figure 3b). Interestingly the U-shaped hormetic response confers the classic pharmacological response of drug on cells as in this case effect of thrombin on activated astrocytes with 10 and 30 μM bringing the astrocytes to the region of homoeostasis (below the threshold for adverse response and low dose deficiency range). Similar effect is being observed when nOM is treated with different doses of argatroban to test for thrombin activity (Figure S9). To test the PAR1 specificity of this nOM effect, we added PAR1-active agents to the astrocyte cultures. The PAR1 antagonist SCH79797 blocked astrocyte activation by nOM in a dose-dependent manner (Figure 3c). The peptide TFLLR binds to the thrombin binding site on PAR1 and prevents thrombin cleavage of the PAR1 extracellular peptide; this treatment also interfered with astrocyte activation by nOM (Figure 3d). Taken together, these data show that pharmacological interference with protease activation of PAR1 blocks the astrocyte-activating effect of nOM, confirming that neurons use thrombin to activate astrocyte PAR1 in a paracrine, dose-dependent manner.
FIGURE 3.
Thrombin activates astrocytes via PAR1. We sought to confirm that PAR1 is necessary during thrombin-evoked astrocyte activation in cultured rat astrocytes. (a) Escalating doses of thrombin (0, 1, 10, 50, and 100 U) were added onto cultured rat astrocytes for 120 min; activation was assessed immediately as described in Figure 1. Illustrative photomicrographs are shown above each condition [GFAP (red); Serpin-A3N (green); scale bar 50 μm]. Activation as measured with GFAP and Serpin-A3N showed an inverse-U shaped dose response to thrombin, with a peak at 50 U. This activation could be blocked by 10 μM of the direct thrombin inhibitor, argatroban. All doses of thrombin other than 100 U elevated activation significantly. (b) Cultured rat neurons were subjected to 120 min OGD and neuronal OGD media (nOM) was collected. Increasing dose of argatroban (1, 10, 50, and 100 μm) and nOM were placed on cultured astrocytes and incubated for 120 min; activation was assessed immediately. In a U-shaped dose-dependent manner, increasing concentrations of argatroban significantly blocked astrocyte activation measured with GFAP and Serpin-A3N. (c) As in panel (b), the PAR1 active antagonist SCH 79797 (1, 10, 30, and 100 μm) blocked the activating effect of nOM in a U-shaped dose-dependent manner. (d) The PAR1 active peptide agonist TFLLR-NH2 interfered with astrocyte activation caused by 120 min nOM. In all four panels, data are presented as mean ± SEM; overall two-way ANOVA was significant (p < .001) with Tukey’s post-hoc test to correct for multiple comparisons (p < .05). OGD, oxygen-glucose deprivation
3.4 |. Astrocyte PAR1 mediates neuronal thrombin activation signaling
To demonstrate the required role of PAR1 mediating astrocyte activation, we conducted MCAo in mice lacking PAR1 constitutively in all cells (PARKO), including neurons and endothelial cells. Constitutive deletion of PAR1 in PARKO mice was confirmed by PCR (Figure 4a). After 120 min MCAo, the area of neuronal injury and severe vascular disruption was smaller in PARKO compared to wt mice (Figure 4b,c). We have shown previously that severe vascular disruption—as labeled by extravasation of 2 MDa FITC-dextran—reliably marks the region of infarction after ischemia (Chen et al., 2009). Further, the area of neuronal injury, as labeled by Fluoro-Jade C, was smaller in the PARKO mice (Figure 4c). The reduced ischemic injury in these mice has been well documented, and may reflect the absence of PAR1 on neurons (Rajput et al., 2014). The expression of astrocyte activation markers was severely reduced in the PARKO mice compared to wt (Figure 4d,e), consistent with our hypothesis that PAR1 is needed for astrocyte activation in response to injury such as ischemia. To further confirm the critical role of PAR1 in mediating astrocyte activation, we treated cultured PARKO astrocytes with thrombin (1–50 U/ml), or nOM from prothrombin deficient or wt neurons. We also stimulated astrocytes directly with other injurious stimuli (reactive oxygen species or glutamate). In cultured PARKO astrocytes, neither nOM nor thrombin caused any activation (Figure 4f). In contrast, both forms of direct injury (H2O2 and glutamate) significantly caused activation, confirming that the PAR1-deficient astrocytes retain the capacity to activate in a non-PAR1-dependent manner. These data confirm the association between ischemia and PAR1 mediated astrocyte activation. The reduced neuronal injury and vascular disruption (Figure 4b,c) in these mice reflect the fact that PAR1 is missing from all cell types, including neurons, and further suggest that PAR1 may mediate multiple contrasting roles in protecting brain during ischemia.
3.5 |. Astrocytes express a protective phenotype in response to neuron-generated thrombin
Upon activation, astrocytes transform from a resting, protoplasmic shape, to an activated stellate shape. Either thrombin or nOM stimulated cultured astrocytes to transform to a stellate, activated shape (Supplemental movies). Limited evidence suggests that activated astrocytes may take on one of two phenotypes, sometimes labeled A1 and A2, although there may not be a strict polarity of activated astrocytes (Liddelow et al., 2017; Zamanian et al., 2012). Previously, a variety of gene expression profiles have been associated with a toxic A1 phenotype and a protective A2 phenotype (Jang et al., 2013; Liddelow et al., 2017). After treating astrocyte cultures with thrombin (1 or 50 U) or nOM (dwell time 60 or 120 min) we extracted RNA and performed RNAseq (Figure 5, Table S5 shows the raw RNA seq data). Of the genes associated with the protective A2 phenotype, thrombin caused an upregulation in many (Figures 5a and S8). No consistent changes were seen in genes typically associated with the toxic, A1 phenotype. Conditioned nOM left to dwell on cultured astrocytes caused downregulation in some A1 genes and upregulation in some A2 genes (Figure 5b). The effect was clearly seen after 120 min dwell time; less so after 60 min. Using PCA and selecting genes with significant expression changes (p < .01), we identified 3,500 significant gene changes following astrocyte exposure to thrombin or nOM (Figures 5c,d and S8). Gene sorting revealed significant overlap comparing thrombin (either dose) against normal media, and less difference between the two doses (Figure 5e). Similarly, the two nOM dwell times differed from normal media, but less so from each other (Figure 5f). These data confirm that astrocyte activation with thrombin or nOM causes gene expression changes consistent with the protective phenotype, although there is not a perfect association.
FIGURE 5.
Expression profile of activated astrocytes. RNA-seq was performed to explore the phenotype of reactive astrocytes treated with thrombin or neuronal OGD media. (a) Heatmap depicting the mean expression of pan-reactive, A1 specific, and A2 specific genes. Most of the pan-reactive genes were upregulated with 50 U of thrombin. A significant increase in A2 specific genes was observed in astrocytes treated with 50 and 1 U of thrombin compared to control. (b) Astrocytes treated with nOM with dwell time of 60 or 120 min. Showed increased reactive astrocyte gene expression. Longer DT appeared to activate more genes. (c, d) Heat maps generated from the total RNA-seq dataset. RNA-seq analysis of differentially expressed genes from in vitro stimulated astrocytes shows 3,500 genes were significantly different (p < .01, ANOVA). (e, f) Venn diagrams showing the number of significantly (p < .05, pair wise analysis) upregulated and downregulated genes. Numbers represent number of genes overlapped between various treatment groups. OGD, oxygen-glucose deprivation
3.6 |. Activated astrocytes protect neurons in a paracrine manner
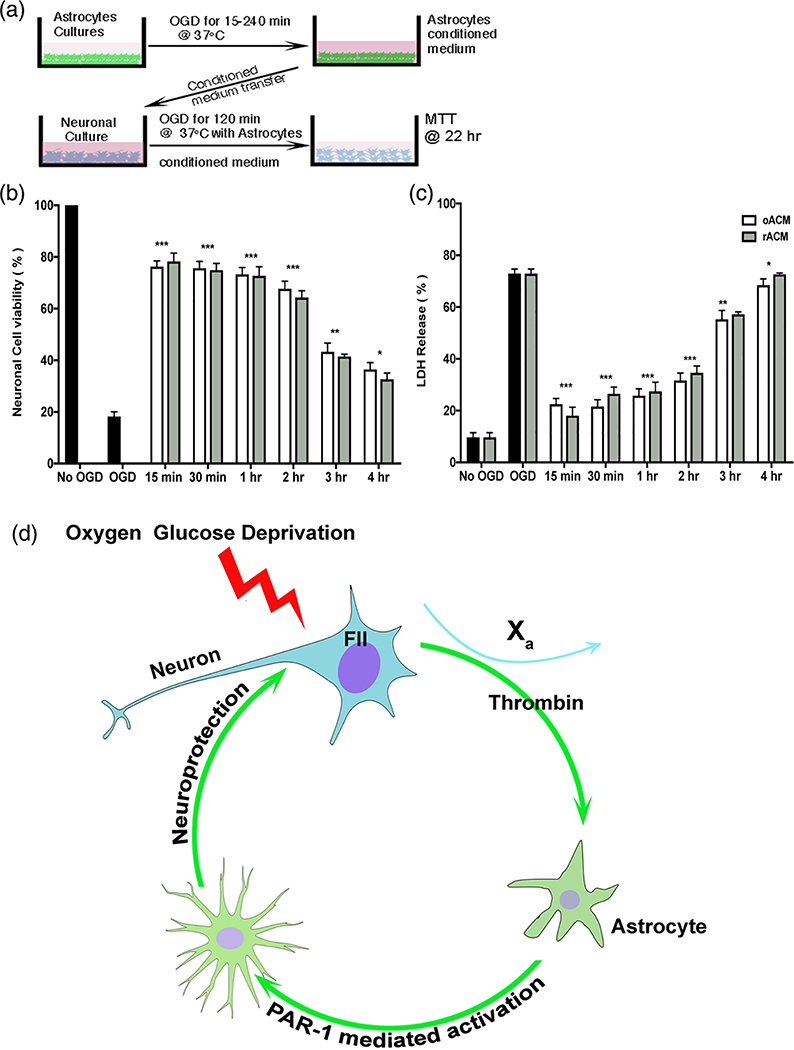
To confirm that astrocyte activation results in neuronal protection, we prepared conditioned media from astrocytes (ACM) after varying durations of OGD (oACM) and after OGD followed by 2 hr reperfusion (rACM, Figure 6a). When oACM or rACM was applied to cultured neurons, up to 80% of OGD-induced neuronal cell death was blocked (Figure 6,c). Astrocyte media prepared after 15, 30, 60, or 120 min OGD showed signification neuronal protection, but 3 or 4 hr OGD produced astrocyte media that was less effective. Astrocyte media remained neuroprotective even after dilution up to 100-fold, suggesting the secretion of large amounts of protective substances. These data suggest a novel hypothesis, shown in Figure 6d, that neurons generate prothrombin which—after conversion to thrombin by local Factor Xa—causes activation of the neighboring astrocytes and the paracrine delivery of protective substances.
FIGURE 6.
Injured astrocyte media protects neurons undergoing OGD. (a) Experimental design to determine whether astrocyte conditioned media protects neurons during OGD. Astrocyte cultures were exposed to OGD for various times (15–240 min) followed by reperfusion for 1 hr. Astrocyte conditioned media after OGD (oACM) or reperfusion (rACM) was added to neuronal cultures prior to 2 hr OGD and 22 hr reperfusion. (b) Cell viability with methyl thiazolyl tetrazolium (MTT) and (c) cell death with the lactate dehydrogenase assay (LDH) were quantified as mean ± SEM. Neurons treated with oAMC showed significantly less cell death and increased cell viability compared to OGD alone. Neurons treated with rACM media were also protected. Two-way ANOVA p < .0001, Sidak’s correction for multiple comparisons, ***p < .0001. (d) Illustration of the hypothesis generated by the data presented here. Neurons exposed to injury (e.g., OGD) release prothrombin (coagulation factor II, or FII) that is converted to thrombin in presence of factor Xa. Thrombin activates nearby astrocytes via PAR1. Activated astrocytes release paracrine neuroprotective factors into the surrounding environment thereby protecting neurons from further injury. OGD, oxygen-glucose deprivation
4 |. DISCUSSION
Our data suggest a central role for astrocyte activation in response to neuron-generated thrombin during ischemia. Astrocytes exerted a powerful protective effect on neurons following thrombin-mediated PAR1 activation, preserving up to 80% of neurons during a standard OGD insult (Figure 6b). Using both an in vivo animal stroke model, and conditioned media transfers in culture, we showed that astrocytes respond to thrombin in a dose-dependent, paracrine manner that requires intact PAR1 on the astrocytes (Figures 2–4). Together, the data suggest that protease/PAR1-mediated communication between neurons and astrocytes constitutes a key mechanism for neuronal protection during ischemia (Figure 6d).
The discovery of the PAR family and their localization to brain cells, has opened a new and increasingly important area of understanding cell–cell communication (Vu et al., 1991). PARs mediate the cell-signaling effect of a variety of serine proteases (Coughlin, 2000; Petersen et al., 2018). In spinal cord, the protease kallikrein-6 appears to mediate communication between oligodendroglia and spinal motor neurons (Burda et al., 2013; Radulovic et al., 2015; Yoon et al., 2015). In brain, the protease/PAR axis appears to play significant roles in development and neurodegeneration (De Luca et al., 2017; Junge et al., 2004; Krenzlin, Lorenz, Danckwardt, Kempski, & Alessandri, 2016; Rohatgi et al., 2004; Xi, Reiser, & Keep, 2013). Recently another protease, urokinase, was shown to activate astrocytes, which then provided a neuroprotective effect (Diaz et al., 2017; Merino & Yepes, 2018), supporting our conceptual formulation.
Neuronal expression of prothrombin, a finding noted previously by others (Dihanich et al., 1991; Riek-Burchardt, Striggow, Henrich-Noack, Reiser, & Reymann, 2002), lacks a clearly delineated purpose in mature brain. The brain also makes an endogenous inhibitor of thrombin, PN-1, which exists in contra-position (Choi et al., 1990; Vaughan & Cunningham, 1993). Exogenously applied thrombin exerts hormetic effects in brain—low thrombin concentration preconditions the brain to resist injury but higher doses kill brain cells (De Luca et al., 2017; Henrich-Noack, Striggow, Reiser, & Reymann, 2006; Vaughan, Pike, Cotman, & Cunningham, 1995)—so we reasoned that neuron-generated thrombin could play a role in initiating brain protective responses. Recent studies have shown major differences in inflammatory response profile with the use of recombinant versus plasma derived thrombin on microglia (Hanisch et al., 2004; Weinstein et al., 2005). Our transcriptomic data confirmed that plasma derived thrombin failed increase gene expression of canonical markers of neuroinflammation in astrocytes (Figure S10). Specifically, we asked whether neuronally derived thrombin acts on astrocyte PAR1 to induce protective responses and whether a dose response effect could be shown. Among the many forms of cell–cell communication in the CNS, proteolysis of the extracellular PAR peptide tail, and subsequent binding of the tethered ligand, is relatively novel (Coughlin, 1999). Our data establish a role for the thrombin/PAR1 axis mediating protective signaling between astrocytes and neurons during ischemia. We are aware of the limitations of Nestin as a driver therefore, we used our conditional knockout animals by treating with tamoxifen well before the stroke. We also confirmed our major findings in vitro and tamoxifen was administered to the neurons in a stable, resting state. As detailed in Figures S4 and S6 there is no thrombin detected in astrocytes in our cultures and was confirmed by RNAseq data, we also confirmed no colocalization of nestin with GFAP and IBA-1 at 24 hr timepoint in mice subjected to MCAo (Figure S11).
More fundamentally, our data provide an explanation for a growing assemblage of data implicating astrocytes as important players in mediating brain protection (De Luca et al., 2017; Liddelow et al., 2017; Nedergaard, Ransom, & Goldman, 2003; Pitt et al., 2017; Sweeney et al., 2017; Vance, Rogers, & Hermann, 2015; Xing et al., 2014). In development, astrocytes support local neuronal circuitry in a domain-specific manner such that neurons are functionally dependent on astrocytes generated from the same progenitor domains; injury does not induce migration for adjacent domains (Tsai et al., 2012). This finding suggested to us that locally associated astrocytes must possess a signaling mechanism to respond to locally injured neurons; our observation that neurons make prothrombin in response to injury suggested a paracrine mechanism. Given the data presented in Figure 2, there are likely other signaling factors that neurons use to activate nearby astrocytes quickly, under 120 min. We have no data to exclude other signaling mechanisms from neurons to activate astrocytes. For example, neurons communicate directly with astrocytes using calcium flux via gap junctions (Simard, Arcuino, Takano, Liu, & Nedergaard, 2003). Astrocytes respond to action potentials in nearby synapses (Simard et al., 2003).
Cell–cell communication via proteases has been documented in the brain (Bushell, Cunningham, McIntosh, Moudio, & Plevin, 2016; Meins et al., 2001; Monard, 1988; Niclou, Suidan, Pavlik, Vejsada, & Monard, 1998; Vance et al., 2015). A possible role for thrombin as an additional help-me signal, joining other putative signaling mechanisms such as LCN2, has recently been published (Xing et al., 2014; Xing & Lo, 2017). Speculations on the role of neuron-generated thrombin have included axon guidance during development (Dihanich et al., 1991; Krenzlin et al., 2016; Weinstein et al., 1995), learning and memory (Maggio et al., 2014; Stein et al., 2015), and synapse remodeling (Ben Shimon et al., 2015). The observation that preconditioning depended on thrombin, coupled with the emerging role of astrocytes in protecting brain cells, suggested a more fundamental link between neurons and astrocytes.
The traditional cell-specific marker for astrocytes, GFAP, is highly expressed in fibrous or reactive astrocytes, but less so in protoplasmic or quiescent cells (Liddelow et al., 2017). The function of GFAP is not fully known, but as an intermediate filament protein, GFAP plays a role in maintaining astrocyte structural integrity. We included GFAP as a well-accepted, but perhaps limited marker of astrocyte activation; we searched for complementary markers of astrocyte activation and found several. During OGD the gene expression of serpin-A3N increased more than other potential markers so we added this maker to our studies (Zamanian et al., 2012). Our data clearly document a significant serpin-A3N response in astrocytes (Figures 3 and 4). Another gene that is significantly upregulated is lipocalin 2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL), but LCN2 is closely associated with the toxic activation phenotype (Jang et al., 2013). We found that LCN2 did not upregulate during astrocyte activation to the extent predicted.
The components of the astrocyte paracrine neuroprotective effect are complex. A large body of data suggests that astrocytes secrete a variety of neuroprotective factors, including growth factors such as VEGF, BDNF, and GDNF (Lin et al., 2006; Shindo et al., 2016), PN-1 (Mirante et al., 2013; Vaughan & Cunningham, 1993), extracellular vesicles (Chaudhuri et al., 2018; Hayakawa et al., 2016), and mitochondria (Hayakawa et al., 2016) to name only a few possibilities. Our data provide a necessary foundation for further studies to identify these mediators of the observed astrocytic neuroprotective effect (Figure 6). Further experiments will seek to explore how the protective factors are made and released. Of special importance, once the PAR1 receptor is activated, a variety of second messenger and signal transduction events are known to occur. Our data will facilitate studies connecting PAR1 induced second messenger events to the release of protective actors, such as protective factors.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Neurological Disorders and Stroke, R01 NS075930 (P.L.), and by the Carmen and Louis Warschaw Family Foundation (P.L.), Barbara Streisand Women’s Heart Center (J.V.E.), The Smidt Heart Institute at Cedars-Sinai Medical Center (J.V.E) and The Erika Glazer Endowed Chair in Women’s Heart Health (J.V.E).
Funding information
National Institute of Neurological Disorders and Stroke, Grant/Award Number: R01 NS075930
Footnotes
DATA AVAILABILITY STATEMENT
Data from all figures will be posted on Figshare. RNAseq data will be uploaded to a publicly available repository. Mice will be made available on request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S, … Yeh LS (2004). UniProt: The universal protein knowledgebase. Nucleic Acids Research, 32(Database issue), D115–D119. 10.1093/nar/gkh131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Hua Y, Keep RF, & Xi G (2018). Thrombin-induced tolerance against oxygen-glucose deprivation in astrocytes: Role of protease-activated receptor-1. Conditioning Medicine, 1(2), 57–63. [PMC free article] [PubMed] [Google Scholar]
- Ben Shimon M, Lenz M, Ikenberg B, Becker D, Shavit Stein E, Chapman J, … Maggio N (2015). Thrombin regulation of synaptic transmission and plasticity: Implications for health and disease. Frontiers in Cellular Neuroscience, 9, 151 10.3389/fncel.2015.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Radulovic M, Yoon H, & Scarisbrick IA (2013). Critical role for PAR1 in kallikrein 6-mediated oligodendrogliopathy. Glia, 61(9), 1456–1470. 10.1002/glia.22534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushell TJ, Cunningham MR, McIntosh KA, Moudio S, & Plevin R (2016). Protease-activated receptor 2: Are common functions in glial and immune cells linked to inflammation-related CNS disorders? Current Drug Targets, 17(16), 1861–1870. [DOI] [PubMed] [Google Scholar]
- Chaudhuri AD, Dastgheyb RM, Yoo SW, Trout A, Talbot CC, Hao H, … Haughey NJ (2018). TNFalpha and IL-1beta modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death & Disease, 9(3), 363 10.1038/s41419-018-0369-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Friedman B, Cheng Q, Tsai P, Schim E, Kleinfeld D, & Lyden PD (2009). Severe blood-brain barrier disruption and surrounding tissue injury. Stroke, 40(12), e666–e674. 10.1161/STROKEAHA.109.551341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Friedman B, Whitney MA, Winkle JA, Lei IF, Olson ES, … Lyden PD (2012). Thrombin activity associated with neuronal damage during acute focal ischemia. Journal of Neuroscience, 32(22), 7622–7631. 10.1523/JNEUROSCI.0369-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Suzuki M, Kim T, Wagner SL, & Cunningham DD (1990). Protease nexin-1. Localization in the human brain suggests a protective role against extravasated serine proteases The American Journal of Pathology, 137(4), 741–747. [PMC free article] [PubMed] [Google Scholar]
- Connolly AJ, Ishihara H, Kahn ML, Farese RV, & Coughlin SR (1996). Role of the thrombin receptor in development and evidence for a second receptor. Nature, 381(6582), 516–519. 10.1038/381516a0 [DOI] [PubMed] [Google Scholar]
- Coughlin SR (1999). How the protease thrombin talks to cells. Proceedings of the National Academy of Sciences of the United States of America, 96(20), 11023–11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR (2000). Thrombin signalling and protease-activated receptors. Nature, 407(6801), 258–264. 10.1038/35025229 [DOI] [PubMed] [Google Scholar]
- De Luca C, Virtuoso A, Maggio N, & Papa M (2017). Neurocoagulopathy: Blood coagulation factors in central nervous system diseases. International Journal of Molecular Sciences, 18(10), 2128 10.3390/ijms18102128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Merino P, Manrique LG, Ospina JP, Cheng L, Wu F, … Yepes M (2017). A cross talk between neuronal urokinase-type plasminogen activator (uPA) and astrocytic uPA receptor (uPAR) promotes astrocytic activation and synaptic recovery in the ischemic brain. Journal of Neuroscience, 37(43), 10310–10322. 10.1523/JNEUROSCI.1630-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihanich M, Kaser M, Reinhard E, Cunningham D, & Monard D (1991). Prothrombin mRNA is expressed by cells of the nervous system. Neuron, 6(4), 575–581. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, … Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 29(1), 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, & Sofroniew MV (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. The Journal of Neuroscience, 24 (9), 2143–2155. 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, & Lo EH (2009). Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke, 40(6), 2244–2250. 10.1161/STROKEAHA.108.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KB, & Paxinos G (1997). The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press. [Google Scholar]
- Hanisch UK, van Rossum D, Xie Y, Gast K, Misselwitz R, Auriola S, … Moller T (2004). The microglia-activating potential of thrombin: The protease is not involved in the induction of proinflammatory cytokines and chemokines. The Journal of Biological Chemistry, 279(50), 51880–51887. 10.1074/jbc.M408318200 [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, … Lo EH (2016). Transfer of mitochondria from astrocytes to neurons after stroke. Nature, 535(7613), 551–555. 10.1038/nature18928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich-Noack P, Striggow F, Reiser G, & Reymann KG (2006). Preconditioning with thrombin can be protective or worsen damage after endothelin-1-induced focal ischemia in rats. Journal of Neuroscience Research, 83(3), 469–475. 10.1002/jnr.20746 [DOI] [PubMed] [Google Scholar]
- Hirayama Y, & Koizumi S (2017). Hypoxia-independent mechanisms of HIF-1alpha expression in astrocytes after ischemic preconditioning. Glia, 65(3), 523–530. 10.1002/glia.23109 [DOI] [PubMed] [Google Scholar]
- Jang E, Kim JH, Lee S, Kim JH, Seo JW, Jin M, … Suk K (2013). Phenotypic polarization of activated astrocytes: The critical role of lipocalin-2 in the classical inflammatory activation of astrocytes. Journal of Immunology, 191(10), 5204–5219. 10.4049/jimmunol.1301637 [DOI] [PubMed] [Google Scholar]
- Jha MK, Seo M, Kim JH, Kim BG, Cho JY, & Suk K (2013). The secretome signature of reactive glial cells and its pathological implications. Biochimica et Biophysica Acta, 1834(11), 2418–2428. 10.1016/j.bbapap.2012.12.006\ [DOI] [PubMed] [Google Scholar]
- Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, … Traynelis SF (2004). Protease-activated receptor-1 in human brain: Localization and functional expression in astrocytes. Experimental Neurology, 188(1), 94–103. 10.1016/j.expneurol.2004.02.018 [DOI] [PubMed] [Google Scholar]
- Kooij V, Venkatraman V, Kirk JA, Ubaida-Mohien C, Graham DR, Faber MJ, & Van Eyk JE (2014). Identification of cardiac myofilament protein isoforms using multiple mass spectrometry based approaches. Proteomics. Clinical Applications, 8(7–8), 578–589. 10.1002/prca.201400039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenzlin H, Lorenz V, Danckwardt S, Kempski O, & Alessandri B (2016). The importance of thrombin in cerebral injury and disease. International Journal of Molecular Sciences, 17(1), 84–105. 10.3390/ijms17010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Zhang JH, & Noble-Haeusslein LJ (2013). RIGOR guidelines: Escalating STAIR and STEPS for effective translational research. Translational Stroke Research, 4(3), 279–285. 10.1007/s12975-012-0209-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, & Dewey CN (2011). RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12, 323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, … Barres BA (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Cheng FC, Lu YZ, Chu LF, Wang CH, & Hsueh CM (2006). Protection of ischemic brain cells is dependent on astrocyte-derived growth factors and their receptors. Experimental Neurology, 201(1), 225–233. 10.1016/j.expneurol.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Luo W, Zhong J, Chang R, Hu H, Pandey A, & Semenza GL (2010). Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but not HIF-2alpha. The Journal of Biological Chemistry, 285(6), 3651–3663. 10.1074/jbc.M109.068577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P, Pereira B, Chen B, Zhao L, Lamb J, Lei IF, & Rajput P (2014). Direct thrombin inhibitor argatroban reduces stroke damage in 2 different models. Stroke, 45(3), 896–899. 10.1161/STROKEAHA.113.004488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P, Lamb J, Kothari S, Toossi S, Boitano P, & Rajput PS (2018). Differential effects of hypothermia on neurovascular unit determine protective or toxic results: Toward optimized therapeutic hypothermia. Journal of Cerebral Blood Flow & Metabolism, 0271678X18814614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Itsekson Z, Ikenberg B, Strehl A, Vlachos A, Blatt I, … Chapman J (2014). The anticoagulant activated protein C (aPC) promotes metaplasticity in the hippocampus through an EPCR-PAR1-S1P1 receptors dependent mechanism. Hippocampus, 24(8), 1030–1038. 10.1002/hipo.22288 [DOI] [PubMed] [Google Scholar]
- Meins M, Piosik P, Schaeren-Wiemers N, Franzoni S, Troncoso E, Kiss JZ, … Monard D (2001). Progressive neuronal and motor dysfunction in mice overexpressing the serine protease inhibitor protease nexin-1 in postmitotic neurons. The Journal of Neuroscience, 21(22), 8830–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino P, & Yepes M (2018). Urokinase-type plasminogen activator induces neurorepair in the ischemic brain. Journal of Neurology and experimental Neuroscience, 4(2), 24–29. 10.17756/jnen.2018-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirante O, Price M, Puentes W, Castillo X, Benakis C, Thevenet J, … Hirt L (2013). Endogenous protease nexin-1 protects against cerebral ischemia. International Journal of Molecular Sciences, 14(8), 16719–16731. 10.3390/ijms140816719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monard D (1988). Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends in Neurosciences, 11(12), 541–544. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, & Goldman SA (2003). New roles for astrocytes: Redefining the functional architecture of the brain. Trends in Neurosciences, 26(10), 523–530. 10.1016/j.tins.2003.08.008 [DOI] [PubMed] [Google Scholar]
- Niclou SP, Suidan HS, Pavlik A, Vejsada R, & Monard D (1998). Changes in the expression of protease-activated receptor 1 and protease nexin-1 mRNA during rat nervous system development and after nerve lesion. The European Journal of Neuroscience, 10(5), 1590–1607. [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (1986). The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press. [Google Scholar]
- Petersen MA, Ryu JK, & Akassoglou K (2018). Fibrinogen in neurological diseases: Mechanisms, imaging and therapeutics. Nature Reviews. Neuroscience, 19(5), 283–301. 10.1038/nrn.2018.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J, Wilcox KC, Tortelli V, Diniz LP, Oliveira MS, Dobbins C, … Klein WL (2017). Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Abeta oligomers. Molecular Biology of the Cell, 28(20), 2623–2636. 10.1091/mbc.E17-06-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Wu J, Mustafa K, Fehlings MG, & Scarisbrick IA (2015). Genetic targeting of protease activated receptor 2 reduces inflammatory astrogliosis and improves recovery of function after spinal cord injury. Neurobiology of Disease, 83, 75–89. 10.1016/j.nbd.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput PS, Lyden PD, Chen B, Lamb JA, Pereira B, Lamb A, … Bai J (2014). Protease activated receptor-1 mediates cytotoxicity during ischemia using in vivo and in vitro models. Neuroscience, 281C, 229–240. 10.1016/j.neuroscience.2014.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riek-Burchardt M, Striggow F, Henrich-Noack P, Reiser G, & Reymann KG (2002). Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neuroscience Letters, 329(2), 181–184. [DOI] [PubMed] [Google Scholar]
- Rohatgi T, Sedehizade F, Reymann KG, & Reiser G (2004). Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: Thrombin as signaling molecule in the brain. The Neuroscientist, 10(6), 501–512. [DOI] [PubMed] [Google Scholar]
- Shikamoto Y, & Morita T (1999). Expression of factor X in both the rat brain and cells of the central nervous system. FEBS Letters, 463(3), 387–389. [DOI] [PubMed] [Google Scholar]
- Shindo A, Maki T, Mandeville ET, Liang AC, Egawa N, Itoh K, … Arai K (2016). Astrocyte-derived pentraxin 3 supports blood-brain barrier integrity under acute phase of stroke. Stroke, 47(4), 1094–1100. 10.1161/STROKEAHA.115.012133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, & Nedergaard M (2003). Signaling at the gliovascular interface. The Journal of Neuroscience, 23 (27), 9254–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein ES, Itsekson-Hayosh Z, Aronovich A, Reisner Y, Bushi D, Pick CG, … Maggio N (2015). Thrombin induces ischemic LTP (iLTP): Implications for synaptic plasticity in the acute phase of ischemic stroke. Scientific Reports, 5, 7912 10.1038/srep07912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney AM, Fleming KE, McCauley JP, Rodriguez MF, Martin ET, Sousa AA, … Scimemi A (2017). PAR1 activation induces rapid changes in glutamate uptake and astrocyte morphology. Scientific Reports, 7, 43606 10.1038/srep43606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenet J, Angelillo-Scherrer A, Price M, & Hirt L (2009). Coagulation factor Xa activates thrombin in ischemic neural tissue. Journal of Neurochemistry, 111(3), 828–836. 10.1111/j.1471-4159.2009.06369.x [DOI] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, … Rowitch DH (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science, 337(6092), 358–362. 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle JA, Chen B, Lei IF, Pereira B, Rajput PS, & Lyden PD (2013). Concurrent middle cerebral artery occlusion and intra-arterial drug infusion via ipsilateral common carotid artery catheter in the rat. Journal of Neuroscience Methods, 213(1), 63–69. 10.1016/j.jneumeth.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance KM, Rogers RC, & Hermann GE (2015). PAR1-activated astrocytes in the nucleus of the solitary tract stimulate adjacent neurons via NMDA receptors. The Journal of Neuroscience, 35(2), 776–785. 10.1523/JNEUROSCI.3105-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan PJ, & Cunningham DD (1993). Regulation of protease nexin-1 synthesis and secretion in cultured brain cells by injury-related factors. The Journal of Biological Chemistry, 268(5), 3720–3727. [PubMed] [Google Scholar]
- Vaughan PJ, Pike CJ, Cotman CW, & Cunningham DD (1995). Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. The Journal of Neuroscience, 15(7 Pt 2), 5389–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, & Coughlin SR (1991). Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell, 64(6), 1057–1068. 10.1152/ajpcell.00001.2002. [DOI] [PubMed] [Google Scholar]
- Wagner SL, Geddes JW, Cotman CW, Lau AL, Gurwitz D, Isackson PJ, & Cunningham DD (1989). Protease nexin-1, an antithrombin with neurite outgrowth activity, is reduced in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 86(21), 8284–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ubl JJ, & Reiser G (2002). Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia, 37(1), 53–63. [DOI] [PubMed] [Google Scholar]
- Wang H, Ubl JJ, Stricker R, & Reiser G (2002). Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. American Journal of Physiology. Cell Physiology, 283(5), C1351–C1364. 10.1152/ajpcell.00001.2002 [DOI] [PubMed] [Google Scholar]
- Wang Y, Luo W, & Reiser G (2007). Activation of protease-activated receptors in astrocytes evokes a novel neuroprotective pathway through release of chemokines of the growth-regulated oncogene/-cytokine-induced neutrophil chemoattractant family. The European Journal of Neuroscience, 26(11), 3159–3168. 10.1111/j.1460-9568.2007.05938.x [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, & Gall CM (1995). Cellular localization of thrombin receptor mRNA in rat brain: Expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. The Journal of Neuroscience, 15(4), 2906–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Hong S, Kulman JD, Bishop C, Kuniyoshi J, Andersen H, … Moller T (2005). Unraveling thrombin’s true microglia-activating potential: Markedly disparate profiles of pharmaceutical-grade and commercial-grade thrombin preparations. Journal of Neurochemistry, 95(4), 1177–1187. 10.1111/j.1471-4159.2005.03499.x [DOI] [PubMed] [Google Scholar]
- Xi G, Reiser G, & Keep RF (2013). The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? Journal of Neurochemistry, 84(1), 3–9. [DOI] [PubMed] [Google Scholar]
- Xing C, & Lo EH (2017). Help-me signaling: Non-cell autonomous mechanisms of neuroprotection and neurorecovery. Progress in Neurobiology, 152, 181–199. 10.1016/j.pneurobio.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Wang X, Cheng C, Montaner J, Mandeville E, Leung W, … Lo EH (2014). Neuronal production of lipocalin-2 as a help-me signal for glial activation. Stroke, 45(7), 2085–2092. 10.1161/STROKEAHA.114.005733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Drucker KL, Wu J, & Scarisbrick IA (2015). The thrombin receptor is a critical extracellular switch controlling myelination. Glia, 63(5), 846–859. 10.1002/glia.22788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, & Barres BA (2012). Genomic analysis of reactive astrogliosis. The Journal of Neuroscience, 32(18), 6391–6410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.