Abstract
Background:
Persons with HIV (PWH) are more likely to smoke and are more susceptible to the harmful effects of smoking than persons without HIV. We examined smoking patterns and use of cessation treatment among PWH and persons without HIV in a U.S. integrated health system.
Methods:
We identified adults (≥18 years) with HIV and demographically-matched persons without HIV between July 2013 and December 2017. Smoking status and cessation treatment were ascertained from health records. We calculated age-standardized annual prevalence of smoking and evaluated trends using Cochran-Armitage tests and Poisson regression. Factors associated with cessation treatment during the study period, and smoking in the last year of the study, were evaluated by HIV status using multivariable Poisson models.
Results:
The study included 11,235 PWH and 227,320 persons without HIV. Smoking prevalence was higher among PWH across all years but declined for both groups (from 16.6% to 14.6% in PWH and 11.6% to 10.5% in persons without HIV). Among smokers, PWH were more likely to initiate cessation treatment compared to persons without HIV (17.9% vs. 13.3%, covariate-adjusted prevalence ratio of 1.31, 95% CI=1.15–1.50), with few differences in cessation treatment across subgroups of PWH. In 2017, smoking prevalence remained higher in PWH, especially among those who were younger or who had diagnoses of depression or substance use disorder.
Conclusion:
In a setting with access to cessation resources, smoking prevalence decreased both in PWH and persons without HIV. PWH had greater uptake of cessation treatment, which is encouraging for smoking reduction and improved health.
Keywords: HIV, smoking, cessation, treatment, tobacco, access-to-care
1. Introduction
Smoking is a major contributor to morbidity and mortality among persons with HIV (PWH) (Lifson and Lando, 2012). In addition to being more likely to smoke than individuals in the general population (Mdege et al., 2017; Murphy et al., 2019; Park et al., 2016; Tron et al., 2014), PWH are more susceptible to the harmful effects of smoking (Rahmanian et al., 2011). Compared to uninfected smokers, PWH who smoke are more likely develop respiratory complications and disease, including smoking-related cancers (Crothers and Tindle, 2011; De et al., 2013; Depp et al., 2016; Pacek and Cioe, 2015). Further, the excess mortality associated with smoking is estimated to be over 3-fold greater among PWH than their uninfected counterparts (Helleberg et al., 2013).
With widespread use of antiretroviral therapy (ART) for treatment of HIV infection, the relevance of smoking as an independent driver of disease and mortality is increasingly apparent. Studies have consistently found that smoking is associated with substantial morbidity and mortality in PWH, even after accounting for HIV-specific clinical factors such as ART exposure, CD4 count, and HIV RNA level (Altekruse et al., 2018; Lifson et al., 2010). In fact, with decreasing AIDS-related mortality, PWH who are effectively treated with ART now lose more life-years from smoking than from HIV infection (Helleberg et al., 2014, 2015; Reddy et al., 2016). Additionally, limited but accumulating data suggest that PWH who smoke experience a greater burden of aging-related functional impairments including neurocognitive impairment (Bryant et al., 2013; Monnig et al., 2016), bone fractures (Hansen et al., 2012; Yin et al., 2010), physical disability, and frailty (Erlandson et al., 2017; Johs et al., 2017).
Given that individuals with well-managed HIV disease are more likely to suffer poor health because of smoking than HIV-related immunodeficiency, smoking cessation should be a critical focus of HIV care. However, despite declines in smoking prevalence in the general U.S. population, PWH remain more likely to smoke and less likely to quit (Mdodo et al., 2015). Barriers to quitting include concomitant use of other substances, psychiatric comorbidity and socioeconomic disadvantage, all of which are more common among PWH (Browning et al., 2013; Calvo-Sanchez and Martinez, 2015; Rahmanian et al., 2011). Several smoking treatment intervention and feasibility studies highlight the importance of tailoring cessation strategies to address these issues (Browning et al., 2013; Mann-Jackson et al., 2019; Moscou-Jackson et al., 2014). However, few studies have evaluated factors associated with uptake of cessation services among PWH in a community-based setting, partly because smoking cessation is not widely integrated into routine HIV care (Fuster et al., 2009; Miles et al., 2019; Torres et al., 2014; Zyambo et al., 2019). Additionally, prior studies have lacked comparison of cessation treatment use by HIV status, which would be important for strategic delivery of health system-level smoking resources and identification of subgroups of PWH that could benefit from more tailored approaches (Fuster et al., 2009; Ladapo et al., 2017; Miles et al., 2019; Torres et al., 2014; Zyambo et al., 2019).
In California, the Patient Protection and Affordable Care Act (ACA) took effect on January 1, 2014, resulting in expanded health coverage options for previously uninsured Californians (Satre et al., 2016). The ACA also expanded cessation benefits, providing comprehensive access to tobacco cessation counseling and pharmacotherapy with no cost-sharing. While this milestone policy ameliorated many financial and access-to-care barriers (Schmittdiel et al., 2017), some studies show persistent sociodemographic disparities in both smoking prevalence and cessation treatment post-ACA (Tan et al., 2018; Young-Wolff et al., 2017). However, data are lacking on post-ACA smoking and cessation treatment among PWH.
Given the unique patterns and impact of smoking in PWH, direct comparison of factors associated with smoking and cessation treatment use among PWH and uninfected controls could inform strategies for tackling remaining smoking reduction challenges in the context of improved access to care. To address these questions, the aims of this study were to examine 1) recent smoking prevalence and trends, 2) factors associated with cessation treatment, and 3) factors associated with current smoking among insured individuals with and without HIV in a setting with both HIV care and cessation resources.
2. Methods
2.1. Study setting
This study was conducted in Kaiser Permanente Northern California (KPNC), an integrated health system in the U.S. which provides inpatient and outpatient services to approximately 4 million members, including over 9,000 with HIV. KPNC has a service area that encompasses the San Francisco Bay Area and the Central Valley from the Sacramento area in the north to Fresno in the south. Following implementation of the ACA, KPNC experienced growth in enrollment of PWH and a significant increase in new enrollment of PWH who smoke (Satre et al. 2016).
Smoking cessation services at KPNC include free-of-charge in-person group classes and one-on-one telephone cessation coaching that are available by provider referral. These services follow evidence-based treatment strategies recommended by the U.S. Public Health Service Clinical Practice Guideline for Treating Tobacco Use and Dependence (Fiore et al. 2008). Approximately 96% of members are enrolled in a KPNC pharmacy plan, which enables them to obtain provider-prescribed smoking cessation medications from any KP pharmacy (Karter et al. 2017).
2.2. Study population
The study included all adults (≥18 years) with HIV infection and a comparator group of adults without HIV who had membership at KPNC at any time between July 2013 and December 2017, with gaps in membership of no more than 90 days. PWH and persons without HIV were frequency-matched 1:20 by age, sex and race/ethnicity. The start of the study in July 2013 coincided with implementation of KPNC’s Alcohol as a Vital Sign program, which involves systematic screening of all patients for unhealthy alcohol use, an important cofactor in evaluations of smoking (Mertens et al. 2015).
Analyses of annual smoking prevalence included patients who had KPNC membership in the year of interest. For analyses of smoking cessation treatment use, we restricted the study population to patients who had at least one instance of “current smoking” documented in the electronic health record (EHR), at least 2 years of membership following report of current smoking, and who were not already engaged in cessation treatment at KPNC, defined as use of any cessation treatment in KPNC during the year prior to first EHR documentation of smoking. For analyses of factors associated with smoking in the final year of the study, we restricted the study population to patients with known smoking status. Thus, for cross-sectional analyses assessing annual smoking prevalence, the study sample was not restricted based on duration of participant follow-up time; however, for analyses of smoking cessation treatment use, each study participant was required to have a minimum of 3 years of follow-up (i.e. 1 year before “current smoking” was reported to allow assessment of KPNC cessation history and 2 years after “current smoking” to assess use of cessation treatment).
2.3. Measures
2.3.1. Smoking status and cessation treatment
Patients’ smoking status and use of cessation treatment were ascertained from screening and service use data in the EHR. Data on use of cessation pharmacotherapy were obtained from KPNC pharmacy databases. Patients were considered to have used cessation treatment if they: 1) filled a cessation-related prescription medication (e.g., nicotine replacement therapy, bupropion or varenicline), 2) attended a group cessation class, or 3) completed an individual cessation coaching session. Since bupropion may be prescribed for indications other than smoking cessation, we required that patients with bupropion prescriptions also have a tobacco dependence diagnosis (International Classification of Diseases [ICD]-9: 305.1x, ICD-10: F17.2x) for their prescription to be counted as a smoking cessation attempt (Young-Wolff et al., 2017).
2.3.2. Covariates
Data on prior clinical diagnoses of substance use disorders, including dependence on tobacco, alcohol or other drugs, were obtained from patients’ EHR (ICD-9 291.x, 292.x, 303.x-305.x; ICD-10 F10.x-F19.x, F55.x). Demographic covariates included sex, age, race/ethnicity and type of health insurance (i.e. commercial insurance, Medicare or Medicaid). Education and income were estimated from Census block group data based on patients’ location of residence. Lower education was defined as ≥25% of the population in the patient’s neighborhood the population in the patient’s neighborhood with household income below poverty level. Clinical covariates included body mass index (BMI), depression, and Charlson score (Charlson et al., 1987) (measure of comorbidity burden, modified to exclude AIDS and calculated based on diagnoses and procedures in the year prior to study entry). The number of outpatient visits in the year prior to study entry served as a measure of patients’ engagement in their healthcare. Data on quantity and frequency of alcohol use were obtained from the EHR. Unhealthy alcohol use was defined as follows, based on daily and weekly drink limits recommended by the National Institute on Alcohol Abuse and Alcoholism: for women of any age and men ≥65-years old, having ≥4 drinks in a day at least once in the past 90 days or ≥8 drinks per week on average; for men <65-years-old, ≥5 drinks in a day at least once in the past 90 days or ≥15 drinks per week on average (Willenbring et al., 2009). HIV-specific covariates included HIV transmission risk (i.e., men who have sex with men [MSM], heterosexual, injection drug use [IDU]), CD4 cell count (cells/μl), HIV RNA level (copies/mL) and history of clinical AIDS.
2.4. Statistical analyses
PWH and persons without HIV were frequency-matched 1:20 by age, sex and race/ethnicity to ensure balance between the two groups. Baseline non-matched characteristics of PWH and persons without HIV were compared using t-tests for continuous variables and χ2 tests for categorical variables.
2.4.1. Smoking prevalence and trends
Using patients’ last reported smoking status in each calendar year, annual prevalence of current smoking was calculated from July 2013 to December 2017, separately by HIV status, and standardized using the age structure of the U.S. population according to the 2010 Census.(Howden and Meyer 2011) Then, trends in smoking prevalence were calculated using Cochran-Armitage tests. Annual percentage change in smoking prevalence and differences in changes by HIV status were evaluated using Poisson regression models.
2.4.2. Use of cessation treatment
Patients entered the study on the date that current smoking was first documented in their EHR. We calculated the proportion of patients who attempted any type of cessation treatment within 2 years of study entry, overall and by type of treatment. Then, we used multivariable Poisson regression with robust error variance to calculate a prevalence ratio (PR) comparing the likelihood of treatment by HIV status, adjusting for sociodemographic, clinical and behavioral factors.
2.4.3. Factors associated with cessation treatment
Sociodemographic, clinical and behavioral factors (listed in section 2.3.2) associated with cessation treatment were evaluated using bivariate and multivariable Poisson regression models with robust error variance, with separate models constructed for PWH and persons without HIV. Each covariate was first evaluated in bivariate models and those significantly associated with treatment at p<0.10 were included in multivariable models. Among PWH, we evaluated whether HIV-specific factors (i.e., most recent CD4 count and HIV RNA level, and any prior clinical AIDS diagnosis) were associated with cessation treatment.
2.4.4. Factors associated with current smoking
Finally, we examined sociodemographic, clinical and behavioral factors (listed in section 2.3.2) associated with current smoking (vs. never or former smoking) among PWH and persons without HIV in 2017, the last year of the study, using the same statistical approach used to evaluate factors associated with cessation treatment. Analyses were conducted in Stata 12 (College Station, TX). This study was approved by the KPNC Institutional Review Board.
3. Results
The overall study population included 11,235 PWH and 227,320 persons without HIV. Study participants were 90.5% male, 52% White, 17% Black, and the mean age was 47 years (Table 1). From July 2013 to December 2017, smoking prevalence was higher among PWH than persons without HIV for all years but declined for both groups over time (from 16.6% to 14.6% among PWH and from 11.6% to 10.5% among persons without HIV; p’s for trend=0.07 for both; Figure 1). Although annual decreases in smoking appeared to be slightly greater among PWH compared to persons without HIV, there was no statistically significant difference (approximately −0.7% annually in PWH and −0.4% annually in persons without HIV; p=0.25). Among 8,432 PWH and 167,391 persons without HIV with active KPNC membership in the last year of follow-up, smoking prevalence remained higher among PWH (PR=1.39, 95% CI=1.33–1.44).
Table 1.
Characteristics of study participants at baseline, by HIV status - Kaiser Permanente Northern California, July 2013 to December 2017
| Characteristic | Persons with HIV N=11,235 n (%) | Persons without HIV N=227,320 n (%) | p |
|---|---|---|---|
| Female1 | 1,069 (9.5) | 21,538 (9.5) | (matched) |
| Mean age (SD), years1 | 47.3 (12.3) | 47.4 (14.0) | (matched) |
| Race/Ethnicity1 | |||
| White, non-Hispanic | 5,864 (52.2) | 118,116 (52.0) | |
| Black, non-Hispanic | 1,873 (16.7) | 38,181 (16.8) | (matched) |
| Hispanic | 1,993 (17.7) | 40,497 (17.8) | |
| Other | 1,055 (9.4) | 21,480 (9.4) | |
| Unknown | 450 (4.0) | 9,046 (4.0) | |
| Lower neighborhood education2 | 1,907 (17.1) | 39,408 (17.4) | 0.40 |
| Lower neighborhood income3 | 1,932 (17.3) | 27,366 (12.1) | <0.001 |
| Insurance | |||
| Commercial/Private | 8,758 (78.0) | 192,757 (84.8) | |
| Medicare | 2,070 (18.4) | 27,857 (12.3) | <0.001 |
| Medicaid | 228 (2.0) | 4,072 (1.8) | |
| Other/Unknown | 179 (4.0) | 2,634 (1.2) | |
| Mean number of outpatient visits in year prior to study entry (SD) | 7.0 (13.7) | 3.4 (7.8) | <0.001 |
| Body mass index category4 | |||
| Normal | 3,543 (31.5) | 37,105 (16.3) | |
| Underweight | 149 (1.3) | 1,089 (0.5) | <0.001 |
| Overweight | 3,515 (31.3) | 65,742 (28.9) | |
| Obese | 1,630 (14.5) | 61,706 (27.1) | |
| Unknown | 2,398 (21.3) | 61,678 (27.1) | |
| Charlson score5 | |||
| 0 | 8,892 (79.1) | 191,875 (84.4) | <0.001 |
| 1 | 1,103 (9.8) | 19,043 (8.4) | |
| ≥2 | 1,240 (11.0) | 16,402 (7.2) | |
| Depression6 | 3,490 (31.0) | 25,614 (11.3) | <0.001 |
| Alcohol use disorder | 1,184 (10.5) | 14,853 (6.5) | <0.001 |
| Tobacco use disorder | 2,977 (26.5) | 38,675 (17.0) | <0.001 |
| Substance use disorder7 | 1,558 (13.9) | 10,524 (4.6) | <0.001 |
| HIV-specific factors | |||
| HIV risk | |||
| Men who have sex with men | 7,846 (69.8) | ||
| Heterosexual | 1,635 (14.6) | - | - |
| Injection drug use | 782 (7.0) | ||
| Other/Unknown | 972 (8.7) | ||
| CD4 T cell count ≥500 cells/μl8 | 5,154 (64.3) | - | - |
| HIV RNA <75 copies/ml9 | 6,592 (82.2) | - | - |
| Prior clinical AIDS diagnosis | 4,872 (43.4) | - | - |
Abbreviation: SD = standard deviation
PWH and persons without HIV were frequency-matched 1:20 by age, sex and race/ethnicity.
% of known; lower neighborhood education defined as ≥25% of the population in the patient’s Census block group having no high school diploma; 82 PWH and 918 persons without HIV had unknown education.
% of known; lower neighborhood income defined as ≥25% of population in the patient’s Census block group with household income below poverty levels; 82 PWH and 926 persons without HIV had unknown income.
Body mass index was defined as follows: Underweight (<18.5 kg/m2), Normal (18.5 to <25 kg/m2), Overweight (25 to <30 kg/m2), Obese (≥30 kg/m2). 23 (0.3%) PWH and 2,091 (1.3%) HIV-uninfected persons had unknown BMI.
Measure of comorbidity burden, modified to exclude AIDS and calculated based on diagnoses and procedures in the year prior to study entry.
Any prior diagnosis of depression.
Other substances besides tobacco and alcohol.
% of known; 3,224 (28.7%) of patients had unknown CD4.
% of known; 3,216 (28.6%) of patient had unknown HIV RNA. 75 copies/mL was the lower limit of detection during the study period.
Figure 1. Annual prevalence and trends in current smoking among persons with and without HIV - Kaiser Permanente Northern California, July 2013 to December 2017.
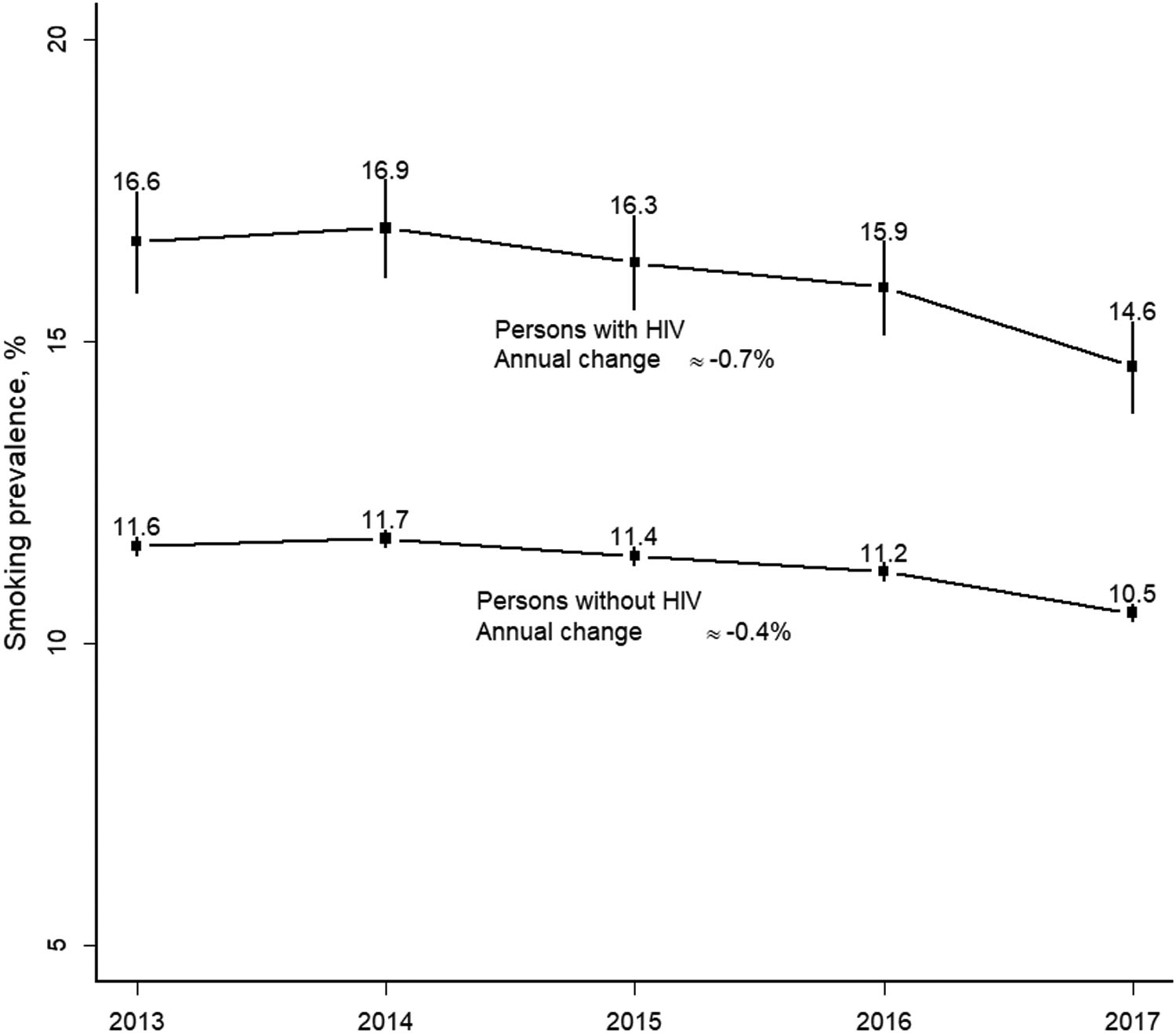
Smoking prevalence shown with 95% confidence intervals.
Of 1,123 HIV-infected smokers and 16,283 HIV-uninfected smokers who were not already engaged in cessation treatment within KPNC (Table 2), 201 (17.9%) PWH and 2,159 (13.3%) persons without HIV initiated at least one type of cessation treatment during 2 years of follow-up. PWH were significantly more likely to initiate any cessation treatment than persons without HIV (adjusted prevalence ratio [aPR]=1.31, 95% CI=1.15–1.50). PWH were also more likely to use cessation pharmacotherapy (15.8% vs. 11.9%; aPR=1.27, 95% CI=1.10–1.46) and individual telephone-based cessation coaching sessions (2.8% vs. 1.6%; aPR=1.65, 95% CI=1.13–2.40), compared with persons without HIV.
Table 2.
Characteristics of persons with and without HIV who reported current smoking and had no cessation treatment in prior year - Kaiser Permanente Northern California, July 2013 to December 2017
| Characteristic | Persons with HIV N=1,123 n (%) | Persons without HIV N=16,283 n (%) | p |
|---|---|---|---|
| Female | 98 (8.7) | 1,320 (8.1) | 0.46 |
| Mean age, years (SD) | 48.1 (11.3) | 49.3 (12.8) | 0.003 |
| Race/Ethnicity | |||
| White, non-Hispanic | 593 (52.8) | 8,770 (53.9) | |
| Black, non-Hispanic | 221 (19.7) | 3,372 (20.7) | |
| Hispanic | 174 (15.5) | 2,565 (15.8) | <0.001 |
| Other | 81 (7.2) | 1,278 (7.8) | |
| Unknown | 54 (4.8) | 298 (1.8) | |
| Lower neighborhood education1 | 205 (18.3) | 3,262 (20.0) | 0.18 |
| Lower neighborhood income2 | 236 (21.1) | 2,230 (13.7) | <0.001 |
| Insurance | |||
| Commercial/Private | 842 (75.0) | 13,275 (81.5) | |
| Medicare | 228 (20.3) | 2,338 (14.4) | <0.001 |
| Medicaid | 47 (4.2) | 566 (3.5) | |
| Other/Unknown | 6 (0.5) | 104 (0.6) | |
| Mean number of outpatient visits in year prior to study entry (SD) | 8.8 (15.8) | 4.8 (10.7) | <0.001 |
| Body mass index category3 | |||
| Normal | 458 (40.8) | 3,754 (23.1) | |
| Underweight | 23 (2.0) | 165 (1.0) | |
| Overweight | 422 (37.6) | 6,034 (37.1) | <0.001 |
| Obese | 213 (19.0) | 5,994 (36.8) | |
| Unknown | 7 (0.6) | 336 (2.1) | |
| Charlson score4 | |||
| 0 | 812 (72.3) | 12,403 (76.2) | |
| 1 | 181 (16.1) | 2,236 (13.7) | 0.01 |
| ≥2 | 130 (11.6) | 1,644 (10.1) | |
| Recent depression5 | 142 (12.6) | 570 (3.5) | <0.001 |
| Unhealthy drinking6 | |||
| No | 864 (76.9) | 12,610 (77.4) | <0.001 |
| Yes | 135 (12.0) | 3,209 (19.7) | |
| Unknown | 124 (11.0) | 464 (2.8) | |
| Tobacco use disorder | 922 (82.1) | 12,665 (77.8) | 0.001 |
| Substance use disorder7 | 299 (26.6) | 2,250 (13.8) | <0.001 |
| HIV-specific factors | |||
| HIV risk | |||
| Men who have sex with men | 734 (65.4) | ||
| Heterosexual | 167 (14.9) | - | - |
| Injection drug use | 127 (11.3) | ||
| Other/Unknown | 95 (8.5) | ||
| CD4 T cell count ≥500 cells/μl8 | 770 (71.2) | - | - |
| HIV RNA <75 copies/ml9 | 937 (86.8) | - | - |
| Prior clinical AIDS diagnosis | 524 (46.7) | - | - |
Abbreviation: SD = standard deviation
% of known; Education estimated from Census block group data; lower education defined as ≥25% of the population in the patient’s Census block group having no high school diploma. 5 (0.4%) PWH and 41 (0.3%) HIV-uninfected persons had unknown education.
% of known; Income estimated from Census block group data; lower income defined as ≥25% of population in the patient’s Census block group with household income below poverty levels. 5 (0.4%) PWH and 41 (0.3%) HIV-uninfected persons had unknown income.
Body mass index was defined as follows: Underweight (<18.5 kg/m2), Normal (18.5 to <25 kg/m2), Overweight (25 to <30 kg/m2), Obese (≥30 kg/m2). 23 (0.3%) PWH and 2,091 (1.3%) HIV-uninfected persons had unknown BMI.
Measure of comorbidity burden, modified to exclude AIDS and calculated based on diagnoses and procedures in the year prior to study entry
Known depression in the year prior to reported current smoking.
Unhealthy drinking was defined as follows, based on daily and weekly drink limits recommended by the National Institute on Alcohol Abuse and Alcoholism: for women of any age and men ≥65-years old, having ≥4 drinks in a day at least once in the past 90 days or ≥8 drinks per week on average; for men <65-years-old, ≥5 drinks in a day at least once in the past 90 days or ≥15 drinks per week on average.
Other substances besides tobacco and alcohol.
% of known; 42 (3.7%) had unknown CD4.
% of known; 43 (3.8%) had unknown HIV RNA level. 75 copies/mL was the lower limit of detection during the study period.
Among both PWH and persons without HIV, cessation treatment was less likely among those who were Hispanic (vs. non-Hispanic White, aPR=0.57, 95% CI=0.36–0.90 in PWH; aPR=0.68, 95% CI=0.60–0.78 in HIV-uninfected; Table 3). Among persons without HIV only, cessation treatment was also significantly less likely among those who were non-Hispanic Black (aPR=0.85, 95% CI=0.76–0.94). In adjusted models of cessation treatment among PWH, sex, age, insurance type, number of outpatient visits in the past year, comorbidity burden, recent depression, unhealthy alcohol use, and other substance use disorders were not significantly associated with lower likelihood of cessation treatment. When examining HIV-specific factors associated with cessation treatment, we found that HIV-infected IDU were more likely to initiate cessation treatment than MSM (aPR=1.48, 95% CI=1.05–2.09). HIV clinical factors were not associated with cessation treatment.
Table 3.
Factors associated with use of cessation treatment within 2 years of follow-up among patients who reported smoking between July 2013 and December 2017 - Kaiser Permanente Northern California
| Persons with HIV N=1,123 | Persons without HIV N=16,283 | |||||
|---|---|---|---|---|---|---|
| Characteristic | n | Adjusted PR1 (95% CI) | p | n | Adjusted PR1 (95% CI) | p |
| Female (Reference: Male) | 22 | 1.14 (0.74–1.76) | 0.54 | 255 | 1.41 (1.25–1.60) | <0.001 |
| Mean age (SD), years2 | 48.0 (10.4) | 0.95 (0.84–1.06) | 0.35 | 51.8 (10.8) | 1.09 (1.05–1.13) | <0.001 |
| Race/Ethnicity3 | ||||||
| White, non-Hispanic | 121 | Reference | 1,306 | Reference | ||
| Black, non-Hispanic | 44 | 0.94 (0.68–1.31) | 0.72 | 464 | 0.85 (0.76–0.94) | 0.002 |
| Hispanic | 19 | 0.57 (0.36–0.90) | 0.02 | 228 | 0.68 (0.60–0.78) | <0.001 |
| Other | 9 | 0.56 (0.29–1.08) | 0.08 | 134 | 0.82 (0.69–0.97) | 0.02 |
| Insurance | ||||||
| Commercial/Private | 141 | Reference | 1,673 | Reference | ||
| Medicare | 45 | 1.12 (0.80–1.59) | 0.51 | 380 | 0.86 (0.75–0.97) | 0.02 |
| Medicaid | 12 | 1.33 (0.80–2.19) | 0.27 | 95 | 1.12 (0.93–1.36) | 0.24 |
| Other/Unknown | 3 | 2.40 (1.01–5.71) | 0.05 | 11 | 0.82 (0.47–1.42) | 0.48 |
| Outpatient visits in year prior to study entry | ||||||
| 0 | 17 | Reference | 556 | Reference | ||
| 1–2 | 26 | 0.90 (0.51–1.59) | 0.72 | 514 | 0.90 (0.81–1.01) | 0.07 |
| 3–9 | 90 | 1.09 (0.67–1.78) | 0.72 | 703 | 0.99 (0.89–1.10) | 0.86 |
| ≥10 | 68 | 1.33 (0.79–2.22) | 0.28 | 386 | 1.11 (0.97–1.27) | 0.15 |
| p for trend = 0.11 | p for trend = 0.17 | |||||
| Charlson score4 | ||||||
| 0 | 141 | Reference | 1,474 | Reference | ||
| 1 | 36 | 0.97 (0.68–1.39) | 0.89 | 378 | 1.23 (1.10–1.37) | <0.001 |
| ≥2 | 24 | 0.89 (0.59–1.36) | 0.60 | 307 | 1.21 (1.06–1.38) | 0.004 |
| p for trend = 0.62 | p for trend <0.001 | |||||
| Recent depression5 | 34 | 1.24 (0.89–1.74) | 0.21 | 132 | 1.44 (1.23–1.70) | <0.001 |
| Unhealthy drinking6 | ||||||
| No | 160 | Reference | 1,758 | Reference | ||
| Yes | 26 | 1.13 (0.78–1.64) | 0.51 | 385 | 0.93 (0.84–1.03) | 0.15 |
| Unknown | 15 | 0.65 (0.40–1.05) | 0.08 | 16 | 0.27 (0.17–0.43) | <0.001 |
| Tobacco use disorder | 177 | 1.52 (1.00–2.30) | 0.05 | 1,938 | 2.11 (1.84–2.42) | <0.001 |
| Substance use disorder7 | 53 | 0.87 (0.69–1.18) | 0.38 | 387 | 1.17 (1.06–1.30) | 0.003 |
Abbreviations: SD = standard deviation; PR = prevalence ratio; CI = confidence interval
Adjusted for all variables in the table.
Adjusted for continuous age in 10-year intervals.
52 (4.8%) PWH and 298 (1.8%) persons without HIV had unknown race/ethnicity.
Measure of comorbidity burden, modified to exclude AIDS and calculated based on diagnoses and procedures in the year prior to study entry.
Known depression in the year prior to reported current smoking.
Unhealthy drinking was defined as follows, based on daily and weekly drink limits recommended by the National Institute on Alcohol Abuse and Alcoholism: for women of any age and men ≥65-years old, having ≥4 drinks in a day at least once in the past 90 days or ≥8 drinks per week on average; for men <65-years-old, ≥5 drinks in a day at least once in the past 90 days or ≥15 drinks per week on average.
Other substances besides tobacco and alcohol.
Among 8,360 PWH and 156,349 persons without HIV with active KPNC membership and known smoking status in the last year of the study, smoking remained more common among PWH (13.5%) than persons without HIV (10.3%), p<0.001. The average age of smokers was similar by HIV status (49 years for PWH and 50 years for persons without HIV) and the likelihood of current smoking declined with older age in both groups (p <0.001, Table 4). Among both PWH and persons without HIV, current smoking was associated with being underweight (vs. having normal BMI; aPR=1.39, 95% CI=1.06–1.83 in PWH; aPR=1.27, 95% CI=1.14–1.43 in persons without HIV), depression in the past year (aPR=1.18, 95% CI=1.06–1.31 in PWH; aPR=1.08, 95% CI=1.03–1.13 in persons without HIV), unhealthy drinking (aPR=1.40, 95% CI=1.17–1.66 in PWH; aPR=1.47, 95% CI=1.42–1.52 in persons without HIV), and having a substance use disorder (aPR=1.67, 95% CI=1.42–1.95 in PWH; aPR=1.34, 95% CI=1.24–1.44 in persons without HIV; Table 4).
Table 4.
Factors associated with smoking among persons with and without HIV - Kaiser Permanente Northern California, 2017
| Persons with HIV N=8,360 | Persons without HIV N=156,349 | |||||
|---|---|---|---|---|---|---|
| Characteristic | n | Adjusted PR1 (95% CI) | p | n | Adjusted PR1 (95% CI) | p |
| Female (Reference: Male) | 114 | 0.97 (0.83–1.13) | 0.70 | 1,199 | 0.72 (0.68–0.76) | <0.001 |
| Mean age, years (SD)2 | 48.5 (12.5) | 0.80 (0.77–0.84) | <0.001 | 49.9 | 0.85 (0.84–0.86) | <0.001 |
| Race/Ethnicity3 | ||||||
| White, non-Hispanic | 572 | Reference | 8,226 | Reference | ||
| Black, non-Hispanic | 188 | 1.01 (0.87–1.16) | 0.93 | 2,635 | 0.97 (0.93–1.01) | 0.10 |
| Hispanic | 208 | 0.98 (0.86–1.12) | 0.79 | 3,367 | 1.16 (1.12–1.20) | <0.001 |
| Other | 108 | 0.99 (0.84–1.17) | 0.94 | 1,523 | 1.00 (0.95–1.04) | 0.90 |
| Lower neighborhood education4 | 227 | 1.08 (0.96–1.22) | 0.21 | 3,485 | 1.18 (1.14–1.22) | <0.001 |
| Lower neighborhood income5 | 245 | 1.13 (1.00–1.27) | 0.05 | 2,500 | 1.16 (1.11–1.20) | <0.001 |
| Insurance | ||||||
| Commercial/Private | 778 | Reference | 12,983 | Reference | ||
| Medicare | 251 | 1.01 (0.88–1.16) | 0.91 | 2,149 | 0.95 (0.91–1.00) | 0.03 |
| Medicaid | 87 | 1.07 (0.90–1.27) | 0.45 | 766 | 1.18 (1.12–1.25) | <0.001 |
| Other/Unknown | 12 | 1.15 (0.77–1.72) | 0.51 | 135 | 1.26 (1.10–1.45) | 0.001 |
| Body mass index category6 | ||||||
| Normal | 487 | Reference | 3,992 | Reference | ||
| Underweight | 36 | 1.39 (1.06–1.83) | 0.02 | 195 | 1.27 (1.14–1.43) | <0.001 |
| Overweight | 402 | 0.77 (0.69–0.85) | <0.001 | 5,856 | 0.79 (0.77–0.82) | <0.001 |
| Obese | 199 | 0.64 (0.56–0.73) | <0.001 | 5,628 | 0.69 (0.67–0.71) | <0.001 |
| Charlson score7 | ||||||
| 0 | 715 | Reference | 11,382 | Reference | ||
| 1 | 190 | 1.01 (0.89–1.16) | 0.86 | 2,431 | 0.90 (0.87–0.93) | <0.001 |
| ≥2 | 223 | 0.90 (0.78–1.03) | 0.12 | 2,220 | 0.81 (0.77–0.84) | <0.001 |
| p for trend = 0.17 | p for trend <0.001 | |||||
| Recent depression8 | 313 | 1.18 (1.06–1.31) | 0.002 | 1,464 | 1.08 (1.03–1.13) | 0.001 |
| Unhealthy drinking9 | ||||||
| No | 882 | Reference | 12,879 | Reference | ||
| Yes | 92 | 1.40 (1.17–1.66) | <0.001 | 2,356 | 1.47 (1.42–1.52) | <0.001 |
| Unknown | 154 | 1.00 (0.87–1.15) | 0.95 | 798 | 1.31 (1.23–1.39) | <0.001 |
| Tobacco use disorder | 910 | 12.4 (10.8–14.2) | <0.001 | 12,393 | 14.6 (14.1–15.1) | <0.001 |
| Substance use disorder10 | 131 | 1.67 (1.42–1.95) | <0.001 | 645 | 1.34 (1.24–1.44) | <0.001 |
Abbreviations: SD = standard deviation; PR = prevalence ratio; CI = confidence interval
Adjusted for all variables in the table.
Adjusted for continuous age in 10-year intervals.
312 (3.7%) PWH and 2,609 (1.7%) persons without HIV had unknown race/ethnicity.
Lower neighborhood education defined as ≥25% of the population in the patient’s Census block group with no high school diploma. 28 (0.3%) PWH and 361 (0.2%) persons without HIV had unknown education.
Lower neighborhood income defined as ≥25% of population in the patient’s Census block group with household income below poverty levels. 28 (0.3%) PWH and 367 (0.2%) persons without HIV had unknown income.
Body mass index was defined as follows: Underweight (<18.5 kg/m2), Normal (18.5 to <25 kg/m2), Overweight (25 to <30 kg/m2), Obese (≥30 kg/m2). 23 (0.3%) PWH and 2,091 (1.3%) HIV-uninfected persons had unknown BMI.
Measure of comorbidity burden, modified to exclude AIDS and calculated based on diagnoses and procedures in the year prior to study entry.
Depression in the year prior to reported current smoking
Unhealthy drinking was defined as follows, based on daily and weekly drink limits recommended by the National Institute on Alcohol Abuse and Alcoholism: for women of any age and men ≥65-years old, having ≥4 drinks in a day at least once in the past 90 days or ≥8 drinks per week on average; for men <65-years-old, ≥5 drinks in a day at least once in the past 90 days or ≥15 drinks per week on average.
Other substances besides tobacco and alcohol.
Among persons without HIV only, smoking was significantly more likely among Hispanic patients (vs. non-Hispanic White patients), patients residing in neighborhoods with lower average education or lower average household income, and patients with Medicaid (vs. commercial insurance/private pay; Table 4). In adjusted models of PWH, smoking was not more likely among Hispanic patients, and associations of smoking with education, income, and insurance coverage were weaker and not statistically significant. Among PWH, smoking was more likely among heterosexuals (aPR=1.31, 95% CI=1.11–1.53) and IDU (aPR=1.49, 95% CI=1.29–1.72) compared to MSM (Table 5). Smoking was more likely among PWH with detectable HIV RNA viral load (≥75 copies/mL vs. <75 copies/mL, aPR=1.39, 95% CI=1.20–1.62). Smoking was not associated with CD4 count or prior clinical AIDS diagnosis.
Table 5.
HIV-specific factors associated with smoking among persons with HIV (N=8,360) - Kaiser Permanente Northern California, 2017
| Characteristic | n | Adjusted PR1 (95% CI) | p |
|---|---|---|---|
| HIV risk | |||
| Men who have sex with men | 706 | Reference | |
| Heterosexual | 183 | 1.31 (1.11–1.53) | 0.001 |
| Injection drug use | 145 | 1.49 (1.29–1.72) | <0.001 |
| Other/Unknown | 94 | 1.15 (0.96–1.38) | 0.12 |
| CD4 count (cells/μl)2 | |||
| ≥500 | 756 | Reference | |
| 200–499 | 227 | 0.96 (0.84–1.09) | 0.54 |
| <200 | 53 | 1.02 (0.80–1.30) | 0.86 |
| HIV RNA3 | |||
| <75 copies/mL | 936 | Reference | |
| ≥75 copies/mL | 144 | 1.39 (1.20–1.62) | <0.001 |
| Prior clinical AIDS diagnosis | 461 | 0.94 (0.84–1.04) | 0.22 |
Abbreviations: PR = prevalence ratio; CI = confidence interval
Adjusted for all variables in the table, and, sex, 10-year age intervals, race/ethnicity, education and income estimated from Census block group data from patient’s primary residence, insurance type, BMI, Charlson score excluding AIDS, recent depression, unhealthy drinking, tobacco use disorder diagnosis and substance use disorder diagnosis.
802 (9.6%) had unknown CD4 count.
245 (2.9%) had unknown HIV RNA. 75 copies/mL was the lower limit of detection during the study period.
4. Discussion
In recent years, smoking prevalence declined for both persons with and without HIV in our study population, consistent with State and national trends (California Department of Public Health. California Tobacco Control Program, Fiore 2016, Jamal et al. 2016), and the gap in smoking prevalence by HIV status was narrower than reported in other studies (Park et al. 2016). In our setting where both patients with and without HIV had access to smoking cessation resources, PWH were more likely than persons without HIV to initiate treatment. Efforts are still needed to address higher smoking prevalence among subgroups of PWH including those who are younger, have depression, or have a substance use disorder.
Prior studies have repeatedly found elevated smoking rates among PWH (Pacek and Cioe 2015). In a meta-analysis of 28 U.S. studies, PWH were 2.5 times more likely to smoke than persons without HIV, a similar but slightly larger effect than in our study where smoking was approximately 1.4 times greater among PWH than persons without HIV in each year from 2013 to 2017 (Park et al. 2016). Reduced differences in smoking prevalence by HIV status may be explained by organized efforts within KPNC to prioritize smoking reduction among patients, including screening of current and former smokers at every outpatient visit. While PWH may be more likely to smoke, they also have a greater number of outpatient visits on average than patients without HIV and therefore have more opportunities to benefit from these resources or get referred for cessation treatment, bringing their smoking prevalence closer to that of HIV-uninfected patients. This finding is consistent with another study within KPNC which found that smokers with psychiatric disorders were more likely to use cessation treatment than smokers without psychiatric disorders, possibly due to greater interactions with the healthcare system (Young-Wolff et al. 2016).
Overall, smoking prevalence in our study population was lower than estimates reported elsewhere regardless of HIV status, which may be partially explained by lower overall smoking prevalence among Californians (10.5% in 2015) (California Department of Public Health, 2020). The California Tobacco Control program has implemented several population-based tobacco control measures including increased taxes on tobacco products, promotion of smoke-free environments and bans on smoking in public places, all of which discourage smoking (Lightwood and Glantz, 2013; Pierce et al., 2018).
Prior studies report that smokers with HIV often report high interest in quitting (Lifson and Lando 2012, Pacek and Cioe 2015, Pacek et al. 2014, Rahmanian et al. 2011), but that this seldom translates into successful cessation, possibly because of limited access to cessation resources or quality healthcare (Yong et al. 2014). Within KPNC, where provider-led discussions on cessation are encouraged and patients have easy and direct linkage to cessation medications and counseling, PWH were more likely to initiate cessation treatments than persons without HIV, and smoking prevalence has consistently declined, paralleling reductions seen in persons without HIV. In addition, differences in initiation of cessation treatment across patient subgroups were either attenuated or absent in PWH compared to uninfected persons, a positive sign that health system-level factors to reduce smoking have had broad impact. One exception was that Hispanic smokers with HIV were less likely to use cessation treatment; however, Hispanic PWH were not more likely to smoke, suggesting differences in cessation treatment preferences or tobacco use behaviors rather than differential access to services.
For the most part, characteristics of patients who remained smokers in the last year of the study were similar to characteristics of smokers in other studies of PWH and in the general U.S. population (Browning et al. 2013, Jamal et al. 2018). PWH who smoked were younger, underweight, reported unhealthy drinking levels, and had diagnoses of depression or a substance use disorder (Lifson and Lando 2012). Consistent with a meta-analysis of sex differences in smoking, men and women with HIV were equally likely to be smokers, indicating that HIV infection is associated with a greater relative increase in smoking for women than men since in the general population, women are less likely to smoke than men (Weinberger et al., 2017). However, unlike other studies, we found no racial differences in smoking prevalence among PWH (Browning et al. 2013). We also found fewer sociodemographic cessation treatment disparities in PWH than uninfected comparators, consistent with other studies of health services use within KPNC and which has been attributed to equal access to care for KPNC members (Lam et al. 2019, Lam et al. 2019, Marcus et al. 2014).
In general, our findings indicate that maximizing access to cessation resources can be effective for smoking reduction but that more is needed to close the remaining gap in smoking prevalence between persons with and without HIV. In settings such as KPNC where there is established infrastructure for providing cessation treatment, proactive outreach and extension of current service models to include additional options for high-priority groups may be a cost-effective way to address this goal. Studies have found that smokers with HIV express interest in cessation modalities that include a social component,(de Dios et al. 2016, Pacek et al. 2014) suggesting that offering group classes specifically for PWH, women or substance users could boost the number of patients willing to attend and likely to quit. Flexible treatment programs, such as intensive cell phone-based interventions with a greater number of structured counseling sessions, are another promising strategy(Gritz et al. 2013, Vidrine et al. 2015) and one that could appeal to both patients and providers, especially with increasing demand to address multiple health issues during clinic encounters. In this study, we were not able to examine in detail how use of cessation treatment directly relates to quit attempts and quit outcomes. Therefore, while our study illustrates the willingness of PWH to engage in cessation treatment, additional research on how patterns of tobacco use over time may be influenced by cessation modality, frequency or duration, could inform strategies to encourage sustained smoking cessation and maximize quit success in those who are already interested and engaged in treatment (Gamarel et al. 2016, Newcomb et al. 2014, Pacek et al. 2016, Savin et al. 2018, Vidrine et al. 2015).
This study had several limitations. First, as smoking status and other data for this study were obtained from EHR, we were limited to information collected for clinical rather than research purposes. Second, as data on smoking intensity, duration and type of tobacco are not recorded in EHR, we were unable to examine outcomes by tobacco use behaviors. Data on use of self-help resources, telephone counseling through local or national quit lines, or tobacco cessation medications purchased outside of KPNC were also not available in EHR. Third, as our study was conducted within a specific region of the U.S., our results may not generalize to other countries or geographic locations. However, since the patient population was highly representative of the insured adult population in the underlying catchment area, results may be generalizable to other health systems in California or those serving diverse populations with a similar model of care. While our HIV patient population was mostly male, possibly limiting generalizability to women with HIV, it is representative of the HIV patient population within KPNC and in California and that is most likely to be seen in clinical practice (California Department of Public Health, 2016; California Department of Public Health, 2018). Fourth, while recent legislation requiring that insurance plans cover the cost of cessation medications was enacted in January 2014, practical implementation of the policy likely occurred more gradually, resulting in some differences in coverage across individual insurance plans which would not have been captured given broad categorization of insurance plan types. Nevertheless, cessation treatment for PWH on Medicare and Medicaid was higher than among PWH with commercial insurance, suggesting there was no underutilization of cessation treatment in these potentially under-insured groups. Lastly, since we were unable to assess patients’ full history of quit attempts, some patients counted as initiating cessation treatment may be doing so after a prior quit attempt. However, we tried to minimize this by excluding individuals with any KPNC tobacco cessation treatment in the year before study entry as we were primarily interested in recent attempts to quit smoking. Also, these limitations would likely have been similar by HIV status and therefore would not have affected our comparisons across groups.
This study had several strengths. By evaluating PWH and persons without HIV in the same setting, we accounted for potential differences in outcomes due to differential access to care. Also, the breadth of individual-level data available in the EHR enabled identification of a well-matched HIV-uninfected comparator population as well as adjustment for many factors shown to adversely impact smoking and cessation, including sociodemographic factors, substance use disorders, clinical depression, and comorbidity burden (Lifson and Lando, 2012).
5. Conclusion
As HIV clinical care increasingly focuses on healthy aging, improving access to and uptake of tobacco treatment resources will be crucial to reduce smoking-related excess morbidity and mortality, and to improve quality of life. In a setting with comprehensive access to smoking cessation resources and an established HIV care system, PWH were more likely than persons without HIV to initiate cessation treatment and declines in smoking prevalence among PWH mirrored trends in persons without HIV. However, additional efforts are needed to address persistently higher smoking prevalence among PWH. To further encourage smoking reduction, health systems should consider tighter integration of cessation treatment into routine care and extension of current cessation resources to better fit treatment preferences of PWH with disproportionately high smoking rates, including women with HIV and PWH who are younger, substance users or who have a diagnosis of depression.
Highlights.
Smoking prevalence among PWH is decreasing, mirroring trends observed in HIV-uninfected persons
Given equal access to care, PWH are more likely to initiate cessation treatment than HIV-uninfected persons
Better integration of smoking cessation into HIV primary care is needed
Role of Funding Source.
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (U01 AA026230, Co-PIs: Silverberg/Satre; K24 AA025703, PI: Satre).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. Drs. Alexeeff, Horberg and Silverberg report a grant from Gilead, Inc., outside the submitted work. No conflicts of interest were declared for the remaining authors.
REFERENCES
- Altekruse SF, Shiels MS, Modur SP, Land SR, Crothers KA, Kitahata MM, Thorne JE, Mathews WC, Fernandez-Santos DM, Mayor AM, Gill JM, Horberg MA, Brooks JT, Moore RD, Silverberg MJ, Althoff KN and Engels EA (2018). “Cancer burden attributable to cigarette smoking among HIV-infected people in North America.” AIDS 32(4): 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KK, Wewers ME, Ferketich AK and Diaz P (2013). “Tobacco use and cessation in HIV-infected individuals.” Clin Chest Med 34(2): 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VE, Kahler CW, Devlin KN, Monti PM and Cohen RA (2013). “The effects of cigarette smoking on learning and memory performance among people living with HIV/AIDS.” AIDS Care 25(10): 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Department of Public Health. California Tobacco Control Program California Tobacco Facts and Figures: A Retrospective Look at 2017. Sacramento, CA, California Department of Public Health. [Google Scholar]
- Calvo-Sanchez M and Martinez E (2015). “How to address smoking cessation in HIV patients.” HIV Med 16(4): 201–210. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL and MacKenzie CR (1987). “A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.” J Chronic Dis 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- Crothers K and Tindle HA (2011). “Prevention of bacterial pneumonia in HIV infection: focus on smoking cessation.” Expert Rev Anti Infect Ther 9(7): 759–762. [DOI] [PubMed] [Google Scholar]
- de Dios MA, Stanton CA, Cano MA, Lloyd-Richardson E and Niaura R (2016). “The Influence of Social Support on Smoking Cessation Treatment Adherence Among HIV+ Smokers.” Nicotine Tob Res 18(5): 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De P, Farley A, Lindson N and Aveyard P (2013). “Systematic review and meta-analysis: influence of smoking cessation on incidence of pneumonia in HIV.” BMC Med 11: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp TB, McGinnis KA, Kraemer K, Akgun KM, Edelman EJ, Fiellin DA, Butt AA, Crystal S, Gordon AJ, Freiberg M, Gibert CL, Rimland D, Bryant KJ and Crothers K (2016). “Risk factors associated with acute exacerbation of chronic obstructive pulmonary disease in HIV-infected and uninfected patients.” Aids 30(3): 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson KM, Wu K, Koletar SL, Kalayjian RC, Ellis RJ, Taiwo B, Palella FJ Jr. and Tassiopoulos K (2017). “Association Between Frailty and Components of the Frailty Phenotype With Modifiable Risk Factors and Antiretroviral Therapy.” J Infect Dis 215(6): 933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC (2016). “Tobacco Control in the Obama Era - Substantial Progress, Remaining Challenges.” N Engl J Med 375(15): 1410–1412. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB and Bailey WC (2008). “A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report.” Am J Prev Med 35(2): 158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster M, Estrada V, Fernandez-Pinilla MC, Fuentes-Ferrer ME, Tellez MJ, Vergas J, Serrano-Villar S and Fernandez-Cruz A (2009). “Smoking cessation in HIV patients: rate of success and associated factors.” HIV Med 10(10): 614–619. [DOI] [PubMed] [Google Scholar]
- Gamarel KE, Brown L, Kahler CW, Fernandez MI, Bruce D and Nichols S (2016). “Prevalence and correlates of substance use among youth living with HIV in clinical settings.” Drug Alcohol Depend 169: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz ER, Danysh HE, Fletcher FE, Tami-Maury I, Fingeret MC, King RM, Arduino RC and Vidrine DJ (2013). “Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS.” Clin Infect Dis 57(4): 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AB, Gerstoft J, Kronborg G, Larsen CS, Pedersen C, Pedersen G and Obel N (2012). “Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study.” Aids 26(3): 285–293. [DOI] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG and Obel N (2013). “Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study.” Clin Infect Dis 56(5): 727–734. [DOI] [PubMed] [Google Scholar]
- Helleberg M, Gerstoft J, Afzal S, Kronborg G, Larsen CS, Pedersen C, Bojesen SE, Nordestgaard BG and Obel N (2014). “Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV.” Aids 28(10): 1499–1508. [DOI] [PubMed] [Google Scholar]
- Helleberg M, May MT, Ingle SM, Dabis F, Reiss P, Fatkenheuer G, Costagliola D, d’Arminio A, Cavassini M, Smith C, Justice AC, Gill J, Sterne JA and Obel N (2015). “Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America.” AIDS 29(2): 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden LM and Meyer JA (2011). Age and Sex Composition 2010: 2010 Census Briefs. U.S. Department of Commerce Economics and Statistics Administration Washington, D.C., U.S. Census Bureau. [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD and Graffunder CM (2016). “Current Cigarette Smoking Among Adults - United States, 2005–2015.” MMWR Morb Mortal Wkly Rep 65(44): 1205–1211. [DOI] [PubMed] [Google Scholar]
- Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA and Neff LJ (2018). “Current Cigarette Smoking Among Adults - United States, 2016.” MMWR Morb Mortal Wkly Rep 67(2): 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johs NA, Wu K, Tassiopoulos K, Koletar SL, Kalayjian RC, Ellis RJ, Taiwo B, Palella FJ Jr. and Erlandson KM (2017). “Disability Among Middle-Aged and Older Persons With Human Immunodeficiency Virus Infection.” Clin Infect Dis 65(1): 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karter AJ, Parker MM, Solomon MD, Lyles CR, Adams AS, Moffet HH and Reed ME (2017). “Effect of Out-of-Pocket Cost on Medication Initiation, Adherence, and Persistence among Patients with Type 2 Diabetes: The Diabetes Study of Northern California (DISTANCE).” Health services research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladapo JA, Richards AK, DeWitt CM, Harawa NT, Shoptaw S, Cunningham WE and Mafi JN (2017). “Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study.” J Am Heart Assoc 6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JO, Hurley LB, Chamberland S, Champsi JH, Gittleman LC, Korn DG, Lai JB, Quesenberry CP Jr., Ready J, Saxena V, Seo SI, Witt DJ, Silverberg MJ and Marcus JL (2019). “Hepatitis C treatment uptake and response among human immunodeficiency virus/hepatitis C virus-coinfected patients in a large integrated healthcare system.” Int J STD AIDS 30(7): 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JO, Hurley LB, Udaltsova N, Alexeeff SE, Klein DB, Corley DA and Silverberg MJ (2019). “Colorectal Cancer Screening in People With and Without HIV in an Integrated Health Care Setting.” J Acquir Immune Defic Syndr 81(3): 284–291. [DOI] [PubMed] [Google Scholar]
- Lifson AR and Lando HA (2012). “Smoking and HIV: prevalence, health risks, and cessation strategies.” Curr HIV/AIDS Rep 9(3): 223–230. [DOI] [PubMed] [Google Scholar]
- Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR and Group ISS (2010). “Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial.” Am J Public Health 100(10): 1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightwood J and Glantz SA (2013). “The effect of the California tobacco control program on smoking prevalence, cigarette consumption, and healthcare costs: 1989–2008.” PLoS One 8(2): e47145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann-Jackson L, Choi D, Sutfin EL, Song EY, Foley KL, Wilkin AM, Morse CG, Rojas NF, Oh TS and Rhodes SD (2019). “A Qualitative Systematic Review of Cigarette Smoking Cessation Interventions for Persons Living with HIV.” J Cancer Educ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JL, Chao CR, Leyden WA, Xu L, Klein DB, Horberg MA, Towner WJ, Quesenberry CP Jr., Abrams DI, Van Den Eeden SK and Silverberg MJ (2014). “Prostate cancer incidence and prostate-specific antigen testing among HIV-positive and HIV-negative men.” J Acquir Immune Defic Syndr 66(5): 495–502. [DOI] [PubMed] [Google Scholar]
- Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J and Siddiqi K (2017). “Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries.” Lancet Glob Health 5(6): e578–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT and Skarbinski J (2015). “Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys.” Ann Intern Med 162(5): 335–344. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Chi FW, Weisner CM, Satre DD, Ross TB, Allen S, Pating D, Campbell CI, Lu YW and Sterling SA (2015). “Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: the ADVISe cluster randomized controlled implementation trial.” Addict Sci Clin Pract 10: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DRB, Bilal U, Hutton HE, Lau B, Lesko CR, Fojo A, McCaul ME, Keruly J, Moore RD and Chander G (2019). “Tobacco Smoking, Substance Use, and Mental Health Symptoms in People with HIV in an Urban HIV Clinic.” J Health Care Poor Underserved 30(3): 1083–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Kahler CW, Lee H, Pantalone DW, Mayer KH, Cohen RA and Monti PM (2016). “Effects of smoking and alcohol use on neurocognitive functioning in heavy drinking, HIV-positive men who have sex with men.” AIDS Care 28(3): 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou-Jackson G, Commodore-Mensah Y, Farley J and DiGiacomo M (2014). “Smoking-cessation interventions in people living with HIV infection: a systematic review.” J Assoc Nurses AIDS Care 25(1): 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JD, Liu B and Parascandola M (2019). “Smoking and HIV in Sub-Saharan Africa: A 25-Country Analysis of the Demographic Health Surveys.” Nicotine Tob Res 21(8): 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb ME, Ryan DT, Greene GJ, Garofalo R and Mustanski B (2014). “Prevalence and patterns of smoking, alcohol use, and illicit drug use in young men who have sex with men.” Drug Alcohol Depend 141: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR and Cioe PA (2015). “Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living With HIV.” Curr HIV/AIDS Rep 12(4): 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Latkin C, Crum RM, Stuart EA and Knowlton AR (2014). “Interest in quitting and lifetime quit attempts among smokers living with HIV infection.” Drug Alcohol Depend 138: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Sweitzer MM and McClernon FJ (2016). “Non-cigarette tobacco and polytobacco use among persons living with HIV drawn from a nationally representative sample.” Drug Alcohol Depend 162: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LS, Hernandez-Ramirez RU, Silverberg MJ, Crothers K and Dubrow R (2016). “Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis.” AIDS 30(2): 273–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Shi Y, Hendrickson EM, White MM, Noble ML, Kealey S, Strong DR, Trinidad DR, Hartman AM and Messer K (2018). “Tobacco control in California compared with the rest of the USA: trends in adult per capita cigarette consumption.” Tob Control 27(e2): e112–e117. [DOI] [PubMed] [Google Scholar]
- Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A and Diaz P (2011). “Cigarette smoking in the HIV-infected population.” Proc Am Thorac Soc 8(3): 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KP, Parker RA, Losina E, Baggett TP, Paltiel AD, Rigotti NA, Weinstein MC, Freedberg KA and Walensky RP (2016). “Impact of Cigarette Smoking and Smoking Cessation on Life Expectancy Among People With HIV: A US-Based Modeling Study.” J Infect Dis 214(11): 1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre DD, Altschuler A, Parthasarathy S, Silverberg MJ, Volberding P and Campbell CI (2016). “Implementation and Operational Research: Affordable Care Act Implementation in a California Health Care System Leads to Growth in HIV-Positive Patient Enrollment and Changes in Patient Characteristics.” J Acquir Immune Defic Syndr 73(5): e76–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin MJ, Frank-Pearce SG, Pulvers K and Vidrine DJ (2018). “The association between lifetime polytobacco use and intention to quit among HIV-positive cigarette smokers.” Drug Alcohol Depend 191: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittdiel JA, Barrow JC, Wiley D, Ma L, Sam D, Chau CV and Shetterly SM (2017). “Improvements in access and care through the Affordable Care Act.” Am J Manag Care 23(3): e95–e97. [PMC free article] [PubMed] [Google Scholar]
- Tan ASL, Young-Wolff KC, Carter-Harris L, Salloum RG and Banerjee SC (2018). “Disparities in the Receipt of Tobacco Treatment Counseling within the US Context of the Affordable Care Act and Meaningful Use Implementation.” Nicotine Tob Res 20(12): 1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres TS, Luz PM, Derrico M, Velasque L, Grinsztejn E, Veloso VG, Cardoso SW, Santini-Oliveira M, Grinsztejn B and De Boni RB (2014). “Factors associated with tobacco smoking and cessation among HIV-infected individuals under care in Rio de Janeiro, Brazil.” PLoS One 9(12): e115900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron L, Lert F, Spire B and Dray-Spira R (2014). “Tobacco smoking in HIV-infected versus general population in france: heterogeneity across the various groups of people living with HIV.” PLoS One 9(9): e107451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ, Kypriotakis G, Li L, Arduino RC, Fletcher FE, Tami-Maury I and Gritz ER (2015). “Mediators of a smoking cessation intervention for persons living with HIV/AIDS.” Drug Alcohol Depend 147: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Funk AP, Rabin S and Shuter J (2017). “Sex Differences in Tobacco Use Among Persons Living With HIV/AIDS: A Systematic Review and Meta-Analysis.” J Acquir Immune Defic Syndr 74(4): 439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbring ML, Massey SH and Gardner MB (2009). “Helping patients who drink too much: an evidence-based guide for primary care clinicians.” Am Fam Physician 80(1): 44–50. [PubMed] [Google Scholar]
- Yin MT, Shi Q, Hoover DR, Anastos K, Sharma A, Young M, Levine A, Cohen MH, Shane E, Golub ET and Tien PC (2010). “Fracture incidence in HIV-infected women: results from the Women’s Interagency HIV Study.” Aids 24(17): 2679–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong LC, Luckhaupt SE, Li J and Calvert GM (2014). “Quit interest, quit attempt and recent cigarette smoking cessation in the US working population, 2010.” Occup Environ Med 71(6): 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Klebaner D, Campbell CI, Weisner C, Satre DD and Adams AS (2017). “Association of the Affordable Care Act With Smoking and Tobacco Treatment Utilization Among Adults Newly Enrolled in Health Care.” Med Care 55(5): 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Kline-Simon AH, Das S, Mordecai DJ, Miller-Rosales C and Weisner C (2016). “Smoking Trends Among Adults With Behavioral Health Conditions in Integrated Health Care: A Retrospective Cohort Study.” Psychiatr Serv 67(9): 996–1003. [DOI] [PubMed] [Google Scholar]
- Zyambo CM, Burkholder GA, Cropsey KL, Willig JH, Wilson CM, Gakumo CA, Westfall AO and Hendricks PS (2019). “Predictors of smoking cessation among people living with HIV receiving routine clinical care.” AIDS Care: 1–9. [DOI] [PubMed] [Google Scholar]


