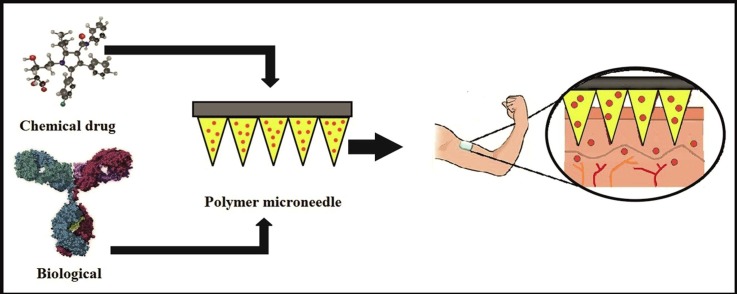
Graphical abstract
Polymer microneedle promotes the delivery of chemical and biological drugs through the skin.
Keywords: Skin, Transdermal route, Microneedle, Polymeric needles, Drug delivery
Abstract
Transdermal drug delivery using microneedles is increasingly gaining interest due to the issues associated with oral drug delivery routes. Gastrointestinal route exposes the drug to acid and enzymes present in the stomach, leading to denaturation of the compound and resulting in poor bioavailability. Microneedle transdermal drug delivery addresses the problems linked to oral delivery and to relieves the discomfort of patients associated with injections to increase patient compliance. Microneedles can be broadly classified into five types: solid microneedles, coated microneedles, dissolving microneedles, hollow microneedles, and hydrogel-forming microneedles. The materials used for the preparation of microneedles dictate the different applications and features present in the microneedle. Polymeric microneedle arrays present an improved method for transdermal administration of drugs as they penetrate the skin stratum corneum barrier with minimal invasiveness. The review summarizes the importance of polymeric microneedle and discussed some of the most important therapeutic drugs in research, mainly protein drugs, vaccines and small molecule drugs in regenerative medicine.
1. Introduction
The skin is the outermost and largest organ of the human body, covering an area of 1.8 m2 and making up close to one-fifth of an average person’s total body mass (Brown & Williams, 2019). Being the first barrier to entry into the body, the skin protects against external threats in the environment, including pathogens, harmful UV rays, toxins, inflammatory agents and dehydration (Yin & Smith, 2016). Due to the large coverage area of the human skin tissue, it offers a convenient, selective, and non-invasive route for drug delivery. Transdermal drug delivery (TDD) refers to the delivery of therapeutic agents across the skin layer. The delivery of drugs via skin overcomes many of the issues associated with oral drug delivery, including the gastric irritation, elimination of hepatic first-pass metabolism, and poor patient compliance. Additionally, it offers better release over time compared to oral drug delivery (Ita, 2015a, Pegoraro et al., 2012). Moreover, transdermal delivery devices are accessible, replaceable, controllable, and could be self-administered in several cases (Merino et al., 2015). Scopolamine, with a primary indication for managing motion sickness (Transderm-Scop®), nicotine for smoking cessation (Nicoderm®), and fentanyl for chronic pain (Lexicomp-Online, 2016) are examples of the first generation trans-delivery drug administration is the remedy for disease.
The greatest challenge in the delivery of active ingredients across the transdermal route is the stratum corneum (SC) that acts as the first protective layer of the skin and limit the drug absorption. This will significantly reduce the effectiveness of delivery of therapeutic agents and limit the types of drugs that can be delivered into the skin. Recently, there have been several studies on microneedles that penetrate the superficial skin barrier (SC) while avoiding contact with important nerves and capillaries in the epidermis to provide a more efficient and quick method for drug delivery compared to available transdermal drug delivery strategies. The novel approach combines between conventional injection and patch system. The drug is delivered transdermally while removing the pain and invasiveness associated with conventional methods in medicine (Kwon et al., 2017).
Microneedles (MNs) have been conceptualized and introduced several years ago but were only successfully fabricated and applied in the 1990 s (Gerstel and Place, 1976, Henry et al., 1998, Prausnitz, 2004). The classification of microneedles has been done according to the function it performs: solid, coated, hollow, dissolving and hydrogel-forming microneedles (Abiandu and Ita, 2019, Shende et al., 2018). The mechanical properties and biocompatibility of the material chosen for the manufacture of MNs are of major importance to the performance of the MNs. Low production cost and high mechanical strength are general considerations for the choice of fabricating materials. In this regard, polymers are the preferred materials for MNs fabrication as it does not elicit an immune response in the body, degrades in the body and can be tailored to perform with different strengths and functions (Lhernould et al., 2015).
Microneedles play an important role in medical field applications for delivering various drugs ranging from small to macromolecules, especially protein drugs that use for the treatment of several diseases. Thus, biotechnology companies give more attention toward research and development of MNs loaded protein drugs (Kochhar et al., 2019, Ma and Wu, 2017, Ryu et al., 2018). However, effective use of biotherapeutics is hindered by the large size, hydrophilic nature, poor absorption, and unstable nature of the drug, which prevent efficient uptake through the skin (Ryu et al., 2018). The progress in MNs research and production technology will lead to the delivery of clinically important drugs in the future (Kochhar et al., 2019).
2. Anatomy of the skin
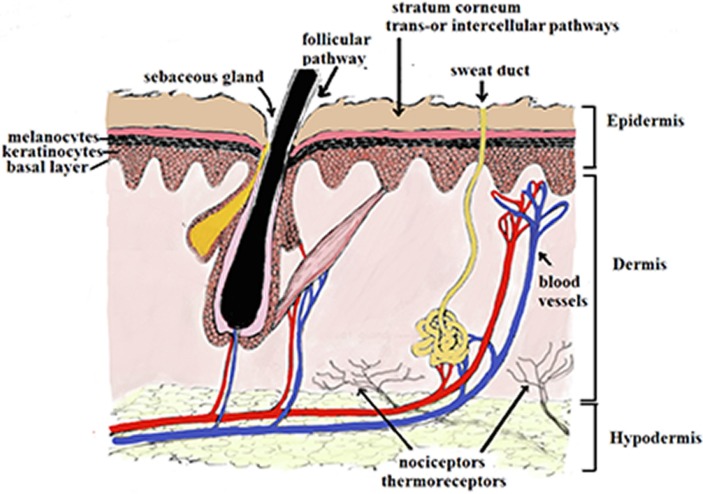
The skin is a multidimensional organ made of three important layers (Lhernould et al., 2015) as presented in Fig. 1 . The layer’s function to protect the internal organs from a host of outside dangers including toxins, external mechanical pressure, and microbial attack from pathogenic species. The skin also functions as a trigger for immune reactions due to the specialized antigen-presenting cells.
Fig. 1.
Structure of Skin and routs of penetration of a molecule across the stratum corneum.
The first skin layer, i.e. the epidermis layer, is approximately 150–200 mm thick and is composed of viable cells. It is made of five layers according to a degree of cell keratinization: stratum corneum (SC, horny layer), stratum lucidum (clear layer), stratum granulosum (granular layer), stratum spinosum (spinous or prickle layer) and stratum germinativum (basal layer) (Yousef et al., 2019). The outermost layer or horny layer SC (10–20 μm) has been referred to as “a brick wall-like structure of corneocytes as “bricks” in a matrix of intercellular lipids, with desmosomes acting as molecular rivets between the corneocytes” (Baroni et al., 2012). Under the SC layer is the viable epidermis layer, which contains the keratinocytes and pigment-producing cells, the melanocytes. This layer is responsible for most drug-related actions, such as drug binding, metabolism, active transport, and surveillance. This layer also contains specialized cells such as the Merkel and Langerhans cells (Monteiro-Riviere, 2010).
The second skin layer is the dermis (3–100 mm), which follows the viable epidermis layer. Here, the skin is made up a more complex mix of cells with different functions such as connective tissue, vascular tissue, lymphatic vessel network, sweat and sebum glands, hair follicles and macrophages (Stojic et al., 2019). This layer not only functions as a host layer for the network of functional tissue but also provides structural support in the skin due to the presence of fibroblasts (Stojic et al., 2019).
The third skin layer, the hypodermis (subcutis) follows the dermis layer, which contains loose connective tissue. The major cells found here are the adipocytes, which function in fat storage for use in body temperature regulation during cold as well as cushioning against outside insults (Sorg et al., 2017). The transfer of molecules via the skin follows an intricate set of steps, involving a number of mechanisms; the intracellular absorption of the molecule passes through the keratin-packed corneocytes by partitioning into and out of the cell membrane; the intercellular absorption where the molecule passes around the corneocytes in the lipid-rich extracellular regions; and appendageal absorption where the molecule moves through the shunts of hair follicles, sweat glands, and sebaceous glands (Tsakovska et al., 2017).
3. Transdermal drug delivery (TDD)
Transdermal delivery is gaining interest as an option for drug administration. The drug reaches the systemic circulation through the skin without losing the drug during reaching its target, enhance the bioavailability, improving sustained drug release, minimizing undesirable side effects, and improving physiological and pharmacological response (Mahmood et al., 2014, Rai et al., 2018). For instance, in testosterone replacement studies, transdermal delivery overcomes the issues associated with oral and intramuscular delivery, it bypasses the hepatic first-pass after oral administration, and thus reduce the required dose. Besides, it eliminates the need for recurring injections and a higher concentration of testosterone in the blood (Hathout et al., 2010, Tajbakhsh et al., 2020). Nevertheless, the delivery of drugs via the transdermal administration route is highly affected by the chemical properties of drugs, which affects absorption through the SC. Hence, few drugs can be delivered in significantly therapeutic amounts via this route (Mittapally et al., 2018).
The drugs applied through transdermal drug delivery system needs to take a tortuous route to bypass through consecutive skin layers containing both aqueous and lipid domains and reach the systemic circulation (Mahmood et al., 2018, Haque and Talukder, 2018). The drug molecule needs to have ideal properties to pass through SC layer; the molecular weight must be less than 600 Da, the value of Log P between 1 and 3, High, but balanced, SC/vehicle partition coefficient, and Low melting point, correlating with good solubility, as predicted by ideal solubility theory (Ye et al., 2018). Olanzapine is one of the drugs that have required physicochemical properties for effective transdermal drug delivery. It is lipophilic (log P 2.8), has a low molecular weight of 312.4 and a low melting point (195 °C). The poor bioavailability of olanzapine orally and susceptibility to loss during delivery means that only 40% of the actual dose remains before entering the circulation system. Together, these characteristics make olanzapine a good candidate for delivery via transdermal drug patches (Alyautdin et al., 2014, Iqbal et al., 2017). Obviously, many medications are unable to follow these stringent requirements for transdermal delivery (Donnelly et al., 2012a, Donnelly et al., 2012b).
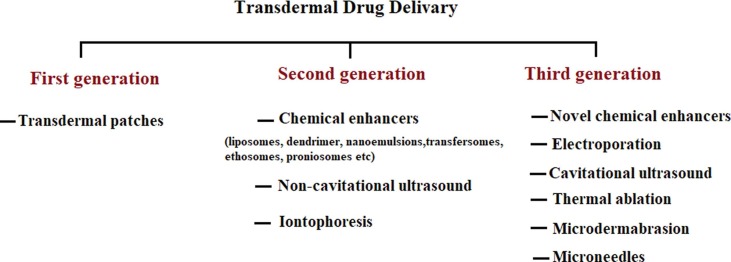
There are three generations of transdermal drug delivery, as illustrated in Fig. 2 . In the first generation, the systems used for transdermal drug delivery was patches, the drug candidate that is appropriate for patch formulation is extremely limited and has to fall into optimal ranges of molecular weight, hydrophilicity and effectiveness at low dosage (Economidou et al., 2018). The second generation of TDDS was implemented with a skin permeability enhancement technique to extend its application in transdermal drug delivery. Improvement methods included iontophoresis, chemical enhancers and non-cavitational ultrasound. Nevertheless, these methods struggle to protect the deeper tissues from any physiological harm and increase the distribution of drug molecules through SC (Lee et al., 2018). In the third generation, novel chemical enhancers, electroporation, cavitational ultrasound, microneedles, thermal ablation and microdermabrasion were introduced, these techniques allowed the biotherapeutics and large molecules to better penetrate the outer corneum layer, resulting in increased efficacy of transdermal delivery in human clinical trials (Nandagopal et al., 2014). However, the techniques used abrasion methods, lasers and heat and exposure to radiofrequency, which harmed the skin, caused discomfort in patients and resulted in the application of the drug and treatment of side effects. Such limitations can be solved by using micrometer size needles called microneedles (Battisti et al., 2019).
Fig. 2.
Generations of transdermal drug delivery.
MNs technology has grown in the last 15 years, permitting drugs to cross the outer corneum layer by puncturing the skin and creation of micro-channels in the outer corneum using micron needles. Compared to other transdermal delivery methods, microneedles are able to deliver molecules of larger sizes without disturbing the nerve endings in the skin, hence minimizing or altogether avoiding pain experienced by patients. Furthermore, microneedles can be used in both solid and liquid preparations according to the required specifications of the disease (Cheung & Das, 2016). The MNs is promising for the future of TDD to facilitate the delivery of drugs with large molecular weight and low lipophilicity, such as proteins, peptides and vaccines, as well as drugs with poor effectiveness at low doses (Waghule et al., 2019, Ye et al., 2018).
3.1. Microneedle drug delivery system
Microneedle (MN) technology employs microscopic needles to deliver drugs across the SC layer into the underlying layers with minimal invasiveness. The microneedles used in these delivery systems differ in length; some are just a few micrometers long but can go up to 2000 μm (Tuan-Mahmood et al., 2013). The short length of MN allows penetration of the SC without touching the nerves in the underlying layers of the skin (Quinn et al., 2014). The use of MNs is preferred over conventional drug delivery methods due to its simple delivery mechanism, pain-free, and minimally invasive devices that offer the simplicity of using transdermal while delivering the effectiveness of invasive needles and syringe (Nayak et al., 2016). Unlike conventional methods, MNs do not require specialized skills or personnel, as they are designed for self-administration by patients. Furthermore, MNs are designed for single-uses; this minimizes the potential for cross-contamination of the drugs (Mahato, 2017).
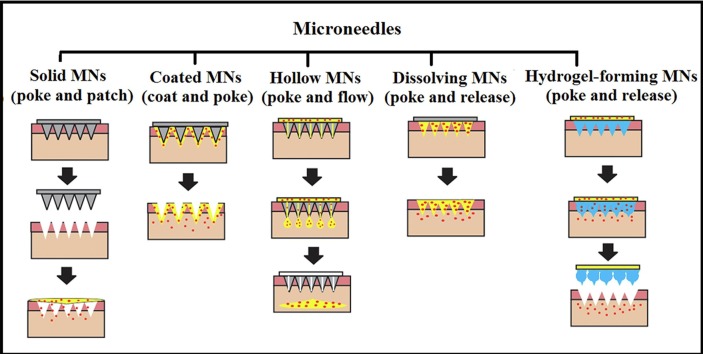
MNs are categorized into five different groups according to the design: solid, hollow, coated, dissolving, and hydrogel-forming microneedles, as illustrated in Fig. 3 . Solid MN delivery consists of two steps and is known as the “poke and patch” approach; first, holes are introduced into the skin using MN arrays; secondly, a conventional drug formulation is delivered via transdermal drug patch (Omolu et al., 2017, Tuan-Mahmood et al., 2013). Coated MNs follow the “coat and patch” approach; here, a drug formulation is coated onto the microneedles prior to application into the skin. Penetration into the skin allows the coating to dissolve; thereafter, the drug is deposited into the skin. In the hollow microneedles approach, the drug is filled into the hollow space in the tip of the microneedle, which is directly deposited into the epidermis or upper dermis layer of the skin upon insertion. In simple terms, this can be described as “poke and flow” (Pamornpathomkul et al., 2017). Dissolving microneedle is made mainly from dissolving or biodegradable polymers and allows for the simple one-step application process, and hydrogel-forming microneedles which absorb water in large quantities into their polymeric network, resulting in swelling (Waghule et al., 2019). The mechanism of dissolving and hydrogel-forming MN drug delivery is termed as poke and release; both strategies eliminate the need to use special measures for discarding of the needle and the risk of inadvertent reuse of the MN (Alkilani et al., 2015, Vandervoort and Ludwig, 2008).
Fig. 3.
The drug delivery mechanism of solid microneedle (solid MN), coated microneedle (coated MN), hollow microneedle (hollow MN), dissolving microneedle (dissolving MN) and hydrogel-forming microneedle (hydrogel-forming MN).
The material used for microneedle can be metal, polymer, glass, and silicon (Bhatnagar et al., 2017). Metal, glass, silicon and ceramics are used in the manufacture of MNs. They are rigid, which allows skin penetration, but brittle, risking breakage inside the skin layers, causing pain, swelling and possibly granulomas. The rigid but brittle properties of these materials have been likened to sea urchin thorns made of mineral calcite. As accidents and issues in the administration of the MNs are inescapable, the best materials for MNs should be biodegradable and biocompatible, to avoid complications that occur from accidents such as when the MN tip breaks inside the first few layers of the skin (Dardano et al., 2019b).
Design and manufacture of the MN focus on the shape and geometry to ensure that the needles are able to function optimally. The strength of the needle allows it to keep intact during penetration and delivery, while tip geometry is crucial to evade the nerve endings. MNs made of metals are able to withhold the force of penetrating the skin, but polymer MNs requires additional strengthening. The MNs need to be able to break the skin barrier without breaking or bending (Yung et al., 2011). MNs can be anywhere between 25 and 2500 μm long, 50 to 250 μm wide, and their tips measure 1 to 25 μm in diameter (Singh et al., 2017, Yung et al., 2011). The overall shape of the microneedle and the geometry of the tip has been used to classify microneedles; into rectangular, pyramidal, cylindrical, conical, or quadrangular with varying dimensions (Singh et al., 2017).
3.2. Polymeric microneedles
Silicon is brittle and does not metabolize in the body; hence the use of other materials such as polymers favoured the production of microneedles. Polymers are preferred due to their inexpensive cost, biocompatibility, biodegradability, hygienic use, swelling and dissolving abilities (Doppalapudi et al., 2014, Hong et al., 2013). Their degradation in vivo in the presence or absence of degrading enzymes yields non- toxic by-products. This property reduces the possibility of infection in the body (Arya et al., 2017). Polymers are used predominantly in the manufacture of dissolving and hydrogel-forming MNs arrays (Demir et al., 2013, McCrudden et al., 2015). Nevertheless, there are few studies using polymers for the production of coated, solid, and hollow MNs which attributed to the weakness of the polymer mechanical strength that is likely to fail during insertion (Ali et al., 2020).
The polymeric MNs can be classified according to materials, formulations, construction of MNs and in vivo performance (Ye et al., 2018); In solid polymer microneedles, the drug is not encapsulated in solid microneedles, and they are effective in generating holes through the SC (Li et al., 2017). Likewise, hollow microneedles act as external drug reservoir applied after creating microchannels in the skin (Yung et al., 2011). Also, the drug formulation and polymers can be coated onto MNs using various coating methods such as dip-coating, casting deposition techniques, spray drying, and Inkjet printing (Chen et al., 2010, Ma and Gill, 2014, McGrath et al., 2011, Uddin et al., 2015). However, the drug loading in coating layers of MNs is restricted due to the limited MN quantity (Chen et al., 2017b).
Dissolving MNs polymers are considered the most effective approach and have many applications; the drug incorporated into dissolvable or degradable polymeric MNs (Ye et al., 2018). As compared to coating MNs, this MNs can significantly enhance the drug loading capacity by encapsulating drug molecules into the whole needle instead of coating on its external surface (Sabri et al., 2019). The release of drugs depends mainly on dissolving and degradations proprieties of polymer in the skin. Dissolvable MNs can be used to deliver and release molecules quickly. This strategy ensures that drugs are delivered to specific targets and taken up immediately, which is plausible for short term applications (Fukushima et al., 2011, Wang et al., 2017).
On the other hand, MNs made of biodegradable polymers are dissolve over a period of time find interesting applications in prolonged/sustained delivery of drugs, the choice of biodegradable polymers is critical to manipulate and control the sustained release profile of drugs according to their degradation rates (Tsioris et al., 2012, Vora et al., 2020). Additionally, the hydrogel-forming MNs prepared mainly from polymer that absorbs interstitial skin fluids and swells to form a hydrogel mass to regulate the release of the drug depending on the crosslinking strength of the hydrogel network. This permits slow drug release over a period of several days (Bhatnagar et al., 2019, Caffarel-Salvador et al., 2015).
The advanced approach of MNs combining between polymer and micro- and nano-particles formulations for the delivery of many different types of therapeutics across the skin (Ye et al., 2018). For instance, the microparticle insulin embedded in MNs arrays provides a greater hypoglycaemic effect comparing with MNs insulin arrays only (Larrañeta et al., 2016). Furthermore, the recent developments focused on the fabrication of smart MNs (bioresponsive) to control drug delivery. In contrast to dissolving and biodegradable MNs, the bioresponsive MNs release the drug smartly according to the change of the physiological signals that achieved by loading of drugs in bioresponsive polymers or encapsulation of drugs in physiological signal sensitive micro- or nanoparticles such as (Du & Sun, 2020) pH-responsive drug release (Ullah et al., 2019), surface activation of nanoparticle that commonly used in cancer treatment (Chen et al., 2020, Singh et al., 2019), glucose that incorporated with insulin in the tips of MNs array (Yu et al., 2015), reactive oxygen species (ROS)-responsive microneedle (MN) patch for anti-acne therapy (Zhang et al., 2018a, Zhang et al., 2018b), and enzymes that triggered or suppress drug release through the inactivity or overexpression of enzymes (Stern, 2005, Yu et al., 2018).
Smart MNs offers opportunities to provide controlled drug delivery based on physiological responses for certain diseases conditions (Kathuria et al., 2018). For instance, Zhang et al. (2017) employed glucose-responsive nanoparticles to encapsulate rosiglitazone as the browning agents that further combined into the polymer MNs array. The pH-sensitive nanoparticle gradually degraded under the physiological glucose condition to release the browning agents into the subcutaneous adipocytes in a sustained manner that leads to increases whole-body energy expenditure and improves type-2 diabetes in a diet-induced obesity mouse model (Zhang et al., 2017).
The most frequently used matrix materials for dissolving polymer MNs are sodium hyaluronate, that is naturally present in the skin (Hiraishi et al., 2013, Matsuo et al., 2012), sodium carboxymethylcellulose (Marin & Andrianov, 2011), poly(vinylalcohol) (PVA) (Nguyen et al., 2018), poly(vinylpyrrolidone) (PVP) (Sun et al., 2013), methylvinylether-co-maleic anhydride (PMVE/MA) (Gantrez AN-139®) (Donnelly et al., 2012a), dextran (Ito et al., 2011), sodium chondroitin sulphate (Ito et al., 2006), hydroxypropyl cellulose (HPC) (Baek et al., 2018), carboxymethyl cellulose (CMC) (Park et al., 2016), hydroxypropyl methylcellulose (HPMC) (He et al., 2018a), sodium alginate (Zhang et al., 2018b), and hyaluronic acid (HA) (Kim et al., 2018b). Meanwhile, other biodegradable polymers used in MN fabrication such as polylactic acid (Na et al., 2020), chitosan (Suzuki et al., 2020), polyglycolic acid, or poly(lactide-co-glycolide) (PLGA) (He et al., 2020) degrade in the skin post-application. In addition to, bioresponsive polymers such as cross-linked methacrylated HA (MeHA) (Yu et al., 2015), cross-linked polyvinyl alcohol (PVA) (Wang et al., 2018a), and cross-linked alginate (W. Chen et al., 2017a).
Ideal polymeric microneedles should be biocompatible, non-immunogenic, mechanically strong, and able to carry large complex drugs without damage (Du & Sun, 2020). Thus the development of polymeric microneedles must consider the type of polymer used, manufacturing process, and design of the MN tip length, width and shape (Wang et al., 2017). Each polymer in studies provides own characterisation in term of strength permeation capability, and drug release either immediate or sustained release.
The major challenge associated with polymeric MNs is the penetration of MNS through the skin layer. Mostly, the mechanical strength is weaker in water-soluble polymers compared to non-dissolving materials such as silicon or metal, and drug encapsulation may further compromise the strength of the MNs (Donnelly et al., 2009, Vora et al., 2020). Mechanical Strength, elastic modulus and fracture toughness of polymer MNs are important; it is reflecting the insertion ability of polymer-based microneedle arrays. Stronger needles will be able to withstand forces without bending and breakage (Juster et al., 2019). Therefore, researchers can combine two or more polymers and additional materials to improve the mechanical strength of MNs (Vora et al., 2017). Besides, target tissue for MN either transdermal or non-transdermal must be considered during select of MNs polymers. Non-transdermal targets such as eye tissue, vascular tissues, and the digestive system are often required MNs that are bendable and simple to use surgically, the right balance of strength and flexibility must be considered when targeting soft tissues that may not be able to handle the pressure of high- strength MN insertion (Lee et al., 2019). Another factor meaningful to consider is environmental humidity because higher moisture levels weakened the MNs strength depend on the polymer used and the level of humidity (Wang et al., 2018b). Furthermore, the active ingredient added into the polymer MNs patch might enhance the mechanical strength but sometimes increase of drug loading led to a decrease in MN mechanical strength (Hiraishi et al., 2013, Permana et al., 2019). Additionally, the mechanical strength of MNs could diminish in case the drug distributed in the needles and baseplates of MNs arrays that indicated by cracking of the baseplate following mechanical evaluation whereas localization the drug in needles not just solve the mechanical strength issue but also results in a reduction in drug wastage as well (Permana et al., 2019, Ramöller et al., 2019).
Microneedle design is an essential aspect determining the effectiveness of the MN form and function (Davis et al., 2003, Wang et al., 2017). Microneedles are organized as arrays of structures either in cone or pyramid form, which function to pierce the human skin to deliver drugs (Tomono, 2019). The material used in MN fabrication is the most important factor in design, as it determines the mechanical strength and drug release properties of the MN. Other factors that need to be considered include the density of the material, height of the microneedle, and diameter width of the tip and base of the microneedle (Loizidou et al., 2016). Microneedles are arrays of cone- or pyramid-shaped structures formed with different materials, generally ranging from 250 to 2000 mm in height could cross the human skin barrier to deliver targeted drug molecules (Tomono, 2019). Several researchers have varied MN length, width, thickness and tip size to optimize penetration (Yan et al., 2010), a sensation of pain compared to conventional needle injection, and effectiveness of drug delivery (Luzuriaga et al., 2018), using different designs/geometries of MNs could lead to maximizing the dose efficiency of the drugs (Permana et al., 2019, Yan et al., 2010).
Polymer MNs are commonly produced using moulding techniques, this method allows for upscaling production, it carries limitations as it usually involves multiple time-consuming steps such as master preparation, mould fabrication, plasticisation of thermoplastic polymers above their glass transition temperature, thus thermo-liable drugs cannot be used (Wang et al., 2016). The researchers solve the issue of thermo-liable drugs by filling the drug and polymer solutions into the mould under vacuum or pressure, and drying under ambient conditions, centrifugation or pressure (Hong et al., 2013, Jagtap et al., 2018, Sullivan et al., 2010, Wang et al., 2015). 3D printing has also been described recently in published reports as an alternative manufacture technique for MNs (Bhatnagar et al., 2019). The fabrication process for microneedles should take into consideration factors such as the sharpness of the MNs tips, take place at ambient temperatures, absence of organic solvents, and preservation of the bioactivity of the loaded drug molecules (Sullivan, 2009).
Polymer MNs are able to improve the flux of the molecules ranging from small hydrophilic molecules such as alendronate to macromolecules, including heparins, insulin and vaccines (Alkilani et al., 2015, Waghule et al., 2019) as illustrated in Table 1, Table 2 . Several studies have shown the effectiveness of MNs array for the transdermal delivery of low molecular weight drugs, biological therapies and vaccines (Gualeni et al., 2018). Biotherapeutics are more complex and expensive than a small molecule pharmaceutical product; they produce through a biological process from living organisms rather than chemical synthesis including biotechnology methods (Nongkhlaw et al., 2020). Opposite to small molecule, the macromolecules and proteins are too large to diffuse into blood capillaries but are able to enter into the lymphatic vessel (Chandran et al., 2019).
Table 1.
Polymeric microneedle for delivering small and macromolecules.
| Compounds | Polymer | Type of MNs | Fabrication method | Geometry of MNs | Main outcome | Reference |
|---|---|---|---|---|---|---|
| Sulforhodamine B |
|
Coated MNs |
|
(5 × 5 arrays, 550, 650, and 750 μm height) | The MNs height and viscosity of coating solution has a significant impact on the drug loading and drug delivery efficiency. The drug loading may enhance by increasing the MNs height and viscosity of coating solution. However, once the viscosity of the coating solution increased, the sharpness of MNs reduced that leads to difficulties in skin penetration and low drug delivery efficiency. | (Chen et al., 2017b) |
| Bleomycin |
|
Coated MNs |
|
Pyramid shape | L-PLA MNs were selected for pre-coated microneedle as they had sufficient mechanical strength for skin penetration regardless of body. Moreover, bleomycin-coated MNs can deliver more concentrated drug dose compared with intralesional injection. | (Lee et al., 2017) |
| Gentamicin | Sodium hyaluronate and poly (vinylpyrrolidone). | Dissolving microneedle | Moulding | Pyramid-shaped (19 × 19 needles 500 μm in height) | The mechanical strength of polymer microneedles was strong enough to penetrate the skin layer. Besides, theses polymer MNs provides sustained release delivery of the drug. | (González-Vázquez et al., 2017) |
| Vitamin D3 (VD3) nano-microparticles | Poly (lactic-co-glycolic acid) (PLGA) to prepared VD3 nano-microparticles and polyvinylpyrrolidone (PVP) | Dissolving MNs(including nano and microparticles) | Laser engineered micromoulds | Conical (19 × 19 and 12 × 12 arrays) and pyramidal (14 × 14) shaped (600 μm hight and 300 μm widths at base). | The polymer MNs shows good mechanical strength and safely inserted to the skin. Moreover, prepared VD3 as nanoparticle provide immediate release profile of the drug. | (Vora et al., 2017). |
| Fluorescein sodium and fluorescein isothiocyanate–dextrans | Polyvinylpyrrolidone (PVP) | Dissolving MNs | Laser-engineered silicone micromoulds | Conical shaped (3 × 3 arrays, 800 μm tall, 300 μm wide at the base, and 50 μm between needles) | Polymer MNs can be also used to deliver the drug to the eye and is considered an effective method in enhancing ocular delivery of both small and macromolecules. | (Thakur et al., 2016). |
| Donepezil | Poly(vinylpyrrolidone) or poly (methyl vinyl ether co-maleic anhydride/acid) (Gantrez®) polymers | Hydrogel-forming MNs | Moulding | Conical shape (600 μm long, 300 μm wide and 150 μm between needles) | The type of polymer used to prepare MNs has impact on permeation of MNs and dissolution rate. By comparison between PVP and Gantrez®, it was found that permeation through the skin was greater in Gantrez®, while PVP MNs had a higher dissolution rate. | (Kearney et al., 2016) |
| Doxorubicin (DOX) | Gelatin methacryloyl (GelMA) | Bioresponsive and biodegradable MNs. | Micro transfer moulding and cross-linked by UV irradiation | (11 × 11 array, 600 μm in Height, 300 μm base width) | The crosslinking degree of the polymer (GelMA) has an influence on drug release behaviour of MNs. Zero polymer crosslinking result in release over 80% of DOX in 30 min while cross-linking with UV irradiation for 1 min leads to about 50% release of DOX in the first 2 h, followed by slow release over 22 h of the remaining 20%. | (Luo et al., 2019) |
| Sulfonhodamine B (SRB) | Hyaluronic acid (HA) andPolyvinyl alcohol (PVA) | Dissolving MNs | Casting | (10 × 10 MN array, 600 μm long, 300 μm wide, and 600 μm between needles) | The drug distribution in the MNs plays a major role in the fabrication and performance of MNs. By comparison between HA and PVA MNs; the drug diffusion in PVA MNs is limited and most of the drug are concentrated in the needle tip. On the other hand, HA MNs show poor control of the drug diffusion and SRB molecules was diffuse into the base of MNs. As a result, the PVA based MNs would be better for MNs fabrication while HA MNs face a great challenge to achieve ideal the controlled distribution of drugs. | (Feng et al., 2020) |
| Diclofenac sodium | Chitosan and PVP | Hydrogel-forming MNs | Moulding | (2.4 mm long, between 780 and 800 μm in base diameter, and 210 μm in tip diameter) | Combine chitosan and PVA for preparation of MNs leads to the improved mechanical strength of MNs and enabled a sustained drug release profile. | (Dathathri et al., 2020) |
| Antifilariasis drugs (Diethylcarbamazine Albendazole, and Doxycycline) | poly(vinyl alcohol) (PVA) and Poly(vinylpyrrolidone) (PVP) | Dissolving MNs (including solid lipid nanoparticles) | Moulding | Pyramidal shape (19 × 19 needles; 500 μm height and 300 μm width at base and 300 μm interspacing). | MNs prepared using PVP and PVA polymers separately, PVP exhibited poor mechanical properties and PVA show adequate mechanical strength. The combination of PVP and PVA could potentially increase the mechanical strength of MNs. Besides, this delivery approach provides effective therapy for lymphatic filariasis. | (Permana et al., 2019) |
Table 2.
Polymeric microneedle for delivering of biotherapeutics.
| Compounds | Polymer | Type of MNs | Fabrication method | Geometry of MNs | Main outcome | Reference |
|---|---|---|---|---|---|---|
| Insulin | Polylactic acid (PLA) | Solid MNs | Moulding | MNs with different with 600, μm heights. | The pretreatment of skin with polymer solid MNs produces microchannels in the skin that mediated insulin delivery in a slow manner to lower the blood glucose level as drug molecules would be difficult to diffuse rapidly into the skin across the micro-holes whereas the subcutaneous injection of insulin can reduce the blood glucose quickly. | (Li et al., 2017) |
| Insulin | Alginate and hyaluronate | Dissolving MNs | Moulding | Pyramids shaped (10 × 10 array, tip width ~10 μm, height ~700 μm and the space between each two MNs is ~600 μm) | Polymer MNs prepared using alginate and hyaluronate displayed good mechanical strength as well as good degradation rate for release of insulin. Also, the relative pharmacological availability of insulin MNs was close to the subcutaneous injection suggesting the potential application of MNs for diabetes treatment. | (Yu et al., 2017) |
| Lysozyme (LYS) | Carboxymethyl cellulose (CMC), polyvinylpyrrolidone (PVP) and hyaluronic acid (HA). | Dissolving MNs | Droplet-born air blowing method | Needle with micro-dimensions and a sharp tip. | The stability of lysozyme (LYS) as a model protein was preserved in the polymer MNs by keeps the fabrication process at low temperature, and mild drying condition. Besides, using specific polymer concentration and add protein stabilizer. These findings highlight the importance of optimizing polymer MNs fabrication parameters to maintain the activity of polymer MNs encapsulated proteins or antigens. | (Lahiji et al., 2018) |
| Parathyroid hormone (PTH) | Hyaluronic acid (HA) | Dissolving MNs | Micro-moulding | MNs were approximately 800 μm long, 160 μm in base diameter, 40 μm tip diameter, and spaced 600 μm wide between each row of needles. | The polymer MNs of PTH show excellent performance for transdermal drug delivery in a rat model of osteoporosis with relative bioavailability reaches to 100 ± 4% compared to normal injection. These findings indicate that the low absorption issue associated with oral dosage form or painful frequent injections could be replaced with self-administration of dissolving MNs. | (Naito et al., 2018) |
| Monoclonal immunoglobulin G (IgG) | Hyaluronic acid (HA) | Dissolving MNs | Moulding | MNs (4 × 4) array and the length was 300 μm. | The polymer MNs of (IgG) using HA provide rapid non-invasive intradermal protein delivery as well as maintain the stability of protein. | (Mönkäre et al., 2015) |
| DNA vaccine for cervical cancer | Polyvinylpyrrolidone (PVP) | Dissolving MNs (including cationic nanoparticles) | Micro-moulding | MNs (19x19) needles were 600 μm long, 300 μm wide at the base and 300 μm between needles. | NPs have sufficient cationic charge to prevent aggregation in order to bind to the negatively charged cancer cell membrane. In general, the vaccine nanoparticles loaded in MNs trigger a robust antigen-specific humoral immune response comparable and even superior to intramuscular injections. | (Ali et al., 2017) |
| Transcribed messenger RNA (mRNA) based cancer vaccination | low molecular weight Polyvinylpyrrolidone (PVP) | Dissolving MNs | Micro-moulding | Pyramidal shape | Polymer MNs triggered more cellular and humoral immune responses comparing with subcutaneous injection. | (Koh et al., 2018) |
| DNA vaccine against antigens of prostate cancer stem cells | Polyvinylpyrrolidone (PVP) | Dissolving MNs (including cationic nanoparticles) | Moulding | – | Vaccine loaded in cationic nanoparticle and integrated in polymer MNs is effectively triggered immune response towards endogenous prostate cancer and demonstrated anti-tumour activity in both prophylactic and therapeutic. | (Cole et al., 2019) |
| Anti-vascular endothelial growth factor (VEGF) aptamer | Polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP) | Dissolving MNs | Moulding | Pyramidal shape consisted of 100 microneedle array (10 × 10) with a 600 μm, height, 200 μm, base, andb500 μm, tip-to-tip distance. | Polymer MNs loaded with aptamer shows high mechanical strength and immediately released to inhibit protein-mediated cell growth. It is interesting prospects as a treatment for diseases caused by protein overexpression. | (Coyne et al., 2017) |
| STAT3 siRNA for melanoma treatment | Dextran 40, polyvinylpyrrolidone (PVP 17), and sodium hyaluronate (HA) | Dissolving MNs | Moulding | Pyramidal shape comprised of 144 (12 × 12) needles 650 μm long, 300 μm wide, 20 μm wide in the tip radius and 300 μm spaced between the arrays | The use of polymer MNs for delivery of STAT3 siRNA shows good initial results for the treatment of skin melanoma with effective inhibition rates and minimal adverse effects. | (Pan et al., 2018) |
| Dermatophagoides farina (D. farinae) extract (Allergen-specific immunotherapy) | Sodium hyaluronate (HA) | Dissolving MNs | Droplet-born air blowing (DAB) | Microneedle array consisted of 76 needles with 0.25 mm needle length. | Polymer MNs were able to immunize against allergic disease at low dose by activating dendritic cells in the skin without significant side effects. | (Kim et al., 2018a) |
| Bovine serum albumin (BSA), | Poly (vinyl alcohol) (PVA) and poly (lactic-co-glycolic acid) (PLGA) | Biodegradable MNs | Dual-nozzle spray delivery and moulding | Pyramidal shape microneedles (10 × 10) arrays 600 μm long and 300 µm wide, | Encapsulation of labile substances (BSA) in polymer MNs using dual-nozzle spray process enhanced the drug stability via minimizing unfavourable processing conditions such as the emulsification process. | (Kim et al., 2017) |
| Rhodamine B (RhB) indocyanine green (ICG) and doxorubicin (DOX) | Sucrose/ Poly-(ethylene glycol) diacrylate (PEGDA) at weight ratios 1:20, 2:20, and 3:20 | Hydrogel- forming MNs | Moulding and UV-induced polymerization | Microneedle patch consisted of (15 × 15) needles. | PEGDA-based microneedles triggered by polymer swelling under the physiological condition that provide sustained release while the higher sucrose content contributed to a more rapid drug dissolution in the hydrogel. | (Gao et al., 2019) |
| Gene therapy (p53 DNA and IR820) for cancer treatment | Sodium hyaluronate (HA) | Dissolving MNs | Casting method | MN patch (5 × 5 array) 904 ± 8 mm long, 8 ± 2 mm wide tip diameter, 313 ± 12 mm wide base and 1094 ± 26 mm measured between tips | The polymer MNs is capable to deliver a gene and photothermal therapies simultaneously and provide a synergistic strategy for cancer treatments. | (Xu et al., 2020) |
| Diphtheria toxoid (DT) vaccine | Sodium hyaluronate, (HA) | Dissolving MNs | Moulding | MN arrays (4 × 4 needles) | The immunization using polymer dissolving MNs shows a higher immune response comparing with hollow MNs. Furthermore, dissolving MNs loading unadjuvanted (DT) provide comparable immune responses after prime immunization comparing with subcutaneous injected DT-AlPO4. | (Leone et al., 2020) |
| Recombinant coronavirus vaccines | Carboxymethyl cellulose (CMC) | Dissolving MNs | Casting –moulding | Microneedles were a 750 µm long, 225 µm wide, and 30° in apex angle | Coronavirus-S1subunit vaccines loaded in polymer MNs trigger potent antigen-specific antibody responses beginning 2 weeks after immunization. | (Kim et al., 2020) |
| vascular endothelial growth factor (VEGF) | Chitosan and (N-isopropylacrylamide) (pNIPAM) | Bioresponsive MNs | Moulding | Conical shape (20 × 20 MN array, 600 μm height with 5 μm tip diameter and 300 μm base diameter) | The temperature-responsive pNIPAM provides control drug release drugs via temperature rising associated with inflammation response at the site of the wound. Besides, chitosan MNs possesses a natural antibacterial property that promotes inflammatory inhibition, collagen deposition, angiogenesis, and tissue regeneration during wound closure. | (Chi et al., 2020). |
The biotherapeutics classified to different types including vaccine-based products, blood component, allergenic, gene therapy, human tissue, proteins and peptides (Nongkhlaw et al., 2020). These biotherapeutics are normally delivered via needle injection due to their poor properties. However, it is well documented that patient compliance with this route is poor due to the pain encountered and potential for contamination and infection (Yamamoto et al., 2020). The introduction of MNs that are able to penetrate the skin at constant depths allows for the introduction of macromolecules to their targets in a rapid and effective manner while avoiding the pain associated with injections due to the absence of contact with nerve endings in the deeper layers of the skin (Ye et al., 2018). Beside, delivers of biotherapeutics such as vaccines through MNs inducing a more potent long-lasting immune response with less vaccine comparing with subcutaneous or intramuscular injection as the vaccines would be closer to antigen-presenting cells present in human skin as well as the biotherapeutics usually do not need to be administered in large doses that means the dose limitations associated with coated or dissolving microneedle systems are not a concern (Lee et al., 2020). Furthermore, improvements in vaccine stabilization technologies, it may be possible to create microneedle vaccines that avoid the need for refrigeration (Chu et al., 2016). Altogether, this suggests that microneedle vaccines should be able to be stockpiled under much less costly conditions and possibly at a greater number of locations.
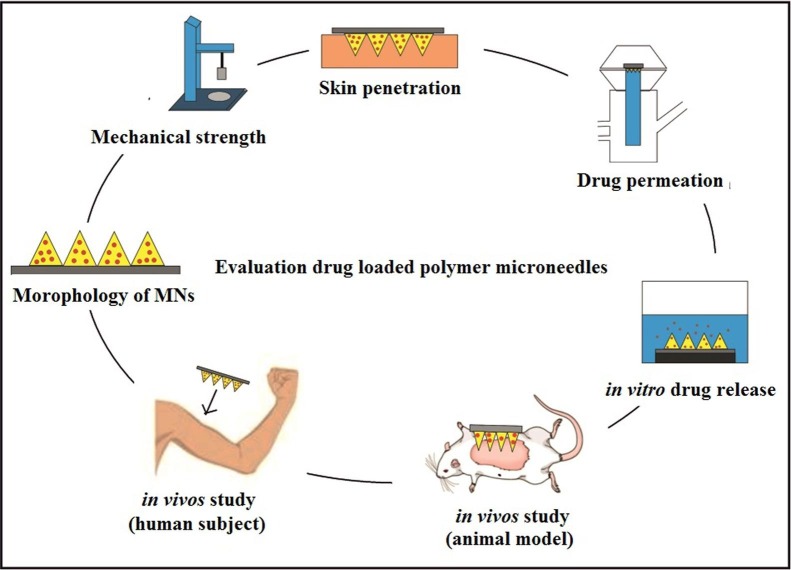
4. Evaluation of drug-loaded polymer microneedles
Designing polymer microneedles, the materials and manufacturing process are important parameters to consider; effective MNs for drug delivery depends on the mechanical strength, skin permeation, and release kinetics that subsequently affect drug delivery as simplifying in Fig. 4 . The morphology and dimensions of MNs including the tip radius, heights, widths, lengths and interspacing of the polymer MNs can be observed using stereomicroscopy, transmission electron microscopy (TEM) or scanning electron microscopy (SEM). The amount of drug encapsulated in the MN is affected by the structural properties of the MN, which is determined by the type and stability of the drug of interest (Lee et al., 2008). The mechanical strength is commonly investigated by using a Texture Analyser in compression mode (Vora et al., 2020) or motorized force measurement test stand (He et al., 2018b). For fracture testing, the MN arrays are subjected to a microscope observation before and after the tests to determine differences in height.
Fig. 4.
The main procedure to evaluate drug-loaded polymer microneedle.
The shape of the microneedle is an important aspect of MN design; it decides how much force can be applied to the MN before the needle breaks. The diameter and angle of the tip, as well as the height and base measurements of the MN, determine safe and reliable insertion of the microneedle into the skin (Bal et al., 2008). Generally, a smaller tip diameter, smaller tip angle, as well as a high ratio of height to base width result in successful needle insertion (Kalluri et al., 2017). Most incomplete insertions occurred due to skin distortions when the length of MNs is short, or aspect ratio is small (Coulman et al., 2011). This situation can be avoided by increasing the length of the needle or manipulating the forces used during manual needle insertion. The force applied during insertion can also be controlled by using special MN applicators that decrease skin distortion and standardize the amount of applied pressure (Daddona et al., 2011, Haq et al., 2009). The skin on different locations of the body have different degrees of distortion; hence the MN design and insertion methods should be individually tailored to achieve successful MN application in these locations (Kalluri et al., 2017).
MNs are applied to the skin surface and pierce the epidermis, creating microscopic holes through which drugs diffuse to the dermal microcirculation. The success of microneedle penetration can be assessed using either parafilm or carefully prepared porcine skin; this porcine skin has close physical properties to human skin, it can be used as a good human skin model (Chen et al., 2018, Lee et al., 2015, Vora et al., 2020). The holes produce by applying MNs in the parafilm, or porcine skin can be visually observed after removal of MNs using methylene blue staining, and the number the blue dots of methylene blue dividing with the number of microneedles on the array, the percentage of successful skin penetration was obtained (Xenikakis et al., 2019). The “penetration success rate” is related to the number of microneedles that penetrate the skin. A 100% success rate indicates that all MN arrays will observe in the skin (Donadei et al., 2019). Generally, parameters such as the tip diameter, base width, length of the microneedle, type of microneedle and its mechanical strength play a critical role in the dimensions of the created microchannel (Kalluri & Banga, 2011).
The microchannel dimensions created by MN can be estimated by histology examination (Sivaraman & Banga, 2017). However, the sample preparation for histological examination may alter skin structure during the freezing and sectioning steps that lead to inaccurate assessment of the microneedle penetration into the skin (Loizidou et al., 2016, Nguyen et al., 2018). Thus, a real-time non-invasive method optical coherence tomography (OCT) can be used for skin characterization (Donnelly et al., 2010). In addition, the measurement of transepidermal water loss (TEWL) is usually carried to evaluate the effect of microneedle application on the skin barrier integrity (Sabri et al., 2019). The common side effect during microneedle insertion includes mild and transient erythema at the site of application that can simply be observed by dermatoscopic or stereo microscopy (Zhu et al., 2016). Besides, the time required for skin recovery to its original state must be noted to evaluate the skin tolerability towards microneedle application (Sabri et al., 2019).
The quantity of drug release through the skin or drug encapsulation in MNs can be assessed using Franz diffusion with the neonatal porcine skin attached facing upwards in the donor compartment of the diffusion cell, and phosphate-buffered saline at pH 7.4 and temperature at 37 °C in the receiver compartment of the cell. The MNs array is applied to the test ‘skin’ and at set time intervals, samples are taken from the sampling arms of the cell and evaluated. Likewise, the MNs are placed inside a beaker or glass vial phosphate-buffered saline (PBS) or any other suitable buffer at pH 7.4 at 37 °C for in vitro drug release, and samples are taken at set intervals, samples are taken at set intervals to determine drug concentrations (González-Vázquez et al., 2017). The drug release also can be evaluated using in vivo animal model; appropriate rat or mice are commonly employed. The anaesthetized animals’ fur is removed, then the underlying skin is pierced firmly using the MN patch. Upon skin penetration, other parameters related to the efficacy of MN can be evaluated such as the MN strength, penetration power, and irritation (Shende et al., 2018).
Most MN studies are performed in vivo in animal models or ex vivo on human skin. However, it has been pointed out that the structure and immune response in the animal model differs significantly from humans. Furthermore, the biochemical properties of human skin ex vivo are different compared to intact human skin (Coulman et al., 2011). Hence, in moving towards clinical trials, human tests need to be included in studies for the results to accurately represent MN function in humans (Arya et al., 2017).
5. Scale-up and manufacturing considerations
The greater acceptance of microneedle-based devices in patients has encouraged an increase in market entry, decreased the cost of innovation and accelerated market growth of MNs. Developments in the field are saturated, as evidenced by the increased number of academic publications and patents on MNs annually. Continuous efforts to transfer scientific research into the clinical application has been ongoing for the past 20 years, with research groups now taking initial commercializing steps (Dardano et al., 2019a). This jump from experimental research to an initial commercialization stage is evidenced in the number of concluded clinical trials in the USA, as can be accessed via https://clinicaltrials.gov/. The global transdermal drug delivery market is estimated to be worth approximately $95.57 billion by 2025 (Businesswire). Currently, there are no MN-containing drug or protein products in the market; only MN-based devices used to administer drug are available (Chandran et al., 2019, Richter-Johnson et al., 2018), Some of MNs devices presented in Table 3 .
Table 3.
Currently marketed microneedle devices for therapeutic use.
| Serial no. | Company | Marketed product | Features | Reference |
|---|---|---|---|---|
| 1 | Valeritas Inc., Bridgewater, NJ, USA | V-Go | A disposable insulin delivery device | (Knutsen et al., 2015, Raina et al., 2017). |
| 2 | Zosano pharma Inc., United States | Adhesive Dermally Applied Microarray (ADAM) | Utilizing for delivery of zolmitriptan | (Kellerman et al., 2017, Spierings et al., 2018) |
| 3 | Sanofi Pasteur, Swiftwater, PA, USA | Fluzone® Intradermal Quadrivalent | Used for delivery of the seasonal influenza vaccine | (US,2020) |
| 4 | Debioject | DebioJect™ | This MN can be connected to any standard syringes to ensure the full penetration of the microneedle into the skin | (Vescovo et al., 2017) |
| 5 | Becton Dickinson, Franklin Lakes, NJ, USA | BD Soluvia™ | The first hollow microneedle product approved for vaccination | (Beran et al., 2009, Donnelly et al., 2012b) |
| 6 | NanoPass Technologies | MicronJet™ | It is a single-use, microneedle-based device for intradermal delivery of drugs, proteins and vaccines | (Donnelly et al., 2012b, Ita, 2015b) |
| 7 | Nano BioSciences | AdminPatch® Microneedle Arrays | This MN allows continuous delivery of drugs by laminated the transdermal patch on the back surface of the microneedles | (Donnelly et al., 2012b) |
| 8 | DermaIndia, Chennai, India | Dermaroller® microneedlerollers | This MN helps to treat some skin conditions | (Nalluri et al., 2015) |
The main focus of MN clinical trials up to this point has been to overcome the skin barrier for initial penetration, improving delivery, assessing immunologic reactions to large molecular drugs, investigating sensory reactions in the skin and general patient attitude towards MN use (Moreira et al., 2019). Recent findings in MN research report the poor biocompatibility of silicon for use in MNs, as well as the possibility of abuse of MNs that do not break off/ dissolve after administration to the patient. As a result, current investigations are directed towards the preparation of MNs from biocompatible polymers approved by the FDA (Donnelly et al., 2012b). The outcomes of human clinical study trial (phase 1) using dissolvable MN arrays to administer a vaccine against influenza, patients had favourable reactions, and the drug delivered initiated favourable therapeutic responses in the human tests (Rouphael et al., 2017).
Moving forward, the biggest hurdle would be the development of straightforward processes that enable efficient, robust and high throughput production of the polymer microneedles at an industrial scale. Current production methods employ batch production processes, which are limiting (McGrath et al., 2014). Polymers used in MN fabrication can be hygroscopic; absorption of water from the production facility compromises the structural integrity and strength of the finished product, affecting the performance of the final MN product (He et al., 2018b). Huge measures will need to be implemented in production methods and facilities for successful fabrication of MNs. As MN array-based product innovation has been in full force, no pharmacopoeia standards are currently in place.
The successful implementation of MNs may be required to make significant investments in equipment and processing technology. Since no pharmacopoeia standards currently exist for MN array-based products, due mainly to the innovative nature of this technology. As companies propose to introduce MN patches into the pharmaceutical market in the future, the need for standardization and regulation regarding sterility, durability, safety, application and disposal of MNs will arise (Larraneta et al., 2016). Thus far, the requirements and restrictions related to sterility and safety of the products up to the disposal of MNs after use depended mainly on the application sector (Dardano et al., 2019b).
Moreover, there is insufficient data on the side effects of polymer MN such as skin irritation, changes in skin barrier function and microbial penetration as well as more investigation are required to select a polymer that minimises the skin irritation (Rodgers et al., 2018). Polymer deposition from MN has great interest currently as the polymers never used before intradermally in spite of are typically approved pharmaceutical excipients. Regulators may require more information on the amounts of polymer left behind in skin after MN removal and information on clearance rates and routes (Donnelly & Woolfson, 2014). The deposition of polymers inside the human body might be a non-issue in case of single MN administration but could be important if a polymer MN was regularly used, it is theorised that repeated application polymer of MN could lead to distribution and deposition of polymer throughout the body, hepatic accumulation, build-up of polymer in the dermal tissue, and immunological reaction (Larraneta et al., 2016, Rodgers et al., 2018).
The study conducted by Vicente-Perez et al. (2017) to investigate the effect of repeated application polymeric microneedle arrays on the skin using an animal model. Two types of MN were prepared; hydrogel-forming MN using Gantrez® S-97 BF, and Polyethylene glycol as well as dissolving MN prepared using Gantrez® S-97 BF and, (polyvinyl pyrrolidone). The result shows that all mice displayed mild erythema after MN removal, but no permanent change in skin observed in dissolving MN. In addition, both dissolving and hydrogel-forming MN arrays do not stimulate the humoral immune system or cause infection or trigger an inflammatory response cascade. Nevertheless, the long-term effects of polymer deposition will require further investigation to ensure they do not represent a toxicity issue (Vora et al., 2020).
In general, widespread application of polymer microneedle for transdermal delivery is still pending due to the lack of human clinical trials, and the need for improvements in production scale and cost. The skin and other target tissues are moist and amenable to distortion and contain nerve endings that are painful when stimulated. In administering MNs to these tissues, MN dissolvability, structure, and application method are important factors to consider for MNs. There is also a need for skilled personnel in the administration of the MNs. Although much has been discovered on successful MN application in the skin, much remains to be discovered for its application in other tissues. Future research and developments in MNs will need to consider the structure of the target tissue and the target disease. This will determine the MN design, type of drug that can be used and amounts for effective delivery into the target tissue (Lee & Prausnitz, 2018).
6. Conclusion
Polymeric microneedles (MNs) are a powerful technology for delivering small chemical molecules to large complex biotherapeutics with established clinical efficacy. MNs based drug delivery through the skin address the shortcoming associated with oral and parental pathways and intended for self-administration at home. The successful development of polymer MNs relies profoundly on the type of polymer used one polymer or mixture of polymers, biocompatibility with drug, design and mechanical strength of the MN. Moreover, animal studies and human studies must be included to evaluate the penetration of MNs through the skin, drug release, pharmacological and toxicity performance. The fundamental studies and commercialization programs must be combined to accelerating the production of polymeric MNs in large-scale, and more effort required to establish guideline regarding the sterilization process, and further awareness of the long-term adverse effects of polymeric MNs in regenerative medicine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to the university Malaysia Pahang for providing support in the form of an internal grant (RDU 180336 and RDU 180371).
References
- Abiandu I., Ita K. Transdermal delivery of potassium chloride with solid microneedles. J. Drug Deliv. Sci. Technol. 2019;53 [Google Scholar]
- Ali A.A., McCrudden C.M., McCaffrey J., McBride J.W., Cole G., Dunne N.J., McCarthy H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. Biol. Med. 2017;13(3):921–932. doi: 10.1016/j.nano.2016.11.019. [DOI] [PubMed] [Google Scholar]
- Ali R., Mehta P., Arshad M., Kucuk I., Chang M., Ahmad Z. Transdermal microneedles—a materials perspective. Aaps Pharmscitech. 2020;21(1):12. doi: 10.1208/s12249-019-1560-3. [DOI] [PubMed] [Google Scholar]
- Alkilani A.Z., McCrudden M.T., Donnelly R.F. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–470. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyautdin R., Khalin I., Nafeeza M.I., Haron M.H., Kuznetsov D. Nanoscale drug delivery systems and the blood–brain barrier. Int. J. Nanomed. 2014;9:795–811. doi: 10.2147/IJN.S52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya J., Henry S., Kalluri H., McAllister D.V., Pewin W.P., Prausnitz M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. doi: 10.1016/j.biomaterials.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek, S. K., Ahn, M. H., & Baek, S. Y. (2018). Method of manufacturing microneedle and microneedle manufactured thereby. In: Google Patents.
- Bal S.M., Caussin J., Pavel S., Bouwstra J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008;35(3):193–202. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Baroni A., Buommino E., De Gregorio V., Ruocco E., Ruocco V., Wolf R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012;30(3):257–262. doi: 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Battisti M., Vecchione R., Casale C., Pennacchio F.A., Lettera V., Jamaledin R., Urciuolo F. Non-invasive production of multi-compartmental biodegradable polymer microneedles for controlled intradermal drug release of labile molecules. Front. Bioeng. Biotechnol. 2019;7:296. doi: 10.3389/fbioe.2019.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran J., Ambrozaitis A., Laiskonis A., Mickuviene N., Bacart P., Calozet Y., Weber F. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7(1):13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S., Dave K., Venuganti V.V.K. Microneedles in the clinic. J. Control. Release. 2017;260:164–182. doi: 10.1016/j.jconrel.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S., Gadeela P.R., Thathireddy P., Venuganti V.V.K. Microneedle-based drug delivery: materials of construction. J. Chem. Sci. 2019;131(9):90. [Google Scholar]
- Brown M.B., Williams A.C. CRC Press; 2019. The Art and Science of Dermal Formulation Development. [Google Scholar]
- Caffarel-Salvador E., Brady A.J., Eltayib E., Meng T., Alonso-Vicente A., Gonzalez-Vazquez P., Jones D.S. Hydrogel-forming microneedle arrays allow detection of drugs and glucose in vivo: potential for use in diagnosis and therapeutic drug monitoring. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran R., Tohit E.R.M., Stanslas J., Mahmood T.M.T. Biomaterials and Bionanotechnology. Elsevier; 2019. Recent advances and challenges in microneedle-mediated transdermal protein and peptide drug delivery; pp. 495–525. [Google Scholar]
- Chen B.Z., Ashfaq M., Zhang X.P., Zhang J.N., Guo X.D. In vitro and in vivo assessment of polymer microneedles for controlled transdermal drug delivery. J. Drug Target. 2018;26(8):720–729. doi: 10.1080/1061186X.2018.1424859. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen B.Z., Wang Q.L., Jin X., Guo X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release. 2017;265:14–21. doi: 10.1016/j.jconrel.2017.03.383. [DOI] [PubMed] [Google Scholar]
- Chen X., Kask A.S., Crichton M.L., McNeilly C., Yukiko S., Dong L., Chen D. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J. Control. Release. 2010;148(3):327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Chen M., Quan G., Sun Y., Yang D., Pan X., Wu C. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J. Control. Release. 2020 doi: 10.1016/j.jconrel.2020.06.039. [DOI] [PubMed] [Google Scholar]
- Chen W., Tian R., Xu C., Yung B.C., Wang G., Liu Y., Wang J. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat. Commun. 2017;8(1):1–11. doi: 10.1038/s41467-017-01764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K., Das D.B. Microneedles for drug delivery: trends and progress. Drug Deliv. 2016;23(7):2338–2354. doi: 10.3109/10717544.2014.986309. [DOI] [PubMed] [Google Scholar]
- Chi J., Zhang X., Chen C., Shao C., Zhao Y., Wang Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020;5(2):253–259. doi: 10.1016/j.bioactmat.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L.Y., Ye L., Dong K., Compans R.W., Yang C., Prausnitz M.R. Enhanced stability of inactivated influenza vaccine encapsulated in dissolving microneedle patches. Pharm. Res. 2016;33(4):868–878. doi: 10.1007/s11095-015-1833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G., Ali A.A., McErlean E., Mulholland E.J., Short A., McCrudden C.M., Coulter J.A. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019;96:480–490. doi: 10.1016/j.actbio.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Coulman S.A., Birchall J.C., Alex A., Pearton M., Hofer B., O’Mahony C., Považay B. In vivo, in situ imaging of microneedle insertion into the skin of human volunteers using optical coherence tomography. Pharm. Res. 2011;28(1):66–81. doi: 10.1007/s11095-010-0167-x. [DOI] [PubMed] [Google Scholar]
- Coyne J., Davis B., Kauffman D., Zhao N., Wang Y. Polymer Microneedle mediated local aptamer delivery for blocking the function of vascular endothelial growth factor. ACS Biomater. Sci. Eng. 2017;3(12):3395–3403. doi: 10.1021/acsbiomaterials.7b00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddona P.E., Matriano J.A., Mandema J., Maa Y.-F. Parathyroid hormone (1–34)-coated microneedle patch system: clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm. Res. 2011;28(1):159–165. doi: 10.1007/s11095-010-0192-9. [DOI] [PubMed] [Google Scholar]
- Dardano P., Battisti M., Rea I., Serpico L., Terracciano M., Cammarano A., De Stefano L. Polymeric microneedle arrays: versatile tools for an innovative approach to drug administration. Adv. Ther. 2019;2(8):1900036. [Google Scholar]
- Dathathri E., Lal S., Mittal M., Thakur G., De S. Fabrication of low-cost composite polymer-based micro needle patch for transdermal drug delivery. Appl. Nanosci. 2020;10(2):371–377. [Google Scholar]
- Davis S., Prausnitz M., Allen M. Paper presented at the TRANSDUCERS'03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems. Digest of Technical Papers (Cat. No. 03TH8664) 2003. Fabrication and characterization of laser micromachined hollow microneedles. [Google Scholar]
- Demir Y.K., Akan Z., Kerimoglu O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadei A., Kraan H., Ophorst O., Flynn O., O'Mahony C., Soema P.C., Moore A.C. Skin delivery of trivalent Sabin inactivated poliovirus vaccine using dissolvable microneedle patches induces neutralizing antibodies. J. Control. Release. 2019;311:96–103. doi: 10.1016/j.jconrel.2019.08.039. [DOI] [PubMed] [Google Scholar]
- Donnelly R.F., Morrow D.I., Singh T.R., Migalska K., McCarron P.A., O'Mahony C., Woolfson A.D. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev. Ind. Pharm. 2009;35(10):1242–1254. doi: 10.1080/03639040902882280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R.F., Garland M.J., Morrow D.I., Migalska K., Singh T.R.R., Majithiya R., Woolfson A.D. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release. 2010;147(3):333–341. doi: 10.1016/j.jconrel.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Donnelly R.F., Singh T.R.R., Garland M.J., Migalska K., Majithiya R., McCrudden C.M., Woolfson A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012;22(23):4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R.F., Singh T.R.R., Morrow D.I., Woolfson A.D. John Wiley & Sons; 2012. Microneedle-Mediated Transdermal and Intradermal Drug Delivery. [Google Scholar]
- Donnelly R.F., Woolfson A.D. Patient safety and beyond: what should we expect from microneedle arrays in the transdermal delivery arena? Ther. Deliv. 2014;5(6):653–662. doi: 10.4155/tde.14.29. [DOI] [PubMed] [Google Scholar]
- Doppalapudi S., Jain A., Khan W., Domb A.J. Biodegradable polymers—an overview. Polym. Adv. Technol. 2014;25(5):427–435. [Google Scholar]
- Du G., Sun X. Current advances in sustained release microneedles. Pharm. Fronts. 2020;2(01):e11–e22. [Google Scholar]
- Economidou S.N., Lamprou D.A., Douroumis D. 3D printing applications for transdermal drug delivery. Int. J. Pharm. 2018;544(2):415–424. doi: 10.1016/j.ijpharm.2018.01.031. [DOI] [PubMed] [Google Scholar]
- Feng Y.H., Liu J.L., Zhu D.D., Hao Y.Y., Guo X.D. Multiscale simulations of drug distributions in polymer dissolvable microneedles. Biointerfaces Colloids Surf. B. 2020:110844. doi: 10.1016/j.colsurfb.2020.110844. [DOI] [PubMed] [Google Scholar]
- Fukushima K., Ise A., Morita H., Hasegawa R., Ito Y., Sugioka N., Takada K. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm. Res. 2011;28(1):7–21. doi: 10.1007/s11095-010-0097-7. [DOI] [PubMed] [Google Scholar]
- Gao Y., Hou M., Yang R., Zhang L., Xu Z., Kang Y., Xue P. Highly porous silk fibroin scaffold packed in PEGDA/sucrose microneedles for controllable transdermal drug delivery. Biomacromolecules. 2019;20(3):1334–1345. doi: 10.1021/acs.biomac.8b01715. [DOI] [PubMed] [Google Scholar]
- Gerstel, M. S., & Place, V. A. (1976). Drug delivery device. In: Google Patents.
- González-Vázquez P., Larrañeta E., McCrudden M.T., Jarrahian C., Rein-Weston A., Quintanar-Solares M., Donnelly R.F. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control. Release. 2017;265:30–40. doi: 10.1016/j.jconrel.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualeni B., Coulman S., Shah D., Eng P., Ashraf H., Vescovo P., Birchall J. Minimally invasive and targeted therapeutic cell delivery to the skin using microneedle devices. Br. J. Dermatol. 2018;178(3):731–739. doi: 10.1111/bjd.15923. [DOI] [PubMed] [Google Scholar]
- Haq M., Smith E., John D.N., Kalavala M., Edwards C., Anstey A., Birchall J.C. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed. Microdevices. 2009;11(1):35–47. doi: 10.1007/s10544-008-9208-1. [DOI] [PubMed] [Google Scholar]
- Haque T., Talukder M.M.U. Chemical enhancer: a simplistic way to modulate barrier function of the stratum corneum. Adv. Pharm. Bull. 2018;8(2):169. doi: 10.15171/apb.2018.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout R.M., Woodman T.J., Mansour S., Mortada N.D., Geneidi A.S., Guy R.H. Microemulsion formulations for the transdermal delivery of testosterone. Eur. J. Pharm. Sci. 2010;40(3):188–196. doi: 10.1016/j.ejps.2010.03.008. [DOI] [PubMed] [Google Scholar]
- He M.C., Chen B.Z., Ashfaq M., Guo X.D. Assessment of mechanical stability of rapidly separating microneedles for transdermal drug delivery. Drug Deliv. Transl. Res. 2018;8(5):1034–1042. doi: 10.1007/s13346-018-0547-z. [DOI] [PubMed] [Google Scholar]
- He M., Yang G., Zhang S., Zhao X., Gao Y. Dissolving microneedles loaded with etonogestrel microcrystal particles for intradermal sustained delivery. J. Pharm. Sci. 2018;107(4):1037–1045. doi: 10.1016/j.xphs.2017.11.013. [DOI] [PubMed] [Google Scholar]
- He M., Yang G., Zhao X., Zhang S., Gao Y. Intradermal implantable PLGA microneedles for etonogestrel sustained release. J. Pharm. Sci. 2020 doi: 10.1016/j.xphs.2020.02.009. [DOI] [PubMed] [Google Scholar]
- Henry S., McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 1998;87(8):922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- Hiraishi Y., Nakagawa T., Quan Y.-S., Kamiyama F., Hirobe S., Okada N., Nakagawa S. Performance and characteristics evaluation of a sodium hyaluronate-based microneedle patch for a transcutaneous drug delivery system. Int. J. Pharm. 2013;441(1–2):570–579. doi: 10.1016/j.ijpharm.2012.10.042. [DOI] [PubMed] [Google Scholar]
- Hong X., Wei L., Wu F., Wu Z., Chen L., Liu Z., Yuan W. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des., Dev. Ther. 2013;7:945. doi: 10.2147/DDDT.S44401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal N., Vitorino C., Taylor K.M. How can lipid nanocarriers improve transdermal delivery of olanzapine? Pharm. Dev. Technol. 2017;22(4):587–596. doi: 10.1080/10837450.2016.1200615. [DOI] [PubMed] [Google Scholar]
- Ita K. Transdermal delivery of drugs with microneedles—potential and challenges. Pharmaceutics. 2015;7(3):90–105. doi: 10.3390/pharmaceutics7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ita K. Transdermal delivery of drugs with microneedles: strategies and outcomes. J. Drug Deliv. Sci. Technol. 2015;29:16–23. [Google Scholar]
- Ito Y., Yoshimitsu J.-I., Shiroyama K., Sugioka N., Takada K. Self-dissolving microneedles for the percutaneous absorption of EPO in mice. J. Drug Target. 2006;14(5):255–261. doi: 10.1080/10611860600785080. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kashiwara S., Fukushima K., Takada K. Two-layered dissolving microneedles for percutaneous delivery of sumatriptan in rats. Drug Dev. Ind. Pharm. 2011;37(12):1387–1393. doi: 10.3109/03639045.2011.576426. [DOI] [PubMed] [Google Scholar]
- Jagtap S., Kshirsagar P., Kore V., Deshmukh M., Shete R. A review: transdermal microneedle. Curr. Pharma Res. 2018;8(2):2357–2367. [Google Scholar]
- Juster H., van der Aar B., de Brouwer H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym. Eng. Sci. 2019;59(5):877–890. [Google Scholar]
- Kalluri H., Banga A.K. Formation and closure of microchannels in skin following microporation. Pharm. Res. 2011;28(1):82–94. doi: 10.1007/s11095-010-0122-x. [DOI] [PubMed] [Google Scholar]
- Kalluri H., Choi S.-O., Guo X.D., Lee J.W., Norman J., Prausnitz M.R. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. Springer; 2017. Evaluation of microneedles in human subjects; pp. 325–340. [Google Scholar]
- Kathuria H., Kochhar J.S., Kang L. Micro and nanoneedles for drug delivery and biosensing. Ther. Deliv. 2018;9:489–492. doi: 10.4155/tde-2018-0012. [DOI] [PubMed] [Google Scholar]
- Kearney M.-C., Caffarel-Salvador E., Fallows S.J., McCarthy H.O., Donnelly R.F. Microneedle-mediated delivery of donepezil: potential for improved treatment options in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2016;103:43–50. doi: 10.1016/j.ejpb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Kellerman D.J., Ameri M., Tepper S.J. Rapid systemic delivery of zolmitriptan using an adhesive dermally applied microarray. Pain Manage. 2017;7(6):559–567. doi: 10.2217/pmt-2017-0036. [DOI] [PubMed] [Google Scholar]
- Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Haagmans B.L. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee J., Shayan F.L., Kim S., Huh I., Ma Y., Jung H. Physicochemical study of ascorbic acid 2-glucoside loaded hyaluronic acid dissolving microneedles irradiated by electron beam and gamma ray. Carbohydr. Polym. 2018;180:297–303. doi: 10.1016/j.carbpol.2017.10.044. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Park S.C., Choi S.-O. Dual-nozzle spray deposition process for improving the stability of proteins in polymer microneedles. RSC Adv. 2017;7(87):55350–55359. [Google Scholar]
- Kim J.H., Shin J.U., Kim S.H., Noh J.Y., Kim H.R., Lee J., Kim J.D. Successful transdermal allergen delivery and allergen-specific immunotherapy using biodegradable microneedle patches. Biomaterials. 2018;150:38–48. doi: 10.1016/j.biomaterials.2017.10.013. [DOI] [PubMed] [Google Scholar]
- Knutsen P.G., Voelker C.Q., Nikkel C.C. Clinical insights into a new, disposable insulin delivery device. Diabetes Spectrum. 2015;28(3):209–213. doi: 10.2337/diaspect.28.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar J.S., Tan J.J., Kwang Y.C., Kang L. Microneedles for Transdermal Drug Delivery. Springer; 2019. Recent trends in microneedle development & applications in medicine and cosmetics (2013–2018) pp. 95–144. [Google Scholar]
- Koh K.J., Liu Y., Lim S.H., Loh X.J., Kang L., Lim C.Y., Phua K.K. Formulation, characterization and evaluation of mRNA-loaded dissolvable polymeric microneedles (RNApatch) Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-30290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon K.M., Lim S.-M., Choi S., Kim D.-H., Jin H.-E., Jee G., Kim J.Y. Microneedles: quick and easy delivery methods of vaccines. Clin. Exp. Vaccine Res. 2017;6(2):156–159. doi: 10.7774/cevr.2017.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiji S.F., Jang Y., Ma Y., Dangol M., Yang H., Jang M., Jung H. Effects of dissolving microneedle fabrication parameters on the activity of encapsulated lysozyme. Eur. J. Pharm. Sci. 2018;117:290–296. doi: 10.1016/j.ejps.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Larraneta E., Lutton R.E., Woolfson A.D., Donnelly R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater. Sci. Eng.: R: Rep. 2016;104:1–32. [Google Scholar]
- Larrañeta E., McCrudden M.T., Courtenay A.J., Donnelly R.F. Microneedles: a new frontier in nanomedicine delivery. Pharm. Res. 2016;33(5):1055–1073. doi: 10.1007/s11095-016-1885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Goudie M.J., Tebon P., Sun W., Luo Z., Lee J., Xue Y. Non-transdermal microneedles for advanced drug delivery. Adv. Drug Deliv. Rev. 2019 doi: 10.1016/j.addr.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-C., He J.-S., Tsai M.-T., Lin K.-C. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J. Mater. Chem. B. 2015;3(2):276–285. doi: 10.1039/c4tb01555j. [DOI] [PubMed] [Google Scholar]
- Lee K.J., Jeong S.S., Roh D.H., Kim D.Y., Choi H.-K., Lee E.H. A practical guide to the development of microneedle systems–in clinical trials or on the market. Int. J. Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118778. [DOI] [PubMed] [Google Scholar]
- Lee J.W., Prausnitz M.R. Taylor & Francis; 2018. Drug Delivery Using Microneedle Patches: Not Just for Skin. [DOI] [PubMed] [Google Scholar]
- Lee J.W., Park J.-H., Prausnitz M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.S., Ryu H.R., Roh J.Y., Park J.-H. Bleomycin-coated microneedles for treatment of warts. Pharm. Res. 2017;34(1):101–112. doi: 10.1007/s11095-016-2042-x. [DOI] [PubMed] [Google Scholar]
- Lee H., Song C., Baik S., Kim D., Hyeon T., Kim D.-H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018;127:35–45. doi: 10.1016/j.addr.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Leone M., Romeijn S., Du G., Le Dévédec S., Vrieling H., O'Mahony C., Kersten G. Diphtheria toxoid dissolving microneedle vaccination: adjuvant screening and effect of repeated-fractional dose administration. Int. J. Pharm. 2020:119182. doi: 10.1016/j.ijpharm.2020.119182. [DOI] [PubMed] [Google Scholar]
- Lhernould M.S., Deleers M., Delchambre A. Hollow polymer microneedles array resistance and insertion tests. Int. J. Pharm. 2015;480(1–2):152–157. doi: 10.1016/j.ijpharm.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Li Q.Y., Zhang J.N., Chen B.Z., Wang Q.L., Guo X.D. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017;7(25):15408–15415. [Google Scholar]
- Loizidou E.Z., Inoue N.T., Ashton-Barnett J., Barrow D.A., Allender C.J. Evaluation of geometrical effects of microneedles on skin penetration by CT scan and finite element analysis. Eur. J. Pharm. Biopharm. 2016;107:1–6. doi: 10.1016/j.ejpb.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Luo Z., Sun W., Fang J., Lee K., Li S., Gu Z., et al. Biodegradable gelatin methacryloyl microneedles for transdermal drug delivery. Adv. Healthcare Mater. 2019;8(3):1801054. doi: 10.1002/adhm.201801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga M.A., Berry D.R., Reagan J.C., Smaldone R.A., Gassensmith J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip. 2018;18(8):1223–1230. doi: 10.1039/c8lc00098k. [DOI] [PubMed] [Google Scholar]
- Ma Y., Gill H.S. Coating solid dispersions on microneedles via a molten dip-coating method: development and in vitro evaluation for transdermal delivery of a water-insoluble drug. J. Pharm. Sci. 2014;103(11):3621–3630. doi: 10.1002/jps.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J. Control. Release. 2017;251:11–23. doi: 10.1016/j.jconrel.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Mahato R. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices. Elsevier; 2017. Microneedles in drug delivery; pp. 331–353. [Google Scholar]
- Mahmood S., Taher M., Mandal U.K. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int. J. Nanomed. 2014;9:4331–4346. doi: 10.2147/IJN.S65408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S., Mandal U.K., Chatterjee B. Transdermal delivery of raloxifene HCl via ethosomal system: formulation, advanced characterizations and pharmacokinetic evaluation. Int. J. Pharm. 2018;542(1–2):36–46. doi: 10.1016/j.ijpharm.2018.02.044. [DOI] [PubMed] [Google Scholar]
- Marin A., Andrianov A.K. Carboxymethylcellulose–Chitosan-coated microneedles with modulated hydration properties. J. Appl. Polym. Sci. 2011;121(1):395–401. [Google Scholar]
- Matsuo K., Yokota Y., Zhai Y., Quan Y.-S., Kamiyama F., Mukai Y., Nakagawa S. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J. Control. Release. 2012;161(1):10–17. doi: 10.1016/j.jconrel.2012.01.033. [DOI] [PubMed] [Google Scholar]
- McCrudden M.T., Alkilani A.Z., Courtenay A.J., McCrudden C.M., McCloskey B., Walker C., Woolfson A.D. Considerations in the sterile manufacture of polymeric microneedle arrays. Drug Deliv. Transl. Res. 2015;5(1):3–14. doi: 10.1007/s13346-014-0211-1. [DOI] [PubMed] [Google Scholar]
- McGrath M.G., Vrdoljak A., O’Mahony C., Oliveira J.C., Moore A.C., Crean A.M. Determination of parameters for successful spray coating of silicon microneedle arrays. Int. J. Pharm. 2011;415(1–2):140–149. doi: 10.1016/j.ijpharm.2011.05.064. [DOI] [PubMed] [Google Scholar]
- McGrath M.G., Vucen S., Vrdoljak A., Kelly A., O’Mahony C., Crean A.M., Moore A. Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014;86(2):200–211. doi: 10.1016/j.ejpb.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Merino S., Martin C., Kostarelos K., Prato M., Vazquez E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano. 2015;9(5):4686–4697. doi: 10.1021/acsnano.5b01433. [DOI] [PubMed] [Google Scholar]
- Mittapally S., Taranum R., Parveen S. Microneedles-a potential transdermal drug delivery. Int. J. Pharma. Res. Health Sci. 2018;6(3):2579–2585. [Google Scholar]
- Mönkäre J., Nejadnik M.R., Baccouche K., Romeijn S., Jiskoot W., Bouwstra J.A. IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery. J. Control. Release. 2015;218:53–62. doi: 10.1016/j.jconrel.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N.A. Toxicology of the Skin. CRC Press; 2010. Structure and function of skin; pp. 15–32. [Google Scholar]
- Moreira A.F., Rodrigues C.F., Jacinto T.A., Miguel S.P., Costa E.C., Correia I.J. Microneedle-based delivery devices for cancer therapy: a review. Pharmacol. Res. 2019;104438 doi: 10.1016/j.phrs.2019.104438. [DOI] [PubMed] [Google Scholar]
- Na Y.-G., Kim M., Han M., Huh H.W., Kim J.-S., Kim J.C., Cho C.-W. Characterization of hepatitis B surface antigen loaded polylactic acid-based microneedle and its dermal safety profile. Pharmaceutics. 2020;12(6):531. doi: 10.3390/pharmaceutics12060531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito C., Katsumi H., Suzuki T., Quan Y.-S., Kamiyama F., Sakane T., Yamamoto A. Self-dissolving microneedle arrays for transdermal absorption enhancement of human parathyroid hormone (1–34) Pharmaceutics. 2018;10(4):215. doi: 10.3390/pharmaceutics10040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N Nalluri, B., Anusha, S. V., R Bramhini, S., Amulya, J., SK Sultana, A., U Teja, C., & B Das, D., 2015. In vitro skin permeation enhancement of sumatriptan by microneedle application. Current drug delivery, 12(6), 761-769. [DOI] [PubMed]
- Nandagopal M.G., Antony R., Rangabhashiyam S., Sreekumar N., Selvaraju N. Overview of microneedle system: a third generation transdermal drug delivery approach. Microsyst. Technol. 2014;20(7):1249–1272. [Google Scholar]
- Nayak A., Babla H., Han T., Das D.B. Lidocaine carboxymethylcellulose with gelatine co-polymer hydrogel delivery by combined microneedle and ultrasound. Drug Deliv. 2016;23(2):658–669. doi: 10.3109/10717544.2014.935985. [DOI] [PubMed] [Google Scholar]
- Nguyen H.X., Bozorg B.D., Kim Y., Wieber A., Birk G., Lubda D., Banga A.K. Poly (vinyl alcohol) microneedles: fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018;129:88–103. doi: 10.1016/j.ejpb.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Nongkhlaw R., Patra P., Chavrasiya A., Jayabalan N., Dubey S. Drug Delivery Aspects. Elsevier; 2020. Biologics: delivery options and formulation strategies; pp. 115–155. [Google Scholar]
- Omolu A., Bailly M., Day R.M. Assessment of solid microneedle rollers to enhance transmembrane delivery of doxycycline and inhibition of MMP activity. Drug Deliv. 2017;24(1):942–951. doi: 10.1080/10717544.2017.1337826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamornpathomkul B., Wongkajornsilp A., Laiwattanapaisal W., Rojanarata T., Opanasopit P., Ngawhirunpat T. A combined approach of hollow microneedles and nanocarriers for skin immunization with plasmid DNA encoding ovalbumin. Int. J. Nanomed. 2017;12:885. doi: 10.2147/IJN.S125945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Ruan W., Qin M., Long Y., Wan T., Yu K., Xu Y. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-19463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.-H., Ha S.K., Choi I., Kim K.S., Park J., Choi N., Sung J.H. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol. Bioprocess Eng. 2016;21(1):110–118. [Google Scholar]
- Pegoraro C., MacNeil S., Battaglia G. Transdermal drug delivery: from micro to nano. Nanoscale. 2012;4(6):1881–1894. doi: 10.1039/c2nr11606e. [DOI] [PubMed] [Google Scholar]
- Permana A.D., Tekko I.A., McCrudden M.T., Anjani Q.K., Ramadon D., McCarthy H.O., Donnelly R.F. Solid lipid nanoparticle-based dissolving microneedles: a promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release. 2019;316:34–52. doi: 10.1016/j.jconrel.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Prausnitz M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004;56(5):581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Quinn H.L., Kearney M.-C., Courtenay A.J., McCrudden M.T., Donnelly R.F. The role of microneedles for drug and vaccine delivery. Expert Opin. Drug Deliv. 2014;11(11):1769–1780. doi: 10.1517/17425247.2014.938635. [DOI] [PubMed] [Google Scholar]
- Rai V.K., Mishra N., Yadav K.S., Yadav N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J. Control. Release. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- Raina P., Patel Brijesh P., Devarshi S., Parikh R. Novel technologies mark the future of insulin. Am. J. Pharmtech Res. 2017;7(1):100–120. [Google Scholar]
- Ramöller I.K., Tekko I.A., McCarthy H.O., Donnelly R.F. Rapidly dissolving bilayer microneedle arrays–a minimally invasive transdermal drug delivery system for vitamin B12. Int. J. Pharm. 2019;566:299–306. doi: 10.1016/j.ijpharm.2019.05.066. [DOI] [PubMed] [Google Scholar]
- Richter-Johnson J., Kumar P., Choonara Y.E., du Toit L.C., Pillay V. Therapeutic applications and pharmacoeconomics of microneedle technology. Expert Rev. Pharmacoecon. Outcomes Res. 2018;18(4):359–369. doi: 10.1080/14737167.2018.1485100. [DOI] [PubMed] [Google Scholar]
- Rodgers A.M., Courtenay A.J., Donnelly R.F. Taylor & Francis; 2018. Dissolving Microneedles for Intradermal Vaccination: Manufacture, Formulation, and Stakeholder Considerations. [DOI] [PubMed] [Google Scholar]
- Rouphael N.G., Paine M., Mosley R., Henry S., McAllister D.V., Kalluri H., Thornburg N.J. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390(10095):649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu Y.C., Kim D.I., Kim S.H., Wang H.-M.D., Hwang B.H. Synergistic transdermal delivery of biomacromolecules using sonophoresis after microneedle treatment. Biotechnol. Bioprocess Eng. 2018;23(3):286–292. [Google Scholar]
- Sabri A.H., Kim Y., Marlow M., Scurr D.J., Segal J., Banga A.K., Lee J.B. Intradermal and transdermal drug delivery using microneedles–fabrication, performance evaluation and application to lymphatic delivery. Adv. Drug Deliv. Rev. 2019 doi: 10.1016/j.addr.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Shende P., Sardesai M., Gaud R. Micro to nanoneedles: a trend of modernized transepidermal drug delivery system. Artif. Cells Nanomed. Biotechnol. 2018;46(1):19–25. doi: 10.1080/21691401.2017.1304409. [DOI] [PubMed] [Google Scholar]
- Singh P., Carrier A., Chen Y., Lin S., Wang J., Cui S., Zhang X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release. 2019;315:97–113. doi: 10.1016/j.jconrel.2019.10.022. [DOI] [PubMed] [Google Scholar]
- Singh T.R.R., McMillan H., Mooney K., Alkilani A.Z., Donnelly R.F. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement. Springer; 2017. Fabrication of microneedles; pp. 305–323. [Google Scholar]
- Sivaraman A., Banga A.K. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv. Transl. Res. 2017;7(1):16–26. doi: 10.1007/s13346-016-0328-5. [DOI] [PubMed] [Google Scholar]
- Sorg H., Tilkorn D.J., Hager S., Hauser J., Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur. Surg. Res. 2017;58(1–2):81–94. doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- Spierings E.L., Brandes J.L., Kudrow D.B., Weintraub J., Schmidt P.C., Kellerman D.J., Tepper S.J. Randomized, double-blind, placebo-controlled, parallel-group, multi-center study of the safety and efficacy of ADAM zolmitriptan for the acute treatment of migraine. Cephalalgia. 2018;38(2):215–224. doi: 10.1177/0333102417737765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. Hyaluronan metabolism: a major paradox in cancer biology. Pathol. Biol. (Paris) 2005;53(7):372–382. doi: 10.1016/j.patbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Stojic M., López V., Montero A., Quílez C., de Aranda Izuzquiza G., Vojtova L., Velasco D. Biomaterials for Skin Repair and Regeneration. Elsevier; 2019. Skin tissue engineering; pp. 59–99. [Google Scholar]
- Sullivan S.P., Koutsonanos D.G., del Pilar Martin M., Lee J.W., Zarnitsyn V., Choi S.-O., Prausnitz M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16(8):915. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, S.P., 2009. Polymer microneedles for transdermal delivery of biopharmaceuticals. PhD diss; Georgia institute of Technology. https://smartech.gatech.edu/handle/1853/33873.
- Sun W., Araci Z., Inayathullah M., Manickam S., Zhang X., Bruce M.A., Rajadas J. Polyvinylpyrrolidone microneedles enable delivery of intact proteins for diagnostic and therapeutic applications. Acta Biomater. 2013;9(8):7767–7774. doi: 10.1016/j.actbio.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Takahashi T., Aoyagi S. Fabrication and characterization of a biodegradable hollow microneedle from Chitosan. J. Robotics Mechatronics. 2020;32(2):401–407. [Google Scholar]
- Tajbakhsh M., Saeedi M., Akbari J., Morteza-Semnani K., Nokhodchi A., Hedayatizadeh-Omran A. An investigation on parameters affecting the optimization of testosterone enanthate loaded solid nanoparticles for enhanced transdermal delivery. Physicochem. Eng. Aspects Colloids Surf. A. 2020:124437. [Google Scholar]
- Thakur R.R.S., Tekko I.A., Al-Shammari F., Ali A.A., McCarthy H., Donnelly R.F. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv. Transl. Res. 2016;6(6):800–815. doi: 10.1007/s13346-016-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomono T. A new way to control the internal structure of microneedles: a case of chitosan lactate. Mater. Today Chem. 2019;13:79–87. [Google Scholar]
- Tsakovska I., Pajeva I., Al Sharif M., Alov P., Fioravanzo E., Kovarich S., Mostrag-Szlichtyng A. Quantitative structure-skin permeability relationships. Toxicology. 2017;387:27–42. doi: 10.1016/j.tox.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Tsioris K., Raja W.K., Pritchard E.M., Panilaitis B., Kaplan D.L., Omenetto F.G. Fabrication of silk microneedles for controlled-release drug delivery. Adv. Funct. Mater. 2012;22(2):330–335. [Google Scholar]
- Tuan-Mahmood T.-M., McCrudden M.T., Torrisi B.M., McAlister E., Garland M.J., Singh T.R.R., Donnelly R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013;50(5):623–637. doi: 10.1016/j.ejps.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M.J., Scoutaris N., Klepetsanis P., Chowdhry B., Prausnitz M.R., Douroumis D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015;494(2):593–602. doi: 10.1016/j.ijpharm.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Ullah A., Khan H., Choi H.J., Kim G.M. Smart microneedles with porous polymer coatings for pH-responsive drug delivery. Polymers. 2019;11(11):1834. doi: 10.3390/polym11111834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US, S. P. Sanofi Pasteur Ships First 2015-2016 Seasonal Influenza Vaccine Doses in United States.
- Vandervoort J., Ludwig A. Microneedles for transdermal drug delivery: a minireview. Front. Biosci. 2008;13:1711–1715. doi: 10.2741/2794. [DOI] [PubMed] [Google Scholar]
- Vescovo P., Rettby N., Ramaniraka N., Liberman J., Hart K., Cachemaille A., Pantaleo G. Safety, tolerability and efficacy of intradermal rabies immunization with DebioJect™. Vaccine. 2017;35(14):1782–1788. doi: 10.1016/j.vaccine.2016.09.069. [DOI] [PubMed] [Google Scholar]
- Vicente-Perez E.M., Larrañeta E., McCrudden M.T., Kissenpfennig A., Hegarty S., McCarthy H.O., Donnelly R.F. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur. J. Pharm. Biopharm. 2017;117:400–407. doi: 10.1016/j.ejpb.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora L.K., Donnelly R.F., Larrañeta E., González-Vázquez P., Thakur R.R.S., Vavia P.R. Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: proof of concept. J. Control. Release. 2017;265:93–101. doi: 10.1016/j.jconrel.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Vora L.K., Courtenay A.J., Tekko I.A., Larrañeta E., Donnelly R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020;146:290–298. doi: 10.1016/j.ijbiomac.2019.12.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G., Singh M., Dua K. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- Wang M., Hu L., Xu C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip. 2017;17(8):1373–1387. doi: 10.1039/c7lc00016b. [DOI] [PubMed] [Google Scholar]
- Wang Q.L., Ren J.W., Chen B.Z., Jin X., Zhang C.Y., Guo X.D. Effect of humidity on mechanical properties of dissolving microneedles for transdermal drug delivery. J. Ind. Eng. Chem. 2018;59:251–258. [Google Scholar]
- Wang Q., Yao G., Dong P., Gong Z., Li G., Zhang K., Wu C. Investigation on fabrication process of dissolving microneedle arrays to improve effective needle drug distribution. Eur. J. Pharm. Sci. 2015;66:148–156. doi: 10.1016/j.ejps.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Wang J., Ye Y., Yu J., Kahkoska A.R., Zhang X., Wang C., Khan S.A. Core–shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12(3):2466–2473. doi: 10.1021/acsnano.7b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.L., Zhu D.D., Chen Y., Guo X.D. A fabrication method of microneedle molds with controlled microstructures. Mater. Sci. Eng., C. 2016;65:135–142. doi: 10.1016/j.msec.2016.03.097. [DOI] [PubMed] [Google Scholar]
- Xenikakis I., Tzimtzimis M., Tsongas K., Andreadis D., Demiri E., Tzetzis D., Fatouros D.G. Fabrication and finite element analysis of stereolithographic 3D printed microneedles for transdermal delivery of model dyes across human skin in vitro. Eur. J. Pharm. Sci. 2019;137 doi: 10.1016/j.ejps.2019.104976. [DOI] [PubMed] [Google Scholar]
- Xu Q., Li X., Zhang P., Wang Y. Rapid dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J. Mater. Chem. B. 2020 doi: 10.1039/d0tb00105h. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Ukai H., Morishita M., Katsumi H. Approaches to improve intestinal and transmucosal absorption of peptide and protein drugs. Pharmacol. Ther. 2020;107537 doi: 10.1016/j.pharmthera.2020.107537. [DOI] [PubMed] [Google Scholar]
- Yan G., Warner K.S., Zhang J., Sharma S., Gale B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharm. 2010;391(1–2):7–12. doi: 10.1016/j.ijpharm.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Ye Y., Yu J., Wen D., Kahkoska A.R., Gu Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018;127:106–118. doi: 10.1016/j.addr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K., Smith A.G. Nuclear receptor function in skin health and disease: therapeutic opportunities in the orphan and adopted receptor classes. Cell. Mol. Life Sci. 2016;73(20):3789–3800. doi: 10.1007/s00018-016-2329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef, H., Alhajj, M., & Sharma, S., 2019. Anatomy, skin (integument), epidermis. [PubMed]
- Yu W., Jiang G., Zhang Y., Liu D., Xu B., Zhou J. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin. Mater. Sci. Eng., C. 2017;80:187–196. doi: 10.1016/j.msec.2017.05.143. [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang Y., Ye Y., DiSanto R., Sun W., Ranson D., Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. 2015;112(27):8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zhang Y., Yan J., Kahkoska A.R., Gu Z. Advances in bioresponsive closed-loop drug delivery systems. Int. J. Pharm. 2018;544(2):350–357. doi: 10.1016/j.ijpharm.2017.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung K., Xu Y., Kang C., Liu H., Tam K., Ko S., Lee T.M. Sharp tipped plastic hollow microneedle array by microinjection moulding. J. Micromech. Microeng. 2011;22(1) [Google Scholar]
- Zhang Y., Liu Q., Yu J., Yu S., Wang J., Qiang L., Gu Z. Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano. 2017;11(9):9223–9230. doi: 10.1021/acsnano.7b04348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Feng P., Yu J., Yang J., Zhao J., Wang J., Gu Z. ROS-responsive microneedle patch for acne vulgaris treatment. Adv. Ther. 2018;1(3):1800035. [Google Scholar]
- Zhang Y., Jiang G., Yu W., Liu D., Xu B. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater. Sci. Eng., C. 2018;85:18–26. doi: 10.1016/j.msec.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Zhu D.D., Wang Q.L., Liu X.B., Guo X.D. Rapidly separating microneedles for transdermal drug delivery. Acta Biomater. 2016;41:312–319. doi: 10.1016/j.actbio.2016.06.005. [DOI] [PubMed] [Google Scholar]