Abstract
G protein-coupled receptors (GPCRs) constitute the largest protein superfamily in the human genome. GPCRs play key roles in mediating a wide variety of physiological events including proliferation and cancer metastasis. Given the major roles that GPCRs play in mediating cancer growth, they present promising targets for small molecule therapeutics. One of the principal goals of our lab is to identify complex natural products (NPs) suitable for ring distortion, or the dramatic altering of the inherently complex architectures of NPs, to rapidly generate an array of compounds with diverse molecular skeletal systems. The overarching goal of our ring distortion approach is to re-program the biological activity of select natural products and identify new compounds of importance to the treatment of disease, such as cancer. Described herein are the results from biological screens of diverse small molecules derived from the indole alkaloid yohimbine against a panel of GPCRs involved in various diseases. Several analogues displayed highly differential antagonistic activities across the GPCRs tested. We highlight the re-programmed profile of one analogue, Y7g, which exhibited selective antagonistic activities against AVPR2 (IC50 = 459 nM) and OXTR (IC50 = 1.16 μM). The activity profile of Y7g could correlate its HIF-dependent anti-cancer activity to its GPCR antagonism since these receptors are known to be upregulated in hypoxic cellular environments. Our findings demonstrate that the ring distortion of yohimbine can lead to the identification of new compounds capable of interacting with distinct cancer-relevant targets.
Keywords: yohimbine, indole alkaloids, ring distortion, drug discovery, GPCR drug targets, cancer
Introduction
GPCRs are integral cell-surface proteins that serve as an interface between the intra- and extracellular environment.1,2 These receptors respond to a plethora of external stimuli (e.g. small molecules, ions) that result in a wide variety of physiological events via downstream signaling cascades predominately activated by heterotrimeric G proteins. GPCRs function by releasing free Gα and Gβγ subunits that initiate intracellular signal transduction pathways. Various GPCRs operate through these heterotrimeric G proteins to induce uncontrolled cellular proliferation and cancer metastasis.3–6 Over 30% of all FDA-approved drugs target GPCRs (implicated in various diseases).7 Despite the successes in targeting certain GPCRs for chemotherapy, a large majority of these proteins have yet to be appropriately classified and successfully targeted with small molecules.8,9 Therefore, success in targeting cancer-relevant GPCRs necessitates the discovery of novel and selective small molecule antagonists that can efficiently inhibit cellular signaling pathways that are critical to cancer.
Natural products have been the main source of therapeutic small molecules because of their exceptional ability to selectively bind and modulate numerous biological targets that play crucial roles in disease.10,11 Architecturally complex natural products (e.g. paclitaxel, vancomycin, morphine) are able to form highly specific binding modes with their respective target due to their unique and multifaceted carbon skeletons, which are decorated with diverse functional groups. These aspects enable natural products to tightly bind their corresponding proteins and induce a desired biological response of therapeutic value. Despite the demonstrated utility of natural products for therapy, a paradigm shift to high-throughput screening (HTS) occurred in the mid-1990’s, which heavily relies on small organic molecules to drive drug discovery efforts.12–14 It has been well documented that HTS libraries are largely populated by structurally simple organic molecules that lack three-dimensionality when compared to natural product therapeutics. Although HTS efforts have served well in drugging certain biological targets (e.g. kinases) these libraries severely lack chemical diversity15 and such compound collections have largely failed to produce viable leads for proteins that are more difficult to target (e.g. transcription factors16,17).
Several elegant strategies to address the lack of chemical diversity in screening have been employed. The most well established approaches are diversity-oriented synthesis (DOS)18,19 and biology-oriented synthesis (BIOS)20 that have been pioneered by Schreiber and Waldmann, respectively. These tactics aim at generating libraries of architecturally complex small molecules by building structural complexity in sequential synthetic reactions, starting from simple building blocks. Screening libraries derived from DOS and BIOS have led to discoveries of several small molecules that modulate diverse targets of therapeutic relevance.21,22 Additionally, other complementary approaches to DOS and BIOS have been developed, which include complexity-to-diversity, also known as the “ring distortion” of natural products.23–26 In a ring distortion approach, select natural products are subjected to a series of chemoselective reactions aimed to dramatically alter the inherently complex ring system to produce diverse and architecturally unique carbon scaffolds. A major goal of ring distortion is to identify complex small molecules that display biological activity that is distinct from the parent natural product.
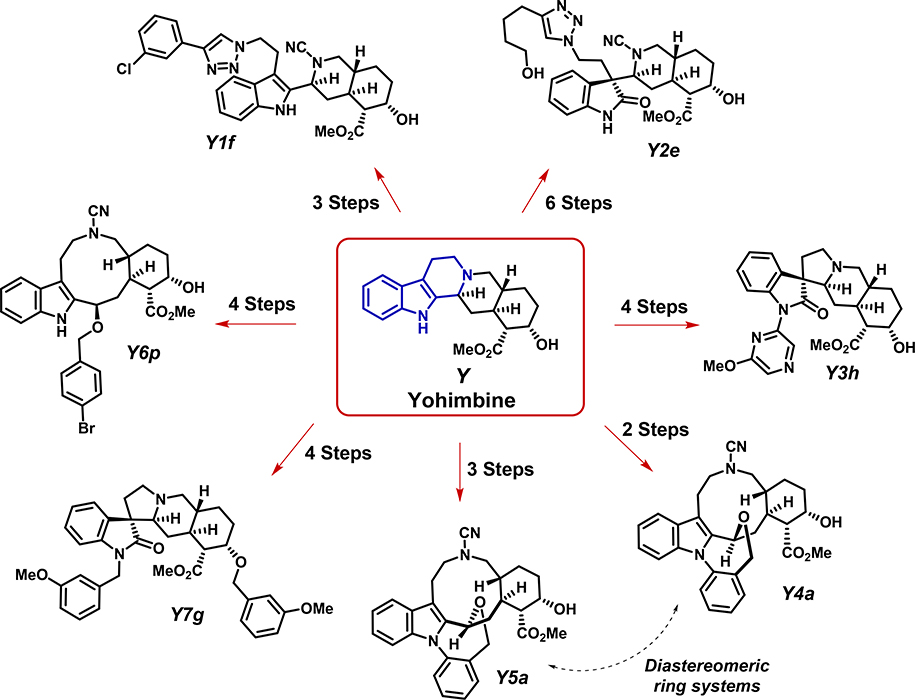
We have recently reported efforts towards establishing a tryptoline-based ring distortion of the complex alkaloid natural product yohimbine (Y).25,27,28 By exploiting the embedded tryptoline substructure of yohimbine, we were able to rapidly generate (two to six synthetic steps) an array of unique small molecules through a series of highly selective ring-cleavage and diastereoselective, oxidative indole rearrangement reactions that afforded cyanamide and spirooxindole products, respectively. Subsequent phenotypic screening of the yohimbine-derived library unveiled analogues that exhibited selective hypoxia-inducible factor (HIF)-dependent anti-cancer activities, and anti-inflammatory properties.25 Additional screening was conducted on the yohimbine-derived compound library and identified new molecules that demonstrated antimalarial activities by preferentially inhibiting Plasmodium falciparum parasites without cytotoxicity toward human cells.28 Several of these yohimbine ring distortion analogues were found to have sub-micromolar anti-plasmodial activity. Furthermore, the parent natural product yohimbine was completely inactive in these biological screening endeavors. Intrigued by the stark contrast in the cellular activity profiles of select ring distortion analogues to yohimbine, we hypothesized that new analogues could demonstrate re-programmed activities towards GPCR targets of relevance to cancer and other disease areas. The work presented here details the screening results of several yohimbine-derived analogues (and yohimbine itself) against a diverse panel of 168 GPCR targets that play important roles in various diseases. From the initial screen and subsequent hit validation, several yohimbine-derived analogues demonstrated differential antagonistic activity profiles against multiple GPCR targets, including several relevant to cancer.
Results and Discussion
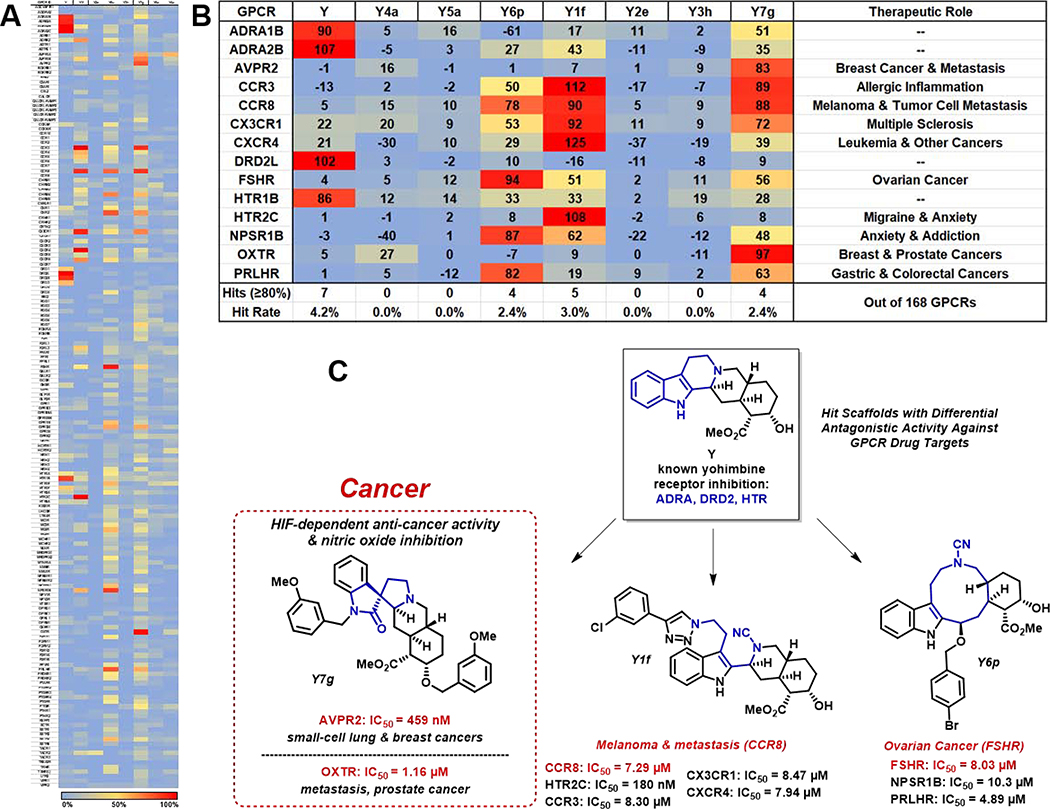
From our initial ring distortion campaign, six analogues (one from each main scaffold class, Figure 1) and yohimbine itself, were chosen to screen for both agonist and antagonist activities against a panel of GPCRs. Our goal was to obtain a detailed activity profile for each analogue against 168 GPCRs using PathHunter β-arrestin cell-based assays.6,29,30 From the primary screen (Figure 2A) at 20 μM, several analogues displayed significant antagonistic activities (≥80% inhibition) against GPCRs while agonist activity was mostly absent during these investigations. Most notably, yohimbine-derived compounds Y6p, Y1f and Y7g all exhibited significant inhibitory activities, whereas Y4a, Y5a, Y2e and Y3h were inactive and did not induce an antagonistic effect for any GPCR in the screen (see supporting information). The parent natural product yohimbine (Y, an adrenoceptor antagonist) unsurprisingly inhibited its native GPCR targets during these investigations, which include: α−2B adrenergic receptor (ADRA2B), dopamine D2b receptor (DRD2L) and hydroxytryptamine receptor (HTR1B).31 A focused heat map of the most significantly inhibited GPCR targets showcases re-programmed activities of the different yohimbine-derived scaffolds when compared to yohimbine (Figure 2B). A loss of activity for Y and a gain of new activities against GPCRs for analogues Y6p, Y1f and Y7g demonstrates proof-of-concept that yohimbine’s activity can be re-programmed to modulate different protein targets using a ring distortion approach.
Figure 1.
Ring distortion of yohimbine (Y) to rapid synthetic access to architecturally diverse analogues for biological screens. These yohimbine-derived compounds were screened against a panel of GPCR drug targets. (Note: color should be used)
Figure 2.
A) Full heatmap of antagonist activity for all 168 GPCRs against Y and ring distortion analogues. B) Focused heatmap of disease-relevant GPCRs that were antagonized during these investigations. C) Chemical structures and IC50 values for yohimbine analogues with diverse “gain-of-function” activity profiles against GPCR targets. (Note: color should be used)
During the course of these investigations, analogue Y6p demonstrated antagonistic activity against four disease-relevant GPCRs at ≥80% inhibition, three of which were advanced to dose-response experiments (Figure 2B, C). Y6p displayed low micromolar potencies for follicle-stimulating hormone receptor (FSHR, IC50 = 8.03 μM), neuropeptide S receptor 1 (NPSR1B, IC50 = 10.3 μM) and prolactin releasing hormone receptor (PRLHR, IC50 = 4.89 μM) upon dose-dependent biochemical validation. FSHR regulates signaling of the follicle-stimulating hormone (FSH), which is essential to maturation and growth of the reproductive system.32 FSHR has been shown to be expressed on most ovarian cancer subtypes and could serve as a promising immunotherapeutic target for chemotherapy.33 NPSR1B, which is primarily found in the brain, is known to modulate anti-anxiety and arousal activities by enhancing dopamine production.34 Selective antagonism of NPSR1B is being probed for its potential to treat cocaine abuse with promising preliminary results.35 PRLHR is the receptor for prolactin releasing peptide (PrRP) and this ligand has been associated with digestive regulation and energy balance. Additionally, PRLHR is involved in the development of uterine fibroids (leiomyomas)36 and has been reported to negatively modulate the effects of opioid stimulation.37
Y1f demonstrated antagonistic activities against five GPCRs (≥80% inhibition), including: the closely related chemokine receptors CCR3 (IC50 = 8.30 μM) and CCR8 (IC50 = 7.29 μM), CX3C (CX3CR1, IC50 = 8.47 μM), CX-C type 4 (CXCR4, IC50 = 7.94 μM) and 5-hydroxytryptamine (serotonin) receptor (HTR2C, IC50 = 180 nM) (Figure 2B, C). Several of these GPCRs are involved in cancer and new antagonists could play a critical role to those suffering from this disease. CCR8 is strongly expressed in malignant melanoma, and antagonism of this target inhibits tumor cell migration.38 CXCR4 is a chemokine receptor that plays a critical role as a co-receptor with CCR5 to enable HIV-1 to enter into CD4+ cells and is involved in leukocyte migration through signaling of its ligand SDF-1.39 It is also known that tumor cell expression of CXCR4 promotes migration and homing to sites where non-malignant cells express SDF-1 (or CXCL12) and drives the metastasis of several cancer types (e.g., leukemia, other blood cancers, breast cancer).40 In addition, HTR2C is a 5-hydroxytryptamine (serotonin) receptor and is involved in a wide range of functions in the central nervous system.41,42 Antagonism of this specific serotonin receptor subtype has been implicated in managing migraine pain relief as well as inducing anxiolytic effects.43–45
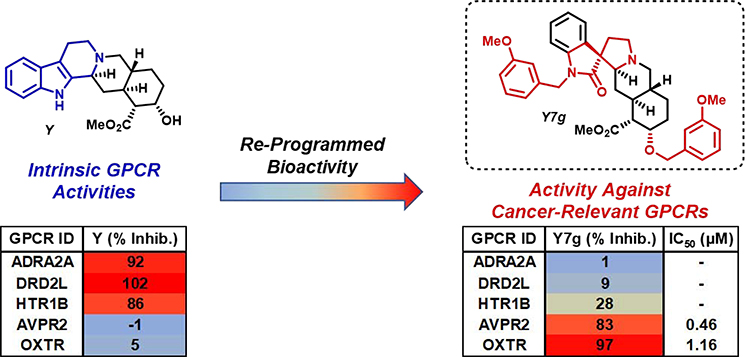
Perhaps the most significant finding from these GPCR-focused investigations so far was the activity profile of Y7g, an analogue that was previously found to exhibit hypoxia-inducible factor (HIF)-dependent anti-cancer and anti-inflammatory activities.25 From the GPCR screen, Y7g inhibited arginine vasopressin receptors (AVPR1A, AVPR1B, AVPR2) and the oxytocin receptor (OXTR), which share a high level of sequence homology (Figure 2B, C).46 AVPRs and OXTR have shown involvement in tumor progression in cancers, including prostate47 and small-cell lung cancer (SCLC).48 The expression of OXTR in prostate cancer cell lines contributes to metastasis.47 Interestingly, vasopressin receptor expression is a selective feature of breast cancers and is also associated with small-cell lung cancer.49–52 Y7g inhibited AVPR2 (83% inhibition at 20 μM) during the initial screen and its antagonistic properties were found to be dose-dependent, reporting an IC50 = 459 nM (compared to AVPR2 antagonist tolvaptan, IC50 = 7.34 pM; see Supporting Information). In addition, Y7g demonstrated antagonistic activities against OXTR in the initial GPCR screen (97% inhibition at 20 μM). Subsequent dose-response studies revealed an IC50 value of 1.16 μM against this receptor (compared to OXTR antagonist L-368,899, IC50 = 4.62 nM; see Supporting Information).
Since AVPR2 is known to be induced by hypoxic cellular environments53,54 and is expressed in a variety of cancers, the anti-cancer properties we previously reported25 for Y7g could be due to its ability to inhibit AVPR2/OXTR. These findings demonstrate a complete re-programing of the innate biological activity of yohimbine through our ring distortion efforts. Yohimbine displayed ≤5% inhibition against AVPR2 and OXTR, while ring distortion compound Y7g gained significant antagonistic activities against these cancer-relevant targets (Figure 3). In addition to the “gain of function” activities demonstrated by Y7g, we also observed a “loss of function” against yohimbine’s known targets ADRA2A, DRD2L and HTR1B as these targets were not inhibited by Y7g.
Figure 3.
Highlighted antagonistic activities for Y and Y7g demonstrate re-programing of activity profiles against select GPCR targets. (Note: color should be used)
The GPCR profiles of active yohimbine-derived scaffolds Y6p, Y1f and Y7g indeed support our initial hypothesis that yohimbine’s innate biological activity could be re-programmed through ring distortion and lead to the identification of new molecules that modulate different proteins of significance to human health. Not only did these analogues modulate GPCRs other than the target proteins yohimbine binds to, but they did so in a selective manner. From the 168 GPCRs tested, Y6p, Y1f and Y7g demonstrated hit rates of 2.4%, 3.0% and 2.4% (compared to yohimbine’s hit rate of 4.2% in this screen, Figure 2B). Collectively, these findings serve as excellent proof-of-concept for the re-programing of yohimbine’s biological activity.
Conclusions
The screening of ring-distorted yohimbine-derived compounds led to the identification of diverse small molecules that demonstrate re-programmed antagonistic activities against cancer-relevant GPCR targets. Analogues Y6p, Y1f and Y7g inhibited GPCRs that mediate cellular proliferation in breast, melanoma and prostate cancers. Additionally, a HIF-dependent anti-cancer agent we previously reported, Y7g, was found to antagonize arginine vasopressin receptors (AVPR1B, AVPR1A, AVPR2) and oxytocin receptor (OXTR), both of which are upregulated in multiple cancer types. Furthermore, hypoxic cellular environments are known to upregulate AVPR expression may be linked to our previous findings regarding Y7g’s antiproliferative activities against cancer cells.25 We believe the results from these investigations could play an important role in the identification of new small molecules that may significantly impact future cancer treatments.
Supplementary Material
Acknowledgements
Robert W. Huigens, PhD was supported by a Research Scholar Grant, RSG-18-013-01 – CDD, from the American Cancer Society. Research was supported, in part, by the National Institutes of Health, NCI grant R01CA172310 (H.L.), NCI Research Specialist Award R50CA211487 (R.R.) and the Debbie and Sylvia DeSantis Chair professorship (H.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).Katritch V, Cherezov V, Stevens RC Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Yu S, Sun L, Jiao Y, Tsz On Lee L The role of G protein-coupled receptor kinases in cancer. Int. J. Biol. Sci. 2018, 14, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).New DC, Wong YH Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J. Mol. Signal 2007, 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Dorsam RT, Gutkind JS G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79. [DOI] [PubMed] [Google Scholar]
- 5).Dubuc C, Savard M, Bovenzi V, Lessard A, Fortier A, Côté J, Neugebauer W, Rizzolio F, Geha S, Giordano A, Chemtob S, Gobeil F Targeting intracellular B2 receptors using novel cell-penetrating antagonists to arrest growth and induce apoptosis in human triple-negative breast cancer. Oncotarget 2018, 9, 9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Al-Awadhi FH, Gao B, Rezaei MA, Kwan JC, Li C, Ye T, Paul VJ, Luesch H Discovery, synthesis, pharmacological profiling, and biological characterization of Brintonamides A-E, novel dual protease and GPCR modulators from a marine cyanobacterium. J. Med. Chem. 2018, 61, 6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Santos R, Ursu O O, Gaulton A, Bento AP, Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI, Overington JP A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Heifetz A, Schertler GFX, Seifert R, Tate CG, Sexton PM, Gurevich VV, Fourmy D, Cherezov V, Marshall FH, Storer RI, Moraes I, Tikhonova IG, Tautermann CS, Hunt P, Ceska T, Hodgson S, Bodkin MJ, Singh S, Law RJ, Biggin PC GPCR structure, function, drug discovery and crystallography: report from Academia-Industry International Conference (UK Royal Society) Chicheley Hall, 1–2 September 2014. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Hothersall JD, Brown AJ, Dale I, Rawlins P Can residence time offer a useful strategy to target agonist drugs for sustained GPCR responses? Drug Discov. Today 2016, 21, 90. [DOI] [PubMed] [Google Scholar]
- 10).Newman DJ, Cragg GM Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 25, 629. [DOI] [PubMed] [Google Scholar]
- 11).Böttcher T, Pitscheider M, Sieber SA Natural products and their biological targets: proteomic and metabolomic labeling strategies. Angew. Chem. Int. Ed. Engl. 2010, 49, 2680. [DOI] [PubMed] [Google Scholar]
- 12).Swinney DC, Anthony J How were new medicines discovered? Nat. Rev. Drug Discov. 2011, 10, 507. [DOI] [PubMed] [Google Scholar]
- 13).Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, Green DV, Hertzberg RP, Janzen WP, Paslay JW, Schopfer U, Sittampalam GS Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011, 10, 188. [DOI] [PubMed] [Google Scholar]
- 14).Hughes JP, Rees S, Kalindjian SB, Philpott KL Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29. [DOI] [PubMed] [Google Scholar]
- 16).Grivas PD, Kiaris H, Papavassiliou AG Tackling transcription factors: challenges in antitumor therapy. Trends Mol. Med. 2011, 17, 537. [DOI] [PubMed] [Google Scholar]
- 17).Grivas PD, Papavassiliou AG Transcriptional corepressors in cancer: emerging targets for therapeutic intervention. Cancer 2013, 119, 1120. [DOI] [PubMed] [Google Scholar]
- 18).Schreiber SL Organic chemistry: molecular diversity by design. Nature 2009, 457, 153. [DOI] [PubMed] [Google Scholar]
- 19).Lenci E, Innocenti R, Menchi G, Trabocchi A Diversity-oriented synthesis and chemoinformatic analysis of the molecular diversity of sp3-rich morpholine peptidomimetics. Front. Chem. 2018, 6, 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Van Hattum H, Waldmann H Biology-oriented synthesis: harnessing the power of evolution. J. Am. Chem. Soc. 2014, 136, 11853. [DOI] [PubMed] [Google Scholar]
- 21).Kato N, Comer E, Sakata-Kato T, Sharma A, Sharma M, Maetani M, Bastien J, Brancucci NM, Bittker JA, Corey V, Clarke D, Derbyshire ER, Dornan GL, Duffy S, Echley S, Itoe MA, Koolen KM, Lewis TA, Lui PS, Lukens AK, Lund E, March S, Meibalan E, Meier BC, McPhail JA, Mitasev B, Moss EL, Sayes M, Van Gessel Y, Wawer MJ, Yoshinaga T, Zeeman AM, Avery VM, Bhatia SN, Burke JE, Catteruccia F, Clardy JC, Clemons PA, Dechering KJ, Duvall JR, Foley MA, Gusovsky F, Kocken CH, Marti M, Morningstar ML, Munoz B, Neafsey DE, Sharma A, Winzeler EA, Wirth DF, Scherer CA, Schreiber SL Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 2016, 538, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Karageorgis G, Reckzeh ES, Ceballos J, Schwalfenberg M, Sievers S, Ostermann C, Pahl A, Ziegler S, Waldmann H Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and −3. Nat. Chem. 2018, 10, 1103. [DOI] [PubMed] [Google Scholar]
- 23).Huigens RW III, Morrison KC, Hicklin RW, Flood TA Jr, Richter MF, Hergenrother PJ A ring-distortion strategy to construct stereochemically complex and diverse compounds from natural products. Nat. Chem 2013, 5, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Llabani E, Hicklin RW, Lee HY, Motika SE, Crawford LA, Weerapana E, Hergenrother PJ Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat. Chem. 2019, 11, 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Paciaroni NG, Ratnayake R, Matthews JH, Norwood IV VM, Arnold AC, Dang LH, Luesch H, Huigens RW III. A tryptoline ring-distortion strategy leads to complex and diverse biologically active molecules from the indole alkaloid yohimbine. Chem. Eur. J. 2017, 28, 4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Norwood VM, Brice-Tutt A, Eans SO, Stacy H, Shi G, Ratnayake R, Rocca JR, Abboud KA, Li C, Luesch H, McLaughlin JP, Huigens RW III. Preventing morphine seeking behavior through the re-engineering of vincamine’s biological activity. J. Med. Chem, published on web: 1–8-2020 DOI: 10.1021/acs.jmedchem.9b01924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Paciaroni NG, Norwood IV VM, Garcia DE, Huigens RW III. Microwave-enhanced, stereospecific ring-closure of medium-ring cyanamide ethers to yohimbine. Tetrahedron Lett. 2019, 60, 1182. [Google Scholar]
- 28).Paciaroni NG, Perry DL, Norwood VM, Solano CM, Collins J, Tenneti S, Chakrabarti D, Huigens RW III. Re-engineering of yohimbine’s biological activity through ring distortion: Identification and structure-activity relationships of a new class of antiplasmodial agents. ACS Infect. Dis. 2020, 6, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).van der Lee MM, Bras M, van Koppen CJ, Zaman GJ Beta-arrestin recruitment assay for the identification of agonists of the sphingosine 1-phosphate receptor EDG1. J. Biomol. Screen. 2008, 13, 986. [DOI] [PubMed] [Google Scholar]
- 30).Liang X, Luo D, Yan J-L, Rezaei MA, Salvador-Reyes LA, Gunasekera SP, Li C, Ye T, Paul VJ, Luesch H Discovery of Amantamide, a Selective CXCR7 Agonist from Marine Cyanobacteria. Org. Lett. 2019, 21, 1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Cogé F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse 2000, 35, 79. [DOI] [PubMed] [Google Scholar]
- 32).Jiang X, Liu H, Chen X, Chen P-H, Fischer D, Sriraman V, Yu HN, Arkinstall S, He X Structure of follicle-stimulating hormone in complex with the entire ectodomain of its receptor. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Perales-Puchalt A, Svoronos N, Rutkowski MR, Allegrezza MJ, Tesone AJ, Payne KK, Wickramasinghe J, Nguyen JM, O’Brien SW, Gumireddy K, Huang Q, Cadungog MG, Connolly DC, Tchou J, Curiel TJ, Conejo-Garcia JR Follice-stimulating hormone receptor is expressed by most ovarian cancer subtypes and is a safe and effective immunotherapeutic target. Clin. Cancer Res. 2017, 23, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Mochizuki T, Kim J, Sasaki K Microinjection of neuropeptide S into the rat ventral tegmental area induces hyperactivity and increases extracellular levels of dopamine metabolites in the nucleus accumbens shell. Peptides 2010, 31, 926. [DOI] [PubMed] [Google Scholar]
- 35).Bonano JS, Runyon SP, Hassler C, Glennon RA, Stevens Negus S Effects of the neuropeptide S receptor antagonist RTI-118 on abuse-related facilitation of intracranial self-stimulation produced by cocaine and methylenedioxypyrovalerone (MDPV) in rats. Eur. J. Pharmacol. 2014, 743, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Varghese BV, Koohestani F, McWilliams M, Colvin A, Gunewardena S, Kinsey WH, Nowak RA, Nothnick WB, Chennathukuzhi VM Loss of the repressor REST in uterine fibroids promotes aberrant G protein-coupled receptor 10 expression and activates mammalian target of rapamycin pathway. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Laurent P, Becker JA, Valverde O, Ledent C, de Kerchove d’Exaerde A, Schiffmann SN, Maldonado R, Vassart G, Parmentier M The prolactin-releasing peptide antagonizes the opioid system through its receptor GPR10. Nat. Neurosci. 2005, 8, 1735. [DOI] [PubMed] [Google Scholar]
- 38).Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D, Condeelis J, Skobe M Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J. Exp. Med. 2013, 210, 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Burger JA, Peled A CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia 2009, 23, 43. [DOI] [PubMed] [Google Scholar]
- 41).Iwamoto K, Bundo M, Kato T Serotonin receptor 2C and mental disorders: genetic, expression and RNA editing studies. RNA Biol. 2009, 6, 248. [DOI] [PubMed] [Google Scholar]
- 42).Sokolov AY, Lyubashina OA, Panteleev SS The role of serotonin receptors in migraine headaches. Neurochem. J 2011, 5, 92. [Google Scholar]
- 43).Yücel Y, Coşkun S, Cengiz B, Özdemir HH, Uzar E, Çim A, Camkurt MA, Aluclu MU Association of Polymorphisms within the Serotonin Receptor Genes 5-HTR1A, 5-HTR1B, 5-HTR2A and 5-HTR2C and Migraine Susceptibility in a Turkish Population. Clin. Psychopharmacol. Neurosci 2016, 14, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Dremencov E, Weizmann Y, Kinor N, Gispan-Herman I, Yadid G Modulation of dopamine transmission by 5HT2C and 5HT3 receptors: a role in the antidepressant response. Curr. Drug. Targets 2006, 7, 165. [DOI] [PubMed] [Google Scholar]
- 45).Obata H, Saito S, Sakurazawa S, Sasaki M, Usui T, Goto F Antiallodynic effects of intrathecally administered 5-HT(2C) receptor agonists in rats with nerve injury. Pain 2004, 108, 163. [DOI] [PubMed] [Google Scholar]
- 46).Chini B, Manning M Agonist selectivity in the oxytocin/vasopressin receptor family: new insights and challenges. Biochem. Soc. Trans. 2007, 35, 737. [DOI] [PubMed] [Google Scholar]
- 47).Zhong M, Boseman ML, Millena AC, Khan SA Oxytocin induces the migration of prostate cancer cells: involvement of the Gi-coupled signaling pathway. Mol. Cancer Res. 2010, 8, 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Péqueux C, Keegan BP, Hagelstein MT, Geenen V, Legros JJ, North WG Oxytocin- and vasopressin-induced growth of human small-cell lung cancer is mediated by the mitogen-activated protein kinase pathway. Endocr. Relat. Cancer 2004, 11, 871. [DOI] [PubMed] [Google Scholar]
- 49).North WG, Pai S, Friedmann A, Yu X, Fay M, Memoli V Vasopressin gene related products are markers of human breast cancer. Breast Cancer Res. Treat. 1995, 34, 229. [DOI] [PubMed] [Google Scholar]
- 50).North WG, Cole B, Akerman B, Pang RH Growth impairment of small-cell cancer by targeting pro-vasopressin with MAG-1 antibody. Front. Oncol. 2014, 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).North WG Gene regulation of vasopressin and vasopressin receptors in cancer. Exp. Physiol. 2000, 85S, 27S. [DOI] [PubMed] [Google Scholar]
- 52).Garona J, Pifano M, Orlando UD, Pastrian MB, Iannucci NB, Ortega HH, Podesta EJ, Gomez DE, Ripoll GV, Alonso DF The novel desmopressin analogue [V4Q5]dDAVP inhibits angiogenesis, tumour growth and metastases in vasopressin type 2 receptor-expressing breast cancer models. Int. J. Oncol. 2015, 46, 2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Takamata A, Nose H, Kinoshita T, Hirose M, Itoh T, Morimoto T Effect of acute hypoxia on vasopressin release and intravascular fluid during dynamic exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol 2000, 279, R161. [DOI] [PubMed] [Google Scholar]
- 54).Kelestimur H, Leach RM, Ward RM, Forsling ML Vasopressin and oxytocin release during prolonged environmental hypoxia in the rat. Thorax 1997, 52, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.