Abstract
Angiotensin-converting enzyme inhibitors (ACEI) are beneficial in heart failure with reduced ejection failure (HFrEF) but are associated with acute declines in eGFR. Prior studies evaluating thresholds of eGFR decline while using ACEIs in HFrEF have not taken into account this medication-driven decline. Using the Studies of Left Ventricular Dysfunction (SOLVD) trials (n=6245), Cox proportional hazards regression models were used to calculate hazard ratios of all-cause mortality and heart failure (HF) hospitalizationassociated with %eGFR decline at 2-weeks and 6-weeks after randomization to enalapril versus placebo. In reference to placebo with equal degree of %eGFR decline, any eGFR decline in the enalapril arm was associated with lower hazard of both outcomes.. Under a conservative estimate using 0% eGFR decline in the placebo arm as the reference,up to a 10% decline with enalapril was associated with mortality benefit (HR=0.87 [0.77, 0.99]) while up to a 35% decline was associated with decreased risk of HF hospitalization (HR=0.78 [0.61, 0.98]). Under the intermediate estimate, up to a 15% decline with enalapril was associated with a mortality benefit (HR=0.86 [0.77, 0.97] and all levels of eGFR decline were associated with decreased risk of HF hospitalization. There was no %eGFR decline, including up to 40%, in any of these models at either 2-weeks or 6-weeks where enalapril was associated with higher mortality risk. In patients with HFrEF, enalapril is associated with decreased risk of mortality and HF hospitalizations, and compelling reasons beyond moderate eGFR decline ought to be considered before its use is withdrawn.
Keywords: SOLVD, ACE inhibitor, Heart failure, Kidney function decline, Cardiorenal syndrome
Introduction
Inhibition of the renin-angiotensin-aldosterone system (RAAS) with either angiotensin-converting enzyme inhibitors (ACEI) or angiotensin-receptor blockers (ARB) has been beneficial in reducing the risk of cardiovascular events in patients with heart failure with reduced ejection fraction (HFrEF)1,2 and is recognized as an integral component of their medical management.3Not only have they been shown to reduce all-cause mortality, they have also been associated with favorable cardiac remodeling,4–6as well as reduction in hospitalization for heart failure (HF).7–9 In the kidney, ACEI/ARB therapy leads to dilation of the efferent arteriole, and thereby can be associated with acute declines in estimated glomerular filtration rate (eGFR) that are considered hemodynamic in nature. While baseline reduced eGFR is a well-established risk factor for worse cardiovascular outcomes in patients with HFrEF,10,11 the prognostic impact of acute eGFR declines, such as those following initiation of ACEI/ARB, is less clear.
Prior studies that have evaluated declines in kidney function in patients with HFrEF have shown mixed results, with some suggesting that an acute decline is a poor prognostic sign,10,12,13 and others finding the opposite.14,15 An eGFR decline of 20%, if occuring in the acute setting of initiation of ACEI, was not found to be associated with an increased risk of mortality in a post-hoc analysis of the Studies of Left Ventricular Dysfunction (SOLVD) trials, in which patients with HFrEF were randomized to enalapril versus placebo.15 In contrast, similar declines among the placebo arm were associated with a higher risk for dying over a median 2-year follow-up. These data suggest that the mechanism underlying the decline may be of importance, hinting that perhaps an ACEI-related hemodynamic decline occuring after ACEI initiation is a benign change. However, the question remains to what degree of eGFR decline can be tolerated before the risk associated with a decline in eGFR outweighs the mortality and cardiovascular benefit associated with ACEI therapy.
The challenge in evaluating this question has been how to account for the two differing mechanisms of decline, either a “benign“ medication-related decline due to the effect of ACEI treatment or a decline that is non ACEI-driven. When the medication-related eGFR decline is not taken into consideration, the inappropriate comparison group will be selected, leading to risk for bias and misguided interpretation of the risk or benefit of the ACEI in the setting of a decline in eGFR. Using the SOLVD trials, we sought to examine the effect of enalapril in relation to varying degrees of eGFR declines with mortality and HF hospitalizations using analyses to address these biases by providing both a magnified as well as conservative estimates of the benefit of enalapril as well an intermediate analysis.
Results
Using the 2-week follow-up time point, a total of 6,245 participants were included in this analysis with a median follow-up of 2.8 years, maximum of 5.2 years. Mean (SD) age was 59.4 (10.2) years, 14% were women and 10.7% were black. Mean (SD) baseline eGFR was 73.4 (19.3) ml/min/1.73 m2.
Baseline Patient Characteristics
Baseline demographics and comorbidities according to categories of %eGFR change at 2-weeks are described in Table 1. In comparing across the groups, participants with greater declines in %eGFR were more likely to be women (p<0.001), have diabetes (p=0.009), hypertension (p=0.011), and NYHA Class III/IV (p<0.001), and use diuretics (p<0.001). Patients with >20% %eGFR decline started at a higher mean (SD) baseline eGFR of 78.0 (21.5) ml/min/1.73 m2 while those who had an improvement in eGFR had a lower mean (SD) baseline eGFR of 65.5 (16.5) ml/min/1.73 m2. Although there was a slightly greater frequency of patients randomized to enalapril among those with greater degrees of decline (51.8% among %eGFR decline >20% as opposed to 47.2% among those with %eGFR increase), there was fairly equal representation of both randomization arms among all categories of %eGFR change.
Table 1.
Baseline Characteristics according to magnitude of eGFR change at 2-week time point after randomization.
| Characteristic | Percent Change in estimated Glomerular Filtration Rate | |||||
|---|---|---|---|---|---|---|
| All | Increase of >5% | Stable −5% to 5% | Decrease of 5% to 20% | Decrease of > 20% | P* | |
| (n = 6245) | (n = 1947) | (n = 1770) | (n = 1928) | (n = 600) | ||
| Age – years | 59.4 ± 10.2 | 59.4 ± 10.3 | 58.4 ± 10.4 | 59.9 ± 9.8 | 61.0 ± 10.0 | <0.001 |
| Female | 891 (14.3) | 269 (13.8) | 243 (13.7) | 240 (12.5) | 139 (23.2) | <0.001 |
| Black | 666 (10.7) | 225 (11.6) | 148 (8.4) | 197 (10.2) | 96 (16.0) | <0.001 |
| Baseline eGFR – ml/min/1.73 m2 | 73.4 ± 19.3 | 65.5 ± 16.6 | 77.7 ± 19.1 | 75.9 ± 19.0 | 78.0 ± 21.5 | <0.001 |
| Diabetes | 1199 (19.2) | 371 (19.1) | 304 (17.2) | 385 (20.0) | 139 (23.2) | 0.009 |
| Hypertension | 2400 (38.4) | 751 (38.6) | 636 (35.9) | 753 (39.1) | 260 (43.3) | 0.011 |
| Ischemia | 4946 (79.3) | 1515 (77.9) | 1429 (80.8) | 1537 (79.8) | 465 (77.6) | 0.102 |
| NYHA Class III/IV | 815 (13.1) | 255 (13.1) | 194 (11.0) | 262 (13.6) | 104 (17.3) | <0.001 |
| Hematocrit– % | 42.7 ± 4.5 | 42.7 ± 4.7 | 42.9 ± 4.5 | 42.6 ± 4.5 | 41.6 ± 4.6 | <0.001 |
| Potassium – mEq/L | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.5 | 0.007 |
| Previous MI | 4703 (75.4) | 1438 (73.9) | 1364 (77.2) | 1472 (76.4) | 429 (71.6) | 0.011 |
| Smoking, current | 1399 (22.4) | 422 (21.7) | 420 (23.8) | 442 (23.0) | 115 (19.2) | 0.095 |
| Diuretic | 2642 (42.3) | 832 (42.8) | 652 (36.8) | 838 (43.5) | 320 (53.3) | <0.001 |
| Prevention Trial | 3865 (61.9) | 1215 (62.4) | 1148 (64.9) | 1187 (61.6) | 315 (52.5) | <0.001 |
| Enalapril Arm | 3085 (49.4) | 918 (47.2) | 859 (48.5) | 997 (51.7) | 311 (51.8) | 0.018 |
values indicate mean ± SD or n (%). GFR estimated using CKD-EPI formula
comparisons performed using analysis of variance or Kruskal-Wallis, or χ2 testing as appropriate
NYHA, New York Heart Association; MI, myocardial infarction
Outcomes in Relation to eGFR Decline
Over the course of follow-up, 1486 (23.8%) patients died. There were 1321 (21.2%) attributed to cardiovascular causes, and 1222 (19.6%) hospitalizations due to HF. Among those with >20% decline in %eGFR at 2-weeks, there were 182 (30.3%) deaths, among which 165 were attributed to cardiovascular causes, and there were 161 (26.8%) HF hospitalizations. In contrast, among those with stable eGFR (<5% decline and <5% increase), there were 378 (21.4%) deaths, 346 (19.6%) cardiovascular deaths, and 299 (16.9%) HF hospitalizations.
Assuming 0% of Decline in the Enalapril Group was due to Medication Effect
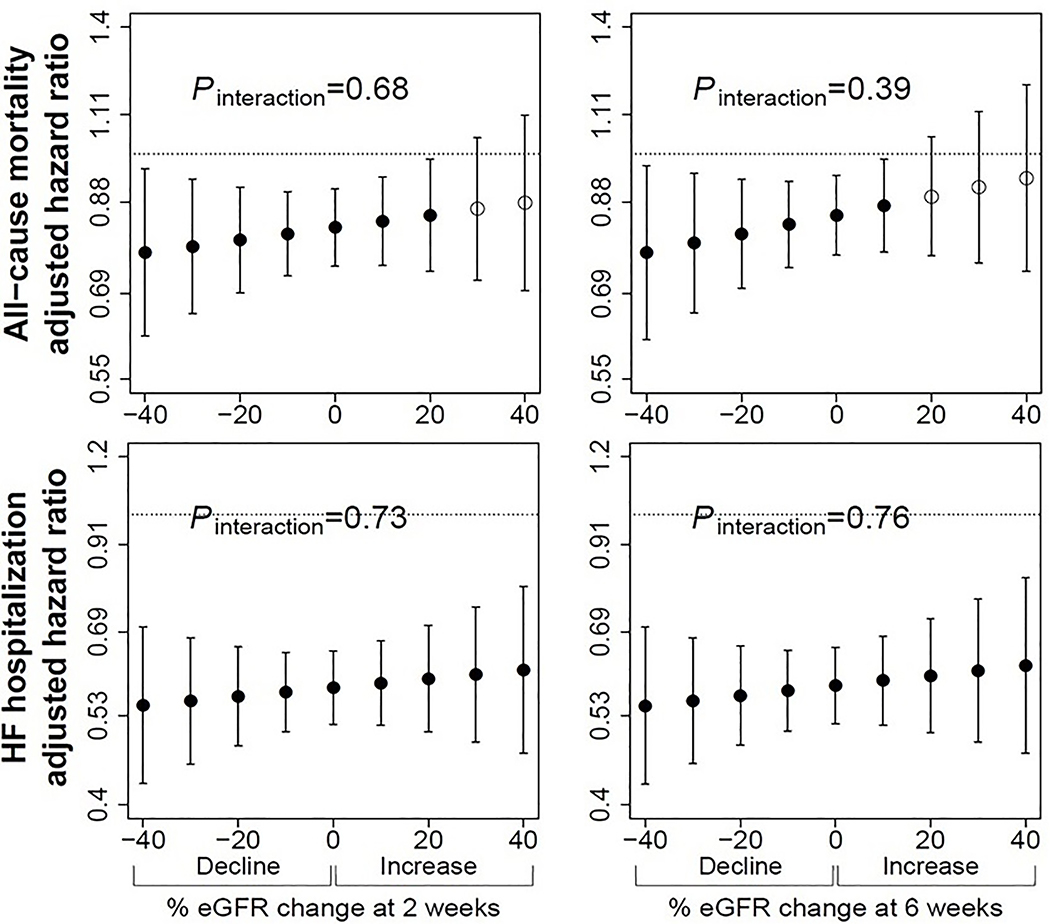
Making the assumption that none of the %eGFR decline was due to medication effect (Figure 1, point a), enalapril was associated with lower hazard for all-cause mortality than placebo at all levels of %eGFR change when using equivalent %eGFR decline as the reference (Figure 2). For a 30% eGFR decline at 2-weeks, the HR for all-cause mortality for enalapril was 0.78 (95% CI 0.65,0.94) (Table 2) with similar results for cardiovascular death (Supplemental Table S1). Using the 6-week time-point, there were similar findings of mortality benefit associated with enalapril at all levels of %eGFR change (Figure 2 and Supplemental Figure S1). A decline of 30% in the enalapril arm was significantly associated with a lower hazard of all-cause mortality when compared to 30% decline in the placebo arm (HR=0.79, 95% CI 0.66, 0.95) (Table 2) with similar results for cardiovascular death (Supplemental Table S2).
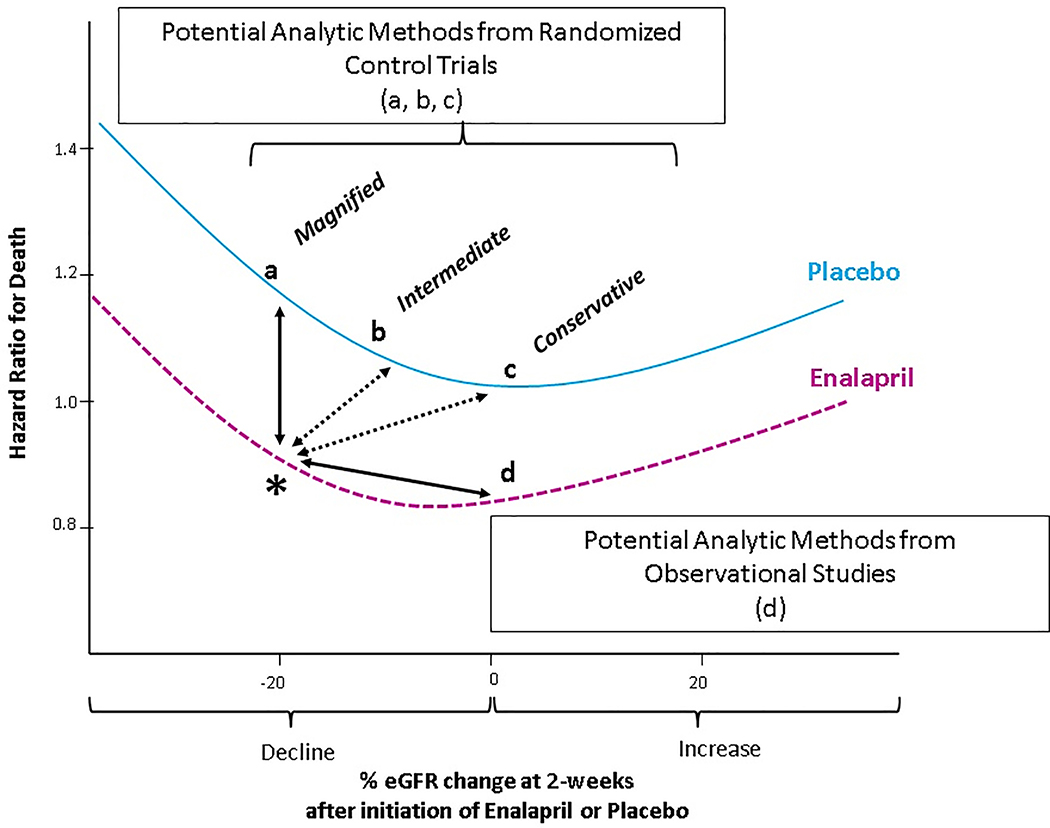
Figure 1. Representation of the potential different reference groups (a, b, c, d) for ascertainment of the hazard ratio for death for a 20% decline in eGFR (*).
Hazard ratio curves for varying changes in eGFR at 2-weeks after randomization to placebo and treatment were estimated by using restricted cubic spline modeling. The hazard ratio for death for a decline of 20% in eGFR at 2-weeks after randomization to enalapril (represented by the asterisk) could differ depending on the reference group,, with options a,b,c as potential reference groups drawn from randomized studies and option d as potentially drawn from observational studies. Option (a) as employed in much of the prior literature,15,20 would be a placebo patient with 20% eGFR decline, under the assumption that none of the decline on enalapril was due to hemodynamic decline; option (b) would be a placebo patient with 10% eGFR decline under the assumption that half of the decline on enalapril was due to hemodynamic effect; option (c) would be a placebo patient with 0% eGFR decline under the assumption that all of the decline on enalapril was due to hemodynamic effect. Option (d) would be an enalapril patient with 0% eGFR decline, as done in prior observational studies.19 HR indicates hazard ratio; eGFR indicates estimated glomerular filtration rate.
Figure 2. Multivariable adjusted hazard ratios for all-cause mortality and heart failure hospitalization for those randomized to enalapril versus placebo according to magnitude of decline at 2-weeks (left), and 6-weeks (right).
Filled circles represent points at which there was a significant hazard ratio for enalapril group versus placebo. There was no significant interaction between decline and treatment at any time point. eGFR indicates estimated glomerular filtration rate, calculated using adjusted serum creatinine and CKD-EPI equation. Pinteraction indicates p-value for interaction. Number of patients at each follow-up time point were 6245 at 2-weeks, and 6055 at 6-weeks. Models adjusted for age, sex, race, baseline kidney function, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, hematocrit, potassium, and trial.
Table 2.
Adjusted hazard ratio for all-cause mortality based on decline in estimated glomerular filtration rate at 2- and 6-week time point following randomization, using 3 different levels of hemodynamic effect of enalapril.
| eGFR Decline | 0% OF DECLINE IN ENALAPRIL GROUP DUE TO HEMODYNAMIC EFFECT Magnified Estimate |
100% OF DECLINE IN ENALAPRIL GROUP TO HEMODYNAMIC EFFECT Conservative Estimate |
50% OF DECLINE IN ENALPRIL GROUP DUE TO HEMODYNAMIC EFFECT Intermediate Estimate |
|||
|---|---|---|---|---|---|---|
| Reference (% Decline with Placebo) |
HR (95% CI) | Reference (% Decline with Placebo) |
HR (95% CI) | Reference (% Decline with Placebo) |
HR (95% CI) | |
| at 2-Weeks | ||||||
| 5% | 5% | 0.82 (0.73, 0.91) | 0% | 0.84 (0.75, 0.94) | 2.5% | 0.83 (0.75, 0.92) |
| 10% | 10% | 0.81 (0.72, 0.90) | 0% | 0.87 (0.77, 0.99) | 5% | 0.84 (0.75, 0.94) |
| 15% | 15% | 0.80 (0.71, 0.91) | 0% | 0.91 (0.79, 1.04) | 7.5% | 0.86 (0.77, 0.97) |
| 20% | 20% | 0.80 (0.69, 0.92) | 0% | 0.95 (0.82, 1.10) | 10% | 0.88 (0.77, 1.01) |
| 25% | 25% | 0.79 (0.67, 0.92) | 0% | 1.00 (0.85, 1.17) | 12.5% | 0.91 (0.78, 1.06) |
| 30% | 30% | 0.78 (0.65, 0.94) | 0% | 1.05 (0.88, 1.26) | 15% | 0.93 (0.78, 1.11) |
| 35% | 35% | 0.78 (0.64, 0.95) | 0% | 1.11 (0.90, 1.36) | 17.5% | 0.95 (0.77, 1.17) |
| 40% | 40% | 0.77 (0.62, 0.96) | 0% | 1.16 (0.91, 1.48) | 20% | 0.97 (0.77, 1.24) |
| at 6-Weeks | ||||||
| 5% | 5% | 0.84 (0.75, 0.93) | 0% | 0.87 (0.78, 0.98) | 2.5% | 0.86 (0.77, 0.96) |
| 10% | 10% | 0.83 (0.74, 0.93 | 0% | 0.90 (0.79, 1.03) | 5% | 0.86 (0.77, 0.97) |
| 15% | 15% | 0.82 (0.72, 0.93) | 0% | 0.92 (0.80, 1.06) | 7.5% | 0.87 (0.77, 0.98) |
| 20% | 20% | 0.81 (0.70, 0.93) | 0% | 0.94 (0.81, 1.09) | 10% | 0.87 (0.76, 0.99) |
| 25% | 25% | 0.80 (0.68, 0.94) | 0% | 0.95 (0.82, 1.11) | 12.5% | 0.87 (0.75, 1.01) |
| 30% | 30% | 0.79 (0.66, 0.95) | 0% | 0.97 (0.82, 1.15) | 15% | 0.87 (0.73, 1.03) |
| 35% | 35% | 0.78 (0.63, 0.96) | 0% | 0.99 (0.81, 1.21) | 17.5 | 0.87 (0.71, 1.06) |
| 40% | 40% | 0.77 (0.61, 0.97) | 0% | 1.01 (0.80, 1.27) | 20% | 0.87 (0.69, 1.10) |
Cox proportional hazard regression models used to evaluate association between enalapril and all-cause mortality at varying levels of %eGFR decline at 2- and 6-weeks after randomization. Analysis performed using 3 different levels of hemodynamic decline from enalapril itself: (1) assuming none (0%) of eGFR decline in the enalapril group was due to hemodynamic effect and thus the reference group comprised placebo patients with equal %eGFR decline; (2) assuming all (100%) of the eGFR decline in the enalapril group was due to hemodynamic effect and thus the reference group comprised placebo patients with 0% eGFR decline; (3) assuming that half (50%) of the eGFR decline in the enalapril group was due to hemodynamic effect and thus the reference group comprised placebo patients with half %eGFR decline. Models adjusted for age, sex, race, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, baseline eGFR, potassium, hematocrit, and trial (Prevention or Treatment).
In terms of HF hospitalization, randomization to enalapril was associated with significantly lower risk regardless of the degree of eGFR decline at 2-weeks (Figure 2). For a 30% decline at 2-weeks, the HR for HF hospitalizations for enalapril was 0.56 (95% CI 0.46, 0.68), with similar results across all levels of eGFR decline at 2-weeks (Table 3). At 6-weeks, results were similar with at least a 45% risk reduction at all levels of eGFR decline in comparison to placebo (Table 3).
Table 3.
Adjusted hazard ratio for hospitalizations due to heart failure based on decline in estimated glomerular filtration rate at 2- and 6-week time point following randomization, using 3 different levels of hemodynamic effect of enalapril.
| eGFR Decline | 0% OF DECLINE IN ENALAPRIL GROUP DUE TO HEMODYNAMIC EFFECT Magnified Estimate |
100% OF DECLINE IN ENALAPRIL GROUP TO HEMODYNAMIC EFFECT Conservative Estimate |
50% OF DECLINE IN ENALPRIL GROUP DUE TO HEMODYNAMIC EFFECT Intermediate Estimate |
|||
|---|---|---|---|---|---|---|
| Reference (% Decline with Placebo) |
HR (95% CI) | Reference (% Decline with Placebo) |
HR (95% CI) | Reference (% Decline with Placebo) |
HR (95% CI) | |
| at 2-Weeks | ||||||
| 5% | 5% | 0.57 (0.51, 0.65) | 0% | 0.60 (0.53, 0.68) | 2.5% | 0.59 (0.52, 0.66) |
| 10% | 10% | 0.57 (0.50, 0.65) | 0% | 0.62 (0.54, 0.72) | 5% | 0.60 (0.53, 0.68) |
| 15% | 15% | 0.57 (0.49, 0.65) | 0% | 0.65 (0.55, 0.76) | 7.5% | 0.61 (0.53, 0.70) |
| 20% | 20% | 0.56 (0.48, 0.66) | 0% | 0.68 (0.58, 0.80) | 10% | 0.62 (0.54, 0.72) |
| 25% | 25% | 0.56 (0.47, 0.67) | 0% | 0.71 (0.59, 0.85) | 12.5% | 0.63 (0.53, 0.75) |
| 30% | 30% | 0.56 (0.46, 0.68) | 0% | 0.74 (0.61, 0.91) | 15% | 0.65 (0.53, 0.79) |
| 35% | 35% | 0.55 (0.44, 0.69) | 0% | 0.78 (0.61, 0.98) | 17.5% | 0.66 (0.52, 0.84) |
| 40% | 40% | 0.55 (0.43, 0.70) | 0% | 0.81 (0.62, 1.07) | 20% | 0.67 (0.51, 0.88) |
| at 6-Weeks | ||||||
| 5% | 5% | 0.58 (0.51, 0.65) | 0% | 0.62 (0.55, 0.71) | 2.5% | 0.60 (0.53, 0.68) |
| 10% | 10% | 0.57 (0.51, 0.65) | 0% | 0.67 (0.58, 0.77) | 5% | 0.62 (0.54, 0.70) |
| 15% | 15% | 0.57 (0.49, 0.65) | 0% | 0.71 (0.60, 0.83) | 7.5% | 0.63 (0.55, 0.72) |
| 20% | 20% | 0.56 (0.48, 0.66) | 0% | 0.74 (0.63, 0.87) | 10% | 0.63 (0.55, 0.73) |
| 25% | 25% | 0.56 (0.47, 0.67) | 0% | 0.77 (0.64, 0.91) | 12.5% | 0.64 (0.54, 0.75) |
| 30% | 30% | 0.56 (0.46, 0.68) | 0% | 0.79 (0.65, 0.96) | 15% | 0.64 (0.53, 0.78) |
| 35% | 35% | 0.55 (0.44, 0.69) | 0% | 0.82 (0.66, 1.02) | 17.5 | 0.65 (0.51, 0.81) |
| 40% | 40% | 0.55 (0.43, 0.70) | 0% | 0.85 (0.66, 1.10) | 20% | 0.65 (0.50, 0.84) |
Cox proportional hazard regression models used to evaluate association between enalapril and heart failure hospitalizations at varying levels of %eGFR decline at 2- and 6-weeks after randomization. Analysis performed using 3 different levels of hemodynamic decline from enalapril itself: (1) assuming none (0%) of eGFR decline in the enalapril group was due to hemodynamic effect and thus the reference group comprised placebo patients with equal %eGFR decline; (2) assuming all (100%) of the eGFR decline in the enalapril group was due to hemodynamic effect and thus the reference group comprised placebo patients with 0% eGFR decline; (3) assuming that half (50%) of the eGFR decline in the enalapril group was due to hemodynamic effect and thus the reference group comprised placebo patients with half %eGFR decline. Models adjusted for age, sex, race, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, baseline eGFR, potassium, hematocrit, and trial (Prevention or Treatment).
The statistical test of interaction between %eGFR change and treatment assignment was not significant at either time-point for all-cause mortality (p of interaction: 0.68 at 2-weeks, 0.39 at 6-weeks). For HF hospitalization, test of interaction also did not reach statistical significance (p of interaction: 0.73 at 2-weeks, 0.76 at 6-weeks) (Figure 2).
Assuming 100% of Decline in the Enalapril Group was due to Medication Effect
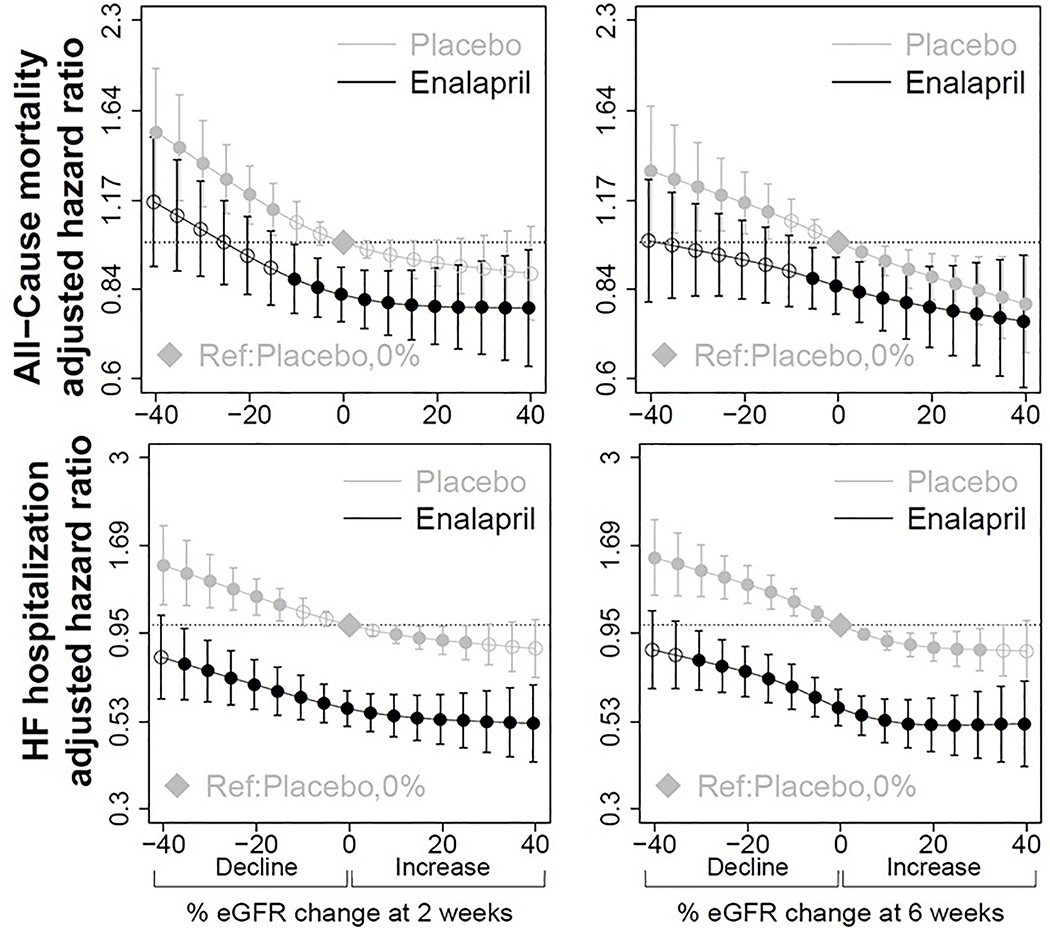
Making the assumption that all of the %eGFR decline with enalapril was due to medication (Figure 1, point c), a much smaller magnitude of decline was significantly associated with a lower hazard of all-cause mortality and cardiovascular mortality. For all-cause mortality, any degree of decline with enalapril was still better than decline on placebo (Figure 3) but was only significantly associated with lower hazards at a decline of up to 10% in reference to 0% on placebo (HR=0.87, 95% CI 0.77,0.99) using the 2-week time-point. Similar results were seen for cardiovascular mortality (Supplemental Table S1 and Figure S1). When using the 6-week time-point, a 5% decline on enalapril had a HR=0.87 (95% CI 0.78, 0.98) for all-cause mortality and a 10% decline was still less than 1 but was not statistically significant (HR=0.90, 95% CI 0.79, 1.03) (Table 2), with similar results for cardiovascular mortality (Supplemental Table S1).
Figure 3. Multivariable hazard ratios for all-cause mortality and heart failure hospitalization for those randomized to enalapril or placebo according to magnitude of decline at 2-weeks (left), and 6-weeks (right).
Placebo, 0% eGFR held as reference point (represented by diamond). Filled circles represent points where hazard ratio for all-cause mortality is significant (p<0.05) compared to the reference point. eGFR indicates estimated glomerular filtration rate, calculated using adjusted serum creatinine and CKD-EPI equation. Number of patients at each follow-up time point were 6245 at 2-weeks, and 6055 at 6-weeks. Models adjusted for age, sex, race, baseline kidney function, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, hematocrit, potassium, and trial.
For HF hospitalization, randomization to enalapril was associated with decreased hazard of hospitalization at all levels of eGFR decline at 2-weeks, reaching statistical significance up to a 35% decline in eGFR [HR 0.78 (95% 0.61, 0.98)] (Table 3). Using the 6-week time point, up to a 30% decline in eGFR on enalapril was associated with decreased HF hospitalizations [HR=0.79 (95% CI 0.65, 0.96)] (Table 3).
Assuming 50% of Decline in the Enalapril Group was due to Medication Effect
Making an assumption that half of the %eGFR decline on enalapril was a hemodynamic effect due to medication itself (Figure 1, point b), the hazard ratio for all-cause mortality remained less than 1 at all levels of decline. At 2-weeks, declines of up to 15% with enalapril were associated with a significantly lower hazard for mortality (HR=0.86, 95% CI 0.77, 0.97). At a decline of 20% with enalapril, the hazard remained below 1 but no longer significant (HR=0.88, 95% CI 0.77, 1.01) (Table 2). Even declines of 40% still had no increase in the hazard for all-cause mortality (HR=0.97, 95% CI 0.77, 1.24). Similar results were seen for the outcome of cardiovascular mortality (Supplemental Table S1). When using the 6-week time-point, a 20% decline on enalapril was associated with a 13% reduction in risk for all-cause mortality (HR=0.87, 95% CI 0.76, 0.99) and declines beyond that were still associated with HR’s than 1 but not statistically significant (Table 2). Similar results were observed for cardiovascular mortality (Supplemental Table S1).
In terms of HF hospitalization, all levels of eGFR decline at 2-weeks on enalapril were significantly associated with reduced risk (Table 3). Even a 40% eGFR decline at 2-weeks was significantly associated with a HR=0.67 (95% CI 0.51, 0.88), and a 40% decline at 6-weeks was associated with HR=0.65 (95% CI 0.50, 0.84).
In Reference to 0% Change with Enalapril
Declines in eGFR after randomization to enalapril, when compared in reference to 0% change on enalapril (Figure 1, point d), were associated with slightly higher risks of all three outcomes. For all-cause mortality, a 15% decline at 2-weeks after starting enalapril was associated with significantly higher risk (HR=1.15, 95% CI 1.00, 1.32) with similar results seen for cardiovascular mortality (Supplemental Table S1). For HF hospitalization, a similar pattern emerged where mild declines were not significantly associated with increased risk, but declines beyond 25% at 2-weeks were associated with significantly increased risk of HF hospitalization (Table 4). When evaluating decline at the 6-week time point, the hazard for all three outcomes was greater than 1, but none of the estimates reached statistical significance for any of the three outcomes (Table 4).
Table 4.
Adjusted hazard ratio for all-cause mortality, cardiovascular mortality, and heart failure hospitalization based on decline in estimated glomerular filtration rate at 2- and 6-weeks, limited to the enalapril arm
| eGFR Decline | Reference (% Decline with Enalapril) | All-Cause Mortality HR (95% CI) | Cardiovascular Mortality HR (95% CI) | Heart Failure Hospitalization HR (95%) |
|---|---|---|---|---|
| At 2 weeks | ||||
| 5% | 0% | 1.06 (0.99, 1.12) | 1.06 (0.99, 1.13) | 1.02 (0.95, 1.10) |
| 10% | 0% | 1.11 (0.99, 1.24) | 1.12 (0.99, 1.27) | 1.05 (0.92, 1.21) |
| 15% | 0% | 1.15 (1.00, 1.32) | 1.17 (1.01, 1.35) | 1.09 (0.93, 1.29) |
| 20% | 0% | 1.18 (1.02, 1.36) | 1.20 (1.03, 1.40) | 1.14 (0.96, 1.36) |
| 25% | 0% | 1.20 (1.02, 1.41) | 1.23 (1.04, 1.46) | 1.20 (1.00, 1.45) |
| 30% | 0% | 1.21 (0.99, 1.48) | 1.26 (1.02, 1.55) | 1.27 (1.01, 1.58) |
| 35% | 0% | 1.23 (0.96, 1.57) | 1.29 (0.99, 1.67) | 1.33 (1.01, 1.75) |
| 40% | 0% | 1.24 (0.92, 1.68) | 1.31 (0.96, 1.80) | 1.40 (1.01, 1.94) |
| At 6 weeks | ||||
| 5% | 0% | 1.04 (0.98, 1.11) | 1.05 (0.98, 1.12) | 0.99 (0.92, 1.06) |
| 10% | 0% | 1.08 (0.96, 1.21) | 1.09 (0.96, 1.24) | 0.98 (0.85, 1.12) |
| 15% | 0% | 1.11 (0.96, 1.28) | 1.12 (0.96, 1.31) | 0.99 (0.83, 1.18) |
| 20% | 0% | 1.12 (0.96, 1.31) | 1.14 (0.96, 1.34) | 1.03 (0.85, 1.24) |
| 25% | 0% | 1.13 (0.96, 1.33) | 1.15 (0.96, 1.37) | 1.08 (0.89, 1.31) |
| 30% | 0% | 1.13 (0.94, 1.36) | 1.15 (0.95, 1.41) | 1.15 (0.93, 1.42) |
| 35% | 0% | 1.13 (0.90, 1.41) | 1.16 (0.91, 1.46) | 1.23 (0.96, 1.57) |
| 40% | 0% | 1.13 (0.86, 1.48) | 1.16 (0.88, 1.54) | 1.31 (0.98, 1.75) |
Analysis performed using Cox proportional hazard regression models with 0% decline on enalapril as the reference. Models adjusted for age, sex, race, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, baseline eGFR, potassium, hematocrit, and trial (Prevention or Treatment).
Discussion
The results of this study suggest that there is a decreased hazard of all-cause mortality as well as cardiovascular mortality and HF hospitalization over median 2.8-year follow-up with enalapril at all levels of eGFR decline in patients with HFrEF, without any evidence of effect modification by eGFR decline. We performed several different analyses to best identify the threshold level of eGFR decline in the treatment group where benefit still occurred. In the most magnified estimate, where none of the decline in the enalapril group was assumed due to medication effects, enalapril was associated with reduced risk for all three outcomes for any degree of eGFR decline. For the most conservative estimate, where all of the decline was assumed due to medication-driven effects, up to a 10% decline with enalapril was associated with significantly decreased risk of death and up to a 35% decline was associated with significantly reduced risk of HF hospitalization.. Lastly, in the intermediate estimate, where half of the decline was assumed medication-driven, an eGFR decline of 15% was associated with significant lower risk of mortality and all levels of decline up to 40% were associated with lower risk of HF hospitalization. Overall, in all analyses, enalapril was never associated with a significantly higher risk of mortality over the course of follow-up regardless of degree of eGFR decline.
It has been argued among the general non-HFrEF population that up to a 30% eGFR decline after initiation of ACEI/ARB can be tolerated.16,17 The European Society of Cardiology guidelines suggest that creatinine increases of up to 50% for patients with HFrEF after ACEI/ARB should be tolerated.18 However, evaluating whether these cut-offs are appropriate has not been rigorously studied and is challenging to evaluate because of biases introduced in prior analyses. There has been selection of several different comparison groups in prior analyses, as described in Figure 1. When addressing this question in the context of an observational study, the reference group includes patients started on ACEI/ARB who do not have a decline in comparison to patients who did have a decline after initiation of ACEI/ARB therapy. One large highly publicized study examined 122,363 patients who were started on ACEI/ARB treatment and found that an increase in serum creatinine by ≥30% detected within 2 months was associated with an increased odds of dying (adjusted incidence rate ratio of 1.84 for death [95% CI of 1.65, 2.05]) compared to those whose creatinine rose by <30% after initiation of ACEI/ARB.19 When performing this comparison in our models, we also found a trend toward worse outcomes among those with eGFR decline after randomization to enalapril when compared to those without any change in eGFR. The concern is that the higher rate of decline may be due to absence of autoregulation or the presence of vascular disease that results in adverse outcomes, rather than the medication effect itself. In the observational nature of these comparisons, statistical analyses are unable to sufficiently adjust for this residual confounding. When addressing this question using randomized controlled trial data, comparisons have been made between individuals who suffer a certain degree of decline with ACEI/ARB therapy versus the same degree of decline with placebo. One meta-analysis examined acute declines in eGFR among patients with reduced systolic function enrolled into five randomized controlled trials of RAAS-blocking agents, and found that among those with an acute decline in kidney function, randomization to RAAS inhibition was associated with a lower risk of mortality (HR 0.72 [95% CI of 0.62, 0.84]) than placebo patients with a similar degree of decline.20 A prior analysis of the SOLVD trial, also comparing those with an eGFR decline of ≥20% after enalapril in reference to patients with ≥20% eGFR decline in the placebo arm, found a similar benefit with enalapril despite the decline in eGFR.15 In the randomized trials, there is, however, a risk of over-estimation of the survival benefit of ACEI/ARB therapy. A similar degree of decline in the placebo arm compared to the treatment arm exaggerates the benefit of the medication because decline in the treatment arm likely reflects a combination of medication-driven effect as well as progression-related effect; while a similar magnitude of decline in the control group only reflects a progression-related effect and is thereby a sicker reference population. We sought to address these biases by incorporating assumptions of levels of medication-driven decline due to the enalapril itself, in order to provide both magnified and conservative estimates of the benefit from enalapril, and also an intermediate analysis which is most likely the closest estimate using the available data.
The current analysis found that when examined across randomized groups, there was a decreased risk for mortality among those randomized to enalapril at all levels of eGFR decline with no evidence of interaction between treatment group and decline. Additionally, we modelled the data in two alternative ways to attempt to evaluate how the hazards for mortality may shift according to three different assumptions regarding the cause of eGFR decline. The estimate was magnified when assuming that none of the eGFR decline in the enalapril group was due to medication-related effects; regardless of the degree of decline, there was always a significant benefit with enalapril. The most conservative estimate was when all of the eGFR decline in the enalapril group was presumed due to medication; the mortality benefit of enalapril was significant only at 10% eGFR decline. We believe that an assumption in between the above two scenarios is likely most accurate, that is, there is partial contribution to the decline from enalapril and partial contribution from non enalapril-related factors contributing to progression. Under this assumption, a 15% decline in the enalapril arm was still significantly beneficial. In addition, higher levels of decline were not associated with harm and the hazard ratio always remained below 1 despite no longer significant.
Regarding heart failure hospitalization outcomes, we observed strong associations between randomization to enalapril and decreased risk for HF hospitalizations. The beneficial effect of enalapril was observed at much greater levels of eGFR decline than were observed for mortality. That is, eGFR declines of up to 40% after randomization to enalapril were associated with significant reduction in risk for HF hospitalization. We believe this additional benefit irrespective of GFR decline may be related to enalapril’s direct effect on improving cardiac remodeling,4–6 leading to less frequent development and progression of HFrEF. The clinical implication of this study is that among patients with HFrEF, RAAS inhibition is beneficial for both survival and reduction in hospitalization for HF, and these results provide support for not discontinuing these medications despite acute eGFR declines. We observed declines of up to 10–15% with enalapril being significantly associated with lower risk of death. For declines greater than 15%, we cannot demonstrate statistical significance of the mortality benefit, but even declines of 40% do not appear to be significantly associated with higher risk of all-cause mortality. Declines of up to 30–40% on enalapril were however associated with reduced risk of HF hospitalization, an important outcome in and of itself, given its tremendous morbidity, as well as association with mortality and costs.21.In combination with the randomized comparisons and other data which suggest that ACEI/ARB have further benefits besides overall survival, such as cardiac remodeling, clinicians should have compelling reasons besides moderate eGFR decline to withdraw these medications.
Strengths and Limitations
There are several strengths to this analysis. It was drawn from a landmark, rigorously conducted randomized controlled trial. Kidney function was measured based on protocol-driven intervals. Our analyses included both the 2-week and 6-week follow-up time points, as drug doses were still being titrated at 2-weeks and relying on the 2-week time point alone as done in prior studies may not be sufficient to appreciate the full medication-related effect. Lastly, our two additional analyses incorporated two unique reference groups that attempt to limit bias by incorporating varying assumptions of differing mechanisms of eGFR decline. As for limitations, patients with a serum creatinine of > 2.5 mg/dl were excluded from the SOLVD trials and thus applying these findings to patients with more advanced CKD may not be appropriate. Dosing of enalapril was encouraged to be maximized in all patients, but without formal protocol regarding incorporation of results of kidney function testing into dosage decisions, it is possible that some clinicians were modifying doses based on changes of serum creatinine while others were not. There is no quantification of hemodynamic parameters such as cardiac output or peripheral resistance as well as congestion parameters, which may influence the response of the kidney to introduction of enalapril. For one of our analyses, the randomization schema of the original trial was maintained, but in other analyses, randomization was not maintained and therefore our results may be prone to other biases such as residual confounding.
Conclusions
In patients with HFrEF, enalapril decreases the risk of mortality and HF hospitalizations when compared with placebo. A moderate eGFR decline is tolerable after introduction of enalapril and clinicians should have compelling reasons beyond these acute declines to withdraw this beneficial class of medications.
Methods
Study Population
The SOLVD studies were two National Heart, Lung and Blood Institute sponsored multicenter, double-blind, randomized controlled trials that evaluated the effect of the ACEI enalapril versus placebo on mortality and cardiovascular outcomes in patients with HFrEF.7,8 Participants with symptomatic heart failure were enrolled in the Treatment Trial, while those without symptoms were enrolled into the Prevention Trial. For the present analysis, patients from the two trials were combined. Exclusion criteria included a serum creatinine >2.5 mg/dl, age >80 years, uncontrollable hypertension, suspected renal artery stenosis, unstable angina, myocardial infarction in the past month, or severe pulmonary disease. The intervention (enalapril) was initiated at 5mg twice daily at randomization and then uptitrated to 10mg twice daily as tolerated at the 2-week follow-up visit post-randomization. Uptitration of enalapril dosing was encouraged unless participants reported dizziness or fainting. Serum creatinine was measured at the time of trial entry, at 2- and 6-weeks post-randomization, and then annually thereafter. The primary end point for the trials was all-cause mortality over the 3–5-year follow-up period of SOLVD, and secondary outcomes included cardiovascular death and HF hospitalizations. Patients without baseline and/or any follow-up serum creatinine measurements were excluded from the present analysis.
Exposure
Kidney function, as reflected by GFR, was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) calculation using measured serum creatinine.22 Serum creatinine values were lowered by 5% as is usual practice for values measured prior to standardization to isotope dilution mass spectometry.23 The exposure of interest was percent change in eGFR (%eGFR) calculated as 100 × (follow-up eGFR – baseline eGFR)/baseline eGFR, where follow-up eGFR was assessed at 2- and 6-weeks post randomization. These two time points were established in the original SOLVD trials as safety measurements following initiation of study drug, and followed usual clinical practice. Both time points were included, since ACEI dose titration continued during the 2-week follow-up visit and thereby the 2-week eGFR may not thoroughly capture the hemodynamic medication-driven changes. The %eGFR change was modeled as a continuous variable. For ease of interpretation of baseline characteristics, %eGFR change was treated as a categorical variable: decrease by >20%, decrease by 5% to 20%, change between −5% to 5%, and increase by >5%. These cutpoints were chosen on the basis of the prior literature that has examined acute changes in eGFR following either RAAS inhibition or blood pressure lowering.15,19,20
Outcome
In keeping with the outcomes from the SOLVD trials, the primary endpoints of interest of this analysis were all-cause mortalityand first hospitalization for HF, as well ascardiovascular mortality. Cause of each patient’s death was determined by the principal investigator at each center on the basis of blinded review. Data for hospitalizations for HF were based on the primary diagnosis at discharge. The time at risk for each endpoint began at the time of the creatinine measurement for each time point until the administrative close of each study (January 31st, 1991 for the Treatment Trial, August 31st, 1991 for the Prevention Trial). Patients who died or were censored between randomization and the time point of interest (2-weeks or 6-weeks) were excluded from each respective analysis.
Covariates
Based on review of the literature and clinical relevance, several covariates were selected for analysis as potential confounding variables in our regression analyses. These included patient demographics (age, sex, race), cardiovascular characteristics (New York Heart Association functional class, ischemic etiology of left ventricular dysfunction, prior myocardial infarction, current smoking, diastolic blood pressure), laboratory findings at the time of study entry (hematocrit, potassium), as well as trial participation (Treatment Trial or Prevention Trial).
Statistical Analysis
We used descriptive statistics to compare the characteristics of patients across categories of %eGFR change, using analysis of variance and the Kruskal-Wallis test as appropriate. Categorical variables were compared using the χ2 test and Fisher’s exact test as appropriate.
Multivariable Cox proportional hazards regression models were used to evaluate the association between %eGFR change based randomization to enalapril or placebo and each outcome. Models were adjusted for the covariates described above. The %eGFR change was entered into the Cox model and given its non-linear log hazards curve, restricted cubic splines with 4 knots using the 5%, 35%, 65% and 95% percentiles of %eGFR change distribution were incorporated. To explore effect modification by treatment assignment, an interaction term was included between treatment (enalapril vs placebo) and the linear component of the restricted cubic spline of %eGFR change.
Because there is no consensus in determining what proportion of eGFR decline can be attributable to medication-driven effect versus non-medication-driven progression of kidney function decline, we further used our regression model to calculate the hazard ratio for each outcome for combinations of %eGFR decline in the enalapril arm against four different reference groups (see Figure 1). Calculations were made using 3 different levels of medication-related decline from enalapril itself: (1) assuming that none (0%) of the eGFR decline in the enalapril group was due to medication effect, allowing use of placebo arm patients with equivalent %eGFR decline as the reference group. This would be the most magnified view of the estimate for the benefit of enalapril and from hereon will be referred as magnified view. (2) Assuming all (100%) of the eGFR decline in the enalapril group was due to its medication effect, which made the reference group comprise placebo patients with 0% eGFR change. This is synonymous to the most conservative view of the estimate for the benefit of enalapril. And (3) assuming that half (50%) of the eGFR decline in the enalapril group was due to its medication effect and the reference group consisted of patients in the placebo arm with half %eGFR decline. Arguably, this third intermediate assumption may be the most reasonable estimate given the spectrum of efferent arteriole response to enalapril. Analyses examining eGFR decline in the enalapril group using a reference group of 0% decline on enalapril were also performed. We repeated the above analyses for each follow-up time point, namely at 2-weeks and at 6-weeks. All analyses were performed using SAS Enterprise Guide (Version 7.12, Cary, NC) and R language (version 3.3.1, R Foundation for Statistical Computing, Vienna Austria).
Supplementary Material
Figure S1. Multivariable adjusted hazard ratios for cardiovascular mortality for those randomized to enalapril versus placebo according to magnitude of decline at 2-weeks (left), and 6-weeks (right). Filled circles represent points at which there was a significant hazard ratio for enalapril group versus placebo. There was no significant interaction between decline and treatment at any time point. eGFR indicates estimated glomerular filtration rate, calculated using adjusted serum creatinine and CKD-EPI equation. Pinteraction indicates p-value for interaction. Number of patients at each follow-up time point were 6245 at 2-weeks, and 6055 at 6-weeks. Models adjusted for age, sex, race, baseline kidney function, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, hematocrit, potassium and trial.
Figure S2. Multivariable adjusted hazard ratios for cardiovascular mortality for those randomized to enalapril versus placebo according to magnitude of decline at 2-weeks (left), and 6-weeks (right). Placebo, 0% eGFR held as reference point (represented by diamond). Filled circles represent points where hazard ratio for all-cause mortality is significant (p<0.05) compared to the reference point. eGFR indicates estimated glomerular filtration rate, calculated using adjusted serum creatinine and CKD-EPI equation. Number of patients at each follow-up time point were 6254 at 2-weeks, and 6055 at 6-weeks. Models adjusted for age, sex, race, baseline kidney function, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, hematocrit, potassium and trial.
Table S1. Adjusted hazard ratio for cardiovascular death based on decline in estimated glomerular filtration rate at 2- and 6-week time point following randomization, using 3 different levels of hemodynamic effect of enalapril.
Acknowledgments
Sources of Funding
NIH Training Grant T32 DK007777; NIH HL 131023
Abbreviations
- SOLVD
Studies Of Left Ventricular Dysfunction
- HFrEF
Heart Failure with reduced Ejection Fraction
- RAAS
renin-angiotensin-aldosterone system
- eGFR
estimated glomerular filtration rate
- ACEI
angiotensin converting enzyme inhibitor
- ARB
angiotensin-receptor blockers
- CI
confidence interval
- HR
hazard ratio
- SD
standard deviation
Footnotes
Supplementary information is available at Kidney International’s website.
Disclosures: none
References
- 1.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The New England journal of medicine. 1987;316(23):1429–1435. [DOI] [PubMed] [Google Scholar]
- 2.Carson P, Tognoni G, Cohn JN. Effect of Valsartan on hospitalization: results from Val-HeFT. Journal of cardiac failure. 2003;9(3):164–171. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327. [DOI] [PubMed] [Google Scholar]
- 4.Konstam MA, Rousseau MF, Kronenberg MW, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86(2):431–438. [DOI] [PubMed] [Google Scholar]
- 5.Konstam MA, Kronenberg MW, Rousseau MF, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (Studies of Left Ventricular Dysfunction) Investigators. Circulation. 1993;88(5 Pt 1):2277–2283. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91(10):2573–2581. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The New England journal of medicine. 1991;325(5):293–302. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The New England journal of medicine. 1992;327(10):685–691. [DOI] [PubMed] [Google Scholar]
- 9.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. The New England journal of medicine. 2001;345(23):1667–1675. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Adams KF Jr., Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. Jama. 2005;293(5):572–580. [DOI] [PubMed] [Google Scholar]
- 11.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. Journal of the American College of Cardiology. 2008;51(13):1268–1274. [DOI] [PubMed] [Google Scholar]
- 12.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. Journal of the American College of Cardiology. 2004;43(1):61–67. [DOI] [PubMed] [Google Scholar]
- 13.Hillege HL, Girbes AR, de Kam PJ, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(2):203–210. [DOI] [PubMed] [Google Scholar]
- 14.Kiernan MS, Gregory D, Sarnak MJ, et al. Early and late effects of high- versus low-dose angiotensin receptor blockade on renal function and outcomes in patients with chronic heart failure. JACC Heart failure. 2015;3(3):214–223. [DOI] [PubMed] [Google Scholar]
- 15.Testani JM, Kimmel SE, Dries DL, et al. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circulation Heart failure. 2011;4(6):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apperloo AJ, de Zeeuw D, de Jong PE. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney international. 1997;51(3):793–797. [DOI] [PubMed] [Google Scholar]
- 17.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Archives of internal medicine. 2000;160(5):685–693. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ (Clinical research ed). 2017;356:j791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark H, Krum H, Hopper I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. European journal of heart failure. 2014;16(1):41–48. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482–1487. [DOI] [PubMed] [Google Scholar]
- 22.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical chemistry. 2007;53(4):766–772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Multivariable adjusted hazard ratios for cardiovascular mortality for those randomized to enalapril versus placebo according to magnitude of decline at 2-weeks (left), and 6-weeks (right). Filled circles represent points at which there was a significant hazard ratio for enalapril group versus placebo. There was no significant interaction between decline and treatment at any time point. eGFR indicates estimated glomerular filtration rate, calculated using adjusted serum creatinine and CKD-EPI equation. Pinteraction indicates p-value for interaction. Number of patients at each follow-up time point were 6245 at 2-weeks, and 6055 at 6-weeks. Models adjusted for age, sex, race, baseline kidney function, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, hematocrit, potassium and trial.
Figure S2. Multivariable adjusted hazard ratios for cardiovascular mortality for those randomized to enalapril versus placebo according to magnitude of decline at 2-weeks (left), and 6-weeks (right). Placebo, 0% eGFR held as reference point (represented by diamond). Filled circles represent points where hazard ratio for all-cause mortality is significant (p<0.05) compared to the reference point. eGFR indicates estimated glomerular filtration rate, calculated using adjusted serum creatinine and CKD-EPI equation. Number of patients at each follow-up time point were 6254 at 2-weeks, and 6055 at 6-weeks. Models adjusted for age, sex, race, baseline kidney function, previous myocardial infarction, smoking, NYHA functional class, diastolic blood pressure, hematocrit, potassium and trial.
Table S1. Adjusted hazard ratio for cardiovascular death based on decline in estimated glomerular filtration rate at 2- and 6-week time point following randomization, using 3 different levels of hemodynamic effect of enalapril.