Abstract
Background
There is limited information describing the presenting characteristics and outcomes of patients with schizophrenia (SCZ) requiring hospitalization for coronavirus disease 2019 (COVID-19).
Aims
We aimed to compare the clinical characteristics and outcomes of COVID-19 SCZ patients with those of non-SCZ patients.
Method
This was a case-control study of COVID-19 patients admitted to 4 AP–HM/AMU acute care hospitals in Marseille, southern France. COVID-19 infection was confirmed by a positive result on polymerase chain reaction testing of a nasopharyngeal sample and/or on chest computed scan among patients requiring hospital admission. The primary outcome was in-hospital mortality. The secondary outcome was intensive care unit (ICU) admission.
Results
A total of 1092 patients were included. The overall in-hospital mortality rate was 9.0%. The SCZ patients had an increased mortality compared to the non-SCZ patients (26.7% vs. 8.7%, P = 0.039), which was confirmed by the multivariable analysis after adjustment for age, sex, smoking status, obesity and comorbidity (adjusted odds ratio 4.36 [95% CI: 1.09–17.44]; P = 0.038). In contrast, the SCZ patients were not more frequently admitted to the ICU than the non-SCZ patients. Importantly, the SCZ patients were mostly institutionalized (63.6%, 100% of those who died), and they were more likely to have cancers and respiratory comorbidities.
Conclusions
This study suggests that SCZ is not overrepresented among COVID-19 hospitalized patients, but SCZ is associated with excess COVID-19 mortality, confirming the existence of health disparities described in other somatic diseases.
Keywords: Schizophrenia, Psychiatry, COVID-19, Observational study
Résumé
Contexte
Il existe peu d’informations décrivant les caractéristiques et les résultats des patients atteints de schizophrénie (SCZ) nécessitant une hospitalisation pour maladie à coronavirus 2019 (COVID-19).
Objectifs
Nous avons cherché à comparer les caractéristiques cliniques et les résultats des patients SCZ atteints de COVID-19 avec ceux des patients non SCZ.
Méthode
Il s’agissait d’une étude cas-témoins de patients COVID-19 admis dans 4 hôpitaux de soins aigus AP–HM/AMU à Marseille, dans le sud de la France. L’infection par COVID-19 a été confirmée par un résultat positif au test d’amplification en chaîne par polymérase d’un échantillon nasopharyngé et/ou au scanner thoracique effectué chez les patients nécessitant une hospitalisation. Le principal critère de jugement a été la mortalité hospitalière. Le critère de jugement secondaire était l’admission en unité de soins intensifs (USI).
Résultats
Un total de 1092 patients a été inclus. Le taux global de mortalité hospitalière était de 9,0 %. Les patients SCZ ont eu une mortalité accrue par rapport aux autres patients (26,7 % contre 8,7 %, p = 0,039), ce qui a été confirmé par l’analyse multivariée après ajustement pour l’âge, le sexe, le tabagisme, l’obésité et les comorbidités (odds ratio ajusté de 4,36 [IC95 % : 1,09–17,44] ; p = 0,038). En revanche, les patients SCZ n’ont pas été plus souvent admis à l’unité de soins intensifs que les patients non SCZ. Il est important de noter que les patients des SCZ étaient pour la plupart institutionnalisés (63,6 %, soit 100 % des décès) et qu’ils étaient plus susceptibles d’avoir des cancers et des comorbidités respiratoires.
Conclusions
Cette étude suggère que les SCZ ne sont pas surreprésentées parmi les patients hospitalisés pour COVID-19, mais que la SCZ est associée à une surmortalité due à COVID-19, confirmant l’existence de disparités de santé décrites dans d’autres maladies somatiques.
Mots clés: Schizophrénie, Psychiatrie, COVID-19, Étude d’observation, Mortalité, Maladie mentale, Santé mentale
Research in context
Evidence before this study
Schizophrenia (SCZ) patients are a population at particular risk of poor COVID-19 outcomes and it remains unclear if they are at increased risk of COVID-19 infection. Among COVID-19 infection risk factors identified in schizophrenia, the presence of comorbidities, poor insight into somatic symptoms, stigma experience, delusions and cognitive impairment leading to a misperception of the risk related to the virus have been identified. Moreover, schizophrenia patients are more frequently hard smokers with lower vitamin D levels and it is unclear how it may impact their chance of surviving to COVID-19. It has been already shown that they do not benefit from the same somatic care than patients without psychiatric disorders in their end-of-life of cancer for example. However, it was unknown whether COVID-19 patients with SCZ benefit from the same care as non-SCZ patient.
Added value of this study
This study is the first large cohort of sequentially hospitalized confirmed COVID-19 patients with and without SCZ. A total of 1092 patients were included in the analysis, with 15 patients with SCZ (frequency = 1.37%, 95% confidence interval [CI] from 0.68 to 2.06). The SCZ patients were more likely to be smokers and to have cancers and respiratory comorbidities than the non-SCZ controls. They were mostly institutionalized (63.6%, 100% of those who died) and elderly.
The overall in-hospital mortality rate was 9.0%. SCZ patients had an increased mortality compared to non-SCZ patients (26.7% vs. 8.7%, P = 0.039), which was confirmed by the multivariable analysis after adjustment for age, sex, smoking status, obesity and Charlson Comorbidity Index (adjusted odds ratio [aOR]: 4.36 [95% CI: 1.09–17.44]; P = 0.038).
A total of 191 patients (17.5%) were admitted to the ICU. The SCZ patients were not more frequently admitted to the ICU than the patients without schizophrenia. None of the deceased SCZ patients were admitted to ICU vs. 28.7% (27/94) for patients without SCZ.
Implications of all the available evidence
The mortality in SCZ patients was 3 times higher than that of the non-schizophrenia patients after adjustment for age, sex, smoking status, obesity and comorbidities confirming the existence of health disparities as already described in other somatic diseases. Contrary to what could have been expected, SCZ is not overrepresented among COVID-19 hospitalized patients compared to the prevalence of SCZ in the general population.
A delay in access to hospital care may be suggested by the higher respiratory rate at admission in patients with SCZ compared to patients without schizophrenia. None of the four non-survivor SCZ patients were admitted to the ICU, which would deserve to be better understood: advanced age and comorbidities, cognitive impairment or death occurring before ICU admission.
Around three-quarters of the SCZ patients were institutionalized, and 100% of the SCZ patients who died were institutionalized. Contrary to what could have been expected, the SCZ patients were not younger than the non-SCZ patients, while their life expectancy is generally reduced compared to the general population. This result is in favor of a risk increase in institutionalized SCZ patients and against an increased risk of COVID-19 infection in the global SCZ population. Our results support a strategy of systematic detection in institutionalized SCZ patients.
Introduction
Only five months after the appearance of COVID-19, the disease caused by the coronavirus that appeared in China in December 2019, France has been bearing the full brunt of the health crisis that has been unleashed across the planet. On January 24th, 2020, the French Ministry of Health reported the first two cases in France; both were infected in China, where they had recently traveled. The first death due to the COVID-19 was registered on February 14th, 2020, in an older Chinese tourist. The next day, a religious demonstration brought 2000 people together in the town of Mulhouse in East France, which was likely to be the starting point for serial contamination throughout the country, from Corsica to French Guiana, and from the Hautes-Alpes to Normandy and the Île-de-France region. On April 4th, France reached an unprecedented milestone: 6838 patients were hospitalized in intensive care unit (ICU), a record “in French medical history” [1].
Among all specific populations that may have a particularly poor COVID-19 outcome, patients with schizophrenia (SCZ) should be given particular attention. Since the Second World War, when SCZ patients died massively in asylums from malnutrition (the so-called “extermination douce” [gentle extermination] [2]), the somatic care of patients with SCZ has lagged behind that of other mentally healthy patients. We have recently found, in a national database study, that patients with schizophrenia received less high-intensity care than those without severe mental disorder [3], and it is unknown whether COVID-19 patients with SCZ benefit from the same care as non-SCZ patients. In summary, COVID-19 may strongly impact SCZ patients and the health and economic disparities they experience [4].
It also remains unknown whether SCZ is a risk factor for COVID-19 infection and/or COVID-19 mortality. Among COVID-19 risk factors, presence of comorbidities, poor insight into somatic symptoms, stigma experience, delusions and cognitive impairment leading to a misperception of the risk related to the virus have been identified [4]. The prevalence of tobacco smoking is higher in patients with SZ than in the general population, with higher rates of heavy smokers and nicotine dependence [5], yet it remains unclear whether smoking is a protective factor or a risk factor for COVID-19 infection and mortality [6]. Hypovitaminosis D has been identified in 27% of patients with SCZ vs. 21% of the general population [7], yet it remains unknown whether hypovitaminosis D may modify the risk for COVID-19 infection and/or mortality [8]. SCZ is also a risk factor for homelessness or institutionalization, which may mediate the risk for increased COVID-19 infection and/or mortality [9]. In addition to these multiple risk factors for poor COVID-19 outcomes, active social withdrawal is one of the most frequent negative symptoms of SCZ that may protect non-institutionalized patients from infection through spontaneous lock-down and social distancing.
The objective of the present study was to compare the presenting characteristics and outcomes of COVID-19 patients with SCZ with COVID-19 patients without SCZ.
Methodology
Setting
This study was conducted at Assistance publique–Hôpitaux Marseille (AP–HM) – Aix-Marseille University (AMU), a quaternary, acute care hospital system in Marseille, southern France. AP–HM/AMU comprises four hospitals (La Timone, La Conception, Sainte-Marguerite, and North), 3400 beds, and 2000 physicians. Approximately 300,000 hospitalizations are recorded every year, involving approximately 200,000 patients. All consecutive patients who were sufficiently medically ill to require hospital admission with confirmed COVID-19 infection by positive result on polymerase chain reaction testing (PCR) of a nasopharyngeal sample and/or by chest computed scan (i.e., extended bilateral ground-glass opacities and area of consolidation) were included. Eligible patients were admitted to any of 4 AP–HM/AMU acute care hospitals from February 27, 2020, to April 15, 2020. Clinical outcomes were monitored until May 4, 2020, the final date of follow-up. The AP–HM/AMU institutional review board (CADS) approved this work as minimal-risk research using data collected for routine clinical practice (CADS identifier: Q7APAN-M9GR8M-2020).
Data sources and collection
Data were obtained from the AP–HM/AMU clinical data warehouse. This warehouse contains all the clinical data available on all inpatient visits to one of the AP–HM/AMU facilities. No data were manually abstracted from the electronic medical record or charts. The data obtained included patients’ sociodemographic data, clinical data (i.e., smoking status, overweight and obesity, symptoms, Charlson Comorbidity Index score [10] and main comorbidities), triage vital signs, initial laboratory test results, initial COVID-19 treatment, ICU admission, Simplified Acute Physiology Score II (SAPS II), length of ICU stay and ICU management (i.e., mechanical ventilation, renal replacement therapy), palliative care, and outcomes (i.e., length of hospital stay and in-hospital mortality). Clinical data, ICU admission and treatments, palliative care and outcomes were based on the 10th revision of the International Statistical Classification of Diseases (ICD10) and procedural codes were based on the classification commune des actes médicaux (CCAM) associated the French from the Programme de Medicalisation des Systèmes d’Information (PMSI) – French medico-administrative database based on diagnosis related-groups (DRGs). The Charlson Comorbidity Index predicts 10-year survival in patients with multiple comorbidities and was used as a measure of total comorbidity burden [10]. The protocol recommended by a medical team from Marseilles for COVID-19 treatment was a combination of hydroxychloroquine (200 mg of oral hydroxychloroquine, three times daily for ten days) and azithromycin (500 mg on day 1 followed by 250 mg daily for the next four days) [11], thus explaining the important amount of this prescription in our study.
Definition of cases and controls
Cases were patients who had a diagnosis of SCZ according to specific ICD10 codes (i.e., F20*, F22*, or F25*). Controls were patients who did not have a diagnosis of mental illness according to specific ICD10 codes in the acute care database and who were not listed in the psychiatry databases.
Outcomes
The primary outcome was in-hospital mortality. The secondary outcome was ICU admission.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges. Categorical variables were summarized as counts and percentages. No imputation was made for missing data.
We used either the Chi2 or Fisher's exact test and Student's t-test or Mann–Whitney test to compare sociodemographic data, clinical data, triage vital signs, initial laboratory test results, initial COVID-19 treatment, ICU admission and treatments, palliative care, and outcomes between cases and controls. Then, multivariable logistic regression models were used to estimate the association between schizophrenia and the two endpoints. An initial multivariable regression model (model 1) included the main known prognostic factors: age, sex, smoking status, overweight and obesity, and Charlson Comorbidity Index. Two additional models were also performed, including prognosis factors previously listed and the two main COVID-19 treatments delivered in our institution (model 2: hydroxychloroquine and model 3: hydroxychloroquine-azithromycin combination). A significance threshold of P < 0.05 was used. All analyses were performed in SAS (version 9.4).
Results
Characteristics of the patients
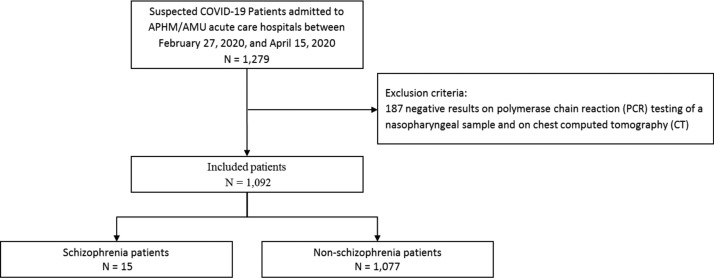
During the study period, a total of 1092 patients were included in the analysis (median age: 63 years [interquartile range (IQR): 51–76]; 46.7% female with no significant differences between SCZ and non-SCZ), with 15 SCZ patients (frequency = 1.37%, 95% confidence interval [CI] from 0.68 to 2.06) and 1077 non-SCZ patients (98.63%) (Fig. 1 , Table 1 ). The SCZ patients were more likely to be smokers (33.3% vs. 11.1%, P = 0.021) and to have cancers (20.0% vs. 5.5%, P = 0.049) and respiratory comorbidities (26.7% vs. 4.9%, P = 0.006) than the non-SCZ controls. The SCZ patients had a higher level of creatinine kinase (213.5 IU/L vs. 98.0 IU/L, P = 0.015) and a lower level of total bilirubin (4.5 μmol/L vs. 7.0 μmol/L, P = 0.006) than the non-SCZ controls. A qualitative analysis of the 15 SCZ patients is presented in Supplementary Table S1. They were mostly institutionalized (63.6%, 100% of those who died) and elderly. The length of hospital stay was similar in the SCZ and non-SCZ patients (median [IQR] 10 days [7–15] vs. 7 [4–14] P = 0.196).
Fig. 1.
Flowchart.
Table 1.
Baseline characteristics of patients hospitalized with COVID-19 (n = 1092).
| Characteristics | Total n = 1092 n (%) |
SCZ patients n = 15 n (%) |
Non-SCZ patients n = 1077 n (%) |
P-value |
|---|---|---|---|---|
| Sociodemographic data | ||||
| Age, median (IQR) — year | 62.5 (51.0–76.0) | 66.0 (63.0–72.0) | 62.0 (51.0–76.0) | 0.345 |
| < 18 year | 19 (1.7) | 0 (0.0) | 19 (1.8) | 0.714 |
| 18–65 year | 587 (53.8) | 7 (46.7) | 580 (53.9) | |
| > 65 year | 486 (44.5) | 8 (53.3) | 478 (44.4) | |
| Male sex — No. (%) | 593 (54.3) | 11 (73.3) | 582 (54.0) | 0.136 |
| Clinical data | ||||
| Smoker | 125 (11.5) | 5 (33.3) | 120 (11.1) | 0.021 |
| Weighta | 0.710 | |||
| Normal weight | 749 (68.6) | 10 (66.7) | 739 (68.6) | |
| Overweight | 128 (11.7) | 1 (6.7) | 127 (11.8) | |
| Obesity | 215 (19.7) | 4 (26.7) | 211 (19.6) | |
| Symptoms | ||||
| Agueusia and/or anosmia | 184 (16.9) | 2 (13.3) | 182 (16.9) | 0.999 |
| Digestive symptoms (diarrhea and/or nausea or vomiting) | 250 (22.9) | 4 (26.7) | 246 (22.8) | 0.758 |
| Respiratory symptoms (cough and/or pharyngitis and/or dyspnea and/or hemoptysis) | 795 (72.8) | 11 (73.3) | 784 (72.8) | 0.999 |
| General symptoms (asthenia and/or headache and/or myalgia/arthralgia) | 532 (48.7) | 3 (20.0) | 529 (49.1) | 0.025 |
| Comorbidities | ||||
| Charlson Comorbidity Index score | 0.389 | |||
| 0 | 540 (49.5) | 8 (53.3) | 532 (49.4) | |
| 1–2 | 372 (34.1) | 3 (20.0) | 369 (34.3) | |
| ≥ 3 | 180 (16.5) | 4 (26.7) | 176 (16.3) | |
| Cancer | 62 (5.7) | 3 (20.0) | 59 (5.5) | 0.049 |
| Hypertension | 393 (36.0) | 0 (0.0) | 393 (36.49) | 0.003 |
| Myocardial infarction | 38 (3.5) | 1 (6.7) | 37 (3.4) | 0.412 |
| Congestive heart failure | 104 (9.5) | 2 (13.3) | 102 (9.5) | 0.647 |
| Chronic renal disease | 90 (8.2) | 1 (6.7) | 89 (8.3) | 0.999 |
| Asthma | 71 (6.5) | 1 (6.7) | 70 (6.5) | 0.999 |
| Chronic pulmonary disease | 57 (5.2) | 4 (26.7) | 53 (4.9) | 0.006 |
| Liver disease | 33 (3.0) | 0 (0.0) | 33 (3.1) | 0.999 |
| Diabetes | 260 (23.4) | 1 (6.7) | 259 (24.1) | 0.138 |
| Vital signs | ||||
| Fever (≥ 38 °C) | 680 (62.3) | 10 (66.7) | 670 (62.2) | 0.723 |
| Heart rate ≥ 100 beats/min | 174 (15.9) | 3 (20.0) | 171 (15.9) | 0.719 |
| Respiratory rate > 24 cycles/min | 229 (21.0) | 7 (47.7) | 222 (20.6) | 0.022 |
| Initial laboratory findings, median (IQR) | ||||
| White-cell count (×10−9/L) | 5.9 (4.5–7.8) | 6.2 (4.9–10.0) | 5.9 (4.5–7.8) | 0.270 |
| Platelet count (×10−9/L) | 205.0 (159.0–255.0) | 224.0 (184.0–277.0) | 204.5 (159.0–255.0) | 0.212 |
| C-reactive protein (mg/L) | 44.3 (9.2–101.1) | 52.0 (9.5–168.0) | 44.2 (9.2–100.7) | 0.378 |
| Creatine kinase (U/L) | 98.0 (60.0–209.0) | 213.5 (134.5–340.0) | 98.0 (60.0–209.0) | 0.015 |
| Lactate dehydrogenase (U/L) | 287.0 (219.0–385.0) | 324.0 (255.0–469.0) | 287.0 (219.0–385.0) | 0.355 |
| Aspartate aminotransferase (U/L) | 37.0 (27.0–55.0) | 43.0 (35.0–59.0) | 37.0 (27.0–55.0) | 0.197 |
| Alanine aminotransferase (U/L) | 27.0 (19.0–41.0) | 30.0 (25.0–36.0) | 27.0 (19.0–41.0) | 0.571 |
| Total bilirubin (μmol/L) | 7.0 (5.0–10.0) | 4.5 (4.0–7.0) | 7.0 (5.0–10.0) | 0.006 |
| Creatinine (μmol/L) | 77.0 (62.8–98.0) | 83.1 (63.2–128.6) | 77.0 (62.8–98.0) | 0.437 |
| Sodium (mmol/L) | 137.0 (135.0–140.0) | 138.0 (135.0–140.0) | 137.0 (135.0–140.0) | 0.642 |
| Potassium (mmol/L) | 4.0 (3.7–4.3) | 4.0 (3.8–4.3) | 4.0 (3.7–4.3) | 0.704 |
| COVID-19 treatment initiated at admission | ||||
| Hydroxychloroquine | 584 (53.5) | 5 (33.3) | 579 (53.8) | 0.115 |
| Hydroxychloroquine–azithromycin combination | 399 (36.5) | 5 (33.3) | 394 (36.6) | 0.795 |
| Antiviral agents | 48 (4.4) | 0 (0.0) | 48 (4.5) | 0.999 |
| Immunosuppressors | 40 (3.7) | 0 (0.0) | 40 (3.7) | 0.999 |
Symptoms and comorbidities based on the 10th revision of the International Statistical Classification of Diseases from the Programme de Médicalisation des Systèmes d’Information (PMSI) – French medico-administrative database based on diagnosis related-groups (DRG).
P-value in bold: statistical significance.
If body mass index (BMI) is 18.5 to < 25: normal weight; if BMI is 25.0 to < 30: overweight; if BMI is 30.0 or higher: obesity.
In-hospital mortality
The overall in-hospital mortality rate was 9.0%. The univariable analysis is presented in Supplementary Table S2. SCZ patients had an increased mortality compared to non-SCZ patients (26.7% vs. 8.7%, P = 0.039) (Table 2 ), which was confirmed by the multivariable analysis after adjustment for age, sex, smoking status, obesity and Charlson Comorbidity Index (model 1) (adjusted odds ratio [aOR]: 4.36 [95% CI: 1.09–17.44]; P = 0.038) (Table 3 ). The additional models (2 and 3) reported the same findings.
Table 2.
Clinical outcomes and management of patients hospitalized with COVID-19 (n = 1092).
| Total n = 1092 n (%) |
SCZ patients n = 15 n (%) |
Non-SCZ patients n = 1077 n (%) |
P-value | |
|---|---|---|---|---|
| Outcomes and management | ||||
| In-hospital mortality | 98 (9.0) | 4 (26.7) | 94 (8.7) | 0.038 |
| ICU admission | 191 (17.5) | 2 (13.3) | 189 (17.6) | 0.999 |
| Recourse to mechanical ventilation | 106 (9.7) | 1 (6.7) | 105 (9.8) | 0.999 |
| Recourse to continuous renal-replacement therapy | 21 (1.9) | 0 (0.0) | 21 (2.0) | 0.999 |
| Palliative care | 49 (4.5) | 1 (6.7) | 48 (4.5) | 0.500 |
| Characteristics of stay | ||||
| Length of ICU stay, median (IQR) — No. of days | 12.0 (4.0–28.0) | –a | 13.0 (4.0–28.0) | – |
| Length of hospital stay, median (IQR) — No. of days | 7.0 (4.0–14.0) | 10 (7.0–15.0) | 7.0 (4.0–14.0) | 0.196 |
Data based on the 10th revision of the International Statistical Classification of Diseases and common classification of medical acts from the Programme de Médicalisation des Systèmes d’Information (PMSI) – French medico-administrative database based on diagnosis related-groups (DRG).
ICU: intensive care unit.
P-value in bold: statistical significance.
Two SCZ patients were admitted to ICU with a length of ICU stay of 3 days and 5 days, respectively.
Table 3.
Associations between schizophrenia and in-hospital mortality and ICU admission (n = 1092).
| In-hospital mortality |
ICU admission |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Without adjustment | 3.80 (1.19–12.20) | 0.025 | 0.72 (0.16–3.20) | 0.671 |
| With adjustment | ||||
| Model 1a | 4.36 (1.09–17.44) | 0.038 | 0.46 (0.10–2.18) | 0.330 |
| Model 2b | 4.28 (1.07–17.20) | 0.040 | 0.53 (0.11–2.61) | 0.439 |
| Model 3c | 4.33 (1.08–17.34) | 0.038 | 0.46 (0.10–2.23) | 0.239 |
OR (95% CI): odds ratios (95% confidence interval); ICU: intensive care unit.
P-value in bold: statistical significance.
Model 1: adjustment for 5 variables associated with COVID-19 outcome (age, sex, smoking status, obesity, Charlson Comorbidity Index).
Model 2: adjustment for 6 variables associated with COVID-19 outcome (age, sex, smoking status, obesity, Charlson Comorbidity Index) and the initial COVID-19 treatment (hydroxychloroquine).
Model 3: adjustment for 6 variables associated with COVID-19 outcome (age, sex, smoking status, obesity, Charlson Comorbidity Index) and the initial COVID-19 treatment (hydroxychloroquine–azithromycin combination).
ICU admission
A total of 191 patients (17.5%) were admitted to the ICU (median SAPS II: 34.5 [IQR]: 29–43; median SAPS II in the non-SCZ-patients: 35.0 [IQR]: 29–43; and SAPS II in the two SCZ patients were 25 and 34, respectively). The univariable analysis is presented in Supplementary Table S3. The SCZ patients were not more frequently admitted to the ICU than the non-SCZ patients (13.3% vs. 17.6%, P = 0.265; aOR: 0.46 [95% CI: 0.10–2.18]; P = 0.330) (model 1) (Table 3). The additional models (2 and 3) reported the same findings. None of the deceased SCZ patients were admitted to ICU vs. 28.7% (27/94) for non-SCZ patients. The median length of ICU stay was 12.0 [IQR]: 4.0–28.0, 13.0 [IQR]: (4.0–28.0) in the non-SCZ patients and the length of ICU stay for the 2 SCZ patients was 3 days and 5 days, respectively.
Discussion
To our knowledge, this study is the first preliminary cohort of sequentially hospitalized confirmed COVID-19 patients with SCZ and non-SCZ. The mortality of the SCZ patients was 3 times higher than that of the non-SCZ patients after adjustment for age, sex, smoking status, obesity and comorbidities.
The first major finding of this study is that SCZ is not overrepresented among COVID-19 hospitalized patients compared to the prevalence of SCZ in the general population (between 0.5 and 1.5% in most countries) [12], [13]. Our data do not suggest that SCZ patients are more at risk of COVID-19 than the general population, contrary to what could have been expected [4]. However, we cannot rule out the possibility of a higher risk of COVID-19 in SCZ patients outside the hospital who have poor access to hospital care [14] and/or out-of-hospital deaths. Future studies should estimate the prevalence and management of COVID-19 for SCZ patients outside the hospital.
SCZ was associated with excess mortality after adjustment for age, sex, smoking status, obesity and comorbidity, underlining the existence of health disparities in COVID-19 as already described in other somatic diseases [3], [15]. Previous studies reported health care disparities in SCZ patients [16]. A delay in access to hospital care may be suggested by the higher respiratory rate at admission in patients with SCZ patients compared to non-SCZ patients. Respiratory rate has been reported as an important indicator of serious illness [17]. However, we have no precise information on the delay between the onset of infection and hospitalization. Further studies will need to explore access to hospital care in SCZ patients with COVID-19. In our study, we found no differences in the treatment protocol administered to SCZ patients compared to non-SCZ patients, and increased mortality remained significant after adjustment for treatment administration. We found no difference in access to the ICU. However, none of the SCZ patients who died were admitted to the ICU, which would deserve to be better understood: advanced age and comorbidities, cognitive impairment or death occurring before ICU admission (for instance massive pulmonary embolism) [3]. Factors consistently found in the literature to be associated with a decision to admit or refuse a patient to the ICU are bed availability, severity of illness, initial ward or team the patient was referred from, patient preference, do not resuscitate order status, age and functional status at baseline [18]. Future studies (based on qualitative studies with ICU clinicians and national medico-administrative studies) should thus explore ICU admission in SCZ patients with COVID-19.
One major finding emerging from the qualitative analysis is that around two thirds of the SCZ patients were institutionalized, and 100% of the SCZ patients who died were institutionalized. We lack national data on the rate of elderly SCZ patients who are institutionalized, yet we can reasonably hypothesize that institutionalization is a risk factor for COVID-19 severe infection in elderly patients with SCZ. Hence, preventive measures should target this population. Contrary to what could have been expected, the SCZ patients were not younger than the non-SCZ patients, while their life expectancy is generally reduced compared to the general population [19]. This result is also in favor of a risk increase in institutionalized SCZ patients and against an increased risk of COVID-19 infection in the global SCZ population. However, these results should be interpreted with caution, as we only identified 15 SCZ patients, and these results should be replicated in future wider databases. Our results support a strategy of systematic detection in institutionalized SCZ patients. This has already been done in a homeless shelter in Boston where 36% of the residents tested positive [20].
Limitations
This study had several limitations. First, the study population only included patients within the Marseilles metropolitan area, which may limit the generalizability of these results. Second, the sample size for the SCZ patients was limited (n = 15). However, this first description of real-life data in patients with SCZ could be shared with other institutions and countries to achieve a larger sample and to provide a more complete picture of SCZ and COVID-19 [21]. Third, the data were collected from the electronic health record database. This precluded the level of detail that would have been possible with a manual medical record review. Additional limitations of our study include missing data for some variables and potential for inaccuracies in the electronic health records.
Strengths
This study is the first to explore the in-hospital mortality of SCZ patients due to COVID-19 infection. The results were adjusted for important confounding factors and suggest a remaining 3-fold increase in in-hospital mortality risk in SCZ patients.
Conclusion
This study suggests that SCZ may not be overrepresented among COVID-19 hospitalized patients compared to the prevalence of SCZ in the general population but that SCZ is associated with increased in-hospital mortality, confirming the existence of health disparities as already described in other somatic diseases. COVID-19 seemed to affect mostly institutionalized and elderly patients. These real-life health data should be shared with other health data producers to achieve a larger sample and to provide a more complete picture of SCZ and COVID-19.
Author contributions
Veronica Orleans and Vanessa Pauly had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Laurent Boyer, Guillaume Fond.
Acquisition, analysis, or interpretation of data: Pascal Auquier, Laurent Boyer, Christophe Lancon, Guillaume Fond, Vanessa Pauly, Veronica Orleans, Sophie Klay, Michel Sanz, Cyprien Fabre, Marc Leone.
Drafting of the manuscript: Laurent Boyer, Guillaume Fond.
Critical revision of the manuscript for important intellectual content: all the authors.
Statistical analysis: Vanessa Pauly.
Administrative, technical, or material support: Veronica Orleans and Michel Sanz.
Supervision: Laurent Boyer.
Funding
This work was funded by Assistance Publique–Hôpitaux Marseille (AP–HM) – Aix-Marseille University (AMU) and the European Health Data & Evidence Network from the European Union's Horizon 2020 research and innovation program.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgment
David Braunstein, Myriam Dubuc, Alexandra Murcia, Gilbert Pironti, Vincent Pradel, Anne Remacle, Fanny Romain, Catherine Seyler, Françoise Volot and all the other members of the Department of Medical Information, Bruno Lacarelle and Amin Ben Lassoued (Biology), Martine Charbit (Pharmacy), Alain Lavit, Christophe Rozier (DSN), and all the health care professionals for their great work during the COVID-19 pandemic.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.encep.2020.07.003.
Appendix A. Supplementary data
References
- 1.Le Monde.fr [Internet]; 2020. Coronavirus : des premiers cas au premier mois de confinement, les principales étapes de l’évolution de l’épidémie en France. [cité 17 mai 2020 ; Disponible sur : https://www.lemonde.fr/planete/article/2020/03/13/coronavirus-des-premiers-cas-aux-annonces-de-macron-les-principales-etapes-de-l-evolution-de-l-epidemie-en-france_6032967_3244.html] [Google Scholar]
- 2.Lafont M. 2000. L’Extermination Douce La cause des fous – 40 000 malades mentaux morts de faim dans les hôpitaux sous Vichy. Étude-Broché. [Google Scholar]
- 3.Fond G., Salas S., Pauly V. End-of-life care among patients with schizophrenia and cancer: a population-based cohort study from the French national hospital database. Lancet Public Health. 2019;4(11):e583–e591. doi: 10.1016/S2468-2667(19)30187-2. [DOI] [PubMed] [Google Scholar]
- 4.Kozloff N., Mulsant B.H., Stergiopoulos V. The COVID-19 global pandemic: implications for people with schizophrenia and related disorders. Schizophr Bull. 2020 doi: 10.1093/schbul/sbaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagud M., Mihaljevic Peles A., Pivac N. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry [Internet] 2019;32(5) doi: 10.1097/YCO.0000000000000529. [cité 19 mai 2020 ; Disponible sur : https://pubmed.ncbi.nlm.nih.gov/31135490/?from_term=schizophrenia+tobacco+smoking&from_size=50&from_pos=8] [DOI] [PubMed] [Google Scholar]
- 6.Cattaruzza M., Zagà V., Gallus S. Tobacco smoking and COVID-19 pandemic: old and new issues. a summary of the evidence from the scientific literature. Acta bio-medica: Atenei Parmensis [Internet] 2020;91(2) doi: 10.23750/abm.v91i2.9698. [cité 19 mai 2020 ; Disponible sur : https://pubmed.ncbi.nlm.nih.gov/32420934/?from_term=covid+tobacco+smoking&from_sort=date&from_size=50&from_pos=1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fond G., Faugere M., Faget-Agius C. Hypovitaminosis D is associated with negative symptoms, suicide risk, agoraphobia, impaired functional remission, and antidepressant consumption in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2018;270:104–110. doi: 10.1016/j.psychres.2018.09.024. [Epub 2018 Sep 13] [DOI] [PubMed] [Google Scholar]
- 8.Grant W.B., Lahore H., McDonnell S.L. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayano G., Tesfaw G., Shumet S. The prevalence of schizophrenia and other psychotic disorders among homeless people: a systematic review and meta-analysis. BMC Psychiatry [Internet] 2019;19(1) doi: 10.1186/s12888-019-2361-7. [cité 19 mai 2020 ; Disponible sur : https://pubmed.ncbi.nlm.nih.gov/31775786/?from_term=schizophrenia+homelessness&from_filter=pubt.systematicreviews&from_size=50&from_pos=3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bannay A., Chaignot C., Blotière P.-O. The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care. 2016;54(2):188–194. doi: 10.1097/MLR.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 11.Gautret P., Lagier J.-C., Parola P. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow-up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Küstner B., Martín C., Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS ONE. 2018;13(4):e0195687. doi: 10.1371/journal.pone.0195687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha S., Chant D., Welham J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence D., Kisely S. Inequalities in healthcare provision for people with severe mental illness. J Psychopharmacol (Oxford) 2010;24(4 Suppl.):61–68. doi: 10.1177/1359786810382058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vancampfort D., Firth J., Correll C.U. The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry. 2019;18(1):53–66. doi: 10.1002/wps.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell A.J., Malone D., Doebbeling C.C. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry. 2009;194(6):491–499. doi: 10.1192/bjp.bp.107.045732. [DOI] [PubMed] [Google Scholar]
- 17.Cretikos M.A., Bellomo R., Hillman K. Respiratory rate: the neglected vital sign. Med J Aust. 2008;188(11):657–659. doi: 10.5694/j.1326-5377.2008.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 18.James F.R., Power N., Laha S. Decision-making in intensive care medicine – A review. J Intensive Care Soc. 2018;19(3):247–258. doi: 10.1177/1751143717746566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samaras K., Correll C.U., Curtis J. Premature mortality and schizophrenia – The need to heal right from the start. JAMA Psychiatry. 2016;73:535–536. doi: 10.1001/jamapsychiatry.2015.3432. [DOI] [PubMed] [Google Scholar]
- 20.Baggett T.P., Keyes H., Sporn N. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA [Internet] 2020 doi: 10.1001/jama.2020.6887. [cité 20 mai 2020 ; Disponible sur : https://jamanetwork.com/journals/jama/fullarticle/2765378] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyer L., Auquier P., Fond G. [Real-life data and COVID-19: the third avenue of research] L’Encéphale. 2020 doi: 10.1016/j.encep.2020.04.003Get. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.