Highlights
-
•
COVID-19 patients with total loss of smell have more olfactory bulb abnormalities at the magnetic resonance imaging than patients without loss of smell.
-
•
The olfactory bulb MRI abnormalities may be objectified through a signal intensity ratio measurement that is calculated between the average signals of the olfactory bulb and the frontal white matter.
-
•
The loss of smell is probably due to olfactory bulb inflammation related to virus spread.
Key words: COVID-19, Coronavirus, Smell, Olfaction, Anosmia, Olfactory, Imaging, MRI
Dear Editor,
We recently read the paper entitled “Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter CORANOSMIA cohort study.1 ” Loss of smell without nasal obstruction was commonly found in the coronavirus disease 2019 (COVID-19), accounting for more than 50% of Western patients.2 The main suspected etiological mechanism consists of the virus spread through the neuroepithelium of the olfactory cleft and a related neuronal cell destruction. However, this mechanism has not been extensively studied through magnetic resonance imaging (MRI) findings on cohort of anosmic COVID-19 patients. In this preliminary study, we investigated the olfactory bulb of COVID-19 patients with or without loss of smell through MRI and we develop a MRI approach to assess the impairment of olfactory bulb.
Adults with laboratory-confirmed COVID-19 diagnosis and self-reported sudden-onset total loss of smell (SOLS) were recruited through the French public call of the COVID-19 Task Force of YO-IFOS (IRB: IJB-0M011–3137). Electronic informed consent was obtained for each patient. The details about the diagnosis procedure were reported in a previous publication.3 The study was conducted according to the ‘Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)’ Statement. Clinical and epidemiological characteristics were electronically collected through an online standardized questionnaire developed with Professional Survey MonkeyⓇ (San Mateo, California, USA). The olfactory and gustatory features were investigated through the smell and taste component of the National Health and Nutrition Examination Survey (NHNES).4 Patients benefited from objective olfactory testing (Sniffin'Sticks tests; Medisense, Groningen, Netherlands), which is a validated test allowing categorization of patients into normosmic (16–12), hyposmic (11–9), and anosmic (8–0).3
Imaging studies were conducted on a 3-Tesla MR imaging system (MR750; GE Healthcare, Milwaukee, WI, USA) with a 20-channel head and neck coil following a protocol including, in order to assess the olfactory bulb signal, a 3D-FLAIR sequence and a 3D-T2 sequence centered on the olfactory bulbs (OB). The 3D-FLAIR sequence was acquired in coronal plane with the following parameters: TR/TE, 8000/133 ms; TSE factor 80; bandwidth, 120 Hz/pixel; section thickness, 2 mm; matrix 240 × 240; FOV, 230 × 230 × 20; voxel size, 0.95 × 0.95 × 2 mm; ETL=220 with variable flip angle; acquisition time=4min36sec; imaging option: Fat Sat, T2 prep. Sections were angled perpendicular to the anterior base of the skull or cribriform plate. Two experienced neuroradiologists independently performed image analyses. A third neuroradiologist resolved potential discordances. Radiologists were blinded regarding the patient olfactory evaluation (anosmic versus normosmic). Qualitative analysis reported the visual analysis of presence of T2/FLAIR OB hyperintensity compared with the signal intensity of the adjacent frontal white matter. Quantitative analysis was performed on T2/FLAIR image by adjusting contours of a ROI centered on the OB on a coronal plane, in order to measure the average OB signal intensity. A signal intensity ratio (SIR) was then calculated between the average signal of the OB and the average signal of a ROI placed in ipsilateral frontal white matter. Statistical analyses were performed using SPSS (version 22.0; IBM Corp, Armonk, NY, USA) according to two subgroups of patients: COVID-19 patients with SOLS and COVID19 without olfactory dysfunction (controls). An intra-class correlation coefficient was performed to compare the reproducibility of signal intensity ratio between two readers. The group outcomes were compared with Mann-Whitney U test.
At the end of the recruitment process, 23 patients were included (14 females). A total of 19 patients composed the SOLS group, while 4 patients did not have anosmia. The clinical and epidemiological characteristics are reported in Table 1 . The most common symptoms developed over the disease were: asthenia, headache and cough. The mean duration of symptoms was 9.6 ± 6.9 days. Patients had normal neurological and general examinations. The fiberoptic was not performed because sanitary recommendations. Regarding the NHNES questions, the loss of smell developed after (N = 12; 63%) the onset of the general symptoms. Taste disorder, which was defined as abnormal sensations of salty, sweet, bitter and sour, was present in 91.3% of patients. The mean sniffin-sticks test was 3.2 ± 4.4. On the 4 patients of the normosmic group (sniffin-sticks test>11), MRI findings reported no abnormal FLAIR hyperintensity of the OB and one patient presented bilateral obstruction of the olfactory cleft.
Table 1.
Clinical and epidemiological features of patients.
| Characteristics | Patients (N-%) |
|---|---|
| Age (Mean - SD) - yo | 39.0 ± 17.1 |
| Gender (Female/Male) | 14/9 |
| Smoker | 5 (21.7) |
| Patients with seasonal allergy | 3 (13.0) |
| Comorbidities | |
| Hypertension | 2 (8.7) |
| Depression | 2 (8.7) |
| Asthma | 2 (8.7) |
| Hypothyroidism | 1 (4.3) |
| Diabetes | 1 (4.3) |
| Heart problems | 1 (4.3) |
| Neurological diseases | 1 (4.3) |
| Autoimmun disease | 1 (4.3) |
| Hypercholesterolemia | 1 (4.3) |
| General Symptoms (N -%) | |
| Asthenia | 21(91.3) |
| Headache | 18 (78.3) |
| Cough | 17 (73.9) |
| Fever (>38C) | 15 (65.2) |
| Myalgia | 14 (60.9) |
| Loss of appetite | 12 (52.2) |
| Arthralgia | 10 (43.5) |
| Chest pain | 9 (39.1) |
| Dyspnea | 5 (21.7) |
| Diarrhea | 5 (21.7) |
| Abdominal pain | 2 (8.7) |
| Conjonctivitis | 2 (8.7) |
| Ear, nose and throat Sympotms (N -%) | |
| Postnasal drip | 21 (91.3) |
| Taste dysfunction | 21 (91.3) |
| Presumed anosmia | 19 (82.6) |
| Nasal obstruction | 18 (78.3) |
| Ear pain | 18 (78.3) |
| Rhinorrhea | 17 (73.9) |
| Face pain/heaviness | 16 (69.6) |
| Throat sputum | 12 (52.2) |
| Sore throat | 6 (26.1) |
| Presumed hyposmia | 4 (17.4) |
| Dysphagia | 2 (8.7) |
Abbreviations: SD=standard deviation.
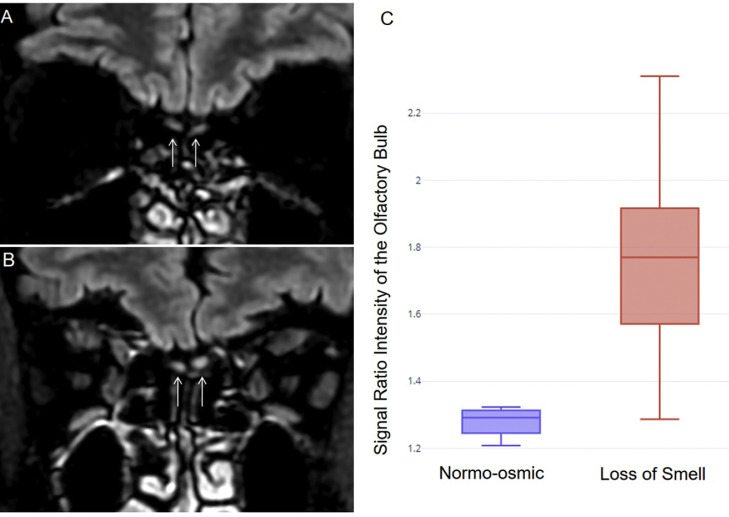
On the 19 patients with SOLS at the time of the MRI, 9 (47%) presented bilateral obstruction of the olfactory clefts. Statistical analysis of Signal Intensity Ratio of the OB showed significant differences between the SOLS group (mean=1.73±0.23) and the normosmic group (mean=1.27±0.04; p<0.0001). The intra-class correlation coefficient for Signal Intensity Ratio measurements was very high (rho=0.94, 95%CI [0.90–0.96], p<0.001). Representative cases are reported in Fig. 1 .
Fig. 1.
MRI findings.
Comparison of T2/FLAIR coronal views centered on the olfactory bulbs showing normal signal in a normosmic (A) and T2/FLAIR hyperintensity of the olfactory bulbs in a patient with anosmia (B). Box plot (C) of signal intensity ratio in loss of smell and in normo-osmic group, revealing a statistically higher T2/FLAIR Signal Intensity Ratio of the olfactory bulb in the loss of smell group (p<0.001).
The present study is the first case-series that describes MRI olfactory abnormalities in COVID-19 patients with SOLS. Three patient profiles were observed: 1) patients with SOLS, OB signal abnormalities (ratio) and no olfactory cleft edema, 2) patients with SOLS, OB signal abnormalities and olfactory cleft edema; and 3) patients without SOLS and normal OB signal. In some cases, the occurrence of concomitant edema of the olfactory cleft mucosa was noted, mainly in the anosmic group but also in one normosmic patient. This could be due to the initial inflammatory reaction of the nasal mucosa since we observed in previous study that mild-to-moderate COVID19 patients with anosmia would have an otolaryngological clinical picture of the disease.2 These findings support that SOLS could be due to a virus-related inflammatory reaction into the OB, which could impair olfactory neural or sustentacullar cells. The inflammatory reaction could start in the neuroepithelium of the olfactory cleft, which appeared obstructed at the MRI over the first day of SOLS.
The originality of the present work is the development of a quantitative approach of the OB of COVID-19 patients. The ratio analysis is easy to analyze the OB involvement and provides a quantitative approach to determine the presence of the neurological pathology as a qualitative analysis alone of the signal of the olfactory bulb can be difficult to appreciate visually, especially on doubtful cases. Visual appreciation may be influenced by the setting of the window level. To be able to adequately measure signal intensity of such small structures as olfactory bulbs, 3D FLAIR sequences must be optimized in terms of spatial resolution. We therefore preferred a coronal acquisition of the sequence, in order to have a submillimetric ‘in plane’ resolution. OB neuropathy related to chronic rhinosinusitis have already been described as presence of T2/FLAIR hyperintensity of olfactory bulbs.5 Presence of MRI OB hyperintensities in acute COVID-19 symptomatic patients suggests a viral neuropathy of the OBs, leading to local inflammatory reaction. The main limitation of this study is the low number of patients in both groups but it was not easy to perform MRI in a context of pandemic.
CRediT authorship contribution statement
Annaelle Chetrit: . Jerome R. Lechien: Writing - review & editing. Amine Ammar: Writing - original draft. Younes Chekkoury-Idrissi: Writing - original draft. Lea Distinguin: Writing - original draft. Marta Circiu: Writing - original draft. Sven Saussez: Writing - original draft. Marie-Christine Ballester: . Marc Vasse: Writing - original draft. Najete Berradja: Writing - original draft. Stephane Hans: . Robert Carlier: . Myriam Edjlali: Writing - review & editing.
Declaration of Competing Interest
The authors have no conflicts of interest.
Acknowledgments
Funding
None.
Data availability
Data are available in the Department of Radiology of AP-HP-Garches Hospital.
Acknowledgment
I. Ducamp for the management of the patient appointment. We also thank Pierre-Frédéric Bourcier for his technical support on the optimization of MR sequence parameters. Florence Toutain, Clément Serpette and all the MRI technicians involved in MRI examinations of COVID-19 patients with neurological symptoms.
References
- 1.Salmon D., Bartier S., Hautefort C., Nguyen Y., Nevoux J., Hamel A.L., Camhi Y., Canouï-Poitrine F., Verillaud B., Slama D., Haim-Boukobza S., Sourdeau E., Cantin D., Corré A., Bryn A., Etienne N., Rozenberg F., Layese R., Papon J.F., Bequignon E., APHP COVID-19 research collaboration Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter CORANOSMIA cohort study. J Infect. 2020 Jul 7 doi: 10.1016/j.jinf.2020.07.005. S0163-4453(20)30463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechien J.R., Chiesa-Estomba C.M., Hans S. Loss of Smell and Taste in 2,013 European Mild-to-Moderation COVID-19 Patients. Ann Int Med. 2020 doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien J.R., Cabaraux P., Chiesa-Estomba C.M. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020 doi: 10.1002/hed.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawal S., Hoffman H.J., Bainbridge K.E., Huedo-Medina T.B., Duffy V.B. Prevalence and Risk Factors of Self-Reported Smell and Taste Alterations: results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES) Chem Sens. 2016;41(1):69–76. doi: 10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung M.S., Choi W.R., Jeong H.Y., Lee J.H., Kim J.H. MR imaging-based evaluations of olfactory bulb atrophy in patients with olfactory dysfunction. AJNR Am J Neuroradiol. 2018;39(3):532–537. doi: 10.3174/ajnr.A5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in the Department of Radiology of AP-HP-Garches Hospital.