Abstract
Mammals have two specialized vascular circulatory systems, the blood vasculature and the lymphatic vasculature. The lymphatic vasculature provides a unidirectional conduit that returns filtered interstitial arterial fluid and tissue metabolites to the blood circulation. It also plays major roles in immune cell trafficking and lipid absorption. As we discuss in this review, the molecular characterization of lymphatic vascular development and our understanding of this vasculature’s role in pathophysiological conditions has greatly improved in recent years, changing conventional views about the roles of the lymphatic vasculature in health and disease. Morphological or functional defects in the lymphatic vasculature have now been uncovered in several pathological conditions. We propose that subtle, asymptomatic alterations in lymphatic vascular function could underlie the variability seen in the body’s response to a wide range of human diseases.
Oliver eTOC blurb
Oliver and colleagues comprehensively review the anatomy, development, and functional roles of the lymphatic vasculature in health and disease. They highlight emerging evidence suggesting the lymphatic system plays more diverse physiological roles that previously appreciated.
Introduction
While blood vessels are essential for oxygen and nutrient delivery, and for the disposal of waste products for detoxification and replenishment, the lymphatic vasculature plays essential roles in immune surveillance, lipid absorption and in the maintenance of tissue fluid balance (Oliver, 2004; Oliver and Alitalo, 2005; Petrova and Koh, 2018; Tammela and Alitalo, 2010). The cellular and molecular characterization of lymphatic vascular development and our understanding of this vasculature’s role in pathophysiological conditions has greatly improved in recent years, changing conventional views about its functional roles in health and disease. Traditionally considered a passive route for the transport of fluid, immune cells and lipoproteins, lymphatics are now known to be active players in major physiological and pathophysiological processes. Until recently, lymphatic vessel dysfunction was associated mainly with primary and secondary lymphedema. Unexpectedly, however, lymphatic vascular defects have been uncovered in conditions including obesity, cardiovascular disease, inflammation, hypertension, atherosclerosis, Crohn’s disease, glaucoma and various neurological disorders, such as Alzheimer’s disease.
In this review, we first provide a brief overview of lymphatic anatomy and of the key molecular and morphological steps underlying formation of the mammalian lymphatic vasculature. Next, we discuss the long-standing conventional views on lymphatic function in normal (immune surveillance) and disease conditions (tumor progression, symptomatic lymphatic disorders). Finally, we assess recent discoveries revealing novel roles of the lymphatic vasculature in a variety of human disorders; findings supporting our hypothesis that subtle, asymptomatic morphological and/or functional alterations in the lymphatic vasculature are responsible for the variability seen in the body’s response to a range of pathologic conditions.
Anatomy of the Lymphatic Vasculature
The lymphatic vasculature consists of a network of thin-walled, blind-ended, highly permeable initial lymphatics (also called lymphatic capillaries although they are functionally distinct to blood vascular capillaries) and larger collecting lymphatic vessels (Figure 1). Initial lymphatics consist of a single layer of loosely connected lymphatic endothelial cells (LECs) that lack a continuous basement membrane and perivascular mural cells, such as pericytes and smooth muscle cells (Figure 1). LECs within initial lymphatics are inter-connected through discontinuous button-like junctions (Figure 1) (Baluk et al., 2007), which facilitate the uptake of interstitial fluid and macromolecules. Blood plasma is continuously filtered from the arterial side of the capillary bed into the interstitial space, where excess fluid and macromolecules are taken up by initial lymphatics (Alitalo, 2011). Initial lymphatics interact with the extracellular matrix through anchoring filaments that facilitate the sensing of changes in interstitial pressure, which in turn modulates the opening of “flap valves” inbetween the button junctions to allow fluid entry (Tammela and Alitalo, 2010) (Figure 1). All of these features make the initial lymphatic vessels highly permeable to large macromolecules, pathogens, and immune cells.
Figure 1: Overview of the main structures forming the mammalian lymphatic system:
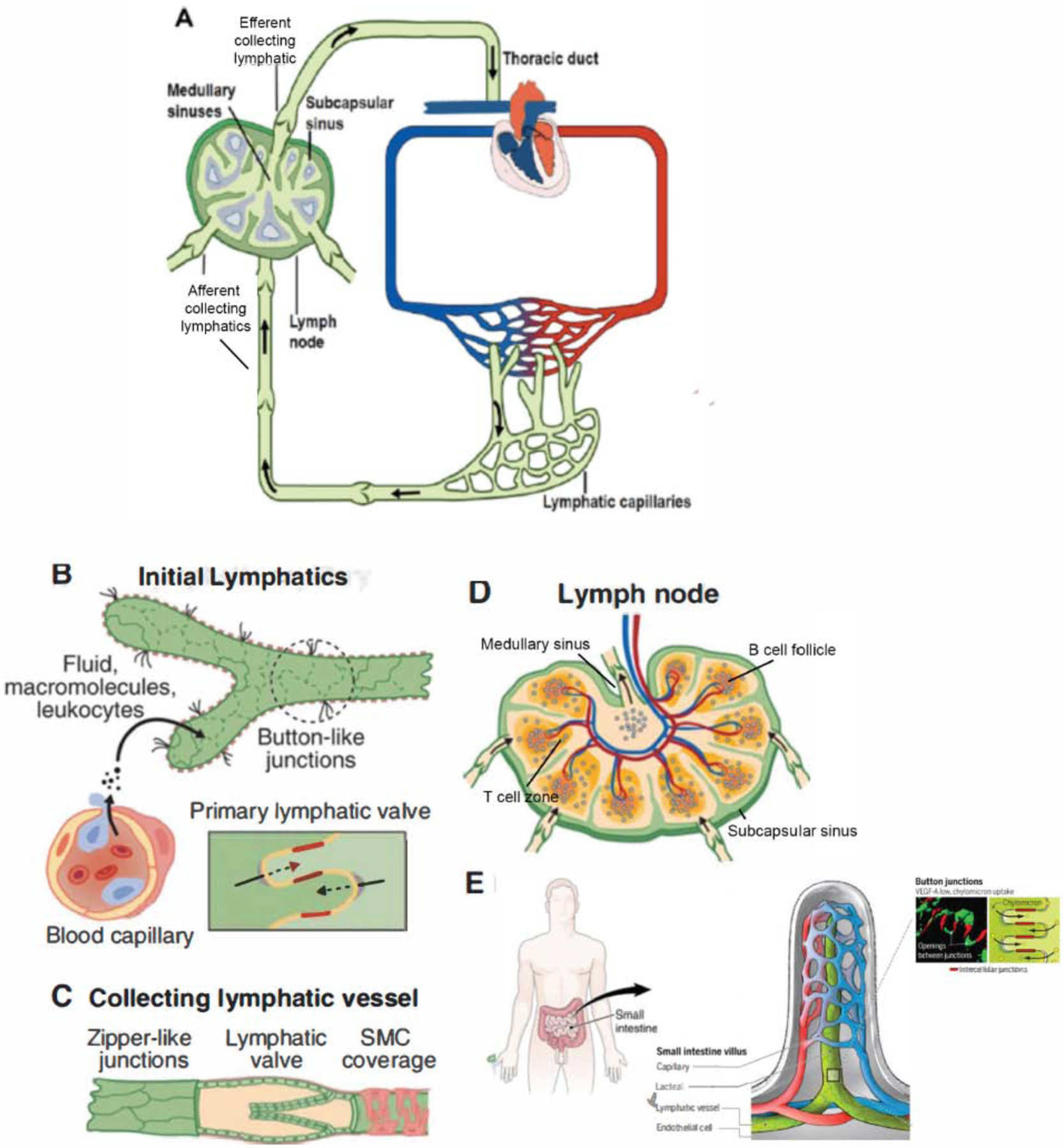
A) The lymphatic vasculature (green) is an arborized network that runs in parallel to the blood vasculature (red and blue). Smaller caliber initial lymphatics absorb the fluid that continuously leaks out from the blood capillary bed and drain their contents (lymph) into larger caliber collecting lymphatics specialized for transport. Lymph is filtered through lymph nodes before entering thoracic or right lymphatic ducts, which returns the lymph to the blood circulation via two pairs of bilaterally located lymphovenous valves. B) Initial lymphatics comprise a single layer of loosely connected lymphatic endothelial cells (LECs) lacking a continuous basement membrane and perivascular mural cells. LECs within initial lymphatics are inter-connected through discontinuous button-like junctions that facilitate the uptake of interstitial fluid and macromolecules released by the blood vasculature. C) In collecting lymphatics, LECs are connected to each other through tighter, continuous zipper-like junctions, and are covered with specialized muscle cells (SMC) that provide contractile activity to assist lymph flow. Collecting lymphatic vessels have valves that regulate the unidirectional flow of lymph, as well as a coordinated contraction of muscle cells that facilitates the transport of lymph back to the blood circulation. D) The lymph nodes (more than 200 in humans), along stretches of collecting lymphatics, are highly organized with segregated compartments of B and T lymphocytes, with lymphatic endothelial cells helping to form the subcapsular and medullary sinuses. The nodes are fed by blood vessels with a specialized postcapillary venule called the high endothelial venule that allows cellular entry into nodes via the blood, in addition to afferent lymphatic cell entry. E) the absorption of dietary fats and fat-soluble vitamins is dependent on intestinal villi, finger-like, enterocyte-lined extensions of the gut wall, containing a blood capillary network (red/blue) and one or two central lymphatic vessels (green) termed lacteals. In mammals, dietary lipids are repackaged in enterocytes into large triglyceride-loaded particles or “chylomicrons” which are taken up by the lacteals.
Initial lymphatics first drain into pre-collecting lymphatic vessels that merge with larger secondary collecting lymphatics, in which LECs are connected to each other through tighter, continuous zipper-like junctions, and are covered with specialized muscle cells that provide contractile activity to assist lymph flow (Figure 1) (Baluk et al., 2007; Muthuchamy and Zawieja, 2008). Collecting lymphatic vessels have valves that regulate the unidirectional flow of lymph (Figure 1). The coordinated contraction of muscle cells facilitates the transport of lymph back to the blood circulation (Norrmen et al., 2009; Sabine et al., 2016). Tissue fluid transported via the collecting lymphatics drains into the thoracic duct and the right lymphatic duct, which in turn, discharge lymph into the large veins at the base of the neck (Figure 1) (Srinivasan and Oliver, 2011; van der Putte, 1975; Wang and Oliver, 2010; Yang and Oliver, 2014).
The lymphatic system also contains lymph nodes (> 200 in humans) (Figure 1), whose close integration with the lymphatic vasculature allows the system to initiate and expand immune responses, while simultaneously serving as a filtration barrier that prevents the return of noxious stimuli to the blood circulation. With the exception of a brief discussion about lymph nodes in inflammation and immunity, this review focuses mostly on the lymphatic vasculature. Lymphatic vessels have not yet been identified in avascular structures such as the epidermis, hair, nails, and cartilage, nor are they present in some vascularized organs such as the brain and retina. Abnormal lymphatic vasculature invasion into bone has been linked to vanishing bone syndrome (Gorham Stout disease, Dellinger et al., 2014), though the bone marrow is normally devoid of lymphatic vessels.
Lymphatic Vasculature Development
Major advances have been made in understanding how the lymphatic vasculature develops, particularly in mouse and zebrafish embryos (Geng et al., 2017; Hogan and Schulte-Merker, 2017; Kazenwadel and Harvey, 2018; Petrova and Koh, 2018; Tammela and Alitalo, 2010; Yang and Oliver, 2014). Also, in humans, mutations in several genes, most of which participate in aspects of lymphatic development, have been identified as responsible for different types of lymphatic disorders (Table 1). This knowledge has advanced our understanding of how defects in lymphangiogenesis contribute to human vascular disease.
Table 1.
Genes and Phenotypes Associated with Symptomatic Lymphatic Disorders
| Gene | Disease and Phenotype | Reference(s) |
|---|---|---|
| VEGFR3 | Nonne-Milroy disease | (Butler et al., 2007; Butler et al., 2009; Ferrell et al., 1998; Irrthum et al., 2000; Karkkainen et al., 2000) |
| Several heterozygous missense mutations impacting the tyrosine kinase activity of vascular endothelial growth factor receptor 3 (VEGFR3) are responsible for this disease, characterized by congenital bilateral lower limb lymphedema. Mutations in VEGFR3 are also responsible for the mutant mouse strain Chy with defective lymphatic vessels, chylous ascites and lymphedematous limb swelling after birth. | ||
| FOXC2 | Lymphedema-distichiasis (LD) syndrome | (Brice et al., 2002; Dagenais et al., 2004; Falls and Kertesz, 1964; Fang et al., 2000; Finegold et al., 2001; Ghalamkarpour et al., 2009; Neel and Schull, 1954; Petrova et al., 2004; van Steensel et al., 2009) |
| This autosomal dominant disorder is characterized by distichiasis (i.e., double row of eyelashes) at birth and bilateral lower limb lymphedema at puberty. The number of lymphatic vessels appears normal in patients with LD; however, they have impaired lymphatic drainage. This defect is likely a consequence of abnormal valve development/function and aberrant mural cell coating in the collecting lymphatic vessels of LD patients and Foxc2 mutant mice. The majority of FOXC2 mutations are insertions, deletions or nonsense mutations, leading to mRNA decay or truncated loss-of-function proteins. | ||
| SOX18 | Hypotrichosis-lymphedema-telangiectasia (HLTS) syndrome | (Francois et al., 2008; Irrthum et al., 2003; Pennisi et al., 2000) |
| A rare disease characterized by the absence of eyebrows and eyelashes, edema of the inferior members or eyelids, and peripheral vein anomalies. Ragged mice carrying point mutations in Sox18 are considered a model for HLTS. These mice exhibit defective vasculogenesis and folliculogenesis, as well as lymphatic vessel malformations, similar to those of humans with HLTS. | ||
| CCBE1 | Hennekam lymphangiectasia-lymphedema syndrome type 1 | (Alders et al., 2009; Alders et al., 2013; Bos et al., 2011; Bui et al., 2016; Connell et al., 2010; Hennekam et al., 1989; Jha et al., 2017; Van Balkom et al., 2002) |
| This syndrome is caused by homozygous and compound heterozygous mutations in the extracellular collagen and calcium-binding EGF domain-1 protein (CCBE1) and is characterized by severe peripheral lymphedema associated with intestinal lymphangiectasias, characteristic facial features, growth and mental retardation and hydrops fetalis. CCBE1 is important to facilitate the proteolytic cleavage and activation of the major VEGFR3 ligand, VEGFC. | ||
| FAT4 | Hennekam lymphangiectasia-lymphedema syndrome 2 | (Alders et al., 2014, Betterman et al., 2020; Pujol et al., 2017) |
| Homozygous and compound heterozygous mutations in FAT4, encoding the giant atypical cadherin FAT4 were identified in Hennekam syndrome patients in whom no CCBE1 mutations were found. FAT4 is important for coordinating LEC polarity in response to flow and as a result, regulates lymphatic vessel valve development. | ||
| ADAMTS3 | Hennekam lymphangiectasia-lymphedema syndrome 3 | (Brouillard et al., 2017; Jeltsch et al., 2014) |
| This syndrome is caused by loss of activity of the protease a disintegrin and metalloproteinase with thrombospondin motifs 3 (ADAMTS3), a protease also required for the proteolytic cleavage and activation of VEGFC. In these patients, bi-allelic missense mutations in ADAMTS3 were identified. | ||
| FBXL7 | Hennekam lymphangiectasia-lymphedema syndrome | (Boone et al., 2020) |
| Is caused by a homozygous single-exon deletion affecting FBXL7 (F-Box and leucine rich repeat protein 7). Data suggests that FBXL7 may be the fourth gene for Hennekam syndrome acting via a shared pathway with FAT4. | ||
| GJC2 | Late-onset autosomal dominant lymphedema | (Ferrell et al., 2010; Lyons et al., 2017; Ostergaard et al., 2011a) |
| Missense mutations in GJC2 (gap junction protein gamma-2) were discovered in a few families with late-onset autosomal dominant lymphedema affecting all 4 extremities; although some families showed reduced penetrance. GJC2 is a key effector of venous valve development, though the precise role of GJC2 in lymphatic vessels remains enigmatic. | ||
| GATA2 | Emberger syndrome | (Geng et al., 2016; Hahn et al., 2011; Kazenwadel et al., 2012; Kazenwadel et al., 2015; Ostergaard et al., 2011b) |
| Heterozygous, loss of function mutations in GATA-binding protein 2 were identified in patients with primary lymphedema with myelodysplasia progressing to acute myeloid leukemia (Emberger syndrome). GATA2 is important for the development and maintenance of lymphovenous and lymphatic vessel valves. | ||
| PTPN14 | Choanal atresia and lymphedema | (Au et al., 2010) |
| An intragenic deletion encompassing both sides of exon 7 of PTPN14 (protein tyrosine phosphatase, non-receptor type 14), a protein that by coimmunoprecipitation was shown to interact with VEGFR3 upon activation by VEGFC, was identified in a consanguineous family with autosomal recessive choanal atresia and lymphedema. | ||
| KIF11 | MCLMR | (Ostergaard et al., 2012) |
| Heterozygous mutations in KIF11 (kinesin family member 11, a DNAinteracting protein encoding the kinesin motor protein EG5) causes MLCRD (microcephaly, lymphedema, chorioretinal dysplasia) and CDMMR (chorioretinal dysplasia, microcephaly and mental retardation), 2 allelic syndromes that have now been regrouped as MCLMR (microcephaly with or without chorioretinopathy, lymphedema, or mental retardation). The role of KIF11 in the lymphatic vasculature remains to be established. | ||
| ITGA9 | Integrin-α9 (ITGA9) is mutated in primary lymphedema; missense mutations in this gene were reported in fetuses with congenital chylothorax. Similar to humans with this condition, Itga9-null mice exhibit chylothorax and die a few days after birth. Characterization of Itga9-conditional mutant embryos revealed that ITGA9 is required for proper lymphatic vessel valve morphogenesis. | (Bazigou et al., 2009; Huang et al., 2000; Ma et al., 2008) |
| REELIN | Congenital lymphedema and accumulation of chylous ascites has also been reported in patients with homozygous mutations in REELIN, which encodes an extracellular matrix protein guiding neuronal cell migration. At least three patients with such mutation exhibited persistent neonatal lymphedema and one has accumulation of chyle. Reelin deletion in mice has been demonstrated to result in impaired maturation of collecting lymphatic vessels, suggesting that collecting vessel dysfunction may underlie the lymphatic defects observed in patients. | (Hong et al., 2000; Lutter et al., 2012) |
| PIEZO1 | Generalized lymphatic dysplasia (GLD) | (Fotiou et al., 2015; Nonomura et al., 2018) |
| Homozygous and compound heterozygous mutations in PIEZO1 (a mechanically activated ion channel) lead to an autosomal recessive form of GLD, a rare form of primary lymphoedema characterized by uniform, widespread edema, with systemic involvement including intestinal and/or pulmonary lymphangiectasia, pleural effusions, chylothorax and/or pericardial effusions. PIEZO1 is important for lymphatic vessel valve development. | ||
| EPHB4 | Lymphatic-related hydrops fetalis | (Martin-Almedina et al., 2016) |
| Ephrin receptor B4 kinase–inactivating missense mutations were identified as responsible for autosomal dominant lymphatic-related hydrops fetalis. Hydrops fetalis is characterized by fluid accumulation in at least 2 fetal compartments. Most cases of hydrops fetalis are nonimmune in nature and approximately 15% are a consequence of a lymphatic abnormality. Functional inactivation of Ephb4 in mice results in defective lymphovenous valve formation and subcutaneous edema. | ||
| CALCRL | Hydrops fetalis with lymphatic dysplasia | (Mackie et al., 2018) |
| Nonimmune hydrops fetalis (NIHF) was associated with a recessive, in frame deletion in the G protein-coupled receptor, Calcitonin Receptor-Like Receptor (hCALCRL). |
Here, we summarize some critical steps guiding lymphatic development in the mouse embryo. In mammals, this starts with the molecular specification and budding of LEC progenitors expressing the master transcription factor Prox1 from the cardinal veins to form lymph sacs (Figure 2) (Oliver, 2004; Sabin, 1902; Wigle and Oliver, 1999). A Prox1-Vegfr3 autoregulatory feedback loop is required to regulate the number of LEC progenitors specified and to maintain their identity (Srinivasan et al., 2014). Prox1 upregulation of fatty acid β-oxidation is also required for maintenance of LEC fate through epigenetic modifications (Wong et al., 2017).
Figure 2: Key events underlying construction of the mammalian lymphatic vasculature during embryonic development.
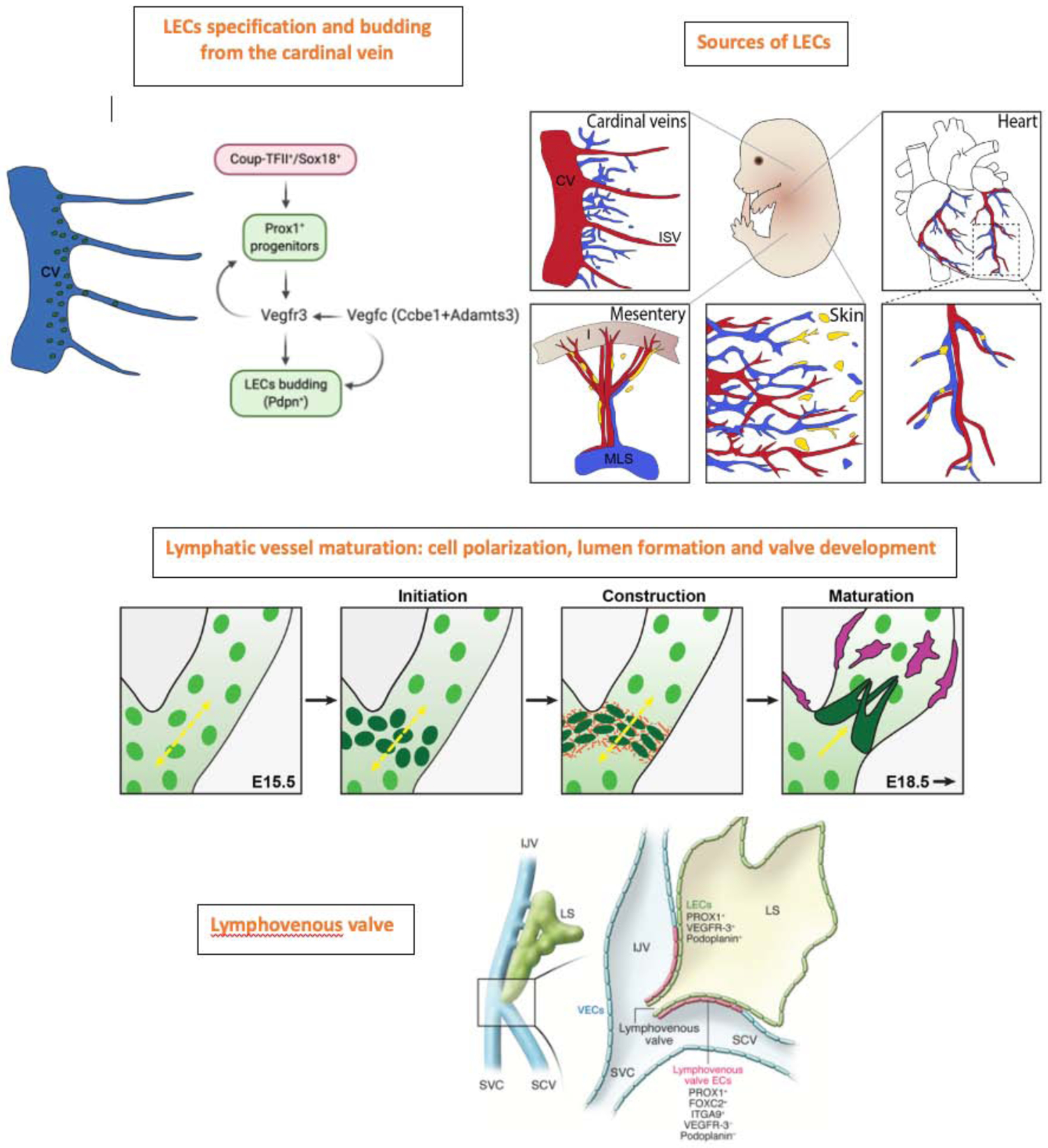
LEC specification and formation of LEC progenitors is initiated upon SOX18 and COUPTFII-mediated induction of Prox1 expression in embryonic venous endothelial cells, initially in the cardinal veins (CV). In turn, Prox1 activates the expression of Vegfr3, and Vegfr3 regulates Prox1 by establishing a feedback loop necessary to maintain the identity of LEC progenitors. Vegfc-mediated activation of Vegfr3 signaling is necessary to maintain Prox1 expression in LEC progenitors. Proteolytic processing of Vegfc by Ccbe1 and Adamts3 is required for lymphatic development to occur. Prox1+ LEC progenitors subsequently bud off from the CV and intersomitic vessels (ISV) and start to express differentiation markers such as podoplanin (Pdpn). Additional LEC progenitor cell sources contributing to lymphatic vasculature formation in other tissues include hemogenic endothelium in the case of mesenteric lymphatics, the second heart field in the case of ventral cardiac lymphatics and a group of cells within the dermal blood capillary bed which contribute to the dorsal dermal lymphatic vasculature. The initial lymphatic plexus is progressively arborized and lumenized and once flow within the network is initiated, valve development, crucial for unidirectional flow begins. Changes in cell polarity and the deposition of extracellular matrix are crucial for the generation of functional valves (shown in organge). An integral stage of lymphatic vessel maturation involves the recruitment of lymphatic muscle cells (magenta to the collecting lymphatics, acting to propel lymph back to the bloodstream. Schematic representing the lymphovenous valves. Most Prox1-expressing LEC progenitors bud off from the veins; however, a small subpopulation remains and forms the lymphovenous valves at the junction of the jugular and subclavian veins (SCV). Each of the valve’s two leaflets has two layers of PROX1+ ECs: an inner PROX1+/ PDPN+ layer continuous with the lymph sac and an outer PROX1+/PDPN− layer continuous with the veins. Left: The region of an E13.5 embryo in which the jugular and subclavian veins join to form the lymphovenous valves. Right: A frontal view of the boxed region shown at left. EJV, external jugular vein; IJV, internal jugular vein; LS, lymph sac; LV, lymphovenous valve; SVC, superior vena cava
The first lymph sacs to develop are the paired jugular lymph sacs, each retaining a connection to the adjacent vein (Kampmeier, 1969; Sabin, 1902; van der Putte, 1975), at which sites lymphovenous valves form (van der Putte, 1975). These sites are where interstitial fluid collected by the lymphatics is returned to the blood circulation (Figure 2). Defects in lymphovenous valve development significantly impact fluid homeostasis (Geng et al., 2017; Srinivasan and Oliver, 2011). The polarized sprouting of LECs from the sacs generates an interconnected network of peripheral lymphatics (van der Putte, 1975) that undergoes continued growth, remodeling and maturation to generate the entire functional lymphatic vascular network (Figure 2). Lymphatic vessel development is intricately linked with lymph node development, a process that is initiated embryonically and is dependent on the lymphatic vasculature (Bovay et al., 2018; Onder et al., 2017). The importance of embryonic events is evident in the developmental etiology of lymphatic vascular diseases, including primary lymphedema (Table 1).
The embryonic origin of lymphatic endothelial progenitor cells
The embryonic origin of LEC progenitors has been explored for over 100 years (Huntington and McClure, 1910; Sabin, 1902). Advances in genetic tools and imaging technology have shed new light on these events in recent years. The first studies to employ genetic lineage tracing techniques to address the embryonic origin of LECs (Srinivasan et al., 2007) confirmed Sabin’s earlier hypothesis that lymphatic vessels originate via the continuous, centrifugal sprouting of lymphatic endothelial cells from embryonic veins and lymph sacs (Sabin, 1902). Subsequent studies in mice using various Cre driver and reporter lines determined that LECs originate from both venous and non-venous progenitors in distinct embryonic tissues. Besides the venous source, hemogenic EC-derived cells were shown to contribute LEC progenitors to the mesenteric lymphatic vessels (Stanczuk et al., 2015). Similarly, in addition to venous-derived LEC progenitors, a group of cells within the dermal blood capillary bed (Pichol-Thievend et al., 2018) and a not yet identified, non-venous derived cell population (Martinez-Corral et al., 2015) contribute to the assembly of the dorsal dermal lymphatic vasculature. Most recently, a second heart field derived progenitor cell population was shown to contribute to the lymphatic vasculature on the ventral side of the heart (Lioux et al., 2020; Maruyama et al., 2019). Intriguingly, recent work has revealed that the overarching progenitor lineage contributing to most Prox1-positive LECs within the mouse embryo originates from the paraxial mesoderm (Stone and Stainier, 2019).
A universal feature of vertebrate LECs is their dependence on Prox1 for the specification and maintenance of LEC identity (Johnson et al., 2008; Wigle et al., 2002). Key roles for the transcription factors Sox18 (Francois et al., 2008), CoupTFII (Srinivasan et al., 2010), Gata2 (Kazenwadel et al., 2012; Kazenwadel et al., 2015) and Hhex (Gauvrit et al., 2018) in regulating Prox1 expression have been uncovered (Figure 2).
Lymphatic vessel growth and maturation
VEGF-C signaling via VEGFR3 is the major signaling axis driving embryonic lymphangiogenesis in vertebrates (Figure 2). The emergence and continued migration of LECs from the cardinal veins to form the initial lymphatic structures of the embryo depends on VEGFC (Karkkainen et al., 2004) and on two factors that control its proteolytic cleavage and activation; the matrix protein Ccbe1 (Bos et al., 2011; Hagerling et al., 2013; Hogan et al., 2009; Le Guen et al., 2014) and the metalloprotease Adamts3 (Bui et al., 2016; Jeltsch et al., 2014). Additional receptors expressed by LECs that contribute to the activity of this pathway include neuropilin 2 (Nrp2), which binds VEGF-C and is important for lymphatic vessel sprouting (Yuan et al., 2002) and β1-integrin, which is activated in response to the mechanical stimulus of increased interstitial volume and facilitates VEGFR3 phosphorylation and activation (Planas-Paz et al., 2012).
Cell polarization and lumen formation and maintenance are key, interdependent events in lymphatic vessel development. Correct cell polarity is important for vessel growth and function; LECs need to respond to sprouting and guidance cues to navigate their appropriate course and to establish luminal and abluminal surfaces, which have a distinct array of cell surface proteins. Cell polarity regulation is also important during valve development. Recent work in mice has identified important mediators of both cell polarity and lumen formation. Key regulators of epithelial planar cell polarity, Celsr1, Vangl2, Pkd1, Pkd2 and Fat4 are also employed to control LEC polarity and are important for valve development (Betterman et al., 2020; Coxam et al., 2014; Outeda et al., 2014; Pujol et al., 2017; Tatin et al., 2013). Intriguingly, FAT4 is redistributed in LECs upon exposure to flow and is important for flow-induced LEC elongation (Betterman et al., 2020), highlighting that mechanical signals, including fluid flow within vessels, are important drivers of vessel morphology. Rasip1, a Ras-interacting protein, is crucial for regulating lymphatic vessel lumen size via regulating the integrity of LEC junctions and is also important for valve development (Liu et al., 2018). Polarity proteins provide a link to the cytoskeleton, underpinning changes in cell shape and orientation that are important for valve construction (Figure 2) (Bazigou and Makinen, 2013).
Transcriptional regulators that drive valve development and are regulated by shear stress include Foxc2 (Sabine et al., 2012; Sabine et al., 2015) and Gata2 (Kazenwadel et al., 2015; Sweet et al., 2015). While the sensors that transduce flow-initiated signals in LECs are incompletely understood, recent work has revealed that channel proteins, including Orai1 (Choi et al., 2017) and Piezo1 (Choi et al., 2019), are shear sensors in LECs and initiate downstream signaling events important for valve development. Recent studies report that canonical Wnt/β-catenin signaling is also activated in LECs in response to fluid flow and is important for lymphovenous and lymphatic vessel valve development (Cha et al., 2016; Cha et al., 2018). Intriguingly, in addition to oscillatory shear stress driving Gata2 elevation in LECs, Gata2 levels are also regulated by ECM stiffness, providing a mechanism by which mechanical signals in the LEC microenvironment drive programs of gene expression important for key events in lymphatic vessel morphogenesis (Frye et al., 2018).
The recruitment of muscle cells to collecting lymphatic vessels is an important aspect of lymphatic vessel maturation, facilitating lymphatic vessel contraction and the return of lymph to the bloodstream. As in blood vessels, PDGFB expression in the LECs of collecting vessels, coupled with ECM-mediated PDGFB tethering, is important for muscle cell recruitment (Wang et al., 2017), although intriguingly, muscle cells are not recruited in the vicinity of valves. Lymphatic muscle cell recruitment is also regulated by semaphorin/neuropilin signaling, specifically via Sema3a and Nrp1 (Bouvree et al., 2012; Jurisic et al., 2012), and by angiopoietin2 (Dellinger et al., 2008; Gale et al., 2002).
Future work focusing on mammalian lymphatic development should conclusively determine the controversial origin/s of LEC progenitors in embryogenesis and during organ repair, and decipher whether LECs derived from distinct progenitor pools have distinct, tissue specific functions. It will be important also to profile organ-associated LECs to gain insight into LEC heterogeneity and specialization to enable the specific targeting of organ-associated vessels for therapeutic purposes. As evident from the compendium of genes in which mutations have been identified in human lymphatic vascular disorders (Table 1), dissecting the cellular and molecular mechanisms underpinning developmental lymphangiogenesis is crucial for understanding the origin of human lymphatic diseases, and will provide new and important opportunities for developing novel therapeutics to treat these pathologic conditions. Accordingly, and as discussed below, a better understanding of how mutations in different genes impact the normal function of the lymphatic vasculature, and the identification of novel pro-lymphangiogenic factors will facilitate the diagnosis and eventually, treatment of patients with symptomatic or asymptomatic lymphatic disorders and associated comorbidities.
Functional Roles of the Lymphatic Vasculature in Health and Disease
Lymphatics have conventionally been considered a passive route for the transport of fluid and immune cells, playing a critical role in adaptive immune responses and also in the absorption of dietary fat in the gastrointestinal tract. Lymphatic vasculature malfunction had been associated with symptomatic pathological conditions such as primary and secondary lymphedema and lymphatic vessels were recognized as an important highway for tumor metastasis (Figure 3). In the last few years this traditional view has expanded, such that morphological or functional defects in the lymphatic vasculature have now been identified in a growing list of medical conditions (Figure 4). Below, we will briefly review traditional roles for lymphatics and discuss novel, recently identified functional roles together with the implications for human disease.
Figure 3. Traditional lymphatic-associated processes.
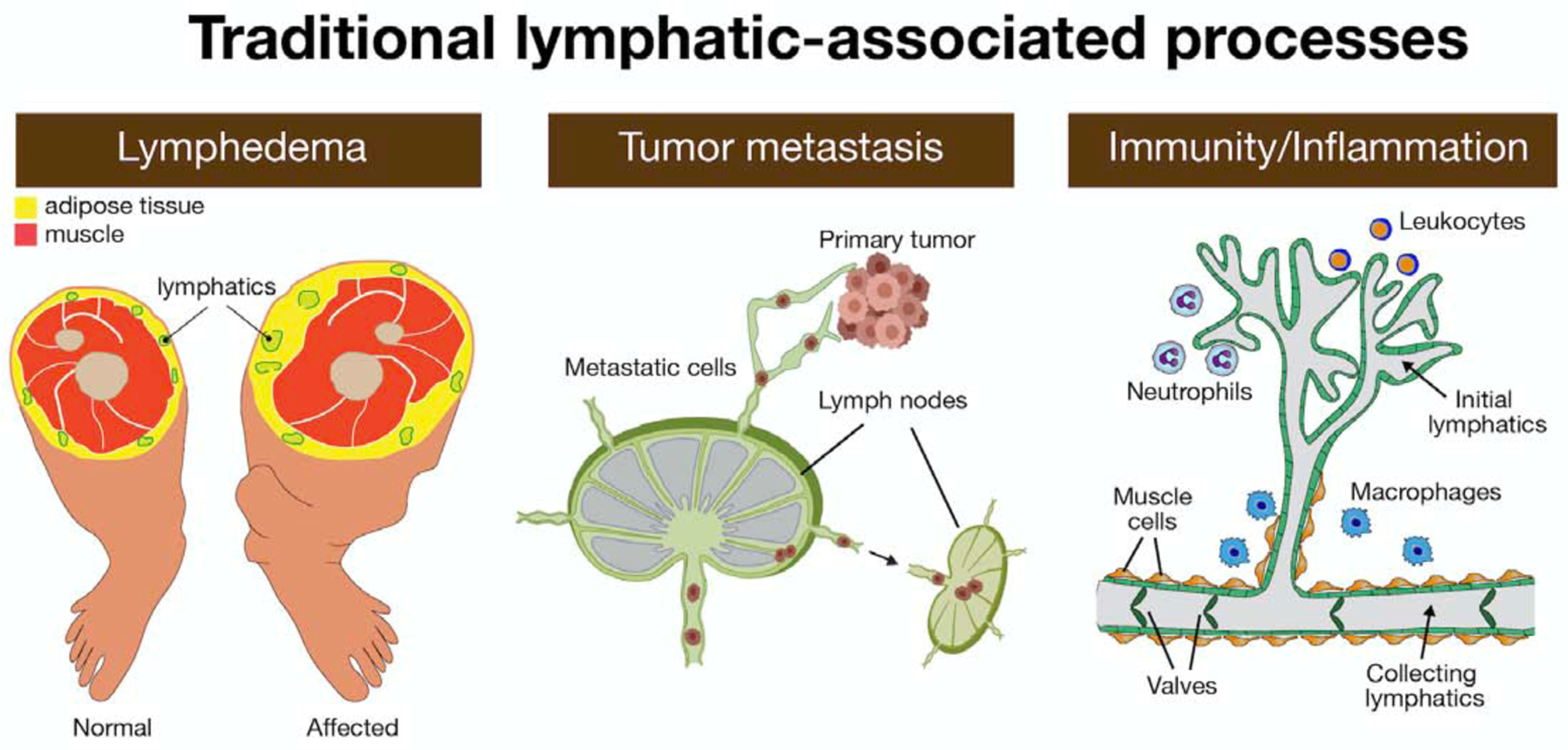
Lymphatic malfunction leads to vascular malformations, and primary and secondary lymphedema. Lymphedema is a disfiguring, disabling, and occasionally life-threatening disease that is characterized by fluid accumulation and the chronic and disabling swelling of the extremities. Dilated damaged and leaky lymphatics do not support the normal flow of lymph and promote unilateral edema and increased adipose tissue accumulation in the affected leg. Lymphatic vessels serve as a route by which tumor cells metastasize. Tumor lymphangiogenesis induced at the location of the primary tumor can facilitate the entry of metastatic tumor cells into lymphatic vessels and lymph nodes, while also supporting immunity to tumors for immune-mediated rejection. Lymphatic function is also important during immune and inflammatory responses. Initial lymphatics composed of a single layer of loosely connected LECs (in green) lack a continuous basement membrane and lymphatic muscle cells. These vessels are highly permeable to interstitial fluid and macromolecules, pathogens, and immune cells (i.e, leukocytes, neutrophils, macrophages). The initial lymphatics drain into larger collecting lymphatics in which LECs are connected to each other through tighter, continuous zipper-like junctions, are covered with muscle-like cells and have valves that regulate the unidirectional flow of lymph.
Figure 4. Schematic representation of the major novel functional roles of the lymphatic vasculature in health and disease.
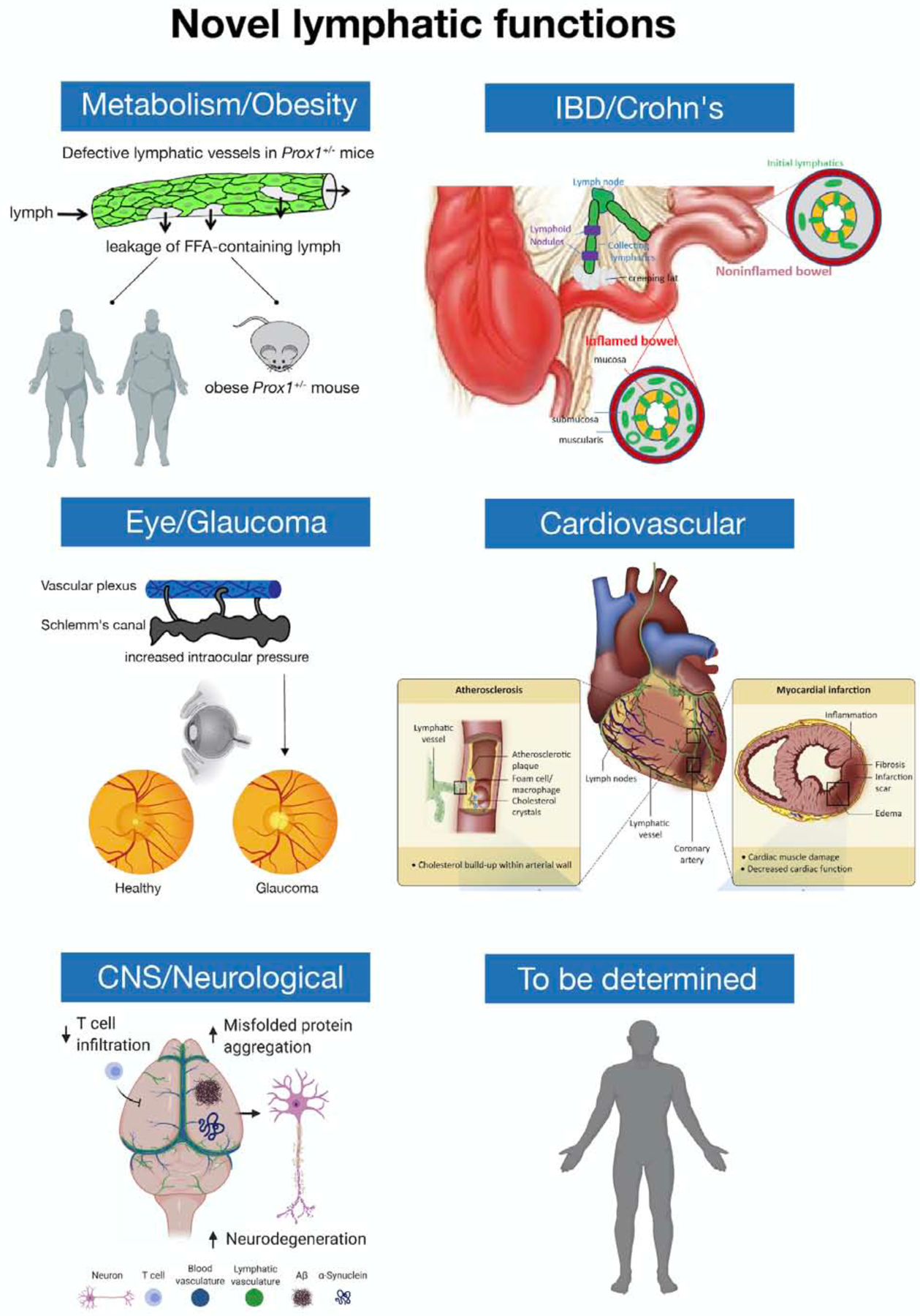
Recent work has uncovered important roles for the lymphatic vasculature in normal and pathological processes. Conditions in which lymphatic vessels are implicated include obesity, IBD/Crohn’s disease, cardiovascular pathologies (atherosclerosis and myocardial infarction), glaucoma and neurological diseases (Alzheimer’s, Parkinson’s, stroke and brain trauma, multiple sclerosis and brain tumors, age-related cognitive decline). In obesity, a direct link between asymptomatic defective and leaky lymphatics and increased adipogenesis and obesity was demonstrated so far in mice (Prox1+/−). It is possible that similar alterations are also responsible in certain forms of obesity in humans. In Crohn’s disease, lymphatics proliferate at the inflamed gut wall where creeping fat is frequently also observed, but this proliferation is counterbalanced by leukocyte-rich obstructions present in the collecting lymphatics. These regions may also be leaky, and leakiness as discussed for obesity, in turn, may drive creeping fat. Crohn’s disease patients with the least lymphatic expansion are more likely to experience relapse after bowel resection. Recent studies suggested a beneficial role for lymphatics in restoring heart function after cardiac injury and that cardiac lymphatic vessels could be therapeutic targets to restore cardiac function after injury. In cardiovascular diseases, atherosclerosis, characterized by the accumulation of plaques comprising fat, cholesterol and immune cells inside the arterial vessel wall, results in the narrowing and hardening of arterial walls, limiting blood flow from the heart. Lymphatics are found at atherosclerotic sites in the adventitial layer of coronary arteries. Myocardial infarction is a life-threatening condition that occurs when blood flow to the heart abruptly cuts off, usually as a consequence of blockage in the coronary arteries, resulting in tissue damage and massive cardiomyocyte death that leads to the formation of fibrotic tissue, pathological remodeling and eventually heart failure. In glaucoma, resistance to aqueous humor outflow is increased, reducing drainage through the Schlemm’s canal and resulting in elevated intraocular pressure and optic neuropathy. Lymphatics are also important novel players in a variety of neurological disorders. It is likely that additional novel functional roles of the lymphatic vasculature in normal and pathological settings will be identified.
Traditional Lymphatic-Associated Pathological Processes
Primary Lymphedema
Although few life-threatening diseases are caused by lymphatic vascular malfunction, defective lymph flow promotes several congenital and acquired disorders, including lymphedema. Lymphedema is a disfiguring, disabling, and occasionally life threatening disease that is characterized by fluid accumulation, t he chronic and disabling swelling of the extremities (Figure 3), tissue fibrosis, subcutaneous fat accumulation, poor immune function, impaired wound healing and susceptibility to infection (Rockson, 2001; Witte et al., 2001). Primary lymphedema has a genetic origin, and secondary lymphedema occurs as a consequence of surgery (such as lymph node resection after breast cancer), infection (as in filariasis) or following radiation therapy (Rockson, 2001; Witte et al., 2001). Nonne-Milroy’s (Milroy, 1892) (Table 1) and Meige (Meige, 1898) disease are examples of primary lymphedemas that appear at birth or puberty, respectively. In general, abnormally dilated lymphatic vessels are seen in Meige’s disease (causes are yet unknown but most likely are genetic and environmental) and underdeveloped lymphatics in Milroy’s. Both result in insufficient lymph transport due to the occlusion of lymphatic drainage caused by the hypoplasia, or impaired lymphatic function of lymphatic vessels, (Witte et al., 2001).
Numerous genetic defects underlying primary lymphedema have been identified in humans in recent years. Among the genes associated with different types of lymphatic disorders are: FLT4 (VEGFR3), FOXC2, SOX18, CCBE1, FAT4, ADAMTS3, FBXL7, GJC2, GATA2, PTPN14, KIF11, ITGA9, REELIN, PIEZO1, EPHB4 and CALCRL. Major phenotypes associated with mutations in these genes are listed in Table 1 and most of those genes participate in different steps of developmental lymphangiogenesis. A full list of the genes identified so far in different lymphatic anomalies is available in Brouillard et al., 2014, Mendola et al., 2013, Jones and Mansour, 2017 and Gordon et al., 2020.
Secondary Lymphedema
Worldwide, most cases of lymphedema are secondary and occur as a consequence of damage to the lymphatic vasculature. Filariasis (i.e., elephantiasis) is the most common cause, and mostly affects people living in tropical regions (Pfarr et al., 2009; Wynd et al., 2007). Filariasis is caused by mosquito-borne worm parasites that invade the lymphatic system, where an inflammatory reaction triggers the production of VEGF, VEGF-C and VEGF-D. Eventually, hyperplasia, obstruction, and extensive damage of the lymphatic vasculature follows, resulting in chronic lymphedema in the lower limbs or genitalia and permanent disability (Pfarr et al., 2009; Rockson, 2001). The leading cause of secondary lymphedema in the industrialized world is lymph node dissection or radiation therapy that damages the lymphatic vasculature after cancer surgery (Rockson, 2001). It affects 15%−20% of women undergoing breast cancer treatment (Vignes et al., 2007). Unfortunately, lymphedema treatment is still based mainly on conservative therapies, such as manual drainage, massage, compression garments, liposuction, and dietary modification (such as limiting the consumption of long-chain fatty acids) (Brorson, 2003; Rockson, 2001). However, the use of pharmacotherapy or pro-lymphangiogenic factors such as VEGFC offers a promising alternative treatment for secondary lymphedema in the near future (Alitalo, 2011; Dayan et al., 2018; Rockson et al., 2018; Tian et al., 2017).
Lymphatics in Immunity
A key feature of the lymphatic system is its critical role in adaptive immune responses and provision for removal of substances or stimuli from tissues that might promote harmful reactions in the circulation. These features are made possible by the inclusion of lymph nodes along every major lymphatic route from tissues to the bloodstream (Figure 1). All lymphatics upstream of lymph nodes are called afferent lymphatics and those emanating from lymph nodes, efferent lymphatics (Figure 1). Because lymph nodes can be found in groups often referred to as chains (but more like beads on a string), the lymphatic vessels connecting one lymph node to another can be efferent to one lymph node while afferent to another.
Briefly, during development, lymphatic vessels supply critical lymph node organizer roles while interacting with specialized lymphocytes called lymph node inducer cells (Bovay et al., 2018; Onder et al., 2017). One of the key signals in the interaction of lymphatics and lymph node inducers is provided through lymphotoxin engagement of the lymphotoxin receptor (De Togni et al., 1994; Drayton et al., 2006; Onder et al., 2017). Mice lacking lymphotoxin or its receptor fail to develop lymph nodes (De Togni et al., 1994), but this ultimately does not prevent lymphatic vasculature development. Flow within nascent lymphatic vessels provides spatial and mechanical cues to form the lymph node capsule (Bovay et al., 2018), allowing sophisticated integration of flow through the lymph node as the vessels and the nodes complete their development. The lymphatic endothelium of the afferent lymphatic collecting vessel forms the floor of the lymph node subcapsular sinus, which serves to elegantly coordinate the entry of cells and molecules into the parenchyma of the lymph node (Martens et al., 2020), where flow is further regulated by the existence of specialized conduits (Roozendaal et al., 2009). Exit of cells and molecules from the lymph node is provided by the medullary sinuses located in the hilum of the lymph nodes, and these sinuses too are comprised of LECs (Cyster and Schwab, 2012).
Through integration with afferent lymphatics, lymph nodes receive antigens derived from the tissues that they drain, ranging from intact micro-organisms to partially degraded material from these organisms. Lymph carries immune cells, such as dendritic cells, that have engulfed and processed antigens for immune presentation in the lymph node, along with an abundance of lymphocytes (Randolph et al., 2017). Given that many antigens consist of non-self, foreign molecules, it is vital for the integrity of the immune response that the lymphatic vasculature does not require receptor engagement for the entry of most cargo. Instead, it relies on the open, button-like junctions of this circulatory system (as discussed above), or on high levels of endocytosis and transcytosis (Kähäri et al., 2019; Triacca et al., 2017).
In mammals, when lymph enters lymph nodes, its cargo is sorted to optimize immune responses. This is largely achieved by the elegant organization of the lymph node conduit system, which separates small from large molecules (Gerner et al., 2017) and allows antigens to be concentrated through the removal of water (Adair et al., 1982; Czepielewski and Randolph, 2018). LECs might also participate in antigen presentation (Randolph et al., 2017). Single-cell RNA sequencing has shown that up to six types of LECs exist in human lymph nodes that correspond to distinct locations in the node (Takeda et al., 2019), with each expressing distinct chemokine profiles and decoy receptors that scavenge chemokines to maintain gradients of chemotactic signals that guide immune cells to areas of their highest functional concentration.
One striking finding from the data defining LEC diversity is the presence of signals in the lymph node that appear to attract neutrophils (Takeda et al., 2019), a cell type of low abundance in resting lymph nodes. Indeed, it is becoming increasingly clear that neutrophil recruitment into reactive lymph nodes plays a role in programming adaptive immunity and the lymph node’s ability to contain infectious agents (Figure 5) (Bogoslowski et al., 2018; Chtanova et al., 2008; Yang and Unanue, 2013). The containment of infectious agents within lymph nodes may have been a major driver in the evolution of the lymphatic vasculature (Miller and Simon, 2018).
Figure 5: A role for lymph nodes in the containment of tumor metastases and infection.
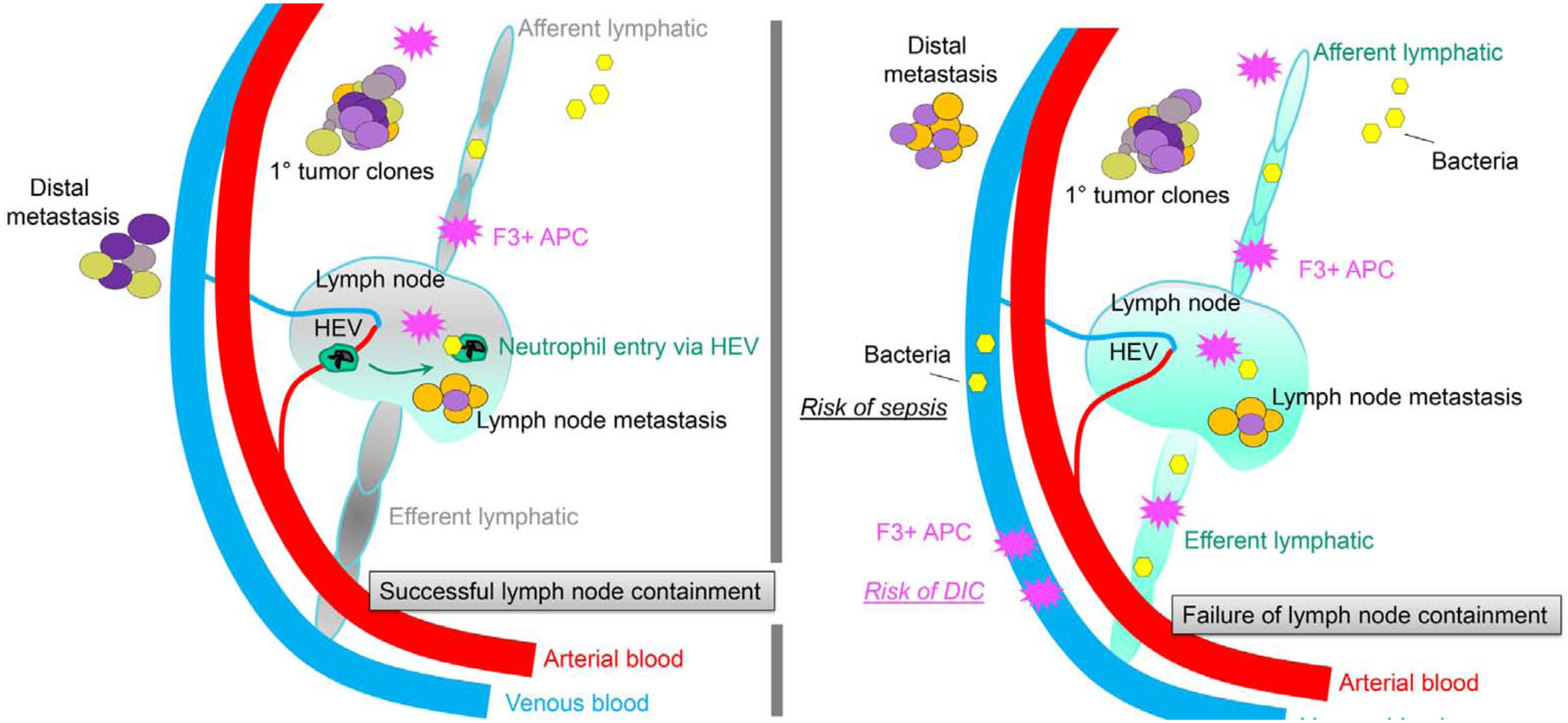
A traditional function of lymphatic vessels is their association with lymph nodes. More detailed structure of lymph nodes is shown in Figures 1 and 3. As shown in the left panel, lymph nodes effectively contain pathogens, in part by coordinating the recruitment of neutrophils through high endothelial venules of the blood stream that, separately from lymphatics, invest lymph nodes. Lymph nodes also serve a key role in containing activated antigen-presenting cells that express tissue factor (factor 3, F3) that functions to initiate coagulation. Finally, lymph nodes often limit metastases that arrive to the lymph node; that is, many metastases to lymph nodes are stopped therein and do not account for distal spread of the tumor. Each putative, distinct tumor clone is shown in a separate color. Right panel depicts outcomes that can occur if lymph node containment dramatically fails. The resulting severe disease risks that emerge under these conditions include enhanced tumor spread, sepsis and spread of infection from organ to organ, and infection-associated disseminated intravascular coagulation (DIC) that results from failed lymph node containment of F3-expressing antigen-presenting cells and their arrival to blood, as seen in Ebola virus infection.
Lymph nodes are also elegant sorting facilities that promote adaptive immunity. Lymphocyte recruitment to lymph nodes, either via a lymph node’s blood supply or afferent lymphatic vasculature, brings lymphocytes into close proximity with antigen-presenting cells (APCs) in a highly efficient manner. Lymphocytes, the TCRs of which engage with cognate peptide-MHC in the node, are retained in the node by sustained loss of the receptor S1P1 for sphingosine 1 phosphate (S1P) from the cell surface (Cyster and Schwab, 2012). One physiologic mechanism for removal of S1P1 from the T cell surface is binding to the activation antigen CD69 that it turns prompts internalization and degradation of S1P1 (Cyster and Schwab, 2012). Eventually, as CD69 expression wanes, lymphocytes can leave the lymph node by sensing S1P produced by LECs at the medullary sinuses of the lymph node (Figure 1) (Cyster and Schwab, 2012). They then recirculate, preferentially to the organ that drains to the lymph nodes where they were stimulated.
In contrast to T cells, most APCs, particularly dendritic cells but also monocytes and macrophages, cannot leave the lymph nodes (Randolph et al., 2005). Indeed, keeping the cells that first phagocytize or become infected with an infectious agent in tissue parenchyma away from the blood vasculature is extremely important in host defense. This is because activated dendritic cells or monocytes/macrophages might otherwise spread infection from organ to organ, as they are known to do during toxoplasmosis (Drewry et al., 2019). When these cells are activated, they also upregulate molecules that initiate blood clotting, such as tissue factor (Grover and Mackman, 2018). Tissue factor, which initiates clotting in the extrinsic pathway of coagulation, expressed on blood-borne monocytes is thought to drive the hemorrhagic, intravascular coagulation complications seen in infectious diseases, such as Ebola (Geisbert et al., 2003). Although egress from lymph nodes is typically low, even the low level that occurs can lead to negative outcomes; the blockade or deficiency of S1P1 aids in reducing such perilous trafficking from the lymph node in Yersinia infection models (St. John et al., 2014).
Sepsis and disseminated intravascular coagulation are two extreme examples of outcomes that the sequestration of myeloid A P C s in lymph nodes might prevent (Figure 5). However, the vertebrate organism zebrafish has lymphatic vessels but no known lymph nodes, which seems inconsistent with the concept that we propose herein that the segregation of activated, procoagulant cells from the bloodstream is a key driver in the evolution of the lymphatic vasculature. Perhaps the zebrafish lymphatic vasculature has other features that limit the dissemination of cellular or molecular cargo to the bloodstream after entering lymphatics from tissues, an interesting question for future research to address.
Lymphatic Vessels in Cancer Progression and Metastasis
Given that the immune system can be harnessed to combat tumor progression, the functionality of the lymphatic vasculature can also markedly impact the control of tumor progression, as discussed below. The relationship between lymphatic vessels and tumor biology has been an area of ongoing research interest, which first took off after evidence emerged that lymphatic vessels supported metastasis (Skobe et al., 2001). The initial interpretation of these findings was that lymphatic vessels simply supported the development of a physical route by which tumor cells might leave one site, mobilize to a draining lymph node, and then beyond. However, evidence later emerged that lymphangiogenesis in tumors was sometimes associated with immunosuppression or immune tolerance (Lund et al., 2016), which might explain why high lymphatic density in tumors can be associated with poor prognosis. This immune tolerance possibly occurs because the transported signals, including peptides derived from cells in the drained tissue, are regulatory or tolerogenic. The presence of tumor-derived products needs to be very high in lymph draining tumor sites to foster immune responses (Broggi et al., 2019).
By contrast, genetic models that have a paucity of lymphatic vessels unexpectedly show greater tumor control compared with controls with normal lymphatics (Steinskog et al., 2016). Also, at odds with the findings discussed above is that various tumor types in different models show greater immune-mediated tumor regression when lymphatic vessels were supported with growth factors like VEGFC (Fankhauser et al., 2017; Song et al., 2020). In a glioblastoma model, tumor rejection was especially robust when VEGFC therapy to support lymphatic vessel expansion was coupled with an anti-PD1 checkpoint blockade (Song et al., 2020). These different responses likely depend on the coupling of lymph transport with signals that either support the development of killer T cells or are more tolerogenic. Furthermore, as some tissue sites are immune-privileged, such as the brain, improved lymphatic drainage can significantly reverse the adverse effects of immune privilege by allowing a tumor to be exposed to the immune system.
In several tumor types, lymphatics are found in association with tertiary lymphoid tissue (TLT) embedded within the tumor. Consistent with the literature cited above, the impact of this lymphoid tissue on immune-mediated tumor tolerance versus rejection, depends on the nature of the tumor environment, as discussed in the references below. In the absence of a checkpoint blockade, TLT can promote tumor growth (Shields et al., 2010), but in the context of checkpoint blockade, TLT in tumors can serve as potent sites of anti-tumor immunity (Cabrita et al., 2020; Helmink et al., 2020; Hiraoka et al., 2015; Petitprez et al., 2020). It is unclear at present if immune cells access these intra-tumoral structures via a lymphatic vessel that supports short-distance travel to the tertiary structure from different parts of the tumor, or if immune cells enter them through migration directly from the surrounding tumor. Likewise, it is unknown if tumor-killing T cells exit the tertiary lymphoid structures via efferent lymphatic vessels and are programmed to return to the tumors via blood vessels, or if T cells directly move out of TLT into the tumor parenchyma. It will be interesting in the future to 3D image tumors that bear tertiary lymphoid structures to determine if they are themselves connected with afferent and efferent lymphatic vessels that serve a draining lymph node, as seen for tertiary lymphoid tissue in Crohn’s disease (Randolph et al., 2016).
In order for lymph nodes to become a supportive metastatic niche, tumors that pass through lymphatic vessels to reach lymph nodes must undergo significant metabolic remodeling (Lee et al., 2019). Interestingly, by tracking the clonal history of the primary tumor and its relationship with different metastases, one can argue that a given site for metastasis possesses properties that render it favorable enough to support multiple, distinct waves of tumor cell arrival to the metastatic location (Heyde et al., 2019). Importantly, however, different sites of metastases arise from different clones in the primary tumor (Naxerova et al., 2017). It is clear that, at least for colorectal cancer metastases, 65% of lymph node metastases are unrelated to distal metastases (Naxerova et al., 2017). This finding casts doubt on the concept that the lymphatic vasculature and associated lymph nodes are robust conduits for distal metastases; they might instead act as barriers to tumor dissemination (Figure 5) (Naxerova et al., 2017).
Nonetheless, at least some distal metastases arise from tumors that have first passed through lymph nodes (Naxerova et al., 2017) (Figure 5). In an experimental model of metastasis in mice, distal metastases of B16 melanoma seeded the lung after passing through skin draining lymphatics and lymph nodes (Pereira et al., 2018). Overall, it appears as though the role of lymphatics in tumor progression is moving away from the great concern early in the 21st century that lymphatics might only promote cancer via enhanced metastasis, towards the current realization that the promotion of lymphangiogenesis, coupled with methods to enhance T cell-mediated immunity, might be especially efficacious for treating cancer, while not increasing the risk of metastasis, at least in certain tumor types. A future focus must be on gaining a better understanding of what manipulations are needed in concert with therapies that support lympangiogenesis to ensure that tumor regression prevails over tumor progression.
Up to here we briefly reviewed our current understanding about the traditional views (Figure 3) of the functional roles of the lymphatic vasculature in health and disease. Great progress has been made in the last years, particularly in the diagnoses and treatment of some of these lymphatic-promoted pathological conditions. It is likely that the identification of reliable biomarkers of lymphatic disease will facilitate a better diagnosis and treatment of patients in the near future, in particular of those with medical conditions not exhibiting obvious lymphatic associated defects.
Novel Functional Roles of the Lymphatic Vasculature
Recent discoveries have changed our conventional views about the roles of lymphatics in health and disease, such that lymphatics are now considered to actively modulate or participate in major physiological and pathophysiological processes. Somewhat unexpectedly, morphological or functional defects in the lymphatic vasculature have been associated with the medical conditions discussed below (Figure 4). These new data argue that the identification of underlying asymptomatic lymphatic defects in a wide array of human diseases could lead to enhanced diagnostics and, potentially, novel therapeutics and preventive strategies.
Obesity
A variety of factors are now accepted as contributing to obesity, in addition to excessive dietary intake or inadequate energy utilization. Obesity is a key risk factor for metabolic and cardiovascular diseases, including type 2 diabetes, hypertension, coronary heart disease, stroke and dyslipidemia (Escobedo and Oliver, 2017; Friedman, 2000; Kopelman, 2000; Roth et al., 2004).
Most nutrients are absorbed by blood vessels, but the absorption of dietary fats and fat-soluble vitamins depends on intestinal villi, finger-like, enterocyte-lined extensions of the gut wall. Gut villi are filled with connective tissue that contains a blood capillary network and one or two central lymphatic vessels, termed lacteals (Figure 1). Lacteals form during late mouse embryogenesis, expand into the villus at early postnatal stages and undergo continuous remodeling (Bernier-Latmani and Petrova, 2017). In mammals, dietary lipids are repackaged in enterocytes into large (200–1,000 nm) triglyceride-loaded particles or “chylomicrons,” which are secreted basally into the intestinal stroma. In addition to triglycerides, chylomicrons also incorporate fat-soluble vitamins and some microbiota components, such as bacterial lipopolysaccharides (Bernier-Latmani and Petrova, 2017; Petrova and Koh, 2018). Lacteals take up chylomicrons and other interstitial fluid components from villi and transport them to the submucosal and mesenteric collecting lymphatic vessels. Intestinal lymph is transported via the mesenteric lymph nodes and thoracic duct to the blood circulation. Lacteal function controls dietary lipid absorption and, therefore, body weight (Cifarelli and Eichmann, 2019; Jiang et al., 2019; McDonald, 2018; Zhang et al., 2018).
Angiogenesis is tightly associated with the outgrowth of adipose tissue, as expanding adipose tissue requires increased nutrient supply from blood vessels. Despite the well-established connections between lymphatics and lipids, and the key roles of intestinal lacteals in dietary fat absorption, evidence emerged only recently indicating that crosstalk occurs between lymphatics and adipose tissue and that lymphatic function is associated with metabolic diseases and obesity (Blum et al., 2014; Escobedo et al., 2016; Escobedo and Oliver, 2017; Harvey et al., 2005). Early studies recognized that lymph nodes and collecting lymph vessels are surrounded by fat, that adipose tissue accumulates in affected tissues of lymphedema patients (Tavakkolizadeh et al., 2001; Wang and Oliver, 2010) and that dermal lipid accumulation is a feature of idiopathic lymphedema patients (Pond, 2005; Rosen, 2002).
Initial data supporting the existence of a relationship between defective lymphatics and obesity was provided by mouse models with lymphatic defects, and by secondary lymphedema patients with associated adipose accumulation. In the Chy mouse model of lymphedema, heterozygous inactivating mutations in Flt4 led to abnormal subcutaneous fat deposition, particularly in the edematous subcutaneous adipose layer adjacent to dysfunctional, hypoplastic lymphatic vessels (Karkkainen et al., 2001; Rutkowski et al., 2010). Severe lymphatic defects and adult onset obesity are also seen in Prox1 heterozygous (Prox1+/−) mice (Harvey et al., 2005), in which excessive fat accumulates and is associated with defective, leaky lymphatic vessels, particularly in the mesentery (Figure 4) (Escobedo et al., 2016; Harvey et al., 2005). These phenotypes led to the proposal that subtle abnormal lymphatic leakage of chyle promotes adipocyte hypertrophy and/or ectopic adipogenesis. Importantly, chyle collected form the thoracic cavity of newborn Prox1+/− pups promoted adipogenesis in vitro (Harvey et al., 2005), and the adipogenic factor within the chyle was identified as a lipid (Escobedo et al., 2016), leading to the conclusion that lymph is adipogenic. These studies provided the first conclusive experimental results that link lymphatic vascular malfunction with obesity.
Support for reciprocal crosstalk occurring between lymphatic vessels and adipose tissue came from the discovery that obese mice have impaired lymphatic function, characterized by leaky lymphatics, and a reduced collecting vessel pumping capacity (Blum et al., 2014; Garcia Nores et al., 2016; Hespe et al., 2016; Nitti et al., 2016; Savetsky et al., 2014; Torrisi et al., 2016). In mice with high fat diet (HFD)-induced obesity, it was found that obesity reduced lymphatic function and increased inflammation, leading to increased subcutaneous adipose deposition, elevated inflammation and fibrosis, resulting in a more severe lymphedema phenotype (Savetsky et al., 2014). Being overweight is also an important risk factor for lymphedema (Swenson et al., 2009); in obese patients the lymphatic drainage of macromolecules is significantly reduced in abdominal subcutaneous adipose tissue (Arngrim et al., 2013). Recent work argues that dietary changes alone are insufficient to induce lymphatic dysfunction, and that this lymphatic malfunction is mainly due to obesity-promoted inflammation (Garcia Nores et al., 2016).
Previous findings have also shown that elevated fatty acids in the plasma of HFDfed mice induced leakiness in lymphatic and vascular structures via apelin depletion, resulting in adipocyte hypertrophy and obesity (Sawane et al., 2013). Apelin is an endogenous ligand of the apelin receptor (APJ), a seven-transmembrane G protein-coupled receptor and has a wide tissue distribution in the brain, as well as in various peripheral organs including heart, lung, vessels and adipose tissue. In cultured cells, it promotes lymphangiogenesis and plays important roles in lymphatic tumor progression and pathological remodeling of the lymphatic endothelium after myocardial infarction in mice (Berta et al., 2014; Karpinich and Caron, 2014; Tatin et al., 2017). Interestingly, adipose tissue from obese human subjects contains more saturated fatty acids that might not only contribute to inflammation, but also be responsible for the lymphatic vascular rupture that in Prox1+/− mice, leads to chyle leakage and obesity (Escobedo and Oliver, 2017). As mentioned above, the fact that lymph can induce adipogenesis, at least in vitro, agrees with clinical studies that demonstrate that secondary lymphedema induces localized fat accumulation in the affected tissue (Boyages et al., 2015; Brorson, 2003, 2010, 2016).
In summary, this growing body of evidence suggests that the leakage of diet-derived, free fatty acids from leaky lymphatic vessels might trigger adipocyte differentiation and obesity and represent a novel risk factor for obesity (Figure 4). The idea that lymph leakage, caused by a subtle defect in the lymphatic vasculature, might contribute to obesity represents a major paradigm shift. It also implies that obesity might be regulated by the local accumulation of factors released from the lymphatic vasculature. If true, this could inform the development of effective therapeutics, such as promoting lymphatic endothelial integrity, preventing the release of adipogenic factors from the lymphatics, or interfering functionally with the adipogenic activity (Schneider et al., 2005).
Cardiovascular Disease
The heart contains a complex network of blood and lymphatic vessels. In mice, cardiac lymphatics derived from the cardinal vein and second heart field become evident at around E14.5, particularly over the dorsal side of the heart (Klotz et al., 2015; Lioux et al., 2020; Srinivasan et al., 2007). As development progresses, lymphatic vessels expand over both the dorsal and ventral surfaces, and into the myocardium during late embryonic and postnatal stages. In humans, cardiac lymphatics span all layers of the heart (Bradham and Parker, 1973; Shimada et al., 1989); although most cardiac lymphatics in mice are found in the subepicardium and outer myocardium (Flaht-Zabost et al., 2014). Cardiac lymphatic vessels play important roles in maintaining tissue fluid balance and immune surveillance and are implicated in myocardial infarction (MI). Although little is known about the role of cardiac lymphatics in the healthy or failing heart, a growing body of evidence indicates that improved cardiac lymphatic vessel growth and function could be a novel therapeutic approach for combatting cardiovascular disease, as we discuss below.
Atherosclerosis
Atherosclerosis, characterized by the accumulation of plaques comprising fat, cholesterol and immune cells inside the arterial vessel wall, results in the narrowing and hardening of arterial walls, limiting blood flow from the heart (Shi et al., 2015) (Figure 4). Atherosclerosis is a leading cause of mortality worldwide, often resulting in heart attack and stroke (Libby and Hansson, 2015). Lymphatics are present at atherosclerotic sites in the adventitial layer of coronary arteries, adjacent to small blood vessels called the vasa vasorum, which are expanded in atherosclerotic plaques (Alitalo, 2011; Brakenhielm and Alitalo, 2019; Nakano et al., 2005). Recent work in mouse models has shown that lymphatics are the main route for the transport of high-density lipoprotein (HDL) particles in cholesterol to the bloodstream (reverse cholesterol transport, RCT) (Lim et al., 2013; Martel et al., 2013). In these models, defective lymphatic function was shown to severely impair RCT (Lim et al., 2013; Martel et al., 2013; Randolph and Miller, 2014), while VEGFC-promoted lymphangiogenesis decreased cholesterol content and improved RCT (Lim et al., 2013; Milasan et al., 2019). Recent work has demonstrated that lymphatics present in the adventitia of human and mouse atherosclerotic lesions increase in density with plaque progression (Rademakers et al., 2017). Furthermore, blocked lymphatic drainage or the inhibition of VEGFR-3-dependent lymphangiogenesis aggravates atherosclerotic plaque formation and increases intimal and adventitial T cell density in atherosclerosis (Rademakers et al., 2017). These results suggest that peri-adventitial lymphatics have a beneficial role in limiting cholesterol accumulation and plaque inflammation during atherosclerosis (Rademakers et al., 2017). It is possible that lymphatics provide a protective pathway for lipid and inflammatory cell efflux from the arterial wall, which could oppose the development of atherosclerotic plaques (Alitalo, 2011). Conversely, defective lymphangiogenesis might contribute to the build-up of atherosclerotic lesions in large arteries as a consequence of lipid accumulation and the recruitment of activated immune cells. Immune cell types, such as macrophages and T and B cells, might participate in the development and progression of atherosclerosis (Hansson and Hermansson, 2011). Future work will determine whether therapeutic lymphangiogenesis that targets the arterial wall can slow down fat deposition and tissue inflammation, thereby conferring protection against atherosclerosis in humans (Brakenhielm and Alitalo, 2019). It might also be important to consider the nature of the extracellular matrix around lymphatic vessels in the artery wall, as the build-up of collagen, with increasing vascular stiffness, can also limit the access of cholesterol to the lymphatic vasculature (Huang et al., 2019).
Myocardial infarction
MI, the most common heart injury, is a life-threatening condition that occurs when blood flow to the heart abruptly cuts off, usually as a consequence of blockage in the coronary arteries, resulting in tissue damage and massive cardiomyocyte (CM) death. This in turn leads to the formation of fibrotic tissue, pathological remodeling and eventually heart failure (Figure 4). MI also produces increased microvascular permeability in the myocardium. As a result, fluids accumulate in the interstitial space of the heart, leading to myocardial edema (Dongaonkar et al., 2010). MI causes a unique reaction of the innate immune system, in which neutrophils influx into the injury site, attracted by apoptotic signals released by dying cells. Concurrently, immune cells contribute to lymphatic remodeling by stimulating or inhibiting lymphangiogenesis.
Defective lymphatic function has been linked to cardiovascular diseases for some time (Bradham and Parker, 1973; Brakenhielm and Alitalo, 2019). Recent findings suggest that abnormal cardiac lymph flow promotes cardiac edema (Henri et al., 2016), and that MI is a trigger for the production of new cardiac lymphatics (Klotz et al., 2015). Other reports indicate that naturally or therapeutically stimulated lymphangiogenesis correlates with improved systolic function after MI by facilitating the resolution of myocardial edema and inflammation (Henri et al., 2016; Klotz et al., 2015; Vuorio et al., 2017) and by delaying atherosclerotic plaque formation (Lim et al., 2013; Milasan et al., 2016; Milasan et al., 2019; Vieira et al., 2018; Vuorio et al., 2014; Vuorio et al., 2017). Thus, all of these processes might facilitate healing after MI (Henri et al., 2016; Klotz et al., 2015; Trincot et al., 2019).
These new findings indicate that the stimulation of lymphangiogenesis in an infarcted heart could be a valuable therapeutic approach to improving cardiac function and preventing adverse cardiac remodeling. However, whether cardiac lymphatic function is important during MI, and the impact it might have on myocardial fluid balance, cardiac inflammation and contractile function remains to be further clarified. It is also unknown whether insufficient or defective lymphangiogenesis contributes to chronic myocardial edema, inflammation and fibrosis.
During MI, early neovascularization is important for recruiting inflammatory cells into the wound and for restoring the supply of oxygen and nutrients to the damaged area. Later on, lymphangiogenesis helps to remove excess fluids, cells, and debris to facilitate tissue remodeling and wound healing (Gancz et al., 2019; Vuorio et al., 2017). Robust lymphangiogenesis after MI is seen in humans (Ishikawa et al., 2007; Nakamura and Rockson, 2008) and mice (Henri et al., 2016; Klotz et al., 2015) in the infarct zone, and also in non-infarcted regions of the heart (Henri et al., 2016; Ishikawa et al., 2007; Klotz et al., 2015; Tatin et al., 2017). These newly formed lymphatic capillaries can be detected in the infarct zone two weeks later.
One of the first reports of lymphangiogenesis in post MI cardiac repair came from rodent MI models (Henri et al., 2016; Klotz et al., 2015). In one study, repeated intraperitoneal injections in mice of recombinant human VEGFR3-selective VEGFC-C156S protein promoted a significant lymphangiogenic response and improved cardiac function (Klotz et al., 2015). In another, recombinant rat VEGFR3-selective designer protein, VEGFC-C152S, was injected intramyocardially in rats using microparticles. This study showed that as myocardial fluid balance improved, cardiac inflammation, fibrosis and dysfunction were attenuated (Henri et al., 2016).
These studies thus indicate that therapeutic lymphangiogenesis could be a new approach for treating heart diseases. However, it remains unclear whether the improved heart function is a direct consequence of increased cardiac lymphatics after MI. MI triggers a robust inflammatory response in which lymphocytes, neutrophils, and monocytes are mobilized, and scavenge dead cells and release chemokines for cardiac remodeling (Henri et al., 2016). It is possible that VEGFC therapy facilitates lymphangiogenesis and lymphatic function, which in turn improves the resolution of cardiac edema and provides a pathway for inflammatory cell efflux, thus favoring wound healing within the injured heart. To further elucidate the mechanism by which VEGFC-induced lymphangiogenesis improves cardiac function after MI, a follow-up study has documented a significant influx of circulating monocytes and activated macrophages that undertake extensive phagocytic activity in the infarcted region after MI; these immune cells in the injured heart depend on lymphatic vessels to circulate back to the lymph nodes (Vieira et al., 2018). These findings show that the therapeutic effects of VEGFC-induced lymphangiogenesis after MI include improving fluid clearance and edema reduction, and facilitating the transmigration of immune cells across the endothelium (Vieira et al., 2018).
In their study, Vuorio et al. extended these findings, using two mouse models with defective Vegfr3 signaling (Vuorio et al., 2018): the sVEGFR3 mice, which express a soluble decoy VEGFR3 (sVEGFR3); and Chy mice, which have an inactivating point mutation in the Vegfr3 gene. In these mice, disrupted Vegfr3 signaling altered the structure of cardiac lymphatics in healthy hearts without affecting cardiac function. Importantly, following MI, the sVEGFR3 mice exhibited higher mortality with intramyocardial hemorrhages and a modified structure of the infarcted area.
Other signaling pathways and factors also reportedly improve cardiac function after MI by regulating lymphangiogenesis. For example, adrenomedullin (AM) is a known cardioprotective peptide and has been previously reported to be essential for proper cardiovascular and lymphatic development in mice (Caron and Smithies, 2001), and its expression increases after cardiac injury (Gibbons et al., 2007). Overexpression of Adm (the gene that encodes the AM protein) in mice increases cardiac lymphatic density and caliber after MI, improves cardiac function and reduces myocardial edema (Trincot et al., 2019). Importantly, the aforementioned work performed in mice and rats argue that improved cardiac function after MI appears to require increased cardiac lymphatic density and also improved vessel integrity (Gancz et al., 2019; Greiwe et al., 2016; Henri et al., 2016). Otherwise, defective lymphatic vasculature could lead to myocardial edema after MI.
Apelin is a bioactive peptide that plays a central role in angiogenesis, lymphangiogenesis and cardiac contractility (Ashley et al., 2005; Dai et al., 2006) and is exclusively expressed on newly formed lymphatics in the ischemic heart (Tatin et al., 2017). In an apelin-knockout mouse, morphological and functional defects in the lymphatic vasculature were observed associated with a proinflammatory status (indicated by the presence of an expanded lymphatic network within inguinal and mesenteric lymph nodes) after MI (Tatin et al., 2017). This study found that apelin deficiency increased the expression of VEGF-C and VEGF-D and exacerbated lymphangiogenesis after MI. Conversely, overexpression of apelin in the ischemic heart was found to restore a functional lymphatic vasculature, reducing matrix remodeling and inflammation.
Together, these studies argue that a defective lymphatic vasculature could be a contributing factor in cardiac diseases. They also show that increased lymphangiogenesis improves heart function after cardiac injury. However, whether increased lymphangiogenesis also improves lymphatic drainage functions remains unknown and will need to be further investigated. Nevertheless, these studies open up additional therapeutic strategies by which to stimulate and restore cardiac lymphatics in cardiac patients, including for example, the therapeutic use of VEGFC.
Neurological Disorders
The human brain is wrapped in a three-layered membranous structure called the meninges. The outmost layer, the dura mater, contains sinuses - venous structures that drain the blood from the brain before it leaves the cranium. Meningeal lymphatic vessels are positioned along the dural sinuses (Figure 6A) and drain brain-derived soluble waste to deep cervical lymph nodes (Aspelund et al., 2015; Da Mesquita et al., 2018a; Louveau et al., 2015; Louveau et al., 2017; Louveau et al., 2018), thus directly connecting the brain with the peripheral immune system. This physical link is of interest as it could mediate both waste removal from the brain and control brain-associated immunity. Recent findings also demonstrated the presence of meningeal lymphatics along the spinal cord (Jacob et al., 2019; Louveau et al., 2018) and suggested that the cerebrospinal fluid is primarily drained via lymphatics and not into the dural sinuses (Ahn et al., 2019; Ma et al., 2019), as was previously assumed (Upton and Weller, 1985).
Figure 6. Lymphatic/glymphatic connection in health and disease.
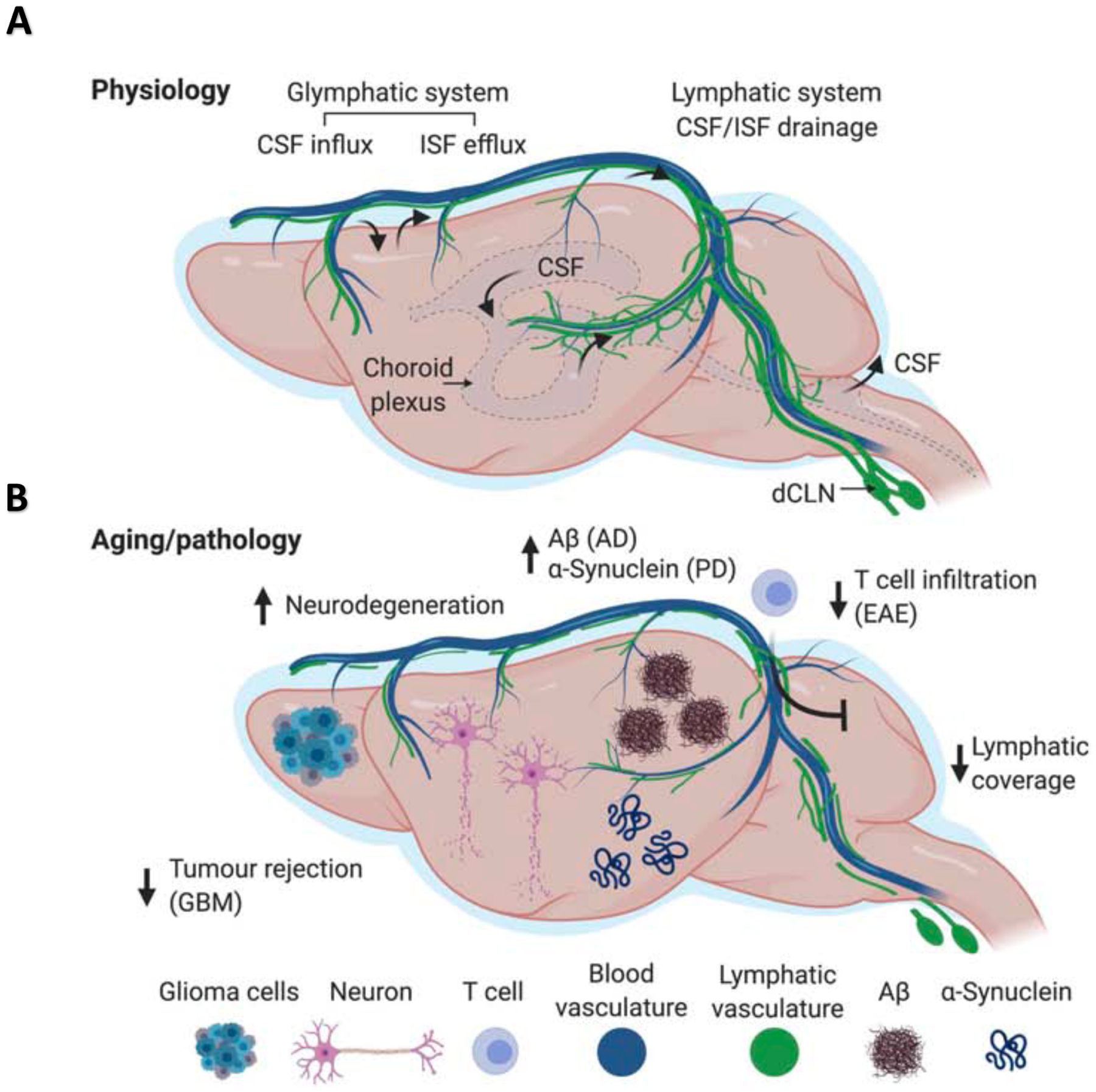
(A) The brain is a highly active organ and its waste products (metabolites, cellular debris, misfolded proteins) need to be removed. The most recently proposed mechanism for brain waste disposal is the glymphatic pathway, which refers to a framework for fluid flow through the brain parenchyma. Cerebrospinal fluid (CSF; depicted in light blue around the brain) is produced by the epithelial cells of the choroid plexus within the brain’s ventricles and circulates within the subarachnoid space. At the brain surface the meningeal vasculature dives into the brain and through these para-arterial spaces the CSF follows a path towards the parenchyma. Pulsating arteries propel CSF through the astroglial endfeet into the parenchyma. This process drives the efflux of brain interstitial fluid, carrying metabolites, protein aggregates, and other waste products from the parenchyma along para-venous walls, back into the CSF. Finally, CSF ends up in the dura mater and this cellular and molecular waste is drained by meningeal lymphatic vessels into the deep cervical lymph nodes. Normal meningeal lymphatic drainage ensures proper clearance of brain waste, through maintaining appropriate glymphatic function. However, disrupted meningeal lymphatics may underlie several neurological disorders. (B) Aging is characterized by dysfunction of many vital systems, including the lymphatic vasculature. A typical characteristic of the age-related deterioration observed in meningeal lymphatics is their reduced diameter and branching, accompanied by impaired drainage into the deep cervical lymph nodes (dCLNs). Impaired meningeal lymphatic function may underlie accumulation and aggregation of proteins, exacerbating conditions such as Alzheimer’s disease, Parkinson’s disease, and others. Poorly functional meningeal lymphatics may also impede immune response against brain tumors. Overactive, meningeal lymphatics, however, may result in break of CNS immune privilege, leading to pathological neuroinflammation, associated with multiple sclerosis
Many neurological diseases involve the aggregation of misfolded proteins and are characterized by immune dysfunction. Given that lymphatic vessels govern both tissue waste disposal and immune surveillance, it is reasonable to hypothesize that globally dysfunctional lymphatics could have a major impact on brain function (Figure 4 and 6B). However, it remains unknown if primary lymphedema is associated with neurological conditions (Berton et al., 2015), as its association with meningeal lymphatic dysfunction has yet to be studied. Meningeal lymphatics are also unlikely to be a driving force in the diseases discussed below. Nevertheless, meningeal lymphatics could plausibly be exploited to facilitate the dispersion of therapy through the brain, to regulate the immune response governing ongoing disease, and to enable the efficient removal of medication-generated metabolites. As such, meningeal lymphatics might facilitate and synergize (and in some cases salvage) existing and new therapies for a range of brain disorders, even those for which meningeal lymphatics are not the central player in disease pathogenesis.
Neurodegenerative disease:
Alzheimer’s, Parkinson’s, stroke and brain trauma Alzheimer’s disease (AD) presents a huge challenge to healthcare worldwide, and is characterized pathologically by amyloid plaques and tau tangles (Bateman et al., 2012). However, both in human disease and in mouse models, plaques do not necessarily correlate well with cognitive dysfunction (Musiek and Holtzman, 2015), although their targeting remains a preferred therapeutic approach (Sevigny et al., 2017). In mouse AD models, antibodies against amyloid and tau proteins lead to the clearance of plaques and tangles (Bacskai et al., 2001; Bard et al., 2000). However, many clinical trials using anti-amyloid antibodies have failed, although the few still in progress appear to hold out some hope of success (Howard and Liu, 2020; Logovinsky et al., 2016; Salloway et al., 2014).
Based on rodent and human evidence, only a small portion (~0.1%) of peripherally injected antibodies reach the brain, presumably by crossing the blood brain barrier (BBB), although the existence of other pathways needs to be further explored (Rustenhoven and Kipnis, 2019). It seems likely that anti-amyloid antibodies, once in the brain, are dispersed via the glymphatic route. The glymphatic system is a fluid conduit through the brain parenchyma (Peng et al., 2016; Yang et al., 2013), which together with meningeal lymphatics represents a system responsible for brain perfusion. In rodents, abstraction of meningeal lymphatic vessels results in impaired glymphatic flow, whereas meningeal lymphatic enhancement via VEGF-C treatment, improves glymphatic perfusion (Da Mesquita et al., 2018a; Da Mesquita et al., 2018b; see Figure 6B for further details). Likewise, the removal of dissociated plaque contents (which might be even more toxic than the plaques) likely occurs via glymphatic/lymphatic pathways (Da Mesquita et al., 2018a; Patel et al., 2019), in addition to its removal via blood-brain barrier (Kisler et al., 2017; Sweeney et al., 2016; Sweeney et al., 2018; Tarasoff-Conway et al., 2015). Given the age-related impairment of both pathways (Da Mesquita et al., 2018a; Kress et al., 2014), it is possible that the toxic remnants of dissolved plaques might not be adequately excreted in aged AD patients. Moreover, the remaining antibodies might cause vascular damage and microglial overactivation, leading to continued synapse loss and neurodegeneration (Hong et al., 2016). In the future, enhanced meningeal lymphatics might be used to support the targeting of Aß and tau, to facilitate the removal of generated neurotoxins, and might be used prophylactically to delay disease onset in those with a family history of early onset AD (Da Mesquita et al., 2018a).
Meningeal lymphatics also play a role in Parkinson’s disease (PD), a neurodegenerative disorder characterized by the death of dopaminergic neurons and by the pathological aggregation of α-synuclein (Irwin et al., 2013; Moore et al., 2005). When meningeal lymphatics were ligated in mice, it worsened the disease phenotype as a result of meningeal lymphatic dysfunction (Zou et al., 2019). Likewise, meningeal lymphatic disruption was accompanied by worsened outcomes in different models of experimental stroke (Chen et al., 2019; Yanev et al., 2020), and lymphatics were recently shown to function also in a brain concussion model, where they seem to regulate the extent of edema, the degree of microglial activation, and overall neuronal degeneration (Bolte et al., 2019). Together, these findings suggest that meningeal lymphatics may be a viable therapeutic target for neurological disorders, associated with protein accumulation/aggregation. Future studies are needed to address the function of meningeal lymphatics in human neurodegenerative disorders, as well as establish a stronger link to human diseases.
Multiple sclerosis and brain tumors
Multiple sclerosis (MS) is an autoimmune disease mediated by autoreactive T cells, which orchestrate a multicellular immune attack on the brain (Steinman, 2014; Yednock et al., 1992). This autoimmune attack is mimicked in mice that have experimental autoimmune encephalomyelitis (EAE), which is used as a model of MS (Wekerle et al., 1994). Surgical resection of cervical lymph nodes (CLN) in EAE mice was found to ameliorate disease development and progression (van Zwam et al., 2009). Interestingly, before inflammation was observed in the EAE brain, it was detectable in the meninges, where many T cells locate in close proximity to the meningeal lymphatics (Louveau et al., 2018). As in the case of deep cervical lymph node (dCLN) resection, the ablation or surgical ligation of meningeal lymphatics inhibited disease development and progression (Louveau et al., 2018). Just as intriguing was the finding that the transcriptional profiles of autoreactive T cells isolated from the dCLNs of mice with ongoing disease differed from those of healthy control dCLNs, and from those that had been disconnected from the meningeal lymphatics. This suggests that molecular cues from the brain/meninges drain into the dCLNs, where they interact with autoreactive T cells and consequently acquire an encephalitogenic phenotype (Louveau et al., 2018). Although the ablation of meningeal lymphatics is unlikely to serve as a therapeutic strategy, determining the molecular mediators that drain through these vessels and endow T cells with destructive phenotypes might lead to therapeutic interventions that can target these molecular mediators.
In general, anti-tumor immunotherapies aim to boost the immune response by preventing immune suppression. In the case of brain tumors, however, the story is more complicated because of the phenomenon of ‘CNS immune privilege’ (Brent, 1997; Medawar, 1948), which renders the peripheral immune system largely blind to brain-derived proteins. For this reason, and because checkpoint inhibitors cannot efficiently access the brain, brain tumors are highly aggressive (Lim et al., 2018). Although the mechanism underlying CNS immune privilege has yet to be elucidated, recent studies in mice have shown that VEGFC-induced enhancement of meningeal lymphatic function leads to improved anti-tumor T cell immunity against brain tumors and the efficient rejection of tumors (Hu et al., 2020; Song et al., 2020). By contrast, the ablation of the meningeal lymphatics further accelerated tumor growth (Hu et al., 2020). An unexpected finding, moreover, was the synergistic effect of VEGFC combined with checkpoint inhibitors on tumor regression (Song et al., 2020). These results are encouraging, given the aggressive nature of brain tumors and the lack of efficient therapies, and may indeed advance therapeutic approaches for treating brain tumors.
Age-related cognitive decline
Aging is characterized by the continual reduction in function of many vital systems. The lymphatic vasculature does not escape the impact of age and deteriorates throughout the body, including in the meninges. A typical characteristic of the age-related deterioration observed in meningeal lymphatics is their reduced diameter and branching, accompanied by impaired drainage into the dCLNs (Ahn et al., 2019; Da Mesquita et al., 2018a). Aging is also associated with impaired glymphatic flow (Kress et al., 2014). Interestingly, however, impaired meningeal lymphatic vessel function, characteristic of aged mice, could be rescued by treatment with VEGFC. Meningeal lymphatics, unlike other tissue lymphatics, depend on VEGFC for both development and maintenance (Ahn et al., 2019; Antila et al., 2017). In old mice, VEGFC treatment resulted in increased lymphatic vessel diameter and their improved function (Da Mesquita et al., 2018a). Interestingly, such therapy also improved glymphatic flow through the brain parenchyma and, most unexpectedly, resulted in cognitive enhancements in aged mice (Da Mesquita et al., 2018a). These benefits were abolished when the meningeal lymphatics were surgically ligated (Da Mesquita et al., 2018a). These findings raise intriguing questions regarding the role of meningeal lymphatics in brain aging and health and should be further explored in animal models and in humans.
Lymphatics in ocular disease
Recent studies revealed that some mammalian vessels exhibit mixed blood endothelial cell and lymphatic endothelial cell molecular features. Among those is the Schlemm’s Canal (SC), an endothelium-lined vascular channel in the eye required to maintain fluid homeostasis by draining aqueous humor from the intraocular chamber into the systemic circulation (Kizhatil et al., 2014). A defective SC elevates ocular pressure leading to glaucoma, a degenerative, age-related eye disease.
Glaucoma
Glaucoma is a group of heterogeneous diseases characterized by chronic degenerative optic neuropathy, and is the second leading cause of irreversible blindness, affecting 3.5% of the population aged 40–80 years worldwide (Kwon et al., 2009; Quigley and Broman, 2006; Weinreb et al., 2014). Primary congenital glaucoma is a severe form of the disease with an unclear etiology, characterized by infant/early-childhood ocular hypertension, enlarged eye globes (buphthalmos) and optic neuropathy, which can result in vision loss and blindness (deLuise and Anderson, 1983; Sarfarazi and Stoilov, 2000; Souma et al., 2016). The most important risk factor for glaucoma is chronic elevated intraocular pressure (IOP), a consequence of impaired aqueous humor outflow (AHO) that eventually leads to the irreversible loss of retinal ganglion cells (Kwon et al., 2009; Weinreb et al., 2014). IOP is determined by the balance between the rate of production and rate of removal of the aqueous humor (AH). AH is constantly produced by the ciliary epithelium in the posterior chamber, flows into the anterior chamber and exits the eye mainly through the trabecular meshwork (TM) into the SC, and is drained into episcleral veins via aqueous veins (Salloway et al.). In humans, 80% of fluid drainage is through the conventional or trabecular AHO pathway (Aihara et al., 2003; Alm and Nilsson, 2009; Aspelund et al., 2014; Kwon et al., 2009; Tamm, 2009).
In glaucoma, aqueous drainage into the SC is reduced, such that aqueous outflow resistance rises and IOP increases, resulting in optic neuropathy (Figure 4) (Almasieh et al., 2012). The absence or hypoplasia of the SC or alterations to its mechanobiology have been implicated in primary congenital glaucoma (Overby et al., 2014; Perry et al., 2012; Smith et al., 2000). A reduced SC area is also seen in primary open angle glaucoma (Kagemann et al., 2010), and is caused by IOP elevation in healthy eyes due to the partial collapse of the canal (Kagemann et al., 2014).
Similar to lymphatic vessels, the SC forms a blind-ended tube that does not contain blood, but transports aqueous humor and antigen presenting cells into the venous circulation through the episcleral vein (Kwon et al., 2009; Ramos et al., 2007; Schlemm, 1830). It has been recently discovered that the SC is a hybrid vessel with both blood and lymphatic molecular and morphological features (Aspelund et al., 2014; Kizhatil et al., 2014; Park et al., 2014; Ramos et al., 2007; Truong et al., 2014). As seen with primary lymphatics, the SC is formed by a continuous, non-fenestrated endothelial cell (EC) monolayer, is surrounded by a discontinuous basement membrane, lacks pericytes or smooth muscle cells, and has a basal-to-apical direction of flow (Aspelund et al., 2014; Hamanaka et al., 1992; Ramos et al., 2007). As shown in mice, the SC originates from the choroidal vein, and its ECs acquire a lymphatic fate via the upregulation of Prox1 (Aspelund et al., 2014; Kizhatil et al., 2014; Park et al., 2014; Thomson et al., 2014).
The molecular regulation of SC development also shares similarities with that of the lymphatic vasculature. For example, as shown in mice, Vegfr3 signaling is required for SC development (Aspelund et al., 2014), and Prox1 and Tie2 (Tek) signaling are required for SC development and maintenance (Kim et al., 2017; Park et al., 2014; Thackaberry et al., 2019; Thomson et al., 2014). Mice lacking ANGPT1, ANGPT2 or TIE2 have no SC and develop glaucoma (Kim et al., 2017; Thomson et al., 2017). The pharmacological reactivation of Tie2-TEK signaling could thus provide new therapeutic approaches to treating high-pressure glaucoma in the future.
Lymphatics in Inflammatory bowel disease
Inflammatory bowel diseases (IBDs) include ulcerative colitis (UC) and Crohn’s disease (CD), and are characterized by chronic inflammation of the gastrointestinal tract in genetically susceptible individuals exposed to environmental risk factors (D’Alessio et al., 2015). Increased lymphangiogenesis and lymphatic vascular dysfunction, such as lymphangiectasia and intralymphatic lymphocyte stasis, have long been described as pathological features of CD.
Crohn’s disease
CD is a major form of IBD that generally affects the terminal portion of the small bowel, is difficult to diagnose and for which there is no cure. Although the pathophysiology of IBD remains unknown, alterations in the intestinal lymphatics are an accepted feature of IBD (Rahier et al., 2013), particularly in CD patients (Van Kruiningen and Colombel, 2008; von der Weid et al., 2011). For example, increased lymphangiogenesis and lymphatic vascular dysfunction, such as lymphangiectasia and intralymphatic lymphocyte stasis, are considered pathological features of CD. In CD, the expansion of mesenteric fat onto the intestinal wall near the terminal ileum (known as creeping fat, Figure 4) characterizes the same sites of the intestine in which strictures form, resulting in surgery in severely affected CD patients (von der Weid et al., 2011). It has been recently proposed that fatty acids released from adipocytes within the creeping fat directly shift the metabolic state of muscle cells in the intestinal muscularis externa to drive muscle proliferation and fibrosis, thereby producing strictures (Mao et al., 2019). A possible source of those fatty acids could be the nearby lymphatics, which serve as the essential route for dietary fat transport in the form of chylomicron particles. This possibility has been recently supported by work identifying abnormal lymphatic patterning in CD patients (Randolph et al., 2016). Prior studies in mice suggested that lymph stasis and/or chylomicron leakage from defective lymphatics into nearby mesenteric tissue could promote fat accumulation (Harvey et al., 2005); therefore, they could be a major contributor to CD and could exacerbate experimental ileitis. It can be argued that lymphatic defects and the leakage of fatty cargo into the mesentery, a defect associated with an inflammatory response, leads to fat accumulation, obesity and also negatively affects the progression of GI pathological alterations, including CD. Such defects are likely caused by defective LEC junction permeability and/or leakiness in the intestinal and mesenteric lymphatics (Harvey et al., 2005; Zhang et al., 2018). As seen in Prox1+/− obese mice (Harvey et al., 2005), defective and leaky lymphatics in CD patients could cause an increase in fat accumulation and inflammation.
The intestine contains only initial lymphatics, while collecting lymphatics are only present in the mesentery. Lacteals (Figure 1) drain into the submucosal lymphatic network and then to the mesenteric collecting lymphatics. Dietary fats and fat-soluble vitamins are released from the lacteals as lipoprotein particles (chylomicrons). Lacteals take up chylomicrons and other interstitial fluid components from the villi, which are then transported through the mesenteric lymphatics to the thoracic duct. This drains into the venous circulation where lymph fluids are metabolized by the liver (Bernier-Latmani and Petrova, 2017; Dixon, 2010; Randolph and Miller, 2014; Zhang et al., 2018). Lacteals respond to high VEGF-A levels by zippering up their endothelial cell junctions, impairing chylomicron passage and making mice resistant to diet-induced obesity (Zhang et al., 2018). Interestingly, button junctions, necessary for initial lymphatics permeability and interstitial fluid uptake, are lost during inflammation in mice (Yao et al., 2012). Thus, although inflammation leads to increased lymphatic vessel density in patients with IBD (Alexander et al., 2010; D’Alessio et al., 2014; Rahier et al., 2013) (Figure 4), some of the functional aspects of these lymphatics might be defective, such that they might be unable to promote efficient draining and inflammation resolution. Given the adipogenic properties of lymph, and the role of inflammation in adipose tissue expansion, it is conceivable that chronic lymph leakage in CD contributes to the generation of creeping fat. In turn, increased visceral adiposity might sustain chronic inflammation and lymphatic dysfunction through the release of pro-inflammatory signals. Leukocyte aggregates that form within the lymphatic vasculature may be sites of leakage due to absence of a capsular structure, or such aggregates may obstruct lymph flow (Randolph et al., 2016). Genomic studies have identified various loci associated with IBD, and most are related to immunity or mucosal barrier functions, but thus far none are related to lymphatic function (Huang et al., 2017). However, impaired lymphatic function in mice generated by blockade of Vegfr3 activity (Becker et al., 2015; Davis et al., 2017; Jurisic et al., 2013) or by lymphatic deletion of the calcitonin receptor-like receptor (Calcrl) (Davis et al., 2017) has been shown to exacerbate IBD and colitis symptoms, whereas delivery of VEGF-C to stimulate lymphatic function improves disease outcomes in mouse models (D’Alessio et al., 2014). Patients with the largest number of lymphatic vessels correlate with those that are most protected from disease recurrence (Rahier et al., 2013) (Figure 4).
Together with lymphatic vasculature dysfunction, macrophages (MΦs) and other immune cells are also major contributors to IBD (Friedrich et al., 2019), such that one can envision that the immune response converges with lymphatic impairments in the pathogenesis of IBD. In this respect, it is important to consider whether foreign antigens that travel to lymph nodes through the lymphatic vasculature contribute to IBD. In Crohn’s disease, for instance, the disease is most common near the terminal ileum where the microbiome begins to become enriched. Food antigens taken up proximally drive immune tolerance as they drain to distinct lymph node compartments, whereas immune responses that may drive inflammatory responses occur in other lymph nodes, and are influenced by the local microbiome or pathogens (Esterházy et al., 2019). With respect to the abundant lipids in lymph, chylomicrons are typically taken up more proximally in the duodenum and jejunum (Esterházy et al., 2019; Wang et al., 2016). However, other lipid- carrying vehicles, such as HDL, which can strongly influence inflammatory reactions in IBD models (Gerster et al., 2015) and impact LEC survival in the presence of pro-inflammatory cytokines, are produced at the ileum (Haberman et al., 2014). It is possible that lipid carrying vehicles interact cooperatively with the immune response to affect lymph cargo, lymphatic vessels, and the course of disease.
This accumulating data involving the lymphatic vasculature as a contributing factor in CD will lead to a more detailed anatomical characterization of these vessels in CD patients. It could also be possible that in the future, promoting lymphatic growth in those patients could improve their condition. Also, the identification of biomarkers of defective lymphatics should facilitate the specific diagnoses of this condition. Overall, while much remains to be understood, therapeutics designed to improve lymphatic function might be beneficial in IBD.
Conclusions
In summary, many novel functional roles for the lymphatic vasculature in health and disease have recently been identified and there is little doubt that this trajectory of discovery will continue in the future. As an example, it was recently reported that superficial dermal lymphatics in mouse and human exhibit a dynamic spatial association with the hair follicle stem cell niche and more importantly, that they play additional functional roles during skin regeneration (Gur-Cohen et al., 2019; Peña-Jimenez et al., 2019; Yoon et al., 2019). A significant implication of recent advances in the field of lymphangiogenesis research is the realization that subtle and sometimes asymptomatic alterations in the optimal function of the lymphatic vasculature could be responsible for pathological conditions ranging from obesity to cardiovascular disease to aging. Progress in deepening our understanding of the genes and signaling pathways important for tissue specific lymphatic vessel development and function has the potential to modify the traditional management of diseases in which lymphatic vessel dysfunction is implicated, as well as provide novel tools able to facilitate the diagnosis, prognosis and treatment options for a range of human pathologies. Accordingly, the identification of easily accessible and reliable biomarkers of symptomatic and asymptomatic lymphatic malfunction would be a valuable asset to facilitate the diagnosis of those pathological conditions in the future.
Acknowledgments
We want to thank Justin Rustenhoven, Kelly Betterman and Nozomu Takata for help with some of the drawings included in the Figures. This work was partially supported by NIH grants RO1HL073402-16 to G.O, R37 AI 049653, DP1 DK109668, R01DK119147 and Rainin Foundation Synergy Award to GJR, and NHMRC APP1146800, APP1146352 to NLH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair TH, Moffatt DS, Paulsen AW, and Guyton AC (1982). Quantitation of changes in lymph protein concentration during lymph node transit. Am J Physiol 243, H351–359. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, et al. (2019). Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66. [DOI] [PubMed] [Google Scholar]
- Aihara M, Lindsey JD, and Weinreb RN (2003). Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci 44, 5168–5173. [DOI] [PubMed] [Google Scholar]
- Alders M, Hogan BM, Gjini E, Salehi F, Al-Gazali L, Hennekam EA, Holmberg EE, Mannens MM, Mulder MF, Offerhaus GJ, et al. (2009). Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet 41, 1272–1274. [DOI] [PubMed] [Google Scholar]
- Alders M, Mendola A, Ades L, Al Gazali L, Bellini C, Dallapiccola B, Edery P, Frank U, Hornshuh F, Huisman SA, et al. (2013). Evaluation of Clinical Manifestations in Patients with Severe Lymphedema with and without CCBE1 Mutations. Mol Syndromol 4, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alders M, Al-Gazali L, Cordeiro I, Dallapiccola B, Garavelli L, Tuysuz B, Salehi F, Haagmans MA, Mook OR, Majoie CB, et al. (2014). Hennekam syndrome can be caused by FAT4 mutations and be allelic to Van Maldergem syndrome. Hum Genet 133, 1161–1167. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Chaitanya GV, Grisham MB, and Boktor M (2010). Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci 1207 Suppl 1, E75–85. [DOI] [PubMed] [Google Scholar]
- Alitalo K (2011). The lymphatic vasculature in disease. Nat Med 17, 1371–1380. [DOI] [PubMed] [Google Scholar]
- Alm A, and Nilsson SF (2009). Uveoscleral outflow--a review. Exp Eye Res 88, 760–768. [DOI] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, and Di Polo A (2012). The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 31, 152–181. [DOI] [PubMed] [Google Scholar]
- Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, et al. (2017). Development and plasticity of meningeal lymphatic vessels. J Exp Med 214, 3645–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arngrim N, Simonsen L, Holst JJ, and Bulow J (2013). Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond) 37, 748–750. [DOI] [PubMed] [Google Scholar]
- Ashley EA, Powers J, Chen M, Kundu R, Finsterbach T, Caffarelli A, Deng A, Eichhorn J, Mahajan R, Agrawal R, et al. (2005). The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res 65, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Tammela T, Antila S, Nurmi H, Leppanen VM, Zarkada G, Stanczuk L, Francois M, Makinen T, Saharinen P, et al. (2014). The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest 124, 3975–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, and Alitalo K (2015). A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au AC, Hernandez PA, Lieber E, Nadroo AM, Shen YM, Kelley KA, Gelb BD, and Diaz GA (2010). Protein tyrosine phosphatase PTPN14 is a regulator of lymphatic function and choanal development in humans. Am J Hum Genet 87, 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, and Hyman BT (2001). Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med 7, 369–372. [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. (2007). Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204, 2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. (2000). Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6, 916–919. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, and Makinen T (2009). Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell 17, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, and Makinen T (2013). Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol Life Sci 70, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker F, Potepalov S, Shehzahdi R, Bernas M, Witte M, Abreo F, Traylor J, Orr WA, Tsunoda I, and Alexander JS (2015). Downregulation of FoxC2 Increased Susceptibility to Experimental Colitis: Influence of Lymphatic Drainage Function? Inflamm Bowel Dis 21, 1282–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier-Latmani J, and Petrova TV (2017). Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol 14, 510–526. [DOI] [PubMed] [Google Scholar]
- Berta J, Hoda MA, Laszlo V, Rozsas A, Garay T, Torok S, Grusch M, Berger W, Paku S, Renyi-Vamos F, et al. (2014). Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget 5, 4426–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton M, Lorette G, Baulieu F, Lagrue E, Blesson S, Cambazard F, Vaillant L, and Maruani A (2015). Generalized lymphedema associated with neurologic signs (GLANS) syndrome: a new entity? J Am Acad Dermatol 72, 333–339. [DOI] [PubMed] [Google Scholar]
- Betterman KL, Sutton DL, Secker GA, Kazenwadel J, Oszmiana A, Lim L, Miura N, Sorokin L, Hogan BM, Kahn ML, et al. (2020). Atypical cadherin Fat4 orchestrates lymphatic endothelial cell polarity in response to flow. J Clin Invest 18, 10–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, and Detmar M (2014). Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9, e94713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoslowski A, Butcher EC, and Kubes P (2018). Neutrophils recruited through high endothelial venules of the lymph nodes via PNAd intercept disseminating Staphylococcus aureus. Proc Natl Acad Sci U S A 115, 2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte AC, Hurt ME, Smirnov I, Kovacs MA, McKee CA, Natale N, Ennerfelt HE, Frost EL, Lammert CE, Kipnis J, et al. (2019). Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. bioRxiv, 817023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone PM, Paterson S, Mohajeri K, Zhu W, Genetti CA, Tai DJC, Nori N, Agrawal PB, Bacino CA, Bi W, et al. (2020). Biallelic mutation of FBXL7 suggests a novel form of Hennekam syndrome. Am J Med Genet A 182, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos FL, Caunt M, Peterson-Maduro J, Planas-Paz L, Kowalski J, Karpanen T, van Impel A, Tong R, Ernst JA, Korving J, et al. (2011). CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ Res 109, 486–491. [DOI] [PubMed] [Google Scholar]
- Bouvree K, Brunet I, Del Toro R, Gordon E, Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, et al. (2012). Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ Res 111, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovay E, Sabine A, Prat-Luri B, Kim S, Son K, Willrodt AH, Olsson C, Halin C, Kiefer F, Betsholtz C, et al. (2018). Multiple roles of lymphatic vessels in peripheral lymph node development. J Exp Med 215, 2760–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyages J, Kastanias K, Koelmeyer LA, Winch CJ, Lam TC, Sherman KA, Munnoch DA, Brorson H, Ngo QD, Heydon-White A, et al. (2015). Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann Surg Oncol 22 Suppl 3, S1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham RR, and Parker EF (1973). The cardiac lymphatics. Ann Thorac Surg 15, 526–535. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, and Alitalo K (2019). Cardiac lymphatics in health and disease. Nat Rev Cardiol 16, 56–68. [DOI] [PubMed] [Google Scholar]
- Brent L (1997). The discovery of immunologic tolerance. Hum Immunol 52, 75–81. [DOI] [PubMed] [Google Scholar]
- Brice G, Mansour S, Bell R, Collin JR, Child AH, Brady AF, Sarfarazi M, Burnand KG, Jeffery S, Mortimer P, et al. (2002). Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet 39, 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggi MAS, Maillat L, Clement CC, Bordry N, Corthesy P, Auger A, Matter M, Hamelin R, Potin L, Demurtas D, et al. (2019). Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. J Exp Med 216, 1091–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson H (2003). Liposuction in arm lymphedema treatment. Scand J Surg 92, 287–295. [DOI] [PubMed] [Google Scholar]
- Brorson H (2010). From lymph to fat: complete reduction of lymphoedema. Phlebology 25 Suppl 1, 52–63. [DOI] [PubMed] [Google Scholar]
- Brorson H (2016). Liposuction in Lymphedema Treatment. J Reconstr Microsurg 32, 56–65. [DOI] [PubMed] [Google Scholar]
- Brouillard P, Boon L, and Vikkula M (2014). Genetics of lymphatic anomalies. J Clin Invest 124, 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillard P, Dupont L, Helaers R, Coulie R, Tiller GE, Peeden J, Colige A, and Vikkula M (2017). Loss of ADAMTS3 activity causes Hennekam lymphangiectasia-lymphedema syndrome 3. Hum Mol Genet 26, 4095–4104. [DOI] [PubMed] [Google Scholar]
- Bui HM, Enis D, Robciuc MR, Nurmi HJ, Cohen J, Chen M, Yang Y, Dhillon V, Johnson K, Zhang H, et al. (2016). Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J Clin Invest 126, 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Dagenais SL, Rockson SG, and Glover TW (2007). A novel VEGFR3 mutation causes Milroy disease. Am J Med Genet A 143A, 1212–1217. [DOI] [PubMed] [Google Scholar]
- Butler MG, Isogai S, and Weinstein BM (2009). Lymphatic development. Birth Defects Res C Embryo Today 87, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, et al. (2020). Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565. [DOI] [PubMed] [Google Scholar]
- Caron KM, and Smithies O (2001). Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci U S A 98, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha B, Geng X, Mahamud MR, Fu J, Mukherjee A, Kim Y, Jho EH, Kim TH, Kahn ML, Xia L, et al. (2016). Mechanotransduction activates canonical Wnt/beta-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev 30, 1454–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha B, Geng X, Mahamud MR, Zhang JY, Chen L, Kim W, Jho EH, Kim Y, Choi D, Dixon JB, et al. (2018). Complementary Wnt Sources Regulate Lymphatic Vascular Development via PROX1-Dependent Wnt/beta-Catenin Signaling. Cell Rep 25, 571–584 e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, He J, Ni R, Yang Q, Zhang Y, and Luo L (2019). Cerebrovascular Injuries Induce Lymphatic Invasion into Brain Parenchyma to Guide Vascular Regeneration in Zebrafish. Dev Cell 49, 697–710 e695. [DOI] [PubMed] [Google Scholar]
- Choi D, Park E, Jung E, Seong YJ, Hong M, Lee S, Burford J, Gyarmati G, Peti-Peterdi J, Srikanth S, et al. (2017). ORAI1 Activates Proliferation of Lymphatic Endothelial Cells in Response to Laminar Flow Through Kruppel-Like Factors 2 and 4. Circ Res 120, 1426–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Park E, Jung E, Cha B, Lee S, Yu J, Kim PM, Lee S, Hong YJ, Koh CJ, et al. (2019). Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, Chan SW, Satija H, Camfield K, Aaron H, et al. (2008). Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29, 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifarelli V, and Eichmann A (2019). The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell Mol Gastroenterol Hepatol 7, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell F, Kalidas K, Ostergaard P, Brice G, Homfray T, Roberts L, Bunyan DJ, Mitton S, Mansour S, Mortimer P, et al. (2010). Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Hum Genet 127, 231–241. [DOI] [PubMed] [Google Scholar]
- Coxam B, Sabine A, Bower NI, Smith KA, Pichol-Thievend C, Skoczylas R, Astin JW, Frampton E, Jaquet M, Crosier PS, et al. (2014). Pkd1 regulates lymphatic vascular morphogenesis during development. Cell Rep 7, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, and Schwab SR (2012). Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30, 69–94. [DOI] [PubMed] [Google Scholar]
- Czepielewski RS, and Randolph GJ (2018). Lymph nodes go with the flow. J Exp Med 215, 2699–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, et al. (2014). VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest 124, 3863–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio S, Tacconi C, and Danese S (2015). Targeting lymphatics in inflammatory bowel disease. Oncotarget 6, 34047–34048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, et al. (2018a). Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Fu Z, and Kipnis J (2018b). The Meningeal Lymphatic System: A New Player in Neurophysiology. Neuron 100, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais SL, Hartsough RL, Erickson RP, Witte MH, Butler MG, and Glover TW (2004). Foxc2 is expressed in developing lymphatic vessels and other tissues associated with lymphedema-distichiasis syndrome. Gene Expr Patterns 4, 611–619. [DOI] [PubMed] [Google Scholar]
- Dai T, Ramirez-Correa G, and Gao WD (2006). Apelin increases contractility in failing cardiac muscle. Eur J Pharmacol 553, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RB, Kechele DO, Blakeney ES, Pawlak JB, and Caron KM (2017). Lymphatic deletion of calcitonin receptor-like receptor exacerbates intestinal inflammation. JCI Insight 2, e92465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan JH, Ly CL, Kataru RP, and Mehrara BJ (2018). Lymphedema: Pathogenesis and Novel Therapies. Annu Rev Med 69, 263–276. [DOI] [PubMed] [Google Scholar]
- Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, and Witte M (2008). Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol 319, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger MT, Garg N, and Olsen BR (2014). Viewpoints on vessels and vanishing bones in Gorham-Stout disease. Bone 63, 47–52. [DOI] [PubMed] [Google Scholar]
- deLuise VP, and Anderson DR (1983). Primary infantile glaucoma (congenital glaucoma). Surv Ophthalmol 28, 1–19. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, and Strauss-Schoenberger J (1994). Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707. [DOI] [PubMed] [Google Scholar]
- Dixon JB (2010). Mechanisms of chylomicron uptake into lacteals. Ann N Y Acad Sci 1207 Suppl 1, E52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongaonkar RM, Stewart RH, Geissler HJ, and Laine GA (2010). Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc Res 87, 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, and Ruddle NH (2006). Lymphoid organ development: from ontogeny to neogenesis. Nature immunology 7, 344–353. [DOI] [PubMed] [Google Scholar]
- Drewry LL, Jones NG, Wang Q, Onken MD, Miller MJ, and Sibley LD (2019). The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nat Microbiol 4, 1951–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, and Oliver G (2016). Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo N, and Oliver G (2017). The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell Metab 26, 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterházy D, Canesso MCC, Mesin L, Muller PA, de Castro TBR, Lockhart A, ElJalby M, Faria AMC, and Mucida D (2019). Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls HF, and Kertesz ED (1964). A New Syndrome Combining Pterygium Colli with Developmental Anomalies of the Eyelids and Lymphatics of the Lower Extremities. Trans Am Ophthalmol Soc 62, 248–275. [PMC free article] [PubMed] [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, and Glover TW (2000). Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet 67, 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, Lund AW, Da Costa E, Hauert S, Rincon-Restrepo M, Tremblay C, et al. (2017). Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med 9. [DOI] [PubMed] [Google Scholar]
- Ferrell RE, Levinson KL, Esman JH, Kimak MA, Lawrence EC, Barmada MM, and Finegold DN (1998). Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum Mol Genet 7, 2073–2078. [DOI] [PubMed] [Google Scholar]
- Ferrell RE, Baty CJ, Kimak MA, Karlsson JM, Lawrence EC, Franke-Snyder M, Meriney SD, Feingold E, and Finegold DN (2010). GJC2 missense mutations cause human lymphedema. Am J Hum Genet 86, 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold DN, Kimak MA, Lawrence EC, Levinson KL, Cherniske EM, Pober BR, Dunlap JW, and Ferrell RE (2001). Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum Mol Genet 10, 1185–1189. [DOI] [PubMed] [Google Scholar]
- Flaht-Zabost A, Gula G, Ciszek B, Czarnowska E, Jankowska-Steifer E, Madej M, Niderla-Bielinska J, Radomska-Lesniewska D, and Ratajska A (2014). Cardiac mouse lymphatics: developmental and anatomical update. Anat Rec (Hoboken) 297, 1115–1130. [DOI] [PubMed] [Google Scholar]
- Fotiou E, Martin-Almedina S, Simpson MA, Lin S, Gordon K, Brice G, Atton G, Jeffery I, Rees DC, Mignot C, et al. (2015). Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non-immune hydrops fetalis. Nat Commun 6, 8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, et al. (2008). Sox18 induces development of the lymphatic vasculature in mice. Nature 456, 643–647. [DOI] [PubMed] [Google Scholar]
- Friedman JM (2000). Obesity in the new millennium. Nature 404, 632–634. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Pohin M, and Powrie F (2019). Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 50, 992–1006. [DOI] [PubMed] [Google Scholar]
- Frye M, Taddei A, Dierkes C, Martinez-Corral I, Fielden M, Ortsater H, Kazenwadel J, Calado DP, Ostergaard P, Salminen M, et al. (2018). Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat Commun 9, 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, et al. (2002). Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 3, 411–423. [DOI] [PubMed] [Google Scholar]
- Gancz D, Perlmoter G, and Yaniv K (2019). Formation and Growth of Cardiac Lymphatics during Embryonic Development, Heart Regeneration, and Disease. Cold Spring Harb Perspect Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, et al. (2016). Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 40, 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvrit S, Villasenor A, Strilic B, Kitchen P, Collins MM, Marin-Juez R, Guenther S, Maischein HM, Fukuda N, Canham MA, et al. (2018). HHEX is a transcriptional regulator of the VEGFC/FLT4/PROX1 signaling axis during vascular development. Nat Commun 9, 2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, and Hensley LE (2003). Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis 188, 1618–1629. [DOI] [PubMed] [Google Scholar]
- Geng X, Cha B, Mahamud MR, Lim KC, Silasi-Mansat R, Uddin MKM, Miura N, Xia L, Simon AM, Engel JD, et al. (2016). Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev Biol 409, 218–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Cha B, Mahamud MR, and Srinivasan RS (2017). Intraluminal valves: development, function and disease. Dis Model Mech 10, 1273–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner MY, Casey KA, Kastenmuller W, and Germain RN (2017). Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J Exp Med 214, 3105–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster R, Eloranta JJ, Hausmann M, Ruiz PA, Cosin-Roger J, Terhalle A, Ziegler U, Kullak-Ublick GA, von Eckardstein A, and Rogler G (2015). Antiinflammatory Function of High-Density Lipoproteins via Autophagy of IkappaB Kinase. Cell Mol Gastroenterol Hepatol 1, 171–187 e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalamkarpour A, Debauche C, Haan E, Van Regemorter N, Sznajer Y, Thomas D, Revencu N, Gillerot Y, Boon LM, and Vikkula M (2009). Sporadic in utero generalized edema caused by mutations in the lymphangiogenic genes VEGFR3 and FOXC2. J Pediatr 155, 90–93. [DOI] [PubMed] [Google Scholar]
- Gibbons C, Dackor R, Dunworth W, Fritz-Six K, and Caron KM (2007). Receptor activity-modifying proteins: RAMPing up adrenomedullin signaling. Mol Endocrinol 21, 783–796. [DOI] [PubMed] [Google Scholar]
- Gordon K, Varney R, Keeley V, Riches K, Jeffery S, van Zanten M, Mortimer P, Ostergaard P, and Mansour S (2020). Update and audit of the St George’s classification algorithm of primary lymphatic anomalies: a clinical and molecular approach to diagnosis. Journal of Medical Genetics, jmedgenet-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiwe L, Vinck M, and Suhr F (2016). The muscle contraction mode determines lymphangiogenesis differentially in rat skeletal and cardiac muscles by modifying local lymphatic extracellular matrix microenvironments. Acta Physiol (Oxf) 217, 61–79. [DOI] [PubMed] [Google Scholar]
- Grover SP, and Mackman N (2018). Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol 38, 709–725. [DOI] [PubMed] [Google Scholar]
- Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, and Fuchs E (2019). Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman Y, Tickle TL, Dexheimer PJ, Kim MO, Tang D, Karns R, Baldassano RN, Noe JD, Rosh J, Markowitz J, et al. (2014). Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest 124, 3617–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, and Kiefer F (2013). A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 32, 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, Babic M, Lin M, Carmagnac A, Lee YK, et al. (2011). Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet 43, 1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka T, Bill A, Ichinohasama R, and Ishida T (1992). Aspects of the development of Schlemm’s canal. Exp Eye Res 55, 479–488. [DOI] [PubMed] [Google Scholar]
- Hansson GK, and Hermansson A (2011). The immune system in atherosclerosis. Nat Immunol 12, 204–212. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, and Oliver G (2005). Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 37, 1072–1081. [DOI] [PubMed] [Google Scholar]
- Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. (2020). B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RC, Geerdink RA, Hamel BC, Hennekam FA, Kraus P, Rammeloo JA, and Tillemans AA (1989). Autosomal recessive intestinal lymphangiectasia and lymphedema, with facial anomalies and mental retardation. Am J Med Genet 34, 593–600. [DOI] [PubMed] [Google Scholar]
- Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards-Levy F, Henry JP, Dumesnil A, Boukhalfa I, Banquet S, et al. (2016). Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation 133, 1484–1497; discussion 1497. [DOI] [PubMed] [Google Scholar]
- Hespe GE, Kataru RP, Savetsky IL, Garcia Nores GD, Torrisi JS, Nitti MD, Gardenier JC, Zhou J, Yu JZ, Jones LW, et al. (2016). Exercise training improves obesity-related lymphatic dysfunction. J Physiol 594, 4267–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyde A, Reiter JG, Naxerova K, and Nowak MA (2019). Consecutive seeding and transfer of genetic diversity in metastasis. Proc Natl Acad Sci U S A 116, 14129–14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, and Shimada K (2015). Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer 112, 1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, and Schulte-Merker S (2009). Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet 41, 396–398. [DOI] [PubMed] [Google Scholar]
- Hogan BM, and Schulte-Merker S (2017). How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev Cell 42, 567–583. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, and Walsh CA (2000). Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet 26, 93–96. [DOI] [PubMed] [Google Scholar]
- Howard R, and Liu KY (2020). Questions EMERGE as Biogen claims aducanumab turnaround. Nat Rev Neurol 16, 63–64. [DOI] [PubMed] [Google Scholar]
- Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, Zhou F, Zhang C, Shao L, Feng J, et al. (2020). Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 30, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fang M, Jostins L, Umicevic Mirkov M, Boucher G, Anderson CA, Andersen V, Cleynen I, Cortes A, Crins F, et al. (2017). Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LH, Zinselmeyer BH, Chang CH, Saunders BT, Elvington A, Baba O, Broekelmann TJ, Qi L, Rueve JS, Swartz MA, et al. (2019). Interleukin-17 Drives Interstitial Entrapment of Tissue Lipoproteins in Experimental Psoriasis. Cell Metab 29, 475–487 e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV Jr., and Sheppard D (2000). Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol 20, 5208–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington GS, and McClure CF (1910). The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica). American Journal of Anatomy 10, 177–312. [Google Scholar]
- Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, and Vikkula M (2000). Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet 67, 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, and Vikkula M (2003). Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Hum Genet 72, 1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, Lee VM, and Trojanowski JQ (2013). Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 14, 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Tanaka M, Shimokawa R, Kimura-Matsumoto M, Morita H, Sato S, Kamata I, et al. (2007). Lymphangiogenesis in myocardial remodelling after infarction. Histopathology 51, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L, Boisserand LSB, Geraldo LHM, de Brito Neto J, Mathivet T, Antila S, Barka B, Xu Y, Thomas JM, Pestel J, et al. (2019). Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun 10, 4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppanen VM, Holopainen T, Kivela R, Ortega S, Karpanen T, and Alitalo K (2014). CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 129, 1962–1971. [DOI] [PubMed] [Google Scholar]
- Jha SK, Rauniyar K, Karpanen T, Leppanen VM, Brouillard P, Vikkula M, Alitalo K, and Jeltsch M (2017). Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1. Sci Rep 7, 4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Tian W, Nicolls MR, and Rockson SG (2019). The Lymphatic System in Obesity, Insulin Resistance, and Cardiovascular Diseases. Front Physiol 10, 1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, and Oliver G (2008). Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22, 3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GE, and Mansour S (2017). An approach to familial lymphoedema. Clin Med (Lond) 17, 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, Ochoa Pereira C, Petrova TV, and Detmar M (2012). An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res 111, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic G, Sundberg JP, and Detmar M (2013). Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm Bowel Dis 19, 1983–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagemann L, Wollstein G, Ishikawa H, Bilonick RA, Brennen PM, Folio LS, Gabriele ML, and Schuman JS (2010). Identification and assessment of Schlemm’s canal by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 51, 4054–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagemann L, Wang B, Wollstein G, Ishikawa H, Nevins JE, Nadler Z, Sigal IA, Bilonick RA, and Schuman JS (2014). IOP elevation reduces Schlemm’s canal cross-sectional area. Invest Ophthalmol Vis Sci 55, 1805–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri L, Fair-Makela R, Auvinen K, Rantakari P, Jalkanen S, Ivaska J, and Salmi M (2019). Transcytosis route mediates rapid delivery of intact antibodies to draining lymph nodes. J Clin Invest 129, 3086–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampmeier OF (1969). Evolution and comparative morphology of the lymphatic system. (Thomas). [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, and Finegold DN (2000). Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet 25, 153–159. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, et al. (2001). A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A 98, 12677–12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. (2004). Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5, 74–80. [DOI] [PubMed] [Google Scholar]
- Karpinich NO, and Caron KM (2014). Apelin signaling: new G protein-coupled receptor pathway in lymphatic vascular development. Arterioscler Thromb Vasc Biol 34, 239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J, Hsu AP, Dyack S, Fernandez CV, Chong CE, et al. (2012). Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood 119, 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, Betterman KL, Chong CE, Stokes PH, Lee YK, Secker GA, Agalarov Y, Demir CS, Lawrence DM, Sutton DL, et al. (2015). GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest 125, 2979–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, and Harvey NL (2018). Lymphatic endothelial progenitor cells: origins and roles in lymphangiogenesis. Curr Opin Immunol 53, 81–87. [DOI] [PubMed] [Google Scholar]
- Kim J, Park DY, Bae H, Park DY, Kim D, Lee CK, Song S, Chung TY, Lim DH, Kubota Y, et al. (2017). Impaired angiopoietin/Tie2 signaling compromises Schlemm’s canal integrity and induces glaucoma. J Clin Invest 127, 3877–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisler K, Nelson AR, Montagne A, and Zlokovic BV (2017). Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 18, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K, Ryan M, Marchant JK, Henrich S, and John SW (2014). Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol 12, e1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, Bollini S, Matsuzaki F, Carr CA, and Riley PR (2015). Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522, 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman PG (2000). Obesity as a medical problem. Nature 404, 635–643. [DOI] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, et al. (2014). Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Fingert JH, Kuehn MH, and Alward WL (2009). Primary open-angle glaucoma. N Engl J Med 360, 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen L, Karpanen T, Schulte D, Harris NC, Koltowska K, Roukens G, Bower NI, van Impel A, Stacker SA, Achen MG, et al. (2014). Ccbe1 regulates Vegfc-mediated induction of Vegfr3 signaling during embryonic lymphangiogenesis. Development 141, 1239–1249. [DOI] [PubMed] [Google Scholar]
- Lee CK, Jeong SH, Jang C, Bae H, Kim YH, Park I, Kim SK, and Koh GY (2019). Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 363, 644–649. [DOI] [PubMed] [Google Scholar]
- Libby P, and Hansson GK (2015). Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 116, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, and Angeli V (2013). Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab 17, 671–684. [DOI] [PubMed] [Google Scholar]
- Lim M, Xia Y, Bettegowda C, and Weller M (2018). Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15, 422–442. [DOI] [PubMed] [Google Scholar]
- Lioux G, Liu X, Temino S, Oxendine M, Ayala E, Ortega S, Kelly RG, Oliver G, and Torres M (2020). A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics. Dev Cell 52, 350–363 e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gu X, Ma W, Oxendine M, Gil HJ, Davis GE, Cleaver O, and Oliver G (2018). Rasip1 controls lymphatic vessel lumen maintenance by regulating endothelial cell junctions. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, Basun H, and Lannfelt L (2016). Safety and tolerability of BAN2401--a clinical study in Alzheimer’s disease with a protofibril selective Abeta antibody. Alzheimers Res Ther 8, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, and Kipnis J (2017). Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127, 3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. (2018). CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21, 1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H, et al. (2016). Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest 126, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter S, Xie S, Tatin F, and Makinen T (2012). Smooth muscle-endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. J Cell Biol 197, 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons O, Saha P, Seet C, Kuchta A, Arnold A, Grover S, Rashbrook V, Sabine A, Vizcay-Barrena G, Patel A, et al. (2017). Human venous valve disease caused by mutations in FOXC2 and GJC2. J Exp Med 214, 2437–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma GC, Liu CS, Chang SP, Yeh KT, Ke YY, Chen TH, Wang BB, Kuo SJ, Shih JC, and Chen M (2008). A recurrent ITGA9 missense mutation in human fetuses with severe chylothorax: possible correlation with poor response to fetal therapy. Prenat Diagn 28, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Ma Q, Decker Y, Muller A, Ineichen BV, and Proulx ST (2019). Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J Exp Med 216, 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie DI, Al Mutairi F, Davis RB, Kechele DO, Nielsen NR, Snyder JC, Caron MG, Kliman HJ, Berg JS, Simms J, et al. (2018). hCALCRL mutation causes autosomal recessive nonimmune hydrops fetalis with lymphatic dysplasia. J Exp Med 215, 2339–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao R, Kurada S, Gordon IO, Baker ME, Gandhi N, McDonald C, Coffey JC, and Rieder F (2019). The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn’s Disease. Inflamm Bowel Dis 25, 421–426. [DOI] [PubMed] [Google Scholar]
- Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, et al. (2013). Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest 123, 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens R, Permanyer M, Werth K, Yu K, Braun A, Halle O, Halle S, Patzer GE, Bošnjak B, and Kiefer F (2020). Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Nature communications 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Almedina S, Martinez-Corral I, Holdhus R, Vicente A, Fotiou E, Lin S, Petersen K, Simpson MA, Hoischen A, Gilissen C, et al. (2016). EPHB4 kinase-inactivating mutations cause autosomal dominant lymphatic-related hydrops fetalis. J Clin Invest 126, 3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Corral I, Ulvmar MH, Stanczuk L, Tatin F, Kizhatil K, John SW, Alitalo K, Ortega S, and Makinen T (2015). Nonvenous origin of dermal lymphatic vasculature. Circ Res 116, 1649–1654. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Miyagawa-Tomita S, Mizukami K, Matsuzaki F, and Kurihara H (2019). Isl1-expressing non-venous cell lineage contributes to cardiac lymphatic vessel development. Dev Biol 452, 134–143. [DOI] [PubMed] [Google Scholar]
- McDonald DM (2018). Tighter lymphatic junctions prevent obesity. Science 361, 551–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB (1948). Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29, 58–69. [PMC free article] [PubMed] [Google Scholar]
- Meige H (1898). Dystrophie oedemateuse hereditaire. Presse méd 6, 341–343. [Google Scholar]
- Mendola A, Schlogel MJ, Ghalamkarpour A, Irrthum A, Nguyen HL, Fastre E, Bygum A, van der Vleuten C, Fagerberg C, Baselga E, et al. (2013). Mutations in the VEGFR3 signaling pathway explain 36% of familial lymphedema. Mol Syndromol 4, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milasan A, Dallaire F, Mayer G, and Martel C (2016). Effects of LDL Receptor Modulation on Lymphatic Function. Sci Rep 6, 27862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milasan A, Smaani A, and Martel C (2019). Early rescue of lymphatic function limits atherosclerosis progression in Ldlr(−/−) mice. Atherosclerosis 283, 106–119. [DOI] [PubMed] [Google Scholar]
- Miller LS, and Simon SI (2018). Neutrophils in hot pursuit of MRSA in the lymph nodes. Proc Natl Acad Sci U S A 115, 2272–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milroy WF (1892). An undescribed variety of hereditary oedema. (Appleton). [Google Scholar]
- Moore DJ, West AB, Dawson VL, and Dawson TM (2005). Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28, 57–87. [DOI] [PubMed] [Google Scholar]
- Musiek ES, and Holtzman DM (2015). Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat Neurosci 18, 800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, and Zawieja D (2008). Molecular regulation of lymphatic contractility. Ann N Y Acad Sci 1131, 89–99. [DOI] [PubMed] [Google Scholar]
- Nakamura K, and Rockson SG (2008). The role of the lymphatic circulation in the natural history and expression of cardiovascular disease. Int J Cardiol 129, 309–317. [DOI] [PubMed] [Google Scholar]
- Nakano T, Nakashima Y, Yonemitsu Y, Sumiyoshi S, Chen YX, Akishima Y, Ishii T, Iida M, and Sueishi K (2005). Angiogenesis and lymphangiogenesis and expression of lymphangiogenic factors in the atherosclerotic intima of human coronary arteries. Hum Pathol 36, 330–340. [DOI] [PubMed] [Google Scholar]
- Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA, et al. (2017). Origins of lymphatic and distant metastases in human colorectal cancer. Science 357, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J, and Schull W (1954). The dominant gene in man Human heredity. University of Chicago Press, Chicago, 41–56. [Google Scholar]
- Nitti MD, Hespe GE, Kataru RP, Garcia Nores GD, Savetsky IL, Torrisi JS, Gardenier JC, Dannenberg AJ, and Mehrara BJ (2016). Obesity-induced lymphatic dysfunction is reversible with weight loss. J Physiol 594, 7073–7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura K, Lukacs V, Sweet DT, Goddard LM, Kanie A, Whitwam T, Ranade SS, Fujimori T, Kahn ML, and Patapoutian A (2018). Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc Natl Acad Sci U S A 115, 12817–12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, et al. (2009). FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol 185, 439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G (2004). Lymphatic vasculature development. Nat Rev Immunol 4, 35–45. [DOI] [PubMed] [Google Scholar]
- Oliver G, and Alitalo K (2005). The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol 21, 457–483. [DOI] [PubMed] [Google Scholar]
- Onder L, Morbe U, Pikor N, Novkovic M, Cheng HW, Hehlgans T, Pfeffer K, Becher B, Waisman A, Rulicke T, et al. (2017). Lymphatic Endothelial Cells Control Initiation of Lymph Node Organogenesis. Immunity 47, 80–92 e84. [DOI] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Brice G, Mansour S, Connell FC, Onoufriadis A, Child AH, Hwang J, Kalidas K, Mortimer PS, et al. (2011a). Rapid identification of mutations in GJC2 in primary lymphoedema using whole exome sequencing combined with linkage analysis with delineation of the phenotype. J Med Genet 48, 251–255. [DOI] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, Dafou D, Kilo T, Smithson S, Lunt P, et al. (2011b). Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet 43, 929–931. [DOI] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Mendola A, Vasudevan P, Connell FC, van Impel A, Moore AT, Loeys BL, Ghalamkarpour A, Onoufriadis A, et al. (2012). Mutations in KIF11 cause autosomal-dominant microcephaly variably associated with congenital lymphedema and chorioretinopathy. Am J Hum Genet 90, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeda P, Huso DL, Fisher SA, Halushka MK, Kim H, Qian F, Germino GG, and Watnick T (2014). Polycystin signaling is required for directed endothelial cell migration and lymphatic development. Cell Rep 7, 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby DR, Zhou EH, Vargas-Pinto R, Pedrigi RM, Fuchshofer R, Braakman ST, Gupta R, Perkumas KM, Sherwood JM, Vahabikashi A, et al. (2014). Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc Natl Acad Sci U S A 111, 13876–13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DY, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, et al. (2014). Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J Clin Invest 124, 3960–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TK, Habimana-Griffin L, Gao X, Xu B, Achilefu S, Alitalo K, McKee CA, Sheehan PW, Musiek ES, Xiong C, et al. (2019). Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener 14, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Jimenez D, Fontenete S, Megias D, Fustero-Torre C, Grana-Castro O, Castellana D, Loewe R, and Perez-Moreno M (2019). Lymphatic vessels interact dynamically with the hair follicle stem cell niche during skin regeneration in vivo. EMBO J 38, e101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, et al. (2016). Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 93, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi D, Gardner J, Chambers D, Hosking B, Peters J, Muscat G, Abbott C, and Koopman P (2000). Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet 24, 434–437. [DOI] [PubMed] [Google Scholar]
- Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, Chin SM, Kitahara S, Bouta EM, Chang J, et al. (2018). Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359, 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry LP, Jakobiec FA, Zakka FR, and Walton DS (2012). Newborn primary congenital glaucoma: histopathologic features of the anterior chamber filtration angle. J AAPOS 16, 565–568. [DOI] [PubMed] [Google Scholar]
- Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, et al. (2020). B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. (2004). Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 10, 974–981. [DOI] [PubMed] [Google Scholar]
- Petrova TV, and Koh GY (2018). Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med 215, 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr KM, Debrah AY, Specht S, and Hoerauf A (2009). Filariasis and lymphoedema. Parasite Immunol 31, 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichol-Thievend C, Betterman KL, Liu X, Ma W, Skoczylas R, Lesieur E, Bos FL, Schulte D, Schulte-Merker S, Hogan BM, et al. (2018). A blood capillary plexus-derived population of progenitor cells contributes to genesis of the dermal lymphatic vasculature during embryonic development. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas-Paz L, Strilic B, Goedecke A, Breier G, Fassler R, and Lammert E (2012). Mechanoinduction of lymph vessel expansion. EMBO J 31, 788–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond CM (2005). Adipose tissue and the immune system. Prostaglandins Leukot Essent Fatty Acids 73, 17–30. [DOI] [PubMed] [Google Scholar]
- Pujol F, Hodgson T, Martinez-Corral I, Prats AC, Devenport D, Takeichi M, Genot E, Makinen T, Francis-West P, Garmy-Susini B, et al. (2017). Dachsous1-Fat4 Signaling Controls Endothelial Cell Polarization During Lymphatic Valve Morphogenesis-Brief Report. Arterioscler Thromb Vasc Biol 37, 1732–1735. [DOI] [PubMed] [Google Scholar]
- Quigley HA, and Broman AT (2006). The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90, 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers T, van der Vorst EP, Daissormont IT, Otten JJ, Theodorou K, Theelen TL, Gijbels M, Anisimov A, Nurmi H, Lindeman JH, et al. (2017). Adventitial lymphatic capillary expansion impacts on plaque T cell accumulation in atherosclerosis. Sci Rep 7, 45263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier JF, Dubuquoy L, Colombel JF, Jouret-Mourin A, Delos M, Ferrante M, Sokol H, Hertogh GD, Salleron J, Geboes K, et al. (2013). Decreased lymphatic vessel density is associated with postoperative endoscopic recurrence in Crohn’s disease. Inflamm Bowel Dis 19, 2084–2090. [DOI] [PubMed] [Google Scholar]
- Ramos RF, Hoying JB, Witte MH, and Daniel Stamer W (2007). Schlemm’s canal endothelia, lymphatic, or blood vasculature? J Glaucoma 16, 391–405. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, and Swartz MA (2005). Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5, 617–628. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, and Miller NE (2014). Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest 124, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Bala S, Rahier JF, Johnson MW, Wang PL, Nalbantoglu I, Dubuquoy L, Chau A, Pariente B, Kartheuser A, et al. (2016). Lymphoid Aggregates Remodel Lymphatic Collecting Vessels that Serve Mesenteric Lymph Nodes in Crohn Disease. Am J Pathol 186, 3066–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Ivanov S, Zinselmeyer BH, and Scallan JP (2017). The Lymphatic System: Integral Roles in Immunity. Annu Rev Immunol 35, 31–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockson SG (2001). Lymphedema. Am J Med 110, 288–295. [DOI] [PubMed] [Google Scholar]
- Rockson SG, Tian W, Jiang X, Kuznetsova T, Haddad F, Zampell J, Mehrara B, Sampson JP, Roche L, Kim J, et al. (2018). Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, Von Andrian UH, and Carroll MC (2009). Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED (2002). The molecular control of adipogenesis, with special reference to lymphatic pathology. Ann N Y Acad Sci 979, 143–158; discussion 188–196. [DOI] [PubMed] [Google Scholar]
- Roth J, Qiang X, Marban SL, Redelt H, and Lowell BC (2004). The obesity pandemic: where have we been and where are we going? Obes Res 12 Suppl 2, 88S–101S. [DOI] [PubMed] [Google Scholar]
- Rustenhoven J, and Kipnis J (2019). Bypassing the blood-brain barrier. Science 366, 1448–1449. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, and Swartz MA (2010). Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol 176, 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin FR (1902). On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. American Journal of Anatomy 1, 367–389. [Google Scholar]
- Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hagerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, et al. (2012). Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 22, 430–445. [DOI] [PubMed] [Google Scholar]
- Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, et al. (2015). FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 125, 3861–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabine A, Saygili Demir C, and Petrova TV (2016). Endothelial Cell Responses to Biomechanical Forces in Lymphatic Vessels. Antioxid Redox Signal 25, 451–465. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, et al. (2014). Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370, 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfarazi M, and Stoilov I (2000). Molecular genetics of primary congenital glaucoma. Eye (Lond) 14 (Pt 3B), 422–428. [DOI] [PubMed] [Google Scholar]
- Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, and Mehrara BJ (2014). Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 307, H165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawane M, Kajiya K, Kidoya H, Takagi M, Muramatsu F, and Takakura N (2013). Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes 62, 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlemm F (1830). Bulbus oculi. Th. Chr. Fr. Enslin Theoretisch-praktisches Handbuch der Chirurgie, mit Einschluß der syphilitischen und Augen-Krankheiten. Berlin, Germany; Carl Gerold, Wien, 331–338. [Google Scholar]
- Schneider M, Conway EM, and Carmeliet P (2005). Lymph makes you fat. Nat Genet 37, 1023–1024. [DOI] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, et al. (2017). Addendum: The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 546, 564. [DOI] [PubMed] [Google Scholar]
- Shi GP, Bot I, and Kovanen PT (2015). Mast cells in human and experimental cardiometabolic diseases. Nat Rev Cardiol 12, 643–658. [DOI] [PubMed] [Google Scholar]
- Shields JD, Kourtis IC, Tomei AA, Roberts JM, and Swartz MA (2010). Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752. [DOI] [PubMed] [Google Scholar]
- Shimada T, Noguchi T, Takita K, Kitamura H, and Nakamura M (1989). Morphology of lymphatics of the mammalian heart with special reference to the architecture and distribution of the subepicardial lymphatic system. Acta Anat (Basel) 136, 16–20. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, and Detmar M (2001). Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7, 192–198. [DOI] [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE, Nishimura DY, Alward WL, Hogan BL, and John SW (2000). Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet 9, 1021–1032. [DOI] [PubMed] [Google Scholar]
- Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, and Iwasaki A (2020). VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577, 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souma T, Tompson SW, Thomson BR, Siggs OM, Kizhatil K, Yamaguchi S, Feng L, Limviphuvadh V, Whisenhunt KN, Maurer-Stroh S, et al. (2016). Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J Clin Invest 126, 2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, and Oliver G (2007). Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21, 2422–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MP, Lagutin O, and Oliver G (2010). The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev 24, 696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, and Oliver G (2011). Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev 25, 2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, Mukatira S, Gil HJ, Nurmi H, Alitalo K, et al. (2014). The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev 28, 2175–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John AL, Ang WXG, Huang MN, Kunder CA, Chan EW, Gunn MD, and Abraham SN (2014). S1P-Dependent trafficking of intracellular yersinia pestis through lymph nodes establishes Buboes and systemic infection. Immunity 41, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Lavina B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, et al. (2015). cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep 10, 1708–1721. [DOI] [PubMed] [Google Scholar]
- Steinman L (2014). Immunology of relapse and remission in multiple sclerosis. Annu Rev Immunol 32, 257–281. [DOI] [PubMed] [Google Scholar]
- Steinskog ES, Sagstad SJ, Wagner M, Karlsen TV, Yang N, Markhus CE, Yndestad S, Wiig H, and Eikesdal HP (2016). Impaired lymphatic function accelerates cancer growth. Oncotarget 7, 45789–45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone OA, and Stainier DYR (2019). Paraxial Mesoderm Is the Major Source of Lymphatic Endothelium. Dev Cell 50, 247–255 e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Ayyadurai S, and Zlokovic BV (2016). Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Kisler K, Montagne A, Toga AW, and Zlokovic BV (2018). The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21, 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DT, Jimenez JM, Chang J, Hess PR, Mericko-Ishizuka P, Fu J, Xia L, Davies PF, and Kahn ML (2015). Lymph flow regulates collecting lymphatic vessel maturation in vivo. J Clin Invest 125, 2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson KK, Nissen MJ, Leach JW, and Post-White J (2009). Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum 36, 185–193. [DOI] [PubMed] [Google Scholar]
- Takeda A, Hollmen M, Dermadi D, Pan J, Brulois KF, Kaukonen R, Lonnberg T, Bostrom P, Koskivuo I, Irjala H, et al. (2019). Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity 51, 561–572 e565. [DOI] [PubMed] [Google Scholar]
- Tamm ER (2009). The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res 88, 648–655. [DOI] [PubMed] [Google Scholar]
- Tammela T, and Alitalo K (2010). Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140, 460–476. [DOI] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, et al. (2015). Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatin F, Taddei A, Weston A, Fuchs E, Devenport D, Tissir F, and Makinen T (2013). Planar cell polarity protein Celsr1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis. Dev Cell 26, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatin F, Renaud-Gabardos E, Godet AC, Hantelys F, Pujol F, Morfoisse F, Calise D, Viars F, Valet P, Masri B, et al. (2017). Apelin modulates pathological remodeling of lymphatic endothelium after myocardial infarction. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakkolizadeh A, Wolfe KQ, and Kangesu L (2001). Cutaneous lymphatic malformation with secondary fat hypertrophy. Br J Plast Surg 54, 367–369. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Zhou Y, Zuch de Zafra CL, Fuh G, Lee CV, Sanowar S, Ridgway JB, Kusi AM, Farman C, Booler H, et al. (2019). Rapid Development of Glaucoma Via ITV Nonselective ANGPT 1/2 Antibody: A Potential Role for ANGPT/TIE2 Signaling in Primate Aqueous Humor Outflow. Invest Ophthalmol Vis Sci 60, 4097–4108. [DOI] [PubMed] [Google Scholar]
- Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, et al. (2014). A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest 124, 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson BR, Souma T, Tompson SW, Onay T, Kizhatil K, Siggs OM, Feng L, Whisenhunt KN, Yanovitch TL, Kalaydjieva L, et al. (2017). Angiopoietin-1 is required for Schlemm’s canal development in mice and humans. J Clin Invest 127, 4421–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Rockson SG, Jiang X, Kim J, Begaye A, Shuffle EM, Tu AB, Cribb M, Nepiyushchikh Z, Feroze AH, et al. (2017). Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci Transl Med 9. [DOI] [PubMed] [Google Scholar]
- Torrisi JS, Hespe GE, Cuzzone DA, Savetsky IL, Nitti MD, Gardenier JC, Garcia Nores GD, Jowhar D, Kataru RP, and Mehrara BJ (2016). Inhibition of Inflammation and iNOS Improves Lymphatic Function in Obesity. Sci Rep 6, 19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triacca V, Guc E, Kilarski WW, Pisano M, and Swartz MA (2017). Transcellular Pathways in Lymphatic Endothelial Cells Regulate Changes in Solute Transport by Fluid Stress. Circ Res 120, 1440–1452. [DOI] [PubMed] [Google Scholar]
- Trincot CE, Xu W, Zhang H, Kulikauskas MR, Caranasos TG, Jensen BC, Sabine A, Petrova TV, and Caron KM (2019). Adrenomedullin Induces Cardiac Lymphangiogenesis After Myocardial Infarction and Regulates Cardiac Edema Via Connexin 43. Circ Res 124, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong TN, Li H, Hong YK, and Chen L (2014). Novel characterization and live imaging of Schlemm’s canal expressing Prox-1. PLoS One 9, e98245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton ML, and Weller RO (1985). The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations. J Neurosurg 63, 867–875. [DOI] [PubMed] [Google Scholar]
- Van Balkom ID, Alders M, Allanson J, Bellini C, Frank U, De Jong G, Kolbe I, Lacombe D, Rockson S, Rowe P, et al. (2002). Lymphedema-lymphangiectasia-mental retardation (Hennekam) syndrome: a review. Am J Med Genet 112, 412–421. [DOI] [PubMed] [Google Scholar]
- van der Putte SC (1975). The early development of the lymphatic system in mouse embryos. Acta Morphol Neerl Scand 13, 245–286. [PubMed] [Google Scholar]
- Van Kruiningen HJ, and Colombel JF (2008). The forgotten role of lymphangitis in Crohn’s disease. Gut 57, 1–4. [DOI] [PubMed] [Google Scholar]
- van Steensel MA, Damstra RJ, Heitink MV, Bladergroen RS, Veraart J, Steijlen PM, and van Geel M (2009). Novel missense mutations in the FOXC2 gene alter transcriptional activity. Hum Mutat 30, E1002–1009. [DOI] [PubMed] [Google Scholar]
- van Zwam M, Huizinga R, Heijmans N, van Meurs M, Wierenga-Wolf AF, Melief MJ, Hintzen RQ, t Hart BA, Amor S, Boven LA, et al. (2009). Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol 217, 543–551. [DOI] [PubMed] [Google Scholar]
- Vieira JM, Norman S, Villa Del Campo C, Cahill TJ, Barnette DN, Gunadasa-Rohling M, Johnson LA, Greaves DR, Carr CA, Jackson DG, et al. (2018). The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J Clin Invest 128, 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes S, Arrault M, and Dupuy A (2007). Factors associated with increased breast cancer-related lymphedema volume. Acta Oncol 46, 1138–1142. [DOI] [PubMed] [Google Scholar]
- von der Weid PY, Rehal S, and Ferraz JG (2011). Role of the lymphatic system in the pathogenesis of Crohn’s disease. Curr Opin Gastroenterol 27, 335–341. [DOI] [PubMed] [Google Scholar]
- Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K, et al. (2014). Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol 34, 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorio T, Tirronen A, and Yla-Herttuala S (2017). Cardiac Lymphatics - A New Avenue for Therapeutics? Trends Endocrinol Metab 28, 285–296. [DOI] [PubMed] [Google Scholar]
- Vuorio T, Yla-Herttuala E, Laakkonen JP, Laidinen S, Liimatainen T, and Yla-Herttuala S (2018). Downregulation of VEGFR3 signaling alters cardiac lymphatic vessel organization and leads to a higher mortality after acute myocardial infarction. Sci Rep 8, 16709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Rong X, Duerr MA, Hermanson DJ, Hedde PN, Wong JS, Vallim TQ, Cravatt BF, Gratton E, Ford DA, et al. (2016). Intestinal Phospholipid Remodeling Is Required for Dietary-Lipid Uptake and Survival on a High-Fat Diet. Cell Metab 23, 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, and Oliver G (2010). Current views on the function of the lymphatic vasculature in health and disease. Genes Dev 24, 2115–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jin Y, Mae MA, Zhang Y, Ortsater H, Betsholtz C, Makinen T, and Jakobsson L (2017). Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity. Development 144, 3590–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, and Medeiros FA (2014). The pathophysiology and treatment of glaucoma: a review. JAMA 311, 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H, Kojima K, Lannes-Vieira J, Lassmann H, and Linington C (1994). Animal models. Ann Neurol 36 Suppl, S47–53. [DOI] [PubMed] [Google Scholar]
- Wigle JT, and Oliver G (1999). Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, and Oliver G (2002). An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21, 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MH, Bernas MJ, Martin CP, and Witte CL (2001). Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech 55, 122–145. [DOI] [PubMed] [Google Scholar]
- Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, Garcia-Caballero M, Missiaen R, Huang H, Bruning U, et al. (2017). The role of fatty acid beta-oxidation in lymphangiogenesis. Nature 542, 49–54. [DOI] [PubMed] [Google Scholar]
- Wynd S, Melrose WD, Durrheim DN, Carron J, and Gyapong M (2007). Understanding the community impact of lymphatic filariasis: a review of the sociocultural literature. Bull World Health Organ 85, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanev P, Poinsatte K, Hominick D, Khurana N, Zuurbier KR, Berndt M, Plautz EJ, Dellinger MT, and Stowe AM (2020). Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab 40, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, and Unanue ER (2013). Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J Exp Med 210, 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, and Nedergaard M (2013). Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 11, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, and Oliver G (2014). Development of the mammalian lymphatic vasculature. J Clin Invest 124, 888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao LC, Baluk P, Srinivasan RS, Oliver G, and McDonald DM (2012). Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol 180, 2561–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, and Karin N (1992). Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 356, 63–66. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Dieterich LC, Karaman S, Proulx ST, Bachmann SB, Sciaroni C, and Detmar M (2019). An important role of cutaneous lymphatic vessels in coordinating and promoting anagen hair follicle growth. PLoS One 14, e0220341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, and Eichmann A (2002). Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 129, 4797–4806. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, Genet G, Boye K, Michon P, Kunzel SE, et al. (2018). Lacteal junction zippering protects against diet-induced obesity. Science 361, 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Pu T, Feng W, Lu M, Zheng Y, Du R, Xiao M, and Hu G (2019). Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl Neurodegener 8, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]


