Abstract
Cenicriviroc (CVC) is a small-molecule chemokine receptor antagonist with highly potent and selective anti-human immunodeficiency virus type 1 (HIV-1) activity through antagonizing C–C chemokine receptor type 5 (CCR5) as a coreceptor of HIV-1. CVC also strongly antagonizes C–C chemokine receptor type 2b (CCR2b), thereby it has potent anti-inflammatory and immunomodulatory effects. CVC is currently under clinical trials in the patients for treatment of nonalcoholic steatohepatitis, in which immune cell activation and dysregulation of proinflammatory cytokines play an important role in its pathogenesis. In this study, CVC was examined for its inhibitory effect on the replication of SARS-CoV-2, the causative agent of COVID-19, in cell cultures and found to be a selective inhibitor of the virus. The 50% effective concentrations of CVC were 19.0 and 2.9 μM in the assays based on the inhibition of virus-induced cell destruction and viral RNA levels in culture supernatants of the infected cells, respectively. Interestingly, the CCR5-specific antagonist maraviroc did not show any anti-SARS-CoV-2 activity. Although the mechanism of SARS-CoV-2 inhibition by CVC remains to be elucidated, CCR2b does not seem to be its target molecule. Considering the fact that the regulation of excessive immune activation is required to treat COVID-19 patients at the late stage of the disease, CVC should be further pursued for its potential in the treatment of SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV-2, Cenicriviroc, Chemokine receptor antagonist, Cytokine storm
Highlights
-
•
Cenicriviroc (CVC), the antagonist of chemokine receptor CCR5/CCR2b, inhibits SARS-CoV-2 replication in vitro.
-
•
CVC has previously been shown to inhibit HIV-1 replication through antagonizing CCR5 (coreceptor of HIV-1).
-
•
CVC has potent anti-inflammatory activity and is currently under clinical trials for treatment of NASH patients.
-
•
The control of excessive immune activation is required for treatment of COVID-19 patients at the late stage.
-
•
CVC may have therapeutic potential for COVID-19 through its antiviral and anti-inflammatory activities.
1. Introduction
The pandemic of severe pneumonia (COVID-19) caused by the transmission of the new coronavirus SARS-CoV-2 is a serious threat to humanity (Di Gennaro et al., 2020; Harapan et al., 2020; Helmy et al., 2020). At present, no vaccines exist, and the nucleoside analog remdesivir (RDV) has recently been approved for treatment of SARS-CoV-2 infection (Grein et al., 2020). RDV has broad-spectrum antiviral activity against several viruses, including Ebola virus and coronaviruses. In addition to RDV, there is an attempt to treat COVID-19 with existing drugs developed for other purposes. These include hydroxychloroquine and chloroquine (Pastick et al., 2020), the anti-influenza virus agent favipiravir (FPV) (Pilkington et al., 2020), and the human immunodeficiency virus type 1 (HIV-1) protease inhibitor lopinavir/ritonavir (Cao et al., 2020). More recently, the anti-parasitic agent ivermectin has been shown to inhibit SARS-CoV-2 replication in cell cultures (Caly et al., 2020). However, these drugs are not optimized for SARS-CoV-2, so that they may have inevitable adverse effects due to the requirement of higher dosages. Furthermore, these antiviral agents must be used at an early stage of infection, since severe deterioration of pneumonia in some patients is considered to be closely associated with not viral replication but “cytokine storm” caused by dysregulated and excessive cytokine release from activated immune cells (Ye et al., 2020). Therefore, the use of anti-inflammatory and immunomodulatory agents is mandatory in the late stage of this disease (Alijotas-Reig et al., 2020).
We have examined several compounds for their inhibitory effect on SARS-CoV-2 replication in cell cultures and found that the chemokine receptor antagonist cenicriviroc (CVC) inhibits the replication of SARS-CoV-2. CVC is a C–C chemokine receptor type 5 (CCR5) antagonist with potent and selective anti-HIV-1 activity (Baba et al., 2005). In addition, CVC antagonizes not only CCR5 but also C–C chemokine receptor type 2b (CCR2b). Since CCR2b is the receptor of monocyte chemoattractant protein 1 (MCP-1), CVC exerts anti-inflammatory and immunomodulatory effects in vivo (Dawson et al., 2003; Xia and Sui, 2009). In fact, CVC is currently under clinical trials for the treatment of nonalcoholic steatohepatitis (NASH), in which immune cell activation and dysregulation of proinflammatory cytokines play an important role in its pathogenesis (Pedrosa et al., 2020).
2. Materials and Methods
2.1. Cells and virus
VeroE6 cell line expressing transmembrane protease serine 2 (VeroE6/TMPRSS2) highly susceptible to SARS-CoV-2 infection (Matsuyama et al., 2020) was obtained from Japanese Collection of Research Bioresources (JCRB) Cell Bank in Japan (JCRB no. JCRB1819) and used for virus propagation and antiviral assays after removal of mycoplasma contamination. Vero cells were also used for experiments. The cells were cultured in Dulbecco's modified Eagle medium (Nacalai Tesque, Kyoto, Japan) supplemented with 5% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin G, 100 μg/ml streptomycin, and 1 mg/ml G418 (Nacalai Tesque). For antiviral assays, the cells were cultured in the absence of G418. SARS-CoV-2 (WK-521 strain, GISAID database ID EPI_ISL_408667), a clinical isolate from a COVID-19 patient, was provided by National Institute of Infectious Diseases, Tokyo, Japan (Matsuyama et al., 2020). The infectious virus titer was determined in VeroE6/TMPRSS2 cells and expressed as 50% cell culture infectious dose (CCID50) per ml.
2.2. Reagents
CVC and maraviroc (MRV) were purchased from MedChemExpress (Monmouth Junction, NJ). The nucleotide/nucleoside analogs RDV and FPV were obtained from ChemScence (Monmouth Junction, NJ) and Selleck Chemicals (Houston, TX), respectively. MCP-1 was purchased from PetpoTech (Rocky Hill, NJ). MRV is the CCR5 antagonist clinically approved for treatment of HIV-1 infection (Woollard and Kanmogne, 2015). Except for MCP-1, these compounds were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 20 mM or higher to exclude the cytotoxicity of DMSO and stored at −20 °C until use. MCP-1 was dissolved in distilled water.
2.3. Antiviral assays
VeroE6/TMPRSS2 cells (2 × 104 cells/well) were cultured in a 96-well microtiter plate and incubated at 37 °C. After 24 h, the cells were mock-infected or infected with SARS-CoV-2 at a multiplicity of infection (MOI) of 0.01 and cultured in the absence or presence of various concentrations of test compounds. After 3 days, the number of viable cells was determined by a tetrazolium dye method. Briefly, 100 μl of culture medium was removed from each well, and 10 μl of water-soluble tetrazolium dye solution (Dojindo, Kumamoto, Japan) was added. After incubating at 37 °C for 2 h, 100 μl of isopropanol acidified with hydrochloric acid was added, and the absorbance was read at two wavelengths (450 and 620 nm) with a microplate reader (Pauwels et al., 1988). The 50% effective concentration (EC50) of each compound was calculated from a dose-dependent curve based on the viability of infected and uninfected cells. All experiments using SARS-CoV-2 were conducted in biosafety level 3 (BSL3) facilities of Kagoshima University.
For immunofluorescence microscopy, Vero cells (2 × 104 cells/well) were cultured in a microtiter plate and incubated. After 24 h, the cells were infected with SARS-CoV-2 at a MOI of 0.1 in the absence of CVC and incubated. After 2 h, the cells were washed with phosphate buffered saline (PBS) to remove unadsorbed virus particles and further incubated in the absence or presence of 40 μM CVC. After 3 days, the cells were fixed with 4% paraformaldehyde in PBS for 15 min. Then, the solution was removed, and the cells were washed with PBS and permeabilized with methanol. After washing with PBS, the cells were treated with PBS containing 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and 0.1% Tween 20 (Fujifilm Wako, Osaka, Japan) and incubated overnight at 4 °C with an anti-SARS-CoV-2 nucleocapsid rabbit antibody (GeneTex, Irvine, CA). After incubation, the cells were washed with PBS and stained with the secondary antibody goat anti-rabbit IgG H&L (Alexa Fluoro® 488; Abcam, Cambridge, UK). The cells were washed with PBS, stained with 4’,6-diamidino-2-phenylindole (DAPI; Bio-Rad, Hercules, CA), and observed under a fluorescent microscope (BZ-X800; Keyence, Osaka, Japan).
2.4. Determination of viral RNA levels
The inhibitory effect of compounds on SARS-CoV-2 replication was also evaluated by the viral RNA levels in culture supernatants of the infected cells. VeroE6/TMPRSS2 cells were infected with the virus and incubated for 3 days in the absence or presence of test compounds, as described above. Ten μl of each culture supernatant was mixed with 10 μl of SideStep Lysis and Stabilization Buffer (Agilent Technologies, Santa Clara, CA) and diluted with 80 μl of distilled water. The amount of viral RNA was measured by real-time reverse transcription polymerase chain reaction (RT-PCR) using TaqMan Gene Expression Cells-to-CT™ Kit (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer's protocol except for its cell lysis step. The primer pair 5′-AAATTTTGGGGACCAGGAAC-3′ and 5′-TGGCAGCTGTGTAGGTCAAC-3′ and the probe 5′-FAM-ATGTCGCGCATTGGCATGGA-TAMRA-3′ were used for real-time PCR (Shirato et al., 2020). The SARS-CoV-2 plasmid control for creating the calibration curve was obtained from Integrated DNA Technologies (Coralville, IA). To examine the virus growth kinetics, culture supernatants were collected on days 1, 2, and 3 after virus infection. Viral RNA was extracted and purified using RNAzol® (Molecular Research Center, Cincinnati, OH) and Direct-zol RNA MicroPrep Kit (Zymo Research, Irvine, CA), according to the manufacturer's protocol. The purified RNA was subjected to reat-time RT-PCR to determine the amount of viral RNA, as described above.
3. Results
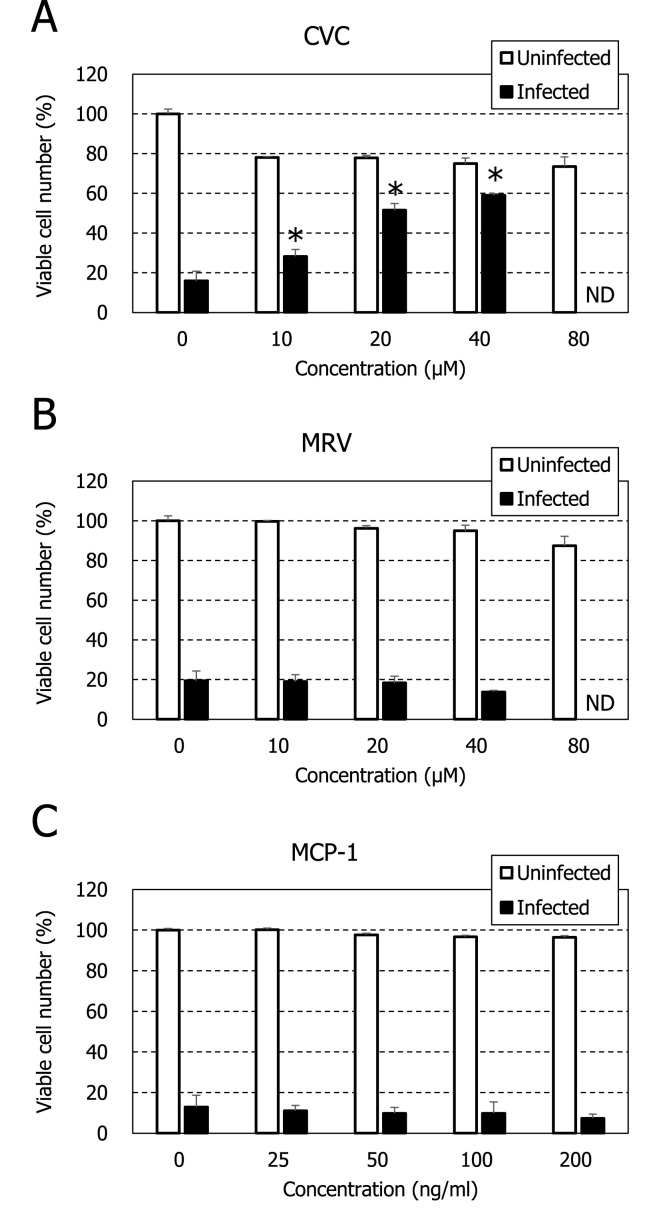
When VeroE6/TMPRSS2 cells were infected with SARS-CoV-2 and incubated in the absence of compounds for 3 days, the cells were completely destroyed by the virus-induced cytopathic effect (Fig. 1 B). Such cell destruction was not observed for the infected cells in the presence of 20 μM CVC, although some morphological changes were identified (Fig. 1D). In contrast, MRV did not inhibit the virus-induced cell destruction even at 40 μM (Fig. 1F). The EC50s of CVC and MRV were 19 ± 0.2 and >40 μM, respectively, based on the inhibition of virus-induced cell destruction (Table 1 ). Both compounds did not show apparent cytotoxicity at concentrations up to 80 μM. Dose-dependent protection of the infected cells from virus-induced cell destruction was observed for CVC but not for MRV (Fig. 2 ). These results indicate that CVC is a selective inhibitor of SARS-CoV-2 replication. As previously reported (Wang et al., 2020), RDV proved to be a potent and selective inhibitor of SARS-CoV-2 replication in our assay, whereas FPV did not show any selective inhibition even at a concentration of 80 μM (Table 1 and Fig. S1).
Fig. 1.
Inhibitory effect of CVC on virus-induced destruction of VeroE6/TMPRSS2 cells. The cells were mock-infected (upper panels) or infected with SARS-CoV-2 at a MOI of 0.01 (lower panels) and cultured in the absence (A, B) or presence of 20 μM CVC (C, D) or 40 μM MRV (E, F). After 3 days, the cells were observed microscopically. Magnification: × 100.
Table 1.
Anti-SARS-CoV-2 activity of CVC, MRV, RDV, and FPV in VeroE6/TMPRSS2 cells.
| Compound | EC50a (μM) | CC50b (μM) | SIc |
|---|---|---|---|
| CVC | 19.0 ± 0.2 | >80 | >4.2 |
| MRV | >40 | >80 | – |
| RDV | 1.1 ± 0.5 | >80 | >72 |
| FPV | >80 | >80 | – |
The data of CVC and RDV represent mean ± range for two separate experiments and mean ± standard deviation for three separate experiments, respectively.
50% Effective concentration, based on the inhibition of virus-induced cell destruction.
50% Cytotoxic concentration, based on the reduction of mock-infected cell viability.
Selectivity index, based on the ratio of CC50 to EC50.
Fig. 2.
Inhibitory effect of CVC on SARS-CoV-2 replication in VeroE6/TMPRSS2 cells. The cells were mock-infected or infected with SARS-CoV-2 at a MOI of 0.01 and cultured in the presence of various concentrations of CVC (A), MRV (B) or MCP-1 (C). After 3 days, the number of viable cells was determined by a tetrazolium dye method (see Materials and Methods). For infected cells, all experiments were conducted in triplicate, and mean values ± standard deviations are shown. Statistical analysis of the data was performed using Student t-test (*p < 0.05). Significance was examined between the number of viable cells in the presence of compound and that in the absence of compound. The number of viable cells was not determined (ND) at a concentration of 80 μM. For uninfected cells, all experiments were conducted in duplicate, and mean values ± ranges are shown.
The anti-SARS-CoV-2 activity was also examined by the inhibition of viral antigen expression in Vero cells. Vero cells is less susceptible to SARS-CoV-2 replication than VeroE6/TMPRSS2 cells. In fact, Vero cells were not destroyed by the virus-induced cytopathic effect on day 3 after infection (Fig. 3 A). However, viral antigen expression was observed for many cells in the absence of CVC (Fig. 3B). In contrast, the antigen expression was completely inhibited in the presence of 40 μM CVC (Fig. 3D). When the anti-SARS-CoV-2 activity of CVC was evaluated by the viral RNA levels in culture supernatants of the infected cells, CVC significantly and dose-dependently reduced the amount of viral RNA in culture supernatants (Fig. 4 A). Its EC50 in this assay was 2.9 μM, and more than 95% inhibition was achieved by CVC at a concentration of 10 μM. In contrast, MRV did not reduce the viral RNA levels at concentrations up to 40 μM (Fig. 4B). To confirm the inhibitory effect of CVC on SARS-CoV-2 replication, the viral growth kinetics was investigated, in which the culture supernatants were collected every day and examined for their viral RNA levels. As shown in Fig. 5 , CVC but not MRV suppressed the replication of SARS-CoV-2 in infected VeroE6/TMPRSS2 cells in a dose dependent manner. On the other hand, it appeared that MRV slightly accelerated the growth of SARS-CoV-2 at a concentration of 20 μM.
Fig. 3.
Inhibitory effect of CVC on viral antigen expression in Vero cells. The cells were infected with SARS-CoV-2 at a MOI of 0.1 and cultured in the absence (A, B) or presence of 40 μM CVC (C, D). After 3 days, the cells were fixed and stained with an anti-SARS-CoV-2 nucleocapsid rabbit antibody followed by an anti-rabbit IgG antibody with fluorescence (see Materials and Methods). The cells were further stained with DAPI and observed with phase-contrast microscopy (A, C) and fluorescent microscopy (B, D). Magnification: × 40.
Fig. 4.
Inhibitory effect of CVC on viral RNA levels in culture supernatants of infected VeroE6/TMPRSS2 cells. The cells were mock-infected or infected with SARS-CoV-2 at a MOI of 0.01 and cultured in the presence of various concentrations of CVC (A) or MRV (B). After 3 days, the amount of viral RNA in culture supernatants was determined by real-time RT-PCR (see Materials and Methods). All experiments were conducted in triplicate, and mean values ± standard deviations are shown. Statistical analysis of the data was performed using Student t-test (*p < 0.05). Significance was examined between the viral RNA level in the presence of compound and that in the absence of compound.
Fig. 5.
Inhibitory effect of CVC on viral growth kinetics in VeroE6/TMPRSS2 cells. The cells were infected with SARS-CoV-2 at a MOI of 0.01 and cultured in the presence of CVC or MRV at the indicated concentrations. On days 1, 2, and 3, the amount of viral RNA in culture supernatants was determined by real-time RT-PCR (see Materials and Methods). All experiments were conducted in triplicate, and mean values ± standard deviations are shown (standard deviations are too small to appear). Statistical analysis of the data was performed using Student t-test (*p < 0.05). Significance was examined between the viral RNA level in the presence of compound and that in the absence of compound.
4. Discussion
In 2005, we have reported that the small molecule and orally bioavailable CCR5 antagonist CVC inhibits HIV-1 replication at subnanomolar concentrations in vitro (Baba et al., 2005). The anti-HIV-1 activity of CVC is attributed to the inhibition of viral entry using CCR5 as a coreceptor. However, different from other anti-HIV-1 CCR5 antagonists such as MRV, CVC also suppresses the binding of MCP-1 to CCR2b-expressing cells. This unique feature of CVC encouraged its manufacturer to develop this compound as an agent for treatment of NASH. A recent randomized, placebo-controlled trial of CVC for treatment of NASH demonstrated that twice as many subjects achieved improvement in fibrosis and no worsening of steatohepatitis compared with placebo (Friedman et al., 2018; Tacke, 2018). Safety and tolerability of CVC were found to be comparable to placebo, suggesting that it can be administered safely to COVID-19 patients for preventing severe deterioration of pneumonia due to the cytokine storm. In fact, alveolar macrophage-borne MCP-1 was reported to be a key agent in the initiation of the systemic inflammation of alveolar hypoxia in rats, and a CCR2b receptor antagonist prevented the mesenteric inflammation of alveolar hypoxia (Chao et al., 2011). Furthermore, a recent study on transcriptome sequencing of the RNAs isolated from the bronchoalveolar lavage fluid and peripheral blood mononuclear cells specimens of COVID-19 patients revealed the association between its pathogenesis and excessive cytokine release including MCP-1 (Xiong et al., 2020).
It will be claimed that the anti-SARS-CoV-2 activity of CVC in vitro is insufficient for the treatment of COVID-19 patients. However, our antiviral assay system using VeroE6/TMPRSS2 cells based on the inhibition of virus-induced cell destruction appears to be aggressive. SARS-CoV-2 uses the SARS-CoV receptor angiotensin-converting enzyme 2 for entry and the serine protease TMPRSS2 for S protein priming. This explains why VeroE6/TMPRSS2 are highly susceptible to SARS-CoV-2 infection (Matsuyama et al., 2020). When the parental cell line Vero was infected with SARS-CoV-2 at a MOI of 0.1 and cultured for 4 days, approximately half of the infected cells survived even in the absence of compounds (Fig. S2). Under such experimental conditions, CVC completely inhibited virus-induced cell destruction at 10 μM in Vero cells. The EC50 values of anti-SARS-CoV-2 compounds reported to date are rather high in VeroE6/TMPRSS2 cells. For instance, the EC50s of RDV and hydroxychloroquine were 1.1 and 22.5 μM, respectively (Table 1 and data not shown). The broad-spectrum anti-RNA virus agent FPV was not inhibitory to SARS-CoV-2 replication even at 80 μM (Table 1 and Fig. S1), which is consistent with a previous report (Choy et al., 2020). The anti-SARS-CoV-2 activity of CVC was more obvious, when determined by the inhibition of viral RNA levels in culture supernatants. Its EC50 was 2.9 μM (Fig. 4), which was similar to those of RDV (1.9 μM) and ivermectin (2.2–2.8 μM) (data not shown and Caly et al., 2020, respectively). Thus, although the anti-SARS-CoV-2 activity of CVC in vitro is modest, we cannot exclude the possibility that CVC exhibits some antiviral efficacy in COVID-19 patients.
The target molecule of CVC remains to be elucidated. Our preliminary studies on its mechanism of action revealed that CVC did not interfere with the entry of SARS-CoV-2 (data not shown). Unlike HIV-1, CCR5 is not the target of CVC for inhibition of SARS-CoV-2 replication, since another potent CCR5 antagonist MRV was totally inactive (Figs. 2B and 4B). Furthermore, MCP-1 could not block SARS-CoV-2 infection (Fig. 2C), suggesting that CCR2b is not the target molecule either. Further studies, such as a time-of-addition experiment and biochemical approaches, are required to elucidate the mechanism of action, and they are currently in progress.
In conclusion, in addition to the potent anti-inflammatory activity of CVC in vivo, the present study has identified its anti-SARS-CoV-2 activity in vitro. Since not only the inhibition of viral replication but also the control of excessive immune activation is mandatory to save COVID-19 patients at the late stage of the disease, CVC should be further pursued for its potential in the treatment of SARS-CoV-2 infection.
Acknowledgments
We thank National Institutes of Biomedical Innovation, Health and Nutrition and National Institute of Infectious Diseases for kindly providing VeroE6/TMPRSS2 cells and SARS-CoV-2 (WK-521 strain), respectively. Kagoshima University is applying for a patent of CVC as a SARS-CoV-2 inhibitor, and M.O., M.T. and M.B. are its inventors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2020.104902.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alijotas-Reiga J., Esteve-Valverded E., Beliznaf C., Selva-O'Callaghana A., Pardos-Geaa J., Quintanaj A., Mekiniank A., Anunciacion-Llunellj A., Miró-Murj F. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmun. Rev. 2020;102569 doi: 10.1016/j.autrev.2020.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Takashima K., Miyake H., Kanzaki N., Teshima K., Wang X., Shiraishi M., Iizawa Y. TAK-652 inhibits CCR5-mediated human immunodeficiency virustype 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob. Agents Chemother. 2005;49(11):4584–4591. doi: 10.1128/AAC.49.11.4584-4591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J., Donham P., van Rooijen N., Wood J.G., Gonzalez N.C. Monocyte chemoattractant protein-1 released from alveolar macrophages mediates the systemic inflammation of acute alveolar hypoxia. Am. J. Respir. Cell Mol. Biol. 2011;45(1):53–61. doi: 10.1165/rcmb.2010-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P., Huang X., Peiris M., Yen H.L. 2020. Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication in Vitro. Iver178, 104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J., Miltz W., Mir A.K., Wiessner C. Targeting monocyte chemoattractant protein-1 signaling in disease. Expert Opin. Ther. Targets. 2003;7(1):35–48. doi: 10.1517/14728222.7.1.35. [DOI] [PubMed] [Google Scholar]
- Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N., Smith L. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Publ. Health. 2020;17(8):E2690. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S.L., Ratziu V., Harrison S.A., Abdelmalek M.F., Aithal G.P., Caballeria J., Francque S., Farrell G., Kowdley K.V., Craxi A., Simon K., Fischer L., Melchor-Khan L., Vest J., Wiens B.L., Vig P., Seyedkazemi S., Goodman Z., Wong V.W., Loomba R., Tacke F., Sanyal A., Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H., Megawati D., Hayati Z., Wagner A.L., Mudatsir M. Coronavirus disease 2019 (COVID-19): a literature review. J. Infect. Publ. Health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9(4):E1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U.S.A. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastick K.A., Okafor E.C., Wang F., Lofgren S.M., Skipper C.P., Nicol M.R., Pullen M.F., Rajasingham R., McDonald E.G., Lee T.C., Schwartz I.S., Kelly L.E., Lother S.A., Mitjà O., Letang E., Abassi M., Boulware D.R. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19) Open Forum Infect. Dis. 2020;7(4) doi: 10.1093/ofid/ofaa130. ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods. 1988;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Pedrosa M., Seyedkazemi S., Francque S., Sanyal A., Rinella M., Charlton M., Loomba R., Ratziu V., Kochuparampil J., Fischer L., Vaidyanathan S., Anstee Q.M. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: study design of the TANDEM trial. Contemp. Clin. Trials. 2020;88:105889. doi: 10.1016/j.cct.2019.105889. [DOI] [PubMed] [Google Scholar]
- Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020;6(2):45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., Katoh H., Sakata M., Nakatsu Y., Mori Y., Kageyama T., Matsuyama S., Takeda M. Development of genetic diagnostic methods for novel voronavirus 2019 (nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020 doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- Tacke F. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis. Expet Opin. Invest. Drugs. 2018;27(3):301–311. doi: 10.1080/13543784.2018.1442436. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard S.M., Kanmogne G.D. Maraviroc: a review of its use in HIV infection and beyond. Drug Des. Dev. Ther. 2015;9:5447–5468. doi: 10.2147/DDDT.S90580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Sui Z. Recent developments in CCR2 antagonists. Expert Opin. Ther. Pat. 2009;19(3):295–303. doi: 10.1517/13543770902755129. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.