Abstract
Background:
Preterm infants are at high risk of infection and have distinct pathogen recognition responses. Suggested mechanisms include soluble mediators that enhance cellular levels of cAMP.
Objective:
To assess the relationship between blood cAMP concentrations and TLR-mediated cytokine production in infants during the first month of life.
Methods:
Cord and serial peripheral blood samples (days of life 1 to 28) were obtained from a cohort of very preterm (<30 weeks geatstational age) and term human infants. Whole blood concentrations of cAMP and FSL-1 and LPS in vitro stimulated cytokine concentrations were measured by ELISA and multiplex bead assay.
Results:
cAMP concentrations were higher in cord than peripheral blood, higher in cord blood of female preterm infants, and lower at days 1 and 7 in infants exposed to chorioamnionitis, even after adjusting for leukocyte counts. TLR2 and TLR4-mediated TNF-α, IL-1β, IL-6, IL-12p70 and IL-10 production in vitro increased over the first month of life in preterm infants and were positively correlated with leukocyte-adjusted cAMP levels and reduced by exposure to choriamnionitis.
Conclusions:
The ontogeny of blood cAMP concentrations and associations with chorioamnionitis and TLR-mediated production of cytokines, suggest that this secondary messenger helps shape distinct neonatal pathogen responses in early life.
Introduction
Newborn infants, especially those born preterm are particularly susceptible to invasive infections; up to 60% of the most premature infants (<28 weeks gestational age; GA) have at least one sepsis episode during their hospital admission (1). Immune responses in preterm infants are distinct from those of term infants, older children and adults and contribute to the increased risk of invasive infection in this population (2). Importantly, dysregulated infection-related inflammation is associated with adverse long-term outcomes in preterm infants (3, 4).
The mechanisms shaping the ontogeny of early life immune responses, particularly in preterm infants, are incompletely understood. Gestational age-dependent maturation of responses to innate immune pattern recognition receptor (PRR) stimulation may be critical for susceptibility to invasive infections and associated outcomes. Prior studies have demonstrated gestational age-specific functional differences in the innate immune response to PRR stimulation (5). In vitro studies have implied a role for soluble mediators, as age-specific differences in response to stimulation were found to be dependent on the use of autologous or age-matched plasma in these experiments (6, 7).
Neonatal plasma contains multiple immunomodulating factors, including adenosine, that is present in markedly elevated concentrations compared to adults (8–10). Adenosine enhances production of cyclic adenosine mono-phosphate (cAMP), a key component of intracellular signaling pathways. Extracellular stimuli such as adeonosine activate adenylate cyclase, which in turn generates cAMP and pyrophosphate (11, 12). cAMP may modulate innate and inflammatory responses to whole microbes, microbial products or vaccine adjuvants (e.g., certain Toll-like Receptor (TLR) agonists) with implications for sepsis pathophysiology as well as adjuvanted vaccine design (9). Whether induced physiologically by adenosine or pharmacologically by phosphodieseterase inhibitors, cAMP inhibits production of pro-inflammatory cytokines (e.g. TNF, IFNs), but preserves or enhances the production of acute-phase cytokines such as IL-6 and regulatory cytokines such as IL-10 (13–16).
Most mechanistic studies of early life immune ontogeny have focused exclusively on cord blood (17), however, risk of infection persists throughout the neonatal period and in preterm infants is highest between 10 and 14 days of age (18). We therefore evaluated the concentrations of circulating cAMP (cellular and extracellular) in the first month of life and their correlation with TLR2 and TLR4-mediated in vitro cytokine production in a prospective longitudinal study.
Methods
Study subjects
This longitudinal observational cohort study was approved by the institutional ethics committee at King Edward Memorial Hospital, Perth, Australia (1627/EW) and written informed consent was obtained from parents prior to study participation. 129 infants born at less than 30 weeks’ GA and 20 term infants (37–41 weeks’ GA) without major congenital abnormalities or chromosomal aberrations were recruited into this study. Serial blood samples were collected from recruited preterm infants from cord and peripheral blood on days of life (DOL) 1, 7, 14, 21 and 28. For ethical reasons, blood collection from recruited healthy term infants was limited to cord and peripheral blood on Days 1 and 28. Infants were determined to have an episode of confirmed late-onset sepsis (LOS; onset >72h after birth) based on a positive blood culture and C-reactive protein (CRP) concentration >15 mg/dL during sepsis evaluation. Those with negative blood cultures but with CRP >15 were deemed to have clinical LOS. Table 1 describes the basic clinical characteristics of the study cohort.
Table 1.
Demographic characteristics of the study cohort.
| Preterm infants n=129 |
Term infants n=20 |
|
|---|---|---|
| Gestational age (weeks) | 27.1 (25.6–28.7) | 39.8 (38.7–41.1) |
| Birthweight (grams) | 920 (725–1175) | 3530 (3045–3890) |
| Male | 72 (55.8%) | 9 (45.0%) |
| Multiple birth | 27 (20.9%) | 0 |
| Caesarean section | 72 (55.8%) | 3 (15%) |
| Histological chorioamnionitis | ||
| yes | 54 (41.8%) | N/A |
| unknown | 17 (13.1%) | |
| Late-onset sepsis | ||
| Confirmed | 5 (3.8%) | 0 |
| Clinical | 4 (3.1%) | 0 |
| Age at septic episode (days) | 12 (9–17) | N/A |
Data are expressed as median (IQR) or n (%), as appropriate.
Blood sampling and preparation
Infant cord and peripheral blood (800 l) for whole blood stimulation and white blood cell extended differential counts was collected by venipuncture or from an existing umbilical catheter (DOL 1 only) on days 7, 14, 21 and 28 into lithium-heparin tubes (Becton Dickinson, North Ryde, Australia).
An additional 400 l of infant blood was collected, as above, into lithium-heparin tubes containing 2 µl of 2mM dipyridamole (in DMSO; Sigma-Aldrich, Castle Hill, Australia) and 2 µl of 200 µM erythro-9-(2-hydroxy-3-nonyl) adenine hydrochloride (in water; Sigma-Aldrich). Samples were centrifuged at 6,000 x g for 2 minutes and plasma was removed. Blood cell pellets were resuspended in 1 ml 0.1M HCL and incubated at room temperature for 20 minutes. These samples were centrifuged at 1,000 x g for 10 minutes and supernatant (blood lysate) was stored at −80°C until cAMP measurement.
Placental histology
Placental histology was examined as part of routine clinical care from pregnancies delivering <30 weeks’ GA. Sections of the chorioamniotic membranes, umbilical cord, chorionic plate and placenta were analyzed using an adaptation of a widely accepted semi-quantitative scoring system, as previously described (19, 20).
cAMP determination
cAMP in blood lysates was quantified using a cyclic AMP ELISA kit in accordance with the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI; Lower limit of detection, 0.3 pmol/mL).
Whole blood stimulation
Twenty-five l of whole blood was cultured in 75 l RPMI 1640 GlutaMAX, supplemented with 0.01M HEPES, 1mM sodium pyruvate, 5.5 M beta-mercaptoethanol (all from Gibco, Life Technologies, Mulgrave, Australia), 24h at 37°C and 5% CO2 with either supplemented RPMI media, 10ng/mL LPS and 100ng/mL FSL-1 (both from Invivogen, San Diego, CA). Following overnight incubation, 100 l of RPMI 1640 supplemented, as above along with the addition of 5% fetal bovine serum (SAFC Biosciences, Lenexa, KS), was added and supernatants harvested and stored at −80°C.
Multiplex cytokine assay
Cytokine production following stimulation was quantified using a validated in-house multiplex bead-based assay (21). Primary antibodies against IL-1 and IL-12p70 (R&D Systems, Minneapolis, MN) and IL-6, IL-10 and TNF- (BD Biosciences, North Ryde, Australia) were covalently conjugated to carboxylated microspheres (Luminex Corporation, Austin, TX). Supernatants were diluted in phosphate-buffered saline (PBS) with 0.05% (v/v) Tween20 and 2% (v/v) newborn bovine serum (all from Sigma-Aldrich). Microspheres, at a concentration of 3,500 per bead region, and diluted samples were transferred to MV Multiscreen plates (Merck-Millipore, Bayswater, Australia) and incubated at room temperature for 30 min on an orbital shaker (500 rpm), protected from light. Biotinylated secondary antibodies against IL-1β, IL-12p70 (R&D Systems) and IL-6, IL-10 and TNF-α (BD Biosciences) were then added, and the plate was incubated for another 30 min at RT (500 rpm, in the dark). Wells were washed with PBS containing 1% (w/v) BSA, 0.25% (v/v) Tween20 and 0.001% (w/v) NaN3 vacuum filtration before a streptavidin-PE conjugate (BD Biosciences) was added for 15 min with shaking. Wells were washed twice as above, and the fluorescence in each bead region was measured on a BioPlex 200 System (Bio-Rad, Gladesville, Australia). Data were acquired electronically in real time and analyzed using BioPlex Manager 5.0 software. Data in pg/mL were generated from seven-point, four- or five-parameter logistic standard curves. All values below the lowest standard were assigned an arbitrary cut-off value of half the lowest detectable standard for analysis.
White blood cell enumeration by extended differential count
Whole blood samples were surface stained with antibodies targeting common leukocyte markers used in the validated method by Faucher et al. (22). In brief, 25μl of whole blood was stained with a cocktail of the following monoclonal antibodies (mAB): phycoerythrin (PE) conjugated CD36 (CD36-PE, clone CD38, mouse anti-human), allophycocyanin (APC) conjugated CD2 (CD2-APC, clone S5.29, mouse), Alexa Fluor 647 (AF647) conjugated CD294 (AF647-CD294, clone BM16, rat anti-human), APC-H7 conjugated CD16 (APC-H7-CD16, clone 3G8, mouse anti-human), BD Horizon V450 (V450) conjugated CD19 (V450-CD19, clone HIB19, mouse anti-human), AmCyan conjugated CD45 (AmCyan-CD45, clone 2D15, mouse anti-human). Following 15 min incubation, red blood cells were lysed with 1ml BD FACS Lysing Solution for 10 min in the dark at room temperature. Cells were then washed with FACS buffer (PBS, 2% w/v bovine serum albumin, 0.01% w/v sodium azide from Sigma-Aldrich and 2% v/v fetal calf serum from SAFC Biosciences) and resuspended in BD Stabilizing Fixative with a known volume transferred to BD TruCOUNT Tubes, to allow for absolute counts of each cell type, and stored at 4 °C in the dark prior to analysis. Spectral compensation for each antibody was achieved using BD CompBeads. All antibody clones and flow cytometry reagents were obtained from BD Biosciences, unless otherwise stated.
White blood cells were analyzed using a three-laser, eight-color BD FACSCanto II flow cytometer and BD FACSDiva software (BD Biosciences,). Prior to acquisition flow cytometer calibration was performed using SPHERO Ultra Rainbow Calibration beads (Spherotech, Lake Forest, IL), in addition to BD Cytometer Setup and Tracking beads (BD Biosciences) to monitor cytometer performance. Post-acquisition cell population analysis was performed using FlowJo software version 8 (Tree Star Inc., Ashland, OR). Various leukocyte populations were identified using the validated gating strategy described by Faucher and colleagues (22). In brief, the following cell types were identified from the white blood cell (WCC; CD45+) population: neutrophils (SSChigh/CD16+), non-cytotoxic T cells (SSClow/CD16−→CD2&CD294+/SSClow), classical monocytes (SSClow/CD16−→CD2&CD294−→CD19−/CD36+).
Statistical analysis
For longitudinal analysis of cAMP concentrations in blood lysates, values were log-transformed to satisfy distributional requirements, and analyzed by fitting a mixed model with the Geisser-Greenhouse correction as implemented in GraphPad Prism version 8.0 (San Diego, CA). This mixed model uses a compound symmetry covariance matrix, and is fit using Restricted Maximum Likelihood (REML). In the presence of random missing values (as was the case in this study), the results can be interpreted as for repeated measures ANOVA. Correlations of cAMP and cell counts were determined using the Spearman correlation test. We controlled for total cell counts when assessing cytokine responses by dividing the cytokine values by the total leukocyte counts and log-transformed this ratio, as well as the cAMP values, and calculated corresponding Spearman correlation coefficients. Comparison of the means between two groups was done using a Mann-Whitney U-test.
Results
cAMP concentrations after birth
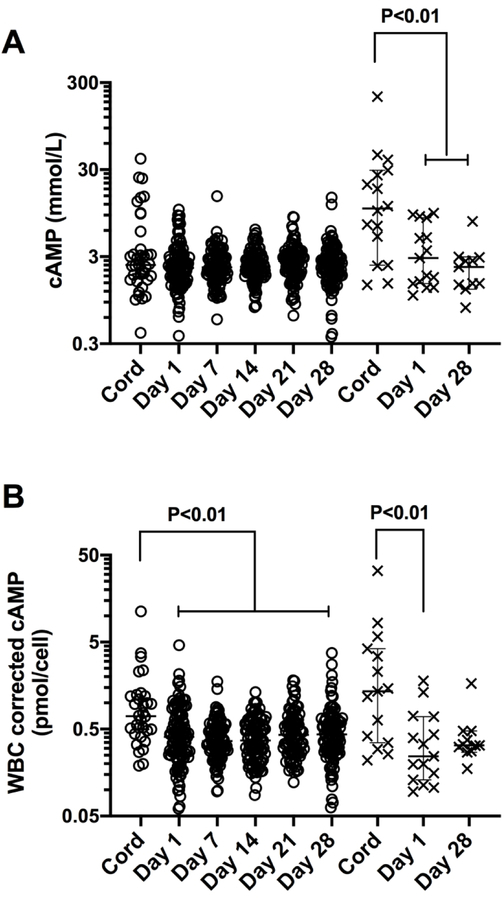
The demographics of the study population are listed in Table 1. cAMP concentrations were significantly higher in cord blood than peripheral blood in term infants but not preterm infants (Figure 1A). The concentration of cAMP in the cord blood of term infants (median 10.7 mmol/L, range 1.4 – 207.3, n=16) was higher than that in preterm infant cord blood (median 2.4 mmol/L, range - 0.4 – 40.05; n=37, P=0.0028), but was not different in peripheral blood on day of life (DOL) 1 (median 2.2 vs 2.8 mmol/L; n=15 and 109, respectively) or DOL28 (median 2.5 vs 2.3 mmol/L; n=11 and 102 respectively).
Figure 1.
cAMP concentrations in whole blood lysates are higher in newborn cord blood (cord) than peripheral blood (Day 1 – 28 of life) after correcting for white cell count. Data show: A) individual cAMP newborn levels and B) individual cell-corrected cAMP levels along with median and 95% CI, n=37–109 for preterm infants (open circles) and 11–16 for term infants (crosses). P values <0.05 shown for mixed effect model with Tukey multiple comparison test.
White cell counts (WCC) in cord blood, and DOL1 peripheral blood, were also higher in term infants compared to preterm infants (6.9×109 cells/L vs 3.4×109 cells/L, n=15 and 32, P<0.0001; and 10×109 cells/l vs 5.5×109 cells/L, n=15 and 109, P=0.0001, for cord and DOL1, respectively). This prompted us to examine the associations between cAMP concentrations and differential WCC (Table 2). cAMP levels in cord blood were positively correlated with the absolute numbers of total white blood cells as well as with the counts for neutrophils, classical monocytes and B cells. cAMP concentrations in peripheral blood showed positive correlations with total white blood cell, neutrophil and inflammatory monocyte counts on DOL1 and DOL7, and with classical monocyte and B cell counts on DOL7. cAMP concentrations on DOL14 were negatively correlated with non-cytotoxic T cell counts.
Table 2.
Spearman rank correlations of cord and peripheral blood (preterm and term) lysate cAMP concentrations versus absolute white cell counts.
| Cell type | Blood sample | Median and range (109 cells/L) | r (95% CI: lower, upper) | p-value (n) |
|---|---|---|---|---|
| WBC | Cord | 4.51 (0.96–16.4) | 0.43 (0.15, 0.64) | 0.001 (47) |
| DOL1 | 6.00 (1.6–47.0) | 0.24 (0.06, 0.40) | 0.004 (124) | |
| DOL7 | 6.57 (1.0–25.0) | 0.45 (0.27, 0.60) | <0.0001 (95) | |
| DOL14 | 6.19 (2.9–26.4) | −0.06 (−0.26, 0.15) | 0.29 (95) | |
| DOL21 | 5.73 (2.1–16.6) | 0.06 (−0.16, 0.26) | 0.30 (89) | |
| DOL28 | 5.86 (1.9–13.1) | 0.03 (−0.16, 0.21) | 0.38 (113) | |
| PMN | Cord | 1.47 (0.006–9.0) | 0.45 (0.18, 0.66) | 0.0007 (47) |
| DOL1 | 2.41 (0.006–15.5) | 0.18 (0.001, 0.35) | 0.02 (124) | |
| DOL7 | 2.15 (0.03–19.8) | 0.41 (0.22, 0.57) | <0.0001 (95) | |
| DOL14 | 2.44 (0.01–19.8) | 0.04 (−0.17, 0.24) | 0.36 (96) | |
| DOL21 | 1.69 (0.01–10.2) | −0.04 (−0.25, 0.18) | 0.37 (89) | |
| DOL28 | 1.46 (0.002–8.6) | 0.03 (−0.16, 0.22) | 0.39 (113) | |
| Classical Monocytes | Cord | 0.24 (0.001–1.2) | 0.3 (0.006, 0.55) | 0.02 (47) |
| DOL1 | 0.05 (0.002–0.38) | 0.08 (−0.10, 0.26) | 0.18 (124) | |
| DOL7 | 0.60 (0.0001–1.6) | 0.30 (0.10, 0.48) | 0.002 (94) | |
| DOL14 | 0.59 (0.0002–1.7) | 0.09 (−0.12, 0.29) | 0.19 (96) | |
| DOL21 | 0.45 (0.0005–1.9) | 0.21 (−0.003, 0.40) | 0.02 (89) | |
| DOL28 | 0.47 (0.0002–0.3) | 0.11 (−0.08, 0.29) | 0.11 (113) | |
| CD16+Monocytes | Cord | 0.04 (0.004–0.2) | 0.14 (−0.16, 0.42) | 0.17 (47) |
| DOL1 | 0.44 (0.0004–26.1) | 0.25 (0.08, 0.41) | 0.002 (124) | |
| DOL7 | 0.06 (0.0009–0.3) | 0.21 (0.003, 0.40) | 0.02 (94) | |
| DOL14 | 0.08 (0.001–0.4) | 0.14 (−0.06, 0.33) | 0.08 (96) | |
| DOL21 | 0.08 (0.008–0.4) | 0.14 (−0.07, 0.34) | 0.10 (89) | |
| DOL28 | 0.08 (0.02–0.3) | 0.12 (−0.07–0.29) | 0.11 (113) | |
| T cells | Cord | 0.88 (0.3–2.9) | 0.21 (−0.08, 0.47) | 0.07 (47) |
| DOL1 | 0.96 (0.17–5.8) | −0.04 (−0.22, 0.14) | 0.34 (124) | |
| DOL7 | 1.37 (0.24–5.2) | 0.15 (−0.06, 0.34) | 0.08 (95) | |
| DOL14 | 1.38 (0.07–6.6) | −0.22 (−0.41, −0.02) | 0.01 (96) | |
| DOL21 | 1.54 (0.2–5.7) | −0.06 (−0.27, 0.15) | 0.28 (89) | |
| DOL28 | 1.64 (0.23–4.6) | 0.03 (−0.16, 0.22) | 0.38 (113) | |
| B cells | Cord | 2.28 (0.005–1.6) | 0.26 (−0.03, 0.51) | 0.04 (47) |
| DOL1 | 2.08 (0.0006–1.1) | 0.05 (−0.13, 0.24) | 0.27 (124) | |
| DOL7 | 0.23 (0.002–1.1) | 0.38 (0.18, 0.54) | <0.0001 (95) | |
| DOL14 | 0.27 (0.002–11.0) | 0.08 (−0.12, 0.28) | 0.22 (96) | |
| DOL21 | 0.30 (0.0005–0.9) | 0.07 (−0.15, 0.27) | 0.27 (89) | |
| DOL28 | 0.34 (0.0006–1.3) | 0.04 (−0.15, 0.23) | 0.34 (113) |
WBC, White blood cells.
Given the positive associations between cAMP concentrations and WCC, we examined WCC corrected cAMP concentrations (pmol/cell) in cord and peripheral blood samples (Figure 1b). The cell corrected concentration of cAMP was still higher in term cord blood than in DOL1 peripheral blood but there was no longer a difference with DOL28 blood. However, the leukocyte-corrected concentration of cAMP in preterm infant cord blood was now higher than peripheral blood throughout the 28 day postnatal period (P<0.01). The cell corrected concentrations of cAMP in cord, DOL1 and DOL28 did not differ between term and preterm infants.
cAMP correlates with TLR2- and TLR4 mediated cytokine production in preterm infants
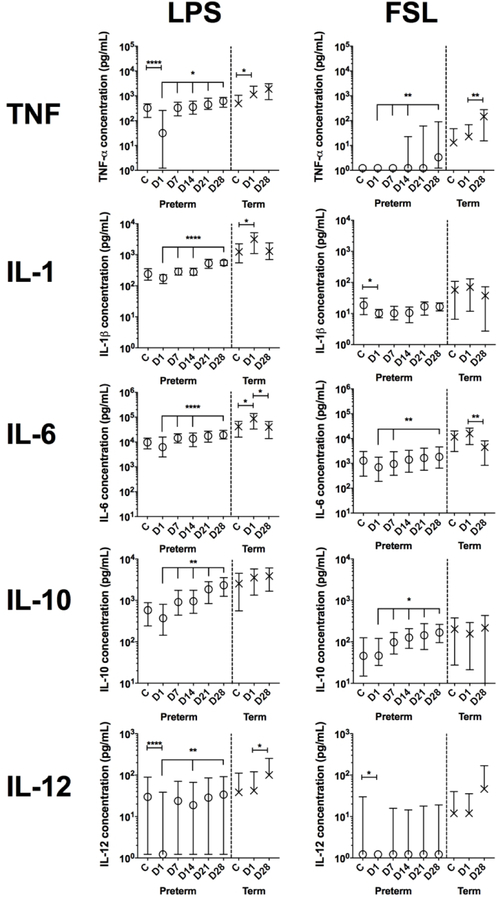
To characterize innate immune responses in early life, cord and peripheral blood was stimulated in vitro with fibroblast-stimulating lipopeptide-1 (FSL-1; TLR2/6 agonist) or lipopolysaccharide (LPS; TLR4 agonist) and cytokines measured in supernatant by multiplex assay (Figure 2). The levels of LPS-induced TNF-α, IL-1β, IL-6 and IL-10 were higher in term infant peripheral blood than the equivalent preterm infant blood samples (P<0.05 for IL-10 at DOL28, P<0.005 for remaining cytokines). Cord blood IL-1β, IL-6 and IL-10 levels were also higher in term infants than preterm infants after LPS stimulation (P<0.001). TNF-α, IL-1β, IL-6 and IL-10 levels were also higher in term infants than preterm infants after FSL stimulation, and this was most marked for IL-6 and IL-10 in cord blood and DOL1 blood (P<0.05), for TNF- on DOL1 and 28 (P<0.05), and for IL-1 on DOL1 (P<0.0001). IL-12p70 levels did not differ between preterm and term infants. LPS consistently induced higher levels of all cytokines than FSL at each time point in both preterm and term infants.
Figure 2.
Cytokine concentrations in preterm and term infant whole-blood from cord blood (cord) or peripheral blood (Day (D) 1 −28 of life) after stimulation with FSL (TLR2/6) or LPS (TLR4). Medians and 95% CI of total cytokine concentrations are depicted. Unstimulated levels of IL-1β, IL12p70 and TNF- were below the limit of detection for nearly all samples (median response = 1.2 pg/mL) while median IL-6 reponses were <17 pg in preterm infants and <42 pg/mL in term infants in the first week of life and then 1.2 pg/mL, thereafter. Median untsimulated IL-10 levels were <6 pg/mL in preterm and term infants in cord and on DOL1 and then 1.2 pg/mL, thereafter. ****, P<0.001; **, P<0.01; *, P<0.05 comparing Day 1 to cord or Day 1 to Days 7–28, using a mixed effect model with Tukey multiple comparison test. N = 56 −121 for preterm infants and 12–19 for term infants.
LPS-stimulated cytokine levels in preterm infants were lower in peripheral blood on DOL1 than in cord blood and then increased over the first month of life (Figure 2). The same pattern was seen for TNF-α, IL-6 after LPS stimulation and for IL-10 after FSL stimulation. IL-12p70 levels were higher in preterm infant cord blood than peripheral blood on DOL1 after LPS and FSL stimulation, as was TNF- after LPS stimulation.
We next assessed if cAMP concentrations in cord and peripheral blood were associated with LPS and FSL-induced cytokine production. Due to the positive correlations of cAMP concentrations with white blood cell counts, we assessed all associations using cytokine and cAMP values after correction of each for leukocyte count. Overall, cell-corrected cAMP levels in preterm infant peripheral blood showed a weak to moderate correlation with cell-corrected cytokine production from DOL1 onwards after FSL stimulation and DOL7 onwards with LPS stimulation (Table 3a/b). Consistent with our previously published studies of term newborns (9), LPS-induced TNF-production in term cord blood was negatively correlated with cAMP whereas DOL1 IL-10 responses were positively correlated. No other term LPS cytokine responses correlated with cAMP and term FSL-induced cytokine reponses did not correlate with cAMP levels.
Table 3a.
Significant spearman rank correlations of cAMP concentrations and LPS-stimulated cytokine concentrations (all WCC-corrected).
| Infant Group | Cytokine | Sample (DOL) | r (95% CI; lower, upper limit) | n | p-value |
|---|---|---|---|---|---|
| Preterm | TNF-α | 7 | 0.35 (0.16 to 0.52) | 96 | 0.0002 |
| 14 | 0.32 (0.12 to 0.49) | 95 | 0.0009 | ||
| 21 | 0.35 (0.15 to 0.53) | 89 | 0.0004 | ||
| 28 | 0.41 (0.23 to 0.56) | 102 | <0.0001 | ||
| IL-1b | 7 | 0.28 (0.08 to 0.46) | 96 | 0.003 | |
| 14 | 0.31 (0.12 to 0.49) | 95 | 0.0009 | ||
| 21 | 0.28 (0.07 to 0.46) | 89 | 0.004 | ||
| 28 | 0.35 (0.16 to 0.52) | 102 | 0.0001 | ||
| IL-6 | 7 | 0.35 (0.16 to 0.52) | 96 | 0.0002 | |
| 14 | 0.39 (0.20 to 0.55) | 95 | <0.0001 | ||
| 21 | 0.39 (0.19 to 0.56) | 89 | <0.0001 | ||
| 28 | 0.43 (0.25 to 0.58) | 102 | <0.0001 | ||
| IL-10 | Cord | 0.38 (0.03 to 0.65) | 32 | 0.02 | |
| 7 | 0.18 (−0.03 to 0.37) | 96 | 0.04 | ||
| 14 | 0.31 (0.11 to 0.49) | 95 | 0.001 | ||
| 21 | 0.32 (0.12 to 0.50) | 89 | 0.001 | ||
| 28 | 0.46 (0.28 to 0.60) | 102 | <0.0001 | ||
| IL-12p70 | 1 | 0.19 (−0.003 to 0.37) | 108 | 0.02 | |
| 7 | 0.19 (−0.01 to 0.38) | 96 | 0.03 | ||
| 14 | 0.21 (0.002 to 0.40) | 95 | 0.02 | ||
| 28 | 0.27 (0.08 to 0.45) | 102 | 0.003 | ||
| Term | TNF-α | Cord | −0.53 (−0.82 to –0.006) | 15 | 0.02 |
| IL-10 | 1 | 0.50 (−0.03 to 0.81) | 15 | 0.03 | |
Table 3b.
Significant spearman rank correlations of cAMP concentrations and FSL-stimulated cytokine concentrations (all WCC-corrected).
| Infant Group | Cytokine | Sample (DOL) | r (95% CI; lower, upper limit) | n | p-value |
|---|---|---|---|---|---|
| Preterm | TNF-α | 1 | 0.43 (0.26 to 0.58) | 108 | <0.0001 |
| 7 | 0.43 (0.24 to 0.58) | 96 | <0.0001 | ||
| 14 | 0.28 (0.076 to 0.46) | 95 | 0.003 | ||
| 21 | 0.28 (0.073 to 0.47) | 89 | 0.004 | ||
| 28 | 0.41 (0.22 to 0.56) | 102 | <0.0001 | ||
| IL-1b | 1 | 0.19 (−0.001 to 0.37) | 108 | 0.02 | |
| 21 | 0.28 (0.07 to 0.46) | 89 | 0.004 | ||
| 28 | 0.20 (−0.004 to 0.38) | 102 | 0.02 | ||
| IL-6 | 14 | 0.19 (−0.02 to 0.38) | 95 | 0.03 | |
| 21 | 0.28 (0.07 to 0.47) | 89 | 0.004 | ||
| 28 | 0.26 (0.06296 to 0.44) | 102 | 0.004 | ||
| IL-10 | 1 | 0.32 (0.14 to 0.49) | 108 | 0.0003 | |
| 7 | 0.20 (−0.004 to 0.39) | 96 | 0.02 | ||
| 14 | 0.28 (0.08 to 0.46) | 95 | 0.003 | ||
| 21 | 0.37 (0.17 to 0.54) | 89 | 0.0002 | ||
| 28 | 0.42 (0.24 to 0.57) | 102 | <0.0001 | ||
| IL-12p70 | 1 | 0.43 (0.26 to 0.58) | 108 | <0.0001 | |
| 7 | 0.36 (0.17 to 0.53) | 96 | 0.0001 | ||
| 14 | 0.24 (0.035 to 0.43) | 95 | 0.009 | ||
| 21 | 0.37 (0.16 to 0.54) | 89 | 0.0002 | ||
| 28 | 0.36 (0.18 to 0.53) | 102 | <0.0001 | ||
cAMP concentrations in preterm infants correlate with sex and chorioamnionitis
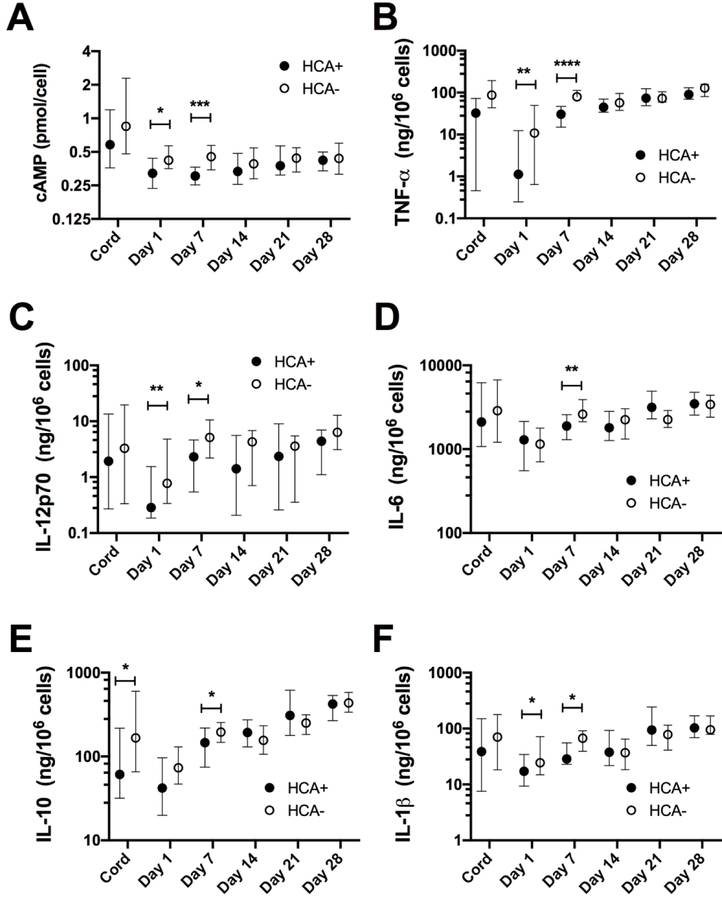
We next examined potential effects of common immunologically relevant clinical parameters on cell-corrected preterm cAMP levels. cAMP concentrations in cord and peripheral blood showed no correlation with GA or birthweight. Further, there was no effect of mode of delivery, multiple birth status or subsequent diagnosis of LOS (P>0.05, data not shown). In contrast, cAMP levels were two-fold higher in female compared to male preterm infant cord blood (median 1.15 vs 0.54 pmol/cell; ranges 0.27–3.73 vs 0.19–11.3 pmol/cell; P=0.04, n =14 and 18 respectively), but these differences were not observed in peripheral blood. cAMP levels were lower in peripheral blood on DOL1 and DOL7 in infants exposed to chorioamnionitis compared to unexposed infants (Figure 3A). In keeping with the positive association between cell-corrected cAMP and cytokine production, LPS-induced cytokine levels were also lower on DOL1 and 7 in infants exposed to chorioamnionitis compared to those who were unexposed (Figure 3B–F). The same pattern was also seen for TNF- and IL-12p70 after FSL stimulation (data not shown).
Figure 3.
White cell count corrected cAMP concentrations and LPS-induced cytokine responses in preterm infant peripheral blood (Day 1 and 7 of life) are associated with exposure to choriamnionitis. Medians and 95% CI are depicted for A) cAMP, B) TNF-α, C) IL-12p70, D) IL-6, E) IL-10 and F) IL-1β. ****, P<0.001; **, P<0.01; *, P<0.05 comparing infants exposed to histiological chorioamnionitis (HCA+) to those unexposed (HCA-) by Mann-Whitney test. n=12–16/group for cord samples and 39–44 per group for peripheral blood samples.
Discussion
To our knowledge, this is the first study to determine total cAMP concentration in human preterm and term infant cord and peripheral blood during the neonatal period and to characterize the association with TLR2 and TLR4-mediated cytokine production. The second messenger cAMP is a key regulator of cellular functions including metabolism, gene expression and immune function (23, 24). Increases in intracellular cAMP generally dampen production of pro-inflammatory/Th1-polarizing cytokines, but in vitro animal and human adult data may not reflect the effects in neonates, due to well recognized age- and species-specific differences (13).
In this unique large longitudinal cohort of preterm and term neonates, we found higher cAMP concentrations in term infant cord blood followed by significantly lower levels in peripheral blood on DOL1 and DOL28. We also observed that cAMP concentrations were positively correlated with WCC, and following adjustment, cAMP concentrations in preterm infant cord blood were also higher than peripheral blood at DOL1–28. This may reflect the dynamics of neonatal immune ontogeny of immunomodulatory molecules that induce cAMP production, including adenosine (8, 9, 15). Exposure to chorioamnionitis was associated with lower cell-corrected cAMP concentrations in peripheral blood at DOL1 and 7 and with lower TLR-induced cytokine concentrations, suggesting that cAMP may play a regulatory role in fetal and early postnatal life (Figure 3). For example, intrauterine inflammation plays a key role in the initiation of preterm delivery and inadequately regulated inflammatory responses are associated with increased risk of common adverse prematurity-related outcomes (25).
Of note, cell-corrected cAMP levels in cord blood were two-fold higher in female than in male neonates. This is in line with emerging evidence that immune function is significantly influenced by sex, but little is known about relevance of sex-specific regulation of neonatal immunity (26, 27). Several sex hormones impact key immune cell functions and the expression of X-linked immune genes contribute to sex-specific immune responses (26, 28). While the mechanisms underlying sex differences in neonatal immunity are largely unknown, our observation of lower cAMP concentrations in male cord blood is consistent with distinct inflammatory responses that could contribute to poorer short- and long-term outcomes in male newborns (29–31).
cAMP concentrations in preterms were positively correlated with peripheral blood TLR2- and TLR4-induced IL-1β, IL-6, IL-12p70, anti-inflammatory IL-10 and TNF- levels. In contrast, in term newborns, cord blood cAMP was negatively correlated with TLR-4-induced TNF- but positively correlated with IL-10, consistent with an inhibitory role of cAMP towards pro-inflammatory/Th1-polarizing cytokines in the term infant (9). This suggests that downstream cAMP signaling responses known to regulate TNF- production in response to LPS, such as protein kinase A activation (32), may be differentialy regulated in preterm infants. Additionally, selective exposure to adenosine receptor modulating substances, such as high-dose caffeine, may alter LPS responses in the peripheral blood of preterm infants (33). Alternatively, such differences between preterm and term infant blood responses could relate to GA-dependent variation in the regulation/maturation of TLR pathways themselves (17).
The positive correlation of IL-6 and IL-10 with cAMP levels suggests that cAMP may have a role in the acute-phase response characteristic of the first days of life. This response mobilizes liver-derived anti-infective proteins to the bloodstream and maintains an anti-inflammatory milieu in the context of rapidly maturing pro-inflammatory capacity with increasing GA (14, 34). The rapid fall in cAMP levels after birth may contribute to regulating the balance of postnatal inflammatory and anti-inflammatory responses to the ubiquitous microbial challenges encountered ex utero. Of note, even with the overall reduction of cAMP concentrations, values at a given DOL varied up to 10-fold, suggesting that in addition to ontogeny, substantial inter-individual differences contribute to concentrations of this signaling metabolite. Differences in the concentrations of cAMP could have potential implications for the effectivness of drugs that influence cAMP concentrations, such as the non-specific phosphodiesterase inhibitor, pentoxifylline whose effects are enhanced in neonates and are dependent on an intact adenosine/cAMP pathway (35, 36).
The strengths of the study include the prospective longitudinal design throughout the first month of life, the sample size and comprehensive analysis of cAMP levels, together with interrogation of immune cell number and capacity for cytokine production in whole blood (where extrinsic factors that may influence cAMP pathways are retained) following stimulation with defined TLR agonists. This study also had some unavoidable limitations; we were unable to determine the predominant source (i.e. intra- vs extracellular, or red blood cell vs white blood cell) of cAMP and its main inducers, due to the limited blood volumes available from preterm infants. We also did not account for maternal factors, such as maternal hypertension which can alter neonatal WCC (37).
In conclusion, cAMP was present in higher concentrations in cord than peripheral blood in preterm and term neonates during the first month of life. Exposure to chorioamnionitis was associated with lower levels of cAMP and TLR-induced cytokine responses. The positive correlation of cAMP levels with TLR-mediated inflammatory and anti-inflammatory cytokine production raises the possibility that cAMP may contribute to shaping early life TLR-mediated cytokine responses. Future studies should further explore the immunomodulatory properties of cAMP pathways in early life, whose distinct functional ontogeny may inform age-specific strategies for treatment or prevention of neonatal infection and inflammation.
Acknowledgments
Statement of financial support:
This project was supported by a National Health & Medical Research Council (NHMRC) of Australia project grant award (572548). SS was supported by a Max-Kade fellowship. DPB is supported by an NHMRC Senior Research Fellowship (1046518). Research at the Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Program. Work in OL’s laboratory was supported by U.S. National Institutes of Health (NIH) National Institutes of Allergy and Infectious Diseases (NIAID) grants 1R01AI100135-01, and U01AI124284-01, as well as Global Health (OPPGH5284) and Grand Challenges Explorations (OPP1035192) awards from the Bill & Melinda Gates Foundation. The Precision Vaccines Program is supported by an internal award from the Boston Children’s Hospital Department of Pediatrics.
Over the past 5 years OL’s lab has received sponsored research support from MedImmune, Crucell (Johnson & Johnson) and reagent support from the Infectious Diseases Research Institute.
The remaining authors have no disclosures to declare.
Footnotes
Disclosure statement:
The authours have no conflicts of interest to declare.
Category of study:
Basic Science
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. 2010. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. PEDIATRICS 126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy O 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature reviews. Immunology 7:379–390. [DOI] [PubMed] [Google Scholar]
- 3.Strunk T, Inder T, Wang X, et al. 2014. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 14:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hibbert JE, Currie A, Strunk T 2018. Sepsis-Induced Immunosuppression in Neonates. Front Pediatr 6:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheid A, Borriello F, Pietrasanta C, et al. 2018. Adjuvant Effect of Bacille Calmette-Guerin on Hepatitis B Vaccine Immunogenicity in the Preterm and Term Newborn. Front Immunol 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philbin VJ, Dowling DJ, Gallington LC, et al. 2012. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 130:195–204 e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Haren SD, Dowling DJ, Foppen W, et al. 2016. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. J Immunol 197:4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belderbos ME, Levy O, Meyaard L, et al. 2013. Plasma-mediated immune suppression: a neonatal perspective. Pediatric Allergy and Immunology 24:102–113. [DOI] [PubMed] [Google Scholar]
- 9.Levy O, Coughlin M, Cronstein BN, et al. 2006. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. Journal of immunology (Baltimore, Md. : 1950) 177:1956–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettengill M, Robson S, Tresenriter M, et al. 2013. Soluble ecto-5’-nucleotidase (5’NT), alkaline phosphatase, and adenosine deaminase (ADA1) activities in neonatal blood favor elevated extracellular adenosine. J Biol Chem. [DOI] [PMC free article] [PubMed]
- 11.Kamenetsky M, Middelhaufe S, Bank EM, et al. 2006. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol 362:623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunahara RK, Taussig R 2002. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2:168–184. [DOI] [PubMed] [Google Scholar]
- 13.Serezani CH, Ballinger MN, Aronoff DM, et al. 2008. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy O 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 7:379–390. [DOI] [PubMed] [Google Scholar]
- 15.Dowling DJ, Levy O 2014. Ontogeny of early life immunity. Trends in Immunology 35:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platzer C, Fritsch E, Elsner T, et al. 1999. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur J Immunol 29:3098–3104. [DOI] [PubMed] [Google Scholar]
- 17.de Jong E, Strunk T, Burgner D, et al. 2017. The phenotype and function of preterm infant monocytes: implications for susceptibility to infection. J Leukoc Biol 102:645–656. [DOI] [PubMed] [Google Scholar]
- 18.Dong Y, Speer CP 2015. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed 100:F257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redline RW, Faye-Petersen O, Heller D, et al. 2003. Amniotic Infection Syndrome: Nosology and Reproducibility of Placental Reaction Patterns. Pediatr Dev Pathol 6:435–448. [DOI] [PubMed] [Google Scholar]
- 20.Strunk T, Doherty D, Jacques A, et al. 2012. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics 129:e134–141. [DOI] [PubMed] [Google Scholar]
- 21.de Jager W, te Velthuis H, Prakken BJ, et al. 2003. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol 10:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faucher JL, Lacronique-Gazaille C, Frebet E, et al. 2007. “6 markers/5 colors” extended white blood cell differential by flow cytometry. Cytometry A 71:934–944. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt B, Roberts RS, Davis P, et al. 2007. Long-Term Effects of Caffeine Therapy for Apnea of Prematurity. New England Journal of Medicine 357:1893–1902. [DOI] [PubMed] [Google Scholar]
- 24.Hofer AM, Lefkimmiatis K 2007. Extracellular calcium and cAMP: second messengers as “third messengers”? Physiology (Bethesda) 22:320–327. [DOI] [PubMed] [Google Scholar]
- 25.Kemp MW 2014. Preterm Birth, Intrauterine Infection, and Fetal Inflammation. Frontiers in Immunology 5:574–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fish EN 2008. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein SL, Marriott I, Fish EN 2015. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 109:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasarhelyi B, Tulassay T 2017. Endocrine Factors Determining Immune Polarization during Perinatal Transition. Klin Padiatr 229:261–266. [DOI] [PubMed] [Google Scholar]
- 29.O’Driscoll DN, Greene CM, Molloy EJ 2017. Immune function? A missing link in the gender disparity in preterm neonatal outcomes. Expert Rev Clin Immunol 13:1061–1071. [DOI] [PubMed] [Google Scholar]
- 30.Carter BM, Holditch-Davis D 2008. Risk factors for necrotizing enterocolitis in preterm infants: how race, gender, and health status contribute. Adv Neonatal Care 8:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace ME, Mendola P, Kim SS, et al. 2017. Racial/ethnic differences in preterm perinatal outcomes. Am J Obstet Gynecol 216:306.e301–306.e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall EA, Zavzavadjian JR, Chang MS, et al. 2009. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal 2:ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavez-Valdez R, Ahlawat R, Wills-Karp M, et al. 2016. Mechanisms of modulation of cytokine release by human cord blood monocytes exposed to high concentrations of caffeine. Pediatr Res 80:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strunk T, Prosser A, Levy O, et al. 2012. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediat Res 72:10–18. [DOI] [PubMed] [Google Scholar]
- 35.Speer EM, Dowling DJ, Ozog LS, et al. 2017. Pentoxifylline inhibits TLR- and inflammasome-mediated in vitro inflammatory cytokine production in human blood with greater efficacy and potency in newborns. Pediatr Res 81:806–816. [DOI] [PubMed] [Google Scholar]
- 36.Konrad FM, Neudeck G, Vollmer I, et al. 2013. Protective effects of pentoxifylline in pulmonary inflammation are adenosine receptor A2A dependent. FASEB J 27:3524–3535. [DOI] [PubMed] [Google Scholar]
- 37.Bolat A, Gursel O, Kurekci E, et al. 2013. Blood parameters changes in cord blood of newborns of hypertensive mothers. Eur J Pediatr 172:1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]