Highlights
-
•
Posterior reversible encephalopathy syndrome may be associated with coronavirus disease.
-
•
Risk factors may include modest blood pressure fluctuations and anakinra.
-
•
All reported patients had clinical improvement.
Key Words: PRES, COVID-19, Immunosuppression
Abstract
Introduction
Encephalopathy is a common complication of coronavirus disease 2019. Although the encephalopathy is idiopathic in many cases, there are several published reports of patients with posterior reversible encephalopathy syndrome in the setting of coronavirus disease 2019.
Objective
To describe the diverse presentations, risk factors, and outcomes of posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019.
Methods
We assessed patients with coronavirus disease 2019 and a diagnosis of posterior reversible encephalopathy syndrome at our institution from April 1 to June 24, 2020. We performed a literature search to capture all known published cases of posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019.
Results
There were 2 cases of posterior reversible encephalopathy syndrome in the setting of coronavirus 2019 at our institution during a 3-month period. One patient was treated with anakinra, an interleukin-1 inhibitor that may disrupt endothelial function. The second patient had an underlying human immunodeficiency virus infection. We found 13 total cases in our literature search, which reported modest blood pressure fluctuations and a range of risk factors for posterior reversible encephalopathy syndrome. One patient was treated with tocilizumab, an interleukin-6 inhibitor that may have effects on endothelial function. All patients had an improvement in their neurological symptoms. Interval imaging, when available, showed radiographic improvement of brain lesions.
Conclusions
Risk factors for posterior reversible encephalopathy syndrome in patients with coronavirus disease 2019 may include underlying infection or immunomodulatory agents with endothelial effects in conjunction with modest blood pressure fluctuations. We found that the neurological prognosis for posterior reversible encephalopathy syndrome in the setting of coronavirus disease 2019 infection is favorable. Recognition of posterior reversible encephalopathy syndrome in this patient population is critical for prognostication and initiation of treatment, which may include cessation of potential offending agents and tight blood pressure control.
Background
Central nervous system (CNS) manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection include encephalopathy, encephalitis, meningitis, acute disseminated encephalomyelitis, and stroke,1 both as a result of direct viral invasion of the central nervous system2, 3, 4, 5 and as a consequence of critical illness and systemic infection.6 Coronavirus disease 2019 (COVID-19) may also cause significant changes in endothelial morphology, including disruption of intercellular junctions, cell swelling, and a loss of contact with the basal membrane.7 However, posterior reversible encephalopathy syndrome (PRES), which may result from such endothelial dysfunction, has rarely been reported in patients with COVID-19.8, 9, 10, 11 The diverse etiologies and risk factors of PRES in this unique population warrant close examination, as they may elucidate strategies to prevent or treat the syndrome in select patients with COVID-19.
Methods
This study protocol was approved by the Boston Medical Center's Institutional Review Board. Two cases were identified through surveillance of patients admitted to the neurocritical care service between April 1, 2020 and June 24, 2020 with positive nasopharyngeal swab testing for SARS-CoV-2 and a clinical diagnosis of PRES.
A literature review for published cases of PRES in patients with COVID-19 was conducted on July 20, 2020 by searching PubMed and Medline using the terms “posterior reversible encephalopathy syndrome AND COVID” and “PRES AND COVID”.
Results
Case 1
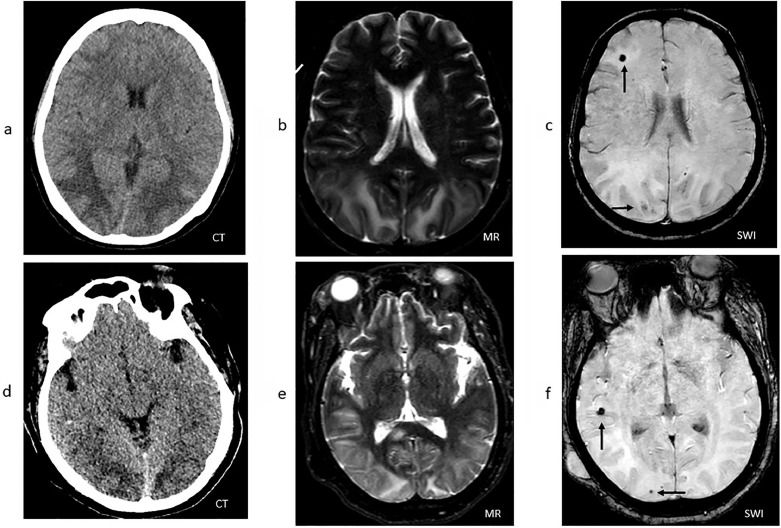
A previously healthy 61-year-old woman presented with one week of dyspnea, headaches, fever, and cough. She was diagnosed with COVID-19 based on a positive nasopharyngeal PCR for SARS-CoV-2 and was treated with remdesivir and anakinra. Her hospital course was complicated by worsening respiratory failure requiring intubation on hospital day 5, subsequent proning for respiratory distress syndrome, ventilator dyssynchrony requiring significant sedation and periodic paralysis, and septic shock with hypotension requiring vasopressors. On hospital day 15, a head CT was obtained because of persistently poor mental status in spite of weaning sedation and revealed significant posterior cortical paramedian hypodensity consistent with cerebral edema (Fig. 1 A). Blood pressure range was 152–187/79–98 mmHg, with no metabolic derangements. CT venogram showed no evidence of venous sinus thrombosis. In order to conserve personal protective equipment and limit healthcare worker exposure and risk, a lumbar puncture was not performed.
Fig. 1.
Patient 1: (a) Head CT demonstrating hypodensities involving the subcortical white matter of the bilateral parietal and occipital lobes. (b) Hyperintensities in the same anatomic distribution as the CT on T2-weighted MRI sequences. (c) Multiple foci of susceptibility artifact in the right parietal and frontal lobes (arrows) on susceptibility-weighted MRI sequences. Patient 2: (d) CT hypodensities involving the cortical and subcortical white matter of the bilateral occipital and temporal lobes. (e) Hyperintensities in the same anatomic distribution as the CT on T2-weighted MRI sequences. (f) Multiple foci of susceptibility artifact in the right.
A subsequent brain MRI on hospital day 18 revealed symmetric white matter T2 hyperintense signal abnormalities involving the parietal and occipital lobes without diffusion restriction consistent with PRES, as well as a focus of susceptibility artifact in the right frontal lobe (Fig. 1B-C). On hospital day 18, she had a clinical seizure characterized by rightward gaze deviation and right arm and leg shaking. She was treated with levetiracetam and valproic acid. Subsequent 24 h continuous video electroencephalography (EEG) revealed rare bursts of repetitive sharp waves and spikes noted over the right hemisphere with a broad field, more prominent over the right frontal-parietal region with no clear ictal evolution. She was extubated on hospital day 21, but was re-intubated on hospital day 24 because of worsening hypoxemia, ultimately undergoing a tracheostomy. Her mental status improved over the subsequent weeks. At time of discharge on hospital day 48, she was alert, oriented, and following commands consistently.
Case 2
A 52-year-old woman with a history of HIV infection (CD4+ lymphocyte count 699/µL, viral load undetected) presented with fever and respiratory distress requiring intubation in the emergency department. Admission labs were remarkable for hyperglycemia to 450 mg/dL with an anion gap of 23 mEq/L, and her course was complicated by oliguric renal failure with a maximum creatinine of 4.33 mg/dL requiring continuous hemodialysis. She was diagnosed with COVID-19 based on a positive nasopharyngeal PCR for SARS-CoV-2 but did not receive specific therapies for COVID-19 because of her renal failure. Her respiratory status continued to worsen, and she underwent proning for respiratory distress syndrome. She subsequently developed ventilator dyssynchrony requiring significant sedation and periodic paralysis. Tracheostomy was placed on hospital day 23 because of an inability to extubate, and her course was further complicated by septic shock with hypotension requiring vasopressors.
On hospital day 34, she had a 2 min clinical seizure characterized by leftward gaze deviation and generalized tonic-clonic movements. Blood pressure range was 140–180/70–97 mmHg. Her head CT revealed bilateral occipital hypodensities (Fig. 1D). A 24 h continuous video EEG revealed a reactive and continuous background without epileptiform discharges. Brain MRI on hospital day 35 revealed diffuse T2 hyperintensities involving the white matter of the bilateral parietal, occipital, frontal, and temporal lobes, with partial sulcal effacement. Similar to case 1, there were punctate microhemorrhages in the temporal and occipital lobes. (Fig. 1E-F). Cerebrospinal fluid (CSF) analysis revealed an elevated protein of 95 mg/dL, glucose 78 mg/dL, elevated total nucleated cell count of 28/µL (89% polymorphonuclear), red blood cell count of 223/µL, and negative infectious studies including a meningitis and encephalitis PCR panel and a cryptococcal antigen. Her mental status gradually improved over the next several days. On hospital day 43, 9 days after onset of symptoms attributable to PRES, she was alert and oriented and began to follow commands consistently. Follow-up head CT demonstrated significant improvement in the hypodensities of the bilateral occipital, parietal, temporal and frontal lobes.
Literature search
We found 13 cases of PRES in patients with COVID-19 in the literature.8 , 9 , 11, 12, 13, 14 There was an additional case of PRES identified via virtuopsy (post-mortem imaging) but this was excluded from our results due to limited clinical details.10 The included patients all had positive nasopharyngeal PCR for SARS-CoV-2. Relevant clinical details for these cases are summarized in Table 1 along with the 2 patients in our series for comparison.
Table 1.
Summary of 13 cases of posterior reversible encephalopathy syndrome in patients with COVID-19. Patients 1 and 2 are described in this case series while patients 3 through 13 are cases reported in the literature. CT: computed tomography; CTA: computer tomography angiography; FLAIR: fluid attenuated inversion recovery; HIV: human immunodeficiency virus; ICU: intensive care unit; MRI: magnetic resonance imaging; PRES: posterior reversible encephalopathy syndrome; SWI: susceptibility weighted imaging.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 | Patient 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 61 | 52 | 48 | 67 | 38 | 58 | 67 | 64 | 64 | 73 | 65 | 74 | 74 |
| Gender | Female | Female | Male | Female | Male | Male | Female | Female | Male | Male | Female | Female | Male |
| Comorbidities | None | HIV | Obesity | Hypertension, diabetes, coronary artery disease, gout, asthma | None | Hyperlipidemia | Hypertension, diabetes, obesity | Hypertension, gastroesophageal reflux disease, hyperuricemia, dyslipidemia, obstructive sleep apnea, paroxysmal atrial fibrillation | Unknown, none reported | Unknown, none reported | Hypertension, diabetes | Hypertension, diabetes, hyperlipidemia | IgG kappa multiple myeloma |
| Blood pressure (mm Hg) | 152-187/79-98 | 140-180/70-97 | 70-180/30-90 | 115-178/72-83 | “high levels for few hours” | 86–189/52–122 | 79-193/44-97 | 150/70 on admission, no others reported | SBP 187, MAP 128 (maximum values) | SBP 212, MAP 135 (maximum values) | SBP 180, MAP 138 (maximum values) | SBP 237, MAP 150 (maximum values) | SBP 140-150 at the time of diagnosis |
| Presentation of COVID-19 | Fever, dyspnea, cough, malaise | Fever, dyspnea, chest pain | Fever, dyspnea, cough | Altered mental status (no respiratory symptoms) | Fever, dyspnea | Fever, dry cough, malaise | Fever, dyspnea, myalgia, vomiting, diarrhea | Fever, dyspnea | Unknown, not described | Dyspnea, cough | Dyspnea, cough | Fever, cough | Fever, dry cough |
| Mechanical Ventilator Status | (+) | (+) | (+) | (-) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Unknown, not reported |
| Chest Radiologic Findings | CT chest with peripheral diffuse ground glass opacities. No pleural effusions. | Chest X-ray with bilateral diffuse airspace opacities | Unknown, not reported | CT chest with bilateral, multifocal ground-glass opacities | CT chest with bilateral multiple, multilobar, peripheral ground glass opacifications | Unknown, not reported | Unknown, not reported | Chest X-ray with reduction of parenchymal transparency in the basal region of right lung | Unknown, not reported | Unknown, not reported | Unknown, not reported | Unknown, not reported | Unknown, not reported |
| Treatment for COVID-19 | Anakinra 200mg IV q12h x 3 days Remdesivir 200mg IV followed by 100mg IV x 4 days | None administereds | Unknown, none reported | Unknown, none reported | Hydroxychloroquine 400mg followed by 200mg/d x 4 days Azithromicin 500mg/d Osetalmivir 150mg/d | Tocilizumab 400mg IV Hydroxychloroquine (dose not reported) Azithromicin (dose not reported) | Hydroxychloroquine (dose not reported) Azithromicin (dose not reported) | Unknown, none reported | Hydroxchloroquine (dose not reported) | Hydroxychloroquine (dose not reported) | Hydroxychloroquine (dose not reported) | Hydroxychloroquine (dose not reported), tocilizumab | Hydroxychloroquine 200 mg q12, lopinavir / ritonavir 400/100 mg q12, dexamethasone 20 mg q12 |
| Symptoms attributable to PRES | Altered mental status | Altered mental status and clinical seizure | Altered mental status | Altered mental status | Bilateral vision loss, apathic, impaired ability to follow commands | Altered mental status | Altered mental status | Altered mental status, blurred vision | Altered mental status, global aphasia | Left gaze preference, subclinical seizures | Stuporous | Persistent confusion with intermittent agitation | Clonic movement of the left limb, focal motor seizures |
| MRI findings | T2 hyperintensities in parietal and occipital lobes; SWI with focus of susceptibility artifact in right frontal lobe | T2 hyperintensities involving right side of splenium of corpus callosum | T2/FLAIR hyperintensities in posterior regions; SWI with extensive petechial hemorrhages throughout corpus callosum | Restricted diffusion with associated edema most extensive in posterior regions; SWI with extensive superimposed hemorrhages in parieto-occipital region | T2/FLAIR hyperintensities and diffusion restriction on DWI in bilateral, especially left occipital, frontal cortical white matter and splenium of corpus callosum | T2/FLAIR hyperintensities in subcortical white matter of bilateral occipital and posterior temporal lobes; SWI with subarachnoid hemorrhage | T2/FLAIR hyperintensities in subcortical white matter of right occipital lobe and left cerebellar hemisphere; SWI with petechial hemorrhage | T2/FLAIR vasogenic edema; GRE with right temporal lobe hypointensity | T2/FLAIR hyperintensities include the white matter of the bilateral occipital lobes, thalamus, and internal capsule | T2/FLAIR bilateral subcortical occipital lobe hyperintensity compatible with vasogenic edema | T2/FLAIR mild subcortical bilateral occipital lobe edema | T2/FLAIR hyperintensities in the white matter of the occipital lobes susceptibility weighted images with signal loss in the right occipital lobe | T2/FLAIR hyperintensities in the parietal lobe and right frontal lobe white matter |
| Cerebrovascular Imaging | CTA/CTV, normal vasculature | None | None | None | None | None | None | CTA without vascular malformation, posterior vessel caliber suggestive of vasoconstriction | None | None | None | None | None |
| Risk factors for PRES | Sepsis | HIV Renal failure | None mentioned | Hypertension | None mentioned | Hypertension Sepsis | Acute kidney injury requiring hemodialysis Sepsis | None mentioned | None mentioned | None mentioned | None mentioned | None mentionted | Multiple myeloma |
| Onset of PRES from hospitalization | Less than 15 days | 34 days | 18 days | 0 days | 5 days | 26 days | 25 days | 25 days | Not reported | 6 weeks | Not reported | Not reported | 15 days |
| SOFA | 6 | 7 | Insufficient data | Insufficient data | Insufficient data | Insufficient data | Insufficient data | Insufficient data | Insufficient data | Insufficient data | Insufficient data | Insufficient data | Insufficient data |
| Outcome | Mental status gradually improved, discharged 33 days after onset of symptoms attributable to PRES | Mental status gradually improved, became interactive and started following commands 9 days after onset of symptoms attributable to PRES | Mental status gradually improved, transferred from ICU to medical floor six days from onset of symptoms attributable to PRES | Mental status gradually improved and discharged | Visual impairment resolved two days after onset following corticosteroid administration, neurocognitive assessment on day 10 of hospitalization was normal, brain MRI two weeks after symptom onset showed complete resolution of prior lesions |
Mentation slowly improved to baseline, discharged 7 days after onset of symptoms attributable to PRES | Mentation slowly improved to nearly baseline, discharged 22 days after onset of symptoms attributable to PRES | Alert, fully oriented; blurry vision normalized | Alert, oriented, inattentive, right homonymous hemianopsia | Alert, following commands, no focal neurological deficits | Mild cognitive deficit, no focal neurological deficit | Alert and oriented to person and time (not to place), consistently following commands | Not reported |
| Reference | n/a | n/a | Franceschi et al (2020)10 | Franceschi et al (2020)10 | Kaya et al (2020)7 | Kishfy et al (2020)8 | Kishfy et al (2020)8 | Cariddi et al (2020)12 | Parauda et al, (2020)13 | Parauda et al, (2020)13 | Parauda et al (2020)13 | Parauda et al (2020)13 | Gomez-Enjuto et al (2020)14 |
Discussion
We cared for 2 patients with COVID-19 and PRES at our institution and identified additional cases through a literature search, finding variable risk factors for developing the syndrome. Although the exact mechanisms underlying PRES are unclear, contributing factors are thought to include hypertension with subsequent cerebral hyperperfusion and endothelial dysfunction.15 SARS-CoV-2 has been found to bind directly to angiotensin-converting enzyme 2 receptors, which can dysregulate the endothelial layer, increase blood pressure, and disrupt cerebral blood flow autoregulation.16 The mechanism by which COVID-19 may be associated with PRES is unclear, although it is notable that neuropathological studies of patients with COVID-19 and central nervous system dysfunction have identified only hypoxic changes without specific brain changes attributable to the virus.17
By contrast to the majority of published cases of PRES,8 , 9 the patients described here had relatively modest blood pressure fluctuations before developing the syndrome. Similar to guidance offered by other authors,8 we advocate for tight blood pressure control in patients with COVID-19 and risk factors for PRES, including renal failure, uncontrolled hypertension, or HIV infection. Two patients who developed PRES in the setting of COVID-19 were treated experimentally for the infection with interleukin inhibitors, one with the interleukin-6 inhibitor tocilizumab and one with the interleukin-1 inhibitor anakinra, which have known effects on endothelial function.18, 19, 20 In particular, the interleukin-6 inhibitor tocilizumab has been described in association with PRES.21 Our findings suggest that caution may be warranted when considering interleukin inhibitors to treat COVID-19 in patients with underlying PRES risk factors, that anakinra may also be associated with an increased risk of PRES in patients with COVID-19, and that these agents should be discontinued if PRES is diagnosed.
One of the patients presented here also had well-controlled HIV. Multiple reports in the literature describe PRES in HIV-infected patients, most often in those with advanced immunodeficiency, though it has been rarely described in patients with well-controlled HIV.22, 23, 24, 25 Possible mechanisms include impaired cerebrovascular reactivity even in virally suppressed patients and long-term exposure to antiretroviral therapies that may lead to mitochondrial damage in endothelial cells.25 The HIV-infected patient presented here had a CSF profile with elevated protein and neutrophilic pleocytosis. The latter finding is rare in PRES and may be associated with a higher risk of ischemic or hemorrhagic events, consistent in this case with the small area of restricted diffusion on imaging.26
The majority of patients had severe respiratory manifestations of COVID-19 requiring intensive care, with just one patient (Patient 4)11 whose primary manifestation of COVID-19 was altered mental status secondary to PRES. Although chest CT findings were concerning for viral pneumonia, she had subclinical respiratory involvement, suggesting that development of PRES is possible without obvious pulmonary manifestations, and that COVID-19 should be considered in patients presenting with PRES without other obvious risk factors.
Five of 7 patients had foci of susceptibility artifact on imaging consistent with various degrees of hemorrhage. In general, PRES is associated with hemorrhage at an estimated rate of 10-30% in patients. Risk factors for developing hemorrhage include bone marrow or solid-organ transplantation and coagulopathy.27 , 28 Reversible cerebral vasoconstriction syndrome, which shares findings of blood-brain barrier breakdown and cerebral edema with PRES, has also been described in association with both subarachnoid and parenchymal hemorrhage.29 , 30 Patients 1 and 8 underwent computed tomography angiography (CTA) of the head and neck to evaluate for underlying vascular abnormalities with no alternative etiologies of hemorrhage found. Patients 2, 3, 4, 6, 7, 8, and 12 all had evidence of hemorrhage on MRI susceptibility sequences but no additional work-up was performed to evaluate for alternative etiologies of hemorrhage. Our observations suggest that hemorrhage may be more likely seen with PRES in patients with COVID-19, but additional study is required to determine the clinical significance of this finding.
Although hemorrhage and cytotoxic edema are risk factors for poor outcome in PRES,31 all seven patients described here had marked clinical recovery. In general, 70–90% of patients with PRES have clinical recovery and resolution of edema on neuroimaging.32 It is unclear if patients with COVID-19 may have a higher likelihood of a favorable outcome following PRES due to its potentially distinct pathophysiology.
Conclusions
Similar to other reports on neurologic complications of COVID-19, ours is limited by a lack of long-term follow-up. It is important, however, to describe cases early to add to the scarce literature on central nervous system injury potentially related to COVID-19. Here, we describe the diverse presentations, risk factors, and outcomes of PRES in patients with COVID-19. Based on these cases, we advocate for tight blood control for patients with COVID-19 and risk factor for PRES, and advise caution regarding the use of interleukin inhibitors in these patients. We also offer hope that COVID-associated PRES may have high likelihood of clinical recovery.
Declaration of Competing Interest
None
Acknowledgements
Dr. Chung is supported by the National Institutes of Health (K08NS112601). Dr. Anand is supported by a Grinspoon Foundation Grant.
References
- 1.Koralnik IJ, Tyler KL. COVID ‐19: a global threat to the nervous system. Ann Neurol. 2020 doi: 10.1002/ana.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duong L, Xu P LA. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in downtown Los Angeles, early April 2020. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye M, Ren Y LT. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L, Wang M, Chen S, He Q, Chang J, Hong C. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. MedRxiv. 2020 doi: 10.1101/2020.02.22.20026500. [DOI] [Google Scholar]
- 7.Huertas A, Montani D, Savale L, Pichon J, Tu L, Parent F. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaya Y, Kara S, Akinci C, Kocaman AS. Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: case report. J Neurol Sci. 2020 doi: 10.1016/j.jns.2020.116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishfya L, Casasola M, Banankhaha P, Parveza A, Jana YJ, Shenoy AM. Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe Covid-19. J Neurol Sci. 2020;41 doi: 10.1016/j.jns.2020.116943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coolen T, Lolli V, Sadeghi N, Rovaï A, Trotta N, Taccone FS. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020 doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- 11.Franceschi AM, Ahmed O, Giliberto L, Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. Am J Neuroradiol. 2020 doi: 10.3174/ajnr.a6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Princiotta Cariddi L, Tabaee Damavandi P, Carimati F, Banfi P, Clemenzi A, Marelli M. Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J Neurol. 2020 doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parauda SC, Gao V, Gewirtz AN. Posterior reversible encephalopathy syndrome in patients with COVID-19 [published online ahead of print, 2020 Jul 9] J Neurol Sci. 2020;416 doi: 10.1016/j.jns.2020.117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gómez-Enjuto S, Hernando-Requejo V, Lapeña-Motilva J, Ogando-Durán G, Fouz-Ruiz D, Domingo-García J. Verapamil as treatment for refractory status epilepticus secondary to PRES syndrome on a SARS-Cov-2 infected patient. Seizure. 2020 doi: 10.1016/j.seizure.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS. Neuropathological Features of Covid-19. N Engl J Med. 2020 doi: 10.1056/nejmc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotani K, Miyamoto M, Ando H. The effect of treatments for rheumatoid arthritis on endothelial dysfunction evaluated by flow-mediated vasodilation in patients with rheumatoid arthritis. Curr Vasc Pharmacol. 2016 doi: 10.2174/1570161114666161013113457. [DOI] [PubMed] [Google Scholar]
- 19.Vallejo S, Palacios E, Romacho T, Villalobos L, Peiró C, Sánchez-Ferrer CF. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2014 doi: 10.1186/s12933-014-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikonomidis I., Tzortzis S., Andreadou I., Dasou P., Katseli C., Katsimbri P., Paraskevaidis I., Parissis J. LJ and A-NM. Inhibition of Interleukin-1 activity by anakinra improves endothelial, coronary and aortic function in patients with CAD and coexistent rheumatoid arthritis by reducing apoptosis and oxidative stress. Eur Heart J. 2012 [Google Scholar]
- 21.Rosa Júnior M., Borges É.I., Fonseca A.P.A., Fiorot J.L., Balarini VV L. Posterior reversible encephalopathy syndrome during treatment with tocilizumab in juvenile idiopathic arthritis. Arq Neuropsiquiatr. 2018;76:720–721. doi: 10.1590/0004-282X20180093. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson J, Taylor C. Posterior reversible encephalopathy syndrome in disseminated histoplasmosis and advanced HIV infection. Int J STD AIDS. 2014 doi: 10.1177/0956462413517670. [DOI] [PubMed] [Google Scholar]
- 23.Sasson SC, Oon A, Chagantri J, Brew BJ, Carr A. Posterior reversible encephalopathy syndrome (PRES) in an HIV-1 infected patient with disseminated varicella zoster virus: a case report. BMC Infect Dis. 2013 doi: 10.1186/1471-2334-13-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerriero S, Ciracì L, Centoducati T, Pignatelli F, Lamargese V, Salvati A. Bilateral visual loss as presenting symptom of posterior reversible encephalopathy syndrome in a patient with HIV/tuberculosis coinfection: a case report. Case Rep Ophthalmol Med. 2012 doi: 10.1155/2012/850176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birner B, Hirzel C, Wagner F, Waldegg G. Posterior reversible encephalopathy syndrome in an HIV-infected patient on antiretroviral treatment: what is the risk factor? BMJ Case Rep. 2018 doi: 10.1136/bcr-2017-221998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis CA, McClelland AC, Mohan S, Kuo E, Kasner SE, Zhang C. Cerebrospinal fluid in posterior reversible encephalopathy syndrome: implications of elevated protein and pleocytosis. Neurohospitalist. 2019 doi: 10.1177/1941874418802061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol. 2012 doi: 10.1007/s00415-011-6152-4. [DOI] [PubMed] [Google Scholar]
- 28.Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: Imaging and clinical features. Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha E, Singhal A. Update on reversible cerebral vasoconstriction syndrome. Curr Treat Options Cardiovasc Med. 2020;22 doi: 10.1007/s11936-020-00819-9. [DOI] [PubMed] [Google Scholar]
- 30.Chung DY, Claassen J, Agarwal S, Schmidt JM, Mayer SA. Assessment of noninvasive regional brain oximetry in posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome. J Intensive Care Med. 2016 doi: 10.1177/0885066615623465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Zhang G, Lerner A, Wang AH, Gao B, Liu J. Risk factors for poor outcome in posterior reversible encephalopathy syndrome: Systematic review and meta-analysis. Quant Imaging Med Surg. 2018 doi: 10.21037/qims.2018.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liman TG, Siebert E, Endres M. Posterior reversible encephalopathy syndrome. Curr Opin Neurol. 2019 doi: 10.1097/WCO.0000000000000640. [DOI] [PubMed] [Google Scholar]