Highlights
-
•
Guidelines for GDM diagnosis during COVID have changed.
-
•
Current guidelines are empirical and do not consider pregnancy outcomes.
-
•
Current guidelines all result in reduced detection of GDM cases.
-
•
Proposed Canadian guidelines reduce GDM by around 80%, but miss many complications.
-
•
Guidelines should consider both GDM frequency and complications detected or missed.
Keywords: Gestational diabetes, Diagnosis, Screening, COVID-19, Pregnancy complications
Abstract
Aims
We assessed how altered diagnostic processes and criteria for gestational diabetes mellitus (GDM) recommended by the United Kingdom (UK), Canada and Australia for use during the COVID-19 pandemic would affect both GDM frequency and related adverse outcomes.
Methods
Secondary analysis of 5974 HAPO study women with singleton pregnancies who underwent 75 g OGTTs and HbA1c assays between 24 and 32 weeks’ gestation and who received no treatment for GDM.
Results
All post COVID-19 modified pathways reduced GDM frequency – UK (81%), Canada (82%) and Australia (25%). Canadian women whose GDM would remain undetected post COVID-19 (missed GDMs) displayed similar rates of pregnancy complications to those with post COVID-19 GDM. Using UK modifications, the missed GDM group were at slightly lower risk whilst the women missed using the Australian modifications were at substantially lower risk.
Conclusions
The modifications in GDM diagnosis proposed for the UK, Canada and Australia result in differing reductions of GDM frequency. Each has both potential benefits in terms of reduction in potential exposure to COVID-19 and costs in terms of missed opportunities to influence pregnancy and postpartum outcomes. These factors should be considered when deciding which protocol is most appropriate for a particular context.
1. Introduction
Despite worldwide variations in processes and criteria, the oral glucose tolerance test (OGTT) is generally considered the “gold standard” in the diagnosis of gestational diabetes mellitus (GDM). Driven by a desire to reduce potential infections during the COVID-19 pandemic, health authorities and professional bodies in the United Kingdom (UK) [1], Canada [2] and Australia [3] have issued urgent statements designed to limit the need for pregnant women to attend prolonged appointments at pathology testing centers for OGTTs. Other national or broader clinical guidelines have been produced, motivated by pragmatism and urgency [4], [5], [6], [7], but none have assessed the impact of suggested changes in terms of pregnancy outcomes. To date, no United States (US) based organizations have recommended a change in policy.
The specific recommendations made by the UK, Canada and Australia are summarized in Table 1 . Clearly, they differ in many ways, both pre and post COVID-19. Each body has acknowledged the limited available evidence supporting the admittedly empirical modified approaches during the pandemic.
Table 1.
Current and modified GDM diagnostic approaches in UK, Canada and Australia, with description of the specific diagnostic approaches evaulated in this paper.
| Country | A) Current process and criteria for GDM diagnosis | B) Modified GDM diagnostic approach during COVID-19 pandemic | C) Post-COVID approach tested in current paper |
|---|---|---|---|
| United Kingdom (1)(published) |
|
|
|
| Canada (2)(published) |
|
|
|
| Australia (3)(published) |
|
|
|
Comparison of (A) Current GDM diagnostic processes and criteria, (B) Proposed modified approach during the COVID-19 pandemic and (C) Approach tested for each country in the current paper.
GDM gestational diabetes mellitus; COVID coronavirus disease; OGTT oral glucose tolerance test; FVPG fasting venous plasma glucose; VPG venous plasma glucose
The aim of our current investigation was to assess, using data from a sub-cohort of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study [8], how each of these recommendations would affect both the number of women diagnosed with GDM with implementation of the new protocols and the number of pregnancy complications potentially captured / missed by each diagnostic approach. To simplify comparisons, we have considered only the approach to “standard” GDM testing, generally recommended between 24 and 28 weeks’ gestation.
2. Material and methods
The original HAPO study protocol was approved by institutional review boards at each center and has been published in detail [9]. All participants gave written informed consent. We included data from blinded 75 g oral glucose tolerance tests (OGTTs), undertaken between 24 and 32 weeks of gestation by women from five HAPO study centers (Bellflower, California and Cleveland, Ohio, United States of America; Brisbane and Newcastle, Australia; and Hong Kong, China). We excluded only participants who were unblinded in the original HAPO study or were missing individual OGTT components or HbA1c. We considered demographic and clinical characteristics, including the following (recorded at the OGTT visit): maternal age, height, and parity (0 or ≥ 1), BMI defined as weight (kg) divided by height (m)2 and grouped as underweight (BMI < 22.5 kg/m2), normal weight (22.6 ≥ BMI < 28.5 kg/m2), overweight (28.5 ≥ BMI < 33.0 kg/m2) and obese (BMI ≥ 33.0 kg/m2) using categories defined for the OGTT visit in the entire HAPO cohort [10], smoking, alcohol consumption, hypertensive disorders in pregnancy (systolic blood pressure [BP] > 140 mmHg and/or diastolic BP > 90 mmHg), family history of diabetes and hypertension (first-degree relative), self-reported ethnicity (white, black, Hispanic, Asian, or other), and HAPO study center. At the time of birth, the following outcome variables were considered: birthweight (grams), large-for-gestational age (LGA; defined as birthweight > 90th centile as per the primary HAPO analyses) [8], primary Cesarean section (CS), neonatal hyperinsulinemia (cord c-peptide > 1.7 μg/L [90th centile for the HAPO cohort])[11], neonatal hypoglycemia (neonatal two-hour glucose < 2.2 mmol/L [10th centile of the HAPO cohort]) [11], neonatal fat mass (grams) and neonatal adiposity (percent fat > 90th centile). HAPO was a blinded epidemiologic study and no participants received GDM treatment during pregnancy.
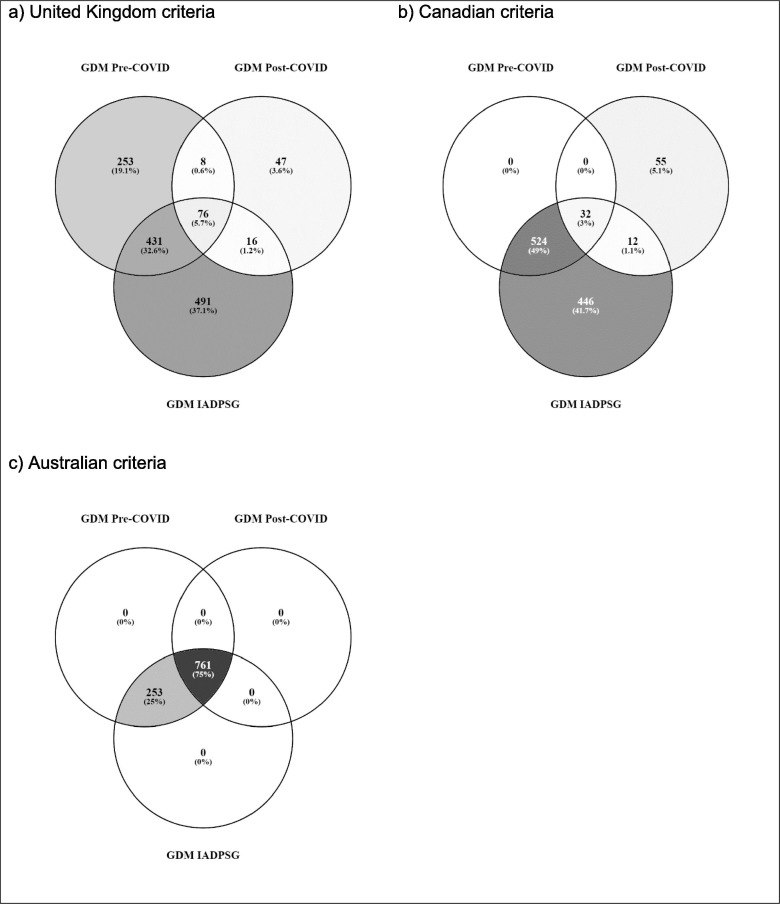
For comparison of GDM frequency using the pre and post COVID-19 diagnostic processes and criteria, we have considered national diagnostic criteria. The Australian diagnostic criteria are numerically equivalent to the IADPSG criteria and yield the largest number of GDM diagnoses. For the UK and Canadian processes and criteria, to allow for illustrative comparisons, we have presumed that all women would undergo testing using a full diagnostic 75 g OGTT. However, we acknowledge that this is not current practice in these countries. All current preferred Canadian diagnostic glucose cut-offs (fasting ≥ 5.3 / 1 h ≥ 10.6 / 2 h ≥ 9.0 mmol/L) are higher than IADPSG cut-offs (fasting ≥ 5.1 / 1 h ≥ 10.0 / 2 h ≥ 8.5 mmol/L). The UK criteria include a higher fasting glucose (≥5.6 mmol/L) and a lower two hour (≥7.8 mmol/L) glucose cut-off than IADPSG. Fig. 1 depicts overlap in these country specific diagnostic groups. We have excluded from consideration diagnostic thresholds based on random glucose measurements, which were not available in the HAPO study. Table 1 shows the current recommendations for GDM testing in each of the three countries, their revised recommendations for use during the COVID-19 pandemic (indicated by the column entitled “Modified GDM diagnostic approach during COVID-19 pandemic”) and the specific diagnostic process and threshold(s) evaluated in the current study (indicated by the column entitled “Post-COVID approach tested in current paper”).
Fig. 1.
GDM frequency by a) United Kingdom, b) Canadian, c) Australian, criteria pre and post. COVID-19 and by IADPSG criteria (assumes universal testing).
2.1. Statistical analysis
Maternal and clinical characteristics of the cohort are presented using appropriate descriptive statistics (mean and standard deviation, median and interquartile range, number and percent) and are compared between those classified as GDM by IADPSG criteria and those who are not using t-tests, Mann-Whitney U tests and Fisher’s exact tests, respectively, using a significance level of 0.05. Each participant was classified into the following groups for each of the UK, Canadian and Australian diagnostic criteria (as specified in Table 1): 1) “Missed GDM” - GDM by local pre COVID-19 criteria but not identified as GDM using local post COVID-19 criteria; 2) GDM by local post COVID-19 criteria; and 3) not GDM by both pre and post COVID-19 local criteria. Comparisons were then undertaken to compare group 2) to groups 1) and 3), using Fisher’s exact test and adjusting for multiple comparisons using Bonferroni’s correction. Statistical significance was accepted at the 0.05 level after correction. Analyses were conducted in StataSE version 14.1 (StataCorp Pty Ltd, College Station, Texas).
3. Results
A total of 5974 singleton pregnancies were included in the analysis, 1802 (30.2%) from Bellflower, 1505 (25.2%) from Hong Kong, 1361 (22.8%) from Brisbane, 720 (12.1%) from Cleveland, and 586 (9.8%) from Newcastle. Overall, 1014 (17.0%) women fulfilled IADPSG criteria for GDM, while 12.9% met current UK criteria and 9.3% current Canadian criteria for GDM. Clinical and demographic characteristics for the cohort are shown in Table 2 .
Table 2.
Characteristics of participants in the current study, presented for the entire cohort and divided by (IADPSG) GDM diagnostic status.
| Variable | Category | All participants | Not GDM by IADPSG Criteria | GDM by IADPSG Criteria | p |
|---|---|---|---|---|---|
| N | 5974 | 4960 | 1014 | ||
| Maternal age (years)^ | 29.4 (5.4) | 29.0 (5.3) | 30.9 (5.6) | <0.001 | |
| Maternal height (cm)^ | 162 (7) | 162 (7) | 161 (7) | <0.001 | |
| Maternal BMI (kg/m2)* | 27.0 (24.2–31.0) | 26.6 (23.9–30.3) | 29.6 (26.1–34.0) | <0.001 | |
| Maternal BMI by WHO category# | Underweight | 751 (12.6%) | 689 (13.9%) | 62 (6.1%) | <0.001 |
| Normal weight | 2886 (48.3%) | 2513 (50.7%) | 373 (36.8%) | ||
| Overweight | 1332 (22.3%) | 1055 (21.3%) | 277 (27.3%) | ||
| Obese | 1005 (16.8%) | 703 (14.2%) | 302 (29.8%) | ||
| Gestational weight gain (kg)* | 14.0 (10.1–18.0) | 14.0 (10.1–18.0) | 14.1 (10.0–18.2) | 0.72 | |
| Gestational weight gain by IOM recommendation# | <IOM recommendation | 1274 (22.4%) | 1121 (23.7%) | 153 (15.9%) | <0.001 |
| =IOM recommendation | 1768 (31.0%) | 1489 (31.5%) | 279 (29.0%) | ||
| >IOM recommendation | 2654 (46.6%) | 2124 (44.9%) | 530 (55.1%) | ||
| Smoking# | 436 (7.3%) | 356 (7.2%) | 80 (7.9%) | 0.430 | |
| Alcohol use# | 463 (7.8%) | 412 (8.3%) | 51 (5.0%) | <0.001 | |
| Multiparous# | 3150 (52.7%) | 2539 (51.2%) | 611 (60.3%) | <0.001 | |
| Ethnicity# | White | 2367 (39.6%) | 2031 (40.9%) | 336 (33.1%) | <0.001 |
| Black | 304 (5.1%) | 243 (4.9%) | 61 (6.0%) | ||
| Hispanic | 1521 (25.5%) | 1165 (23.5%) | 356 (35.1%) | ||
| Asian | 1575 (26.4%) | 1345 (27.1%) | 230 (22.7%) | ||
| Other | 207 (3.5%) | 176 (3.5%) | 31 (3.1%) | ||
| Family history of diabetes# | 1475 (24.7%) | 1140 (23.0%) | 335 (33.0%) | <0.001 | |
| Family history of hypertension# | 2375 (39.8%) | 1952 (39.4%) | 423 (41.7%) | 0.170 | |
| Mean arterial blood pressure (mmHg)^ | 81.6 (8.5) | 81.0 (8.4) | 84.3 (8.7) | <0.001 | |
| Fasting glucose (mmol/L)^ | 4.5 (0.4) | 4.4 (0.30) | 5.0 (0.4) | <0.001 | |
| One-hour glucose (mmol/L)^ | 7.5 (1.7) | 7.1 (1.4) | 9.5 (1.6) | <0.001 | |
| Two-hour glucose(mmol/L)^ | 6.3 (1.3) | 6.0 (1.0) | 7.7 (1.5) | <0.001 | |
| Mean OGTT z-scorê | 0.05 (0.77) | −0.18 (0.57) | 1.17 (0.60) | <0.001 | |
| HbA1c (mmol/mol)^ | 29.3 (4.1) | 28.8 (4.0) | 31.4 (4.2) | <0.001 | |
| HbA1c (%)^ | 4.8 (0.4) | 4.8 (0.4) | 5.0 (0.4) | <0.001 | |
Table of participant characteristics.
P values refer to comparison of GDM and non (IADPSG) GDM women.
IADPSG International Association of Diabetes and Pregnancy Study Group; GDM gestational diabetes mellitus; BMI body mass index; WHO World Health Organization; IOM Institute of Medicine; OGTT oral glucose tolerance test.
mean (standard deviation).
median (interquartile range).
n (%).
Using the newly recommended national COVID-19 processes and criteria would result in markedly reduced GDM frequency in the UK (81% reduction to 2.5%) and Canada (82% reduction to 1.7%), with a less marked 25% reduction to 12.7% in Australia (Fig. 1).
Examination of Tables 3 (UK), 4 (Canada) and 5 (Australia) clearly demonstrate that, in terms of absolute numbers on a population level, the majority of adverse clinical outcomes are found amongst women without a GDM diagnosis, though the frequency of these outcomes is higher among women who have GDM. This is not unexpected as the non-GDM group is numerically larger and the outcomes described are not specific to GDM pregnancy and are strongly influenced (for example) by the presence of maternal overweight / obesity without GDM. [12]
Table 3.
Frequency of pregnancy complications in all women using United Kingdom criteria to classify women as: missed GDMs (pre-COVID GDM, post-COVID non-GDM), post-COVID GDM, non-GDM (according to pre-COVID criteria). The column “% GDM outcomes missed post COVID” lists the percentage of each outcome which occurred amongst women classified as GDM using the pre COVID process and criteria, but would remain undetected using the post COVID process and criteria.
| Outcome | Missed GDM | Post-COVID GDM | % GDM Outcomes Missed Post-COVID& | Non-GDM by both pre and post-criteria |
|---|---|---|---|---|
| N | 684 | 147 | – | 5143 |
| Pregnancy-related hypertension# | 118 (18.5%) | 41 (29.1%)** | 48.4% | 785 (15.7%)*** |
| Preterm# | 62 (9.1%) | 16 (10.9%) | 79.5% | 271 (5.3%)*** |
| Birthweight (g)^ | 3404 (541) | 3493 (547) | – | 3373 (515)*** |
| Large-for-gestational age# | 95 (13.9%) | 24 (16.6%) | 79.8% | 447 (8.7%)*** |
| Primary Cesarean section# | 145 (24.4%) | 28 (21.9%) | 83.8% | 786 (17.3%)*** |
| Neonatal hyperinsulinemia# | 97 (16.0%) | 26 (20.8%) | 78.9% | 336 (7.4%)*** |
| Neonatal hypoglycemia# | 99 (19.4%) | 27 (26.7%) | 78.6% | 662 (17.1%) |
| Neonatal fat mass (g)^ | 415 (170) | 458 (185)* | – | 393 (167)*** |
| Neonatal adiposity# | 74 (13.4%) | 25 (22.1%)* | 74.7% | 363 (8.8%)*** |
n (%);
mean (standard deviation);
calculated as number of missed outcomes / total number of outcomes;
p < 0.05 in comparison to missed GDMs;
p < 0.01 in comparison to missed GDMs;
p < 0.001 in comparison to missed GDMs; GDM gestational diabetes mellitus; COVID coronavirus disease.
Considering the UK situation (Table 3), the 684 women currently classified as GDM but who would not be detected (“missed”) under the post COVID-19 guidelines demonstrate statistically significantly higher rates of preterm birth, LGA, primary CS, neonatal hyperinsulinemia and neonatal adiposity than non GDM women. Those still classified as GDM using the revised guidelines demonstrate, in addition, an elevated risk of pregnancy related hypertension and numerically higher risks for other outcomes.
Considering the Canadian criteria (Table 4 ), the 524 women currently classified as GDM but considered non GDM under post COVID-19 guidelines demonstrate increased risks (compared to non-GDM women) for all the outcomes listed for the UK, including pregnancy related hypertension.
Table 4.
Frequency of pregnancy complications in all women using Canadian criteria to classify as: missed GDMs (pre-COVID GDM, post-COVID non-GDM), post-COVID GDM, non-GDM (according to pre-COVID criteria). The column “% GDM outcomes missed post COVID” lists the percentage of each outcome which occurred amongst women classified as GDM using the pre COVID process and criteria, but would remain undetected using the post COVID process and criteria.
| Outcome | Missed GDM | Post-COVID GDM | % GDM Outcomes Missed Post-COVID& | Non-GDM by both pre and post-criteria |
|---|---|---|---|---|
| N | 524 | 99 | – | 5351 |
| Pregnancy-related hypertension# | 128 (26.3%) | 25 (26.3%) | 83.7% | 791 (15.2%)*** |
| Preterm# | 52 (9.9%) | 8 (8.1%) | 86.7% | 289 (5.4%)*** |
| Birthweight (g)^ | 3485 (561) | 3478 (559) | – | 3367 (513) *** |
| Large-for-gestational age# | 90 (17.2%) | 16 (16.3%) | 84.9% | 460 (8.6%)*** |
| Primary Cesarean section# | 110 (24.9%) | 21 (25.3%) | 84.0% | 828 (17.5%)*** |
| Neonatal hyperinsulinemia# | 85 (18.4%) | 18 (20.9%) | 82.5% | 356 (7.6%)*** |
| Neonatal hypoglycemia# | 87 (21.1%) | 18 (26.1%) | 82.9% | 683 (17.1%) |
| Neonatal fat mass (g)^ | 459 (185) | 440 (182) | – | 390 (165) *** |
| Neonatal adiposity# | 76 (18.3%) | 18 (22.8%) | 80.9% | 368 (8.5%)*** |
n (%);
mean (standard deviation);
calculated as number of missed outcomes / total number of outcomes;
p < 0.001 in comparison to missed GDMs; GDM gestational diabetes mellitus; COVID coronavirus disease.
The Australian process and criteria (Table 5 ) yield a different pattern of outcomes. The 253 women with IADPSG GDM, who would be “missed” under the proposed Australian post COVID-19 process (fasting glucose followed by selective OGTT, no change in diagnostic thresholds) have outcome frequencies similar to those of non GDM women, whilst those who would remain in the GDM group with post COVID-19 testing show significantly higher risks for all listed outcomes. This finding is likely related, at least in part, to the lower fasting glucose threshold used in Australia, which follows the IADPSG recommendations [13].
Table 5.
Frequency of pregnancy complications in all women using Australian criteria to classify as: missed GDMs (pre-COVID GDM, post-COVID non-GDM), post-COVID GDM, non-GDM (according to pre-COVID criteria)The column “% GDM outcomes missed post COVID” lists the percentage of each outcome which occurred amongst women classified as GDM using the pre COVID process and criteria, but would remain undetected using the post COVID process and criteria.
| Outcome | Missed GDM | Post-COVID GDM | % GDM Outcomes Missed Post-COVID& | Non-GDM by both pre and post-criteria |
|---|---|---|---|---|
| N | 253 | 761 | – | 4960 |
| Pregnancy-related hypertension# | 23 (9.5%) | 212 (30.0%)*** | 9.8% | 709 (14.6%) |
| Preterm# | 18 (7.1%) | 67 (8.8%) | 21.2% | 264 (5.3%) |
| Birthweight (g)^ | 3367 (532) | 3535 (552)*** | – | 3356 (509) |
| Large-for-gestational age# | 28 (11.1%) | 140 (18.4%)* | 16.7% | 398 (8.0%) |
| Primary Cesarean section# | 33 (15.3%) | 168 (26.5%)*** | 16.4% | 758 (17.2%) |
| Neonatal hyperinsulinemia# | 27 (11.8%) | 121 (18.0%) | 18.2% | 311 (7.1%)* |
| Neonatal hypoglycemia# | 33 (17.0%) | 121 (20.4%) | 21.4% | 634 (17.2%) |
| Neonatal fat mass (g)^ | 400 (159) | 474 (185)*** | – | 386 (163) |
| Neonatal adiposity# | 20 (9.8%) | 111 (18.8%)** | 15.3% | 331 (8.2%) |
n (%);
mean (standard deviation);
calculated as number of missed outcomes / total number of outcomes;
p < 0.05 in comparison to missed GDMs; * p < 0.05 in comparison to missed GDMs GDM gestational diabetes mellitus; COVID coronavirus disease.
Table 3, Table 4, Table 5 also document the proportion of pregnancy complications which occurred amongst GDM women using pre COVID-19 diagnostic processes, but which would be “missed” post COVID-19 as these women would no longer be classified as GDM and therefore would no longer receive any additional care. This represents the “opportunity cost” of revising the GDM diagnostic strategies. For example, the UK strategy reduces GDM numbers by 81%, but misses 48% of GDM related pregnancy hypertension, whilst the comparable figures for Canada are an 82% reduction in GDM frequency, with 84% of GDM related pregnancy hypertension missed.
4. Discussion
The three different current approaches to GDM testing yield different results. Perhaps unsurprisingly, the three different suggested modifications in diagnostic strategies proposed for GDM testing in response to the COVID-19 pandemic also appear to have different potential advantages and disadvantages [14]. The UK (1) and Canadian (2) strategies both prioritize avoidance of OGTTs with the intention of reducing potential COVID-19 exposure. Both approaches would reduce the frequency of GDM diagnoses by over 80%, but in each case the women, currently diagnosed with GDM, who would become “Missed GDMs” using the revised approaches still appear to be at higher risk of a range of pregnancy complications commonly associated with GDM. In the case of the Canadian criteria (Table 4), the “Missed GDM” women carry risks which are comparable to those who would still be detected under post COVID-19 guidelines and the percentage of GDM outcomes missed generally exceeds the overall reduction in GDM prevalence.
The proposed Australian modified testing protocol would reduce GDM frequency by 25% and these “Missed GDMs” appear to carry lower risks of complications. However, this approach would still require OGTTs in the 1405 women (23.5%) whose initial fasting venous plasma glucose (VPG) falls in the range 4.7–5.0 mmol/L. Further, this testing protocol requires an initial fasting VPG, which may be associated with increased patient numbers at collection centers in the early morning, potentially making appropriate physical distancing more difficult. It also brings back (at least for Australia) the issue of “two step testing”, where an initial result must be reviewed and a decision made as to whether a full OGTT is required. This approach has been variably reported as fraught with the potential for process errors (36% adherence) [15] or widely implementable (95% adherence) [16], even within the well-funded Canadian system.
A major strength of our approach is application of various diagnostic criteria to a large and ethnically diverse cohort, whose glucose results remained unblinded and glycemia untreated during pregnancy. Thus we have been able to assess not only the potential GDM prevalence using the three strategies, but also the impact of these changes in terms of pregnancy complications. This type of analysis is possible because of the blinded nature of the HAPO study and the fact that participants received no interventions.
However, we acknowledge that, for the UK and Canada, our processes do not precisely match current or proposed (post COVID-19) testing recommendations for GDM. The UK uses a selective, risk factor based check list to determine which women should receive biochemical testing and Canada’s preferred pathway involves two step testing with an initial non fasting “glucose screen”. This approach is estimated to reduce overall GDM diagnoses by approximately 25% [17]. Thus, in both countries, actual current detection of GDM cases is likely to be lower than would be the case with universal testing.
Further, our approach, in particular in the Australian context, assumes that results of a second glucose test (fasting or OGTT) would yield similar results to the first test performed, Actual reproducibility of OGTT results is known to be poor both outside pregnancy [18] and during pregnancy [19] and sample processing is also critically important [20].
Our cohort is multiethnic and represents five diverse HAPO centers. However, the findings in other countries (or even centers) may vary, especially as HAPO has reported marked geographic and ethnic differences in the proportion of women diagnosed with GDM based on fasting vs. post glucose load values across differing locations [21].
The COVID-19 pandemic poses a variety of serious challenges to pregnancy care [22] and GDM detection and management are only one part of a complex and rapidly evolving set of adaptations to care (e.g. use of telehealth or telephone visits) designed to both optimize pregnancy outcomes and protect pregnant women and health care staff. Pathology collection centers are able to utilize other strategies to reduce infection risk, including physical distancing, hand washing and the use of face masks. A woman who contracts COVID-19 infection during pregnancy clearly may acquire this through household or community transmission, rather than specifically at a pathology collection centre, so our primary clinical aim should be to reduce avoidable exposure and to encourage general protective measures in other settings.
Australia has experienced far fewer COVID-19 cases than the UK or Canada, and testing recommendations have already been modified, with routine OGTTs again recommended where COVID-19 transmission risk is considered low [23], the strategy described in this paper recommended for moderate risk situations and fasting glucose testing alone preferred if COVID-19 risk is considered high.
We hope that our analysis, rather than defining a single “correct” approach to the diagnosis of GDM in the context of the COVID-19 pandemic, will provide objective data to allow clinicians and health policy makers to balance the risk of potential exposure to COVID-19 during a diagnostic OGTT against the consequences of missing both a large number of potential GDM diagnoses the subsequent opportunity to provide women with interventions to reduce pregnancy complications and the postpartum health risks for both mother and baby.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest relevant to this work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Royal College of Obstetricians and Gynaecologists. Guidance for maternal medicine services in the evolving coronavirus (COVID-19) pandemic; 2020. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-05-13-guidance-for-maternal-medicine-services-in-the-evolving-coronavirus-covid-19-pandemic.pdf [accessed 21 May 2020].
- 2.Yamamoto JM DL, Reig DS, Berger H. Urgent update - temporary alternative screening strategy for gestational diabetes screening furing the COVID-19 pandemic; 2020. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/jcjd/JCJD_COVID_guidelines_020420-1585856697530.pdf [accessed 21 May 2020]. [DOI] [PMC free article] [PubMed]
- 3.Australasian Diabetes in Pregnancy Society. Diagnostic testing for gestational diabetes mellitus (GDM) during the COVID 19 pandemic. Antenatal and postnatal testing advice; 2020. https://www.adips.org/documents/COVID-19GDMDiagnosis030420ADIPSADSADEADAforWebsite.pdf [accessed 21 May 2020].
- 4.Thangaratinam S., Cooray S.D., Sukumar N., Huda M.S.B., Devlieger R., Benhalima K. ENDOCRINOLOGY IN THE TIME OF COVID-19: Diagnosis and management of gestational diabetes mellitus. Europ J Endocrinol/ Europ Federat Endocrine Soc. 2020;183:G49–G56. doi: 10.1530/eje-20-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Codina M., Corcoy R., Goya M.M. Update of the hyperglycemia Gestational diagnosis during the COVID-19 pandemic. Endocrinol Diabetes Nutr. 2020 doi: 10.1016/j.endinu.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasuga Y., Saisho Y., Ikenoue S., Ochiai D., Tanaka M. A new diagnostic strategy for gestational diabetes during the COVID-19 pandemic for the Japanese population. Diabetes/Metabolism Res Rev. 2020 doi: 10.1002/dmrr.3351. e3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vambergue A., Jacqueminet S., Lamotte M.F., Lamiche-Lorenzini F., Brunet C., Deruelle P. Three alternative ways to screen for hyperglycaemia in pregnancy during the COVID-19 pandemic. Diabetes Metabolism. 2020 doi: 10.1016/j.diabet.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 9.Contreras M., Sacks D., Watson W., Dooley S., Foderaro M., Niznik C. The hyperglycemia and adverse pregnancy outcome (HAPO) study. Int J Gynecol Obstetrics. 2002;78:69–77. doi: 10.1016/s0020-7292(02)00092-9. [DOI] [PubMed] [Google Scholar]
- 10.Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. Bjog. 2010;117:575–84. [DOI] [PubMed]
- 11.Metzger B.E., Persson B., Lowe L.P., Dyer A.R., Cruickshank J.K., Deerochanawong C. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010;126:e1545–e1552. doi: 10.1542/peds.2009-2257. [DOI] [PubMed] [Google Scholar]
- 12.Catalano P.M., McIntyre H.D., Cruickshank J.K., McCance D.R., Dyer A.R., Metzger B.E. The hyperglycemia and adverse pregnancy outcome study associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzger B.E., Gabbe S.G., Persson B., Buchanan T.A., Catalano P.A., Damm P. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntyre H.D., Moses R.G. The diagnosis and management of gestational diabetes mellitus in the context of the COVID-19 pandemic. Diabetes Care. 2020 doi: 10.2337/dci20-0026. [DOI] [PubMed] [Google Scholar]
- 15.Sievenpiper J.L., McDonald S.D., Grey V., Don-Wauchope A.C. Missed follow-up opportunities using a two-step screening approach for gestational diabetes. Diabetes Res Clin Pract. 2012;96:e43–e46. doi: 10.1016/j.diabres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Donovan L.E., Savu A., Edwards A.L., Johnson J.A., Kaul P. Prevalence and timing of screening and diagnostic testing for gestational diabetes mellitus: a population-based study in Alberta, Canada. Diabetes Care. 2016;39:55–60. doi: 10.2337/dc15-1421. [DOI] [PubMed] [Google Scholar]
- 17.van Leeuwen M., Louwerse M.D., Opmeer B.C., Limpens J., Serlie M.J., Reitsma J.B. Glucose challenge test for detecting gestational diabetes mellitus: a systematic review. BJOG. 2012;119:393–401. doi: 10.1111/j.1471-0528.2011.03254.x. [DOI] [PubMed] [Google Scholar]
- 18.Mooy J.M., Grootenhuis P.A., de Vries H., Kostense P.J., Popp-Snijders C., Bouter L.M. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39:298–305. doi: 10.1007/BF00418345. [DOI] [PubMed] [Google Scholar]
- 19.Catalano P.M., Avallone D.A., Drago N.M., Amini S.B. Reproducibility of the oral glucose tolerance test in pregnant women. Am J Obstet Gynecol. 1993;169:874–881. doi: 10.1016/0002-9378(93)90019-f. [DOI] [PubMed] [Google Scholar]
- 20.Potter J.M., Hickman P.E., Oakman C., Woods C., Nolan C.J. Strict preanalytical oral glucose tolerance test blood sample handling is essential for diagnosing gestational diabetes mellitus. Diabetes Care. 2020 doi: 10.2337/dc20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks D.A., Hadden D.R., Maresh M., Deerochanawong C., Dyer A.R., Metzger B.E. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poon L.C., Yang H., Kapur A., Melamed N., Dao B., Divakar H. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int J Gynaecol Obstet. 2020;149:273–286. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Australasian Diabetes in Pregnancy Society Diagnostic testing for gestational diabetes mellitus (GDM) during the COVID-19 pandemic: Antenatal and postnatal testing advice. Australia; 2020. https://www.adips.org/documents/ADIPSADSCOVID-19GDMDiagnosisUpdated250420Website.pdf [accessed July 10 2020].