Abstract
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2. Our understanding of this new disease continues to grow. The impact of the disease on immunocompromised transplant recipients is largely unknown. We present a case of a solid organ transplant recipient on immunosuppressive therapy who successfully recovered from COVID-19 infection. We also review 10 similar cases found in the literature and describe the clinical course and management, including immunosuppressive therapy.
A pneumonia caused by a novel coronavirus designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first noted in Wuhan, a city in Hubei Province, China in December 2019. It spread rapidly in China and later in the entire world and was recognized by the World Health Organization as a pandemic on March 11, 2020 [1]. Investigations are under way to better understand the disease.
We present a case of a renal transplant recipient who developed respiratory distress because of COVID-19 infection and successfully recovered. To the date of this paper, the literature search for previous reports of COVID-19 infection in immunocompromised transplant recipients resulted in 8 publications describing 10 cases.
We discuss the clinical course and the management of the disease, including immunosuppressive therapy. We speculate a possible mechanism of the potential effect of immunosuppressants in the course of this disease.
Case Report
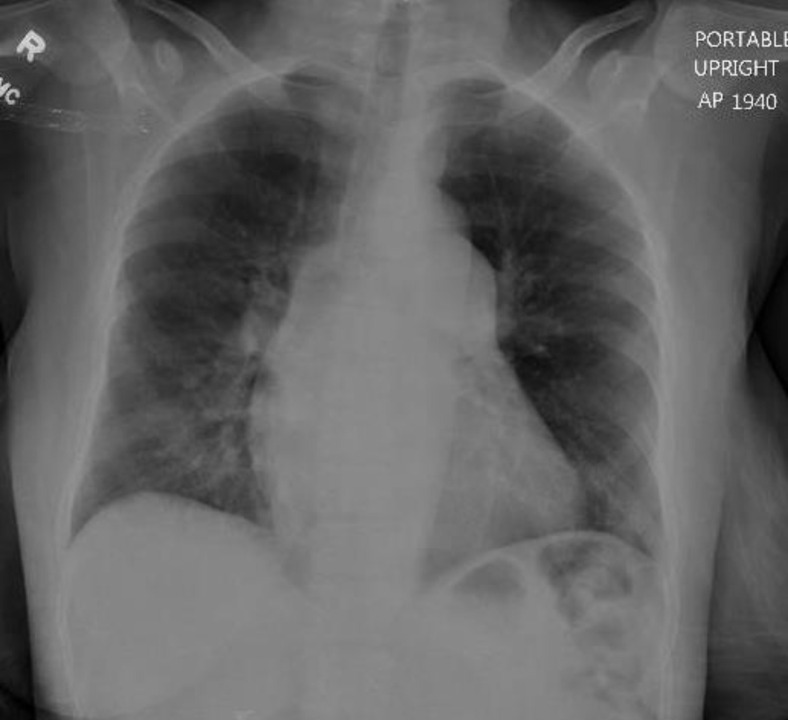
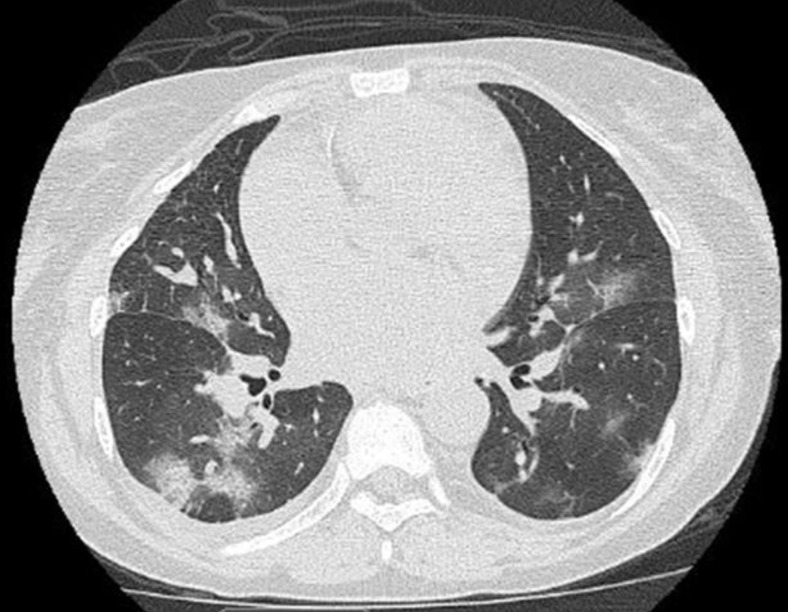
A 43-year-old woman who received a kidney transplant 12 years ago because of sickle cell nephropathy presented with a 1-day history of nonproductive cough, shortness of breath, subjective fever, and generalized musculoskeletal pain. Besides sickle cell disease, she has history of recurrent deep venous thrombosis treated with apixaban, pulmonary hypertension on 2 L of home oxygen, and essential hypertension. She was on maintenance immunosuppressive therapy with tacrolimus 3 mg/d and prednisone 5 mg/d. On admission, the patient was saturating well on 2 L by nasal cannula without distress. Other vital signs were within normal limits. On physical examination, the patient was found to have bronchial lung sounds on the right side. The rest of the examination was unremarkable. Laboratory tests showed acute kidney injury (creatinine 2.2 mg/dL, with baseline 1.8 mg/dL), neutrophilia, and lymphopenia (Table 1 ). Chest radiography showed opacity in right lower lobe (Fig 1 ), a finding consistent with pneumonia. Rapid nucleic acid amplification tests for influenza A and B and respiratory syncytial virus through nasal swab were reported as negative. Treatment with ceftriaxone and azithromycin for community-acquired pneumonia was initiated. The patient was started on intravenous opioids for vaso-occlusive pain crisis of sickle cell disease. On day 2 through 4 of hospitalization, the patient musculoskeletal pain improved but continued to report a nonproductive cough. On hospital day 5, the patient developed intermittent fevers and her oxygen requirement increased to 5 L by nasal cannula (Table 1). Other vital signs remained largely stable. Antibiotics coverage was broadened to vancomycin, cefepime, and azithromycin. Subsequent chest computed tomography (CT) revealed multiple bilateral ground-glass opacities (Fig 2 ). A concern for acute chest syndrome with sickle cell disease was raised, and the patient received 2 units of packed red blood cells. Blood and sputum cultures were obtained and showed no growth. Given the radiographic finding, persistent fever, increased supplemental oxygen requirement, and the recent concern for COVID-19 in other states in the United States, the patient was tested for COVID-19 with a nasal swab nucleic acid test and the result was positive. The patient was 1 of the first COVID-19 patients discovered in Detroit metro area. The patient denied recent travel or any contact with sick people. The patient’s acute kidney injury on admission improved to baseline after 5 days (Table 1). Her immunosuppressive therapy of tacrolimus 3 mg/d and prednisone 5 mg/d were never stopped and resumed during hospitalization. The tacrolimus trough level was within normal limits during hospitalization (Table 1). The patient was not treated with any specific novel therapy for COVID-19. Laboratory test results reflected neutrophilia and lymphopenia during hospitalization (Table 1). Testing for C-reactive protein and lactate dehydrogenase was not done. The patient remained hemodynamically stable during hospitalization. On hospital day 7, the patient’s clinical condition improved. Vancomycin and cefepime were discontinued on day 8, and the patient’s fever and cough resolved. The oxygen requirement improved back to baseline (2 L by nasal cannula). The patient was discharged home after 10 days of hospitalization.
Table 1.
Vital Signs and Laboratory Findings
| Variable | On Admission | Hospital Day 2 | Hospital Day 5 | Hospital Day 8 | Reference Ranges |
|---|---|---|---|---|---|
| Oxygen supplement | NC/2 L | NC/2 L | NC/5 L | NC/2 L | — |
| Hematocrit, % | 19.7 | 23 | 18.2 | 20.1 | 38.9-49.7 |
| Hemoglobin, mg/dL | 6.4 | 7.4 | 5.5 | 6.4 | 13.3-17.1 |
| White blood cell count, K/CUMM | 6.0 | 11.6 | 15.2 | 9.5 | 3.5-10.6 |
| Differential count, K/CUMM | |||||
| Neutrophils | 13.5 | 8.8 | 8.3 | 6.8 | 1.58-7.13 |
| Lymphocytes | 0.6 | 1.1 | 1.4 | 0.8 | 1.0-3.8 |
| Platelet count, K/CUMM | 251 | 124 | 114 | 140 | 150-450 |
| Sodium, mmol/L | 140 | 139 | 137 | 138 | 136-145 |
| Potassium, mmol/L | 5.1 | 5.0 | 4.4 | 4.3 | 3.5-5.1 |
| Chloride, mmol/L | 112 | 111 | 111 | 109 | 98-107 |
| Bicarbonate, mmol/L | 18 | 20 | 17 | 22 | 21-31 |
| Anion gap, mmol/L | 10 | 8 | 9 | 12 | 5-15 |
| Glucose, mg/dL | 94 | 123 | 79 | 120 | 75-105 |
| Blood urea nitrogen, mg/dL | 36 | 27 | 20 | 18 | 7-25 |
| Creatinine, mg/dL | 2.2 | 1.96 | 1.94 | 1.85 | 0.70-1.30 |
| Calcium, mg/dL | 9.5 | 9.2 | 8.8 | 9.1 | 8.6-10.8 |
| Alanine aminotransferase, U/L | — | 4 | 5 | 14 | 7-52 |
| Aspartate aminotransferase, U/L | — | 19 | 18 | 25 | 13-39 |
| Tacrolimus level, ng/mL | 6.7 | 7.2 | 5.1 | 9.5 | 5.0-15.0 |
Abbreviations: K/CUMM, thousand cells per cubic millimeter; NC, nasal cannula.
Fig 1.
Chest radiography on day 1.
Fig 2.
Chest computed tomography scan on day 5.
Results
After reviewing the literature and to the date of this report, 10 cases of COVID-19 infection in immunocompromised transplant recipients have been reported in the literature, including the current case (Table 2 ). Patients range in age from 37 to 75 years. All patients in the series, except ours, were men. Presentation is typical in most cases, with fever and cough being the most frequently presented symptoms. Diagnosis of COVID-19 was accomplished by nasal swab nucleic acid test. Most patients in the series had a kidney transplant and were on mycophenolate, tacrolimus, or both for immunosuppressive therapy. CT findings were similar in most cases and showed bilateral ground-glass opacities. During hospitalization, immunosuppressive therapy was with held in 5 patients and continued in 3 patients, including ours. Two case reports did not comment about immunosuppressive therapy. All patients, except 2, received antiviral therapy. Other therapies, such as methylprednisolone, hydroxychloroquine, and intravenous immunoglobulin (IVIg) were administered in other cases.
Table 2.
Previous Case Reports of COVID-19 Infection in Immunocompromised Transplant Recipients
| Author | Age/ Sex | Organ Transplanted Years/Months Ago | Immunosuppression | Immunosuppression During Hospitalization | Presenting Signs/Symptoms | CT Findings | Intubation | Treatment | Disposition | Transplant Rejection |
|---|---|---|---|---|---|---|---|---|---|---|
| Guillen [11] | 50 M | Kidney: 4 y ago | Tacrolimus, everolimus, and prednisone | Held | Fever, productive cough | Bilateral ground-glass opacities | Yes | Hydroxychloroquine, lopinavir/ritonavir, interferon beta | Extubated, recovering on medical floor | No |
| Huang [3] | 58 M | Kidney: 12 y ago | Mycophenolate mofetil and corticosteroid | Unknown | Fever, cough | Unknown | Yes | Methylprednisolone | Deceased | No |
| Gandolfini [2] | 75 M | Kidney: 10 y ago | Mycophenolate mofetil, tacrolimus, and corticosteroid | Held | Cough, myalgia, and fever | Bilateral ground-glass opacities | None | Hydroxychloroquine, lopinavir + ritonavir, or darunavir + cobicistat | Deceased | No |
| Gandolfini [2] | 52 M | Kidney: 8 mo ago | Mycophenolate mofetil, tacrolimus, and corticosteroid | Held | Cough, myalgia, and fever | Bilateral ground-glass opacities | None | Hydroxychloroquine, lopinavir + ritonavir or darunavir + cobicistat and colchicine | Still in hospital, on noninvasive ventilation | No |
| Li [12] | 51 M | Heart: 15 y ago | Mycophenolate mofetil and tacrolimus | Held | Fever, diarrhea | Bilateral ground-glass opacities | None | Moxifloxacin and ganciclovir, then human gamma globulin + methylprednisolone | Discharged | No |
| Li [12] | 43 M | Heart: 3 y ago | Mycophenolate mofetil and tacrolimus | Unknown | Fever | Unknown | None | Umifenovir + ganciclovir | Discharged | No |
| Qin [13] | 37 M | Liver: perioperative | Tacrolimus and corticosteroids | Continued: lower dose | Fever | Bilateral ground-glass opacities | None | Oseltamivir, recombinant human granulocyte colony-stimulating factor and intravenous immunoglobin | Discharged | No |
| Seminari [14] | 50 M | Kidney: 4 y ago | Mycophenolate mofetil and tacrolimus | Continued | Fever and cough | Bilateral reticular opacities | None | None | Discharged | No |
| Zhu [15] | 52 M | Kidney: 12 y ago | Mycophenolate mofetil, tacrolimus, and corticosteroid | Held | Dry cough, fever, dyspnea, and gastrointestinal symptoms | Bilateral ground-glass opacities | None | Umifenovir + moxifloxacin, then methylprednisolone, intravenous immunoglobulin, and interferon alfa | Discharged | No |
| Current study | 43 F | Kidney: 12 y ago | Tacrolimus and corticosteroids | Continued | Dry cough, fever, and shortness of breath | Bilateral ground-glass opacities | None | None | Discharged | No |
Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography.
Six patients experienced resolution of symptoms, did not require mechanical ventilation, and discharged from hospital. Two patients were still in hospital while their cases were reported. One was stable on noninvasive ventilation, and the other one, according to further communication with the author, was extubated successfully and recovering on a medical floor. Two patients died; one was a 75-year-old man [2] who developed acute worsening of respiratory status and died before mechanical intubation, and the other one was a 58-year-old man [3] who had severe respiratory distress requiring intubation and extracorporeal membrane oxygenation (ECMO) but had developed multiorgan failure and died. In our review, the age of patients who recovered and were discharged or still in hospital were relatively younger than the 2 patients who died.
Discussion
It is well known that infections after transplantation may manifest in atypical fashion and might promote transplant rejection [4]; however, our patients presented with typical symptoms and none of the cases reported transplant rejection during hospitalization.
Advanced age and comorbidities such as hypertension, diabetes mellitus, and chronic lung and kidney disease have been associated with higher severity and mortality in COVID-19 infection [[5], [6], [7]]. However, specific data regarding risks associated with immunocompromised conditions are limited and further studies are needed. Generally, these patients are more susceptible to bacterial, fungal, viral, and parasitic infections and have poor outcomes compared to immunocompetent subjects [4]. However, this is not always the case; our experience from other types of coronavirus such as severe acute respiratory syndrome-related coronavirus [8] and Middle East respiratory syndrome (MERS) [9] showed that immunosuppressed status was not a risk factor for disease severity. One hypothesis that might explain this is that immunosuppression therapies mitigate the inflammatory reaction triggered by the virus. This reaction, also called cytokine storm or hyperinflammation, may play a major role in COVID-19 severity and mortality [10]. Although our review showed a high mortality rate, with 2 of 10 patients having died, our sample is small and not sufficient to predict mortality and severity of illness in immunocompromised transplant recipients and further large studies are needed.
Data regarding resuming or holding immunosuppressive therapy in COVID-19 are limited. Immunosuppressants may diminish antiviral host immunity, so some clinicians tend to hold or decrease the dose of immunosuppressive therapy and use corticosteroids instead as the only antirejection drug. On the other hand, some clinicians tend to resume immunosuppressive therapy because stopping immunosuppression, besides increasing the risk for transplant rejection, might increase the immune surge reaction “cytokine storm,” leading to poor outcomes.
Conclusion
In COVID-19 infection, clinicians should not label immunosuppressed status in transplant recipients as a poor prognostic factor until further large-scale epidemiologic investigations are conducted. In addition, further large studies are needed to elaborate on the management of immunosuppressive therapy during hospitalization with COVID-19 infection.
References
- 1.World Health Organization Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020
- 2.Gandolfini I., Delsante M., Fiaccadori E., Zaza G., Manenti L., Degli Antoni A. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20:1941–1943. doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J., Lin H., Wu Y. COVID-19 in posttransplant patients: report of 2 cases. Am J Transplant. 2020;20(7):1879–1881. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman J.A. Infection in organ transplantation. Am J Transplant. 2017;17:856–879. doi: 10.1111/ajt.14208. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020;395(10229):1038. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020:2648. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Leung G.M., Hedley A.J., Ho L.M. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med. 2004;141(9):662–673. doi: 10.7326/0003-4819-141-9-200411020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Garbati M.A., Fagbo S.F., Fang V.J. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to MERS coronavirus or other causes. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guillen E., Pineiro G.J., Revuelta I. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875–1878. doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li F., Cai J., Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020;39(5):496–497. doi: 10.1016/j.healun.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin J., Wang H., Qin X. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020:31257. doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 14.Seminari E., Colaneri M., Sambo M., the COVID19 IRCCS San Matteo Pavia Task Force SARS CoV-2 infection in a renal-transplanted patients: a case report. Am J Transplant. 2020;20:1882–1884. doi: 10.1111/ajt.15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L., Xu X., Ma K. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20(7):1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]