Abstract
Liver transplant recipients may be at increased risk for adverse outcomes with coronavirus disease 2019 (COVID-19) infection because of chronic immunosuppression and associated comorbidities. There is a paucity of literature describing clinical presentation, treatments, and outcomes in liver transplant recipients with COVID-19. A systematic search was performed for articles published up to June 15, 2020, revealing 223 liver transplant recipients with COVID-19 in 15 studies. Patients most commonly presented with fever (66.7%), dyspnea (34.0%), and diarrhea (28.4%). Of these, 77.7% required hospitalization, 24% had mild disease, 40% had moderate disease, and 36% had severe disease. Immunosuppression was modified in 32.8% of recipients. The case fatality rate was 19.3%. Dyspnea on presentation, diabetes mellitus, and age 60 years or older were significantly associated with increased mortality (P ≤ .01) with a trend to higher mortality rate observed in those with hypertension and those receiving corticosteroids at the time of COVID-19 diagnosis. The median time from symptoms to death was 11.5 days (2-45 days). In conclusion, liver transplant recipients with severe acute respiratory syndrome coronavirus 2 are overrepresented with regard to severe disease and hospitalizations. Older liver transplant patients with diabetes mellitus or hypertension, who are on maintenance corticosteroids, with a diagnosis of COVID-19 and describing breathlessness should be aggressively monitored for signs of deterioration because of the risk for mortality.
Highlights
-
•
Hospitalization and mortality rates in liver transplants recipients with coronavirus disease 2019 (COVID-19) are disproportionately high when compared to nontransplant counterparts regardless of age, time after transplant, or country of residence.
-
•
Older age and diabetes are significant risk factors for death among liver transplant recipients with COVID-19.
-
•
Liver transplant recipients presenting with dyspnea in the context of confirmed COVID-19 are at an independently increased risk for mortality and should undergo intensive monitoring for signs of clinical deterioration.
-
•
An immunosuppression regimen including corticosteroids at the time of COVID-19 infection and a history of hypertension demonstrate a trend to increased mortality in recipients of liver transplant with COVID-19.
-
•
Further data are required before standardized recommendations regarding the utility of COVID-19–directed therapy or immune therapy modulation in the context of COVID-19 can be made.
The clinical presentation of coronavirus disease 2019 (COVID-19) cases and the case-fatality rate has varied significantly between both patient subgroups and countries [1]. Various predictors of disease severity have been identified, including age, cardiovascular disease, cancer, chronic kidney disease, and diabetes [[2], [3], [4], [5], [6], [7]]. However, data on solid organ transplant recipients has been limited to case reports and series [[2], [3], [4], [5], [6], [7], [8]]. The case-fatality rate from COVID-19 ranges widely from 1% to 7.2%, although the rate appears to be much higher for solid organ transplant recipients [9,10].
The clinical course of COVID-19 in liver transplant recipients is variable, with biologically plausible reasons to favor both a reduced and an intensified immunosuppression strategy based on the stage of infection [11]. Further complicating transplant-associated complications is the prevalence of cardiovascular risk in transplant recipients, including hypertension and metabolic syndrome, both of which confer increased risk for COVID-19–associated mortality [3,4,6]. Collating and analyzing rapidly emerging data is vital to identify modifiable risk factors to optimize standardized management. We conducted a systematic review to consolidate current literature on clinical presentation, treatment, and outcomes in liver transplant recipients with COVID-19.
Methods
Data Sources
A literature search was performed through the EMBASE, PubMed, and Web of Science databases up to June 15, 2020. Keywords using Medical Subject Heading, where available, and Emtree Index terms were developed from main subject headings of “COVID-19,” “coronavirinae,” and “liver transplantation.” The search was limited to articles in English. Reference lists of reviewed articles were screened to identify further relevant studies. The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [12].
Study Selection
Studies were included if they reported outcomes in liver transplant recipients with confirmed diagnosis of COVID-19 defined as detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on a specimen from the respiratory tract from a symptomatic patient. Studies that did not delineate liver transplantation patients from a cohort of solid organ transplantation recipients, multiorgan transplants, and in vitro/animal studies were excluded. Studies reporting the same case were consolidated into 1 data entry.
Data Extraction
Two investigators (J.F. and J.M.) independently searched and extracted relevant articles, which were subsequently verified by the senior investigator (A.N.K.); discrepancies were resolved by consensus. Data were entered into detailed forms and included study design and characteristics, sample size, patient demographics, interval after transplantation, duration of symptoms before diagnosis, baseline immunosuppression, severity of disease, treatment administered, and mortality. Patients were stratified into mild, moderate, or severe, based on a published classification of COVID-19 severity [11]. Quality of data was anticipated to be variable because we predicted the majority of study designs to be case reports or case series.
Clinical Outcome Assessment
The primary objective was to characterize the presenting features of COVID-19 in liver transplant recipients and the case-fatality rate. Secondary objectives were to assess for clinical risk predictors of mortality and the management of baseline immunosuppression strategies with COVID-19 disease severity.
Statistical Analysis
Descriptive statistics were presented as absolute and relative frequencies for categorical variables. Comparisons between 2 groups were performed with the χ2 test for categorical data and the Student’s t test or Mann-Whitney U test as appropriate for continuous data. A P value of < .05 was considered statistically significant. Statistical analysis was performed using Stata 13/MP (StataCorp, College Station, Tex, United States). Denominators for baseline characteristics and mortality data varied because of differences in data reporting across studies (Table 1, Table 2, Table 3 ).
Table 1.
Clinical Characteristics and Mortality in Liver Transplant Recipients With COVID-19
| Overall (N = 223) | Deceased (n = 43) | Survived (n = 180) | P value | |
|---|---|---|---|---|
| Age (years), mean (IQR) | 59.6 (61-65) | 64.7 (63-67) | 58.1 (58-65) | .009 |
| Male (%) | 69.3 | 74.4 | 66.1 | .44 |
| % Aged ≥ 60 y | 67.8 | 90.7 | 45.0 | <.00001 |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range.
Table 2.
Clinical Characteristics and Mortality in Liver Transplant Recipients With COVID-19
| Overall† | Deceased∗ (n = 27) | Survived (n = 93) | P value | |
|---|---|---|---|---|
| Comorbidities | ||||
| Hypertension (%) | 51.4 | 69.2 | 47.6 | .05 |
| Diabetes mellitus (%) | 42.1 | 36.2 | 15.0 | .01 |
| Chronic kidney disease (%) | 29.1 | 40 | 30.4 | .44 |
| Overweight (BMI ≥ 25 kg/m2, %) | 52.5 | 61.9 | 46.5 | .21 |
| Cardiovascular disease (%) | 14.3 | 31.8 | 18.7 | .18 |
| Symptoms on Presentation | ||||
| Fever (%) | 66.7 | 57.1 | 68.3 | .43 |
| Dyspnea (%) | 34.0 | 88.9 | 36.7 | <.001 |
| Diarrhea (%) | 28.4 | 42.9 | 36.7 | .94 |
| Immunosuppression Used | ||||
| Corticosteroid use (%) | 42.2 | 56.5 | 38.4 | .09 |
| Tacrolimus/cyclosporin (%) | 87.5 | 88.5 | 89.9 | .83 |
| Mycophenolate (%) | 53.2 | 60.9 | 51.2 | .41 |
| mToR inhibitor (%) | 9.9 | 6.2 | 10.9 | .58 |
Abbreviations: BMI, Body mass index; COVID-19, coronavirus disease 2019; mToR, mammalian target of rapamycin.
Data from Pereira et al [9] with “severe disease - defined as ICU admission, intubation or death,” were presumed deceased in this analysis (n = 4).
Data from Belli et al [15] reported overall outcomes and did not stratify comorbidities, symptoms on presentation, and immunosuppression used based on mortality. Denominators used are included in supplementary table 6.
Table 3.
Duration Since Transplantation and Mortality in Liver Transplant Recipients With COVID-19
| Overall† (N = 203) | Deceased∗ (n = 43) | Survived (n = 160) | P Value | |
|---|---|---|---|---|
| Duration Since Transplant | ||||
| < 2 y since transplant (%) | 14.7 (n = 30) | 11.6 (n = 5) | 15.6 (n = 25) | .51 |
| > 2 y since transplant (%) | 85.2 (n = 173) | 23.8 (n = 38) | 84.4 (n = 135) | .51 |
Abbreviation: COVID-19, coronavirus disease 2019.
Data from Belli et al [15] reported overall outcomes, and did not stratify comorbidities, symptoms on presentation and immunosuppression used based on mortality.
Data from Pereira et al [9] with severe disease - defined as ICU admission, intubation or death’, were presumed deceased in this analysis. (n = 4).
Results
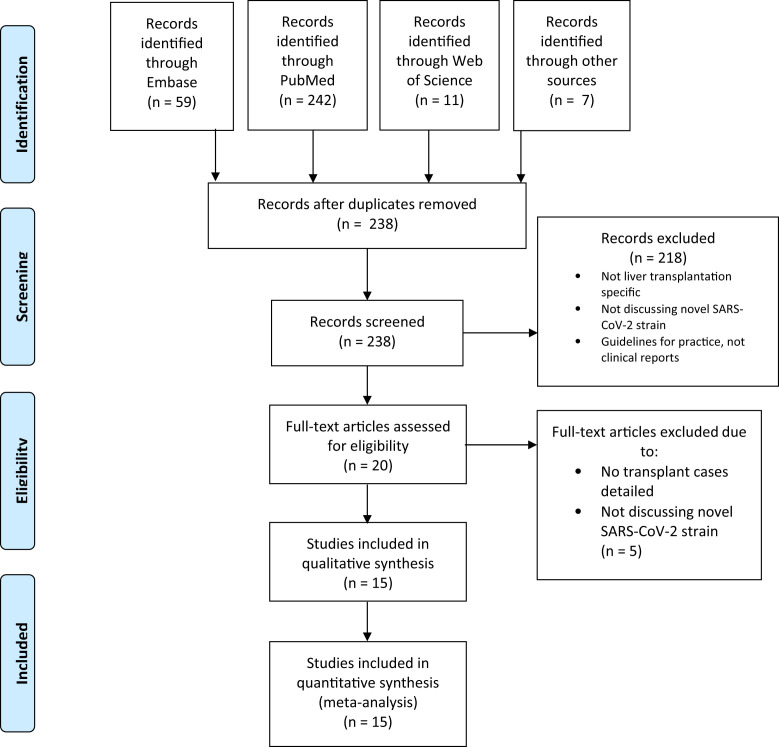
The initial search yielded 238 studies. We eliminated 218 studies after initial screening; 15 studies with a total of 223 patients were included in the final analysis. Details of the literature search are reported in Fig 1 . These included 4 multicenter registry studies [9,[13], [14], [15]], 5 single-center case series [10,[16], [17], [18], [19]], and 5 case reports [[20], [21], [22], [23], [24], [25]]. Studies included reports from Italy, Spain, France, the United States, and China.
Fig 1.
Flow diagram of systematic search process.
Clinical Characteristics of Liver Transplant Recipients With COVID-19
Among the 223 patients included, 67.8% were over 60 years old and 69.3% were male. The mean age of patients was 59.5 ± 11 years. Of cases that reported on transmission, SARS-CoV-2 was community acquired in 35% of cases, nosocomially acquired in 5%, and unclear or not reported in the remaining. The majority (85.2%) of patients experienced infection ≥ 2 years after transplantation, with only 6 patients [13,19,20,22,23] diagnosed within the first month after transplant (Table 3). One case was thought to represent a potential donor-derived infection, with the recipient developing symptoms on post-transplant day 2 [13,20]. Clinical characteristics of patients are summarized in Table 2. Advanced age (60 years or older) and diabetes mellitus were significantly associated with mortality (P < .001 and P = .01, respectively). Hypertension, body mass index ≥ 25 kg/m2, and an immunosuppression regimen that includes corticosteroids at the time of COVID-19 presentation demonstrated a trend toward increased mortality (Table 2).
Clinical Presentation of Liver Transplant Recipients With COVID-19
The majority of patients (77.7%) were hospitalized during the course of illness, with the remainder of patients either managed in the outpatient or setting lacking documentation of hospitalization status. Duration of symptoms before presentation was incompletely reported, although the majority underwent testing for SARS-CoV-2 within 7 days of symptom onset. Using the classifications proposed by Siddiqi and Mehra [11], data on disease severity was available in 76 patients. Of these, 64% of patients had mild-moderate disease and 36% had severe disease. The most common presenting symptoms were fever (67.1%), dyspnea (34.3%), and diarrhea (28.6%) (Table 2). Liver function tests (LFTs) were described only in case reports, which limited quantification and pooling of data. Data pertaining to thoracic imaging were reported in 42.5% of cases. Of these, 94% demonstrated radiologic evidence of COVID-19 on either chest x-ray examination or chest computed tomography (CT).
Treatment in Hospitalized Patients With COVID-19
Baseline immune suppression regimen was detailed in 52.0% (116/223) of recipients (Table 2) and was subsequently augmented in 17.0% (38/223) of cases. Of these, there was either a reduction or cessation of antimetabolite in 76.3% (29/38) or calcineurin inhibitor in 52% (20/38) [9,10,14,[18], [19], [20], [21], [22], [23], [24],26]. A total of 93 of 141 (66%) patients were prescribed hydroxychloroquine, with 35 cases documenting patient survival outcome and no survival benefit trend observed. Six patients received immune modulatory therapy, with 9 patients prescribed interleukin 6 (IL-6) inhibitors and 4 patients receiving systemic interferon (Table 4 ). Details of therapies, including antivirals, corticosteroids, immunotherapy, and antibiotics against survival outcome are summarized in Table 4.
Table 4.
Therapies Instituted for Liver Transplant Patients Diagnosed With COVID-19
| Hydroxychloroquine (n = 35) | Author | Deceased∗ |
Author | Survived |
|---|---|---|---|---|
| (n = 11) | (n = 24) | |||
| Fernández-Ruiz et al [10] (n = 1) | Administered day 1 after symptom onset | Fernández-Ruiz et al [10] (n = 3) | Administered days 1, 3, and 8 after symptom onset | |
| Pereira et al [9] (n = 4) | Day administered not stated. Duration of treatment 5 days | Lagana et al [20] (n = 1) | Administered day 1 of diagnosis | |
| Lee et al [18] (n = 6) | Administered hydroxychloroquine | Pereira et al [9] (n = 8) | Day administered not stated. Duration of treatment 5 days | |
| Lee et al [18] (n = 12) | Administered hydroxychloroquine |
| Antivirals (n = 5) | Author (n = cases) | n = 2 | Author (n = cases) | n = 3 |
|---|---|---|---|---|
| Huang et al [24] (n = 1) | Lopinavir/ritonavir + Umifenovir | Qin et al [14] Zhong et al [23] (n = 1) |
Oseltamivir | |
| Fernández-Ruiz et al [10] (n = 1) | Lopinavir/ritonavir | Fernández-Ruiz et al [10] (n = 1) | Lopinavir/ritonavir | |
| Liu et al [25] (n = 1) | Oseltamivir Umifenovir + Lopinavir/ritonavir |
| Antibiotics and Antifungals (n = 36) | Author (n = cases) | n = 7 | Author (n = cases) | n = 29 |
|---|---|---|---|---|
| Huang et al [24] (n = 1) | Piperacillin/tazobactam Cefoperazone-sulbactam + caspofungin Meropenem + Voriconazole |
Qin et al [14] (n = 1) Zhong et al [23] (n=1) | Cefoperazone + Sulbactam sodium | |
| Pereira et al [9] (n = 3) | Azithromycin | Liu et al [25] (n = 1) | Cefoperazone | |
| Lee et al [18] (n = 3) | Azithromycin | Pereira et al [9] (n = 6) | Azithromycin | |
| Lee et al [18] (n = 12) | Azithromycin | |||
| Donato et al [16] (n = 9) | Unspecified “antibiotic therapy” |
| Corticosteroid Therapy (n = 8) | Author (n = cases) | n = 6 | Author (n = cases) | n = 2 |
|---|---|---|---|---|
| Fernández-Ruiz et al [10] (n = 1) | Methylprednisolone | Liu et al [25] (n = 1) | Methylprednisolone | |
| Pereira et al [9] (n = 1) | Bolus dose of corticosteroid | Pereira et al [9] (n = 1) | Bolus dose of “steroid” | |
| Lee et al [18] (n = 4) | Commenced IV corticosteroid therapy |
| Immunotherapy (n = 6) | Author (n = cases) | n = 3 | Author (n = cases) | n = 3 |
|---|---|---|---|---|
| Fernández-Ruiz et al [10] (n = 1) | Interferon alfa | Liu et al [25] (n = 1) | IVIg, Interferon alfa |
|
| Fernández-Ruiz et al [10] (n = 1) | Interferon beta | Fernández-Ruiz et al [10] (n = 1) | Interferon beta | |
| Pereira et al [9] (n = 1) | Tocilizumab (IL-6 antagonist) | Pereira et al [9] (n = 1) | Tocilizumab (IL-6 antagonist) |
Abbreviations: IL, interleukin; IVIg, intravenous immunoglobulin.
Four patients recorded by Pereira et al [9] as having “severe disease - defined as ICU admission, intubation or death,” were assumed deceased.
Predictors of Mortality or Severe Illness in Liver Transplant Recipients With COVID-19
Overall, 43 (19.3%) patients died as a result of complications of COVID-19 [9,10,[13], [14], [15],18,19,24]. The median duration of time from symptoms to death in hospital was 11.2 days (2-45 days). Age of 60 years or older and diabetes mellitus were significantly associated with increased mortality (P ≤ .01). Hypertension and an immunosuppression regimen that included corticosteroids at the time of COVID-19 presentation demonstrated a trend toward increased mortality (Table 2). Liver transplant recipients presenting with dyspnea were at a significantly higher risk for subsequent COVID-19 related mortality (P < .001). Of the 43 patients who died, all but 3 were 60 years of age or older with multiple comorbidities and all but 5 died at ≥ 1 year after liver transplant.
Discussion
Multiple case series support the concept that recipients of solid organ transplant present with more severe COVID-19 disease, are more likely to require hospitalization, and have a higher mortality rate from COVID-19 compared to their nontransplant counterparts [10,27,28]. This systematic review and quantitative analysis indicates a high mortality rate among liver transplant patients with COVID-19 compared to published reports in the general population [7,29]. Importantly, we found that when recipients of liver transplant with COVID-19 present with dyspnea, a history of diabetes mellitus, and age of 60 years or older, they are at increased risk for mortality (P ≤ .01). These risk factors for subsequent deterioration should serve as critical markers for any clinical teams evaluating recipients of liver transplant with COVID-19 to initiate aggressive monitoring for signs of deterioration.
Liver transplant recipients with COVID-19 experience more severe disease (36%) compared with approximately 6% reported in the general patient population [30,31]. The hospitalization rate in this liver transplant cohort was 77.7%, which is in contrast to rates of age-adjusted hospitalization in non–transplant recipients aged 20 to 29 years from 1.1% up to 18.4% in those older than 80 years [32]. This may be accounted for by the older age of the liver transplant cohort (mean = 59.6 years). The observed case-fatality rate in our liver transplant cohort with COVID-19 was 19.3%, similar to that seen in thoracic transplant recipients [28]. This may be partly because of their older age (mean age = 59.6 years). However, this remains substantially higher than even the 8% mortality rate in an all-comer patient population aged 70 to 79 years [29]. This is substantially higher than the mean case-fatality rate of approximately 1% to 4% in the general population [32,33]. Given the likelihood of severe disease in this patient population, even simple interventions that reduce the risk for COVID-19 acquisition, including social distancing, reduced contact with health services with telemedicine, working from home when possible, and good sanitation, should form an essential component of every transplant program’s anti–COVID-19 response [34].
The most common presenting symptoms reported in liver transplant recipients with COVID-19 are fever and dyspnea, which is comparable with other transplant subgroups and the broader nontransplant population [27,33]. Importantly, however, in liver transplant patients, dyspnea is a significant risk factor for mortality. Although this may seem intuitive because severe COVID-19 disease is defined by pneumonia and acute respiratory distress syndrome, for which the primary symptom would be expected to be breathlessness, this should serve as an important clinical marker for any clinician reviewing a liver transplant patient with COVID-19, particularly in the early phase of illness. Patients who report any shortness of breath in the context of a SARS-CoV-2 infection should be monitored closely, with consideration for admission to specialized centers for observation and access to intensive care services.
Gastrointestinal symptoms are more prominent in liver transplant recipients with COVID-19 (28.6%) compared with the general population (2%-4%) but are comparable to reports from other solid organ transplant cohorts [9,35,36]. For patients presenting with predominant upper gastrointestinal symptoms, including nausea and vomiting, direct invasion of the central nervous system from the upper respiratory tract with SARS-CoV-2 has been proposed as the mechanism [37]. Lower gastrointestinal tract symptoms and diarrhea are a frequent symptom of other coronaviruses; for example, it is reported in up to 30% of Middle East respiratory syndrome cases [38]. Direct viral invasion of enterocytes causing alteration of intestinal permeability and malabsorption is one of multiple biologically plausible mechanisms [38]. Clinicians assessing liver transplant patients at risk for SARS-CoV-2 should remain vigilant when questioning patients and include gastrointestinal symptoms as part of COVID clinical assessment algorithms.
Because of the heterogeneous nature of reporting in the context of the pandemic, meaningful conclusions regarding the utility of radiologic investigations for the diagnostic evaluation of liver transplant patients presenting with suspected COVID-19 could not be made in this analysis. Of the 51 patients who had radiologic findings reported, 94% of these had radiographic or CT findings “suggestive” of a diagnosis of COVID-19. However, these radiologic changes included unilateral or bilateral patchy consolidation, pleural effusions, or hypostatic changes, the differential diagnoses of which remain broad, particularly in the immunocompromised. Whether radiologic scans, particularly CT, maintain the high sensitivity and specificity for COVID-19 in the setting of immunosuppression is also unclear. Although radiologic evaluation remains an important part of a diagnostic workup in the immunocompromised patient presenting with fevers or symptoms of a lower respiratory tract infection, the issues surrounding infection control, resource allocation, and safe patient transportation in the COVID-19 context limited the practicability of performing these studies routinely. For this reason and because rates of pathogen coinfection in COVID-19 appear to be low, it may well be that in liver transplant recipients with confirmed COVID-19, imaging specificity beyond a mobile chest x-ray examination in the initial evaluation of patients may have limited diagnostic utility [39].
Only 6 studies detailed the results of liver biochemistry, with no measurable trends reportable [19,20,[22], [23], [24], [25]]. The prevalence of abnormal liver function tests in 1267 COVID-19–positive patients pooled from 12 studies was 19%, with more severe disease associated with liver injury [40]. There are plausible mechanisms for liver injury because the angiotensin-converting enzyme inhibitor 2 receptor used by the SARS-CoV-2 virus for cellular entry is expressed on hepatocytes and cholangiocytes [20,41]. Deranged alanine aminotransferase, platelets, and albumin have been associated with higher mortality in COVID-19 in nontransplant patients, but whether these derangements are directly or indirectly virally mediated or similarly associated with mortality risk in liver transplant is unknown [42]. Four patients included in the review were considered clinically to have developed acute cellular rejection as evidenced by LFT derangement, but only 1 underwent allograft biopsy. Lagana et al [20] described a 5-month-old infant who underwent transplant from a living donor who was found to be COVID-19 positive in the days after donation. The recipient then became febrile on post-transplant day 4 with hypoxia, necessitating noninvasive ventilatory support with subsequent SARS-CoV-2 detected from the respiratory tract. On post-transplant day 6, when hepatitic LFT derangement was noted, an allograft biopsy identified histologic features thought to be most consistent with acute cellular rejection and immunosuppression. Paradoxically, LFTs worsened with immunosuppression and only improved with rapid corticosteroid taper and discontinuation of mycophenolate mofetil (MMF). Although it is unproven, the authors speculated that in the absence of other alternative cause the deranged LFTs observed were more likely to represent SARS-CoV-2–mediated hepatitis rather than rejection based on the atypical clinical response to immunosuppression. Although hepatocellular injury in the context of severe COVID-19 infection has been well described elsewhere, determining the exact mechanisms mediating hepatocellular injury in the context of multiple possible confounders at time of critical illness remains challenging [43]. Interestingly, similar nonspecific hepatic injury as described in the allograft biopsy of this case has been noted in the histopathologic examination of liver tissue on postmortem tissue from deceased COVID-19 patients [44].
Recipients of liver transplant who are 60 years of age or older and presenting with a diagnosis of COVID-19 are associated with a 3-fold greater risk for COVID-19–related mortality compared to their counterparts younger than 60. Older age is a well-described risk factor for both severe disease and in-hospital mortality in COVID-19 [[2], [3], [4], [5]]. A diagnosis of diabetes mellitus at presentation with COVID-19 is also associated with a statistically significant 2-fold greater risk for mortality in liver transplant recipients, which is consistent with prior reports in nontransplant patients [3,4]. Diabetes is a well-described risk factor for mortality in infections and in the context of COVID-19 has been associated with a more than doubled risk for intensive care unit admission and tripled risk for death in hospitalized COVID-19 patients in meta-analysis [45,46]. Because liver transplant recipients are overrepresented with regard to cardiometabolic risk profile, attention should be paid by clinicians to glycemic control in the context of the COVID-19 pandemic as a possible modifiable risk factor in this patient cohort [47,48].
An immune suppression regimen that included corticosteroids at the time of COVID-19 diagnosis demonstrated a trend to increased mortality in liver transplant recipients; however, it is difficult to draw conclusions because of confounding factors. Whether a corticosteroid-containing regimen is a surrogate marker for patients who are earlier post-transplant and therefore more immunosuppressed and thus more likely to have metabolic complications known to increase COVID-19 mortality or there is some alternative reason is unclear. There is a preference to reduce immunosuppression in liver transplant recipients at time of COVID-19 diagnosis, with nearly a quarter of patients prescribed a reduction of baseline immunosuppression. Interestingly, there are in-vitro data to support the anti-coronavirus properties of various antirejection medications. While cyclosporin has demonstrated anti-SARS coronavirus 2003 (SARS-CoV) properties by blocking translocation of nuclear factors from T cells into the cytosol [11,49], 6-mercaptopurine (6-MP), 6-thioguianine (6-TG), and MMF have demonstrated anti–Middle East respiratory syndrome proteolytic properties [50,51]. Although various institutional approaches to immune suppression management have been described [27], there presently remains insufficient evidence to guide any standardized approach to the augmentation of immunosuppression in transplant recipients diagnosed with COVID-19.
There are several limitations of this review, including the variability in the detail of data recorded, the extrapolation of data from cohort studies, and the inability to use meta-analysis techniques. Furthermore, reporting bias is likely to lead to an overestimation of transplant recipients with a heightened disease severity. We report a low proportion with COVID-19 diagnosed early after liver transplant. This may be due to both donor and recipient COVID-19 peri-transplant testing protocols in addition to reduction in the net number of transplants undertaken during the pandemic. A further limitation is our assumption that the liver transplantation patients with severe disease reported by Pereira et al [9] died in the intensive care unit. Although this accounts for only 4 patients, this may skew the interpretation of therapeutic interventions and survival benefit given the small sample sizes. The COVID-19 outcome data are continually changing, and thus reports of any delayed mortality in liver transplant recipients have yet to be published. A clear need for systematic and centralized data collection and analysis is highlighted in this study. Transplant centers are encouraged to collaborate with international registries to facilitate further study in this patient population [14] (https://covidcirrhosis.web.unc.edu and https://covid-hep.net).
Conclusion
COVID-19 manifests with a similar illness in liver transplant as it does in other transplant recipients and in the non–immune suppressed, but it is more likely to manifest with concurrent diarrheal symptoms. Older patients with dyspnea and diabetes mellitus are at a higher risk for mortality, and in these patients more intensive monitoring for deterioration should be undertaken because case fatality reaches nearly 23%. Data are currently insufficient to guide optimal immunosuppression and COVID-19–directed therapeutic strategies.
Footnotes
Olivia Catherine Smibert and Anoop Ninan Koshy contributed equally to this work.
A.N.K. and O.C.S. are co-senior authors.
References
- 1.Johns Hopkins University Coronavirus Resource Center Mortality analyses. 2020. https://coronavirus.jhu.edu/data/mortality [accessed 23.05.20]
- 2.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lian J., Jin X., Hao S. Analysis of epidemiological and clinical features in older patients with corona virus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis. 2020;71(150):740–747. doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8.Wu J., Li W., Shi X. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020;288(1):128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 9.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Ruiz M., Andrés A., Loinaz C. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;2(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maggi U., De Carlis L., Yiu D. The impact of the COVID-19 outbreak on liver transplantation programmes in Northern Italy. Am J Transplant. 2020;20(7):1840–1848. doi: 10.1111/ajt.15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5(7):643–644. doi: 10.1016/s2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belli L.S., Duvoux C., Karam V. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;59(8):724–725. doi: 10.1016/S2468-1253(20)30183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donato M.F., Invernizzi F., Lampertico P., Rossi G. Health status of liver transplanted patients during the coronavirus outbreak in Italy: a large single center experience from Milan. Clin Gastroenterol Hepatol. 2020;18(9):2131–2133. doi: 10.1016/j.cgh.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 18.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;59(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BT, Perumalswami PV, Im GY, Florman S, Schiano TD. COVID-19 in liver transplant recipients: an initial experience from the U.S. epicenter. Gastroenterology, in press. https://doi.org/10.1053/j.gastro.2020.05.050. [DOI] [PMC free article] [PubMed]
- 20.Lagana S.M., De Michele S., Lee M.J. COVID-19 associated hepatitis complicating recent living donor liver transplantation. Arch Pathology Laboratory Med. 2020 doi: 10.5858/arpa.2020-0186-SA. in press. [DOI] [PubMed] [Google Scholar]
- 21.Kates O.S., Fisher C.E., Stankiewicz-Karita H.C. Earliest cases of coronavirus disease 2019 (COVID-19) identified in solid organ transplant recipients in the United States. Am J Transplant. 2020;20(7):1885–1890. doi: 10.1111/ajt.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin J., Wang H., Qin X. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020 doi: 10.1002/hep.31257. in press. [DOI] [PubMed] [Google Scholar]
- 23.Zhong Z., Zhang Q., Xia H. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916–1921. doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J.F., Zheng K.I., George J. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020;20(7):1907–1910. doi: 10.1111/ajt.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B., Wang Y., Zhao Y., Shi H., Zeng F., Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20(7):1891–1895. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonato G., Dioscoridi L., Mutignani M. Faecal-oral transmission of SARS-COV-2: practical implications. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberici F., Delbarba E., Manenti C. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5(5):580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latif F., Farr M.A., Clerkin K.J. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.2159. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 30.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verity R., Okell L., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aylward B., Liang W. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf [accessed 28.02.20]
- 34.Ren Z.-L., Hu R., Wang Z.-W. Epidemiologic and clinical characteristics of heart transplant recipients during the 2019 coronavirus outbreak in Wuhan, China: a descriptive survey report. J Heart Lung Transpl. 2020;39(5):412–417. doi: 10.1016/j.healun.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakodkar P., Kaka N., Baig M.N. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020;12(4) doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol. 2020;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawson T.M., Moore L.S.P., Zhu N. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa530. ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao R., Qiu Y., He J.-S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7) doi: 10.1016/S2468-1253(20)30126-6. 30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Association for the Study of Liver Diseases Clinical best practice advice for hepatology and liver transplant providers during the covid-19 pandemic: AASLD Expert Panel Consensus Statement. 2020. https://www.aasld.org/sites/default/files/2020-05/AASLD-COVID19-ClinicalInsights-May42020-FINAL.pdf [accessed 05.05.20] [DOI] [PMC free article] [PubMed]
- 42.Boettler T., Newsome P.N., Mondelli M.U. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper [review] JHEP Rep. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burgueno J.F., Reich A., Hazime H. Expression of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26(6):797–808. doi: 10.1093/ibd/izaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cariou B., Hadjadj S., Wargny M. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koshy A.N., Gow P.J., Han H.-C. Cardiovascular mortality following liver transplantation: predictors and temporal trends over 30 years. Eur Heart J. 2020:qcaa009. doi: 10.1093/ehjqcco/qcaa009. [DOI] [PubMed] [Google Scholar]
- 48.Koshy A.N., Farouque O., Cailes B. Prediction of perioperative cardiovascular events in liver transplantation. Transplantation. 2020 doi: 10.1097/tp.0000000000003306. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka Y., Sato Y., Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. 2013;5(5):1250–1260. doi: 10.3390/v5051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mubarak A., Alturaiki W., Hemida M.G. Middle East respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019:6491738. doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Wilde A.H., Zevenhoven-Dobbe J.C., van der Meer Y. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92(Pt 11):2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]